Abstract
AIM
To perform a meta-analysis on the risk of developing Mycobacterium tuberculosis (TB) infection in Crohn’s disease (CD) patients treated with tumor necrosis factor-alpha (TNFα) inhibitors.
METHODS
A meta-analysis of randomized, double-blind, placebo-controlled trials of TNFα inhibitors for treatment of CD in adults was conducted. Arcsine transformation of TB incidence was performed to estimate risk difference. A novel epidemiologically-based correction (EBC) enabling inclusions of studies reporting no TB infection cases in placebo and treatment groups was developed to estimate relative odds.
RESULTS
Twenty-three clinical trial studies were identified, including 5669 patients. Six TB infection cases were reported across 5 studies, all from patients receiving TNFα inhibitors. Eighteen studies reported no TB infection cases in placebo and TNFα inhibitor treatment arms. TB infection risk was significantly increased among patients receiving TNFα inhibitors, with a risk difference of 0.028 (95%CI: 0.0011-0.055). The odds ratio was 4.85 (95%CI: 1.02-22.99) with EBC and 5.85 (95%CI: 1.13-30.38) without EBC.
CONCLUSION
The risk of TB infection is higher among CD patients receiving TNFα inhibitors. Understanding the immunopathogenesis of CD is crucial, since using TNFα inhibitors in these patients could favor mycobacterial infections, particularly Mycobacterium avium subspecies paratuberculosis, which ultimately could worsen their clinical condition.
Keywords: Tuberculosis, Tumor necrosis factor-alpha inhibitors, Crohn’s Disease, Meta-analysis, Systematic review
Core tip: The increased risk of Mycobacterium tuberculosis (TB) and other Mycobacterium species when on tumor necrosis factor-alpha (TNFα) inhibitor treatments has been a problem in patients with autoimmune disorders, such as Crohn’s disease (CD). This meta-analysis examines in detail the clinical trials that involve CD patients on TNFα inhibitors and their risk of developing TB infections. Our data concludes that, out of twenty-three studies examined, TNFα inhibitors are indeed associated with an increased risk of TB infection in CD patients. Knowledge of this data could help re-analyze what medications autoimmune patients should be prescribed to and evaluate possible linkages to Mycobacterium avium subspecies paratuberculosis infection. Thus, this information should be used to further inform clinical decision making and research.
INTRODUCTION
The prevalence of Crohn’s disease (CD) is growing globally, especially in places adapting western lifestyles[1]. The most accepted hypothesis for CD pathogenesis is that a dysregulated immune response to opportunistic intestinal pathogens leads to persistent inflammation[2]. Studies have shown that the levels of circulating pro-inflammatory cytokines are highly elevated in CD patients, including tumor necrosis factor-alpha (TNFα)[3]. Therefore, there is an increase in the development and use of TNFα inhibitors, which are monoclonal antibodies designed to antagonize this specific cytokine in order to reduce the symptoms of CD[4]. Currently, there are three European Medicines Agency and United States Food and Drug Association approved TNFα inhibitors indicated for CD treatment: Adalimumab, certolizumab pegol, and infliximab.
Although these medications have shown efficacy in alleviating CD symptoms in some patients, 10%-30% of CD patients had no initial response to TNFα inhibitors, and about 50% of the initial responders have lost their response over time[5]. TNFα inhibitor treatment for other inflammatory autoimmune disorders, such as rheumatoid arthritis (RA), show similar effects, where roughly 30%-40% of RA patients have a poor response to the medications[6,7]. Moreover, about 40% of CD patients are at high risk of disease relapse after discontinuing TNFα inhibitor treatment[8].
Additionally, inhibiting TNFα increased risk of infections as an adverse effect, especially opportunistic infections[9]. However, it is uncertain whether there is sufficient evidence indicating an increase in risk of developing Mycobacterium tuberculosis (TB) infection from using TNFα inhibitors among CD patients. A 2016 meta-analysis reported a significant increase in opportunistic infections overall and a moderate increase in other types of infections; however, that study did not find a significant increase in the risk of TB infection development among CD patients who received TNFα inhibitors[10].
Our primary focus on TB infection is pertinent, since there is an irrefutable growing evidence relating CD to Mycobacterium avium subspecies paratuberculosis (MAP), an intracellular TB-like bacterium[11]. More importantly, TNFα is necessary for formation and maintenance of granuloma in order to limit mycobacterial infections like MAP[12]. Thus, targeting TNFα can not only disrupt the body’s ability to contain and respond to TB but also to MAP, which could further increase the patient’s susceptibility to MAP or worsen their disease condition[2]. Therefore, the effect of TNFα inhibitors on CD patients-associated with MAP infection requires further investigation.
Other meta-analyses of TB infection risk potentially associated with anti-TNFα therapy excluded trials reporting zero-event data from both anti-TNFα treatment and placebo groups (“double-zero studies”)[13,14]. The number of such “double-zero studies” was high as the incidence of TB infection was very low. Additionally, data from trials reporting zero-event data from one group (“single-zero studies”) were subject to modification (“continuity correction”) that lacks biological basis. These analytical approaches cast uncertainty about whether there is sufficient evidence indicating an increase in risk of developing TB infection from using TNFα inhibitors. Numerous double-blind, randomized, placebo-controlled trials (RCTs) of TNFα inhibitor use in CD patients provide data to estimate the risk of TB development in CD patients receiving those medications. This will open more insights into selecting the most appropriate treatment plan and antibiotics for CD patients[15]. Thus, we conducted a meta-analysis of randomized controlled trials to quantitatively analyze the altered risk for developing TB in CD patients treated with TNFα inhibitors.
MATERIALS AND METHODS
This systematic review and meta-analysis was registered in the prospective register of systematic reviews (PROSPERO) international database on February 8th, 2018 (ID: CRD42018087548)[16]. The work followed the preferred reporting items for the systematic reviews and meta-analyses (PRISMA) checklist[17].
Data source and search strategy
A search in the PubMed database up to January 21, 2018 was conducted. The following search terms were used: Tuberculosis, biologic(s), adalimumab, certolizumab, infliximab, anti-TNFα, TNFα inhibitors, or TNFα in conjunction with Crohn’s disease. The search results were further restricted to double-masked, randomized, placebo-controlled trials. ClinicalTrials.gov supplemented the searches, in the event of clinical trials that did not yet have published data in PubMed. Irrelevant studies were screened out after title and abstract review. Full text and abstracts of studies that made it past the initial screening were evaluated more closely.
Selection
Only studies and sources in English were considered. Studies were included if they were randomized, placebo-controlled, double-masked trials with appropriate exposure in adult populations. Exposure was defined as the patient receiving treatment of TNFα inhibitors (adalimumab, certolizumab pegol, and infliximab), which were approved for the treatment of CD in adult patients (18 years or older) by the EMA and the FDA. Non-approved drugs and biosimilars of TNFα inhibitors were excluded from the study. All doses of drugs were included. Observational studies and duplicates were screened out. Single studies that had both an induction and maintenance phase but reported distinct patient groups were analyzed as two separate trials.
Data extraction
Data was extracted onto a Microsoft Excel spreadsheet. First author, year of publication, study duration, number of participants in treatment and control groups, patient characteristics, treatment parameters (i.e., TNFα inhibitor and placebo), events in treatment and control groups, and screening method were obtained from each study. Studies found from ClinicalTrials.gov were also analyzed for the aforementioned criteria. Cases of TB infection were the primary outcome assessed in this analysis. TB infection was defined as diagnosis of active TB by the clinician or other medical professional.
Risk difference
Arcsine differences (ASD) were used as the measure of risk differences. For a trial with NT subjects in the anti-TNFα treatment group, NC subjects in the control group, and a and b being the number of reported TB cases, respectively, the ASD can be calculated with Equation 1 below:
Math 1
Math 1.
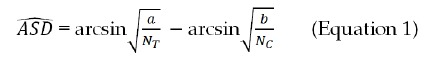
Math(A1).
The use of arcsine transformation can be dated back to the 1940s[18,19]. The main advantages of using ASD are that the variance of the point estimate (i.e., ASD) is determined solely by the sample size and that it handles occurrences of 0 counts, allowing for incorporation of trials with 0 events in both control and treatment groups into meta-analyses[20].
Relative odds
Odds ratio (ORs) were calculated using the Yusuf-Peto method[21]. Although widely used, the Mantel-Haenszel method cannot include trials with zero events from either one or both groups without substituting zero with a non-zero number. The Yusuf-Peto method can include single-zero studies; thus, the Yusuf-Peto odds ratio (ORpeto) has been recognized as a relatively efficient estimator, especially when treatment effects from trials are not large or the sample size is similar between two groups[22]. The ORpeto can be calculated with Equation 2:
Math 2
Math 2.
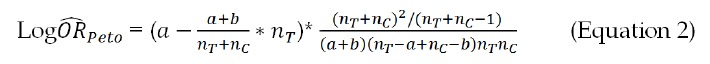
Math(A1).
Where nT, nC, a and b denote the same as in Equation 1.
However, the Yusuf-Peto method cannot include double-zero studies. Existing approaches that have been used for meta-analysis on TNFα inhibitors are to exclude double-zero studies and, for single-zero studies, to change zero counts by adding either 0.5 or a similar number that is inversely proportional to the relative size of the opposite group[9,10,23]. These analytical treatments are not biologically supported. In the case of excluding double-zero studies, the results will bias away from the null hypothesis. Thus, we proposed an epidemiologically-based background correction (EBC). Our approach was to estimate the expected number of incidence cases (e.g., if 0.01 TB cases were expected from an experimental arm, such TB cases could not be “observed” but using 0.01 to replace 0 would reflect the underlying epidemiology). Our correction assumed that the incidence of TB infection was 20 cases/100000 person-years as reported for patients with inflammatory bowel disease (IBD) in the United Kingdom[24]. EBC was then calculated according to Equation 3 below.
Math 3
Math 3.
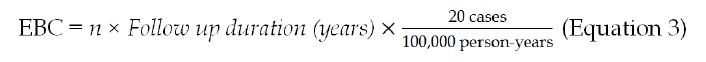
Math(A1).
Where n is the number of subjects in either the treatment or the placebo group. The EBC was added into counts for both TB and non-TB for any trials reporting zero occurrences.
Statistical analysis
Statistical analysis followed the intent-to-treat principle. R version 3.4.3[25] with the “meta” package was used to make plots and calculate the Yusuf-Peto odds ratio and ASD along with the corresponding confidence intervals (calculation accuracy was verified through manual calculation on a Microsoft Excel spreadsheet). Weight of contribution from individual studies to the pooled estimate was based on the inverse variance of the point estimate for individual studies. Inter-study variance was estimated using the DerSimonian-Laird method[26]. Two-sided P values of less than 0.05 and the 95% confidence interval (95%CI) excluding the null indicated statistical significance.
RESULTS
Search results
The selection process was summarized in Figure 1. A total of 748 articles were located from PubMed. Based on the review of titles and abstracts, 706 studies were not relevant or were duplicates, and they were subsequently excluded. The remaining 42 articles were more closely examined to determine inclusion in the analysis. Six studies were excluded because they were head-to-head, 3 were not placebo-controlled, 5 did not study EMA and FDA approved drugs, and 3 studies were duplicates (measured the same sample). Two trials were located through clinicaltrials.gov; of which, one (NCT00291668) did not post the results and was excluded. Thus, a total of 23 studies were included in the meta-analysis[27-48].
Figure 1.
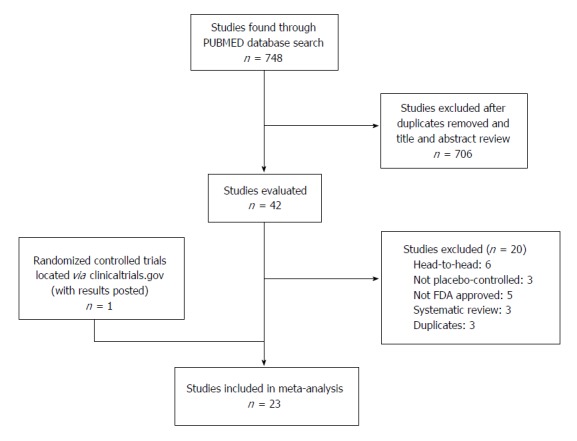
Evidence collection and selection.
The 23 studies (Table 1) has evaluated adalimumab (7 studies; 1726 patients), certolizumab pegol (6 studies; 2,008 patients), and infliximab (10 studies; 1935 patients). Both induction and maintenance studies were included (5 induction; 18 maintenance). A total of 5669 patients underwent the clinical trials, with 3275 patients in the treatment group and 2394 patients in the control group. Follow-up duration ranged from 4 to 104 wk (mean follow-up duration = 32 wk). A total of about 3558 person-years were exposed to TNFα inhibitors or placebo (2033 person-years in the treatment, 1525 in person-years in the control). Publication dates ranged from 1997 to 2016. A total of 6 cases of TB were reported; all were from the anti-TNFα treatment groups. Two cases of TB were reported with adalimumab (one study), 1 with certolizumab pegol, and 3 with infliximab (three studies).
Table 1.
Summary of randomized, placebo-controlled, double-masked trials included
| Study | Follow-up duration (wk) |
Anti-TNFα treatment |
Placebo |
||
| N1 | N2 | N1 | N2 | ||
| Adalimumab | |||||
| Hanauer et al[44], 2006 | 4 | 0 | 225 | 0 | 74 |
| Colombel et al[47], 2007 | 52 | 2 | 517 | 0 | 261 |
| Sandborn et al[35], 2007a | 52 | 0 | 37 | 0 | 18 |
| Sandborn et al[34], 2007b | 4 | 0 | 159 | 0 | 166 |
| Rutgeerts et al[37], 2012 | 48 | 0 | 64 | 0 | 65 |
| Watanabe et al[28], 2012 | 52 | 0 | 25 | 0 | 25 |
| Watanabe et al[28], 2012 | 4 | 0 | 67 | 0 | 23 |
| Total | 1094 | 632 | |||
| Certolizumab pegol | |||||
| Winter et al[27], 2004 | 12 | 0 | 66 | 0 | 24 |
| Schreiber et al[30], 2005 | 20 | 0 | 145 | 0 | 73 |
| Sandborn et al[36], 2007c | 26 | 0 | 331 | 0 | 329 |
| Schreiber et al[31], 2007 | 20 | 1 | 216 | 0 | 212 |
| Sandborn et al[33], 2011 | 6 | 0 | 223 | 0 | 215 |
| UCB Pharma[42], 2014 | 36 | 0 | 87 | 0 | 87 |
| Total | 1068 | 940 | |||
| Infliximab | |||||
| Targan et al[29], 1997 | 12 | 0 | 83 | 0 | 25 |
| D’Haens et al[46], 1999 | 4 | 0 | 22 | 0 | 8 |
| Present et al[41], 1999 | 18 | 0 | 63 | 0 | 31 |
| Rutgeerts et al[38], 1999 | 36 | 0 | 37 | 0 | 36 |
| Hanauer et al[45], 2002 | 44 | 1 | 385 | 0 | 188 |
| Sands et al[32], 2004 | 40 | 0 | 139 | 0 | 143 |
| Lémann et al[43], 2006 | 52 | 0 | 57 | 0 | 58 |
| Colombel et al[48], 2010 | 30 | 1 | 169 | 0 | 170 |
| Regueiro et al[40], 2011 | 52 | 0 | 11 | 0 | 13 |
| Regueiro et al[39], 2016 | 104 | 1 | 147 | 0 | 150 |
| Total | 1113 | 822 | |||
Number of TB cases;
Number of total subjects.
Risk difference
The risk difference between TNFα inhibitors and placebo was 0.028 (95%CI: 0.0011-0.055; P < 0.05) (Table 2). Random model results are presented, although the inter-study variance did not additionally contribute to the total variance of the pooled ASD (i.e., the DerSimonian-Laird estimate of inter-study variance was zero). The weights for each drug - adalimumab, certolizumab pegol, and infliximab - were 28.9%, 36.9%, and 34.2% respectively. The respective risk differences were 0.028, 0.015, and 0.042 (Figure 2).
Table 2.
Risk of Mycobacterium tuberculosis infection associated with the use of tumor necrosis factor-alpha inhibitors in patients with Crohn’s disease
| n | Risk estimate | 95%CI | P value | |
| Risk difference odds ratio | 23 | 0.028 | (0.0011, 0.055) | 0.042 |
| Including Double-Zero Studies | 23 | 4.85 | (1.02, 22.99) | 0.047 |
| Excluding Double-Zero Studies | 5 | 5.85 | (1.13, 30.38) | 0.036 |
n: Number of individual clinical studies pooled together.
Figure 2.
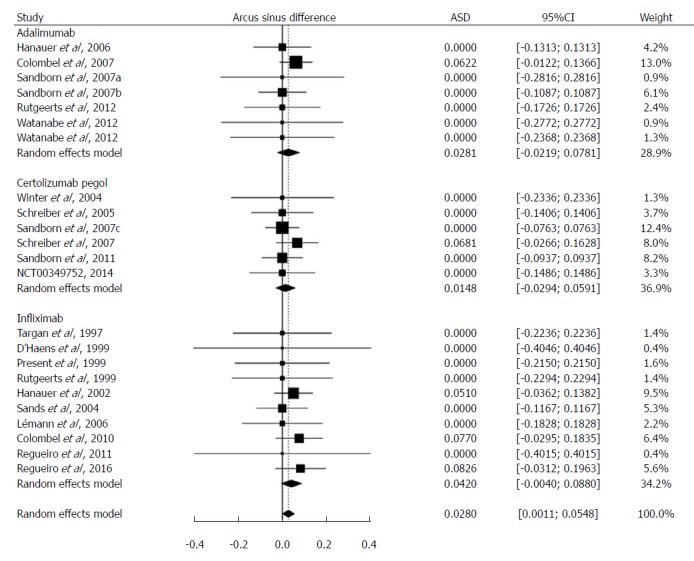
Difference of the risk of Mycobacterium tuberculosis infection between patients with Crohn’s disease treated with tumor necrosis factor-alpha inhibitors and those treated with placebo. Risk difference was calculated after arcsine transformation of Mycobacterium tuberculosis incidence (Arcus sinus difference, ASD) and indicated by the numbers on x-axis. Weight: the percentage contribution of an individual study to the overall estimation. The x-axis indicates the risk difference. The vertical dashed line indicates the overall point estimate. The solid horizontal lines show the confidence interval (CI). The size of the black box and diamond is proportional to the corresponding weight.
The funnel plot of the ASDs (Figure 3) indicates that trials showing no difference were apparently more likely published. This did not suggest publication bias, since risk of TB infection was neither the reason for publishing those trials nor the primary objective of those studies. In fact, the funnel plot reflects the fact that larger studies provided better chance to detect rare risks than smaller ones.
Figure 3.
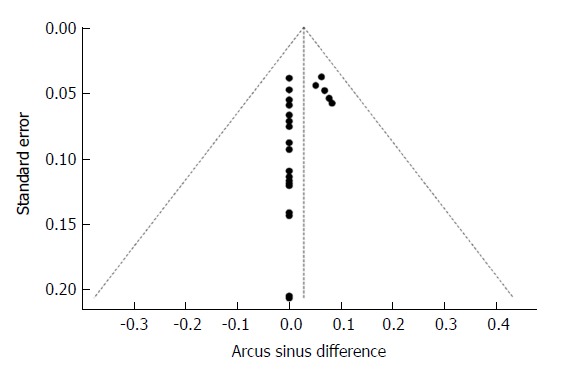
Relationship between the estimated Mycobacterium tuberculosis infection risk difference and the corresponding standard error of the estimate. Risk difference was calculated after arcsine transformation of Mycobacterium tuberculosis incidence (Arcus sinus difference, ASD). The dashed vertical line indicates the overall arcsine difference found, and the diagonal lines indicate the expected 95% confidence intervals associated with the expected mean ASD for clinical trials with various numbers of study subjects.
Relative odds
The sizes of the treatment arms were mostly similar to the sizes of the control arms, with the median ratio being 1.03. However, particular studies had very unbalanced arm sizes (maximum ratio = 3.32:1, average ratio = 1.64:1). The odds ratio was 4.85 (95%CI: 1.02-22.99; P < 0.05) with EBC and 5.85 (95%CI: 1.13-30.38; P < 0.05) without EBC (Table 2). The random effects model was not used because the Yusuf-Peto odds ratio was calculated under the assumption of a fixed effects model[21]. Weights for adalimumab, certolizumab pegol, and infliximab were 31.1%, 18.2%, and 50.7%, respectively, in the analysis with EBC (Figure 4). Without EBC, only 5 studies could be included (with the weight of 31.5% for adalimumab, 17.7% for certolizumab pegol, and 50.8% for infliximab).
Figure 4.
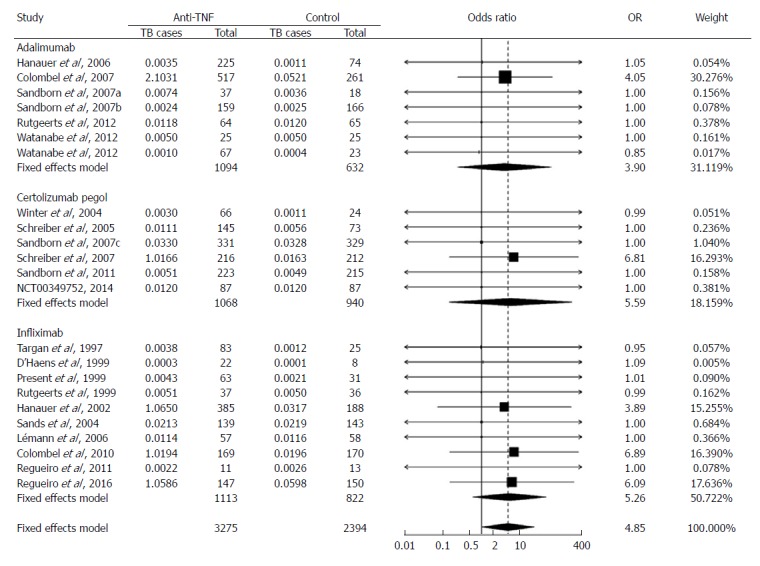
Odds of patients developing active Mycobacterium tuberculosis when treated with tumor necrosis factor-alpha inhibitors for Crohn’s disease relative to those treated with placebo. Odds ratio (OR) was calculated using the Yusuf-Peto method and indicated by the numbers on the x-axis. Number of Mycobacterium tuberculosis cases was modified using the background Mycobacterium tuberculosis incidence, i.e., adding to the reported number of Mycobacterium tuberculosis cases with a number (less than 1) that might be expected from a given number of patients (listed under “Total”). Weight: the percentage contribution of an individual study to the pooled estimate. The vertical dashed line indicates the pooled odds ratio. The solid horizontal lines show the confidence interval (CI). The size of the black box and diamond is proportional to the corresponding weight. Mycobacterium tuberculosis here denotes tuberculosis.
Expected number of anti-TNFα treated cases to observe a tuberculosis case
If the background TB infection incidence in patients was 20 cases/100000 person-years, one TB case might be expected from a community of 5000 CD patients within a year. An ASD of 0.028 would be translated into a TB incidence of 177 cases/100000 person-years; thus, 1 TB case may be expected when treating 565 patients with TNFα inhibitors for one year (Table 3). If the harm effect of TNFα inhibitors can be described on a multiplicative scale as shown from the pooled Yusuf-Peto odds ratio, the numbers of patients to be treated to expect 1 TB case might be around 855 to 1031 (Table 3).
Table 3.
Estimated absolute incidence of Mycobacterium tuberculosis infection in patients with Crohn’s disease treated with tumor necrosis factor-alpha inhibitors and the number of patients with Crohn’s disease who need to be treated with tumor necrosis factor-alpha inhibitors to expect one Mycobacterium tuberculosis case
| Incidence of TB with TNFα treatment (cases/person-years)1 | Number of patients treated to see one TB Case in one year | |
| Based on risk difference | 177/100000 | 565 |
| Based on relative odds estimated with background correction | 97/100000 | 1031 |
| Based on relative odds estimated without background correction | 117/100000 | 855 |
The background incidence was assumed to be 20 cases/100000 person-years as reported by Aberra et al[24]. TB: Mycobacterium tuberculosis.
DISCUSSION
One of the most common complications following the use of TNFα inhibitors is increasing frequency of opportunistic infections[49]. Particularly, there is strong evidence linking mycobacterial infection to TNFα inhibitors, and TB infection risk is higher among patients receiving infliximab in comparison to controls[50]. Interestingly, TNFα-deficient animal models were more susceptible to mycobacterial infections compared to wild-type controls, although there was no survival rate difference in a healthy environment[51]. This indicates that TNFα plays a critical role in the immune response against mycobacterial infections.
Several studies have shown that there is a microbial factor affecting CD patients, and MAP was isolated from intestinal tissues, blood, and milk samples of not only CD patients but also patients with RA and type 1 diabetes[52-58]. Since MAP shares molecular homology and activity similar to TB, inducing TB infection susceptibility is an alarming sign for the immune response against MAP infection[58-60].
This study advances knowledge and awareness of the association between TNFα inhibitors and TB among CD patients. First, a non-biased estimation of TB infection risk associated with TNFα inhibitors for CD treatment was performed through arcsine transformation of TB incidence, which enabled the inclusion of all qualified studies including double-zero studies in the analysis. Second, a novel, epidemiologically-based background correction to adjust for zero counts was developed to enable the inclusion of double-zero studies into the estimation of the relative effect (odds ratio in this study). Lastly, with the use of these analytical approaches, a significant increase of TB infection risk associated with using TNFα inhibitors to treat CD was shown from existing evidence, challenging findings of previous studies.
In our study, all 23 qualified trials were included. Among these 23 studies, 18 (78%) did not report TB cases from either the anti-TNFα treatment or the control group; these double-zero studies would have been excluded if we had followed the methods that previous meta-analyses in this area took. The double-zero observation was not a surprise. TB infection was rare in the Americas, Europe, Japan, Austria, and South Africa, where these RCTs were conducted. The median sample size of the control group across these 23 studies was 73 people; the median follow-up duration was 30 wk. Mathematically, only about 0.0084 TB cases would be expected in these control patients if the background TB infection incidence was 20 cases/100000 person-years as reported by Aberra et al[24]. If TNFα inhibitors had increased the TB infection risk by 5 times, there might have been about a 4% chance to observe 1 TB case in the anti-TNFα treatment arm. Meta-analysis provides excellent opportunities to pool multiple studies together to improve the estimation of the chance of observing a TB case. Discarding these double-zero studies (78% of the studies in our analysis) might decrease the value of meta-analysis.
We regarded the risk difference calculated after arcsine transformation of incidence as the primary results. The ASD method does not call for any correction for zero counts. Additionally, the robustness of an ASD estimate is not contingent on the effect size and the treatment-to-control balance of sample size. These analytical features provided a distinct advantage over either the Yusuf-Peto method or the Mantel-Haenszel method. However, from a biological perspective, it is possible that the TB infection risk from the use of TNFα inhibitors in patients with CD may be better described on a multiplicative scale (relative scale). Thus, a risk difference of 0.028 could be translated into increasing the risk of TB by a factor of 8, as the number needed to harm was calculated at 565 patients compared to the background number needed to harm of 5000 patients (Table 3). However, the 8 times increased risk of TB (based off ASD) only applies if the incidence of TB remains constant (20 cases/100000 person-years). We thus performed further analysis to better describe this multiplicative scale, for which we chose the Yusuf-Peto method because, as compared to the Mantel-Haenszel method, it can handle single-zero studies[61]. Unfortunately, the Yusuf-Peto method cannot handle double-zero studies.
We proposed an epidemiologically-based background correction (i.e., EBC) to mathematically replace zeros. If metrics other than ASD (e.g., odds ratio, hazard ratio or rate ratio) have to be estimated and the risk of interest is so rare that even one occurrence is not expected, we recommend that EBC be used for continuity correction instead of adding 0.5 (or a similar number depending on the ratio of sample size between treatment and control groups) or statistical-model based estimates[62,63]. The latter approaches lack biological considerations, and in the case of adding a number around 0.5, artificially make a much larger background incidence than there actually is (it would have boosted the background incidence by ~60 times in this meta-analysis).
There are major limitations with the use of EBC. The EBC was based off TB incidence rate in the UK IBD populations. Although the RCTs in this study were largely conducted in Western countries, the TB incidence of Crohn’s patients in the UK may not represent the TB incidence of the countries in which the clinical trials were conducted, let alone the patients who participated in the clinical trial. Furthermore, the TB incidence rate was found in populations with IBD, which may not be representative of TB incidence in the population with CD.
Aside from the analytical approach to avoid Simpson’s paradox, the validity to pool results from individual studies in this meta-analysis largely resides in the fact that each study had a placebo-treatment arm. The impact from the difference of study populations was therefore minimized, as the end point (risk difference or odds ratio) mainly reflected the effect of TNFα inhibitors [the effect of confounders was either subtracted out (for ASD) or normalized (for ORs)]. Thus, common factors restricting the use of meta-analysis, such as geographic location, population characteristics, exposure, maintenance vs induction trials, status (e.g., phase 3 vs phase 4) of the clinical trials, secular trend, and TB screening methods could be assumed to be not of major concern.
Perhaps the fact that only studies in English were included in the analysis may limit the generalizability of the results, considering that the demographics and trends of CD and TB infection differ among different regions or different populations[64]. The included studies were mainly EMA- and FDA-regulated clinical trials conducted in western countries. In fact, only one study that contained strictly Asian populations was included in this meta-analysis[28]. EBC may also be compromised, as the prevalence and incidence of TB infection was higher in Asian countries[65]. Thus, caution should be taken when extrapolating the results from this analysis to predict TB infection risk of TNFα inhibitors treating CD in non-western countries.
Additional attention should be paid to TB screening. Patients could have had either latent TB infection that was reactivated or acquired TB infection through exposure. The screening methods of trials varied and often went unreported. Furthermore, screening out patients based on a positive tuberculin skin test may have different impacts on the TB infection occurrence due to the different practices of Bacillus Calmette-Guérin vaccinations[66]. Lastly, two studies did not report screening methods[39,45]. Close examination of the additional details of TB screening may provide further insight on the nature of TB infection - whether it was acquired or reactivated.
We conclude that there is sufficient evidence to assert that using TNFα inhibitors increases the risk of developing TB infection in patients with CD. Twenty-three studies were analyzed, and multiple statistical methods repeatedly gave significant risk. To our knowledge, these 23 studies represented all appropriate literature available for the topic at hand, with an extensive and careful review conducted. No studies were excluded, provided that they used a placebo control and were randomized and masked. The randomization minimized potential confounding such as age, duration of IBD, and disease activity. These results challenge findings of previous studies, which reported no significantly increased risk of TB infection when TNFα inhibitors were used to manage patients with CD[10,14]. Based on the risk difference found in this study, on average 565 patients treated with TNFα inhibitors may result in 1 patient getting infected with TB, vs 5000 patients not treated with TNFα inhibitors producing 1 case of TB, if the background incidence of TB infection in moderately severe CD is similar to the rates found in the UK IBD population.
The etiology of CD remains uncertain. Evidence suggests that CD may be caused by an immune response to commensal enteric bacteria[67]. Recent research also suggests that CD is intimately linked to MAP, which is a TB-like bacterium[11,55,57]. The use of TNFα inhibitors in these patients could favor MAP infection and worsen the patient condition. It is currently difficult to come to conclusions considering that the RCTs did not test for MAP infection - much less reported it. Further research could be done on looking at patient outcomes and determining which patients had MAP infection and what their susceptibility to infection was.
ARTICLE HIGHLIGHTS
Research background
Recent literature has identified many adverse effects from the use of tumor necrosis factor alpha (TNFα) inhibitors. Among these are an increased risk of opportunistic infections and serious adverse events. However, previous meta-analyses have not identified a significantly increased risk of tuberculosis (TB) infection, which is especially pertinent considering that Mycobacterium avium subspecies paratuberculosis (MAP) is suspected to have an intimate role in the etiology of Crohn’s disease (CD).
Research motivation
Due to the suspected role of MAP in the etiology of CD and previous literature on the topic, TNFα inhibitors likely increase the risk of TB infection, which would guide future clinical decisions. However, such an association has not been adequately quantified. Additionally, current statistical models commonly used in meta-analyses fail to adequately analyze data where events are rare (defined as less than 1 case per 1000 person-years). Our research would not only bring additional considerations when making clinical decisions about anti-TNFα therapy but also introduce existing statistical models and novel corrections that can help deal with rare events.
Research objectives
In this study, we seek to advance the awareness of and quantify the association between TNFα inhibitors and TB in CD patients. We seek to include all qualified studies - including studies with zero events in the treatment and control groups - without using corrections, which previous meta-analyses have failed to do. Finally, we seek to introduce a novel, epidemiologically-based background correction (EBC) that can adjust for zero counts.
Research methods
The Preferred Reporting Items for the Systematic reviews and Meta-Analyses (PRISMA) protocol was followed. Only randomized, placebo-controlled trials (RCTs) were considered. Arcsine differences were used to calculate risk differences in a non-biased way. Odds ratios were calculated using the Yusuf-Peto method both with and without corrections (EBC).
Research results
Twenty-three RCTs were analyzed, and all the statistical methods repeatedly provided significantly increased risk of TB infection. A risk difference (RD) of 0.028 (95%CI: 0.0011-0.055) was calculated. The odds ratio (OR) was 4.85 (95%CI: 1.02-22.99) when all studies were included using EBCs and 5.85 (95%CI: 1.13-30.38) when studies reporting zero tuberculosis cases were excluded.
Research conclusions
There is an increased risk of TB infection in patients with Crohn’s disease who use TNFα inhibitors. This risk could range from 5 times (OR) to as high as 8 times (RD). Alternative therapy such as using more antibiotics and less immunosuppressive agents may be evaluated.
Research perspectives
This study provided us with additional approaches that can be considered when conducting future meta-analyses. ASD is a particularly useful method that can contribute to future meta-analyses. The relationship between MAP and TB is still unclear. Further research on the validity of the EBC should be pursued.
ACKOWLEDGEMENTS
The authors wish to thank members of Dr. Saleh A Naser’s lab who provided insightful comments and suggestions.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare that they have no competing interests.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Peer-review started: April 13, 2018
First decision: April 27, 2018
Article in press: June 2, 2018
P- Reviewer: Ogata H S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
Contributor Information
Brent L Cao, Division of Molecular Microbiology, Burnett School of Biomedical Sciences, College of Medicine, University of Central Florida, Orlando, FL 32816, United States.
Ahmad Qasem, Division of Molecular Microbiology, Burnett School of Biomedical Sciences, College of Medicine, University of Central Florida, Orlando, FL 32816, United States.
Robert C Sharp, Division of Molecular Microbiology, Burnett School of Biomedical Sciences, College of Medicine, University of Central Florida, Orlando, FL 32816, United States.
Latifa S Abdelli, Division of Molecular Microbiology, Burnett School of Biomedical Sciences, College of Medicine, University of Central Florida, Orlando, FL 32816, United States.
Saleh A Naser, Division of Molecular Microbiology, Burnett School of Biomedical Sciences, College of Medicine, University of Central Florida, Orlando, FL 32816, United States. saleh.naser@ucf.edu.
References
- 1.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Qasem A, Naser AE, Naser SA. The alternate effects of anti-TNFα therapeutics and their role in mycobacterial granulomatous infection in Crohn’s disease. Expert Rev Anti Infect Ther. 2017;15:637–643. doi: 10.1080/14787210.2017.1328276. [DOI] [PubMed] [Google Scholar]
- 3.Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- 4.Swaminath A, Lebwohl B, Capiak KM, Present DH. Practice patterns in the use of anti-tumor necrosis factor alpha agents in the management of Crohn’s disease: a US national practice survey comparing experts and non-experts. Dig Dis Sci. 2011;56:1160–1164. doi: 10.1007/s10620-010-1530-9. [DOI] [PubMed] [Google Scholar]
- 5.Roda G, Jharap B, Neeraj N, Colombel JF. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin Transl Gastroenterol. 2016;7:e135. doi: 10.1038/ctg.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707–718. doi: 10.1038/nrrheum.2017.187. [DOI] [PubMed] [Google Scholar]
- 7.Hyrich KL, Watson KD, Silman AJ, Symmons DP; British Society for Rheumatology Biologics Register. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2006;45:1558–1565. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 8.Gisbert JP, Marín AC, Chaparro M. The Risk of Relapse after Anti-TNF Discontinuation in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Am J Gastroenterol. 2016;111:632–647. doi: 10.1038/ajg.2016.54. [DOI] [PubMed] [Google Scholar]
- 9.Minozzi S, Bonovas S, Lytras T, Pecoraro V, González-Lorenzo M, Bastiampillai AJ, Gabrielli EM, Lonati AC, Moja L, Cinquini M, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf. 2016;15:11–34. doi: 10.1080/14740338.2016.1240783. [DOI] [PubMed] [Google Scholar]
- 10.Bonovas S, Fiorino G, Allocca M, Lytras T, Nikolopoulos GK, Peyrin-Biroulet L, Danese S. Biologic Therapies and Risk of Infection and Malignancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1385–1397.e10. doi: 10.1016/j.cgh.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 11.Naser SA, Sagramsingh SR, Naser AS, Thanigachalam S. Mycobacterium avium subspecies paratuberculosis causes Crohn’s disease in some inflammatory bowel disease patients. World J Gastroenterol. 2014;20:7403–7415. doi: 10.3748/wjg.v20.i23.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–4627. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 13.Williams CJ, Peyrin-Biroulet L, Ford AC. Systematic review with meta-analysis: malignancies with anti-tumour necrosis factor-α therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:447–458. doi: 10.1111/apt.12624. [DOI] [PubMed] [Google Scholar]
- 14.Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013;108:1268–1276. doi: 10.1038/ajg.2013.138. [DOI] [PubMed] [Google Scholar]
- 15.Qasem A, Safavikhasraghi M, Naser SA. A single capsule formulation of RHB-104 demonstrates higher anti-microbial growth potency for effective treatment of Crohn’s disease associated with Mycobacterium avium subspecies paratuberculosis. Gut Pathog. 2016;8:45. doi: 10.1186/s13099-016-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao B, Naser SA. Systematic review and meta-analysis on the risk of tuberculosis in patients with Crohn’s disease who take TNFα inhibitors. PROSPERO: International prospective register of systematic reviews; 2018. [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman M, Tukey J. Transformations related to the angular and the square root. Annals of Mathematical Statistics. 1950;21:607–611. [Google Scholar]
- 19.Anscombe FJ. The transformation of Poisson, binomial and negative-binomial data. Biometrika. 1948;35:246–254. [Google Scholar]
- 20.Rücker G, Schwarzer G, Carpenter J, Olkin I. Why add anything to nothing? The arcsine difference as a measure of treatment effect in meta-analysis with zero cells. Stat Med. 2009;28:721–738. doi: 10.1002/sim.3511. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–371. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 22.Brockhaus AC, Bender R, Skipka G. The Peto odds ratio viewed as a new effect measure. Stat Med. 2014;33:4861–4874. doi: 10.1002/sim.6301. [DOI] [PubMed] [Google Scholar]
- 23.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 24.Aberra FN, Stettler N, Brensinger C, Lichtenstein GR, Lewis JD. Risk for active tuberculosis in inflammatory bowel disease patients. Clin Gastroenterol Hepatol. 2007;5:1070–1075. doi: 10.1016/j.cgh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 25.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: http://www.r-project.org/
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Winter TA, Wright J, Ghosh S, Jahnsen J, Innes A, Round P. Intravenous CDP870, a PEGylated Fab’ fragment of a humanized antitumour necrosis factor antibody, in patients with moderate-to-severe Crohn’s disease: an exploratory study. Aliment Pharmacol Ther. 2004;20:1337–1346. doi: 10.1111/j.1365-2036.2004.02285.x. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe M, Hibi T, Lomax KG, Paulson SK, Chao J, Alam MS, Camez A; Study Investigators. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn’s disease. J Crohns Colitis. 2012;6:160–173. doi: 10.1016/j.crohns.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber S, Rutgeerts P, Fedorak RN, Khaliq-Kareemi M, Kamm MA, Boivin M, Bernstein CN, Staun M, Thomsen OØ, Innes A; CDP870 Crohn’s Disease Study Group. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology. 2005;129:807–818. doi: 10.1053/j.gastro.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OØ, Hanauer SB, McColm J, Bloomfield R, Sandborn WJ; PRECISE 2 Study Investigators. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239–250. doi: 10.1056/NEJMoa062897. [DOI] [PubMed] [Google Scholar]
- 32.Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 33.Sandborn WJ, Schreiber S, Feagan BG, Rutgeerts P, Younes ZH, Bloomfield R, Coteur G, Guzman JP, D’Haens GR. Certolizumab pegol for active Crohn’s disease: a placebo-controlled, randomized trial. Clin Gastroenterol Hepatol. 2011;9:670–678.e3. doi: 10.1016/j.cgh.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 34.Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Colombel JF, Panaccione R, D’Haens G, Li J, Rosenfeld MR, Kent JD, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838. doi: 10.7326/0003-4819-146-12-200706190-00159. [DOI] [PubMed] [Google Scholar]
- 35.Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, Panaccione R, Wolf D, Kent JD, Bittle B, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, Bloomfield R, Schreiber S; PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228–238. doi: 10.1056/NEJMoa067594. [DOI] [PubMed] [Google Scholar]
- 37.Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, Reinisch W; EXTEND Investigators, Kumar A, Lazar A, Camez A, Lomax KG, Pollack PF, D’Haens G. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102–1111.e2. doi: 10.1053/j.gastro.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 38.Rutgeerts P, D’Haens G, Targan S, Vasiliauskas E, Hanauer SB, Present DH, Mayer L, Van Hogezand RA, Braakman T, DeWoody KL, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology. 1999;117:761–769. doi: 10.1016/s0016-5085(99)70332-x. [DOI] [PubMed] [Google Scholar]
- 39.Regueiro M, Feagan BG, Zou B, Johanns J, Blank MA, Chevrier M, Plevy S, Popp J, Cornillie FJ, Lukas M, et al. Infliximab Reduces Endoscopic, but Not Clinical, Recurrence of Crohn’s Disease After Ileocolonic Resection. Gastroenterology. 2016;150:1568–1578. doi: 10.1053/j.gastro.2016.02.072. [DOI] [PubMed] [Google Scholar]
- 40.Regueiro M, El-Hachem S, Kip KE, Schraut W, Baidoo L, Watson A, Swoger J, Schwartz M, Barrie A, Pesci M, et al. Postoperative infliximab is not associated with an increase in adverse events in Crohn’s disease. Dig Dis Sci. 2011;56:3610–3615. doi: 10.1007/s10620-011-1785-9. [DOI] [PubMed] [Google Scholar]
- 41.Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 42.UCB Pharma. Corticosteroid Sparing Effect of Certolizumab in Crohn’s Disease (COSPAR1) ( NCT00349752; Last Update Posted on September 5, 2014). Available at ClinicalTrials.gov. Accessed on March 27, 2018. [Google Scholar]
- 43.Lémann M, Mary JY, Duclos B, Veyrac M, Dupas JL, Delchier JC, Laharie D, Moreau J, Cadiot G, Picon L, Bourreille A, Sobahni I, Colombel JF; Groupe d’Etude Therapeutique des Affections Inflammatoires du Tube Digestif (GETAID) Infliximab plus azathioprine for steroid-dependent Crohn’s disease patients: a randomized placebo-controlled trial. Gastroenterology. 2006;130:1054–1061. doi: 10.1053/j.gastro.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 44.Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333; quiz 591. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 45.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 46.D’haens G, Van Deventer S, Van Hogezand R, Chalmers D, Kothe C, Baert F, Braakman T, Schaible T, Geboes K, Rutgeerts P. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn’s disease: A European multicenter trial. Gastroenterology. 1999;116:1029–1034. doi: 10.1016/s0016-5085(99)70005-3. [DOI] [PubMed] [Google Scholar]
- 47.Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 48.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 49.Antoni C, Braun J. Side effects of anti-TNF therapy: current knowledge. Clin Exp Rheumatol. 2002;20:S152–S157. [PubMed] [Google Scholar]
- 50.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 51.Keane J, Gershon SK, Braun MM. Tuberculosis and treatment with infliximab. The New England journal of medicine. 2002;346:2. [Google Scholar]
- 52.Schwartz D, Shafran I, Romero C, Piromalli C, Biggerstaff J, Naser N, Chamberlin W, Naser SA. Use of short-term culture for identification of Mycobacterium avium subsp. paratuberculosis in tissue from Crohn’s disease patients. Clin Microbiol Infect. 2000;6:303–307. doi: 10.1046/j.1469-0691.2000.00093.x. [DOI] [PubMed] [Google Scholar]
- 53.Qasem A, Abdel-Aty A, Abu-Suwa H, Naser SA. Oxidative stress due to Mycobacterium avium subspecies paratuberculosis (MAP) infection upregulates selenium-dependent GPx activity. Gut Pathog. 2016;8:12. doi: 10.1186/s13099-016-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naser SA, Schwartz D, Shafran I. Isolation of Mycobacterium avium subsp paratuberculosis from breast milk of Crohn’s disease patients. Am J Gastroenterol. 2000;95:1094–1095. doi: 10.1111/j.1572-0241.2000.01954.x. [DOI] [PubMed] [Google Scholar]
- 55.Sharp RC, Beg SA, Naser SA. Role of PTPN2/22 polymorphisms in pathophysiology of Crohn's disease. World J Gastroenterol. 2018;24:657–670. doi: 10.3748/wjg.v24.i6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharp RC, Beg SA, Naser SA. Polymorphisms in Protein Tyrosine Phosphatase Non-receptor Type 2 and 22 (PTPN2/22) Are Linked to Hyper-Proliferative T-Cells and Susceptibility to Mycobacteria in Rheumatoid Arthritis. Front Cell Infect Microbiol. 2018;8:11. doi: 10.3389/fcimb.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharp RC, Abdulrahim M, Naser ES, Naser SA. Genetic Variations of PTPN2 and PTPN22: Role in the Pathogenesis of Type 1 Diabetes and Crohn’s Disease. Front Cell Infect Microbiol. 2015;5:95. doi: 10.3389/fcimb.2015.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naser SA, Thanigachalam S, Dow CT, Collins MT. Exploring the role of Mycobacterium avium subspecies paratuberculosis in the pathogenesis of type 1 diabetes mellitus: a pilot study. Gut Pathog. 2013;5:14. doi: 10.1186/1757-4749-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bach H, Papavinasasundaram KG, Wong D, Hmama Z, Av-Gay Y. Mycobacterium tuberculosis virulence is mediated by PtpA dephosphorylation of human vacuolar protein sorting 33B. Cell Host Microbe. 2008;3:316–322. doi: 10.1016/j.chom.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 60.Bach H, Ko HH, Raizman EA, Attarian R, Cho B, Biet F, Enns R, Bressler B. Immunogenicity of Mycobacterium avium subsp. paratuberculosis proteins in Crohn’s disease patients. Scand J Gastroenterol. 2011;46:30–39. doi: 10.3109/00365521.2010.513061. [DOI] [PubMed] [Google Scholar]
- 61.Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26:53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- 62.Bhaumik DK, Amatya A, Normand SL, Greenhouse J, Kaizar E, Neelon B, Gibbons RD. Meta-Analysis of Rare Binary Adverse Event Data. J Am Stat Assoc. 2012;107:555–567. doi: 10.1080/01621459.2012.664484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bai O, Chen M, Wang X. Bayesian Estimation and Testing in Random Effects Meta-analysis of Rare Binary Adverse Events. Stat Biopharm Res. 2016;8:49–59. doi: 10.1080/19466315.2015.1096823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ng SC. Emerging Trends of Inflammatory Bowel Disease in Asia. Gastroenterol Hepatol (NY) 2016;12:193–196. [PMC free article] [PubMed] [Google Scholar]
- 65.WHO. 2018. Global tuberculosis report 2017. Accessed on March 27. Available from: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 66.Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012. doi: 10.1371/journal.pmed.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]


