Abstract
Rationale: The ARDS Network (ARDSNet) used a positive end-expiratory pressure (PEEP)/FiO2 model in many studies. In general, pediatric intensivists use less PEEP and higher FiO2 than this model.
Objectives: To evaluate whether children managed with PEEP lower than recommended by the ARDSNet PEEP/FiO2 model had higher mortality.
Methods: This was a multicenter, retrospective analysis of patients with pediatric acute respiratory distress syndrome (PARDS) managed without a formal PEEP/FiO2 protocol. Four distinct datasets were combined for analysis. We extracted time-matched PEEP/FiO2 values, calculating the difference between PEEP level and the ARDSNet-recommended PEEP level for a given FiO2. We analyzed the median difference over the first 24 hours of PARDS diagnosis against ICU mortality and adjusted for confounding variables, effect modifiers, or factors that may have affected the propensity to use lower PEEP.
Measurements and Main Results: Of the 1,134 patients with PARDS, 26.6% were managed with lower PEEP relative to the amount of FiO2 recommended by the ARDSNet protocol. Patients managed with lower PEEP experienced higher mortality than those who were managed with PEEP levels in line with or higher than recommended by the protocol (P < 0.001). After adjustment for hypoxemia, inotropes, comorbidities, severity of illness, ventilator settings, nitric oxide, and dataset, PEEP lower than recommended by the protocol remained independently associated with higher mortality (odds ratio, 2.05; 95% confidence interval, 1.32–3.17). Findings were similar after propensity-based covariate adjustment (odds ratio, 2.00; 95% confidence interval, 1.24–3.22).
Conclusions: Patients with PARDS managed with lower PEEP relative to FiO2 than recommended by the ARDSNet model had higher mortality. Clinical trials targeting PEEP management in PARDS are needed.
Keywords: acute respiratory distress syndrome, acute lung injury, positive end expiratory pressure, ARDS Network, pediatrics
At a Glance Commentary
Scientific Knowledge on the Subject
Optimal methods to titrate positive end-expiratory pressure (PEEP) remain an active area of controversy in the management of patients with acute respiratory distress syndrome (ARDS). Although a PEEP/FiO2 protocol inspired by the ARDS Network has been used in a variety of clinical trials, many intensive care practitioners use less PEEP and higher FiO2 than this protocol, particularly in pediatric ARDS.
What This Study Adds to the Field
Through secondary analysis of four previously published datasets containing more than 1,000 patients with pediatric ARDS managed without a formal PEEP/FiO2 protocol, we found that patients managed with lower PEEP than would be recommended by the PEEP/FiO2 protocol for a given FiO2 experienced higher mortality than those who were managed either per protocol or with higher relative PEEP. These findings held in stratified analyses and after adjustment for confounding variables, effect modifiers, and propensity-based covariate adjustment. Clinical trials are needed.
Although adequate positive end-expiratory pressure (PEEP) is essential to prevent atelectrauma in patients with acute respiratory distress syndrome (ARDS), observational data in both adults and children highlight that many patients with ARDS are on PEEP lower than recommended (1–3). Although PEEP titration methods are controversial, previous ARDS studies have used a PEEP/FiO2 titration table popularized by the ARDS Network (ARDSNet) (3–5). This table recommends combinations of PEEP and FiO2, such that both are escalated or deescalated in tandem as hypoxemia worsens or improves. Although other approaches may have advantages, degree of hypoxemia is an important consideration in PEEP management (4, 6, 7).
There is substantial variability in PEEP/FiO2 combinations chosen during usual care ventilation, with a proclivity toward increasing FiO2 over PEEP for hypoxemia (2, 8–11). Hence, although PEEP/FiO2 tables are standard in clinical trials, clinical practice appears quite discrepant. This may be because there are limited data directly evaluating whether patients managed congruent with these PEEP/FiO2 combinations have better outcomes than those managed with different combinations.
This issue becomes even more important in pediatrics, as there are virtually no studies examining the relationship between PEEP and mortality in ARDS, although several confirm the PEEP/FiO2 table recommendations from ARDSNet are rarely followed in children (8, 9, 11–14). In general, there is reluctance to escalate PEEP above 10 cm H2O, particularly for younger children (8, 11). Through secondary analysis of aggregated data from four previously published studies of children with pediatric ARDS (PARDS) (11, 15–18), we sought to determine if patients managed with PEEP levels lower than recommended by the ARDSNet table for a given FiO2 in actual practice had higher mortality than patients who were managed with PEEP levels consistent with or higher than recommended by the ARDSNet table.
Methods
We combined four previously published datasets of invasively mechanically ventilated children with PARDS (15–18). Two of the datasets were gathered retrospectively by reviewing the electronic health records of pediatric ICU (PICU) patients admitted to Children’s Hospital Los Angeles (CHLA) from 2000 to 2007 (15) and 2009 to 2013 (16). One dataset was gathered prospectively from patients admitted to the Children’s Hospital of Philadelphia (CHOP) from 2011 to 2016 (17, 18). The fourth dataset was gathered prospectively across eight hospitals in the Collaborative Pediatric Critical Care Research Network (CPCCRN) from 2011 to 2012 (11). We excluded CHOP and CHLA patients from the CPCCRN dataset to prevent any potential patient overlap. The parent studies were approved by their respective institutional review boards, and anonymous data were aggregated for this study.
PARDS Eligibility
Although inclusion criteria for each of the four studies differed slightly, we included patients who met the Pediatric Acute Lung Injury Consensus Conference definition of PARDS while on invasive mechanical ventilation (1). Hypoxemia for the PARDS definition was based on oxygenation index (OI) greater than or equal to 4 or oxygen saturation index greater than or equal to 5. The CHLA dataset from 2000 to 2007 and the CHOP dataset included patients only on the basis of OI, whereas the CHLA dataset from 2009 to 2013 and the CPCCRN dataset included patients using oxygen saturation index when OI was not available. All patients had a PARDS trigger (i.e., pneumonia, sepsis), with pulmonary parenchymal disease on chest radiograph. Patients whose respiratory failure was believed to be primarily due to cardiac disease were excluded. Although required to meet Pediatric Acute Lung Injury Consensus Conference criteria for inclusion, because our question of interest focused on PEEP (which affects mean airway pressure in the OI calculation), we chose to group hypoxemia severity on the basis of the Berlin definition (PaO2/FiO2 [PF] ratio), imputing PF ratio from the ratio of oxygen saturation as measured by pulse oximetry to FiO2 using previously published equations (19) when an arterial blood gas measurement was not available.
PEEP/FiO2 Scoring
We extracted time-matched PEEP and FiO2 values every 6 hours over the first 24 hours after diagnosis. FiO2 measurements were categorized into intervals of 0.05, ranging from 0.21 to 1.0. For each FiO2/PEEP pair, we calculated how far the set PEEP for a given FiO2 was from the “low PEEP” ARDSNet protocol–recommended PEEP. For example, for a patient on 0.6 FiO2, the ARDSNet PEEP/FiO2 table recommends a PEEP of 10 cm H2O. If the patient was actually on a PEEP of 5 cm H2O, a PEEP discordance score of −5 would be assigned. For FiO2 values in which multiple PEEP levels are permitted by ARDSNet, we used the level closest to the clinical PEEP (i.e., clinical PEEP = 6 cm H2O, ARDSNet PEEP allowed 8–10 cm H2O, PEEP discordance score = 6 − 8 = −2 cm H2O). We subsequently took the median score over the first 24 hours of PARDS diagnosis (using the initial time point plus four 6-hr blocks [when available]) for analysis. A composite variable deemed “low PEEP” was categorized for patients with PEEP below protocol for a given FiO2 and was used for multivariable analysis. None of the hospitals had a formal protocol for PEEP/FiO2 management, although CHOP used a strategy in which an inability to wean FiO2 below 0.60 after intubation warranted escalation of PEEP to 8 to 10 cm H2O.
Additional Variables
From each dataset (when available), we extracted demographic data, diagnoses, ventilator settings, use of nitric oxide, comorbidities, use of inotrope/vasopressor medications within 24 hours of PARDS diagnosis, and outcomes. These additional variables were homogenized among datasets to ensure similar case definitions through discussion among the principal investigators of each study and on the basis of case report forms. Many of these supplementary data were not available from the CPCCRN dataset, so these patients were excluded from multivariable analyses. PICU mortality was the primary outcome.
Outcome Measures and Analysis
The primary objective was to evaluate whether patients managed with lower PEEP for a given FiO2 than recommended by the ARDSNet table in actual practice had higher mortality than patients managed with PEEP in line with or higher than what would be recommended from ARDSNet. We considered this combination variable because on a univariate basis, higher PEEP and higher FiO2 are associated with mortality (they are both increased in the setting of hypoxemia). This combination variable allowed us to examine the potential impact of ventilator strategies that prioritize one value over the other to achieve an oxygenation target, because these variables are changed to a different extent by providers in similar patient states. Secondary objectives were to determine whether this relationship was consistent among all datasets and whether it was modified by variables that may affect PEEP choice, such as hypoxemia (i.e., PF ratio), demographics and diagnoses, nitric oxide, or other factors.
We report PICU mortality as a function of PEEP discordance relative to the ARDSNet table. We next explored factors that may be associated with PICU mortality or PEEP lower than recommended by the protocol for a given FiO2. Continuous variables were analyzed against mortality with a Mann-Whitney U test, as data were often not normally distributed. Categorical data were analyzed with a chi-square test with Yates correction. ICU mortality was also examined as a function of PEEP lower than the protocol, with survival analysis, stratified by initial PF ratio. We then constructed a multivariate logistic regression model retaining variables that were either associated with PICU mortality or resulted in a greater than 15% change in the parameter estimates for PEEP lower than the protocol, also considering multiplicative interaction terms (retaining those with P < 0.1). Given the high correlation among many ventilator and hypoxemia variables, for multivariable models we used PF ratio (imputed from oxygen saturation as measured by pulse oximetry to FiO2 ratio when PaO2 not available) instead of OI because of the overlap of mean airway pressure with PEEP and other ventilator settings. We selected other variables, which were highly correlated with one another on the basis of a correlation matrix (i.e., driving pressure and peak inspiratory pressure), retaining the variable with the highest univariate association with the outcome, to avoid issues of colinearity. Finally, a propensity score was created to model clinical and severity-of-illness factors associated with PEEP lower than the protocol, considering all variables with a univariate relationship (P < 0.2), retaining variables that maintained independent relationships with low PEEP (P < 0.1). This propensity score was used as covariate adjustment when further analyzing the independent relationship between PEEP lower than the protocol and PICU mortality. Analysis was performed using Microsoft Excel (Microsoft Corporation), Statistica version 12 (StatSoft), and Stata version 10 (StataCorp).
Sensitivity Analysis
We performed a sensitivity analysis stratifying by those with persistent hypoxemia (PF ≤ 200) at 24 hours. In addition, because of notable differences between CHOP and CHLA datasets, we performed subgroup analyses within datasets and did comparative analyses between datasets. For the CHLA patients only, we examined whether the relationship was similar on Day 2 and 3 of mechanical ventilation. Additional subgroup analyses can be found in the online supplement.
Results
Description of Cohort
From the four datasets, 1,134 patients were included (358 CHLA 2000–2007, 254 CHLA 2009–2013, 453 CHOP, and 69 CPCCRN). Overall mortality was 18.6%. Using Berlin classifications, 44 had PF greater than 300 (3.9%; 15.9% mortality), 264 had PF 200 to 300 (23.3%; 13.3% mortality), 500 had PF 100 to 200 (44.1%; 14.6% mortality), and 326 had PF less than or equal to 100 (28.8%; 29.5% mortality). Patient characteristics of the aggregate cohort stratified by mortality are summarized in Table 1. The mode of conventional ventilation was pressure control in 63% of patients (>97% of CHLA patients), and pressure-regulated volume control in 36.7% of patients (93% of CHOP patients).
Table 1.
Characteristics and Outcomes of Study Cohort, Stratified by Pediatric ICU Mortality
| Variable | Overall (N = 1,134) | Survivors (n = 923) | Nonsurvivors (n = 211) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mo | 46.6 (13.2–137.6) | 44.4 (12.6–128.4) | 75 (16.2–164.6) | 0.01 |
| Sex, male | 621 (54.8) | 503 (54.5) | 118 (55.9) | 0.70 |
| Race (n = 1,065) | 0.16 | |||
| White | 305 (28.6) | 245 (28.4) | 60 (29.7) | |
| Hispanic | 332 (31.2) | 267 (30.9) | 65 (32.2) | |
| Black | 199 (18.7) | 172 (19.9) | 27 (13.4) | |
| Other race | 229 (21.5) | 179 (20.7) | 50 (24.8) | |
| ARDS trigger (n = 1,065) | ||||
| Pneumonia | 660 (58.2) | 549 (59.4) | 111 (52.6) | 0.07 |
| Sepsis | 323 (28.5) | 242 (26.2) | 81 (38.4) | <0.001 |
| Drowning | 20 (1.9) | 16 (1.9) | 4 (2.0) | 0.9 |
| Aspiration | 82 (7.7) | 65 (7.5) | 17 (8.4) | 0.67 |
| Trauma | 72 (6.8) | 53 (6.1) | 19 (9.4) | 0.09 |
| Dataset | 0.005 | |||
| CHLA 2000–2007 | 358 (31.6) | 281 (78.5) | 77 (21.5) | |
| CHLA 2009–2013 | 254 (22.4) | 194 (76.4) | 60 (23.6) | |
| CHOP | 453 (40.0) | 388 (85.6) | 65 (14.4) | |
| CPCCRN | 69 (6.1) | 60 (87.0) | 9 (13.0) | |
| Comorbidities (n = 1,065) | ||||
| Immunodeficiency | 248 (23.3) | 162 (18.7) | 86 (42.6) | <0.001 |
| Cancer | 161 (15.1) | 107 (12.4) | 54 (26.7) | <0.001 |
| Stem cell transplant | 64 (6.0) | 27 (3.1) | 37 (18.3) | <0.001 |
| Solid organ transplant | 41 (3.8) | 38 (4.4) | 3 (1.5) | 0.05 |
| Neurologic disease | 260 (24.4) | 219 (25.4) | 41 (20.3) | 0.13 |
| Data at PARDS diagnosis | ||||
| ARDS severity | <0.001 | |||
| PF > 300 | 44 (3.9) | 37 (4) | 7 (3.3) | |
| PF 200–300 | 264 (23.3) | 229 (24.8) | 35 (16.6) | |
| PF 100–200 | 500 (44.1) | 427 (46.2) | 73 (34.6) | |
| PF ≤ 100 | 326 (28.8) | 230 (24.9) | 96 (45.5) | |
| PRISM III raw score | 11 (6–17) | 9 (5–15) | 17 (11–27) | <0.001 |
| PF ratio | 147 (91–207) | 153 (101–212) | 114 (65–180) | <0.001 |
| OI | 10.0 (6.3–17.7) | 9.3 (6.0–15.6) | 15.2 (7.4–25.8) | <0.001 |
| FiO2 | 0.60 (0.44–0.90) | 0.55 (0.40–0.80) | 0.78 (0.50–1.00) | <0.001 |
| PEEP, cm H2O | 8.0 (6.0–10.0) | 8.0 (6.0–10.0) | 8.5 (6.0–10.0) | 0.03 |
| Data over first 24 h (n = 1,005) | ||||
| ARDS severity | <0.001 | |||
| PF > 300 | 115 (10.8) | 189 (10.3) | 26 (13.2) | |
| PF 200–300 | 408 (38.5) | 362 (41.9) | 46 (23.4) | |
| PF 100–200 | 420 (39.6) | 345 (39.9) | 75 (38.1) | |
| PF ≤ 100 | 118 (11.2) | 68 (7.9) | 50 (25.4) | |
| PaO2, mm Hg (n = 938) | 89 (76–108) | 89 (77–106) | 91 (71–114) | 0.94 |
| Day 1 average PaO2 range | n = 938 | n = 750 | n = 188 | <0.001 |
| PaO2 ≤ 60 mm Hg | 53 (5.7) | 30 (4) | 23 (12.2) | |
| PaO2 60–80 mm Hg | 253 (27) | 207 (27.6) | 46 (24.5) | |
| PaO2 80–100 mm Hg | 314 (33.5) | 269 (35.9) | 45 (23.9) | |
| PaO2 > 100 mm Hg | 318 (33.9) | 244 (32.5) | 74 (39.4) | |
| PF ratio | 198 (141–253) | 205 (148–253) | 170 (99–242) | <0.001 |
| OI | 7.6 (5.3–13.0) | 7.2 (5.2–11.4) | 11.2 (6.2–22.8) | <0.001 |
| FiO2 | 0.50 (0.40–0.60) | 0.46 (0.40–0.57) | 0.60 (0.46–0.80) | <0.001 |
| PEEP, cm H2O | 8.8 (6.7–10.8) | 8.4 (6.6–10.3) | 10.0 (7.5–12.0) | <0.001 |
| Vt, ml/kg | 7.5 (6.5–8.6) | 7.5 (6.5–8.6) | 7.4 (6.1–8.5) | 0.31 |
| PIP, cm H2O | 28 (24.5–33) | 28 (24–32) | 31 (26.7–36) | <0.001 |
| Driving pressure, cm H2O | 19.5 (16–23) | 19 (16–22.6) | 20.5 (18–24.5) | <0.001 |
| Inotropes | 630 (59.2) | 474 (54.9) | 156 (77.2) | <0.001 |
| Nitric oxide | 223 (21) | 159 (18.4) | 64 (31.6) | <0.001 |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; CHLA = Children’s Hospital Los Angeles; CHOP = Children’s Hospital of Philadelphia; CPCCRN = Collaborative Pediatric Critical Care Research Network; OI = oxygenation index; OSI = oxygen saturation index; PARDS = pediatric acute respiratory distress syndrome; PEEP = positive end-expiratory pressure; PF = PaO2/FiO2; PIP = peak inspiratory pressure; PRISM = Pediatric Risk of Mortality.
Data are presented as median (interquartile range) or n (%). When a PaO2 metric was not available, PF was calculated from the ratio of oxygen saturation measured by pulse oximetry to FiO2, and OI was calculated from OSI using previously published formulae (19). Race, ARDS triggers, and comorbidities were not available in the CPCCRN data, so the total number is reduced to 1,065. Some patients did not have available data 24 hours after PARDS diagnosis (died, extubated, or no PF ratio, OI, or OSI available), so the number is reduced to 1,005. Driving pressure was calculated as PIP minus PEEP, as most patients were on pressure-regulated modes of ventilation, and inspiratory holds were not routinely performed. The percentages refer to the percentage of overall patients, survivors, or nonsurvivors with a given variable. P values compare the difference between survivors and nonsurvivors, among variables, or among groupings of variables (i.e., P < 0.001 for PARDS severity implies difference in PARDS severity categories between survivors and nonsurvivors, without post hoc comparison of which groups are different).
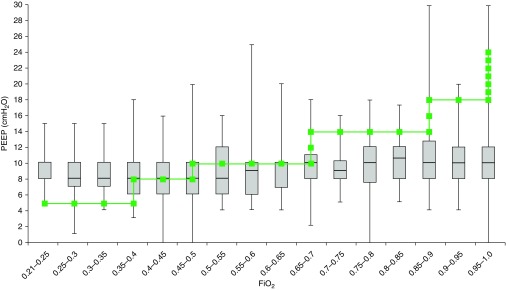
There was variability in PEEP/FiO2 combinations chosen in actual practice (Figure 1). In general, FiO2 was prioritized over PEEP as hypoxemia worsened. Overall, 302 (26.6%) patients were managed with PEEP lower than ARDSNet protocol recommendations, 351 (31.0%) were managed per protocol, 394 (34.7%) were managed with PEEP higher than protocol, and 87 (7.7%) did not have adequate PEEP data for Day 1 of ARDS (5.7% of CHOP, 14.5% of CHLA 2000–2007, 3.1% of CHLA 2009–2013, 1.4% CPCCRN).
Figure 1.
All pediatric acute respiratory distress syndrome (PARDS) positive end-expiratory pressure (PEEP)/FiO2 combinations. Actual PEEP values as a function of actual FiO2 levels (median [bar] and interquartile range [box]) for all patients with PARDS for the first day of mechanical ventilation after PARDS diagnosis. The superimposed line represents the ARDS Network protocol target combinations of PEEP/FiO2. In general, clinicians used more PEEP than recommended when FiO2 was less than 0.4 and used less PEEP than recommended when FiO2 was more than 0.5. Median PEEP level did not exceed 10 cm H2O, regardless of FiO2.
PEEP Discordance
On unadjusted analysis, there was a dose-dependent trend that patients managed with PEEP less than recommended by the protocol for a given FiO2 for the first 24 hours of PARDS diagnosis had higher mortality, with the highest survival appearing to be with PEEP levels 1 to 4 cm H2O above what would be recommended by the protocol (Figure 2A). When stratifying by initial PF ratio, this pattern was consistent, with the largest effect seen in those with PF ratio between 100 and 200 (Figures 2B–2D). Overall, patients managed with PEEP lower than recommended by the protocol experienced higher mortality (26.5% vs. 14.9%, P < 0.001). Although this trend was consistent among all initial PF ratio groups, it was only statistically significant in those with PF ratio between 100 and 200 (PF < 100, 31% vs. 26%, P = 0.3; PF 100–200, 22.6% vs. 11.8%, P = 0.004; PF 200–300, 17.1% vs. 12.3%, P = 0.4; Figures 3A–3D).
Figure 2.
Unadjusted pediatric acute respiratory distress syndrome (PARDS) mortality as a function of positive end-expiratory pressure (PEEP) discordance from the ARDS Network (ARDSNet) PEEP/FiO2 protocol for all patients, and stratified by initial PaO2/FiO2 (PF) ratio group. Total N = 1,134 with 18.6% mortality. Total number of patients with PARDS in each PEEP range is represented by the bars, and ICU mortality (with SE) is represented by the squares. (A) All patients with PARDS. There was approximately an even split between patients managed with PEEP below protocol, per protocol, and above protocol. PEEP lower than recommended by the ARDSNet protocol for a given FiO2 was associated with higher mortality. The lowest mortality occurred when PEEP was 1 to 4 cm H2O above protocol. (B) PF 200 to 300; (C) PF 100 to 200; (D) PF less than or equal to 100. The general trend that mortality is higher for those with PEEP lower than protocol is consistent across all initial PF subgroups. As initial PF ratio worsens, the number of patients managed with PEEP lower than protocol increases.
Figure 3.
Survival curves for patients on positive end-expiratory pressure (PEEP) lower than recommended by the protocol versus PEEP in line or higher than recommended by the protocol for a given FiO2, stratified by initial PaO2/FiO2 (PF) ratio. (A) All patients; (B) PF 200 to 300; (C) PF 100 to 200; (D) PF less than or equal to 100. For all patients, use of PEEP lower than recommended by the protocol for a given FiO2 was associated with higher mortality (P < 0.0001). When subgrouping by initial PF ratio, the trends were consistent, but only statistically significant for PF between 100 and 200 (PF < 100, P = 0.3; PF 100–200, P = 0.004; PF 200–300, P = 0.4). PARDS = pediatric acute respiratory distress syndrome.
Because higher FiO2 relative to PEEP may be a reflection of disease severity or patient-specific factors, we sought to identify variables that may be associated with use of lower PEEP. For this analysis, we restricted the cohort to the 1,047 patients with complete PEEP and FiO2 values. Variables found to be associated with PEEP use lower than recommended by the protocol for a given FiO2 (all P < 0.05) included Hispanic race, dataset, lower initial and 24-hour PF ratio and worse PF group, higher OI, lower use of inotropes, sepsis, higher Pediatric Risk of Mortality III score, and higher ventilator driving pressure (peak inspiratory pressure − PEEP). Age and Vt were not associated with PEEP lower than the protocol (P > 0.2). PF ratio group improved 24 hours after PARDS diagnosis in 38% of patients, although this did not appear to be related to PEEP use in relation to the protocol (Table 2).
Table 2.
Variables Associated with the Propensity to Use Lower Positive End-Expiratory Pressure Relative to the ARDS Network Model
| Variable | Overall (N = 1,047) | High PEEP/On-Protocol PEEP (n = 745) | Low PEEP (n = 302) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mo | 46.8 (13.2–141.0) | 49.2 (13.2–132.4) | 42.0 (11.9–154.0) | 0.99 |
| Sex, male | 574 (54.8) | 404 (54.2) | 170 (56.3) | 0.54 |
| Race (n = 979) | 0.002 | |||
| White | 284 (29.0) | 223 (31.7) | 61 (22.2) | |
| Hispanic | 299 (30.5) | 193 (27.4) | 106 (38.6) | |
| Black | 182 (18.6) | 135 (19.2) | 47 (17.1) | |
| Other race | 214 (21.9) | 153 (21.7) | 61 (22.2) | |
| ARDS trigger (n = 979) | ||||
| Pneumonia | 611 (58.4) | 443 (59.5) | 168 (55.6) | 0.25 |
| Sepsis | 303 (28.9) | 202 (27.1) | 101(33.4) | 0.04 |
| Drowning | 19 (1.9) | 14 (2.0) | 5 (1.8) | 0.86 |
| Aspiration | 70 (7.1) | 55 (7.8) | 15 (5.4) | 0.20 |
| Trauma | 62 (6.4) | 43 (6.1) | 20 (7.3) | 0.5 |
| Dataset | <0.001 | |||
| CHLA 2000–2007 | 306 (29.3) | 162 (52.9) | 144 (47.1) | |
| CHLA 2009–2013 | 246 (23.5) | 168 (68.3) | 78 (31.7) | |
| CHOP | 427 (40.8) | 374 (87.6) | 53 (12.4) | |
| CPCCRN | 68 (6.5) | 41 (60.3) | 27 (39.7) | |
| Comorbidities (n = 979) | ||||
| Immunodeficiency | 230 (23.5) | 170 (24.1) | 60 (21.9) | 0.44 |
| Cancer | 150 (15.3) | 115 (16.3) | 35 (12.7) | 0.15 |
| Stem cell transplant | 57 (5.8) | 45 (6.4) | 12 (4.3) | 0.22 |
| Solid organ transplant | 40 (4.1) | 31 (4.4) | 9 (3.3) | 0.42 |
| Neurologic disease | 237 (24.2) | 164 (23.3) | 73 (26.6) | 0.29 |
| Data at PARDS diagnosis | ||||
| PARDS severity | <0.001 | |||
| PF > 300 | 32 (2) | 24 (3.2) | 8 (2.7) | |
| PF 200–300 | 252 (24.1) | 211 (28.3) | 41 (13.6) | |
| PF 100–200 | 472 (45.1) | 357 (47.9) | 115 (38.15) | |
| P ≤ 100 | 291 (27.8) | 153 (20.5) | 138 (45.7) | |
| PRISM III raw score | 11 (6–17) | 11 (5–17) | 11 (6–19) | 0.05 |
| PF ratio | 147 (91–207) | 156 (109–217) | 109 (66–162) | <0.001 |
| OI | 10 (6.3–17.7) | 9.1 (6.0–14.3) | 12.2 (6.7–24.2) | <0.001 |
| FiO2 | 0.60 (0.44–0.90) | 0.50 (0.40–0.75) | 0.79 (0.50–1.00) | <0.001 |
| PEEP, cm H2O | 8.0 (6.0–10.0) | 8.0 (6.6–10.0) | 6.0 (5.0–10.0) | <0.001 |
| Data over first 24 h (n = 974) | ||||
| PARDS severity | <0.001 | |||
| PF > 300 | 113 (11) | 92 (12.5) | 21 (7.2) | |
| PF 200–300 | 400 (39) | 341 (46.5) | 59 (20.2) | |
| PF 100–200 | 405 (39.5) | 264 (40) | 141 (48.3) | |
| PF ≤ 100 | 108 (10.5) | 37 (5.1) | 71 (24.3) | |
| PF category improved | 387 (37.7) | 281 (38.2) | 106 (36.6) | 0.8 |
| PaO2, mm Hg (n = 862) | 89 (76–108) | 89 (78–106) | 89 (73–111) | 0.49 |
| Day 1 average PaO2 range | n = 862 | n = 624 | n = 238 | <0.001 |
| PaO2 ≤ 60 mm Hg | 40 (4.6) | 19 (3) | 21 (8.8) | |
| PaO2 60–80 mm Hg | 240 (27.8) | 167 (26.8) | 73 (30.7) | |
| PaO2 80–100 mm Hg | 300 (34.8) | 246 (39.4) | 54 (22.7) | |
| PaO2 > 100 mm Hg | 282 (32.7) | 192 (30.8) | 90 (37.8) | |
| OI | 7.6 (5.3–13.0) | 7.0 (5.2– 10.5) | 10.0 (5.4–19.3) | <0.001 |
| FiO2 | 0.47 (0.40–0.60) | 0.44 (0.38–0.52) | 0.61 (0.50–0.80) | <0.001 |
| PEEP, cm H2O | 8.8 (6.7–10.8) | 9.4 (8.0–10.9) | 7.0 (5.0–10.0) | <0.001 |
| Vt, ml/kg | 7.5 (6.5–8.6) | 7.7 (6.5–9.0) | 7.5 (6.5–8.6) | 0.50 |
| PIP, cm H2O | 28 (24.5–33) | 28 (24.6–32) | 29.5 (24–34) | 0.32 |
| Driving pressure, cm H2O | 19.5 (16–23) | 19 (15.6–22) | 20.1 (17.2–25.5) | <0.001 |
| Inotropes | 598 (61.0) | 446 (63.0) | 152 (55.2) | 0.02 |
| Nitric oxide | 195 (19.9) | 132 (18.8) | 63 (22.9) | 0.14 |
| Outcome | ||||
| Mortality | 191 (18.2) | 111 (14.9) | 80 (26.5) | <0.001 |
| 28-day ventilator-free days | 17.0 (0–22) | 17.4 (3.0–22.1) | 15.6 (0–21.2) | 0.02 |
For definition of abbreviations, see Table 1.
Data are presented as median (interquartile range) or n (%). When a PaO2 metric was not available, PF was calculated from ratio of oxygen saturation measured by pulse oximetry to FiO2, and OI was calculated from OSI using previously published formulae (19). A total of 1,047 patients had PEEP/FiO2 data available for analysis. Race, ARDS triggers, and comorbidities were not available in the CPCCRN data, so the total number is reduced to 979. Some patients did not have available data 24 hours after PARDS diagnosis (died, extubated, or no PF ratio, OI, or OSI available), so the number is reduced to 974. Twenty-eight–day ventilator-free days defined as the number of days in the first 28 days after ARDS diagnosis in which the patient was alive and not on invasive mechanical ventilation. The percentages refer to the percent overall patients, high/on-protocol PEEP, or low PEEP with a given variable. P values are comparing the difference between two PEEP groups, among variables or groupings of variables (i.e., P < 0.001 for PARDS severity implies difference in PARDS severity categories between high/on-protocol PEEP and low PEEP without post hoc comparison of which groups are different).
Multivariable and Propensity Models
In general, more hypoxemic patients with higher severity of illness were more likely to be managed with lower PEEP relative to FiO2 (Table 2). To account for these factors, we first constructed a multivariable logistic regression model on the outcome of PICU mortality. After controlling for PF ratio over the first 24 hours, inotrope use, immunodeficiency, stem cell transplant, Pediatric Risk of Mortality III score, nitric oxide, driving pressure, and dataset, patients managed with PEEP levels lower than recommended by the ARDSNet for a given FiO2 were more likely to die (odds ratio [OR], 2.05; 95% confidence interval [CI], 1.32–3.17; Table 3). There were two significant multiplicative interactions that were included (CHOP dataset × nitric oxide and inotrope × stem cell transplant). The interaction between CHOP dataset and PEEP in relation to the protocol was not significant (P = 0.4). In addition, we created a propensity model for using PEEP lower than recommended by the protocol. Similarly, after adjusting for this propensity to use lower PEEP and other covariates, we found that lower PEEP relative to FiO2 proposed in the ARDSNet model was independently associated with higher mortality (OR, 2.0; 95% CI, 1.24–3.22) (see Tables E1–E3 in the online supplement).
Table 3.
Multivariable Model on ICU Mortality
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| PEEP lower than ARDSNet (vs. on-protocol/high PEEP) | 2.05 (1.32–3.17) | 0.001 |
| PRISM III* | 1.08 (1.06–1.11) | <0.001 |
| Immunodeficiency (vs. no immunodeficiency) | 2.00 (1.27–3.13) | 0.003 |
| Stem cell transplant, no inotropes | 4.64 (2.29–9.39) | <0.001 |
| Stem cell transplant, yes inotropes | 12.4 (5.12–30.1) | 0.013 |
| No stem cell transplant, yes inotropes | 2.67 (1.66–4.30) | <0.001 |
| CHOP dataset (vs. all other datasets) | 0.46 (0.28–0.77) | 0.001 |
| Nitric oxide not at CHOP | 3.28 (1.70–6.35) | 0.001 |
| Nitric oxide at CHOP | 1.29 (0.52–3.23) | NS |
| Driving pressure* | 1.05 (1.009–1.087) | 0.015 |
| PF ratio (Day 1)* | 1.0 (0.998–1.002) | 0.91 |
Definition of abbreviations: ARDSNet = ARDS Network; CHOP = Children’s Hospital of Philadelphia; CI = confidence interval; NS = not significant; PEEP = positive end-expiratory pressure; PF = PaO2/FiO2; PRISM = Pediatric Risk of Mortality.
There was an independent association between PEEP lower than recommended by the ARDSNet protocol for a given FiO2 and higher mortality, after controlling for PRISM III score, immunodeficiency, stem cell transplant, inotrope use, nitric oxide, driving pressure, PF ratio, and dataset. There were multiplicative interactions between inotrope use and stem cell transplant, and CHOP and nitric oxide use. PF ratio was retained in model because it had an important confounding effect on the relationship between PEEP lower than the protocol and mortality.
Variables treated as continuous in the multivariable model.
Sensitivity Analysis
When limiting the analysis to only those who had PF ratio less than or equal to 200 24 hours after PARDS diagnosis, the multivariable OR was similar, although no longer statistically significant (OR, 1.64; 95% CI, 0.97–2.77; P = 0.06).
There were notable differences among the datasets, which prompted us to perform subgroup analysis and directly compare CHOP and CHLA patients. CHLA had a higher percentage of patients with initial PF ratio less than 100 (33% CHLA vs. 23% CHOP, P < 0.001). PF ratio improved in a similar percentage of patients by 24 hours between datasets (36% CHLA vs. 39% CHOP, P = 0.4). By 24 hours into PARDS diagnosis, 14.6% of patients at CHLA had PF less than 100 compared with 6.6% at CHOP (P < 0.001). Patients at CHOP were more likely to be on inotropes and vasopressors or have pneumonia or aspiration. Patients at CHLA were more likely to have sepsis, immunodeficiency, and solid organ transplant (Table 4, all P < 0.05; stratified by PF, Table E4).
Table 4.
Children’s Hospital of Philadelphia versus Children’s Hospital Los Angeles Cohort Demographics, Comorbidities, Ventilator Management, and Outcomes among All Patients with Acute Respiratory Distress Syndrome
| CHLA (N = 612) | CHOP (N = 453) | P Value | |
|---|---|---|---|
| Age, mo | 46 (11.2 to 141) | 49 (16.8 to 143) | 0.16 |
| ARDS trigger | |||
| Pneumonia | 361 (59) | 299 (66) | 0.02 |
| Sepsis | 225 (36.8) | 98 (21.6) | <0.001 |
| Neurological disease | 165 (27) | 95 (21) | 0.02 |
| Trauma | 43 (7) | 29 (6.4) | 0.7 |
| Drowning | 9 (1.5) | 11 (2.4) | 0.255 |
| Aspiration | 28 (4.6) | 54 (11.9) | <0.001 |
| Comorbidities | |||
| Immunodeficiency | 161 (26.3) | 87 (19) | 0.007 |
| Stem cell transplant | 31 (5) | 33 (7.3) | 0.13 |
| Cancer | 100 (16.3) | 61 (13.4) | 0.19 |
| Solid transplant | 35 (5.7) | 6 (1.3) | <0.001 |
| PRISM III raw score | 11 (6 to 17) | 11 (5 to 17) | 0.47 |
| Day 1 ventilator settings | |||
| FiO2 | 0.49 (0.41 to 0.65) | 0.45 (0.37 to 0.56) | <0.0001 |
| Mean airway pressure, cm H2O | 13.3 (10.2 to 17.7) | 16 (14 to 19) | <0.0001 |
| PEEP, cm H2O | 8.0 (6 to 10) | 10 (8 to 11.4) | <0.0001 |
| PF ratio | 190 (128 to 258) | 210 (162 to 251) | 0.01 |
| OI | 6.6 (4.4 to 11.8) | 8.4 (6.2 to 13.5) | <0.0001 |
| Driving pressure, cm H2O | 19.5 (16 to 22.5) | 19.5 (16 to 23.5) | 0.18 |
| Vt, ml/kg | 7.6 (6.2 to 9.1) | 7.4 (6.6 to 8.2) | 0.06 |
| PEEP discordance | 0 (−2 to 0) | 2 (0 to 3) | <0.0001 |
| Low | 222 (40.2) | 53 (12.4) | <0.0001 |
| On | 196 (35.3) | 123 (28.8) | |
| High | 134 (24.3) | 251 (58.8) | |
| Inotropes or vasopressors | 270 (44.1) | 360 (79) | <0.0001 |
| Nitric oxide | 62 (10.1) | 161 (35) | <0.0001 |
| Outcomes | |||
| Mortality | 137 (22.4) | 65 (14.4) | 0.001 |
| 28-day VFDs | 16.7 (0 to 23) | 16 (3 to 21) | 0.01 |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; CHLA = Children’s Hospital Los Angeles; CHOP = Children’s Hospital of Philadelphia; IQR = interquartile range; OI = oxygenation index; PEEP = positive end-expiratory pressure; PF = PaO2/FiO2; PRISM = Pediatric Risk of Mortality; VFD = ventilator-free days.
Data are presented as median (IQR) and n (%). See online supplement for subgroups by PF ratio.
Regarding ventilator management, CHOP used higher PEEP when FiO2 was between 0.21 and 0.6 compared with CHLA (Figures E1A–E1D). PEEP at CHOP follows a near-normal distribution, whereas PEEP at CHLA has more variability (Figure 4). CHOP used PEEP lower than protocol only 12.4% of the time (79% PF < 100; 17.5% PF 100–200; 2.9% PF 200–300), compared with 40% at CHLA (63% PF < 100; 45.1% PF 100–200; 28.4% PF 200–300). As a result, patients with PF ratio greater than 100 24 hours after PARDS diagnosis at CHOP were on a higher mean airway pressure, higher PEEP, and had a higher OI, with similar or higher PF ratio. CHOP patients were more likely to be on inhaled nitric oxide (97% PF < 100; 51% PF 100–200; 20% PF 200–300) than CHLA patients (26% PF < 100; 10% PF 100–200; 5% PF 200–300). Driving pressure and Vt were similar between CHOP and CHLA (Figures 5 and E2 and Tables 4 and E4).
Figure 4.
Positive end-expiratory pressure (PEEP) distribution for Children’s Hospital of Philadelphia (CHOP) (blue, top) versus Children’s Hospital Los Angeles (CHLA) (orange, bottom). There is a relatively normal distribution of PEEP in the CHOP dataset, which is shifted slightly to the right when FiO2 is increased over 0.5 (top right). In contrast, CHLA data have more variability in PEEP, which is retained, although shifted to the right, when FiO2 is increased over 0.5 (bottom right). obs = observations.
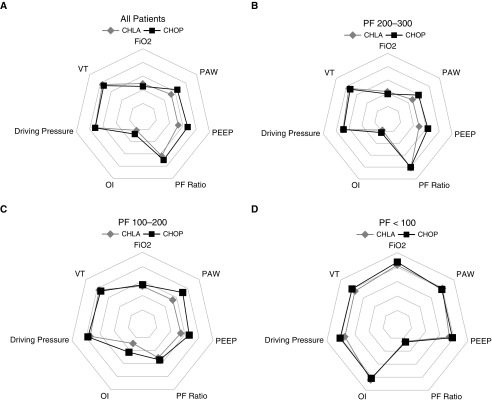
Figure 5.
Differences in ventilator strategy on Day 1 of acute respiratory distress syndrome (ARDS) between Children’s Hospital of Philadelphia (CHOP) and Children’s Hospital Los Angeles (CHLA), stratified by PaO2/FiO2 (PF) ratio 24 hours after pediatric ARDS (PARDS) diagnosis. All variables are scaled to a maximum value of 1 for each variable, to highlight relative differences between datasets. Actual values for these parameters can be found in Tables 4 and E4. The individual squares and diamonds represent the median value for the dataset for a given variable. (A) For all patients, CHOP generally used a higher mean airway pressure (PAW), higher positive end-expiratory pressure (PEEP), and slightly lower FiO2 than CHLA, with slightly higher PF ratio and oxygenation index (OI), and similar driving pressure and Vt. (B and C) For patients with PF ratio (B) 200 to 300 or (C) 100 to 200, PAW and PEEP are higher at CHOP than CHLA. (D) For those with PF less than or equal to 100, PAW, PEEP, OI, and PF ratio are similar between datasets, although FiO2, Vt, and driving pressure are slightly higher at CHOP.
Nonconventional ventilation (NCV) strategies were used in 24.7% of patients within the first 24 hours of PARDS diagnosis at CHOP, compared with 9.6% at CHLA (P < 0.001). The median OI at initiation of NCV was 20 (IQR, 15–28), with a median PEEP of 12 (IQR, 10–12) at CHOP compared with a median OI of 35 (IQR, 18–48) with a median PEEP of 14 (IQR, 10–15) at CHLA (both P < 0.01). Mortality was similar between those who did and did not receive NCV within the first 24 hours at CHOP (14.3% vs. 14.4%, P = 0.99), but those who received NCV within the first 24 hours at CHLA had much higher mortality (44% vs. 18.9%, P < 0.001). At CHOP, the first NCV mode was airway pressure release ventilation in 12.9%, extracorporeal membrane oxygenation in 1.3%, high-frequency oscillatory ventilation in 43%, and high-frequency percussive ventilation in 43%. High-frequency oscillatory ventilation was the first NCV mode used at CHLA in all patients.
On a univariate basis in both institutions, PEEP lower than protocol on Day 1 was associated with higher mortality (Figure E3). This relationship was retained in the CHLA cohort after controlling for potential confounding factors (OR, 2.09; 95% CI, 1.26–3.46; Table 5). However, after controlling for confounding factors in the CHOP dataset, PEEP lower than the protocol was not associated with mortality (OR, 0.87; 95% CI, 0.32–2.35; Table 6).
Table 5.
Children’s Hospital Los Angeles Patients Only, Multivariable Model for ICU Mortality Including Data from Day 1 of Mechanical Ventilation (n = 535)
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| PEEP lower than ARDSNet (vs. on-protocol/high PEEP) | 2.09 (1.26–3.46) | 0.004 |
| PRISM III* | 1.08 (1.05–1.11) | <0.001 |
| Immunodeficiency (vs. no immunodeficiency) | 2.71 (1.19–6.21) | 0.018 |
| Stem cell transplant, no inotrope | 4.97 (2.49–9.95) | 0.001 |
| Stem cell transplant, yes inotrope | 16.00 (3.3–77.1) | <0.001 |
| No stem cell transplant, yes inotrope | 2.23 (1.40–3.56) | 0.001 |
| Nitric oxide (vs. no nitric oxide) | 3.37 (1.70–6.64) | <0.001 |
| Driving pressure* | 1.07 (1.02–1.12) | 0.009 |
| PF ratio (Day 1)* | 1.001 (0.998–1.004) | 0.42 |
Definition of abbreviations: ARDSNet = ARDS Network; CI = confidence interval; PEEP = positive end-expiratory pressure; PF = PaO2/FiO2; PRISM = Pediatric Risk of Mortality.
PEEP lower than recommended by the ARDS Network Protocol for a given FiO2 remains independently associated with mortality.
Variables treated as continuous in the multivariable model.
Table 6.
Children’s Hospital of Philadelphia Patients Only, Multivariable Model for ICU Mortality Including Data from Day 1 of Mechanical Ventilation (n = 427)
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| PEEP lower than ARDSNet (vs. on-protocol/high PEEP) | 0.87 (0.32–2.35) | 0.78 |
| PRISM III* | 1.10 (1.07–1.14) | <0.001 |
| Immunodeficiency (vs. no immunodeficiency) | 2.80 (1.26–6.24) | 0.012 |
| Stem cell transplant (vs. no stem cell transplant) | 4.70 (1.69–13.2) | 0.003 |
| PF ratio (Day 1)* | 0.995 (0.989–1.0001) | 0.06 |
For definition of abbreviations, see Table 5.
PEEP lower than recommended by the ARDS Network Protocol for a given FiO2 did not remain independently associated with mortality after multivariable adjustment.
Variables treated as continuous in the multivariable model.
Day 2 and 3 PEEP data were available from the CHLA dataset, and the multivariable odds ratios were similar, although findings were not statistically significant, likely because of smaller sample size (Tables E5 and E6).
Discussion
Using the ARDSNet PEEP/FiO2 protocol as a framework to analyze observational data from more than 1,100 patients with PARDS, we have found that patients managed with PEEP levels lower than recommended by the ARDSNet model for a given FiO2 had higher mortality. This is consistent when stratifying by PF ratio and holds after controlling for confounding variables directly in multivariable modeling, as well as in propensity-based covariate adjustment.
Although we chose to combine datasets for the primary analysis using interaction terms to account for differences among the institutions, notable differences in comorbidities and ventilator management between datasets prompted us to perform subgroup analysis stratified by institution, namely CHOP and CHLA. The sensitivity analysis highlights that the relationship between PEEP lower than the protocol and mortality is most relevant in the CHLA data. We believe this is for several reasons. 1) CHOP patients are generally managed with PEEP in line with or higher than recommended by the protocol. Only 12% of patients have PEEP levels lower than recommended by the protocol at CHOP, compared with 40% at CHLA and 40% in the CPCCRN dataset. This resulted in higher PEEP and mean airway pressure for a similar PF ratio. 2) PEEP use does not vary as much at CHOP as a function of hypoxemia severity. At CHOP, nearly all patients with PF ratio greater than 100 had PEEP above protocol, and nearly all patients with PF less than 100 had PEEP below protocol. These extremes (<20% of patients below protocol when PF > 100 and 20% on or above protocol when PF < 100) make it difficult to draw conclusions about PEEP management from the CHOP dataset alone, because using observational data to compare outcomes of patients who are managed with different PEEP/FiO2 combinations is dependent on variability in PEEP level for a given FiO2. This variability was present in CHLA and CPCCRN datasets, but not at CHOP. 3) Patients at CHOP were three to four times more likely to receive inhaled nitric oxide than those at CHLA, which may also alter hypoxemia severity. 4) CHOP uses nearly three times more NCV in the first 24 hours than CHLA, with a median OI of 20 at initiation of NCV at CHOP, compared with 35 at CHLA. 5) CHOP primarily uses pressure-regulated volume control, whereas CHLA uses primarily pressure control, although Vt and driving pressure were similar between datasets. 6) Finally, CHOP included only patients with bilateral infiltrates on chest imaging, whereas the other datasets included patients with unilateral or bilateral disease. We chose not to stratify analysis on the basis of chest imaging interpretation, because each dataset used different methods for gauging bilateral versus unilateral disease (i.e., radiologist, intensivist, multiple practitioners, and timing of the films). Future studies need to standardize these interpretations before we understand their relevance.
Overall mortality was lower at CHOP than CHLA. Part of this relates to different inclusion criteria and methods for screening for PARDS, different PARDS triggers, differences in comorbidities between datasets, and differences in adjuvant therapies, such as inhaled nitric oxide and inotropes and vasopressors. However, ventilator strategies between institutions were similar with respect to Vt and driving pressure but were notably different with oxygenation strategies (particularly higher PEEP at CHOP for those with mild or moderate ARDS and sooner transition to alternative modes of ventilation rather than further escalating PEEP for severe ARDS). These data highlight a reality of multicenter practice and research, that ventilator management is institution and practitioner dependent in the absence of an agreed-on protocol. There are few validated pediatric protocols, making this an important area for research. PEEP management at CHOP is less variable than CHLA, with significantly fewer patients managed with PEEP lower than recommended by ARDSNet (12% vs. 40%). These findings may contribute to the mortality differences between datasets.
Our findings could have significant implications. As our data and previous investigations have highlighted (2, 8, 9, 11), there is reluctance among pediatric intensivists to escalate PEEP in response to hypoxemia, preferentially increasing FiO2. On average, PEEP plateaus around 10 cm H2O, even with severe hypoxemia. The reasons are likely multifactorial and may relate to concerns about high PEEP levels in infants and neonates with low chest wall elastance, concerns about cardiopulmonary interactions, or a belief that high FiO2 is not dangerous (20, 21). Interestingly, we found that patients with hyperoxia (i.e., PaO2 > 100 mm Hg) had higher mortality than those with more normal PaO2 (60–100 mm Hg, Table 1), although this finding did not hold in multivariable modeling. However, it provides indirect evidence of ill effects of high concentrations of oxygen, particularly if it leads to hyperoxia. Although the general principles of lung-protective ventilation with use of higher PEEP to promote alveolar recruitment are supported among pediatric intensivists, and in fact specifically recommended in expert-based guidelines for PARDS management (1, 10, 22, 23), our data highlight that these principles are not executed at the bedside for most children.
In truth, there are no clear data that demonstrate that PEEP level matters in PARDS. There have been no randomized controlled trials and, like many other pieces of ventilator management in ARDS, direct extrapolation from adults has limitations (3, 15, 20, 24–27). In our analysis, for patients with initially similar levels of hypoxemia, management with PEEP that is escalated in conjunction with FiO2 is associated with lower mortality than those for whom FiO2 is primarily increased. Interestingly we found this pattern most evident in those with moderate hypoxemia (PF, 100–200). Although one could speculate as to physiologic reasons for this finding, it likely relates to sample size and variability in PEEP management in this range. The range of PEEP (below, on protocol, and higher than protocol) is well represented in this subgroup, making it more possible to find a relationship. A large proportion of patients with PF less than 100 were managed with PEEP below the protocol, and many were transitioned to alternative modes of ventilation, making it difficult to draw conclusions in this group specifically.
However, our observational data cannot and should not imply a causal relationship that management on the basis of the ARDSNet PEEP/FiO2 table for PARDS would result in improved mortality, although there is strong biological plausibility for this (6). Moreover, there are many reasons to believe that the ARDSNet PEEP/FiO2 table is suboptimal in PARDS, as a more individualized approach using transpulmonary pressure, lung compliance, dead space, or other methods have strong theoretical advantages (5, 6, 28–34). Nevertheless, our data do highlight that there may be problems with usual care PEEP management in PARDS and that clinical trials in this area should be a priority for research. This is particularly important in light of the recent findings from the Alveolar Recruitment for ARDS Trial (ART), that adult patients with ARDS managed with lung recruitment and PEEP titration on the basis of respiratory system compliance had higher mortality than those managed with the ARDSNet PEEP/FiO2 protocol (35).
We are limited by the data available, and it may be that our findings reflect patient severity of illness or residual unmeasured confounding. Because of the nature of the ARDSNet PEEP/FiO2 titration model, patients with very negative PEEP discordance values must be on high levels of FiO2. Indeed, the largest proportion of patients who were managed with lower PEEP relative to the amount of FiO2 had severe hypoxemia. To mitigate these concerns, we performed a variety of stratified analyses as well as two methods for adjustment and multivariable modeling. Our findings held after multivariable and propensity covariate adjustment incorporating oxygenation metrics, PARDS triggers, comorbidities, inotropes and vasopressors, admission severity of illness, other ventilator settings, and inhaled nitric oxide. However, we did not have data on other potential confounders (i.e., other cointerventions like neuromuscular blockade, prone positioning, air leak syndrome or pneumothorax, etc.), and there is potential selection bias on the basis of individual practices for arterial line placement and potential differences on the basis of bilateral versus unilateral infiltrates. These limitations can likely only be overcome with a well-designed randomized control trial.
In conclusion, through secondary analysis of data from more than 1,100 patients with PARDS, we have found that patients managed with PEEP levels lower than recommended by the ARDSNet PEEP/FiO2 model experienced higher mortality, even after covariate adjustment. Randomized controlled trials targeting PEEP management in PARDS are needed.
Footnotes
Supported by University of Southern California Office of the Provost and NIH/National Institute of Child Health and Human Development grant R21 HD061870 (C.J.L.N.).
Author Contributions: R.G.K., N.Y., A.K.B., N.J.T., and C.J.L.N. were involved in the conceptualization and design of the study and discussion of results, and helped with consideration of analytic approaches. R.G.K., N.Y., and C.J.L.N. provided anonymous original data sources for aggregation. R.G.K. and K.P. were responsible for data transformation, database management, statistical analysis, and writing of the manuscript. All authors edited and approved the final manuscript. R.G.K. is the guarantor of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201707-1404OC on January 26, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 5.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Expiratory Pressure (Express) Study Group. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 6.Chiumello D, Cressoni M, Carlesso E, Caspani ML, Marino A, Gallazzi E, et al. Bedside selection of positive end-expiratory pressure in mild, moderate, and severe acute respiratory distress syndrome. Crit Care Med. 2014;42:252–264. doi: 10.1097/CCM.0b013e3182a6384f. [DOI] [PubMed] [Google Scholar]
- 7.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 8.Khemani RG, Sward K, Morris A, Dean JM, Newth CJL NICHD Collaborative Pediatric Critical Care Research Network (CPCCRN) Variability in usual care mechanical ventilation for pediatric acute lung injury: the potential benefit of a lung protective computer protocol. Intensive Care Med. 2011;37:1840–1848. doi: 10.1007/s00134-011-2367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santschi M, Jouvet P, Leclerc F, Gauvin F, Newth CJL, Carroll CL, et al. PALIVE Investigators; Pediatric Acute Lung Injury and Sepsis Investigators Network (PALISI); European Society of Pediatric and Neonatal Intensive Care (ESPNIC) Acute lung injury in children: therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med. 2010;11:681–689. doi: 10.1097/PCC.0b013e3181d904c0. [DOI] [PubMed] [Google Scholar]
- 10.Thomas NJ, Jouvet P, Willson D. Acute lung injury in children--kids really aren’t just “little adults”. Pediatr Crit Care Med. 2013;14:429–432. doi: 10.1097/PCC.0b013e31827456aa. [DOI] [PubMed] [Google Scholar]
- 11.Newth CJL, Sward KA, Khemani RG, Page K, Meert KL, Carcillo JA, et al. Eunice Kennedy Shriver National Institute for Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) Variability in usual care mechanical ventilation for pediatric acute respiratory distres syndrome: time for a decision support protocol? Pediatr Crit Care Med. 2017;18:e521–e529. doi: 10.1097/PCC.0000000000001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Fernández Y, Azagra AM, de la Oliva P, Modesto V, Sánchez JI, Parrilla J, et al. Pediatric Acute Lung Injury Epidemiology and Natural History (PED-ALIEN) Network. Pediatric acute lung injury epidemiology and natural history study: incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med. 2012;40:3238–3245. doi: 10.1097/CCM.0b013e318260caa3. [DOI] [PubMed] [Google Scholar]
- 13.Erickson S, Schibler A, Numa A, Nuthall G, Yung M, Pascoe E, et al. Paediatric Study Group; Australian and New Zealand Intensive Care Society. Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8:317–323. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 14.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 15.Khemani RG, Conti D, Alonzo TA, Bart RD, III, Newth CJL. Effect of tidal volume in children with acute hypoxemic respiratory failure. Intensive Care Med. 2009;35:1428–1437. doi: 10.1007/s00134-009-1527-z. [DOI] [PubMed] [Google Scholar]
- 16.Parvathaneni K, Belani S, Leung D, Newth CJ, Khemani RG. Evaluating the performance of the pediatric acute lung injury consensus conference definition of acute respiratory distress syndrome. Pediatr Crit Care Med. 2017;18:17–25. doi: 10.1097/PCC.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 17.Yehya N, Bhalla AK, Thomas NJ, Khemani RG. Alveolar dead space fraction discriminates mortality in pediatric acute respiratory distress syndrome. Pediatr Crit Care Med. 2016;17:101–109. doi: 10.1097/PCC.0000000000000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yehya N, Servaes S, Thomas NJ. Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Crit Care Med. 2015;43:937–946. doi: 10.1097/CCM.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 19.Khemani RG, Thomas NJ, Venkatachalam V, Scimeme JP, Berutti T, Schneider JB, et al. Pediatric Acute Lung Injury and Sepsis Network Investigators (PALISI) Comparison of SpO2 to PaO2 based markers of lung disease severity for children with acute lung injury. Crit Care Med. 2012;40:1309–1316. doi: 10.1097/CCM.0b013e31823bc61b. [DOI] [PubMed] [Google Scholar]
- 20.Khemani RG, Newth CJL. The design of future pediatric mechanical ventilation trials for acute lung injury. Am J Respir Crit Care Med. 2010;182:1465–1474. doi: 10.1164/rccm.201004-0606CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingaramo OA, Ngo T, Khemani RG, Newth CJ. Impact of positive end-expiratory pressure on cardiac index measured by ultrasound cardiac output monitor. Pediatr Crit Care Med. 2014;15:15–20. doi: 10.1097/PCC.0b013e3182976251. [DOI] [PubMed] [Google Scholar]
- 22.Tamburro RF, Kneyber MC Pediatric Acute Lung Injury Consensus Conference Group. Pulmonary specific ancillary treatment for pediatric acute respiratory distress syndrome: proceedings from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med. 2015;16:S61–S72. doi: 10.1097/PCC.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 23.Rimensberger PC, Cheifetz IM Pediatric Acute Lung Injury Consensus Conference Group. Ventilatory support in children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:S51–S60. doi: 10.1097/PCC.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 24.Curley MAQ, Hibberd PL, Fineman LD, Wypij D, Shih M-C, Thompson JE, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005;294:229–237. doi: 10.1001/jama.294.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 26.Kneyber MC, Zhang H, Slutsky AS. Ventilator-induced lung injury: similarity and differences between children and adults. Am J Respir Crit Care Med. 2014;190:258–265. doi: 10.1164/rccm.201401-0168CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Jager P, Burgerhof JG, van Heerde M, Albers MJ, Markhorst DG, Kneyber MC. Tidal volume and mortality in mechanically ventilated children: a systematic review and meta-analysis of observational studies. Crit Care Med. 2014;42:2461–2472. doi: 10.1097/CCM.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 28.Albaiceta GM, Luyando LH, Parra D, Menendez R, Calvo J, Pedreira PR, et al. Inspiratory vs. expiratory pressure-volume curves to set end-expiratory pressure in acute lung injury. Intensive Care Med. 2005;31:1370–1378. doi: 10.1007/s00134-005-2746-6. [DOI] [PubMed] [Google Scholar]
- 29.Chelucci GL, Dall’Ava-Santucci J, Dhainaut JF, Chelucci A, Allegra A, Lockhart A, et al. Association of PEEP with two different inflation volumes in ARDS patients: effects on passive lung deflation and alveolar recruitment. Intensive Care Med. 2000;26:870–877. doi: 10.1007/s001340051275. [DOI] [PubMed] [Google Scholar]
- 30.David M, Karmrodt J, Bletz C, David S, Herweling A, Kauczor HU, et al. Analysis of atelectasis, ventilated, and hyperinflated lung during mechanical ventilation by dynamic CT. Chest. 2005;128:3757–3770. doi: 10.1378/chest.128.5.3757. [DOI] [PubMed] [Google Scholar]
- 31.Gattinoni L, Pelosi P, Suter PM, Pedoto A, Vercesi P, Lissoni A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease: different syndromes? Am J Respir Crit Care Med. 1998;158:3–11. doi: 10.1164/ajrccm.158.1.9708031. [DOI] [PubMed] [Google Scholar]
- 32.Talmor D, Sarge T, Malhotra A, O’Donnell CR, Ritz R, Lisbon A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tugrul S, Cakar N, Akinci O, Ozcan PE, Disci R, Esen F, et al. Time required for equilibration of arterial oxygen pressure after setting optimal positive end-expiratory pressure in acute respiratory distress syndrome. Crit Care Med. 2005;33:995–1000. doi: 10.1097/01.ccm.0000163402.29767.7b. [DOI] [PubMed] [Google Scholar]
- 34.Villar J, Kacmarek RM, Pérez-Méndez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34:1311–1318. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
- 35.Cavalcanti AB, Suzumura ÉA, Laranjeira LN, Paisani DM, Damiani LP, Guimarães HP, et al. Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]