Abstract
Objectives
Cathepsin K is expressed by osteoclasts and synovial fibroblasts and degrades key components of bone and cartilage. Inhibition of cathepsin K protease activity may be beneficial for the prevention of bone erosion and cartilage degradation in rheumatoid arthritis (RA). The collagen-induced arthritis (CIA) rat model is well established for studying the pathology and treatment of RA. We investigated the effect of ONO-KK1-300-01, a cathepsin K inhibitor (CKI), on arthritis and bone mineral density (BMD) in rats with CIA.
Methods
Seven-month-old female Sprague Dawley rats were divided into 5 groups: rats without CIA (CNT); CIA rats that underwent ovariectomy (OVX) and were treated with CKI; CIA rats that underwent OVX and were treated with vehicle (Veh); CIA rats that underwent sham surgery and were treated with CKI; and CIA rats that underwent sham surgery and were treated with Veh. CKI was orally administered at a dose of 15 mg/kg, thus initiating collagen sensitization, until death at 4 weeks. We evaluated hind paw thickness and the arthritis score every week until death. Radiographs of the resected left foot were obtained with a soft X-ray apparatus. Destruction of bone and cartilage was classified and scored as previously described by Engelhardt et al. BMD was measured by bone densitometry at the halfway point between the distal metaphysis and the diaphysis of the resected right femur. We also performed histomorphometry of the proximal left tibia, histological evaluation of arthritis, and a bone strength test.
Results
CKI administration significantly reduced hind paw thickness and the arthritis score, and prevented a decrease in BMD. The radiographic score was significantly lower in the CKI group than in the Veh group. In the histomorphometric analysis, bone-resorption parameters were significantly lower in the CKI groups than in the Veh groups. CKI significantly inhibited synovial proliferation in the CIA rats. In the bone strength test, the ultimate stress was significantly higher in the CKI groups than in the Veh groups.
Conclusion
Our findings indicate that cathepsin K inhibitors may inhibit systemic and local bone loss, ameliorate arthritis, and attenuate the decrease of bone strength in an animal model of arthritis.
Keywords: Cathepsin K inhibitor, CIA rat, Arthritis, Bone marrow density
Highlights
-
•
We investigated the effect of a cathepsin K inhibitor (CKI) on arthritis and bone mineral density (BMD) in rats with CIA.
-
•
CKI administration significantly reduced hind paw thickness and the arthritis score, and prevented a decrease in BMD.
-
•
Bone-resorption parameters were significantly lower in the CKI groups than in the Vehicle groups.
-
•
CKI may inhibit bone loss, ameliorate arthritis, and attenuate the decrease of bone strength in an animal model of arthritis.
1. Introduction
Cathepsin K, which is expressed by osteoclasts and synovial fibroblasts, degrades key components of bone and cartilage, such as type I and type II collagen, osteonectin, and aggrecan (Salminen-Mankonen et al., 2007). Since cathepsin K inhibitors (CKIs) selectively inhibit bone resorption with a minor effect on bone formation (Ochi et al., 2011), CKIs have been used to treat osteoporosis in previous studies. Cathepsin K is highly expressed in synovial fibroblasts and macrophages in rheumatoid arthritic joints (Hou et al., 2001; Hummel et al., 1998). A positive correlation has been observed between the extent of radiological destruction and serum levels of cathepsin K (Skoumal et al., 2005). Inhibition of cathepsin K protease activity may be beneficial for the prevention of bone erosion and cartilage degradation in rheumatoid arthritis (RA) (Salminen-Mankonen et al., 2007; Weidauer et al., 2007; Yasuda et al., 2005).
Osteoporosis is often a complication of RA, resulting in an increased risk of fracture. Furthermore, osteoporosis is exacerbated by estrogen deficiency (Saville and Kharmosh, 1967; Teshima et al., 1987; Reid et al., 1982). In our previous studies, we evaluated the effects of estrogen replacement therapy on arthritis severity and bone mineral density (BMD) in ovariectomized rats with collagen-induced arthritis (CIA), an established model for studying the pathology and treatment of RA (Fukata et al., 2004; Yamane et al., 2003; Yamasaki et al., 2001; Yoshioka et al., 2008). In these studies, OVX in CIA rats worsened arthritis severity and bone loss.
Two previous studies examined the effects of CKIs on arthritis, but both assessed only arthritis symptoms (Asagiri et al., 2008; Svelander et al., 2009). This is the first study to investigate the effect of a CKI not only on arthritis but also on BMD, bone histomorphometry, and bone strength. The aim of this study was to evaluate the effect of ONO-KK1-300-01, a CKI, on arthritis and BMD in CIA rats.
2. Materials and methods
2.1. Animals
Seven-month-old female Sprague-Dawley rats (retired breeder animals with a body weight of 278–410 g; Shimizu Laboratory Supplies, Kyoto, Japan) were used. This experiment was conducted at the animal research facilities of Tottori University, with approval by the Animal Experiment Ethical Committee of Tottori University. Animals were given tap water and solid food (calcium content 1.18 g/100 g, phosphorus content 1.09 g/100 g, vitamin D3 content 250 IU/100 g) (CE-2; CLEA Japan, Tokyo, Japan) ad libitum. Animals were maintained in an animal room, which was illuminated for 12 h daily (07:00–19:00), at a temperature of 24 °C. Animals were used in experiments after a 4-week acclimation period.
Animals were divided into the following 5 groups, with mean body weight equalized across groups during randomization: injection of saline only + vehicle administration (CNT; n = 11); collagen sensitization + ovariectomy (OVX) + CKI (CIA + OVX + CKI; n = 11); collagen sensitization + OVX + vehicle administration (CIA + OVX + Veh; n = 11); collagen sensitization + sham surgery + CKI administration (CIA + sham + CKI; n = 11); and collagen sensitization + sham surgery + vehicle administration (CIA + sham + Veh; n = 11).
Rats in the 5 experimental groups were examined weekly for body weight, arthritis score (Svelander et al., 2009), and hind paw thickness. Four weeks after initial collagen sensitization, rats were killed under anesthesia with intraperitoneal injection of pentobarbital at 40 mg/kg, just before the collection of blood by cardiac puncture. We assessed the success of ovariectomy by evaluating uterine atrophy. The left knee joint, the left femur, and both feet were resected and fixed in 70% alcohol at 4 °C for analysis.
2.2. Preparation of the CIA model
Under intraperitoneal anesthesia with pentobarbital at a dose of 35 mg/kg, 1.0 ml of an emulsion containing 500 μg of bovine type II collagen in 0.3% acetic acid solution (catalog K-41; Cosmo-Bio, Tokyo, Japan) and 500 μg of Freud's incomplete adjuvant (catalog 263910; Difco, Detroit, MI, USA) were injected intracutaneously at 2 sites on the back of each rat. For additional collagen sensitization, 0.5 ml of the same emulsion was injected intracutaneously at 2 sites on the posterior aspects of both hip joints at 1 week after initial sensitization; OVX or sham surgery was performed at time of the second injection. In the CNT group, physiologic saline was injected intracutaneously, using the same volume and methods applied for the other 4 groups.
2.3. Methods of drug administration
A CKI (ONO-KK1-300-01; Ono Pharmaceutical, Osaka, Japan) was dissolved in 0.5% methyl cellulose (catalog 155496; Wako Pure Chemical Industries, Osaka, Japan) and administered orally at a dose of 15 mg/kg daily, thus initiating sensitization, until death at 4 weeks. The dose used was based on a previous observation and is considered to be appropriate in experimental animals (Ochi et al., 2014). We selected this dose in order to investigate the effect of this CKI on arthritis during a 4-week observational period. In the groups receiving vehicle, only the 0.5% methyl cellulose was administered orally, using the same volume and methods applied for the CKI groups.
2.4. Clinical evaluation of arthritis
Rats were examined for body weight, arthritis score, and hind paw thickness every week until death. Arthritis was evaluated according to the degree of severity and the extent of erythema and edema of the periarticular tissue, scored from 0 to 3 as follows: 0 = no arthritis, 1 = one side of joint affected, 2 = both sides of joints affected, and 3 = entire paw affected (Svelander et al., 2009). The total arthritis score was the sum of the individual scores for digits, metacarpophalangeal joint and wrist in the forepaw, and ankle joint in the hind paw (maximum possible score 12). If both the right and left hind paws showed arthritis, the score was 2 points. If both right and left hind paws showed severe arthritis, the score was 3 points. We evaluated both the right and left joints, and the maximum total score was 24 points.
Hind paw thickness was evaluated by measuring the ankle width from the medial malleolus to the lateral malleolus using a constant-tension caliper. Hind paw thickness was expressed as the mean of both hind legs. These parameters were measured by an investigator blinded to the experimental groups.
2.5. Radiographic examination
Radiographs of the resected left foot were obtained with a soft X-ray apparatus (DigitalDiagnost; Philips, Amsterdam, The Netherlands). Destruction of bone and cartilage was classified and scored according to the method described by Engelhardt et al. (1995). The overall radiographic score was the sum of the scores for spongiosa, periosteum, and compact bone (maximum score 8.5). Radiographs were evaluated by 3 observers who were blinded to the study groups. The final radiographic score was the mean of the scores determined by the 3 observers.
2.6. Measurement of BMD
Areal BMD was measured by bone densitometry (Discovery; Hologic, Waltham, MA, USA) at the halfway point between the distal metaphysis and the diaphysis of the resected right femur. The pixel size was 0.1512 cm in length and 0.0640 cm in width.
2.7. Bone histomorphometry
Bone labeling by intraperitoneal injection of calcein at 10 mg/kg was performed twice: at 7 days and 2 day before death (the schedule involved 1 day for the first labeling; 5 days between first and second labelings; 1 day for the second labeling; and 2 day between the second labeling and sacrifice). The left knee joint (from the distal one-third of the femur to one-third of the proximal tibia), resected at death, was fixed in 70% alcohol. The knee joints were then stained with Villanueva bone stain and embedded in methylmethacrylate resin without decalcification. The resulting blocks of knee joint specimens were sectioned in the frontal plane at a thickness of 5 μm with a microtome (RM2255; Leica Biosystems, Nussloch, Germany).
For histomorphometric analysis, the following parameters were measured in the secondary spongiosa, extending 1.3–3.9 mm distally from the proximal growth cartilage of the tibia: bone volume/total volume (BV/TV, %); osteoid volume/total volume (OV/TV, %); osteoblast surface/bone surface (Ob.S/BS, %); osteoid surface/bone surface (OS/BS, %); trabecular thickness (Tb.Th, μm); osteoclast surface/bone surface (Oc.S/BS, %); eroded surface/bone surface (ES/BS, %); osteoclast number/bone surface (N.Oc/BS, mm−1); mineralizing surface/bone surface (MS/BS, %); mineral apposition rate (MAR, μm/day); bone formation rate/bone volume (BFR/BV, %/year); and erosion depth (μm). Each parameter was expressed according to the classification of Parfitt et al. (1987) Dempster et al., 2013). For each specimen, one section was prepared from the same site and 25 visual fields were evaluated.
Bone histomorphometric analysis was performed using semiautomatic image analysis software (System Supply, Nagano, Japan) and an optical fluorescent microscope (BX51; Olympus, Tokyo, Japan). These parameters were measured by an investigator blinded to the experimental groups.
2.8. Histological evaluation of arthritis
Arthritis and erosion were evaluated histologically by preparing non-decalcified specimens from the knee joints used in bone histomorphometry. The following parameters were measured in the medial half of the medial compartment using a microscope with a magnifying power of 40: total tissue area (in mm2), pannus area (in mm2), and synovial tissue area (in mm2). The total tissue area was obtained by tracing the tissue perimeter or the borders of the area. The pannus area was measured at the tibial and femoral sides. The synovial tissue area included the total tissue area of the synovium, infiltrating inflammatory cells, and proliferating fibroblasts, as well as the inflammatory granulation tissue (pannus) with scar-like fibrous tissue (including the eroded areas of the bone). Each parameter was measured using a semiautomatic digitizer connected to a personal computer (System Supply) (Hayashi et al., 2011). These parameters were measured by an investigator blinded to the experimental groups.
2.9. Bone strength test
With the right femur, a 3-point bending test was performed at a test speed of 1 mm/min using an AGS-H Shimadzu Autograph (Shimadzu, Kyoto, Japan). The bones were placed on the posterior surface on 2 rounded bars with a distance of 20 mm between the bars. The load-deformation curve was recorded. The ultimate load (in N) was the load at the ultimate failure point. Stiffness (in N/mm) was calculated from the maximum slope of the curve.
The ultimate stress (in N/mm2) was calculated using the formula δ (ultimate stress) = 4I (ultimate load × L × b / 2), where I is the second moment of inertia (in mm4), L is the distance between the supporting bars (in mm), and b is the width of the femur in the anterior-posterior direction (in mm). The value for the second moment of inertia used in the stress analysis was calculated under the assumption that the femoral cross-sections were elliptically shaped, using the formula I = π (ab3 − [a − 2 t][b − 2 t]3) / 64, where a is the width of the cross-section in the medial-lateral direction (in mm), b is the width of the femur in the anterior-posterior direction (in mm), and t is the average cortical thickness (in mm). Average cortical thickness was calculated from thickness measurements made in each of the 4 quadrants of the femoral cross-sections using a constant-tension caliper. Widths a and b were measured at the location of the femur where the top loader contacted the bone. The modulus of elasticity of the femur was calculated using the following formula: modulus of elasticity = (L3 × stiffness) / (48 × second moment of inertia). These parameters were measured by an investigator blinded to the experimental groups.
2.10. Statistical analysis
Changes of body weight and hind paw thickness were analyzed by 2-way repeated analysis of variance (ANOVA) for the effects of CKI administration and OVX and their interactions. The data for BMD, bone histomorphometry, histological evaluation, and bone strength test were compared by 2-way ANOVA. Fisher's protected least significance difference (PLSD) procedure was performed after ANOVA. The incidence of arthritis was compared among the 4 collagen-sensitized groups using the chi-squared test. For comparison of the arthritis score and radiographic score, the Kruskal-Wallis test was performed among the 4 collagen-sensitized groups. Scheffe's test was performed after the Kruskal-Wallis test. Data from rats that developed arthritis were used for body weight, arthritis score, hind paw thickness, radiographic score, BMD, bone histomorphometry, histologic evaluation, and bone strength test. CIA rats sometimes do not develop arthritis; 5 in this study did not (3 in the CIA + OVX + Veh group, 2 in the CIA + sham + Veh group), and were excluded from the analysis, along with 2 rats that died under anesthesia at the beginning (one in the CIA + OVX + CKI, one in the CIA + sham + CKI). Statistical analysis was performed using StatView software version 5.0 (SAS Institute, Cary, NC, USA); p < 0.05 was considered statistically significant.
3. Results
3.1. Changes in body weight
Mean body weight values were measured at 0, 2, and 4 weeks after sensitization (Supplementary Information). CIA rats showed a significant reduction in body weight compared with CNT rats at 4 weeks. There were no significant differences between the CKI and Veh groups at 4 weeks (2-way ANOVA, p = 0.77).
3.2. Changes in arthritis
The incidences of arthritis at 3 and 4 weeks after sensitization are shown in Table 1. In the 4 experimental groups with CIA, joint swelling started to occur at 2 weeks after sensitization, and developed rapidly until 4 weeks. The incidence of arthritis was not significantly different between the 4 groups with CIA (chi-squared test, p = 0.59). CKI administration significantly suppressed hind paw thickness (2-way ANOVA, p = 0.04) and there were significant differences between the CIA + OVX + CKI group and the CIA + OVX + Veh group at 4 weeks (Fisher's PLSD test, p = 0.04) (Fig. 1, Fig. 2).
Table 1.
Arthritis scores, radiographic scores, and bone mineral density.
| CIA |
||||
|---|---|---|---|---|
| OVX |
Sham |
|||
| CKI | Vehicle | CKI | Vehicle | |
| Incidence of arthritis (%) | ||||
| 3 weeks | 45.5 | 63.6 | 54.5 | 63.6 |
| 4 weeks | 90.9 | 72.7 | 90.9 | 81.8 |
| Arthritis score (points)a | ||||
| 3 weeks⁎ | 2.0 (0.5, 3.5) | 4.5 (3.0, 6.0) | 1.5 (0, 3.0) | 4.0 (1.5, 4.75) |
| 4 weeks⁎ | 4.5 (3.0, 6.5)b | 7.0 (5.0, 8.0)b | 4.0 (2.0, 4.0)c | 6.0 (5.25, 6.75)c |
| Radiographic score (points)a | ||||
| 4 weeks⁎ | 3.88 (3.25, 4.0) | 5.63 (4.25, 6.25) | 2.63 (2.375, 2.875)d | 4.50 (3.0, 6.25)d |
| BMD (g/cm2) | 0.155 ± 0.016 | 0.148 ± 0.014 | 0.169 ± 0.019e | 0.148 ± 0.006e |
CIA = collagen-induced arthritis, OVX = ovariectomy, CKI = cathepsin K inhibitor, BMD = bone mineral density.
BMD was measured at the halfway point of the distal resected right femur. Values of BMD represent means ± SD. BMD was significantly higher in the CKI groups (2-way ANOVA, p = 0.006). Post hoc analyses with Fisher's PLSD test were performed among the 4 groups.
p < 0.05 (by Kruskal-Wallis test).
Data are represented as follows: median (75th percentile, 25th percentile).
p < 0.05 (CIA + OVX + CKI vs. CIA + OVX + Vehicle by Scheffe's test).
p < 0.05 (CIA + Sham + CKI vs. CIA + Sham + Vehicle by Scheffe's test).
p < 0.05 (CIA + Sham + CKI vs. CIA + Sham + Vehicle by Scheffe's test).
p < 0.05 (CIA + Sham + CKI vs. CIA + Sham + Vehicle).
Fig. 1.
Typical photographs of the hind paw.
A, swollen hind paw (CIA group). B, normal hind paw (Control group).
Hind paw thickness was evaluated by measuring the ankle width from the medial malleolus to the lateral malleolus using a constant-tension caliper.
CIA = collagen-induced arthritis.
Fig. 2.

Hind paw thickness.
Data represent the mean. Time-dependent development of hind paw thickness elicited by collagen sensitization. CKI administration significantly inhibited hind paw thickening (p < 0.05, by 2-way repeated ANOVA). There was no significant effect of OVX. There was no significant interaction between CKI administration and OVX. Post hoc comparisons performed among the 4 groups each week by Fisher's PLSD test. #p < 0.05 (vs. CIA + OVX + Veh) CIA = collagen-induced arthritis, OVX = ovariectomy, CKI = cathepsin K inhibitor, Veh = vehicle, PLSD = protected least significance difference.
There was no significant effect of OVX on hind paw thickness, and there was no significant interaction between OVX and CKI.
The arthritis score showed a rapid early increase between 2 and 4 weeks, and there were significant differences at 3 and 4 weeks between the 4 collagen-sensitized groups (Kruskal-Wallis test, p = 0.03 at 3 weeks and p = 0.0008 at 4 weeks) (Table 1). At 4 weeks, the score in the CIA + OVX + CKI group was significantly lower than in the CIA + OVX + Veh group (Scheffe's test, p = 0.03). The score in the CIA + sham + CKI group was significantly lower than in the CIA + sham + Veh group (Scheffe's test, p = 0.008).
3.3. Radiographic findings
Joint destruction was observed in all collagen-sensitized groups. There was a significant difference between the 4 experimental groups (Kruskal-Wallis test, p = 0.002). The score in the CIA + sham + CKI group was significantly lower than in the CIA + sham + Veh group (Scheffe's test, p = 0.02) (Table 1 and Fig. 3).
Fig. 3.
Radiographic appearance of the left foot.
A, CIA + OVX + CKI group. B, CIA + OVX + Veh group. C, CIA + Sham + CKI group. D, CIA + Sham + Veh group.
The score in the CIA + sham + CKI group was significantly lower than that in the CIA + sham + Veh group.
CIA = collagen-induced arthritis, OVX = ovariectomy, CKI = cathepsin K inhibitor, Veh = vehicle.
3.4. BMD
BMD was significantly higher in the CKI groups (2-way ANOVA, p = 0.006) and there was a significant difference between the CIA + sham + CKI group and the CIA + sham + Veh group (Fisher's PLSD test, p = 0.004) (Table 1). There was no significant effect of OVX, nor was there an interaction between OVX and CKI.
3.5. Bone histomorphometry
BV/TV was higher in the CKI groups than in the Veh groups (2-way ANOVA, p = 0.02) and the effect of CKI was significant. ES/BS, Oc.S/BS, N·Oc/BS, and erosion depth were lower in the CKI groups, and there was a significant CKI effect (2-way ANOVA, p = 0.02, 0.007, 0.006, <0.0001, respectively). On the other hand, OVX significantly increased ES/BS, Oc.S/BS, and N·Oc/BS (2-way ANOVA, p = 0.02, 0.003, 0.03, respectively).
There were significant differences in Tb.Th, Ob.S/BS, OS/BS, Oc.S/BS, and ES/BS among the 4 groups (Table 2 and Fig. 4). There was no significant interaction between OVX and CKI administration. There was no significant difference between groups with respect to OV/TV, MS/BS, MAR, or BFR/BS.
Table 2.
Data obtained from bone histomorphometry.
| CIA |
||||||
|---|---|---|---|---|---|---|
| OVX |
Sham |
p value |
||||
| CKI | Vehicle | CKI | Vehicle | CKI | OVX | |
| BV/TV (%) | 26.9 ± 5.2 | 22.5 ± 2.9 | 29.1 ± 3.8 | 25.7 ± 6.0 | 0.02 | 0.09 |
| OV/TV (%) | 0.101 ± 0.162 | 0.047 ± 0.055 | 0.015 ± 0.030 | 0.198 ± 0.381 | 0.35 | 0.64 |
| Ob.S/BS (%) | 2.8 ± 4.0 | 1.6 ± 1.4 | 0.5 ± 0.8 | 4.8 ± 7.2b | 0.28 | 0.75 |
| OS/BS (%) | 3.8 ± 5.4 | 2.2 ± 1.8 | 1.1 ± 1.9 | 6.4 ± 8.7b | 0.30 | 0.67 |
| Tb.Th (μm) | 86.8 ± 18.6 | 69.9 ± 13.4a | 91.5 ± 15.0 | 72.6 ± 8.9b | 0.0007 | 0.45 |
| Oc.S/BS (%) | 4.9 ± 3.6 | 10.3 ± 4.7a | 2.6 ± 1.2 | 5.5 ± 3.3 | 0.0007 | 0.003 |
| ES/BS (%) | 15.0 ± 9.4 | 27.0 ± 10.9a | 10.7 ± 4.9 | 17.6 ± 8.4 | 0.002 | 0.02 |
| N·Oc/BS (%) | 1.1 ± 0.9 | 2.0 ± 1.1 | 0.5 ± 0.3 | 1.3 ± 0.9b | 0.006 | 0.03 |
| MS/BS (%) | 3.4 ± 5.1 | 1.6 ± 1.4 | 1.6 ± 2.6 | 6.5 ± 8.5 | 0.36 | 0.36 |
| MAR (μm/day) | 1.3 ± 1.1 | 1.3 ± 0.8 | 0.8 ± 1.0 | 1.3 ± 1.1 | 0.52 | 0.52 |
| BFR/BS (mm3/mm2/year) | 0.028 ± 0.045 | 0.010 ± 0.011 | 0.012 ± 0.021 | 0.053 ± 0.085 | 0.49 | 0.42 |
| Erosion depth (μm) | 14.7 ± 1.8 | 25.2 ± 3.1a | 5.9 ± 0.8 | 19.6 ± 2.6b | <0.0001 | <0.0001 |
CIA = collagen-induced arthritis, OVX = ovariectomy, CKI = cathepsin K inhibitor, BV/TV = bone volume/total volume, OV/TV = osteoid volume/total volume, Ob.S/BS = osteoblast surface/bone surface, OS/BS = osteoid surface/bone surface, Tb.Th = trabecular thickness, Oc.S/BS = osteoclast surface/bone surface, ES/BS = eroded surface/bone surface, N.Oc/BS = osteoclast number/bone surface, MS/BS = mineralizing surface/bone surface, MAR = mineral apposition rate, BFR/BV = bone formation rate/bone volume.
Values represent means ± SD. There was no significant interaction between CKI administration and OVX by 2-way ANOVA. Post hoc analyses with Fisher's PLSD test were performed among the 4 groups.
p < 0.05 (vs. CIA + OVX + CKI).
p < 0.05 (vs. CIA + sham + CKI).
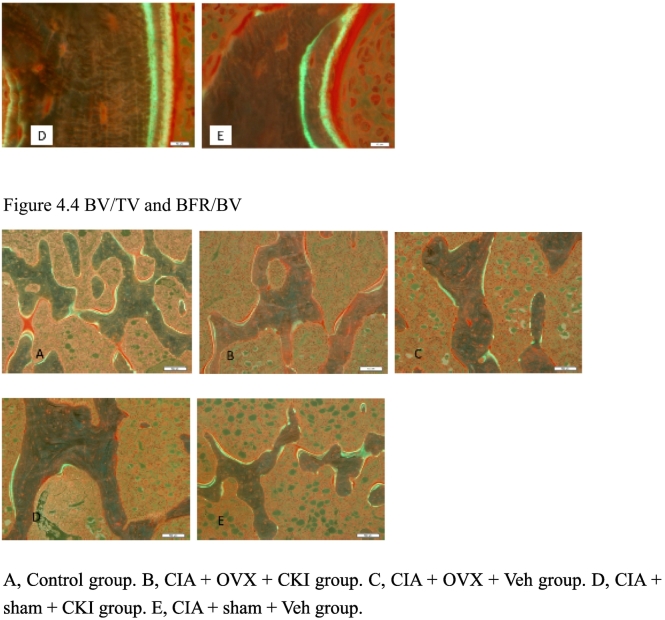
Fig. 4.
Histologic sections of a knee joint stained with Villanueva bone stain.
4.1, Osteoclasts are shown. Erosion depth and N. Oc/BS were lower in the CKI groups.
4.2, Osteoblasts are shown. Ob.S/BS did not differ significantly between the 5 groups.
4.3, Mineral apposition rates are shown. There were no significant differences between the 5 groups.
4.4, BV/TV and BFR/BV. BV/TV were higher in the CKI groups than in the Veh groups.
A, Control group. B, CIA + OVX + CKI group. C, CIA + OVX + Veh group. D, CIA + Sham + CKI group. E, CIA + Sham + Veh group.
BV/TV was higher in the CKI groups than in the Veh groups.
CIA = collagen-induced arthritis, OVX = ovariectomy, CKI = cathepsin K inhibitor, Veh = vehicle, BV/TV = bone volume/tissue volume, BFR/BV = bone formation rate/bone volume.
3.6. Histological evaluation of arthritis and erosion
The CIA groups exhibited proliferating joint synovium and eroding joint cartilage. CKI administration had a significant effect on inhibition of synovial proliferation (2-way ANOVA, p = 0.01), with significant differences among the 4 groups (Fig. 5, Fig. 6). The pannus area/total tissue area in the CKI groups was significantly lower than in the Veh groups (2-way ANOVA, p = 0.02). OVX had no effect on proliferating joint synovium or eroding joint cartilage. There was no significant interaction between CKI administration and OVX.
Fig. 5.
Histologic evaluation of arthritis.
Data represent the mean ± SD. CKI administration significantly inhibited synovium proliferation and pannus area (p < 0.05 by 2-way ANOVA).
OVX did not influence synovium proliferation or pannus area. There was no significant interaction between CKI administration and OVX. Post-hoc comparisons performed among the 4 groups by Fisher's PLSD test. ⁎p < 0.05 (vs. CIA + OVX + Veh).
CIA = collagen-induced arthritis, OVX = ovariectomy, CKI = cathepsin K inhibitor, Veh = vehicle, PLSD = protected least significance difference.
Fig. 6.
Histological sections of a knee joint stained with Villanueva bone stain.
CKI administration had a significant effect on inhibition of synovial proliferation, with significant differences among the 5 groups. The ratio of the pannus area to total tissue area in the CKI groups was significantly lower than in the Veh groups.
CIA = collagen-induced arthritis, OVX = ovariectomy, CKI = cathepsin K inhibitor, Veh = vehicle.
3.7. Bone strength test
The ultimate stress at break was higher in the CKI groups than in the Veh groups (2-way ANOVA, p = 0.02). However, there were no significant differences between these groups in any other parameters (Table 3).
Table 3.
Bone strength test.
| CIA |
||||||
|---|---|---|---|---|---|---|
| OVX |
Sham |
p value |
||||
| CKI | Vehicle | CKI | Vehicle | CKI | OVX | |
| Structural properties | ||||||
| Ultimate load (N) | 121 ± 8 | 115 ± 21⁎ | 130 ± 9 | 118 ± 16 | 0.93 | 0.27 |
| Stiffness (102 N/mm) | 283 ± 60 | 253 ± 70 | 279 ± 67 | 261 ± 63 | 0.29 | 0.94 |
| Material properties | ||||||
| Ultimate stress (MPa) | 165 ± 21 | 159 ± 25 | 192 ± 28 | 155 ± 24⁎ | 0.02 | 0.19 |
| Modulus of elasticity (102 MPa) | 81 ± 22 | 72 ± 19 | 86 ± 19 | 70 ± 20 | 0.07 | 0.79 |
| Second moment of inertia (mm4) | 6.0 ± 0.8 | 5.9 ± 0.4 | 5.3 ± 1.0 | 6.4 ± 0.8⁎ | 0.09. | 0.72 |
CIA = collagen-induced arthritis, OVX = ovariectomy, CKI = cathepsin K inhibitor.
Values are means ± SD. There was no significant interaction between CKI administration and OVX by 2-way ANOVA. Post hoc analyses with Fisher's PLSD test were performed among the 4 groups.
p < 0.05 versus CIA + sham + CKI.
4. Discussion
In this study, CKI administration significantly suppressed hind paw thickness, arthritis score, and histological arthritis in CIA rats. The radiographic score was significantly lower in the CKI group than in the Veh group. Histomorphometry demonstrated that bone-resorption parameters were significantly lower in the CKI groups than in the Veh groups. There was no significant interaction between CKI administration and OVX. Thus, OVX and CKI independently affected arthritis and BMD. To the best of our knowledge, there have been 2 reports on the effect of CKIs on arthritis, but both evaluated only arthritis symptoms (Asagiri et al., 2008; Svelander et al., 2009). This study is the first to assess the impact of a CKI on not only arthritis but also BMD, bone histomorphometry, and bone strength. Since RA is often accompanied by osteoporosis, it is useful to study the effect of CKIs on arthritis.
In this study, BMD was not significantly lower in the OVX groups, but the mean BMD of the OVX groups was lower than that of the sham groups (p = 0.15). We think the reason why there was no significant difference was that the sample size was small and the analysis period was short. ONO-5334 has shown potent selective inhibition of cathepsin K in vitro and has resulted in improvement in both BMD and bone strength in osteoporosis models (Ochi et al., 2014; Eastell et al., 2014; Engelke et al., 2014; Yamada et al., 2016). In the first clinical study of ONO-5334, Eastell et al. (2011) investigated the efficacy and safety of the compound in postmenopausal women with osteoporosis. The ONO-5334 group and the alendronate group showed a significant increase in BMD of the lumbar spine, total hip, and femoral neck. Also, Ochi et al. reported that ONO-KK1-300-01, which is the prototype compound of ONO-5334, had an additive effect on the preventive action of parathyroid hormone on bone loss in OVX rats.
In this study, CKI prevented arthritis and reduced the pannus area. Hou et al. demonstrated that expression of cathepsin K was localized at the site of cartilage erosion in RA (Hou et al., 2002). It was also reported that inhibition of cathepsin K protease activity was beneficial for the prevention of bone erosion and cartilage degradation in RA (Salminen-Mankonen et al., 2007; Weidauer et al., 2007; Yasuda et al., 2005). These studies indicated that inhibition of cathepsin K reduced the pannus area. CKI showed a cartilage-protective effect in several models of RA and osteoarthritis (Asagiri et al., 2008; Svelander et al., 2009; Tanaka et al., 2016). One of these studies demonstrated that prophylactic treatment with CKI reduced the severity of CIA in mice (Svelander et al., 2009). Asagiri et al. (2008) found that inhibition of cathepsin K potently suppressed both autoimmune joint inflammation and osteoclastic bone resorption in autoimmune arthritis. These authors also showed that expression of interleukin (IL)-6, IL-12, and IL-23 in bone-marrow dendritic cells was down-regulated at the mRNA level, and interferon-β production by bone-marrow dendritic cells was suppressed by CKI. These results suggest that cathepsin K inactivation leads to the blockade of all Toll-like receptor 9 downstream signaling pathways in dendritic cells, and as a result, secretion of cytokines to Th17 cells and macrophages is suppressed, which may reduce inflammation. We consider that the mechanisms underlying our results involve the prevention of cathepsin K expression at the site of cartilage erosion, synovial fibroblasts, and macrophage suppression and blockade of Toll-like receptor 9 signaling pathways in dendritic cells (Asagiri et al., 2008; Svelander et al., 2009).
OVX is considered to produce a physiological state that is equivalent to menopause. In this study, there were significant differences between the CIA + OVX + CKI group and the CIA + OVX + Veh group in hind paw thickness, arthritis score, histological evaluation of arthritis, Tb.Th, Oc.S/BS, ES/BS, and erosion depth. There were no significant differences between the CIA + sham + CKI group and the CIA + sham + Veh group in hind paw thickness, histological evaluation of arthritis, and ES/BS. Comparing the OVX groups, the radiographic score in the CIA + OVX + CKI group was lower than that in the CIA + OVX + Veh group, although the difference was not statistically significant. This may be because there was not enough time for OVX to have an effect. Also, the OVX group showed severe destruction of bone and cartilage, and the median radiographic score of this group was higher than that of the sham group. However, a greater difference between the OVX groups and the sham groups would be expected. We think the lack of such a difference was due to the small sample size or to the possibility that CKI could not adequately suppress severe joint destruction when severe arthritis occurred.
In both OVX and sham animals, CKI affected the arthritis score, but the sham-operated animals showed no significant difference in hind paw thickness. However, the mean hind paw thickness of the CIA + sham + Veh group was higher than that of the CIA + sham + CKI group. The arthritis score evaluated eight joints, while assessment of hind paw thickness score involved only the ankle joints. This may explain why there was no significant difference in hind paw thickness in the sham-operated animals.
CIA rats are generally used as a model of RA, but not of osteoporosis. We used the OVX model to create a state of postmenopausal osteoporosis. Furthermore, Holmdahl et al. showed that OVX increased the incidence of arthritis and accelerated inflammation caused by collagen type II immunization (Holmdahl et al., 1986, Holmdahl et al., 1987). Their results suggest that treatment with low doses of beta-oestradiol exerts a suppressive effect on both the development of collagen arthritis and on T cell-dependent immune reactivity towards type II collagen. We implemented the OVX model since by exacerbating arthritis, it might amplify the effect of CKI.
The primary limitation of this study is that the effects of CKI were evaluated in a CIA rat model, and the results may therefore not be clinically relevant to humans with RA. Also, rats were given tap water and solid food ad libitum. Weight loss may have affected bone mineral density, but the body weights of the CIA groups were not significantly different.
In conclusion, a cathepsin K inhibitor inhibited clinically and histologically proven arthritis as well as bone loss in rats with CIA, and attenuated the decrease of bone strength.
The following are the supplementary data related to this article.
Body weight changes.
Transparency document
Transparency document.
Acknowledgements
Ono Pharmaceutical supplied the ONO-KK1-300-01. We also acknowledge Ryohei Fukui for his advice on bone mineral density analysis and Akemi Ito for her assistance in preparing and staining tissue sections and conducting histomorphometry.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Asagiri M., Hirai T., Kunigami T., Kamano S., Gober H.J., Okamoto K., Nishikawa K., Latz E., Golenbock D.T., Aoki K., Ohya K., Imai Y., Morishita Y., Miyazono K., Kato S., Saftig P., Takayanagi H. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science. 2008;319(5863):624–627. doi: 10.1126/science.1150110. [DOI] [PubMed] [Google Scholar]
- Dempster D.W., Compston J.E., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R., Parfitt A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastell R., Nagase S., Ohyama M., Small M., Sawyer J., Boonen S., Spector T., Kuwayama T., Deacon S. Safety and efficacy of the cathepsin K inhibitor ONO-5334 in postmenopausal osteoporosis: the OCEAN study. J. Bone Miner. Res. 2011;26(6):1303–1312. doi: 10.1002/jbmr.341. [DOI] [PubMed] [Google Scholar]
- Eastell R., Nagase S., Small M., Boonen S., Spector T., Ohyama M., Kuwayama T., Deacon S. Effect of ONO-5334 on bone mineral density and biochemical markers of bone turnover in postmenopausal osteoporosis: 2-year results from the OCEAN study. J. Bone Miner. Res. 2014;29(2):458–466. doi: 10.1002/jbmr.2047. [DOI] [PubMed] [Google Scholar]
- Engelhardt G., Homma D., Schnitzler C. Meloxicam: a potent inhibitor of adjuvant arthritis in the Lewis rat. Inflamm. Res. 1995;44(12):548–555. doi: 10.1007/BF01757360. [DOI] [PubMed] [Google Scholar]
- Engelke K., Nagase S., Fuerst T., Small M., Kuwayama T., Deacon S., Eastell R., Genant H.K. The effect of the cathepsin K inhibitor ONO-5334 on trabecular and cortical bone in postmenopausal osteoporosis: the OCEAN study. J. Bone Miner. Res. 2014;29(3):629–638. doi: 10.1002/jbmr.2080. [DOI] [PubMed] [Google Scholar]
- Fukata S., Hagino H., Okano T., Yamane I., Kameyama Y., Teshima R. Effect of intermittent administration of human parathyroid hormone on bone mineral density and arthritis in rats with collagen-induced arthritis. Arthritis Rheum. 2004;50(12):4060–4069. doi: 10.1002/art.20728. [DOI] [PubMed] [Google Scholar]
- Hayashi I., Hagino H., Okano T., Enokida M., Teshima R. Effect of raloxifene on arthritis and bone mineral density in rats with collagen-induced arthritis. Calcif. Tissue Int. 2011;88(2):87–95. doi: 10.1007/s00223-010-9432-6. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Klareskog L., Rubin K. Role of T lymphocytes in murine collagen induced arthritis. Agents Actions. 1986;19(5):295–305. doi: 10.1007/BF01971231. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Jansson L., Meyerson B. Oestorogen induced suppression of collagen arthritis: I. Long term oestradiol treatment of DBA/1 mice reduces severity and incidence of arthritis and decreases the anti type II collagen immune response. Clin. Exp. Immunol. 1987;70(2):372–378. [PMC free article] [PubMed] [Google Scholar]
- Hou W.S., Li Z., Gordon R.E., Chan K., Klein M.J., Levy R., Keysser M., Keyszer G., Brömme D. Cathepsin k is a critical protease in synovial fibroblast-mediated collagen degradation. Am. J. Pathol. 2001;159(6):2167–2177. doi: 10.1016/S0002-9440(10)63068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W.S., Li W., Keyszer G., Weber E., Levy R., Klein M.J., Gravallese E.M., Goldring S.R., Brömme D. Comparison of cathepsins K and S expression within the rheumatoid and osteoarthritic synovium. Arthritis Rheum. 2002;46(3):663–674. doi: 10.1002/art.10114. [DOI] [PubMed] [Google Scholar]
- Hummel K.M., Petrow P.K., Franz J.K., Müller-Ladner U., Aicher W.K., Gay R.E., Brömme D., Gay S. Cysteine proteinase cathepsin K mRNA is expressed in synovium of patients with rheumatoid arthritis and is detected at sites of synovial bone destruction. J. Rheumatol. 1998;25(10):1887–1894. [PubMed] [Google Scholar]
- Ochi Y., Yamada H., Mori H., Nakanishi Y., Nishikawa S., Kayasuga R., Kawada N., Kunishige A., Hashimoto Y., Tanaka M., Sugitani M., Kawabata K. Effects of ONO-5334, a novel orally-active inhibitor of cathepsin K, on bone metabolism. Bone. 2011;49(6):1351–1356. doi: 10.1016/j.bone.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Ochi Y., Yamada H., Mori H., Kawada N., Kayasuga R., Nakanishi Y., Tanaka M., Imagawa A., Ohmoto K., Kawabata K. ONO-5334, a cathepsin K inhibitor, improves bone strength by preferentially increasing cortical bone mass in ovariectomized rats. J. Bone Miner. Metab. 2014;32(6):645–652. doi: 10.1007/s00774-013-0542-x. [DOI] [PubMed] [Google Scholar]
- Parfitt A.M., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Reid D.M., Kennedy N.S., Smith M.A., Tothill P., Nuki G. Total body calcium in rheumatoid arthritis: effects of disease activity and corticosteroid treatment. Br. Med. J. (Clin. Res. Ed.) 1982;285(6338):330–332. doi: 10.1136/bmj.285.6338.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen-Mankonen H.J., Morko J., Vuorio E. Role of cathepsin K in normal joints and in the development of arthritis. Curr. Drug Targets. 2007;8(2):315–323. doi: 10.2174/138945007779940188. [DOI] [PubMed] [Google Scholar]
- Saville P.D., Kharmosh O. Osteoporosis of rheumatoid arthritis: influence of age, sex and corticosteroids. Arthritis Rheum. 1967;10(5):423–430. doi: 10.1002/art.1780100504. [DOI] [PubMed] [Google Scholar]
- Skoumal M., Haberhauer G., Kolarz G., Hawa G., Woloszczuk W., Klingler A. Serum cathepsin K levels of patients with longstanding rheumatoid arthritis: correlation with radiological destruction. Arthritis Res. Ther. 2005;7(1):R65–R70. doi: 10.1186/ar1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svelander L., Erlandsson-Harris H., Astner L., Grabowska U., Klareskog L., Lindstrom E., Hewitt E. Inhibition of cathepsin K reduces bone erosion, cartilage degradation and inflammation evoked by collagen-induced arthritis in mice. Eur. J. Pharmacol. 2009;613(1–3):155–162. doi: 10.1016/j.ejphar.2009.03.074. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Yamada H., Nishikawa S., Mori H., Ochi Y., Horai N., Li M., Amizuka N. Joint degradation in a monkey model of collagen-induced arthritis: role of cathepsin K based on biochemical markers and histological evaluation. Int. J. Rheumatol. 2016;2016:893–896. doi: 10.1155/2016/8938916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima R., Yamamoto K., Kishimoto H., Morio Y., Hagino H., Maeyama I. Osteoporotic changes in rheumatoid arthritis. Nihon Seikeigeka Gakkai Zasshi. 1987;61(4):289–297. [PubMed] [Google Scholar]
- Weidauer E., Yasuda Y., Biswal B.K., Cherny M., James M.N., Brömme D. Effects of disease-modifying anti-rheumatic drugs (DMARDs) on the activities of rheumatoid arthritis-associated cathepsins K and S. Biol. Chem. 2007;388(3):331–336. doi: 10.1515/BC.2007.037. [DOI] [PubMed] [Google Scholar]
- Yamada H., Ochi Y., Mori H., Nishikawa S., Hashimoto Y., Nakanishi Y., Tanaka M., Bruce M., Deacon S., Kawabata K. Effects of 16-month treatment with the cathepsin K inhibitor ONO-5334 on bone markers, mineral density, strength and histomorphometry in ovariectomized cynomolgus monkeys. Bone. 2016;86:43–52. doi: 10.1016/j.bone.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Yamane I., Hagino H., Okano T., Enokida M., Yamasaki D., Teshima R. Effect of minodronic acid (ONO-5920) on bone mineral density and arthritis in adult rats with collagen-induced arthritis. Arthritis Rheum. 2003;48(6):1732–1741. doi: 10.1002/art.10987. [DOI] [PubMed] [Google Scholar]
- Yamasaki D., Enokida M., Okano T., Hagino H., Teshima R. Effects of ovariectomy and estrogen replacement therapy on arthritis and bone mineral density in rats with collagen-induced arthritis. Bone. 2001;28(6):634–640. doi: 10.1016/s8756-3282(01)00426-4. [DOI] [PubMed] [Google Scholar]
- Yasuda Y., Kaleta J., Brömme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv. Drug Deliv. Rev. 2005;57(7):973–993. doi: 10.1016/j.addr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Hagino H., Yamasaki D., Okano T., Teshima R. Effect of estrogen replacement therapy on arthritis and bone mineral density in estrogen-replete rats with collagen-induced arthritis. Mod. Rheumatol. 2008;18(1):23–28. doi: 10.1007/s10165-007-0011-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Body weight changes.
Transparency document.