Since the last update in this series was published in 2015, pulmonary vascular medicine has seen important advances from the molecular to population levels (1). Basic, translational, and epidemiologic science have rapidly advanced the understanding of how genetic, endocrine, immune, and metabolic factors contribute to pulmonary vascular disease. Preclinical and early-stage clinical efforts highlight promising treatments addressing emerging targets in the pathophysiology of pulmonary arterial hypertension (PAH).
Mechanisms of Sex Bias
Prior work exploring the molecular basis of sex bias in PAH, namely increased female susceptibility and survival in PAH, has been focused on the regulation and activity of estrogen in women. The importance of sex hormone activity was recently extended to men with PAH, in whom worse hemodynamic and functional parameters correlate with higher concentrations of estradiol and lower concentrations of dehydroepiandrosterone sulfate (2). Indeed, the observed low concentrations of dehydroepiandrosterone sulfate associated with pulmonary hypertension (PH) risk have been reproduced (3). In mice carrying a pathogenic BMPR2 (bone morphogenetic protein receptor type 2) mutant gene, inhibition of estrogen activity mitigated experimental PH and normalized critical metabolic signaling axes (4). The aryl hydrocarbon receptor regulates downstream estrogen production in pulmonary vascular cells via both CYP1A1 (cytochrome P450 family 1 subfamily A member 1) and aromatase. The aryl hydrocarbon receptor can be targeted in BMPR2-overexpressing mice (5), increasing microRNA (miR)-29, which recapitulates elevated miR-29 found in heritable pulmonary arterial hypertension (HPAH) lung tissue. By contrast, anti–miR-29 attenuates experimental PH and restores PPAR-γ (peroxisome proliferator-activated receptor-γ) (6). Finally, the Y chromosome appears to protect against experimental PH (7), offering a novel genetic explanation for sex bias in PAH.
Signaling
Genetic, experimental, and pharmacologic lines of evidence continue to reveal mechanisms by which BMPR2 mutations and dysregulated BMP (bone morphogenetic protein) and TGF-β (transforming growth factor-β) receptor family signaling drive PAH. TNF-α (tumor necrosis factor-α) inhibits BMPR2 expression and promotes post-translational cleavage via ADAM (a disintegrin and metalloproteinase domain-containing protein)-10 and ADAM17 in pulmonary arterial smooth muscle cells (PASMCs), favoring BMP-mediated proliferation via ACVR2 (activin receptor type 2), whereas anti–TNF-α treatment restores BMP signaling and reverses disease progression in experimental PH (8). Linking metabolic dysfunction and idiopathic pulmonary arterial hypertension (IPAH), exposure of PASMCs to high glucose concentrations increases expression of SMURF1, an E3 ubiquitin–protein ligase that dampens BMP signaling and decreases phospho-SMAD1/5/8 (fusion of Caenorhabditis elegans Sma genes and the Drosophila Mad, Mothers against decapentaplegic), mimicking signaling patterns in mutation-negative IPAH-derived PASMCs that are normalized by blocking glucose uptake (9). As a potential regulator of the balance between protective BMPR2 and deleterious TGF-β signaling, elevated miR-130a/301b, previously found in PAH tissues (10), acts downstream of TGF-β1 in PASMCs to downregulate protective BMPR2 and PPAR-γ signaling, consistent with amelioration of experimental PH in TGF-β1–transgenic mice by the PPAR-γ agonist pioglitazone (11, 12). Consistent with the pathogenic role of TGF-β, a selective TGF-β1/3 trap dampened TGF-β–Smad2–plasminogen activator inhibitor 1 signaling and inhibited pulmonary vascular remodeling in experimental PH without cardiotoxicity previously associated with anaplastic lymphoma kinase (ALK) 4/5/7 inhibitors, suggesting a translatable approach (13). SMAD3, typically considered an effector of TGF-β signaling and fibrosis, was paradoxically decreased in PAH lungs and pulmonary arteries (PAs) derived from experimental PH, whereas SMAD3 depletion caused proliferation and migration of PASMCs and pulmonary artery endothelial cells (PAECs) and increased expression of smooth muscle actin in PASMCs mediated by myocardin-related transcriptional factor (14). Expression of HMGA1 (high mobility group AT hook 1) is elevated in PAH versus control PAECs and is coexpressed with smooth muscle markers in obstructive and plexiform lesions, suggesting endothelial-to-mesenchymal transition (15). VEGF3 (vascular endothelial growth factor 3) and hypermethylation of BMPR2 emerged as potential penetrance factors in HPAH. BMP-mediated receptor endocytosis and SMAD signaling are impaired in VEGFR3-deficient zebrafish and mice, the latter of which develop exaggerated experimental PH (16). Hypermethylation of the BMPR2 promoter was more frequent in affected versus unaffected mutation carriers from 11 families (17). The notion that BMPR2 mutations influence vascular structure and development was supported by the observation of dilated and thickened bronchial arteries in BMPR2 mutation–positive (HPAH) versus BMPR2 mutation–negative (IPAH) lungs (18).
MicroRNA Biology
Noncoding RNAs including miRNAs continue to emerge as critical regulators of PAH pathogenesis (19), and an endocrine function of active extracellular miRNAs packaged in microvesicles as a means of cell-to-cell communication in experimental PH was recently supported (20). Specific miRNAs are regulatory factors in metabolic dysregulation, BMP signaling, and cellular plasticity in PAH. A miR-124/PTBP1 (polypyrimidine tract binding protein 1)/PKM (pyruvate kinase muscle isozyme) axis regulates glycolysis in endothelial cells (21) and adventitial fibroblasts (22) to promote cellular proliferation and PH. miR-138– and miR-25–dependent signaling contributes to PH in monocrotaline-exposed rats through impairment of the mitochondrial calcium uniporter complex (23). BMP signaling in PAH appears to be regulated by miR-140-5p via its effects on SMURF1 (24), and miR-204 induces transdifferentiation of smooth muscle to osteoblast-like cells, potentially enhancing calcification of diseased PAs (25).
Stem Cell Technology
Inducible pluripotent stem cells (iPSCs) are stem cells that can be reprogrammed directly from adult cells and subsequently can be differentiated in vitro into a variety of mature cell types, including cardiopulmonary vascular cells. iPSCs retain their original genomic sequences from their individual donors and thus have been used advantageously to model the role of BMPR2 in HPAH (26, 27). iPSCs hold promise for future strategies for “retuning” BMP signaling that are difficult to test in traditional cellular or animal models, thus potentially facilitating high-throughput drug screening in PAH.
Vascular Stiffness
Vascular stiffness has emerged as a key pathogenetic driver in PAH (28, 29), with evidence that vascular matrix stiffness is an early, pervasive driver of many types of PAH (30, 31) that is regulated via mechanoactivation by two related transcriptional coactivators, YAP (Yes-associated protein) and TAZ (transcriptional co-activator with PDZ-binding motif), members of the Hippo signaling pathway. Their activation initiates vascular cell proliferation in PAH (32), in part via anaplerosis, a metabolic pathway that replenishes tricarboxylic acid cycle intermediates, providing cellular biomass for hyperproliferative vascular phenotypes in PAH (33).
Vascular Tone
Identification of loss-of-function mutations in the KCNK3 (potassium two-pore domain channel subfamily K member 3) gene in HPAH led to the elucidation of its impact in plasma membrane depolarization and regulation of pulmonary arterial tone, vascular remodeling, and consequent PH in vivo (34). Omega-3 fatty acids such as docosahexaenoic acid regulate pulmonary vascular tone in PH (35) via activation of the calcium-activated potassium (KCa) currents in PASMCs and pulmonary vasodilation. Hemoglobin-α, an avid scavenger of nitric oxide in diseased vascular endothelium, also contributes to altered pulmonary vasomotor tone in PAH (36).
Other Signaling Pathways
Emerging targets in PAH include Notch signaling, a system of ligands and transmembrane receptors that regulate embryonic development and vascular homeostasis. Whereas Notch3 is known to control proliferation and differentiation in PAH (37), Notch1 is a crucial regulator of endothelial proliferation and survival (38), and Notch2 is a key effector bridging BMPR2 and TNF signaling in PAH (8). The important role of osteoprotegerin in PAH continues to be further elucidated (39). The therapeutic potential of rescuing expression of SERCA2A (sarcoplasmic reticulum Ca2+-ATPase 2a) in pulmonary vascular remodeling and right ventricular (RV) dysfunction has been reported (40).
The effect of pulmonary vascular tone on RV energy use is increasingly recognized. Among treatment-naive patients with PAH, the administration of prostacyclin improved RV stroke volume through a reduction in end-systolic volume that was modulated predominantly via improved RV contractility and decreased diastolic stiffness (41). These data and findings derived from other similar studies (42) imply an opportunity to enhance RV energy use efficiency, and thus performance, by normalized pulmonary vascular stiffness and tone (reviewed in detail in [43]). In summary, new insights into the molecular and cellular regulation of pulmonary vascular tone and vascular cell fate, as well as the diverse contributions of sex hormones, glucose metabolism, and inflammatory cytokines, reveal a remarkable degree of convergence of these influences on common effectors of the BMP/TGF-β, PPAR-γ, and other pivotal axes of PAH (Figure 1).
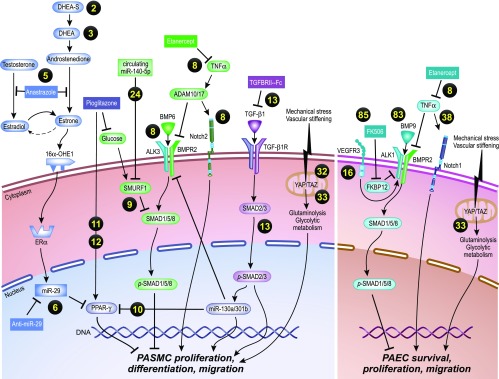
Figure 1.
Novel molecular and cellular insights into pulmonary arterial hypertension signaling reveals convergence of sex hormone, glucose metabolism, and inflammatory signaling on common effectors of the BMP (bone morphogenetic protein)/TGF-β (transforming growth factor) and PPAR-γ (peroxisome proliferator-activated receptor-γ) axes and other signaling axes. The yellow numbers indicate references. Illustration by Jacqueline Schaffer. 16α-OHE1 = 16α hydroxyestrone 1; ADAM = a disintegrin and metalloprotease; ALK = activin-like kinase; BMPR2 = BMP receptor type 2; DHEA = dehydroepiandrosterone; DHEA-S = dehydroepiandrosterone sulfate; ERα = estrogen receptor α; Fc = fragment crystallizable region; FK506 = tacrolimus; FKBP12 = FK506 binding protein; miR = microRNA; PAEC = pulmonary artery endothelial cell; PASMC = pulmonary artery smooth muscle cell; p-SMAD = phosphorylated SMAD; SMAD = fusion of Caenorhabditis elegans Sma genes and the Drosophila Mad, Mothers against decapentaplegic; SMURF1 = E3 ubiquitin-protein ligase SMURF1; TAZ = transcriptional co-activator with PDZ-binding motif; TGF-β1 = transforming growth factor β-1; TGF-β1R = TGF-β1 receptor; TNFα = tumor necrosis factor α; VEGFR3 = vascular endothelial growth factor receptor 3; YAP = Yes-8 associated protein.
Immunity and Inflammation in PAH
The long-standing notion that inflammation and immunity contribute to remodeling of the pulmonary vascular bed in PAH (44–47) has been extended by observations that pulmonary vascular lesions in patients with PAH and animal models include perivascular inflammatory infiltrates comprised of T and B lymphocytes, macrophages, dendritic cells, and mast cells (48–51). In addition, PAH is newly associated with IL-1R1/MyD88 signaling, CCL2 (CC chemokine ligand 2), CXCL-1 (chemokine [C-X-C motif] ligand 1), and PHD (prolyl 4-hydroxylase) deficiency; and regulatory T-cell dysfunction (52–55). Macrophages, bone marrow–derived cells, and myeloid-derived suppressor cells may represent important local sources of factors that regulate pulmonary vascular remodeling (49, 56, 57) which may initiate maladaptive immune and inflammatory responses through immune cell recruitment and feedback regulation. Factors further contributing to the activation of an immune response are aging of the pulmonary circulation, chronic exposure to hypoxia, dysregulation in BMPR2-mediated signaling, shear stress, metabolic derangements, and circulating autoantibodies and immune complexes (58–61).
Genetics and Genomics
Mutations in the gene encoding BMPR2 account for over 80% of HPAH and approximately 10 to 20% of sporadic IPAH (62). Mutations in other genes have recently been identified in PAH and overlap syndromes, including ACVRL1 (ALK-1), BMPR1B (ALK-6), GDF2 (growth differentiation factor 2) (BMP-9), TBX4 (T-box 4), ENG (endoglin), SMAD9 (Smad-8), CAV (caveolin), and KCNK3 (34, 61–66). A recent, large case–control analysis revealed overrepresentation of several novel rare variants, including ATP13A3 (ATPase 13A3), AQP1 (aquaporin 1), and SOX17 (transcription factor SOX-17), and independently validated GDF2 in PAH (67).
The potential for rare variant analyses to add precision to the characterization of patients with PAH was demonstrated in the prediction of acute vasodilator responses (68). Likewise, biallelic EIF2AK4 (eukaryotic translation initiation factor 2 α kinase 4) mutations, shown to associate with pulmonary venoocclusive disease/pulmonary capillary hemangiomatosis spectrum disease (69, 70), were discovered in 1% of patients diagnosed with IPAH or HPAH, suggesting that genetic testing could reclassify these patients and facilitate early referral for lung transplant (71). Although such patients frequently had abnormal imaging, a substantial portion of these patients’ diagnoses would not be confirmed by computed tomography alone. The understanding of the complex genetic and genomic factors involved in PAH continues to expand as genomic medicine plays a rapidly growing role in guiding PAH diagnosis and therapy.
Metabolism/Mitochondrial Function
In human and experimental PAH, metabolic dysregulation resembling the Warburg effect (described in cancer cells) has been observed in pulmonary arterial cells, RV cardiomyocytes, skeletal myocytes, and immune cells (72). Increased glycolysis is found in platelets isolated from patients with PAH, extending metabolic dysregulation to the hematologic compartment (73). Abnormalities in fatty acid metabolism in blood and steatosis in the RV myocardium are observed in human and experimental PH (74, 75), attributed to interplay between glucose and fatty acid metabolism via the Randle cycle.
As a potential driver of these adaptations, miR-124 was shown to regulate polypyrimidine tract binding protein and promote an increase in the PKM2/PKM1 ratio associated with increased glycolysis in adventitial fibroblasts (22) and PAECs (21) in PH, suggesting therapeutic potential of PKM2 inhibition. Severe obliterative vascular remodeling and severe PH were described in PHD2-deficient mice in vivo, which was due to PASMC proliferation in PHD2-deficient endothelial cells through a mechanism involving hypoxia-inducible factor (HIF)-2α–dependent CXCL12 release (55). The HIPPO central component, large tumor suppressor 1,is inactivated in PAH, resulting in upregulation of its effector YAP associated with coordinated shifts in cell metabolism favoring cell growth and proliferation (32). YAP and TAZ are also mechanoactivated by vascular extracellular matrix stiffness: By modulating the expression of glutaminase, pyruvate carboxylase, and lactate dehydrogenase A, YAP-TAZ stimulates a glutaminolytic metabolic phenotype as well as glycolytic metabolism in PAECs and PASMCs exposed to vascular stiffness in PAH (33).
Providing novel insight into the pathobiological basis of stimulant-associated PAH, in vitro exposure to amphetamine under hypoxia induces HIF-1α deacetylation and degradation through the protein phosphatase 2A/AKT/Sirtuin 1 axis, limiting adaptive cellular responses to oxidative stress, whereas administration of amphetamine to mice under hypoxia suppresses Hif-1α to exacerbate DNA damage and pulmonary vascular remodeling (76). Glycolytic reprogramming is associated with an increase in the reduced coenzyme NAD+ reduced (NADH). Accompanying increases in NADH production, NADH sensor CtBP (C-terminal binding protein) was activated in PAH, resulting in suppressed expression of the cyclin-dependent genes p15 and p21, the proapoptotic genes NOXA (phorbol-12-myristate-13-acetate-induced protein 1) and PERP (TP53 apoptosis effector), and the antiinflammatory gene HMOX1 (heme oxygenase 1) in lung fibroblasts (77). These findings suggest 4-methylthio-2-oxobutyric acid (a pharmacologic agent decreasing NADH) and CtBP1 inhibitors as potential therapeutic strategies in PAH. NAMPT (nicotinamide phosphoribosyltransferase), which regulates intracellular NAD concentrations, is expressed at higher concentrations in the plasma and lungs of patients with PAH (78). Consistent with an important pathophysiologic role, NAMPT+/− mice are protected from hypoxia-mediated PH, and inhibition of NAMPT activity prevented and partially reversed experimental PH.
These findings highlight the potential of mitochondria- and metabolism-targeting therapies, which have now been explored in human studies. Dichloroacetate, a pyruvate dehydrogenase kinase inhibitor, was assessed in a 4-month, open-label clinical trial in patients with IPAH, with improved hemodynamics reported in a subset of them (79). Dichloroacetate responses were associated with genetic variation in Sirtuin 3 and/or uncoupling protein 2, highlighting a potential precision medicine approach to metabolic therapy in PAH.
Clinical Trials and Repurposing Existing Drugs for the Treatment of PAH
Interventions and outcomes of key clinical trials are summarized in Table 1. Inhibition of aromatase, the key enzyme in estrogen synthesis, with anastrozole improves experimental PH in multiple models (80). The researchers in the AIPH (Anastrozole in Pulmonary Arterial Hypertension) study randomized 18 postmenopausal women or men with PAH to 1 mg of anastrozole or placebo (2:1 ratio) (81). Anastrozole significantly reduced 17β-estradiol concentrations compared with placebo (−40% vs. −4%; P = 0.003) and was well tolerated without deleterious effects on RV function. Anastrozole modestly increased 6-minute-walk distance (6MWD) compared with placebo (+26 vs. −12 m; P = 0.023) without altering functional class, quality of life, or serum NT-proBNP (N-terminal pro–brain natriuretic peptide) concentration. The mechanism for improvement in 6MWD is unclear because another study suggested that 17β-estradiol improves skeletal muscle mitochondrial function (82).
Table 1.
Summary of Key Clinical Trials in 2016 and 2017
| Study | Population | Characteristics | Intervention | Key Findings |
|---|---|---|---|---|
| AIPH (81) | PAH (n = 18) | 12-wk multicenter double-blind RCT | 1 mg of anastrozole vs. placebo (2:1) | • 29-m improvement in 6MWD |
| • No change in functional class, quality of life, or serum NT-proBNP levels | ||||
| TransformPAH (86) | PAH (n = 23) | 16-wk double-blind RCT | Tacrolimus at three target serum levels vs. placebo | • No improvement in 6MWD, NT-proBNP, RV function, or time to clinical worsening |
| PAHTCH (88) | PAH (n = 24) | 24-wk double-blind RCT | Low fixed dose, dose-escalating carvedilol, or placebo (1:1:1) | • Carvedilol well tolerated |
| • No improvement in 6MWD, RV function, or quality of life | ||||
| RESPITE (89) | PAH with inadequate response to PDE5I (n = 61) | 24-wk multicenter open-label uncontrolled trial | Replacing PDE5I with riociguat | • Improved functional class, 6MWD, and NT-proBNP |
| GRIPHON-CTD (90) | CTD-associated PAH (n = 334) | Post hoc analysis of multicenter RCT | Selexipag vs. placebo | • Selexipag reduced the combined morbidity and mortality endpoint by 41% in patients with CTD-PAH |
| • Selexipag was well tolerated | ||||
| SIOVAC (91) | Patients with mPAP ≥30 mm Hg ≥1 yr after valve surgery (n = 200) | Multicenter parallel-group double-blind RCT | Sildenafil 40 mg three times daily vs. placebo | • Sildenafil was associated with increased risk of composite endpoint of death, heart failure–related hospitalization, functional class, and patient global self-assessment |
| • No change in 6MWD, NT-proBNP, or Doppler estimated pulmonary artery pressures |
Definition of abbreviations: 6MWD = 6-minute-walk distance; AIPH = Anastrozole in Pulmonary Arterial Hypertension trial; CTD = connective tissue disease; GRIPHON = Selexipag for the Treatment of Pulmonary Arterial Hypertension; mPAP = mean pulmonary artery pressure; NT-proBNP = N-terminal pro–brain natriuretic peptide; PAH = pulmonary arterial hypertension; PAHTCH = PAH Treatment with Carvedilol for Heart Failure trial; PDE5I = phosphodiesterase 5 inhibitor; RCT = randomized controlled trial; RESPITE = Riociguat Clinical Effects Studies in Patients with Insufficient Treatment Response to Phosphodiesterase-5-Inhibitors; RV = right ventricular; SIOVAC = Sildenafil for Improving Outcomes after Valvular Correction; TransformPAH = FK506 (Tacrolimus) in Pulmonary Arterial Hypertension.
BMPR2 is reduced in experimental and human PAH (83, 84), whereas activation of BMPR2 using tacrolimus reverses PH in multiple experimental models (85). To translate this approach, the researchers in the double-blind TransformPAH (FK506 [Tacrolimus] in Pulmonary Arterial Hypertension) trial examined the safety and tolerability of tacrolimus in 23 patients with stable PAH and good functional capacity (median 6MWD, 526 m) on baseline therapy who were randomized to three target serum tacrolimus trough concentrations or placebo for 16 weeks (86). Tacrolimus did not improve 6MWD, NT-proBNP, echocardiographic markers of RV function, or time to clinical worsening. Some patients receiving high-dose tacrolimus had a significant increase in peripheral blood mononuclear BMPR2 expression and improvement in 6MWD, supporting further studies to identify potential responders.
On the basis of beneficial effects of β-blockade on RV function in experimental PH (87), the researchers in the PAHTCH (PAH Treatment with Carvedilol for Heart Failure) trial randomized 30 patients in a 1:1:1 ratio to placebo, low fixed dose, or dose-escalating carvedilol for 24 weeks (88). Carvedilol was well tolerated, with promising effects of decreased heart rate and RV fluorodeoxyglucose–positron emission tomography uptake and increased β-adrenergic receptor expression in peripheral leukocytes. However, it did not change 6MWD, RV function, or quality of life. This study suggests that carvedilol may be safe in PAH, but further studies are needed to determine its efficacy.
The researchers in the RESPITE (Riociguat Clinical Effects Studies in Patients with Insufficient Treatment Response to Phosphodiesterase-5-Inhibitors) study evaluated the safety and efficacy of replacing phosphodiesterase 5 inhibitors (PDE5Is) with sGC (soluble guanylate cyclase) activator riociguat in patients with PAH with inadequate responses to PDE5Is in a 24-week, multicenter, open-label, uncontrolled study of 61 patients (89). Switching to riociguat significantly improved functional class in 52%, increased 6MWD by 31 m, and reduced NT-proBNP. However, adverse events (52%) and serious adverse effects (16%) were common. The superiority of switching versus adding nitric oxide/sGC–targeted therapies will require confirmation in randomized controlled trials.
In a post hoc analysis of the GRIPHON (Selexipag for the Treatment of Pulmonary Arterial Hypertension) trial, the effects of the oral prostacyclin analog selexipag were examined in 334 patients with connective tissue disease (CTD)–associated PAH (90). There was no difference in maximum tolerated selexipag dose between CTD-PAH patients and the overall GRIPHON study cohort, with a similar side effect profile and frequency in patients with CTD versus other PAH etiologies. Selexipag reduced the combined morbidity and mortality endpoint by 41% in patients with CTD-PAH, with similar risk reduction regardless of underlying CTD (P = 0.89) and independent of background PAH-specific therapies (P = 0.87), and this was driven by a reduction in hospitalization and disease progression.
The researchers in the SIOVAC (Sildenafil for Improving Outcomes after Valvular Correction) trial examined the effect of sildenafil versus placebo on clinical outcomes in subjects with residual PH after valve surgery (91). There was no difference between groups in clinical outcomes, 6MWD, or BNP. These results argue against the use of sildenafil in patients with this PH phenotype.
Epidemiology and Clinical Outcomes
The putative classification of borderline PH was considered at the 2013 World Symposium on Pulmonary Hypertension, but its recognition as a clinical entity was deferred owing to lack of robust epidemiologic data. Recently, three reports involving more than 26,000 individuals have shown that borderline PH is present in 18 to 36% of patients referred for right heart catheterization (RHC) (92–94). Mean pulmonary pressure values between 19 and 24 mm Hg were consistently associated with increased adjusted mortality in these studies, and retrospective data suggest that many patients with borderline PH subsequently develop overt PH (93). Although it is unknown if interventions against PH risk factors can forestall or prevent disease (95), these observations suggest that the current diagnostic framework and hemodynamic criteria for PH do not capture a subset of vulnerable patients.
Advances in group I PAH have included data on medical use and associated PAH phenotypes. An analysis of the Medicare National Inpatient Sample showed that PAH-related hospitalizations declined between 2001 and 2012, whereas hospital charges and length of stay have increased, with no change in in-hospital mortality (96). In the largest prospective cohort of patients with presumed methamphetamine-associated PAH, patients with methamphetamine-associated PAH had worse hemodynamics and a twofold increase in adjusted mortality compared with IPAH, were less adherent to medications, and were less likely to receive parental therapy, highlighting several challenges in this population while strengthening the epidemiologic link between methamphetamine exposure and PAH (97).
Advances in group 2 PH include two reports focused on combined pre- and postcapillary pulmonary hypertension (pre- and post-CPH). First, patients with typical PAH, “atypical” PAH (PAH hemodynamics with multiple risk factors for left heart disease), or CPH from the COMPERA registry (Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension) were found to have similar survival; however, PAH therapies were less effective in improving 6MWD and NT-proBNP among patients with CPH (98). This study suggested the existence of a disease spectrum spanning typical PAH and CPH and showed that cardiometabolic comorbidities influence the response to PAH therapies among patients with atypical PAH or CPH. Second, patients with CPH develop pulmonary vascular disease similar to that of patients with PAH despite a severity and chronicity of left heart disease similar to that of patients with isolated post-CPH. Authors of an exploratory genetic analysis in patients with CPH found overrepresentation of genes and biologic pathways known to be perturbed in PAH (99). Thus, some patients with CPH may harbor a predisposition for pulmonary vascular disease, suggesting the potential to identify patients who might respond to PAH therapies and highlighting the need for further investigation into CPH pathobiology.
In a study analyzing the burden of echocardiographic PH among individuals with HIV infection, the combination of HIV and PH was associated with high mortality after adjusting for cardiovascular risk factors and left ventricular function and after excluding individuals with any chronic disease (100). When examined as a continuous variable, adjusted mortality risk in HIV began at PA pressure values currently considered to be normal, suggesting that current screening thresholds may not adequately capture risk related to rising pulmonary pressure in patients with HIV.
Two large prospective studies in subjects with chronic thromboembolic pulmonary hypertension (CTEPH) demonstrated excellent short- and long-term outcomes among patients who underwent pulmonary endarterectomy (3-yr survival, 84 to 91%) (101, 102). Persistent severe PH and elevated pulmonary vascular resistance (PVR) were associated with worse long-term survival.
Biomarkers
Agnostic “omics” approaches to biomarker identification allow insight into disease diagnosis and prognosis that is unconstrained by current knowledge of disease pathology. Metabolomic profiling (n = 1,416 metabolites) of patients with PAH versus healthy control subjects revealed that constituents of nucleoside, lipid, and amino acid pathways distinguished patients with PAH from healthy control subjects, independent of previously identified prognostic biomarkers (NT-proBNP, red cell distribution width) (3). The same group performed proteomic profiling (n = 1,129 proteins) in discovery and validation cohorts with IPAH or HPAH, identifying nine proteins involved in myocardial stress, inflammation, iron metabolism, and coagulation that improved the prognostic ability of NT-proBNP and the REVEAL (Registry to Evaluate Early and Long-Term PAH Disease Management) score (103). In a targeted analysis, hepatoma-derived growth factor, an angiogenic factor expressed by PAECs, was associated with worse clinical status and reduced survival in two independent PAH cohorts (104, 105). Relating metabolomic profiling to RV-PA function, indoleamine 2,3-dioxygenase–dependent tryptophan metabolites were associated with RV–pulmonary vascular dysfunction (resting increases in right-sided pressure/resistance, and abnormal pressure–flow relationship during exercise) in subjects undergoing rest/exercise RHC and RV radionuclide ventriculography (106). Finally, increased endothelin 1 was associated with elevated echocardiographic RV systolic pressure in African American individuals in the Jackson Heart Study (107). These studies identified exciting new biomarkers of prognostic and mechanistic significance, highlighting biologic pathways with therapeutic potential in PAH.
Two studies examined the role of TAFI (thrombin-activatable fibrinolysis inhibitor) in patients with CTEPH. TAFI is increased in the plasma and is highly expressed in PAs and thrombus from patients with CTEPH. TAFI inhibition reduces 4-hour clotting in the whole-blood clot lysis assay and improves experimental PH, suggesting a potential new therapeutic target in CTEPH (108, 109).
Imaging
An important step in the diagnostic algorithm for PH is the performance of / scintigraphy. It was recently shown that a considerable number of patients with an abnormal / single-photon emission computed tomographic scan are ultimately diagnosed with nonthromboembolic PAH after undergoing pulmonary angiography (110). In these patients, global perfusion abnormalities in the / single-photon emission computed tomographic scan were associated with poor outcome. It is possible that occult thromboembolic lesions could be revealed by using specialized methods such as optical frequency domain imaging or pulmonary angioscopy (111).
The feasibility of echocardiography for assessment and monitoring of RV function during exercise continues to be debated. Exercise stress echocardiography was shown to be feasible and identified RV pathology with reasonable accuracy (112). In patients with systemic sclerosis (SSc), speckle-derived strain determinations revealed a heterogeneous pattern of regional heart strain independent of RV systolic pressure not detected by conventional echocardiographic measures (113). Using cardiac magnetic resonance, the therapeutic superiority of combination therapy over monotherapy in PAH was related to a greater improvement in RV ejection fraction (114). In large derivation and validation cohorts, stiffness of the proximal PA and RV end-systolic volume index were the strongest independent predictors of mortality in PAH (115). A machine learning approach predicted outcomes in PAH on the basis of three-dimensional RV motion via cardiac magnetic resonance imaging (116).
Physiology
Age was found to be associated with different hemodynamic responses to exercise, with subjects older than 50 years of age exhibiting higher PVR and lower pulmonary vascular compliance at peak exercise than subjects 50 years old or younger (117). Another study showed that 7 ml/kg saline fluid challenge at RHC reclassified 6% and 8% of non-PH and pre-CPH, respectively, to a post-procedural diagnosis of post-CPH (118). These data suggest that an integrated approach including provocative maneuvers (e.g., exercise or fluid challenge) is useful for diagnosing abnormal pulmonary vascular responses (119). Abnormal RV function has emerged as a central part of the HPAH phenotype, although the underlying mechanisms for this effect could not be explained fully by the germline mutation (120). In preclinical physiologic studies, neurohormonal overactivation (121), myocardial stiffness (122), abnormal shear responses (60), and genetic modifiers (123) were identified as key contributors to PAH and right heart failure pathophysiology.
In PAH, the right ventricle may be affected differentially across different disease subtypes and independent of RV afterload. For example, RV–PA uncoupling occurs in SSc-PAH but not IPAH, despite similar levels of pulmonary hypertension and PVR between these cohorts (124). This suggests a primary defect in the SSc-PAH right ventricle, such as impaired sarcomere function (125). The disease-specific mechanisms regulating sarcomere dysfunction in SSc-PAH (or IPAH) remain unknown, but they may involve SERCA2A, phospholamban, or other calcium-regulating proteins (40, 126).
Pediatric Pulmonary Vascular Disease
The diagnosis and clinical management of patients with pediatric PH was advanced by parallel efforts in North America and Europe to create comprehensive care guidelines (127–129). These guidelines 1) highlight the varied nature of pediatric PH subtypes, including the prevalence of developmental lung disease and complex syndromic conditions; 2) emphasize the potential utility of genetic counseling and testing, as well as screening of relatives at specialized PH centers; and 3) provide algorithms for management of PH-specific therapies. In September 2017, the U.S. Food and Drug Administration approved bosentan as the first chronic therapeutic for PAH care in children in the United States.
The guidelines also emphasized the importance of specialty centers for PH diagnosis and care, including the performance of cardiac catheterization with acute vasodilator testing (AVT). In a multinational registry study, the conduct and interpretation of AVT was found to be tremendously variable, even among experienced pediatric centers (130). Only 70% of centers employed inhaled nitric oxide in the AVT protocol, and only 23% of patients with PAH determined to “respond” were trialed on a calcium channel blocker single-therapy regimen, contrary to international guidelines. In addition, evaluation of different criteria for response showed that the Sitbon criteria were most predictive of a successful long-term response to calcium channel blocker monotherapy (131).
Improving precision in treatment will require improved endpoints for clinical trials and observational studies. The utility of accelerometry for measurement of physical activity and its correlation with clinical severity markers was demonstrated in pediatric PAH, with affected children exhibiting dramatically decreased physical activity compared with their healthy peers (132). Accelerometry was associated with functional class, 6MWD, and adverse PAH-related events, supporting potential utility in future studies. Comprehensive clinical guidelines, multicenter and multinational collaborations, and novel approaches to monitoring response to therapy are among the advances driving continued progress in treatment of children with PH.
Conclusions
These recent studies demonstrate accelerated progress in basic and clinical PAH science, heralding expanded treatment options in coming years. Future directions will include enhanced understanding of the genetic and molecular bases of pulmonary vascular remodeling (133, 134), molecular classification of PH phenotypes (135, 136), leveraging of underlying biology to personalize therapy (137, 138), and understanding the determinants of RV compensation versus failure.
Footnotes
Supported by NIH grant R34HL136989-01, American Heart Association grant 13FTF16070002, and Gilead Sciences Scholars Program in Pulmonary Arterial Hypertension (E.L.B.); American Heart Association grant 15SDG25560048 (T.T.); NIH grants 1K08HL11207-01A1, R56HL131787, and 1R01HL139613-01, American Heart Association grant 15GRNT25080016, the Pulmonary Hypertension Association, and the Systemic Sclerosis Foundation (B.A.M.); NIH grants R01 HL124021, R01 HL122596, R01 HL138437, and UH2TR002073 (S.Y.C.); NIH grants R01 HL134802, P01 HL108800, P01 HL092870, R01 HL111259, and U01 HL121518 (E.D.A.); NIH grants K08 HL107450 and R01 HL128734, U.S. Department of Defense grant PR161256, and a Pulmonary Hypertension Association/American Heart Association career development grant (E.S.); the Netherlands CardioVascular Research Initiative, the Dutch Heart Foundation, the Dutch Federation of University Medical Centers, the Netherlands Organization for Health Research and Development, and the Royal Netherlands Academy of Sciences (H.J.B.); the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada (R.P.); NIH grants R01 HL127342, R01 HL111656, and R01 HL133951 (R.F.M.); and NIH grants R01 HL131910 and R42 HL132742 and the Boston Biomedical Innovation Center (P.B.Y.).
Author Contributions: All authors made substantial contributions to the conception or design of the work. All authors participated in drafting or revising the manuscript, and all authors approved of the final version to be published.
Originally Published in Press as DOI: 10.1164/rccm.201801-0062UP on March 13, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gladwin MT. Translational advances in the field of pulmonary hypertension bench to bedside: how fundamental discoveries in science are advancing our understanding and therapy of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;195:1–3. doi: 10.1164/rccm.201608-1637ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, et al. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med. 2016;193:1168–1175. doi: 10.1164/rccm.201509-1785OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhodes CJ, Ghataorhe P, Wharton J, Rue-Albrecht KC, Hadinnapola C, Watson G, et al. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation. 2017;135:460–475. doi: 10.1161/CIRCULATIONAHA.116.024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Austin ED, Talati M, Fessel JP, Farber-Eger EH, Brittain EL, et al. Oestrogen inhibition reverses pulmonary arterial hypertension and associated metabolic defects. Eur Respir J. 2017;50:1602337. doi: 10.1183/13993003.02337-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dean A, Gregorc T, Docherty CK, Harvey KY, Nilsen M, Morrell NW, et al. Role of the aryl hydrocarbon receptor in Sugen 5416–induced experimental pulmonary hypertension. Am J Respir Cell Mol Biol. 2018;58:320–330. doi: 10.1165/rcmb.2017-0260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Talati M, Fessel JP, Hemnes AR, Gladson S, French J, et al. Estrogen metabolite 16α-hydroxyestrone exacerbates bone morphogenetic protein receptor type II–associated pulmonary arterial hypertension through microRNA-29–mediated modulation of cellular metabolism. Circulation. 2016;133:82–97. doi: 10.1161/CIRCULATIONAHA.115.016133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Umar S, Cunningham CM, Itoh Y, Moazeni S, Vaillancourt M, Sarji S, et al. The Y chromosome plays a protective role in experimental hypoxic pulmonary hypertension [letter] Am J Respir Crit Care Med 2018197952–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurst LA, Dunmore BJ, Long L, Crosby A, Al-Lamki R, Deighton J, et al. TNFα drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat Commun. 2017;8:14079. doi: 10.1038/ncomms14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes JW, Kucera ET, Tian L, Mellor NE, Dvorina N, Baldwin WW, III, et al. Bone morphogenic protein type 2 receptor mutation-independent mechanisms of disrupted bone morphogenetic protein signaling in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2016;55:564–575. doi: 10.1165/rcmb.2015-0402OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertero T, Lu Y, Annis S, Hale A, Bhat B, Saggar R, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest. 2014;124:3514–3528. doi: 10.1172/JCI74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvier L, Chouvarine P, Legchenko E, Hansmann G. Transforming growth factor β1– and bone morphogenetic protein 2/PPARγ–regulated microRNAs in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;196:1227–1228. doi: 10.1164/rccm.201705-0923LE. [DOI] [PubMed] [Google Scholar]
- 12.Calvier L, Chouvarine P, Legchenko E, Hoffmann N, Geldner J, Borchert P, et al. PPARγ links BMP2 and TGFβ1 pathways in vascular smooth muscle cells, regulating cell proliferation and glucose metabolism. Cell Metab. 2017;25:1118–1134.e7. doi: 10.1016/j.cmet.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Yung LM, Nikolic I, Paskin-Flerlage SD, Pearsall RS, Kumar R, Yu PB. A selective transforming growth factor-β ligand trap attenuates pulmonary hypertension. Am J Respir Crit Care Med. 2016;194:1140–1151. doi: 10.1164/rccm.201510-1955OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zabini D, Granton E, Hu Y, Miranda MZ, Weichelt U, Breuils Bonnet S, et al. Loss of SMAD3 promotes vascular remodeling in pulmonary arterial hypertension via MRTF disinhibition. Am J Respir Crit Care Med. 2018;197:244–260. doi: 10.1164/rccm.201702-0386OC. [DOI] [PubMed] [Google Scholar]
- 15.Hopper RK, Moonen JR, Diebold I, Cao A, Rhodes CJ, Tojais NF, et al. In pulmonary arterial hypertension, reduced BMPR2 promotes endothelial-to-mesenchymal transition via HMGA1 and its target slug. Circulation. 2016;133:1783–1794. doi: 10.1161/CIRCULATIONAHA.115.020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwangbo C, Lee HW, Kang H, Ju H, Wiley DS, Papangeli I, et al. Modulation of endothelial bone morphogenetic protein receptor type 2 activity by vascular endothelial growth factor receptor 3 in pulmonary arterial hypertension. Circulation. 2017;135:2288–2298. doi: 10.1161/CIRCULATIONAHA.116.025390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu D, Yan Y, Chen JW, Yuan P, Wang XJ, Jiang R, et al. Hypermethylation of BMPR2 promoter occurs in patients with heritable pulmonary arterial hypertension and inhibits BMPR2 expression. Am J Respir Crit Care Med. 2017;196:925–928. doi: 10.1164/rccm.201611-2273LE. [DOI] [PubMed] [Google Scholar]
- 18.Ghigna MR, Guignabert C, Montani D, Girerd B, Jaïs X, Savale L, et al. BMPR2 mutation status influences bronchial vascular changes in pulmonary arterial hypertension. Eur Respir J. 2016;48:1668–1681. doi: 10.1183/13993003.00464-2016. [DOI] [PubMed] [Google Scholar]
- 19.Chun HJ, Bonnet S, Chan SY. Translating microRNA biology in pulmonary hypertension: it will take more than “miR” words. Am J Respir Crit Care Med. 2017;195:167–178. doi: 10.1164/rccm.201604-0886PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aliotta JM, Pereira M, Wen S, Dooner MS, Del Tatto M, Papa E, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. 2016;110:319–330. doi: 10.1093/cvr/cvw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caruso P, Dunmore BJ, Schlosser K, Schoors S, Dos Santos CC, Perez-Iratxeta C, et al. Identification of microRNA-124 as a major regulator of enhanced endothelial cell glycolysis in pulmonary arterial hypertension via PTBP1 (polypyrimidine tract binding protein) and pyruvate kinase M2. Circulation. 2017;136:2451–2467. doi: 10.1161/CIRCULATIONAHA.117.028034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Wang D, Li M, Plecitá-Hlavatá L, D’Alessandro A, Tauber J, et al. Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a microRNA-124/PTBP1 (polypyrimidine tract binding protein 1)/pyruvate kinase muscle axis. Circulation. 2017;136:2468–2485. doi: 10.1161/CIRCULATIONAHA.117.028069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong Z, Chen KH, DasGupta A, Potus F, Dunham-Snary K, Bonnet S, et al. MicroRNA-138 and microRNA-25 down-regulate mitochondrial calcium uniporter, causing the pulmonary arterial hypertension cancer phenotype. Am J Respir Crit Care Med. 2017;195:515–529. doi: 10.1164/rccm.201604-0814OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman AM, Arnold ND, Pickworth JA, Iremonger J, Ciuclan L, Allen RM, et al. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest. 2016;126:2495–2508. doi: 10.1172/JCI83361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruffenach G, Chabot S, Tanguay VF, Courboulin A, Boucherat O, Potus F, et al. Role for Runt-related transcription factor 2 in proliferative and calcified vascular lesions in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;194:1273–1285. doi: 10.1164/rccm.201512-2380OC. [DOI] [PubMed] [Google Scholar]
- 26.Sa S, Gu M, Chappell J, Shao NY, Ameen M, Elliott KA, et al. Induced pluripotent stem cell model of pulmonary arterial hypertension reveals novel gene expression and patient specificity. Am J Respir Crit Care Med. 2017;195:930–941. doi: 10.1164/rccm.201606-1200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu M, Shao NY, Sa S, Li D, Termglinchan V, Ameen M, et al. Patient-specific iPSC-derived endothelial cells uncover pathways that protect against pulmonary hypertension in BMPR2 mutation carriers. Cell Stem Cell. 2017;20:490–504.e5. doi: 10.1016/j.stem.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boucherat O, Bonnet S, Paulin R. The HIPPO-thesis of pulmonary HYPERtension [editorial] Am J Respir Crit Care Med. 2016;194:787–789. doi: 10.1164/rccm.201604-0741ED. [DOI] [PubMed] [Google Scholar]
- 29.Dabral S, Pullamsetti SS. Vascular stiffness and mechanotransduction: back in the limelight. Am J Respir Crit Care Med. 2017;196:527–530. doi: 10.1164/rccm.201611-2254LE. [DOI] [PubMed] [Google Scholar]
- 30.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, et al. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Reports. 2015;13:1016–1032. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu F, Haeger CM, Dieffenbach PB, Sicard D, Chrobak I, Coronata AM, et al. Distal vessel stiffening is an early and pivotal mechanobiological regulator of vascular remodeling and pulmonary hypertension. JCI Insight. 2016;1:e86987. doi: 10.1172/jci.insight.86987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kudryashova TV, Goncharov DA, Pena A, Kelly N, Vanderpool R, Baust J, et al. HIPPO–integrin-linked kinase cross-talk controls self-sustaining proliferation and survival in pulmonary hypertension. Am J Respir Crit Care Med. 2016;194:866–877. doi: 10.1164/rccm.201510-2003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest. 2016;126:3313–3335. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antigny F, Hautefort A, Meloche J, Belacel-Ouari M, Manoury B, Rucker-Martin C, et al. Potassium channel subfamily K member 3 (KCNK3) contributes to the development of pulmonary arterial hypertension. Circulation. 2016;133:1371–1385. doi: 10.1161/CIRCULATIONAHA.115.020951. [DOI] [PubMed] [Google Scholar]
- 35.Nagaraj C, Tang B, Nagy BM, Papp R, Jain PP, Marsh LM, et al. Docosahexaenoic acid causes rapid pulmonary arterial relaxation via KCa channel-mediated hyperpolarisation in pulmonary hypertension. Eur Respir J. 2016;48:1127–1136. doi: 10.1183/13993003.01814-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez RA, Miller MP, Hahn SA, Galley JC, Bauer E, Bachman T, et al. Targeting pulmonary endothelial hemoglobin α improves nitric oxide signaling and reverses pulmonary artery endothelial dysfunction. Am J Respir Cell Mol Biol. 2017;57:733–744. doi: 10.1165/rcmb.2016-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, et al. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med. 2009;15:1289–1297. doi: 10.1038/nm.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dabral S, Tian X, Kojonazarov B, Savai R, Ghofrani HA, Weissmann N, et al. Notch1 signalling regulates endothelial proliferation and apoptosis in pulmonary arterial hypertension. Eur Respir J. 2016;48:1137–1149. doi: 10.1183/13993003.00773-2015. [DOI] [PubMed] [Google Scholar]
- 39.Jia D, Zhu Q, Liu H, Zuo C, He Y, Chen G, et al. Osteoprotegerin disruption attenuates HySu-induced pulmonary hypertension through integrin αvβ3/FAK/AKT pathway suppression. Circ Cardiovasc Genet. 2017;10:e001591. doi: 10.1161/CIRCGENETICS.116.001591. [DOI] [PubMed] [Google Scholar]
- 40.Aguero J, Ishikawa K, Hadri L, Santos-Gallego CG, Fish KM, Kohlbrenner E, et al. Intratracheal gene delivery of SERCA2a ameliorates chronic post-capillary pulmonary hypertension: a large animal model. J Am Coll Cardiol. 2016;67:2032–2046. doi: 10.1016/j.jacc.2016.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanderpool RR, Desai AA, Knapp SM, Simon MA, Abidov A, Yuan JX, et al. How prostacyclin therapy improves right ventricular function in pulmonary arterial hypertension. Eur Respir J. 2017;50:1700764. doi: 10.1183/13993003.00764-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanderpool RR, Rischard F, Naeije R, Hunter K, Simon MA. Simple functional imaging of the right ventricle in pulmonary hypertension: can right ventricular ejection fraction be improved? Int J Cardiol. 2016;223:93–94. doi: 10.1016/j.ijcard.2016.08.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69:236–243. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 44.Nicolls MR, Voelkel NF. The roles of immunity in the prevention and evolution of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;195:1292–1299. doi: 10.1164/rccm.201608-1630PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huertas A, Perros F, Tu L, Cohen-Kaminsky S, Montani D, Dorfmüller P, et al. Immune dysregulation and endothelial dysfunction in pulmonary arterial hypertension: a complex interplay. Circulation. 2014;129:1332–1340. doi: 10.1161/CIRCULATIONAHA.113.004555. [DOI] [PubMed] [Google Scholar]
- 47.Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:897–908. doi: 10.1164/rccm.201202-0335OC. [DOI] [PubMed] [Google Scholar]
- 48.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197:104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aiello RJ, Bourassa PA, Zhang Q, Dubins J, Goldberg DR, De Lombaert S, et al. Tryptophan hydroxylase 1 inhibition impacts pulmonary vascular remodeling in two rat models of pulmonary hypertension. J Pharmacol Exp Ther. 2017;360:267–279. doi: 10.1124/jpet.116.237933. [DOI] [PubMed] [Google Scholar]
- 51.Breitling S, Hui Z, Zabini D, Hu Y, Hoffmann J, Goldenberg NM, et al. The mast cell–B cell axis in lung vascular remodeling and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2017;312:L710–L721. doi: 10.1152/ajplung.00311.2016. [DOI] [PubMed] [Google Scholar]
- 52.Parpaleix A, Amsellem V, Houssaini A, Abid S, Breau M, Marcos E, et al. Role of interleukin-1 receptor 1/MyD88 signalling in the development and progression of pulmonary hypertension. Eur Respir J. 2016;48:470–483. doi: 10.1183/13993003.01448-2015. [DOI] [PubMed] [Google Scholar]
- 53.Amsellem V, Abid S, Poupel L, Parpaleix A, Rodero M, Gary-Bobo G, et al. Roles for the CX3CL1/CX3CR1 and CCL2/CCR2 chemokine systems in hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol. 2017;56:597–608. doi: 10.1165/rcmb.2016-0201OC. [DOI] [PubMed] [Google Scholar]
- 54.Huertas A, Phan C, Bordenave J, Tu L, Thuillet R, Le Hiress M, et al. Regulatory T cell dysfunction in idiopathic, heritable and connective tissue-associated pulmonary arterial hypertension. Chest. 2016;149:1482–1493. doi: 10.1016/j.chest.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Dai Z, Li M, Wharton J, Zhu MM, Zhao YY. Prolyl-4 hydroxylase 2 (PHD2) deficiency in endothelial cells and hematopoietic cells induces obliterative vascular remodeling and severe pulmonary arterial hypertension in mice and humans through hypoxia-inducible factor-2α. Circulation. 2016;133:2447–2458. doi: 10.1161/CIRCULATIONAHA.116.021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan L, Chen X, Talati M, Nunley BW, Gladson S, Blackwell T, et al. Bone marrow–derived cells contribute to the pathogenesis of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;193:898–909. doi: 10.1164/rccm.201502-0407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bryant AJ, Shenoy V, Fu C, Marek G, Lorentsen KJ, Herzog EL, et al. Myeloid-derived suppressor cells are necessary for development of pulmonary hypertension. Am J Respir Cell Mol Biol. 2018;58:170–180. doi: 10.1165/rcmb.2017-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saito T, Miyagawa K, Chen SY, Tamosiuniene R, Wang L, Sharp O, et al. Upregulation of human endogenous retrovirus-K is linked to immunity and inflammation in pulmonary arterial hypertension. Circulation. 2017;136:1920–1935. doi: 10.1161/CIRCULATIONAHA.117.027589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang S, Zeng H, Xie XJ, Tao YK, He X, Roman RJ, et al. Loss of prolyl hydroxylase domain protein 2 in vascular endothelium increases pericyte coverage and promotes pulmonary arterial remodeling. Oncotarget. 2016;7:58848–58861. doi: 10.18632/oncotarget.11585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szulcek R, Happé CM, Rol N, Fontijn RD, Dickhoff C, Hartemink KJ, et al. Delayed microvascular shear adaptation in pulmonary arterial hypertension: role of platelet endothelial cell adhesion molecule-1 cleavage. Am J Respir Crit Care Med. 2016;193:1410–1420. doi: 10.1164/rccm.201506-1231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guignabert C, Bailly S, Humbert M. Restoring BMPRII functions in pulmonary arterial hypertension: opportunities, challenges and limitations. Expert Opin Ther Targets. 2017;21:181–190. doi: 10.1080/14728222.2017.1275567. [DOI] [PubMed] [Google Scholar]
- 62.Evans JD, Girerd B, Montani D, Wang XJ, Galiè N, Austin ED, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med. 2016;4:129–137. doi: 10.1016/S2213-2600(15)00544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eyries M, Soubrier F. Molecular genetic diagnosis of pulmonary arterial hypertension: an increased complexity. Rev Esp Cardiol (Engl Ed) 2016;69:1003–1004. doi: 10.1016/j.rec.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 64.Machado RD, Southgate L, Eichstaedt CA, Aldred MA, Austin ED, Best DH, et al. Pulmonary arterial hypertension: a current perspective on established and emerging molecular genetic defects. Hum Mutat. 2015;36:1113–1127. doi: 10.1002/humu.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang G, Fan R, Ji R, Zou W, Penny DJ, Varghese NP, et al. Novel homozygous BMP9 nonsense mutation causes pulmonary arterial hypertension: a case report. BMC Pulm Med. 2016;16:17. doi: 10.1186/s12890-016-0183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 2013;369:351–361. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gräf S, Morrell NW. Towards a molecular classification of pulmonary arterial hypertension. Eur Respir J. 2016;48:987–989. doi: 10.1183/13993003.01550-2016. [DOI] [PubMed] [Google Scholar]
- 68.Hemnes AR, Zhao M, West J, Newman JH, Rich S, Archer SL, et al. Critical genomic networks and vasoreactive variants in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;194:464–475. doi: 10.1164/rccm.201508-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Best DH, Sumner KL, Austin ED, Chung WK, Brown LM, Borczuk AC, et al. EIF2AK4 mutations in pulmonary capillary hemangiomatosis. Chest. 2014;145:231–236. doi: 10.1378/chest.13-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eyries M, Montani D, Girerd B, Perret C, Leroy A, Lonjou C, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet. 2014;46:65–69. doi: 10.1038/ng.2844. [DOI] [PubMed] [Google Scholar]
- 71.Hadinnapola C, Bleda M, Haimel M, Screaton N, Swift A, Dorfmüller P, et al. NIHR BioResource–Rare Diseases Consortium; UK National Cohort Study of Idiopathic and Heritable PAH. Phenotypic characterization ofEIF2AK4 mutation carriers in a large cohort of patients diagnosed clinically with pulmonary arterial hypertension. Circulation. 2017;136:2022–2033. doi: 10.1161/CIRCULATIONAHA.117.028351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res. 2014;115:148–164. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 73.Nguyen QL, Corey C, White P, Watson A, Gladwin MT, Simon MA, et al. Platelets from pulmonary hypertension patients show increased mitochondrial reserve capacity. JCI Insight. 2017;2:e91415. doi: 10.1172/jci.insight.91415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brittain EL, Talati M, Fessel JP, Zhu H, Penner N, Calcutt MW, et al. Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation. 2016;133:1936–1944. doi: 10.1161/CIRCULATIONAHA.115.019351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Talati MH, Brittain EL, Fessel JP, Penner N, Atkinson J, Funke M, et al. Mechanisms of lipid accumulation in the bone morphogenetic protein receptor type 2 mutant right ventricle. Am J Respir Crit Care Med. 2016;194:719–728. doi: 10.1164/rccm.201507-1444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen PI, Cao A, Miyagawa K, Tojais NF, Hennigs JK, Li CG, et al. Amphetamines promote mitochondrial dysfunction and DNA damage in pulmonary hypertension. JCI Insight. 2017;2:e90427. doi: 10.1172/jci.insight.90427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li M, Riddle S, Zhang H, D’Alessandro A, Flockton A, Serkova NJ, et al. Metabolic reprogramming regulates the proliferative and inflammatory phenotype of adventitial fibroblasts in pulmonary hypertension through the transcriptional corepressor C-terminal binding protein-1. Circulation. 2016;134:1105–1121. doi: 10.1161/CIRCULATIONAHA.116.023171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J, Sysol JR, Singla S, Zhao S, Yamamura A, Valdez-Jasso D, et al. Nicotinamide phosphoribosyltransferase promotes pulmonary vascular remodeling and is a therapeutic target in pulmonary arterial hypertension. Circulation. 2017;135:1532–1546. doi: 10.1161/CIRCULATIONAHA.116.024557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, Howard L, et al. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med. 2017;9:eaao4583. doi: 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- 80.Mair KM, Wright AF, Duggan N, Rowlands DJ, Hussey MJ, Roberts S, et al. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med. 2014;190:456–467. doi: 10.1164/rccm.201403-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawut SM, Archer-Chicko CL, DeMichele A, Fritz JS, Klinger JR, Ky B, et al. Anastrozole in Pulmonary Arterial Hypertension: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2017;195:360–368. doi: 10.1164/rccm.201605-1024OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Torres MJ, Kew KA, Ryan TE, Pennington ER, Lin CT, Buddo KA, et al. 17β-Estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab. 2018;27:167–179.e7. doi: 10.1016/j.cmet.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21:777–785. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, III, Loyd JE, et al. International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 85.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123:3600–3613. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spiekerkoetter E, Sung YK, Sudheendra D, Scott V, Del Rosario P, Bill M, et al. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur Respir J. 2017;50:1602449. doi: 10.1183/13993003.02449-2016. [DOI] [PubMed] [Google Scholar]
- 87.Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang PJ, Chau VQ, et al. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med. 2010;182:652–660. doi: 10.1164/rccm.201003-0335OC. [DOI] [PubMed] [Google Scholar]
- 88.Farha S, Saygin D, Park MM, Cheong HI, Asosingh K, Comhair SA, et al. Pulmonary arterial hypertension treatment with carvedilol for heart failure: a randomized controlled trial. JCI Insight. 2017;2:95240. doi: 10.1172/jci.insight.95240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoeper MM, Simonneau G, Corris PA, Ghofrani HA, Klinger JR, Langleben D, et al. RESPITE: switching to riociguat in pulmonary arterial hypertension patients with inadequate response to phosphodiesterase-5 inhibitors. Eur Respir J. 2017;50:1602425. doi: 10.1183/13993003.02425-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gaine S, Chin K, Coghlan G, Channick R, Di Scala L, Galiè N, et al. Selexipag for the treatment of connective tissue disease-associated pulmonary arterial hypertension. Eur Respir J. 2017;50:1602493. doi: 10.1183/13993003.02493-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bermejo J, Yotti R, Garcia-Orta R, Sanchez-Fernandez PL, Castano M, Segovia-Cubero J, et al. Sildenafil for Improving Outcomes after VAlvular Correction (SIOVAC) investigators Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial Eur Heart J 2018391255–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation. 2016;133:1240–1248. doi: 10.1161/CIRCULATIONAHA.115.020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Assad TR, Maron BA, Robbins IM, Xu M, Huang S, Harrell FE, et al. Prognostic effect and longitudinal hemodynamic assessment of borderline pulmonary hypertension. JAMA Cardiol. 2017;2:1361–1368. doi: 10.1001/jamacardio.2017.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Douschan P, Kovacs G, Avian A, Foris V, Gruber F, Olschewski A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509–516. doi: 10.1164/rccm.201706-1215OC. [DOI] [PubMed] [Google Scholar]
- 95.Maron BA, Abman SH. Focusing on developmental origins and disease inception for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2017;195:292–301. doi: 10.1164/rccm.201604-0882PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anand V, Roy SS, Archer SL, Weir EK, Garg SK, Duval S, et al. Trends and outcomes of pulmonary Arterial Hypertension-Related Hospitalizations in the United States: Analysis of the Nationwide Inpatient Sample Database From 2001 Through 2012. JAMA Cardiol. 2016;1:1021–1029. doi: 10.1001/jamacardio.2016.3591. [DOI] [PubMed] [Google Scholar]
- 97.Zamanian RT, Hedlin H, Greuenwald P, Wilson DM, Segal JI, Jorden M, et al. Features and outcomes of methamphetamine-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;197:788–800. doi: 10.1164/rccm.201705-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Opitz CF, Hoeper MM, Gibbs JS, Kaemmerer H, Pepke-Zaba J, Coghlan JG, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol. 2016;68:368–378. doi: 10.1016/j.jacc.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 99.Assad TR, Hemnes AR, Larkin EK, Glazer AM, Xu M, Wells QS, et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol. 2016;68:2525–2536. doi: 10.1016/j.jacc.2016.09.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brittain EL, Duncan MS, Chang J, Patterson OV, DuVall SL, Brandt CA, et al. Increased echocardiographic pulmonary pressure in HIV-infected and -uninfected individuals in the Veterans Aging Cohort Study Am J Respir Crit Care Med 2018197923–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cannon JE, Su L, Kiely DG, Page K, Toshner M, Swietlik E, et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the United Kingdom national cohort. Circulation. 2016;133:1761–1771. doi: 10.1161/CIRCULATIONAHA.115.019470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Delcroix M, Lang I, Pepke-Zaba J, Jansa P, D’Armini AM, Snijder R, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation. 2016;133:859–871. doi: 10.1161/CIRCULATIONAHA.115.016522. [DOI] [PubMed] [Google Scholar]
- 103.Rhodes CJ, Wharton J, Ghataorhe P, Watson G, Girerd B, Howard LS, et al. Plasma proteome analysis in patients with pulmonary arterial hypertension: an observational cohort study. Lancet Respir Med. 2017;5:717–726. doi: 10.1016/S2213-2600(17)30161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kameny RJ, Fineman JR. The prescient prognosticator? Hepatoma-derived growth factor in pulmonary hypertension. Am J Respir Crit Care Med. 2016;194:1186–1187. doi: 10.1164/rccm.201606-1159ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang J, Nies MK, Fu Z, Damico R, Korley FK, Hassoun PM, et al. Hepatoma-derived growth factor predicts disease severity and survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;194:1264–1272. doi: 10.1164/rccm.201512-2498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lewis GD, Ngo D, Hemnes AR, Farrell L, Domos C, Pappagianopoulos PP, et al. Metabolic profiling of right ventricular-pulmonary vascular function reveals circulating biomarkers of pulmonary hypertension. J Am Coll Cardiol. 2016;67:174–189. doi: 10.1016/j.jacc.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jankowich MD, Wu WC, Choudhary G. Association of elevated plasma endothelin-1 levels with pulmonary hypertension, mortality, and heart failure in African American individuals: the Jackson Heart Study. JAMA Cardiol. 2016;1:461–469. doi: 10.1001/jamacardio.2016.0962. [DOI] [PubMed] [Google Scholar]
- 108.Satoh T, Satoh K, Yaoita N, Kikuchi N, Omura J, Kurosawa R, et al. Activated TAFI promotes the development of chronic thromboembolic pulmonary hypertension: a possible novel therapeutic target. Circ Res. 2017;120:1246–1262. doi: 10.1161/CIRCRESAHA.117.310640. [DOI] [PubMed] [Google Scholar]
- 109.Yaoita N, Satoh K, Satoh T, Sugimura K, Tatebe S, Yamamoto S, et al. Thrombin-activatable fibrinolysis inhibitor in chronic thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2016;36:1293–1301. doi: 10.1161/ATVBAHA.115.306845. [DOI] [PubMed] [Google Scholar]
- 110.Chan K, Ioannidis S, Coghlan JG, Hall M, Schreiber BE. Pulmonary arterial hypertension with abnormal / single-photon emission computed tomography. JACC Cardiovasc Imaging. doi: 10.1016/j.jcmg.2017.07.026. [online ahead of print] 16 Oct 2017; DOI: 10.1016/j.jcmg.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 111.Chibana H, Tahara N, Itaya N, Sasaki M, Sasaki M, Nakayoshi T, et al. Optical frequency-domain imaging and pulmonary angioscopy in chronic thromboembolic pulmonary hypertension. Eur Heart J. 2016;37:1296–1304. doi: 10.1093/eurheartj/ehv736. [DOI] [PubMed] [Google Scholar]
- 112.Claessen G, La Gerche A, Voigt JU, Dymarkowski S, Schnell F, Petit T, et al. Accuracy of echocardiography to evaluate pulmonary vascular and RV function during exercise. JACC Cardiovasc Imaging. 2016;9:532–543. doi: 10.1016/j.jcmg.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 113.Mukherjee M, Chung SE, Ton VK, Tedford RJ, Hummers LK, Wigley FM, et al. Unique abnormalities in right ventricular longitudinal strain in systemic sclerosis patients. Circ Cardiovasc Imaging. 2016;9:e003792. doi: 10.1161/CIRCIMAGING.115.003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van de Veerdonk MC, Huis In T Veld AE, Marcus JT, Westerhof N, Heymans MW, Bogaard HJ, et al. Upfront combination therapy reduces right ventricular volumes in pulmonary arterial hypertension. Eur Respir J. 2017;49:1700007. doi: 10.1183/13993003.00007-2017. [DOI] [PubMed] [Google Scholar]
- 115.Swift AJ, Capener D, Johns C, Hamilton N, Rothman A, Elliot C, et al. Magnetic resonance imaging in the prognostic evaluation of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;196:228–239. doi: 10.1164/rccm.201611-2365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dawes TJW, de Marvao A, Shi W, Fletcher T, Watson GMJ, Wharton J, et al. Machine learning of three-dimensional right ventricular motion enables outcome prediction in pulmonary hypertension: a cardiac MR imaging study. Radiology. 2017;283:381–390. doi: 10.1148/radiol.2016161315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oliveira RK, Agarwal M, Tracy JA, Karin AL, Opotowsky AR, Waxman AB, et al. Age-related upper limits of normal for maximum upright exercise pulmonary haemodynamics. Eur Respir J. 2016;47:1179–1188. doi: 10.1183/13993003.01307-2015. [DOI] [PubMed] [Google Scholar]
- 118.D’Alto M, Romeo E, Argiento P, Motoji Y, Correra A, Di Marco GM, et al. Clinical relevance of fluid challenge in patients evaluated for pulmonary hypertension. Chest. 2017;151:119–126. doi: 10.1016/j.chest.2016.08.1439. [DOI] [PubMed] [Google Scholar]
- 119.Kovacs G, Avian A, Olschewski H. Proposed new definition of exercise pulmonary hypertension decreases false-positive cases. Eur Respir J. 2016;47:1270–1273. doi: 10.1183/13993003.01394-2015. [DOI] [PubMed] [Google Scholar]
- 120.van der Bruggen CE, Happé CM, Dorfmüller P, Trip P, Spruijt OA, Rol N, et al. Bone morphogenetic protein receptor type 2 mutation in pulmonary arterial hypertension: a view on the right ventricle. Circulation. 2016;133:1747–1760. doi: 10.1161/CIRCULATIONAHA.115.020696. [DOI] [PubMed] [Google Scholar]
- 121.da Silva Gonçalves Bos D, Happé C, Schalij I, Pijacka W, Paton JFR, Guignabert C, et al. Renal denervation reduces pulmonary vascular remodeling and right ventricular diastolic stiffness in experimental pulmonary hypertension. JACC Basic Transl Sci. 2017;2:22–35. doi: 10.1016/j.jacbts.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rain S, Andersen S, Najafi A, Gammelgaard Schultz J, da Silva Gonçalves Bós D, Handoko ML, et al. Right ventricular myocardial stiffness in experimental pulmonary arterial hypertension: relative contribution of fibrosis and myofibril stiffness. Circ Heart Fail. 2016;9:e002636. doi: 10.1161/CIRCHEARTFAILURE.115.002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jiang B, Deng Y, Suen C, Taha M, Chaudhary KR, Courtman DW, et al. Marked strain-specific differences in the SU5416 rat model of severe pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2016;54:461–468. doi: 10.1165/rcmb.2014-0488OC. [DOI] [PubMed] [Google Scholar]
- 124.Hsu S, Houston BA, Tampakakis E, Bacher AC, Rhodes PS, Mathai SC, et al. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation. 2016;133:2413–2422. doi: 10.1161/CIRCULATIONAHA.116.022082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hsu S, Kokkonen-Simon KM, Kirk JA, Kolb TM, Damico RL, Mathai SC, et al. Right ventricular myofilament functional differences in humans with systemic sclerosis-associated versus idiopathic pulmonary arterial hypertension Circulation 20181372360–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maron BA. Independence day: separating right ventricular function from pulmonary arterial hypertension in systemic sclerosis. Circulation. 2016;133:2345–2347. doi: 10.1161/CIRCULATIONAHA.116.023237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015;132:2037–2099. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 128.Abman SH, Ivy DD, Archer SL, Wilson K AHA/ATS Joint Guidelines for Pediatric Pulmonary Hypertension Committee. Executive summary of the American Heart Association and American Thoracic Society Joint Guidelines for Pediatric Pulmonary Hypertension. Am J Respir Crit Care Med. 2016;194:898–906. doi: 10.1164/rccm.201606-1183ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hansmann G, Apitz C, Abdul-Khaliq H, Alastalo TP, Beerbaum P, Bonnet D, et al. Executive summary. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension: the European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart. 2016;102(Suppl 2):ii86–ii100. doi: 10.1136/heartjnl-2015-309132. [DOI] [PubMed] [Google Scholar]
- 130.Douwes JM, Humpl T, Bonnet D, Beghetti M, Ivy DD, Berger RM TOPP Investigators. Acute vasodilator response in pediatric pulmonary arterial hypertension: current clinical practice from the TOPP Registry. J Am Coll Cardiol. 2016;67:1312–1323. doi: 10.1016/j.jacc.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 131.Sitbon O, Humbert M, Jaïs X, Ioos V, Hamid AM, Provencher S, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 132.Zijlstra WMH, Ploegstra MJ, Vissia-Kazemier T, Roofthooft MTR, Sarvaas GDM, Bartelds B, et al. Physical activity in pediatric pulmonary arterial hypertension measured by accelerometry; a candidate clinical endpoint. Am J Respir Crit Care Med. 2017;196:220–227. doi: 10.1164/rccm.201608-1576OC. [DOI] [PubMed] [Google Scholar]
- 133.Austin ED, West J, Loyd JE, Hemnes AR. Molecular medicine of pulmonary arterial hypertension: from population genetics to precision medicine and gene editing. Am J Respir Crit Care Med. 2017;195:23–31. doi: 10.1164/rccm.201605-0905PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pullamsetti SS, Savai R, Seeger W, Goncharova EA. From cancer biology to new pulmonary arterial hypertension therapeutics: targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med. 2017;195:425–437. doi: 10.1164/rccm.201606-1226PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hemnes AR, Beck GJ, Newman JH, Abidov A, Aldred MA, Barnard J, et al. PVDOMICS Study Group. PVDOMICS: a multi-center study to improve understanding of pulmonary vascular disease through phenomics. Circ Res. 2017;121:1136–1139. doi: 10.1161/CIRCRESAHA.117.311737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barnes JW, Dweik RA. Pulmonary hypertension and precision medicine through the “omics” looking glass. Am J Respir Crit Care Med. 2017;195:1558–1560. doi: 10.1164/rccm.201704-0750ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Newman JH, Rich S, Abman SH, Alexander JH, Barnard J, Beck GJ, et al. Enhancing insights into pulmonary vascular disease through a precision medicine approach: a joint NHLBI-Cardiovascular Medical Research and Education Fund workshop report. Am J Respir Crit Care Med. 2017;195:1661–1670. doi: 10.1164/rccm.201701-0150WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bonnet S, Provencher S, Guignabert C, Perros F, Boucherat O, Schermuly RT, et al. Translating research into improved patient care in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;195:583–595. doi: 10.1164/rccm.201607-1515PP. [DOI] [PMC free article] [PubMed] [Google Scholar]