Abstract
Rationale: Loss of the peripheral pulmonary vasculature, termed vascular pruning, is associated with disease severity in patients with chronic obstructive pulmonary disease.
Objectives: To determine if pulmonary vascular pruning is associated with asthma severity and exacerbations.
Methods: We measured the total pulmonary blood vessel volume (TBV) and the blood vessel volume of vessels less than 5 mm2 in cross-sectional area (BV5) and of vessels less than 10 mm2 (BV10) in cross-sectional area on noncontrast computed tomographic scans of participants from the Severe Asthma Research Program. Lower values of the BV5 to TBV ratio (BV5/TBV) and the BV10 to TBV ratio (BV10/TBV) represented vascular pruning (loss of the peripheral pulmonary vasculature).
Measurements and Main Results: Compared with healthy control subjects, patients with severe asthma had more pulmonary vascular pruning. Among those with asthma, those with poor asthma control had more pruning than those with well-controlled disease. Pruning of the pulmonary vasculature was also associated with lower percent predicted FEV1 and FVC, greater peripheral and sputum eosinophilia, and higher BAL serum amyloid A/lipoxin A4 ratio but not with low-attenuation area or with sputum neutrophilia. Compared with individuals with less pruning, individuals with the most vascular pruning had 150% greater odds of reporting an asthma exacerbation (odds ratio, 2.50; confidence interval, 1.05–5.98; P = 0.039 for BV10/TBV) and reported 45% more asthma exacerbations during follow-up (incidence rate ratio, 1.45; confidence interval, 1.02–2.06; P = 0.036 for BV10/TBV).
Conclusions: Pruning of the peripheral pulmonary vasculature is associated with asthma severity, control, and exacerbations, and with lung function and eosinophilia.
Keywords: severe asthma, eosinophilia, pulmonary vascular, pruning
At a Glance Commentary
Scientific Knowledge on the Subject
Loss of the peripheral pulmonary vasculature seen on computed tomography, often termed vascular pruning, has been shown to be associated with emphysema and with disease severity in patients with chronic obstructive pulmonary disease. It is unknown if it is present in asthma.
What This Study Adds to the Field
In this study, we demonstrate that not only is pulmonary vascular pruning present in the computed tomography scans of individuals with asthma, it is also associated with disease severity and is independently associated with an increased odds and rate of asthma exacerbations.
Although asthma and chronic obstruction pulmonary disease (COPD) are often thought of as diseases of the airways, both are also associated with changes in the other lung compartments including the pulmonary vasculature (1–15). COPD in particular is associated with radiologic and pathologic changes in the pulmonary vasculature that are, in turn, associated with mortality and disease severity (1–6). For example, we have shown that a smaller relative volume of the peripheral pulmonary vasculature, often termed vascular pruning, measured using computed tomographic (CT), is associated with COPD disease severity and cardiac dysfunction (16, 17).
Although there are fewer studies on pulmonary vascular involvement in asthma, in certain cases asthma is associated with significant vascular involvement, such as in eosinophilic granulomatous polyangiitis (18). In addition, several animal models of asthma develop inflammation and remodeling of the pulmonary vessels, raising the question of whether vascular involvement may be present in patients with asthma who do not meet the clinical criteria for eosinophilic granulomatous polyangiitis (7–13). Based on these findings and our prior work on COPD, we hypothesized that CT measures of pruning of the pulmonary vasculature may be associated with disease severity in asthma.
Some of these results have previously been reported in the form of an abstract (19).
Methods
Cohort and Clinical Measurements
The Severe Asthma Research Program (SARP) is an NIH-sponsored multicenter study designed to improve the understanding of severe asthma (20, 21). For this study we used data from adult participants with both severe and nonsevere asthma from the third phase of SARP (SARP III) and from a smaller group of participants characterized as healthy control subjects (20). Severe asthma was defined based on the American Thoracic Society/European Respiratory Society guidelines, and mild-moderate asthma was defined based on the National Asthma Education and Prevention Program guidelines (20–23). Individuals were defined as having well-controlled asthma if they had an asthma control test (ACT) score greater than or equal to 20, an asthma control questionnaire score less than or equal to 0.75, and one or fewer exacerbations in the past year. They were defined as being suboptimally controlled if they had an ACT score of between 16 and 19 and/or two to three exacerbations in the past year, and were defined as poorly controlled if their ACT score was less than or equal to 15 or their asthma control questionnaire score was greater than or equal to 1.5 or if they had three or more exacerbations in the past year. All subjects had fewer than 5 pack-years of smoking history and none had smoked within the prior 5 years (20). All participants provided informed consent and the study was approved by the institutional review board at each center.
At the baseline visit, participants provided an extensive questionnaire-based history that included asthma control surveys. They also underwent lung function testing including measurement of the fractional exhaled nitric oxide and a bronchodilator challenge (24–34). Peripheral (blood) eosinophilia and sputum eosinophilia were also measured, and those individuals without a contraindication underwent methacholine challenge and bronchoscopy (20, 35, 36). At the second baseline visit, adult participants were given 40 mg of intramuscular triamcinolone to determine their response to systemic steroids (37). The asthma control surveys, spirometry, bronchodilator response, and sputum and peripheral eosinophilia measures were then repeated 18 ± 3 (mean ± SD) days later, and for this study changes in these measures are expressed as absolute differences from baseline (24). Participants returned for annual follow-up visits that included spirometry and an updated questionnaire-based history with questions regarding interval asthma exacerbations, which were defined as respiratory events that required at least 3 days of an oral or intravenous steroid burst (a dose >10 mg or double the individual’s chronic daily steroid dose). For our study, a participant was defined as having an asthma exacerbation if at least one was reported during follow-up.
CT Imaging and Analysis
Volumetric CT scans of the chest were performed on a subgroup of individuals from three clinical centers (the Universities of Pittsburgh and Wisconsin and Washington University in St. Louis). CT scanning was performed using the protocol established by the SPIROMICS (Subpopulations of Intermediate Outcome Measures in Chronic Obstructive Pulmonary Disease) study, which includes contiguous images to enable vascular segmentation. For this study, the primary analyses, including the measurements of the pulmonary vasculature, were performed using baseline scans acquired at TLC (38, 39). Expiratory CT images were obtained in a subset of individuals and were acquired at FRC for consistency with prior SARP studies (39). We applied previously described fully automated methods to segment the lungs from the chest wall and other thoracic structures and to segment the pulmonary vasculature from the lung parenchyma (40, 41).
We also performed automated measurements of the percentage of lung occupied by low-attenuation area (LAA) (on images acquired at TLC), which is a densitometric measure of airspace dilation and destruction correlated with emphysema, and of the percentage of lung with air trapping (on images acquired at FRC). For the purposes of this study LAA was defined as area with an attenuation less than −950 Hounsfield units based on prior work suggesting that tissue with a density less than this threshold is most closely associated with the histopathologic presence of emphysema, and air trapping was defined as the percentage of lung with an attenuation of less than −856 Hounsfield units (42–47).
Summary measures of the pulmonary vasculature included the total blood vessel volume (TBV) and the blood vessel volume of the smaller pulmonary blood vessels using two different thresholds: those less than 5 mm2 in cross-sectional area and those less than 10 mm2 in cross-sectional area (BV5 and BV10, respectively) (16, 48, 49). These volumes are the combined volumes of the pulmonary arteries and veins, and because of variations in blood vessel volume based on overall body size, all analyses were performed using the ratio of either BV5 or BV10 to TBV (BV5/TBV and BV10/TBV, respectively). Lower values of these ratios suggest CT imaging evidence of vascular pruning (i.e., a smaller proportion of the blood vessel volume comprised of small peripheral pulmonary blood vessels) (16). All of the quantitative image analyses were performed using the Chest Imaging Platform (https://chestimagingplatform.org/) and implemented using 3D Slicer (https://www.slicer.org/), both of which are open source image processing tools (50, 51).
Statistical Analysis
Summary statistics are presented as means and SD where appropriate. The measures of vascular pruning were analyzed as continuous variables except for the analyses of the odds of a prospective asthma exacerbation as discussed later. Pearson correlation and multivariable linear regression were used to determine the associations between the measures of vascular pruning and other continuous clinical and radiographic measurements. The latter analyses were adjusted for age, sex, race, body mass index, LAA, CT measured lung volume, and prebronchodilator percent predicted FEV1 (FEV1 pp), except for the models for the baseline spirometric measures, which were not adjusted for prebronchodilator FEV1 pp and the models for the densitometric CT measures (LAA and air trapping), which were not adjusted for LAA. Bonferroni correction was applied to the multivariable analyses to account for multiple testing as indicated, but not to the univariate correlations because these were believed to be descriptive.
Because we hypothesized that greater asthma severity and worse asthma control would be associated with more pulmonary vascular pruning, the Jonckheere-Terpstra test, a rank based nonparametric test for ordered differences among classes, was used to test the associations between asthma severity category (ordered as healthy control, mild-moderate asthma, severe asthma) and vascular pruning, and for the association between and asthma control category (ordered as well-controlled, suboptimally controlled, poorly controlled) and vascular pruning (52). In addition, pair-wise comparisons were made between asthma severity categories and asthma control categories using the Steel-Dwass-Critchlow-Fligner method, a nonparametric approach based on pair-wise two-sample Wilcoxon comparisons corrected for multiple testing (53).
Multivariable logistic regression was used to assess the associations between BV5/TBV and BV10/TBV and the odds of reporting a prospective asthma exacerbation, and zero inflated negative binomial regression was used to assess the associations between BV5/TBV and BV10/TBV and the rate of prospective asthma exacerbations (54). The latter was chosen to account for the large number of participants who reported no exacerbation during follow-up. For these analyses the odds ratios and incidence rate ratios are expressed as the odds or rate of those with the most vascular pruning (those in the lowest 25% of BV5/TBV or BV10/TBV) versus those with less vascular pruning (those in the highest 75% of BV5/TBV or BV10/TBV). The multivariable exacerbation analysis models were adjusted for age, sex, race, body mass index, prebronchodilator FEV1 pp, baseline ACT score, and a reported history of exacerbation in the year before the baseline visit. All analyses were performed using SAS 9.4 or JMP 13 Pro (SAS Institute), all statistical tests were two-sided, and P less than 0.05 were considered statistically significant.
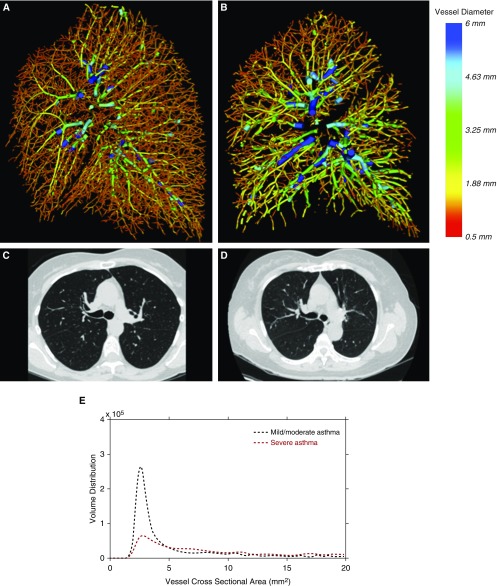
Results
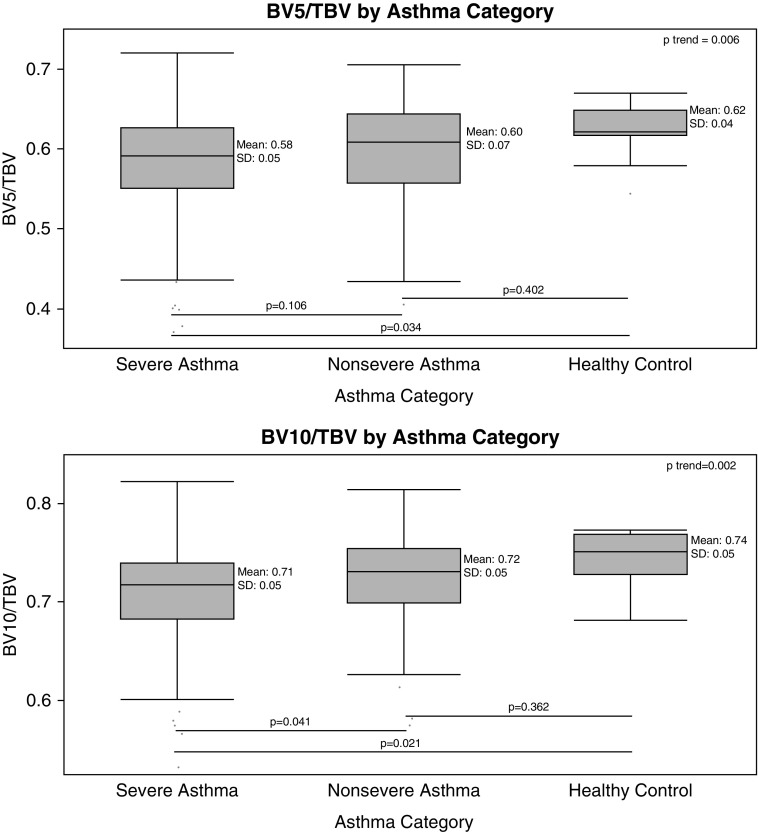
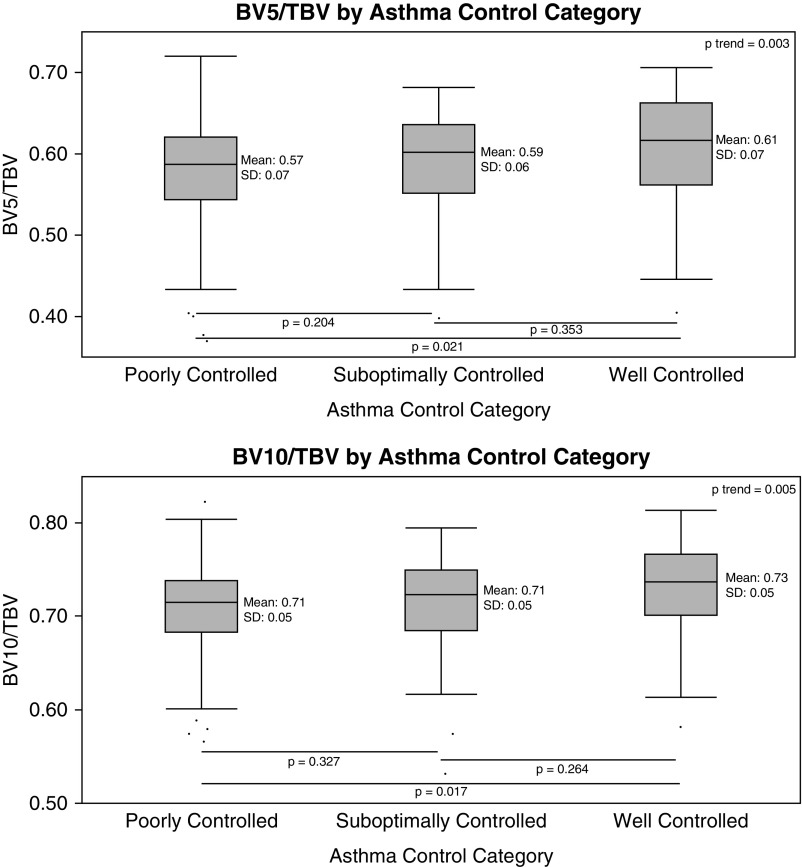
The clinical and imaging characteristics of the 237 participants who had CT imaging and clinical data available are shown in Table 1. Of note, the cohort was largely comprised of individuals with severe asthma who had a relatively small amount (mean ± SD, 2.0 ± 2.3%) of LAA or emphysema-like tissue on CT. Figure 1 provides examples of the pulmonary vasculature of the right lung from individuals with mild and severe asthma, and demonstrates loss of the peripheral pulmonary vasculature in the individual with more severe disease. As shown in Figure 2, greater asthma severity was associated with a trend toward greater pruning of the pulmonary vasculature (i.e., lower BV5/TBV and BV10/TBV; P for trend = 0.006 and 0.002 for BV5/TBV and BV10/TBV, respectively), but pairwise differences were only present between those with severe disease and healthy control subjects (P = 0.034 and 0.021 for BV5/TBV and BV10/TBV, respectively) and between those with severe asthma and those with mild-moderate disease (BV10/TBV only, P = 0.041). Similarly, as shown in Figure 3 worse asthma control was associated with a trend toward more vascular pruning (P for trend = 0.003 and 0.005 for BV5/TBV and BV10/TBV, respectively), but pairwise differences were only present between those with well-controlled asthma and those with poorly controlled asthma (P = 0.021 and P = 0.017 for BV5/TBV and BV10/TBV, respectively).
Table 1.
Cohort Characteristics
| n Available | ||
|---|---|---|
| Clinical characteristics | ||
| Age, yr, mean ± SD | 46.0 ± 14.8 | 237 |
| Female, n (%) | 157 (66.2) | 237 |
| Nonwhite, n (%) | 93 (39.2) | 237 |
| Body mass index, kg/m2, mean ± SD | 32.8 ± 9.0 | 237 |
| Spirometric characteristics | ||
| Percent predicted FEV1, mean ± SD | 73.2 ± 20.8 | 237 |
| Percent predicted FVC, mean ± SD | 84.9 ± 18.1 | 237 |
| Percent change in FEV1 with bronchodilator, mean ± SD | 17.4 ± 17.3 | 237 |
| Methacholine challenge PC20, mean ± SD | 2.9 ± 3.5 | 103 |
| Asthma category, n (%) | ||
| Healthy control | 11 (4.6) | 237 |
| Nonsevere | 68 (28.7) | 237 |
| Severe | 158 (66.7) | 237 |
| Asthma control | ||
| Asthma control test score, mean ± SD | 16.4 ± 5.0 | 226 |
| Poor asthma control, n (%) | 131 (58.0) | 226 |
| Eosinophilia | ||
| Peripheral eosinophil, %, mean ± SD | 4.1 ± 3.5 | 233 |
| Sputum eosinophil, %, mean ± SD | 4.2 ± 9.4 | 176 |
| Fractional exhaled nitric oxide, mean ± SD | 29.7 ± 27.4 | 234 |
| Steroid response | ||
| Change in peripheral eosinophil, %, mean ± SD | −1.9 ± 2.9 | 121 |
| Change in sputum eosinophil, %, mean ± SD | −2.6 ± 8.1 | 141 |
| Change in fractional exhaled nitric oxide, mean ± SD | −7.1 ± 20.6 | 218 |
| Change in FEV1, mean ± SD | 0.09 ± 0.27 | 223 |
| Other sputum characteristics | ||
| Sputum neutrophil, %, mean ± SD | 55.0 ± 24.7 | 176 |
| BAL | ||
| BAL neutrophil, %, mean ± SD | 2.0 ± 3.0 | 93 |
| BAL eosinophil, %, mean ± SD | 1.6 ± 5.4 | 93 |
| BAL serum amyloid A/lipoxin A4 ratio, mean ± SD | 109.1 ± 340.3 | 58 |
| Quantitative CT measures | ||
| CT measured lung volume, L, mean ± SD | 5.0 ± 1.2 | 237 |
| Percent low-attenuation area, mean ± SD | 2.0 ± 2.3 | 237 |
| Percent air trapping on expiratory CT, mean ± SD | 10.8 ± 2.8 | 187 |
| Measures of pulmonary vasculature | ||
| BV5, ml, mean ± SD | 111.0 ± 28.4 | 237 |
| BV10, ml, mean ± SD | 134.8 ± 31.6 | 237 |
| TBV, ml, mean ± SD | 188.7 ± 42.6 | 237 |
| BV5/TBV, mean ± SD | 0.587 ± 0.066 | 237 |
| BV10/TBV, mean ± SD | 0.714 ± 0.050 | 237 |
| Longitudinal exacerbation analyses | ||
| Duration of follow-up, yr, mean ± SD | 2.6 ± 1.2 | 214 |
| Number of exacerbations per yr, mean ± SD | 1.2 ± 1.6 | 214 |
| Participants who reported at least 1 exacerbation during follow-up, n (%) | 87 (40.7) | 214 |
Definition of abbreviations: BV5 = aggregate vessel volume for vessels less than 5 mm2 in cross-sectional area; BV10 = aggregate vessel volume for vessels less than 10 mm2 in cross-sectional area; CT = computed tomography; PC20 = provocative concentration causing a 20% fall in FEV1; TBV = total pulmonary blood vessel volume.
Low-attenuation area is defined as the percentage of lung occupied by tissue with attenuation less than −950 Hounsfield units on inspiratory CT obtained.
Percentage of area with air trapping is defined as the percentage of lung occupied by tissue with attenuation less than −856 Hounsfield units on expiratory CT.
Figure 1.
Sagittal view of the three-dimensional quantitative reconstruction of the pulmonary vasculature in the right lung of (A) a subject without evidence of pruning and normal lung function (FEV1 percent predicted, 97%), and (B) a subject with evidence of pruning and impaired lung function (FEV1 percent predicted, 75%). The color red corresponds to smaller vessels, and blue corresponds to larger vessels. (C and D) Representative axial computed tomography images from the same two participants. (E) Quantitative profiles of blood volume distribution of the same two participants.
Figure 2.
Pruning by asthma disease severity. Severe asthma was defined on the basis of American Thoracic Society/European Respiratory Society guidelines, and mild–moderate asthma was defined on the basis of National Asthma Education and Prevention Program guidelines (20–23). In box plots, the box portion represents the 25th to the 75th percentile with the solid mid-line representing the mean, and the whisker portion represents the 2.5th to the 97.5th percentile. P values for pairwise comparisons shown below the box plots are based on the Steel-Dwass-Critchlow-Fligner method (53). P trend values are based on the Jonckheere-Terpstra test. BV5 = blood vessel volume of vessels less than 5 mm2 in cross-sectional area; BV10 = blood vessel volume of vessels less than 10 mm2 in cross-sectional area; TBV = total pulmonary blood vessel volume.
Figure 3.
Pruning by asthma control category. The categories are: well controlled—ACT ≥20 and ACQ ≤0.75 and one or fewer exacerbations in the past year; suboptimally controlled—ACT 16–19 and/or 2–3 exacerbations in the past year; and poorly controlled—ACT ≤15 or ACQ ≥1.5 or three or more exacerbations in the past year. In box plots, the box portion represents the 25th to the 75th percentile with the solid mid-line representing the mean, and the whisker portion represents the 2.5th to the 97.5th percentile. P values for pairwise comparisons shown below the box plots are based on the Steel-Dwass-Critchlow-Fligner method (53). P trend values are based on the Jonckheere-Terpstra test. ACQ = asthma control questionnaire; ACT = asthma control test; BV5 = blood vessel volume of vessels less than 5 mm2 in cross-sectional area; BV10 = blood vessel volume of vessels less than 10 mm2 in cross-sectional area; TBV = total pulmonary blood vessel volume.
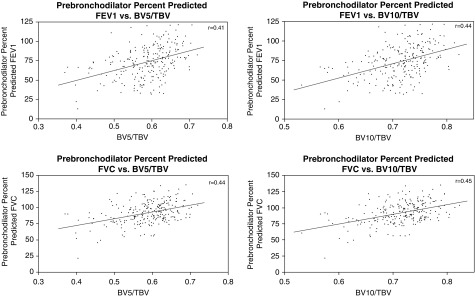
The univariate correlations between vascular pruning and continuous clinical and radiologic measures are shown in Table 2 and Figure 4. Notably, loss of the peripheral pulmonary vasculature was correlated with lower lung function, lower ACT score, more air trapping (BV10/TBV only), greater peripheral eosinophilia and sputum eosinophilia (BV10/TBV only for sputum eosinophilia), higher BAL white blood cell count, and a higher BAL serum amyloid A to lipoxin A4 ratio (SAA/LXA4).
Table 2.
Univariate Correlations
| Parameter | BV5/TBV |
BV10/TBV |
||
|---|---|---|---|---|
| r | P Value | R | P Value | |
| Prebronchodilator FEV1 pp | 0.41 | <0.001 | 0.44 | <0.001 |
| Prebronchodilator FVC pp | 0.44 | <0.001 | 0.45 | <0.001 |
| Percent change in FEV1 with bronchodilator | −0.12 | 0.057 | −0.12 | 0.061 |
| Methacholine challenge PC20 | 0.10 | 0.333 | 0.09 | 0.387 |
| Asthma control test score | 0.25 | <0.001 | 0.22 | 0.001 |
| Low-attenuation area | 0.06 | 0.320 | 0.07 | 0.280 |
| Percent air trapping on expiratory CT | −0.14 | 0.063 | −0.16 | 0.033 |
| Peripheral eosinophil, % | −0.15 | 0.022 | −0.22 | 0.001 |
| Sputum eosinophil, % | −0.16 | 0.033 | −0.21 | 0.005 |
| Fractional exhaled nitric oxide | −0.01 | 0.831 | −0.05 | 0.449 |
| Sputum neutrophil, % | −0.03 | 0.682 | −0.06 | 0.413 |
| BAL neutrophil, % | −0.07 | 0.520 | −0.09 | 0.384 |
| BAL eosinophil, % | 0.14 | 0.180 | 0.14 | 0.186 |
| BAL serum amyloid A/lipoxin A4 ratio | −0.57 | <0.001 | −0.59 | <0.001 |
| Steroid response | ||||
| Change in prebronchodilator FEV1 | 0.00 | 0.975 | −0.01 | 0.877 |
| Change in peripheral eosinophil, % | 0.20 | 0.028 | 0.30 | 0.001 |
| Change in sputum eosinophil, % | 0.05 | 0.552 | 0.12 | 0.163 |
| Change in fractional exhaled nitric oxide | −0.07 | 0.315 | −0.04 | 0.523 |
Definition of abbreviations: BV5 = blood vessel volume for vessels less than 5 mm2 in cross-sectional area; BV10 = blood vessel volume for vessels less than 10 mm2 in cross-sectional area; CT = computed tomography; PC20 = provocative concentration causing a 20% fall in FEV1; pp = percent predicted; TBV = total pulmonary blood vessel volume.
n = 237 for all analyses except for: methacholine challenge (n = 103), sputum eosinophil % (n = 176), BAL neutrophil % (n = 92), BAL eosinophil % (n = 92), BAL serum amyloid A/lipoxin A4 (n = 58), FEV1 steroid response (n = 223), peripheral eosinophil steroid response (n = 121), sputum eosinophil steroid response (n = 141), fractional exhaled nitric oxide steroid response (n = 218), and air trapping (n = 187).
Figure 4.
Pruning and lung function. r values are based on Pearson correlation. For definition of abbreviations, see Figure 2.
In the multivariable analyses, loss of the peripheral pulmonary vasculature was associated with lower FEV1 pp and percent predicted FVC (FVC pp), and higher BAL SAA/LXA4. It was also associated with greater peripheral and sputum eosinophilia, and a greater reduction in peripheral eosinophils in response to triamcinolone (BV10/TBV only). The largest changes in vascular pruning were associated with the SAA/LXA4 ratio. For every 1 SD increase in BAL SAA/LXA4 BV5/TBV decreased by 0.0233 (confidence interval [CI], 0.0124–0.0341; P < 0.001) and BV10/TBV decreased by 0.0208 (CI, 0.0123–0.0294; P < 0.001) (Table 3). Strong associations were also seen with lung function and peripheral and sputum eosinophilia. For example, for every 1 SD decrease in FEV1 pp BV5/TBV decreased by 0.0170 (CI, 0.0078–0.0261; P < 0.001) and BV10/TBV decreased by 0.0143 (CI, 0.0071–0.0214; P = 0.001), and for every 1 SD increase in peripheral eosinophilia BV5/TBV decreased by 0.0138 (CI, 0.0039–0.0237; P = 0.001) and BV10/TBV decreased by 0.0130 (CI, 0.0053–0.0206; P < 0.001).
Table 3.
Multivariable Associations
| Parameter | BV5/TBV |
BV10/TBV |
||||
|---|---|---|---|---|---|---|
| Change Per 1 SD | Confidence Interval | P Value | Change Per 1 SD | Confidence Interval | P Value | |
| Effect on clinical parameter expressed per 1 SD change in pruning | ||||||
| Prebronchodilator FEV1 pp | 7.10 | 3.28 to 10.92 | <0.001 | 7.28 | 3.55 to 10.73 | 0.001 |
| Prebronchodilator FVC pp | 4.71 | 1.59 to 7.84 | <0.001 | 4.77 | 1.83 to 7.71 | <0.001 |
| Percent change in FEV1 with bronchodilator | 2.05 | −1.01 to 5.10 | 0.370 | 2.18 | −0.72 to 5.08 | 0.237 |
| Methacholine challenge PC20 | −0.15 | −1.37 to 1.06 | 1.000 | −0.20 | −1.37 to 0.96 | 1.000 |
| Asthma control test score | 0.23 | −0.71 to 1.17 | 1.000 | 0.18 | −0.72 to 1.07 | 1.000 |
| Low-attenuation area | 0.12 | −0.34 to 0.58 | 1.000 | 0.23 | −0.21 to 0.67 | 0.732 |
| Percent air trapping on expiratory CT | −2.09 | −4.87 to 0.69 | 0.238 | −1.55 | −4.24 to 1.13 | 0.584 |
| Peripheral eosinophil, % | −1.12 | −1.91 to −0.32 | 0.001 | −1.27 | −2.02 to −0.52 | <0.001 |
| Sputum eosinophil, % | −3.05 | −5.73 to −0.38 | 0.015 | −3.28 | −5.82 to −0.74 | 0.004 |
| BAL eosinophil, % | 0.52 | −2.01 to 3.05 | 1.000 | 0.45 | −1.92 to 2.82 | 1.000 |
| Fractional exhaled nitric oxide | −3.73 | −10.39 to 2.92 | 0.985 | −4.60 | −10.92 to 1.72 | 0.367 |
| Sputum neutrophil, % | 0.03 | −6.97 to 7.03 | 1.000 | −0.49 | −7.19 to 6.21 | 1.000 |
| BAL neutrophil, % | 0.13 | −1.21 to 1.48 | 1.000 | −0.01 | −1.27 to 1.25 | 1.000 |
| BAL serum amyloid A/lipoxin A4 ratio | −269.7 | −441.3 to −98.1 | <0.001 | −268.6 | −418.9 to −118.2 | <0.001 |
| Steroid response | ||||||
| Change in prebronchodilator FEV1 | −0.01 | −0.08 to 0.06 | 1.000 | −0.01 | −0.07 to 0.06 | 1.000 |
| Change in peripheral eosinophil, % | 0.72 | −0.16 to 1.60 | 0.197 | 0.94 | 0.12 to 1.76 | 0.014 |
| Change in sputum eosinophil, % | 1.46 | −1.33 to 4.26 | 1.000 | 1.96 | −0.64 to 4.56 | 0.306 |
| Change in fractional exhaled nitric oxide | 1.04 | −4.01 to 6.09 | 1.000 | 1.77 | −3.04 to 6.58 | 1.000 |
| Effect on pruning measure expressed per 1 SD change in clinical parameter | ||||||
| Prebronchodilator FEV1 pp | 0.0170 | 0.0078 to 0.0261 | <0.001 | 0.0143 | 0.0071 to 0.0214 | 0.001 |
| Prebronchodilator FVC pp | 0.0173 | 0.0058 to 0.0288 | <0.001 | 0.0147 | 0.0056 to 0.0237 | <0.001 |
| Percent change in FEV1 with bronchodilator | 0.0069 | −0.0034 to 0.0172 | 0.370 | 0.0061 | −0.0020 to 0.0142 | 0.237 |
| Methacholine challenge PC20 | −0.0017 | −0.0148 to 0.0115 | 1.000 | −0.0018 | −0.0121 to 0.0085 | 1.000 |
| Asthma control test score | 0.0020 | −0.0063 to 0.0103 | 1.000 | 0.0013 | −0.0052 to 0.0078 | 1.000 |
| Low-attenuation area | 0.0023 | −0.0069 to 0.0116 | 1.000 | 0.0039 | −0.0034 to 0.0111 | 0.732 |
| Percent air trapping on expiratory CT | −0.0080 | −0.0186 to 0.0026 | 0.238 | −0.0048 | −0.0131 to 0.0035 | 0.584 |
| Peripheral eosinophil, % | −0.0138 | −0.0237 to −0.0039 | 0.001 | −0.0130 | −0.0206 to −0.0053 | <0.001 |
| Sputum eosinophil, % | −0.0119 | −0.0223 to −0.0015 | 0.015 | −0.0104 | −0.0185 to −0.0023 | 0.004 |
| BAL eosinophil, % | 0.0022 | −0.0086 to 0.0130 | 1.000 | 0.0016 | −0.0070 to 0.0102 | 1.000 |
| Fractional exhaled nitric oxide | −0.0051 | −0.0143 to 0.0040 | 0.985 | −0.0052 | −0.0123 to 0.0019 | 0.367 |
| Sputum neutrophil, % | 0.00004 | −0.0100 to 0.0100 | 1.000 | −0.0006 | −0.0084 to 0.0072 | 1.000 |
| BAL neutrophil, % | 0.0013 | −0.0117 to 0.0142 | 1.000 | −0.0001 | −0.0104 to 0.0103 | 1.000 |
| BAL serum amyloid A/lipoxin A4 ratio | −0.0233 | −0.0380 to −0.0085 | <0.001 | −0.0208 | −0.0325 to −0.0092 | <0.001 |
| Steroid response | ||||||
| Change in prebronchodilator FEV1 | −0.0012 | −0.0106 to 0.0083 | 1.000 | −0.0007 | −0.0081 to 0.0067 | 1.000 |
| Change in peripheral eosinophil, % | 0.0117 | −0.0026 to 0.0259 | 0.197 | 0.0126 | 0.0016 to 0.0236 | 0.014 |
| Change in sputum eosinophil, % | 0.0058 | −0.0052 to 0.0168 | 1.000 | 0.0065 | −0.0021 to 0.0152 | 0.306 |
| Change in fractional exhaled nitric oxide | 0.0020 | −0.0078 to 0.0119 | 1.000 | 0.0028 | −0.0049 to 0.0105 | 1.000 |
For definition of abbreviations, see Table 2.
For effect on clinical parameter expressed per 1 SD change in pruning, change is expressed as an absolute amount per 1 SD increase in BV5/TBV or BV10/TBV. For instance, for 1 SD increase in BV5/TBV the prebronchodilator FEV1 pp increases by 7.10.
For effect on pruning measure expressed per 1 SD change in clinical parameter, change is expressed as an absolute amount per 1 SD increase in clinical, laboratory, or imaging parameter. For instance, for 1 SD increase in the prebronchodilator FEV1 pp the BV5/TBV increases by 0.0170.
Models were adjusted for age, sex, race, body mass index, percentage of lung occupied by low-attenuation area, CT-measured lung volume, and prebronchodilator FEV1 pp except for the models for the baseline spirometric measures, which were not adjusted for prebronchodilator FEV1 pp, and the models for the densitometric CT measurements, which were not adjusted for low-attenuation area.
P values and confidence intervals given are corrected for multiple comparisons within each group/family as represented by boldface, using the Bonferroni correction. For example, for the spirometric analyses the P values given are adjusted for a total of four comparisons (BV5/TBV with FEV1 and FVC, and BV10/TBV with FEV1/FVC). Confidence intervals are similarly corrected. For example, for the spirometric analyses, the confidence intervals given are the 98.75% confidence intervals.
n = 237 for all analyses except for: methacholine challenge (n = 103), sputum eosinophil % (n = 176), BAL neutrophil % (n = 92), BAL eosinophil % (n = 92), BAL serum amyloid A/lipoxin A4 (n = 58), peripheral eosinophil steroid response (n = 121), sputum eosinophil steroid response (n = 141), fractional exhaled nitric oxide steroid response (n = 218), and air trapping (n = 187).
Finally, compared with individuals with less pruning, individuals with the most vascular pruning (i.e., those in the lowest quartile of BV10/TBV) had a 150% greater odds of reporting an asthma exacerbation during follow-up (BV10/TBV only: odds ratio, 2.50; CI, 1.05–5.98; P = 0.039) (Table 4), and had a 45% higher exacerbation rate during follow-up (BV5/TBV incidence rate ratios, 1.45; CI, 1.00–2.11; P = 0.049) (BV10/TBV incidence rate ratios, 1.45; CI, 1.02–2.06; P = 0.036) (Table 5).
Table 4.
Association between Vascular Pruning and Prospective Asthma Exacerbations
| Odds Ratio for Reporting Asthma Exacerbation during Follow-up | Confidence Interval | P Value | |
|---|---|---|---|
| BV5/TBV (low vs. high) | 1.51 | 0.65 to 3.54 | 0.341 |
| BV10/TBV (low vs. high) | 2.50 | 1.05 to 5.98 | 0.039 |
Definition of abbreviations: BV5 = aggregate vessel volume for vessels less than 5 mm2 in cross-sectional area; BV10 = aggregate vessel volume for vessels less than 10 mm2 in cross-sectional area; TBV = total pulmonary blood vessel volume.
BV5/TBV and BV10/TBV dichotomized at 25th percentile. That is, effect estimates are expressed as odds of those with the most pruning (top 25%) vs. those with less pruning (bottom 75%).
All models were adjusted for baseline values of age, sex, race, body mass index, prebronchodilator FEV1 percent predicted, and asthma control test score as well as for a reported history of exacerbation in the year before the baseline visit.
n with longitudinal data available = 214. n who reported exacerbation during follow-up = 87 (40.7%).
Table 5.
Association between Vascular Pruning and Prospective Asthma Exacerbation Rate
| Incidence Rate Ratio for Asthma Exacerbations during Follow-up | Confidence Interval | P Value | |
|---|---|---|---|
| BV5/TBV (low vs. high) | 1.45 | 1.00 to 2.11 | 0.049 |
| BV10/TBV (low vs. high) | 1.45 | 1.02 to 2.06 | 0.036 |
For definition of abbreviations, see Table 4.
BV5/TBV and BV10/TBV dichotomized at 25th percentile. That is, effect estimates are expressed as the rate of those with the most pruning (top 25%) vs. those with less pruning (bottom 75%).
All models were adjusted for baseline values of age, sex, race, body mass index, prebronchodilator FEV1 percent predicted, and asthma control test score as well as for a reported history of exacerbation in the year before the baseline visit.
n with longitudinal data available = 214. n who reported exacerbation during follow-up = 87 (40.7%).
Discussion
In this study of patients with severe and mild-moderate asthma, we found that loss of the peripheral pulmonary vasculature on CT scan was associated with a trend toward worse asthma severity and control. In addition, pulmonary vascular pruning, measured as a lower small vessel volume fraction, was associated with worse lung function, greater eosinophilic inflammation, and a higher BAL SAA/LXA4 ratio. Finally, compared with those with less pruning, those who had the most pruning had a higher odds of having an asthma exacerbation during follow-up and had a higher rate of asthma exacerbations during follow-up.
Our data suggest that pulmonary vascular pruning is associated with increased asthma severity and reduced lung function as well as eosinophil specific inflammation. Whether pruning contributes to this severity or is a physiologic or pathophysiologic result of the severity is unclear. Much of the prior work on the vasculature within the lung in patients with asthma has focused on the bronchial circulation, whereas the involvement of the pulmonary circulation is less well characterized (7, 14, 15, 18, 55–59). In a small autopsy study, Saetta and coworkers (60) examined the muscular pulmonary arteries adjacent to peripheral airways from patients with fatal asthma and found that the pulmonary arteries did not differ in size, intimal thickness, medial thickness, or adventitial thickness compared with those from control subjects, but did have more adventitial eosinophilic inflammation. In a series of studies, Rydell-Törmänen and coworkers (8, 12) found that allergic airway inflammation was associated with increased eosinophilic vascular inflammation, vascular smooth muscle remodeling, perivascular alterations in collagen synthesis, and increase in endothelial cell proliferation in allergen-exposed BALB/C mice. Their work also suggests that some of this vascular remodeling may be irreversible (10, 11).
Although these studies generally examined vasculature much smaller than described in our current work, they suggest possible biologic plausibility for our findings. Namely, severe asthma may have a previously unappreciated “small vessel” component, even in the absence of clinically apparent eosinophilic granulomatous polyangiitis. This may be in addition to or associated with the “small airways” aspect of the disease. Additional studies that include both CT imaging and biopsy or autopsy specimens are needed to demonstrate if this is the case. If it is, then it would be important to determine if in severe asthma these vascular changes are present in the small vessels of other organ systems as they are in other chronic diseases (61, 62).
The very strong association between the SAA/LXA4 ratio and pulmonary vascular pruning is also intriguing. SAA is associated with BAL neutrophilia, and recently a high SAA, low LXA4 endotype has been described that is associated with neutrophilic lung inflammation and poor asthma control (63, 64). However, SAA is also increased in severe allergic asthma, and may function as an adjuvant to promote allergy to an innocuous inhaled allergen (65, 66). LXA4 is an arachidonic acid–derived specialized proresolving mediator, which is protective in murine models of lung inflammation and is decreased in the sputum and BAL of patients with severe asthma (67). Moreover, LXA4 administration in ovalbumin-allergic mice decreases airway hyperresponsiveness and reduces lung tissue eosinophilia and vascular injury (68). It is therefore conceivable that increased SAA induced inflammation in the setting of low LXA4 in severe asthma is associated with pulmonary vascular injury and pruning.
Although these pathophysiologic mechanisms for our finding are possible, alternative mechanisms for our observation of increased vascular pruning in severe asthma certainly exist and include functional rather than anatomic causes of decreased regional blood flow. For instance, airway constriction or occlusion, possibly caused by mucus plugging, may result in regional hypoventilation and hypoxia, which in turn may lead to hypoxic vasoconstriction of the small pulmonary vessels in that region. Although this likely occurs to some extent, recent work using positron emission tomography/CT imaging suggests that it is not the sole mechanism for regional perfusion redistribution in bronchoconstricted patients with asthma, and other functional explanations are also likely to exist (60, 69). For instance, regional hypoventilation results in regional hyperinflation that may lead to increased alveolar pressure and compression of the pulmonary vasculature similar to what occurs in West’s zone 1 (69–71). The lack of association between measures of vascular pruning and CT densitometry measured air trapping on expiratory imaging in the adjusted analyses in this study argue against this being the primary explanation for our findings. However, BV10/TBV was associated with CT-measured air trapping in the univariate analyses, a trend existed for the association between BV5/TBV and CT air trapping in the adjusted analyses, and expiratory imaging was only available in a subset of the participants. In addition, although correlated, air trapping on expiratory imaging and regional hyperinflation do not always overlap (72). Thus, further work that combines measurements of the pulmonary vasculature with regional measures of ventilation, air trapping, and hyperinflation, such as magnetic resonance imaging, parametric response mapping, and positron emission tomography, is needed to investigate these functional possibilities more fully (69, 70, 73–76).
Other potential explanations for our findings include a direct mechanical effect of airway constriction on the pulmonary vasculature or that asthma and pulmonary vascular remodeling share certain signaling pathways (69, 77). For instance, activation of the transcription factor NFAT (nuclear factor of activated T cells) has been implicated in the pathogenesis of both asthma and pulmonary arterial hypertension (7). If these diseases do share this mechanism of pathogenesis then stimulation of vasoactive intestinal peptide, or another inhibitor of NFAT activation, could be useful in the treatment of both (7).
One striking finding from our study is the lack of association between vascular pruning and a radiologic measure of emphysema, LAA (78). Although we were unable to definitively demonstrate a lack of emphysema in patients with severe asthma using histopathology, the absence of an association between LAA and pruning suggests that loss of the distal pulmonary vasculature in severe asthma is unlikely to be caused by clinically occult emphysema, or by misclassification of COPD. That said, in the absence of direct evaluation of patients’ histopathology we are unable to say definitively that emphysema is not present. In addition, more broadly, without pathologic evaluation we are unable to definitely answer the question of whether there are pathologic changes to the distal pulmonary vasculature in the individuals with severe asthma who had vascular pruning. Future work that involves pathologic evaluation in combination with additional functional imaging is needed to better determine the precise mechanisms of our findings. Additional work is also needed using arterial-venous segmentation to determine if the associations seen in our study are differentially driven by arterial or venous pruning.
With regard to other limitations, the resolution of the CT scanning may limit the detection of the very small vasculature, which may partially explain our more robust findings with BV10/TBV than with BV5/TBV. Finally, the differences in pruning measures between asthma groups were relatively small and given the cohort size we were unable to demonstrate pairwise differences in pruning between each of the asthma severity and control groups. Similarly, although many of the correlations between pruning and the continuous measures of disease severity were statistically significant, the correlation coefficients themselves were relatively low. This may be caused in part by the heterogeneity of the disease and the imprecision of some of the measurement techniques. Further work is needed in other cohorts to determine if this is the case.
Using quantitative CT analysis, in the SARP III cohort, we found that individuals with severe asthma had more pruning of the pulmonary vasculature than those with mild-moderate asthma or healthy control subjects. In addition, pulmonary vascular pruning was associated with worse lung function and eosinophilia, and increased odds of asthma exacerbation. If these findings are replicated and found to have pathologic correlates they may suggest that asthma is not only an airways disease but a pulmonary vascular one as well.
Footnotes
Supported by NHLBI grants to the Severe Asthma Research Program Principal Investigators, Clinical Centers, and Data Coordinating Center as follows: U10 HL109164 (E.R.B.), U10 HL109257 (M.C.), U10 HL109250 (B.M.G.), U10 HL109146 (J.V.F.), U10 HL109250 (B.M.G.), U10 HL109172 (E.I. and B.D.L.), U10 HL109168 (N.N.J.), U10 HL109152 (S.E.W.), and U10 HL109086 (D.T.M.). In addition, this program is supported through the following NIH National Center for Advancing Translational Sciences awards: UL1 TR001420 (Wake Forest University), UL1 TR000427 (University of Wisconsin), and UL1 TR001102 (Harvard University). Additional support for the development of the vascular analysis tool was provided by R01 HL116473 (R.S.J.E. and G.R.W.). Additional support was provided by K23 HL136905 (F.N.R.), K23 HL114735 (C.E.C.), and T32 HL007633 (S.Y.A.).
Author Contributions: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. S.Y.A., F.N.R., B.D.L., R.S.J.E., E.I., and G.R.W. designed the initial study and wrote the initial manuscript. S.Y.A. and F.N.R performed the literature search and the statistical analyses, and created the figures. F.N.R. and R.S.J.E. developed the vascular analysis tool and deployed it in the available data. S.Y.A., F.N.R., C.E.C., J.C.R., A.G.C., J.C.C.-G., E.M.D., E.R.B., M.C., J.V.F., S.B.F., B.M.G., E.A.H., N.N.J., D.T.M., and S.E.W. contributed to the final study design and to the production of the final manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201712-2426OC on April 19, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: SARP Investigators
References
- 1.Cordasco EM, Beerel FR, Vance JW, Wende RW, Toffolo RR. Newer aspects of the pulmonary vasculature in chronic lung disease. A comparative study. Angiology. 1968;19:399–407. doi: 10.1177/000331976801900703. [DOI] [PubMed] [Google Scholar]
- 2.Hale KA, Niewoehner DE, Cosio MG. Morphologic changes in the muscular pulmonary arteries: relationship to cigarette smoking, airway disease, and emphysema. Am Rev Respir Dis. 1980;122:273–278. doi: 10.1164/arrd.1980.122.2.273. [DOI] [PubMed] [Google Scholar]
- 3.Minai OA, Chaouat A, Adnot S. Pulmonary hypertension in COPD: epidemiology, significance, and management: pulmonary vascular disease: the global perspective. Chest. 2010;137(Suppl. 6):39S–51S. doi: 10.1378/chest.10-0087. [DOI] [PubMed] [Google Scholar]
- 4.Wright JL, Lawson L, Paré PD, Hooper RO, Peretz DI, Nelems JM, et al. The structure and function of the pulmonary vasculature in mild chronic obstructive pulmonary disease. The effect of oxygen and exercise. Am Rev Respir Dis. 1983;128:702–707. doi: 10.1164/arrd.1983.128.4.702. [DOI] [PubMed] [Google Scholar]
- 5.Magee F, Wright JL, Wiggs BR, Paré PD, Hogg JC. Pulmonary vascular structure and function in chronic obstructive pulmonary disease. Thorax. 1988;43:183–189. doi: 10.1136/thx.43.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J. 2008;32:1371–1385. doi: 10.1183/09031936.00015608. [DOI] [PubMed] [Google Scholar]
- 7.Said SI, Hamidi SA, Gonzalez Bosc L. Asthma and pulmonary arterial hypertension: do they share a key mechanism of pathogenesis? Eur Respir J. 2010;35:730–734. doi: 10.1183/09031936.00097109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rydell-Törmänen K, Uller L, Persson CG, Erjefält JS. Allergen exposure of mouse airways evokes remodeling of both bronchi and large pulmonary vessels. Am J Respir Crit Care Med. 2005;171:19–25. doi: 10.1164/rccm.200406-698OC. [DOI] [PubMed] [Google Scholar]
- 9.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med. 2008;205:361–372. doi: 10.1084/jem.20071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rydell-Törmänen K, Uller L, Erjefält JS. Allergic airway inflammation initiates long-term vascular remodeling of the pulmonary circulation. Int Arch Allergy Immunol. 2009;149:251–258. doi: 10.1159/000199721. [DOI] [PubMed] [Google Scholar]
- 11.Rydell-Törmänen K, Johnson JR, Fattouh R, Jordana M, Erjefält JS. Induction of vascular remodeling in the lung by chronic house dust mite exposure. Am J Respir Cell Mol Biol. 2008;39:61–67. doi: 10.1165/rcmb.2007-0441OC. [DOI] [PubMed] [Google Scholar]
- 12.Rydell-Törmänen K, Uller L, Erjefält JS. Remodeling of extra-bronchial lung vasculature following allergic airway inflammation. Respir Res. 2008;9:18. doi: 10.1186/1465-9921-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harkavy J. Vascular allergy. Clinics. 1946;5:504–549. [PubMed] [Google Scholar]
- 14.Harkness LM, Ashton AW, Burgess JK. Asthma is not only an airway disease, but also a vascular disease. Pharmacol Ther. 2015;148:17–33. doi: 10.1016/j.pharmthera.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Harkness LM, Kanabar V, Sharma HS, Westergren-Thorsson G, Larsson-Callerfelt AK. Pulmonary vascular changes in asthma and COPD. Pulm Pharmacol Ther. 2014;29:144–155. doi: 10.1016/j.pupt.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Estépar RS, Kinney GL, Black-Shinn JL, Bowler RP, Kindlmann GL, Ross JC, et al. COPDGene Study. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188:231–239. doi: 10.1164/rccm.201301-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahaghi FN, Wells JM, Come CE, De La Bruere IA, Bhatt SP, Ross JC, et al. and the COPDGene Investigators. Arterial and venous pulmonary vascular morphology and their relationship to findings in cardiac magnetic resonance imaging in smokers. J Comput Assist Tomogr. 2016;40:948–952. doi: 10.1097/RCT.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avdalovic M. Pulmonary vasculature and critical asthma syndromes: a comprehensive review. Clin Rev Allergy Immunol. 2015;48:97–103. doi: 10.1007/s12016-014-8420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ash SY, Rahaghi FN, Cardet JC, Come CE, Ross J, Diaz AA, et al. Associations between pruning of the pulmonary vasculature, impaired lung function and fixed obstruction in asthmatics: the SARP cohort [abstract] Am J Respir Crit Care Med. 2017;195:A4867. [Google Scholar]
- 20.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. National Heart, Lung, Blood Institute’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, et al. NHLBI Severe Asthma Research Program (SARP) Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185:356–362. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 23.National Asthma Education and Prevention Program. National Asthma Education and Prevention Program. Expert panel report: guidelines for the diagnosis and management of asthma update on selected topics--2002. J Allergy Clin Immunol. 2002;110(Suppl. 5):S141–S219. [PubMed] [Google Scholar]
- 24.Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, et al. Severe Asthma Research Program. Effects of age and disease severity on systemic corticosteroid responses in asthma. Am J Respir Crit Care Med. 2017;195:1439–1448. doi: 10.1164/rccm.201607-1453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 26.Juniper EF, Gruffydd-Jones K, Ward S, Svensson K. Asthma Control Questionnaire in children: validation, measurement properties, interpretation. Eur Respir J. 2010;36:1410–1416. doi: 10.1183/09031936.00117509. [DOI] [PubMed] [Google Scholar]
- 27.Brusasco V, Crapo R, Viegi G American Thoracic Society; European Respiratory Society. Coming together: the ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J. 2005;26:1–2. doi: 10.1183/09031936.05.00034205. [DOI] [PubMed] [Google Scholar]
- 28.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. ATS/ERS Task Force. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 29.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 30.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 31.Brand PL, Quanjer PH, Postma DS, Kerstjens HA, Koëter GH, Dekhuijzen PN, et al. The Dutch Chronic Non-Specific Lung Disease (CNSLD) Study Group. Interpretation of bronchodilator response in patients with obstructive airways disease. Thorax. 1992;47:429–436. doi: 10.1136/thx.47.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popa V. ATS guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med. 2001;163:292–293. doi: 10.1164/ajrccm.163.1.16310b. [DOI] [PubMed] [Google Scholar]
- 34.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Teague WG, Erzurum S, Fitzpatrick A, Mantri S, Dweik RA, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program (SARP) Determinants of exhaled breath condensate pH in a large population with asthma. Chest. 2011;139:328–336. doi: 10.1378/chest.10-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125:1028–1036. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. “Refractory” eosinophilic airway inflammation in severe asthma: effect of parenteral corticosteroids. Am J Respir Crit Care Med. 2004;170:601–605. doi: 10.1164/rccm.200404-440OC. [DOI] [PubMed] [Google Scholar]
- 38.Sieren JP, Newell JD, Jr, Barr RG, Bleecker ER, Burnette N, Carretta EE, et al. SPIROMICS Research Group. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194:794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi S, Hoffman EA, Wenzel SE, Castro M, Fain S, Jarjour N, et al. Quantitative computed tomographic imaging-based clustering differentiates asthmatic subgroups with distinctive clinical phenotypes. J Allergy Clin Immunol. 2017;140:690–700. doi: 10.1016/j.jaci.2016.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross JC, San Jose Estepar R, Kindlmann G, Diaz A, Westin CF, Silverman EK, et al. Automatic lung lobe segmentation using particles, thin plate splines, and maximum a posteriori estimation. Med Image Comput Comput Assist Interv. 2010;13:163–171. doi: 10.1007/978-3-642-15711-0_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Estepar RS, Ross JC, Krissian K, Schultz T, Washko GR, Kindlmann GL. Computational vascular morphometry for the assessment of pulmonary vascular disease based on scale-space particles; Proc IEEE Int Symp Biomed Imaging; 2012. pp. 1479–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madani A, Van Muylem A, Gevenois PA. Pulmonary emphysema: effect of lung volume on objective quantification at thin-section CT. Radiology. 2010;257:260–268. doi: 10.1148/radiol.10091446. [DOI] [PubMed] [Google Scholar]
- 43.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 44.Gevenois PA, De Vuyst P, de Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154:187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 45.Gevenois PA, Yernault JC. Can computed tomography quantify pulmonary emphysema? Eur Respir J. 1995;8:843–848. [PubMed] [Google Scholar]
- 46.Wang Z, Gu S, Leader JK, Kundu S, Tedrow JR, Sciurba FC, et al. Optimal threshold in CT quantification of emphysema. Eur Radiol. 2013;23:975–984. doi: 10.1007/s00330-012-2683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mets OM, van Hulst RA, Jacobs C, van Ginneken B, de Jong PA. Normal range of emphysema and air trapping on CT in young men. AJR Am J Roentgenol. 2012;199:336–340. doi: 10.2214/AJR.11.7808. [DOI] [PubMed] [Google Scholar]
- 48.Matsuoka S, Washko GR, Dransfield MT, Yamashiro T, San Jose Estepar R, Diaz A, et al. Quantitative CT measurement of cross-sectional area of small pulmonary vessel in COPD: correlations with emphysema and airflow limitation. Acad Radiol. 2010;17:93–99. doi: 10.1016/j.acra.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuoka S, Washko GR, Yamashiro T, Estepar RS, Diaz A, Silverman EK, et al. National Emphysema Treatment Trial Research Group. Pulmonary hypertension and computed tomography measurement of small pulmonary vessels in severe emphysema. Am J Respir Crit Care Med. 2010;181:218–225. doi: 10.1164/rccm.200908-1189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Estepar RS, Ross JC, Harmouche R, Onieva J, Diaz AA, Washko GR. Chest Imaging Platform: an open-source library and workstation for quantitative chest imaging [abstract] Am J Respir Crit Care Med. 2015;191:A4975. [Google Scholar]
- 52.Mahrer JM, Magel RC. A comparison of tests for the k-sample, non-decreasing alternative. Stat Med. 1995;14:863–871. doi: 10.1002/sim.4780140814. [DOI] [PubMed] [Google Scholar]
- 53.Spurrier JD. Additional tables for Steel-Dwass-Critchlow-Fligner distribution-free multiple comparisons of three treatments. Commun Stat Simul Comput. 2006;35:441–446. [Google Scholar]
- 54.Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary AJ, et al. COPDGene Investigators; ECLIPSE Study Investigators. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367:913–921. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156:229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- 56.Van der Velden J, Barker D, Barcham G, Koumoundouros E, Snibson K. Increased vascular density is a persistent feature of airway remodeling in a sheep model of chronic asthma. Exp Lung Res. 2012;38:307–315. doi: 10.3109/01902148.2012.697975. [DOI] [PubMed] [Google Scholar]
- 57.McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–S45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- 58.Hashimoto M, Tanaka H, Abe S. Quantitative analysis of bronchial wall vascularity in the medium and small airways of patients with asthma and COPD. Chest. 2005;127:965–972. doi: 10.1378/chest.127.3.965. [DOI] [PubMed] [Google Scholar]
- 59.Carroll NG, Cooke C, James AL. Bronchial blood vessel dimensions in asthma. Am J Respir Crit Care Med. 1997;155:689–695. doi: 10.1164/ajrccm.155.2.9032214. [DOI] [PubMed] [Google Scholar]
- 60.Saetta M, Di Stefano A, Rosina C, Thiene G, Fabbri LM. Quantitative structural analysis of peripheral airways and arteries in sudden fatal asthma. Am Rev Respir Dis. 1991;143:138–143. doi: 10.1164/ajrccm/143.1.138. [DOI] [PubMed] [Google Scholar]
- 61.Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, et al. Diabetic microvascular disease: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2017;102:4343–4410. doi: 10.1210/jc.2017-01922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Agtmaal MJM, Houben AJHM, Pouwer F, Stehouwer CDA, Schram MT. Association of microvascular dysfunction with late-life depression: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:729–739. doi: 10.1001/jamapsychiatry.2017.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ricklefs I, Barkas I, Duvall MG, Cernadas M, Grossman NL, Israel E, et al. National Heart Lung, Blood Institute’s Severe Asthma Research Program 3 Investigators. ALX receptor ligands define a biochemical endotype for severe asthma. JCI Insight. [online ahead of print] 2017 Jul 20; DOI: 10.1172/jci.insight.93534. [Google Scholar]
- 64.Bozinovski S, Uddin M, Vlahos R, Thompson M, McQualter JL, Merritt AS, et al. Serum amyloid A opposes lipoxin A4 to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc Natl Acad Sci USA. 2012;109:935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ather JL, Ckless K, Martin R, Foley KL, Suratt BT, Boyson JE, et al. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J Immunol. 2011;187:64–73. doi: 10.4049/jimmunol.1100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Büyüköztürk S, Gelincik AA, Genç S, Koçak H, Oneriyidogan Y, Erden S, et al. Acute phase reactants in allergic airway disease. Tohoku J Exp Med. 2004;204:209–213. doi: 10.1620/tjem.204.209. [DOI] [PubMed] [Google Scholar]
- 67.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt B, et al. Lipoxins and aspirin-triggered lipoxins in airway responses. Adv Exp Med Biol. 2003;525:19–23. doi: 10.1007/978-1-4419-9194-2_5. [DOI] [PubMed] [Google Scholar]
- 68.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 69.Kelly VJ, Hibbert KA, Kohli P, Kone M, Greenblatt EE, Venegas JG, et al. Hypoxic pulmonary vasoconstriction does not explain all regional perfusion redistribution in asthma. Am J Respir Crit Care Med. 2017;196:834–844. doi: 10.1164/rccm.201612-2438OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harris RS, Winkler T, Tgavalekos N, Musch G, Melo MF, Schroeder T, et al. Regional pulmonary perfusion, inflation, and ventilation defects in bronchoconstricted patients with asthma. Am J Respir Crit Care Med. 2006;174:245–253. doi: 10.1164/rccm.200510-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.West JB, Dollery CT, Naimark A. Distribution of blood flow in isolated lung; relation to vascular and alveolar pressures. J Appl Physiol. 1964;19:713–724. doi: 10.1152/jappl.1964.19.4.713. [DOI] [PubMed] [Google Scholar]
- 72.Smith BM, Hoffman EA, Basner RC, Kawut SM, Kalhan R, Barr RG. Not all measures of hyperinflation are created equal: lung structure and clinical correlates of gas trapping and hyperexpansion in COPD: the Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study. Chest. 2014;145:1305–1315. doi: 10.1378/chest.13-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Svenningsen S, Guo F, Kirby M, Choy S, Wheatley A, McCormack DG, et al. Pulmonary functional magnetic resonance imaging: asthma temporal-spatial maps. Acad Radiol. 2014;21:1402–1410. doi: 10.1016/j.acra.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 74.Svenningsen S, Kirby M, Starr D, Coxson HO, Paterson NA, McCormack DG, et al. What are ventilation defects in asthma? Thorax. 2014;69:63–71. doi: 10.1136/thoraxjnl-2013-203711. [DOI] [PubMed] [Google Scholar]
- 75.Martinez CH, Diaz AA, Meldrum C, Curtis JL, Cooper CB, Pirozzi C, et al. SPIROMICS Investigators. Age and small airway imaging abnormalities in subjects with and without airflow obstruction in SPIROMICS. Am J Respir Crit Care Med. 2017;195:464–472. doi: 10.1164/rccm.201604-0871OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Capaldi DP, Zha N, Guo F, Pike D, McCormack DG, Kirby M, et al. Pulmonary imaging biomarkers of gas trapping and emphysema in COPD: (3)He MR imaging and CT parametric response maps. Radiology. 2016;279:597–608. doi: 10.1148/radiol.2015151484. [DOI] [PubMed] [Google Scholar]
- 77.Hiorns JE, Bidan CM, Jensen OE, Gosens R, Kistemaker LE, Fredberg JJ, et al. Airway and parenchymal strains during bronchoconstriction in the precision cut lung slice. Front Physiol. 2016;7:309. doi: 10.3389/fphys.2016.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Müller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest. 1988;94:782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]