Abstract
AIM
To evaluate recent trends in gastric cancer incidence, response to treatment, and overall survival among Alaska Native (AN) people.
METHODS
A retrospective analysis of the Alaska Native Medical Center patient database was performed. Patient history, clinical, pathological, response to treatment and patient outcomes were collected from one-hundred and thirty-two AN gastric cancer patients. The Surveillance, Epidemiology and End Result database 18 was used to collect comparison United States non-Hispanic White (NHW) and AN gastric cancer patient data between 2006-2014.
RESULTS
AN gastric cancer patients have a higher incidence rate, a poorer overall survival, and are diagnosed at a significantly younger age compared to NHW patients. AN patients differ from NHW patients in greater prevalence of non-cardia, diffuse subtype, and signet ring cell carcinomas. AN females were more likely to be diagnosed with later stage cancer, stage IV, compared to AN males. Diminished overall survival was observed among AN patients with increasing stage, O+ blood type, < 15 lymph nodes examined at resection, and no treatment. This study is the first report detailing the clinicopathologic features of gastric cancer in AN people with outcome data.
CONCLUSION
Our findings confirm the importance of early detection, treatment, and surgical resection for optimizing AN patient outcomes. Further research on early detection markers are warranted.
Keywords: Alaska Native, Gastric cancer, Helicobacter pylori, Gender, Health disparities
Core tip: Gastric cancer (GC) is a leading cancer health disparity among the Alaska Native (AN) people. The aim of this study was to evaluate recent trends in AN gastric cancer incidence and survival. AN patients differ from non-Hispanic White patients in increased incidence, younger age at diagnosis, a higher presence of non-cardia, diffuse subtype, signet ring cell carcinomas, Helicobacter pylori, and greater proportion of GC among women. AN patients diagnosed at an early stage and whom receive surgical treatment have better overall survival compared to later stage patients. Therefore, additional screening programs and early detection measures for AN people, may improve patient outcomes.
INTRODUCTION
Worldwide, there are significant racial disparities in incidence and mortality of gastric cancer. The highest incidence and mortality rates occur in Eastern Asia, Eastern Europe, Central America, and South America populations, whereas North America and Africa populations have the lowest incidence[1,2]. In recent years, gastric cancer incidence has declined in Eastern Asia and Asian patients have been shown to have better outcomes compared to other ethnic groups such as in South American, United States Hispanic, and African American patients[2,3]. In the United States (US), gastric cancer incidence rates are also declining in the general population[4] and recently in racial ethnic subgroups such as Asian Americans who have had a historically high prevalence of gastric cancer[5]. In contrast, gastric cancer incidence and mortality rates have remained the same among the Alaska Native (AN) population, becoming one of the leading cancer health disparities[6-8].
The AN population has a 3-fold higher incidence and mortality rate of gastric cancer when compared to the US non-Hispanic White (NHW) population[6,7,9]. AN patients are often diagnosed with advanced stage disease, and have a poor overall 5-year survival rate of less than 20%[10,11]. Gastric cancer etiology differs between the NHW and AN populations. NHW patients are most often diagnosed with gastric cancers in the cardia, gastroesophageal junction or distal esophagus, while gastric cancers in AN patients are localized to the central and distal stomach[9,12]. Further, differences in tumor subtype have been observed between the two populations, with the diffuse subtype being most common among AN patients[7]. The high incidence and prevalence of non-cardia gastric cancers among AN patients has been associated with the high seropositivity rates of Helicobacter pylori (H. pylori) among the general AN population[13-15]. In addition to H. pylori, multiple risk factors could contribute to this cancer health disparity among the AN people, including socioeconomic factors and biological differences, such as access to treatment, genetic influences, lifestyle differences, and environmental exposures.
A greater understanding of gastric cancer incidence and response to treatment among the AN people may allow for the design of screening programs or the identification of early detection measures to potentially reduce incidence and improve patient outcomes. In order to further investigate how to reduce gastric cancer incidence and mortality rates among the AN population, we sought to evaluate recent trends in gastric cancer incidence, as well as to report on clinical response to treatment and overall survival outcomes in this high incidence population.
MATERIALS AND METHODS
Alaska Native gastric cancer patients
The University of Alaska Anchorage Institutional Review Board (IRB), Alaska Area IRB, Southcentral Foundation Review Board, and the Alaska Native Tribal Health Consortium Health Research Review Committee approved this study. The medical records were reviewed from one-hundred and thirty-two AN patients with histologically confirmed gastric adenocarcinoma presenting at the Alaska Native Medical Center (ANMC), a referral hospital for the Alaska Tribal Health System, from January 2006 to December 2014. Demographic and clinicopathologic variables obtained from medical records included: sex, age at diagnosis, region of Alaska where patient resides, tumor grade, stage, primary location, metastatic site, histologic type according to Lauren classification[16], histological appearance, stage, type of therapy, order of treatment, surgical resection, lymph nodes examined, recurrence site, overall survival, presence of H. pylori at time of biopsy/resection, chronic gastritis, gastroesophageal reflux disease (GERD), gastric ulcer, blood type, self-reported family history of gastrointestinal cancers and tobacco use. Patients were classified into five regions: far north, interior, southwest, southcentral, and southeast designated by the Alaska Department of Labor and Workforce Development 2010 census. Vital status was obtained through Social Security Disability Insurance program or through the Alaska Department of Health and Social Services.
SEER database
Data collected on US NHW and AN gastric adenocarcinoma patients were obtained from the US National Institute’s SEER Program of the National Cancer Institute 18 dataset for the period 2006-2014. The SEER program collects information on incidence, prevalence, survival, and cancer mortality from cancer registries representing approximately 28% of the US population. The SEER database captures all cancer cases among the AN population, approximately 150000 people, through the Alaska Native Tumor Registry. SEER*Stat software (www.seer.cancer.gov/seerstat) Version 8.2.1 was used for analysis of data.
Data classification and coding
Overall survival was calculated from the date of gastric cancer diagnosis until death from any cause or date of last follow-up. Patient vital status was confirmed through the ANMC tumor registry. Survival times of patients with stable disease were censored at the last follow-up date. Anatomical subsite and histological conditions were grouped using the International Classification of Disease for Oncology 3 (ICD-O-3) codes. ICD-O-3 codes used in the study were: 8140 Adenocarcinoma, not otherwise specified (NOS); 8142, Linitis plastica; 8144/3, adenocarcinoma, intestinal type; 8145/3, adenocarcinoma, diffuse type; 8211/3, tubular adenocarcinoma; 8255/3, adenocarcinoma with mixed subtypes; 8260/3, papillary adenocarcinoma, NOS; 8480/3, mucinous adenocarcinoma; and 8490/3, signet ring carcinoma. For anatomic subsite analysis the four-digit topography site codes (C15, esophageal cancer and C16, stomach cancer) were used to extract and analyze the incident cases of gastric cancer. Four anatomic subsites were formed: Cardia, C15.5 lower third esophagus and C16.0 cardia; Non-Cardia, C16.1 fundus, C16.2 body, C16.3 gastric antrum, C16.4 pylorus, C16.5 lesser curvature of stomach NOS, C16.6 greater curvature of stomach NOS; Overlapping, C16.8 overlapping lesion of stomach; and Unspecified. C16.9 stomach NOS. Pathological stage was classified according to the American Joint Committee on Cancer (AJCC) 7th edition manual for stomach cancer[17]. The number of lymph nodes examined following resection were dichotomized into < 15 or ≥ 15 based on recommendation from the National Comprehensive Cancer Network[18].
Statistical analysis
Raw frequencies and percentages of cases for available data from the US SEER database and the ANMC hospital are reported. Data were analyzed using software SPSS 23.0 (SPSS Inc, Chicago, IL, United States). Patient demographics and clinicopathological characteristics between NHW versus AN and AN male versus AN female were compared using chi-square tests for categorical variables and Student t tests for continuous variables. Wilcoxon rank-sum (Mann-Whitney) test was used for variables that were not normally distributed. Association between various clinicopathological characteristics and overall survival were examined with Cox proportional hazard models. The Kaplan-Meier method was used for survival analysis, and differences in survival between groups were evaluated using the log-rank test. Variables with a P value < 0.1 on univariate analysis were included in the multivariate Cox proportional hazards regression model analysis. A two-sided P value of < 0.05 was considered significant.
RESULTS
Gastric cancer in AN people is distinct from non-Hispanic white people
Between 2006-2014, a total of 132 AN patients with adenocarcinoma of the gastroesophageal junction or stomach were identified from the ANMC hospital database. In the same period of time using the US SEER database, we identified 40717 NHW patients and 164 AN patients with adenocarcinoma of the gastroesophageal junction or stomach. Similar trends were observed between the AN Hospital and AN SEER data. As shown in Table 1, there were significant differences in the clinicopathological characteristics between the NHW and AN patients. Compared to the US NHW population, AN patients (AN SEER and AN Hospital) have a higher incidence rate and were significantly younger at time of diagnosis (59.9 years vs 69.2 years; P < 0.0001) (Figure 1). Also, the AN patients had significant differences in tumor location and appearance, with a higher prevalence of non-cardia tumors (60.6% vs 21.3%; P < 0.0001, Table 1) and signet ring cell carcinomas (39.4% vs 12.7%; P < 0.0001). AN patients were significantly more likely to be diagnosed with stage IV disease (50.0% vs 37.6%; P = 0.03, Table 1). We also observed among the AN patients a larger proportion of females (37.8% vs 26.0%; P = 0.01, Table 1). AN females had a higher prevalence of signet ring cell carcinoma compared to NHW females (46% vs 35.4%).
Table 1.
Comparative epidemiology of gastric cancer in United States Non-Hispanic White and Alaska Native populations n (%)
|
United States |
P1 value | |||
| Non-Hispanic White | Alaska Native-SEER | Alaska Native Hospital | ||
| Population (in millions)23 | 274.6 | 0.14 | 0.14 | |
| Cancer registry summary | ||||
| Registry type (number) | Population based (SEER 18) | Population based (SEER 18) | Hospital based (1) | |
| Years included | 2006-2014 | 2006-2014 | 2006-2014 | |
| Incident cases | 40717 | 164 | 132 | |
| Calculated incidence rate4 | ||||
| Male | 12.1 | 26.7 | 22.8 | |
| Female | 3.4 | 18.7 | 13.6 | |
| Gender | 0.01 | |||
| Male | 30141 (74.0) | 95 (57.9) | 82 (62.1) | |
| Female | 10576 (26.0) | 69 (42.1) | 50 (37.8) | |
| Age | < 0.00015 | |||
| Median (yr) | 69.2 ± 0.07 | 60.4 ± 1.3 | 59.9 ± 1.2 | |
| < 20-34 | 258 (0.6) | 4 (2.4) | 3 (2.3) | |
| 35-44 | 926 (2.3) | 9 (5.5) | 9 (6.8) | |
| 45-54 | 4150 (10.2) | 44 (26.8) | 39 (29.5) | |
| 55-64 | 9224 (22.7) | 41 (25.0) | 28 (21.2) | |
| 65-74 | 11089 (27.2) | 38 (23.2) | 30 (22.7) | |
| 75-84 | 10144 (24.9) | 23 (14.0) | 19 (14.4) | |
| > 84 | 4927 (12.1) | 5 (3.0) | 4 (3.0) | |
| Anatomic site | < 0.00017 | |||
| Cardia | 26387 (64.8) | 38 (23.2) | 22 (16.7) | |
| GE JX | 14502 (35.6) | 14 (8.5) | 12 (9.1) | |
| Non-cardia | 8659 (21.3) | 86 (52.4) | 80 (60.6) | |
| Fundus | 970 (2.4) | 4 (2.4) | 12 (9.1) | |
| Body6 | 3726 (9.2) | 52 (30.0) | 33 (25.0) | |
| Antrum | 3483 (8.6) | 22 (13.4) | 18 (13.6) | |
| Pylorus | 480 (1.2) | 11 (6.7) | 17 (12.9) | |
| Overlap (multifocal) | 1523 (3.7) | 9 (5.5) | 27 (20.5) | |
| Unspecified | 4148 (10.2) | 31 (18.9) | 3 (2.3) | |
| Histological appearance | < 0.00018 | |||
| Adenocarcinoma, NOS | 33,921 (83.3) | 126 (76.8) | 80 (60.6) | |
| Linitis Plastica, AC | 172 (0.4) | 1 (0.6) | 2 (1.5) | |
| Mucinous, AC | 626 (1.5) | 3 (1.8) | 5 (3.8) | |
| Tubular, AC | 168 (0.5) | 1 (0.6) | 1 (0.8) | |
| Papillary, AC | 91 (0.2) | 1 (0.6) | 1 (0.8) | |
| Mixed Cell, AC | 560 (1.4) | 1 (0.6) | 0 (0) | |
| Signet Ring | 5179 (12.7) | 31 (18.9) | 52 (39.4) | |
| Stage | 0.002 | |||
| I | 8901 (21.9) | 35 (21.3) | 28 (21.1) | |
| II | 5662 (13.9) | 24 (14.6) | 24 (18.0) | |
| III | 5327 (13.1) | 14 (8.5) | 14 (11.4) | |
| IV | 15323 (37.6) | 75 (45.7) | 66 (50.0) | |
| Unspecified | 5504 (13.5) | 16 (9.8) | 0 (0) | |
Bold type indicates statistical significance (P < 0.05), statistics were performed on SEER Non-Hispanic White and Hospital based Alaska Native populations;
Population for US White 2010, US Census;
Population for AK Native 2010, Alaska Department of Labor and workforce development;
Incidence rates, per 100000 person years and are age-adjusted using the 2000 US standard population;
Wilcoxon rank sum test;
Body includes body, lesser curvature, and greater curvature;
Chi-square test between Cardia, Non-Cardia, Overlapping, and Unspecified;
Chi-square test between adenocarcinoma and Signet Ring. NOS: Not otherwise specified; AC: Adenocarcinoma; GE JX: Gastroesophageal junction; SEER: Surveillance and Epidemiology End Results Program (SEER) 18 dataset; AC: Adenocarcinoma; JX: Junction.
Figure 1.
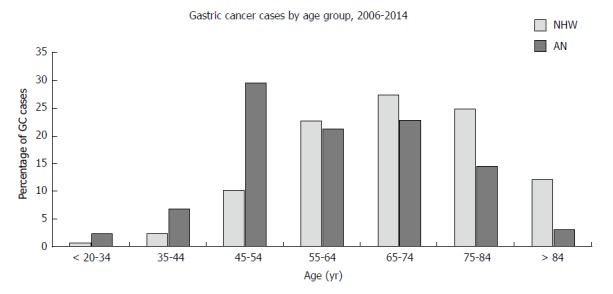
Age group distribution for non-Hispanic White and Alaska Native patients diagnosed with gastric cancer 2006-2014. AN: Alaska Native; NHW: Non-Hispanic White.
Epidemiology and clinical features of gastric cancer in AN people
The median age of AN cancer patients was 58.3 years for men and 62.4 years for women (Table 2). AN males diagnosed with gastric cancer had significantly higher rates of tobacco use compared to females (87.8% vs 72.0% P = 0.04). In addition, we observed gastric cancer in AN males were more likely to metastasize to multiple sites compared to females (29.4% vs 18.8%). AN female and male patients did not differ in their geographical location, of which the majority of patients resided in three regions of Alaska: North (28.8%), Southwest (30.3%), and Southcentral (30.3%) (Table 2). AN females were more likely to be diagnosed with stage IV gastric cancer (64% vs 41.5%) and choose not to seek treatment (36% vs 24.4% respectively) compared to AN males. AN male and female gastric patients had similar distribution for blood type, grade, primary tumor location, treatment, H. pylori positive tumor, chronic gastritis, GERD, gastric ulcers, and family history of gastric and/or colorectal cancer.
Table 2.
Descriptive epidemiology of Alaska Native gastric cancer patients n (%)
| Overall | Female | Male | |
| Patients | 132 | 50 (37.9) | 82 (62.1) |
| Mean age (yr) | 59.8 ± 1.2 | 62.4 ± 2.0 | 58.2 ± 1.4 |
| Histological type | |||
| Diffuse | 75 (56.8) | 29 (58.0) | 46 (56.1) |
| Intestinal | 51 (38.6) | 16 (32.0) | 35 (42.7) |
| NOS | 6 (4.5) | 5 (10.0) | 1 (1.2) |
| Blood type | |||
| A+ | 55 (41.7) | 23 (46.0) | 32 (39.0) |
| AB+ | 8 (6.1) | 3 (6.0) | 5 (6.1) |
| B+ | 9 (6.8) | 5 (10.0) | 4 (4.9) |
| O+ | 30 (22.7) | 10 (20.0) | 20 (24.4) |
| Unknown | 30 (22.7) | 9 (18.0) | 21 (25.6) |
| Histological appearance | |||
| Signet Ring | 52 (39.4) | 23 (46.0) | 29 (35.4) |
| Adenocarcinoma | 80 (59.8) | 27 (54.0) | 53 (64.6) |
| Linitis Plastica, AC | 2 (2.5) | 0 (0) | 2 (3.8) |
| Mucinous, AC | 2 (2.5) | 0 (0) | 2 (3.8) |
| Tubular, AC | 1 (1.3) | 0 (0) | 1 (1.9) |
| Papillary | 1 (1.3) | 0 (0) | 1 (1.9) |
| NOS, AC | 75 (93.8) | 27 (100) | 46 (86.8) |
| Stage | |||
| I | 28 (21.2) | 8 (16.0) | 20 (24.4) |
| II | 24 (18.2) | 6 (12.0) | 18 (22.0) |
| III | 14 (11.4) | 4 (8.0) | 10 (12.2) |
| IV | 66 (50.0) | 32 (64.0) | 34 (41.5) |
| Grade | |||
| Well/moderately differentiated | 35 (26.5) | 9 (18.0) | 26 (31.7) |
| Poorly differentiated | 88 (66.7) | 36 (72.0) | 52 (63.4) |
| Unknown | 9 (6.8) | 5 (10.0) | 4 (4.9) |
| Anatomic site | |||
| GE JX | 12 (9.1) | 5 (10.0) | 7 (8.5) |
| Cardia | 10 (7.6) | 5 (10.0) | 5 (6.1) |
| Fundus | 12 (9.1) | 6 (12.0) | 6 (7.3) |
| Body | 33 (25.0) | 10 (20.0) | 23 (28.0) |
| Antrum | 18(13.6) | 8 (16.0) | 10 (12.2) |
| Pylorus | 17 (12.9) | 7 (14.0) | 10 (12.2) |
| Overlap (multifocal) | 27 (20.5) | 9 (18.0) | 18 (22.0) |
| Unspecified | 3 (2.3) | 0 (0) | 3 (3.7) |
| Regions of Alaska | |||
| North | 38 (28.8) | 17 (34.0) | 21 (25.6) |
| Interior | 11 (8.3) | 1 (2.0) | 10 (12.2) |
| Southwest | 40 (30.3) | 15 (30.0) | 25 (30.5) |
| Southcentral | 40 (30.3) | 17 (34.0) | 23 (28.0) |
| Southeast | 3 (2.3) | 0 (0) | 3 (3.7) |
| Treatment2 | |||
| Chemotherapy only | 40 (30.3) | 12 (24.0) | 28 (34.1) |
| Neoadjuvant | 21 (15.9) | 8 (16.0) | 13 (15.9) |
| Adjuvant | 12 (9.1) | 4 (8.0) | 8 (9.8) |
| Resection only | 12 (9.1) | 5 (10.0) | 7 (8.5) |
| Neoadjuvant, resection, adjuvant | 9 (6.8) | 3 (6.0) | 6 (7.3) |
| None | 38 (28.8) | 18 (36.0) | 20 (24.4) |
| Metastasis site1 | |||
| Omentum/peritoneium/diaphram | 27 (41.5) | 14 (43.8) | 13 (38.2) |
| Liver | 14 (21.5) | 6 (18.8) | 8 (23.5) |
| Lung | 1 (1.5) | 0 (0) | 1 (2.9) |
| Bone | 2 (3.1) | 0 (0) | 2 (5.9) |
| Ovary | 6 (9.2) | 6 (18.8) | 0 (0) |
| Multiple Sites | 15 (23.1) | 6 (18.8) | 10 (29.4) |
| Additional clinical and pathological variables | |||
| H. pylori | 52 (41.3) | 20 (41.7) | 32 (41.0) |
| Chronic gastritis | 101 (76.5) | 38 (76.0) | 63 (76.8) |
| GERD | 51 (39.5) | 18 (36.0) | 33 (41.3) |
| Gastric ulcer | 91 (68.9) | 35 (70.0) | 56 (68.3) |
| Tobacco | 108 (81.8) | 36 (72.0) | 72 (87.8) |
| Family history GI cancer | 38 (28.8) | 16 (32.0) | 21 (25.6) |
Patients with stage 4 gastric cancer were used for analysis;
Neoadjuvant and adjuvant treatments include chemotherapy and chemoradiation regimens based on NCCN guidelines. NOS: Not otherwise specified; AC: Adenocarcinoma; GE JX: Gastroesophageal junction; H. pylori: Helicobacter pylori; GERD: Gastroesophageal reflux disease; GC: Gastric cancer.
Clinical response to treatment and overall survival in AN gastric cancer patients
Results of univariate Cox proportional regression analysis for factors associated with overall survival are shown in Table 3. In this study, 109 deaths (82.6%) were observed among the AN patients (n = 132) within a five-year study period with greater than 90% of patients dying from gastric cancer. Anatomic site, AJCC stage, treatment, the number of lymph nodes examined during resection, and blood type were associated with diminished overall survival. Patients with tumors diagnosed at stage IV, and poorly/undifferentiated histology had a higher risk of death. Patients whose tumors involved multiple regions of the stomach (overlap), upper third, and GE junction had decreased survival times when compared to patients with distal tumors. Patients with O+ blood type had significantly poorer survival compared to A+ and AB+ patients (Table 3). Patients were treated with chemotherapy, surgery, and radiation according to standard NCCN guidelines[18]. Those who received a gastric resection with or without additional therapy had significantly better survival compared to patients that received only chemotherapy. Patients who received neoadjuvant chemotherapy with or without radiation, followed by surgery, and adjuvant chemotherapy had the best survival.
Table 3.
Univariate analysis of Alaska Native gastric cancer patients to identify variables associated with overall survival
| HR1 | 95%CI | P2 value | |
| Age, ≥ 55 yr vs < 55 yr | 0.73 | 0.49-1.07 | 0.11 |
| Sex, male vs female | 0.86 | 0.59-1.27 | 0.45 |
| Geographical Region | 0.53 | ||
| North | 1.00 | ||
| Interior | 0.99 | 0.50-1.96 | |
| Southwest | 0.68 | 0.42-1.11 | |
| Southcentral | 1.00 | 0.61-1.62 | |
| Southeast | 0.65 | 0.15-2.69 | |
| Signet ring, present or absent | 0.78 | 0.52-1.17 | 0.22 |
| Diffuse vs intestinal | 1.01 | 0.68-1.49 | 0.97 |
| Anatomic site | 0.07 | ||
| Noncardia Cardia | 1.00 | ||
| Cardia3 | 1.10 | 0.66-1.84 | |
| Overlap/NOS | 1.74 | 1.08-2.81 | |
| Grade | 0.22 | ||
| Poorly differentiated | 1.00 | ||
| Well/moderately | 0.70 | 0.66-1.84 | |
| Unknown | 0.82 | 0.37-1.84 | |
| AJCC Stage | < 0.0001 | ||
| I | 1.00 | ||
| II | 1.42 | 0.74-2.75 | |
| III | 1.67 | 0.79-3.54 | |
| IV | 4.91 | 2.80-8.61 | |
| Treatment4 | < 0.0001 | ||
| Chemo | 1.00 | ||
| None | 2.07 | 1.29-3.32 | |
| Neoadjuvant, surgery | 0.23 | 0.12-0.45 | |
| Surgery only | 0.29 | 0.14-0.61 | |
| Surgery, adjuvant | 0.27 | 0.13-0.59 | |
| Neoadjuvant, surgery, adjuvant | 0.15 | 0.06-0.39 | |
| No. of nodes examined, ≥ 15 vs < 155 nodes3 | 0.37 | 0.19-0.75 | < 0.0001 |
| Blood Type | 0.04 | ||
| A+ | 1.00 | ||
| AB+ | 0.88 | 0.42-1.82 | |
| B+ | 1.72 | 0.76-3.96 | |
| O+ | 1.78 | 1.05-3.01 | |
| Unknown | 2.35 | 1.24-4.45 | |
| Multiple primaries, yes vs no | 0.73 | 0.45-1.17 | 0.22 |
| Tobacco | 0.51 | ||
| Yes | 1.00 | ||
| None | 0.83 | 0.49-1.42 | |
| Chew/Iqmik | 0.78 | 0.31-1.95 | |
| Former user6 | 1.26 | 0.80-1.97 | |
| Gastric ulcer, yes vs no | 1.02 | 0.67-1.54 | 0.93 |
| Chronic Gastritis, yes vs no | 0.62 | 0.39-0.98 | 0.04 |
| H. pylori, tumor positive vs negative | 0.90 | 0.61-1.34 | 0.62 |
| GERD, yes vs no | 0.75 | 0.51-1.13 | 0.17 |
Univariate analysis was performed using Kaplan-Meier analysis model and log-rank test;
Bold type indicates statistical significance (P < 0.05);
Cardia includes gastroesophageal junction and cardia gastric cancers;
Neoadjuvant and adjuvant treatments include chemotherapy and chemoradiation regimens based on NCCN guidelines;
Patients that had a resection were included in analysis;
Patients discontinued smoking before time of diagnosis. HR: Hazard ratio; CI: Confidence interval; AJCC: American Joint Committee on Cancer; Chemo: Chemotherapy; H. pylori: Helicobacter pylori; GERD: Gastroesophageal reflux disease.
In univariate analysis, patients who were diagnosed with chronic gastritis at the time of gastric cancer diagnosis had better survival than people without gastritis (Table 3). Upon further investigation, patients were more likely to be diagnosed with stage IV cancer without gastritis (75%) compared to patients with chronic gastritis (43%). We did not observe differences in survival in patients who had H. pylori positive tumors, the presence of signet ring cells, diffuse type tumors, or gastric ulcers at the time of diagnosis. The results of the multivariable analysis for association with overall survival are shown in Table 4. This analysis revealed that the variables independently associated with overall survival included AJCC stage and treatment modality, with neoadjuvant chemotherapy or chemoradiation followed by surgery and adjuvant therapy having the greatest association with overall survival.
Table 4.
Multivariable Cox regression analysis for variables associated with overall survival
| HR | 95%CI | P1 value | |
| AJCC stage | 0.004 | ||
| I | 1.00 | ||
| II | 1.59 | 0.74-3.32 | |
| III | 3.09 | 1.32-7.27 | |
| IV | 3.08 | 1.53-6.19 | |
| Treatment | 0.007 | ||
| Chemotherapy | 1.00 | ||
| None | 2.80 | 1.57-5.00 | |
| Neoadjuvant, surgery | 0.28 | 0.10-5.31 | |
| Surgery only | 0.48 | 0.15-12.61 | |
| Surgery, adjuvant | 0.19 | 0.06-5.17 | |
| Neoadjuvant, surgery, adjuvant | 0.18 | 0.5-5.31 | |
| Blood type | 0.160 | ||
| A+ | 1.00 | ||
| AB+ | 0.81 | 0.34-2.23 | |
| B+ | 1.01 | 0.45-2.24 | |
| O+ | 1.40 | 0.84-2.54 | |
| Unknown | 0.63 | 0.31-1.17 | |
| Chronic gastritis, yes vs no | 1.01 | 0.59-1.69 | 0.970 |
Bold type indicates statistical significance (P < 0.05). HR: Hazard ratio; CI: Confidence interval; AJCC: American Joint Committee on Cancer.
DISCUSSION
Evaluating the clinicopathological features of gastric adenocarcinoma in AN patients, we found significant diverse outcomes in epidemiological factors and survival outcomes compared to NHW patients. Our study showed that age-adjusted incidence rates were higher among AN patients compared to US NHW patients. Further, AN patients were more likely to be younger at time of diagnosis, develop non-cardia gastric cancer, and have gastric cancers with signet ring cell histological features. These findings correlate to what has been observed in gastric cancer patients in developing countries and in Asian American populations[3,5]. However, gastric cancer patients in developing countries and in the US tend to be more often male (3:1 male to female), which was not seen represented in our patient population (1.6:1 male to female). A male to female sex ratio of 1:1 has been reported amongst young NHW gastric cancer patients[19,20] and more recent data indicates non-cardia cancers are on the rise in patients younger than 50, particularly among females[21]. A unique observation from our study was that AN females had a higher rate of signet ring cell carcinoma, compared to NHW patients and AN males. High incidence of signet ring cell carcinoma has been reported in other ethnic groups (African American, Asian, AI/AN, and Hispanic) as well as in female patients[22,23], however, the details of these associations have not been well investigated.
The younger age at diagnosis among AN patients with gastric cancer could be driven by multiple etiologies. One factor is earlier exposure to particular gastric cancer risk factors such as H. pylori infection and tobacco use. Previous research revealed 40% of AN children have been infected with H. pylori by age 4, 70% by age 10, and 78% by age 14[24]. This study and our results suggest the likelihood of long term exposure to systemic inflammation due to the early age of acquisition of H. pylori may play an important role in the high incidence of non-cardia cancer, younger age at diagnosis, and the overall gastric cancer health disparity among the AN people. Further, the high prevalence of tobacco use among the AN people may also contribute to the younger age of diagnosis of gastric cancer patients. Another variable associated with gastric cancer in younger individuals is genetic predisposition such as CDH1 germline mutations that result in hereditary diffuse gastric cancers. Approximately 30% of AN patients had a family history of gastrointestinal cancers and there was no difference in age of diagnosis. Further, other types of cancer among the AN people such as lung, kidney, and colorectal cancer are also associated with younger age of diagnosis suggesting earlier age of diagnosis of cancer is a general characteristic in AN cancer patients compared to NHW patients. Often cancers diagnosed at a younger age are more aggressive and are found at a later stage, which may also contribute to cancer health disparities among the AN people.
AN patients were more likely to be diagnosed with non-cardia gastric adenocarcinoma compared to NHW patients. Multiple epidemiological studies have shown non-cardia to be associated with other ethnic populations, such as Hispanics in Central America, US Hispanics, and Eastern Asians[4,25,26]. A commonality between these ethnic groups is the presence of H. pylori in non-cardia gastric cancer[27]. Of the AN patients, approximately half of them had active H. pylori infections at time of diagnosis, of which 65% of the positive cases were in patients diagnosed with non-cardia gastric cancer. However, it is unknown as to how many of the patients have been previously infected or treated for H. pylori during their lifetime. Fock et al[26] reported that the incidence of gastric adenocarcinoma in Asia tends to mirror the seroprevalence rate of H. pylori infection. The CDC has reported H. pylori seroprevalence rate of 75% among AN people[28], rates which are similar to or higher than the rates reported in Eastern Asia[26]. Further, the majority of H. pylori strains in the AN people are CagA and VacA positive[29], both of which are associated with an increased risk of developing severe gastritis, atrophic gastritis, peptic ulcer disease and distal gastric cancers[27,30-32]. Along with the high prevalence of H. pylori, both AN and Eastern Asians share a similar diet and lifestyle: High intake of salty and smoked foods, low vegetable intake and high rates of tobacco use, all of which may contribute to increased risk of gastric cancer[33]. Chronic inflammation and infection are of particular interest in the AN population due to the high levels of chronic gastritis, endemic rates of H. pylori infection, and an increased incidence of EBV-driven cancers in AN people, such as nasopharyngeal carcinoma and lymphoepithelial tumors of the parotid gland[34,35]. Understanding how environmental and dietary factors play a role in increased risk of gastric cancer among AN people requires further investigation.
This study also revealed differences between AN male and female gastric cancer patients. AN males were diagnosed at an earlier age, while AN females were more likely to be diagnosed at a later stage. Many of AN female patients also elected not to receive treatment 36%, compared to 24% of males, regardless of stage of diagnosis. In our study, AN males were more likely to use tobacco compared to the AN females, which has been reported by Alaska’s Behavioral Risk Factor Surveillance System (BRFSS) in the general AN population[36]. Further, 82% of AN patients were current or former smokers, which is higher than the reported 42% of the total AN population[36]. We also observed similar trends between AN males and females with regards to concurrent H. pylori infection, gastritis, GERD, and gastric ulcer. The diagnosis of gastritis, GERD, and gastric ulcers are all known risk factors for developing gastric cancer. Approximately a third of AN patients reported a family history of a first-degree relative with gastric or colorectal cancer, which is higher than reported in other studies[19,33,37]. Previous studies reported that gastric cancers within a population share similar pathological characteristics, suggesting the association of genetic, environmental and lifestyle factors with gastric carcinogenesis[7,38]. However, further research is needed in order to evaluate how genetic, environmental, and lifestyle factors play a role in AN gastric cancer.
Our study is the first study to evaluate clinical outcomes in AN gastric cancer patients. We observed that overall survival was influenced by AJCC stage, blood type, chronic gastritis and treatment. While stage and treatment have been previously reported in the literature as significant prognostic factors of survival[4,37,39], blood type and chronic gastritis have never been associated with overall survival. AN patients with the blood group O had lower overall survival compared to the A/AB groups. Previous studies have reported that patients with blood type A are at a higher risk for developing gastric cancer[40-42]. AN patients with the presence of chronic gastritis were shown to have a more favorable prognosis, which was also associated with an earlier stage at diagnosis. This result suggests that AN patients presenting with symptoms of chronic gastritis may be at high risk for gastric cancer and may benefit from an endoscopy at time of initial presentation. There are currently no standard guidelines on screening for gastric cancer in the US[18], whereas Asian countries with a high incidence of gastric cancer have implemented screening programs using a variety of modalities. However, the most effective gastric cancer screening modality and the screening interval remains controversial. Furthermore, an improved understanding of the composition of immune cells present within the tumor and surrounding microenvironment as well as their function may further elucidate this observation.
AN patients are more likely to present with later stage disease resulting in worse outcomes and the inability of patients to obtain surgical resection, the only curative therapy for gastric cancer. The Alaska Tribal Health System is a unique health system with 58 tribal health centers, 160 tribal community health aide clinics, and 6 regional hospitals dispersed throughout a vast land mass that covers more than 25% of the contiguous US. Patients with cancer are referred to the Alaska Native Medical Center (ANMC), a tertiary hospital in Anchorage, Alaska where they receive cancer therapies according to standard international guidelines[18]. Many of the AN patients included in this study must travel to ANMC to receive their care and medical treatments. The average patient distance from ANMC and its effect on patient care and outcomes has not been studied but is worthy of further investigation. Our study revealed AN patients who received surgery with or without chemotherapy had a better overall survival compared to patients who received chemotherapy alone. Further, patients who received neoadjuvant, surgery, and adjuvant treatment had the best overall survival. Our data supports recent studies that have shown perioperative chemotherapy and/or adjuvant treatment significantly improves overall survival in patients with resectable gastric cancer[39,43,44]. Because no clinical trials addressing the benefits of perioperative and adjuvant treatment included AN patients, our study suggest AN patients also benefit from having perioperative and/or adjuvant treatment with surgery. However, a randomized clinical trial that included AN patients would be necessary to confirm this finding. In addition to surgery, patients who had more than 15 regional lymph nodes examined at the time of resection had a better overall survival when compared to patients who had less than 15 lymph nodes examined. Previous studies have shown lower recurrence and increased survival in patients who received extended lymph node dissection with a D2 lymphadenectomy of 15 or more lymph nodes[45,46]. However, these results have not been consistent across all studies[47,48], and this issue remains controversial.
There are limitations to our study. First, the small number of AN gastric cancer cases diagnosed at ANMC from 2006-2014 limited our power to detect modest associations. The AN population is relatively small- consisting of 150000 people. In order to conduct this study, we reviewed all AN gastric cancer cases diagnosed at the ANMC between 2006-2014, approximately 132 cases. Records from this timespan had the epidemiological information needed to conduct this study, which is why we focused on these individuals. Even with the small number of cases, we were able to detect significant differences in the results. Although the AN population is small, we feel this population is worthy of study because of the poor clinical outcomes and gastric cancer mortality rates that are unique to this population within Alaska. Second, as a retrospectively assembled surveillance study, some relevant confounders were not documented and could not be assessed; for example, blood type has been shown to correlate with gastric cancer prognosis, but was not assessed in 20% of our patients. Future studies need to include relevant confounders, particularly blood type. In addition, family history and tobacco use are captured in AN medical records as patient-reported, which may result in artificially lower percentages due to under reporting[49,50]. Finally, our retrospective surveillance study of AN gastric patients was selected from the ANMC hospital-based registry, which represents approximately 80% of AN gastric cancer cases reported to the SEER-funded AN Tumor Registry. The 20% of patients not in the hospital registry are most likely living outside of Alaska, receiving care at private hospitals, or traveling to other regions of the US for treatment. For accuracy, it would be beneficial to have data on all AN patients, but due to our limitations, the current study uses only patients who were cared for in Alaska at ANMC. It is possible that by not including all AN people we are introducing an element of selection bias into our results, however similar trends in clinical or pathological characteristics were observed between the AN SEER and AN ANMC Hospital-based registries. By utilizing the ANMC hospital-based registry we were able to further evaluate clinicopathological and treatment outcomes that were not collected by the SEER registry.
In summary, gastric cancer in AN people is distinct from the NHW population. AN patients were observed to have increased incidence, poorer prognosis, earlier age of diagnosis, and variation in location, and subtype of gastric cancer. These clinicopathological characteristics could be driven by multiple variables including, socioeconomic factors and biological differences, such as lifestyle differences, genetic alterations, and environmental exposures. Our findings confirm the importance of early detection, treatment, and surgical resection for AN patients with resectable gastric adenocarcinoma in order to optimize patient outcomes. This study highlights the need for further investigation into understanding the basis for the increased incidence and poorer prognosis of this devastating cancer in AN people.
ARTICLE HIGHLIGHTS
Research background
Gastric cancer is a leading cancer health disparity among the Alaska Native (AN) people, with a 3-fold higher incidence and mortality rate compared to United States non-Hispanic White (NHW) people. There are currently a paucity of studies investigating the clinicopathologic features of this disease in AN people, and their relationship to clinical outcomes.
Research motivation
This study was conducted to gain a deeper understanding of AN gastric cancer patient characteristics, pathologic variables, clinical patterns of care, and patient outcomes to gain insights into to this cancer health disparity.
Research objectives
In order to further investigate how to reduce gastric cancer incidence and mortality rates among the AN population, we sought to evaluate recent trends in gastric cancer incidence, response to treatment, and overall survival outcomes in this high incidence population. A greater understanding of gastric cancer incidence and response to treatment among the AN people may facilitate the design of screening programs or the identification of early detection measures, and elucidate new areas for future investigation to potentially reduce incidence and improve patient outcomes.
Research methods
We performed a retrospective analysis of 132 AN gastric cancer patients treated at the Alaska Native Medical Center (ANMC) from 2006-2014, utilizing the ANMC Tumor Registry and manual patient chart reviews. We compared our findings to data on United States (US) NHW and AN gastric adenocarcinoma patients obtained from the US National Institute’s SEER Program of the National Cancer Institute 18 dataset for the period 2006-2014. Data were analyzed using software SPSS 23.0.
Research results
AN patients differ from NHW patients in that they have a higher prevalence of non-cardia tumors, unique histological features with a higher incidence of the diffuse subtype, and a higher incidence of signet ring cell carcinomas. AN females were more likely to be diagnosed with stage IV cancers compared to AN males. We observed a decreased overall survival among AN patients with advanced stage disease, O+ blood type, < 15 lymph nodes examined at resection, and no treatment. AN gastric cancer patients have a higher incidence rate, a poorer overall survival, and are diagnosed at a significantly younger age compared to NHW patients. This study is the first report detailing the clinicopathologic features of gastric cancer in AN people, as well as information on patterns of care, and clinical outcome data.
Research conclusions
Gastric cancer in AN people is distinct from the NHW population. AN patients were observed to have increased incidence, poorer prognosis, earlier age of diagnosis, and variation in location, and histological subtype of gastric cancer. These clinicopathological characteristics could be driven by multiple variables including, socioeconomic factors and biological differences, such as lifestyle differences, genetic alterations, and environmental exposures. Our findings confirm the importance of early detection, treatment, and surgical resection for AN patients with resectable gastric adenocarcinoma in order to optimize patient outcomes. This study highlights the need for further investigation into understanding the basis for the increased incidence and poorer prognosis of this devastating cancer in AN people.
Research perspectives
Our work highlights the unique clinical and pathologic features of gastric cancer in the AN population. The high incidence of this cancer warrants prompt referral for endoscopic evaluation of AN patients presenting with gastrointestinal symptoms. Of particular concern is the finding that younger women present more frequently with stage IV disease, emphasizing the need to consider a diagnosis of gastric cancer earlier in this population. Clinical outcomes are poor in this population, despite the fact that patients are treated according to standard guidelines. An important area for future study will be investigations into the molecular features of gastric cancer in AN people, with the goal of identifying new prognostic and predictive markers that may improve treatment regimens, and possibly identify new targets for precision medicine.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Linda O’Brien for providing the ANMC tumor registry data; Dr. Aravind Sanjeevaiah and Dr. Benjamin Koziner for helpful discussions; Dr. Max Kullberg and Dr. Janet Johnston for their critical review of the manuscript.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): E
Institutional review board statement: This study was reviewed and approved by the University of Alaska Anchorage Institutional Review Board (IRB), Alaska Area IRB, Southcentral Foundation Research Review Board, and the Alaska Native Tribal Health Consortium Health Research Review Committee.
Informed consent statement: Patients were not required to give informed consent to this study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: The authors have no conflicting financial interests.
Data sharing statement: No additional data are available.
Peer-review started: April 2, 2018
First decision: April 19, 2018
Article in press: June 2, 2018
P- Reviewer: Milone M, Nagahara H, Tomizawa M, Ziogas DE S- Editor: Gong ZM L- Editor: Logan S E- Editor: Huang Y
Contributor Information
Holly A Martinson, WWAMI School of Medical Education, University of Alaska Anchorage, Anchorage, AK 99508, United States. hamartinson@alaska.edu.
Nancy J Shelby, WWAMI School of Medical Education, University of Alaska Anchorage, Anchorage, AK 99508, United States.
Steven R Alberts, Department of Oncology, Mayo Clinic Cancer Center, Rochester, MN 55905, United States.
Matthew J Olnes, Hematology and Medical Oncology Department, Alaska Native Medical Center, Anchorage, AK 99508, United States.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Sierra MS, Cueva P, Bravo LE, Forman D. Stomach cancer burden in Central and South America. Cancer Epidemiol. 2016;44 Suppl 1:S62–S73. doi: 10.1016/j.canep.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483–4490. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee E, Liu L, Zhang J, Stern MC, Barzi A, Hwang A, Kim AE, Hamilton AS, Wu AH, Deapen D. Stomach Cancer Disparity among Korean Americans by Tumor Characteristics: Comparison with Non-Hispanic Whites, Japanese Americans, South Koreans, and Japanese. Cancer Epidemiol Biomarkers Prev. 2017;26:587–596. doi: 10.1158/1055-9965.EPI-16-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmack AM, Schade TL, Sallison I, Provost EM, Kelly JJ. Cancer in Alaska Native People: 1969-2013: The 45 Year Report. Anchorage, AK: Alaska Native Tumor Registry, Alaska Native Epidemiology Center, Alaska Native Tribal Health Consortium; 2015. [Google Scholar]
- 7.Arnold M, Moore SP, Hassler S, Ellison-Loschmann L, Forman D, Bray F. The burden of stomach cancer in indigenous populations: a systematic review and global assessment. Gut. 2014;63:64–71. doi: 10.1136/gutjnl-2013-305033. [DOI] [PubMed] [Google Scholar]
- 8.Alberts SR, Kelly JJ, Lanier AP, Sacco F. Occurrence of esophageal and gastric cancer in Alaska Natives, 1969-2003. Alaska Med. 2006;48:2–11. [PubMed] [Google Scholar]
- 9.Wiggins CL, Perdue DG, Henderson JA, Bruce MG, Lanier AP, Kelley JJ, Seals BF, Espey DK. Gastric cancer among American Indians and Alaska Natives in the United States, 1999-2004. Cancer. 2008;113:1225–1233. doi: 10.1002/cncr.23732. [DOI] [PubMed] [Google Scholar]
- 10.Lanier AP, Kelly JJ, Maxwell J, McEvoy T, Homan C. 2006. Cancer in Alaska Natives 1969-2003 35-year Report. Alaska Native Tribial Health Consortium. Anchorage, AK. [Google Scholar]
- 11.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 12.Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 13.Camargo MC, Murphy G, Koriyama C, Pfeiffer RM, Kim WH, Herrera-Goepfert R, Corvalan AH, Carrascal E, Abdirad A, Anwar M, et al. Determinants of Epstein-Barr virus-positive gastric cancer: an international pooled analysis. Br J Cancer. 2011;105:38–43. doi: 10.1038/bjc.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keck JW, Miernyk KM, Bulkow LR, Kelly JJ, McMahon BJ, Sacco F, Hennessy TW, Bruce MG. Helicobacter pylori infection and markers of gastric cancer risk in Alaska Native persons: a retrospective case-control study. Can J Gastroenterol Hepatol. 2014;28:305–310. doi: 10.1155/2014/892084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacco F, Bruce MG, McMahon BJ, Bruden D. A prospective evaluation of 200 upper endoscopies performed in Alaska Native persons. Int J Circumpolar Health. 2007;66:144–152. doi: 10.3402/ijch.v66i2.18245. [DOI] [PubMed] [Google Scholar]
- 16.Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 17.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 18.Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531–546. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 19.Dhobi MA, Wani KA, Parray FQ, Wani RA, Wani ML, Peer GQ, Abdullah S, Wani IA, Wani MA, Shah MA, et al. Gastric cancer in young patients. Int J Surg Oncol. 2013;2013:981654. doi: 10.1155/2013/981654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theuer CP, de Virgilio C, Keese G, French S, Arnell T, Tolmos J, Klein S, Powers W, Oh T, Stabile BE. Gastric adenocarcinoma in patients 40 years of age or younger. Am J Surg. 1996;172:473–476; discussion 476-477. doi: 10.1016/S0002-9610(96)00223-1. [DOI] [PubMed] [Google Scholar]
- 21.Anderson WF, Rabkin CS, Turner N, Fraumeni JF Jr, Rosenberg PS, Camargo MC. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J Natl Cancer Inst. 2018;110:608–615. doi: 10.1093/jnci/djx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taghavi S, Jayarajan SN, Davey A, Willis AI. Prognostic significance of signet ring gastric cancer. J Clin Oncol. 2012;30:3493–3498. doi: 10.1200/JCO.2012.42.6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Cai H, Sheng W, Yu L, Long Z, Shi Y, Wang Y. Clinicopathological Characteristics and Survival Outcomes of Primary Signet Ring Cell Carcinoma in the Stomach: Retrospective Analysis of Single Center Database. PLoS One. 2015;10:e0144420. doi: 10.1371/journal.pone.0144420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parkinson AJ, Gold BD, Bulkow L, Wainwright RB, Swaminathan B, Khanna B, Petersen KM, Fitzgerald MA. High prevalence of Helicobacter pylori in the Alaska native population and association with low serum ferritin levels in young adults. Clin Diagn Lab Immunol. 2000;7:885–888. doi: 10.1128/cdli.7.6.885-888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corral JE, Delgado Hurtado JJ, Domínguez RL, Valdez de Cuéllar M, Balmore Cruz C, Morgan DR. The descriptive epidemiology of gastric cancer in Central America and comparison with United States Hispanic populations. J Gastrointest Cancer. 2015;46:21–28. doi: 10.1007/s12029-014-9672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol. 2010;25:479–486. doi: 10.1111/j.1440-1746.2009.06188.x. [DOI] [PubMed] [Google Scholar]
- 27.Kang SY, Han JH, Ahn MS, Lee HW, Jeong SH, Park JS, Cho YK, Han SU, Kim YB, Kim JH, et al. Helicobacter pylori infection as an independent prognostic factor for locally advanced gastric cancer patients treated with adjuvant chemotherapy after curative resection. Int J Cancer. 2012;130:948–958. doi: 10.1002/ijc.26081. [DOI] [PubMed] [Google Scholar]
- 28.Tveit AH, Bruce MG, Bruden DL, Morris J, Reasonover A, Hurlburt DA, Hennessy TW, McMahon B. Alaska sentinel surveillance study of Helicobacter pylori isolates from Alaska Native persons from 2000 to 2008. J Clin Microbiol. 2011;49:3638–3643. doi: 10.1128/JCM.01067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miernyk K, Morris J, Bruden D, McMahon B, Hurlburt D, Sacco F, Parkinson A, Hennessy T, Bruce M. Characterization of Helicobacter pylori cagA and vacA genotypes among Alaskans and their correlation with clinical disease. J Clin Microbiol. 2011;49:3114–3121. doi: 10.1128/JCM.00469-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 31.Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Doorn LJ, Figueiredo C, Mégraud F, Pena S, Midolo P, Queiroz DM, Carneiro F, Vanderborght B, Pegado MD, Sanna R, et al. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology. 1999;116:823–830. doi: 10.1016/s0016-5085(99)70065-x. [DOI] [PubMed] [Google Scholar]
- 33.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley JJ, Schade TL, Starkey BM, White S, Ashokkumar R, Lanier AP. 2012. Cancer in Alaska Native People 1969-2008 a 40-Year Report. Alaska Native Tribial Health Consortium. Anchorage, AK. [Google Scholar]
- 35.Lanier AP, Alberts SR. Cancers of the buccal cavity and pharynx in Circumpolar Inuit. Acta Oncol. 1996;35:545–552. doi: 10.3109/02841869609096986. [DOI] [PubMed] [Google Scholar]
- 36.Walker B, Davidson V, Butler J, Allely K. 2015. Alaska Tobacco Facts 2015 Update. [Google Scholar]
- 37.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med. 2008;47:1077–1083. doi: 10.2169/internalmedicine.47.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group, Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, Van Cutsem E, Buyse M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 40.Edgren G, Hjalgrim H, Rostgaard K, Norda R, Wikman A, Melbye M, Nyrén O. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: a cohort study. Am J Epidemiol. 2010;172:1280–1285. doi: 10.1093/aje/kwq299. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Liu L, Ji J, Zhang J, Yan M, Zhang J, Liu B, Zhu Z, Yu Y. ABO blood group system and gastric cancer: a case-control study and meta-analysis. Int J Mol Sci. 2012;13:13308–13321. doi: 10.3390/ijms131013308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu MZ, Zhang DS, Ruan DY, Luo HY, Wang ZQ, Zhou ZW, Wang FH, Li YH, Xu RH. A relationship between ABO blood groups and clinicopathologic characteristics of patients with gastric adenocarcinoma in China. Med Oncol. 2011;28 Suppl 1:S268–S273. doi: 10.1007/s12032-010-9735-5. [DOI] [PubMed] [Google Scholar]
- 43.Knight G, Earle CC, Cosby R, Coburn N, Youssef Y, Malthaner R, Wong RK; Gastrointestinal Cancer Disease Site Group. Neoadjuvant or adjuvant therapy for resectable gastric cancer: a systematic review and practice guideline for North America. Gastric Cancer. 2013;16:28–40. doi: 10.1007/s10120-012-0148-3. [DOI] [PubMed] [Google Scholar]
- 44.Ikoma N, Cormier JN, Feig B, Du XL, Yamal JM, Hofstetter W, Das P, Ajani JA, Roland CL, Fournier K, et al. Racial disparities in preoperative chemotherapy use in gastric cancer patients in the United States: Analysis of the National Cancer Data Base, 2006-2014. Cancer. 2018;124:998–1007. doi: 10.1002/cncr.31155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biondi A, D’Ugo D, Cananzi FC, Papa V, Borasi A, Sicoli F, Degiuli M, Doglietto G, Persiani R. Does a minimum number of 16 retrieved nodes affect survival in curatively resected gastric cancer? Eur J Surg Oncol. 2015;41:779–786. doi: 10.1016/j.ejso.2015.03.227. [DOI] [PubMed] [Google Scholar]
- 46.Datta J, Lewis RS Jr, Mamtani R, Stripp D, Kelz RR, Drebin JA, Fraker DL, Karakousis GC, Roses RE. Implications of inadequate lymph node staging in resectable gastric cancer: a contemporary analysis using the National Cancer Data Base. Cancer. 2014;120:2855–2865. doi: 10.1002/cncr.28780. [DOI] [PubMed] [Google Scholar]
- 47.Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069–2077. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 48.El-Sedfy A, Dixon M, Seevaratnam R, Bocicariu A, Cardoso R, Mahar A, Kiss A, Helyer L, Law C, Coburn NG. Personalized Surgery for Gastric Adenocarcinoma: A Meta-analysis of D1 versus D2 Lymphadenectomy. Ann Surg Oncol. 2015;22:1820–1827. doi: 10.1245/s10434-014-4168-6. [DOI] [PubMed] [Google Scholar]
- 49.Murff HJ, Spigel DR, Syngal S. Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. JAMA. 2004;292:1480–1489. doi: 10.1001/jama.292.12.1480. [DOI] [PubMed] [Google Scholar]
- 50.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11:12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]


