Abstract
Purpose
The aim of this study was to review the genetics, epidemiology, clinical findings, and management of BRCA1-associated protein-1 (BAP1) cancer predisposition syndrome, particularly focusing on the development of uveal melanoma (UM).
Methods
This is a review article based on eligible studies identified by systematically searching PubMed, Web of Science, and reference lists.
Results
UM is the most common primary intraocular malignancy. Most UM cases are sporadic, but a small percentage has been documented with familial tendency. Until recently, there was little information regarding the genetics of this malignant tumor, and we have now begun to understand the pathways of development. BAP1 is a scavenger protein that regulates cell cycle, cellular differentiation, and DNA damage response. Patients and families with germline BAP1 mutation are predisposed to familial cancers including UM, mesothelioma, cutaneous melanoma (CM), renal cell carcinoma (RCC), and others. Clinicians should be aware of the implications of germline BAP1 mutation and advise genetic testing and assessment for BAP1 germline mutation in suspected patients and families.
Conclusions
The ability of BAP1 gene mutation to cause multiple tumor types and high penetrance in carriers suggests that this gene has an important role for influencing cancer cell growth. With progress in understanding the molecular landscape of UM and the development of treatments targeted to the pathways involving BAP1 and other gene mutations, it is possible to improve the outcome of this malignant cancer.
Keywords: Uveal melanoma, Mesothelioma, Renal cell carcinoma, BAP1 cancer predisposition syndrome, BRCA1-associated protein-1, BAP1
Introduction
Uveal melanoma (UM) is the most common primary intraocular malignancy in adults and most commonly found in light complexion Caucasians with an age-adjusted incidence of 4.3 per million people.1, 2, 3 It is estimated that approximately 2500 North Americans develop UM annually.1 While the disease is relatively rare, the chance for two or more first-degree relatives with UM is exquisitely low, estimated to be less than 0.0002.4 However, approximately 1% of all UM patients demonstrate some degree of familial uveal melanoma (FUM),5, 6 and it has been suggested in the past that there could be an autosomal dominant (AD) mode of inheritance for familial form of UM.4
Since 1971, several reports have described the association between UM and other cancers,7 especially cutaneous melanoma (CM), breast cancer, and prostate cancer.8, 9, 10 Abdel-Rahman et al estimated that approximately 11.6% of all patients with UM are at risk for a hereditary cancer predisposition.5 In a prospective analysis of 2320 cases of UM in the Collaborative Ocular Melanoma Study (COMS), second cancers were found in 222 (10%) patients, excluding basal or squamous cell carcinoma. The most common second malignancies were cancer of the prostate (2.2%), breast (1.6%), lung (1.2%), genitourinary (1%), gastrointestinal (0.9%), and leukemia/lymphoma (0.8%). In that cohort, the 5-year cumulative risk for second primary cancer was 8% at 5 years and 15% at 10 years.10
Patients with hereditary predisposition to UM could have higher risk for development of other cancers related to germline genetic alterations. In a 1996 analysis of FUM in 27 families from our department, we concluded that most affected patients were first-degree relatives, and underlying genetic alterations, yet to be discovered, were likely responsible for this relationship.4 Since then, now over 20 years later, genetic alterations important for UM development and progression have been identified and include Guanine nucleotide-binding protein G (GNAQ/11), Eukaryotic translation initiation factor (EIF1AX), Splicing factor 3B subunit 1 (SF3B1), and BRCA1-associated protein-1(BAP1).11, 12
BAP1 is a highly-penetrant germline mutation that has been recognized as an important predisposing factor for hereditary cancers, including UM.12 BAP1 tumor predisposition syndrome (BAP1-TPDS) is a newly-recognized cancer syndrome that predisposes the patient to UM, malignant mesothelioma (MMe), CM, renal cell carcinoma (RCC), and possibly to a range of other cancers as well.12, 13, 14 Compared to non-predisposed patients with equivalent cancers, most of the BAP1-related cancers tend to be more aggressive and triggered earlier in life.13, 14 Therefore, patients with BAP1 germline mutation are at risk for several malignant tumors and should be counseled regarding cancer risk for patient and family members as well as routinely monitored.
BAP1 gene structure and function
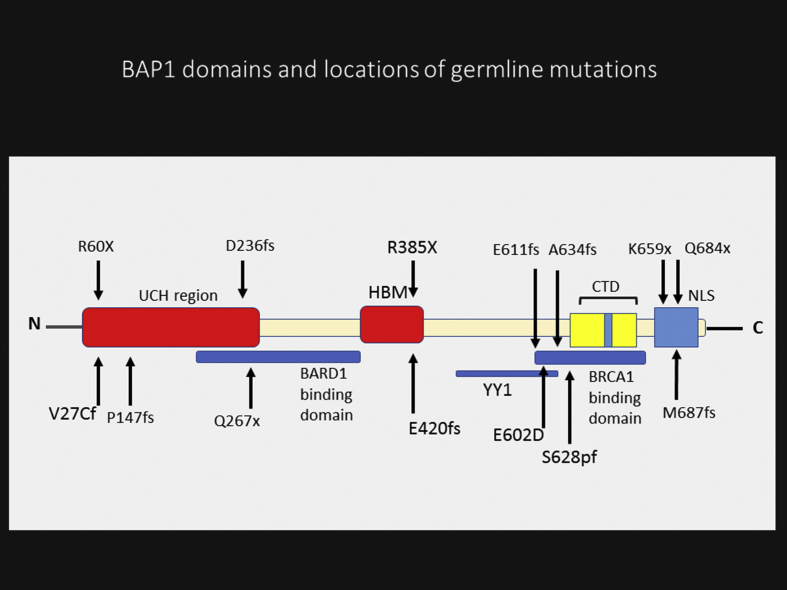
BAP1 is a deubiquitinating enzyme, with the gene located on the short arm of chromosome 3 (3p21.1), and contains 729 amino acids.14, 15 This protein has three main domains including N-terminal catalytic domain, which removes ubiquitin from ubiquitylated substrates; the middle portion with host cell factor 1 (HCF1) binding domain; and the C-terminal domain (CTD) which is important for interaction with additional sex combs like (ASXL1/2) and other proteins.14 (Fig. 1).
Fig. 1.
Schematic structure of BRCA1-associated protein-1 (BAP1) domains and locations of reported germline mutations. Consists of: ubiquitin carboxyl hydrolase (UCH) domain; HBM, host cell factor 1 (HCF1) binding domain; nuclear localization signals (NLS); C-terminal domain (CTD), additional sex combs like (ASXL1/2) binding domain; BRCA1-associated RING domain protein 1 (BARD1) binding region; Breast Cancer type 1 (BRCA1) binding region and Ying Yang 1 (YY1) binding region.
BAP1 functions as a tumor suppressor protein through its deubiquitinase activity that regulates target genes in cell cycle control, cellular differentiation, and DNA damage repair. This protein has been shown to form a ternary complex with HCF1 and transcription factor Ying Yang 1 (YY1) for cell proliferation and cell cycle control.15 BAP1 is an essential DNA damage repair enzyme, through a complex with several recombination proteins including Breast Cancer type 1 (BRCA1) and BRCA1-associated RING domain protein 1 (BARD1), which promotes E3 ubiquitin ligase activity to regulate DNA damage response.14 The first reports demonstrated that tumor suppressor effect of BAP1 results from nuclear localization and deubiquitinating activity,15 but new findings revealed that extra-nuclear BAP1 was specifically present in the endoplasmic reticulum (ER) fraction. It binds, deubiquitylates, and stabilizes type 3 inositol-1,4,5-trisphosphate receptor (IP3R3), modulating calcium release from the ER into the cytosol and mitochondria, promoting apoptosis.16 Nevertheless, the structural architecture of the details of BAP1 complexes have not been completely characterized, so the impact of various mutations is still unclear.14, 15
History and epidemiology
BAP1 is a deubiquitinating hydrolase enzyme that was identified in 1998, and initial data suggested that BAP1 suppressed the growth of human breast cancer cells in soft agar.17 Earlier reports had shown that BAP1 tumor suppressor function was in cooperation with BRCA-1 in cultured cells, so this enzyme was initially named BAP1, but later shortened to BAP1.17 Over a 10-year period, the true clinical value of the impact of BAP1 was realized.
Some of the understanding of BAP1 came through clinical observations of familial cancers.4, 5 One sentinel example is the story of familial mesothelioma. MMe in the western world is often associated with asbestos exposure.18 This is a relatively rare cancer causing 2500 deaths yearly in the United States. In contrast, in Cappadocia, a semi-arid region in central Turkey, a mesothelioma epidemic was observed in the early 2000s.18 Among people living in 3 small villages, 50% of all deaths were caused by this malignant tumor.18, 19 Pedigree studies of these villages revealed that mesothelioma was prevalent in some families but not others, and this malignancy was transmitted in an AD fashion.18, 19 At the same time, in the United States, two unrelated families, L (from Louisiana) and W (from Wisconsin), were found with high incidence of mesothelioma, and each had only minimal exposure to asbestosis.20 Further important clinical observations disclosed that two members in the L family developed UM.20 In the United States, approximately 3000 patients with mesothelioma and 2500 patients with UM are diagnosed annually; hence, chance for simultaneous occurrence of these rare malignancies in more than one individual in the same family was estimated at 36 per trillion population.20 This linkage suggested a common genetic factor. Genetic assessment and chromosome microarray on the L and W families disclosed alteration in chromosome region 3p21 in both mesothelioma and UM cases.20 Sequencing this region of chromosome 3 led to the identification of germline BAP1 as the mutated gene in the L and W families.21 Since then, mutations in BAP1 gene has been confirmed in mesothelioma,20 UM,21 CM,22 and RCC.23
BAP1 tumor predisposition syndrome
BAP1 tumor predisposition syndrome (BAP1-TPDS) is a novel cancer syndrome that has been identified from three independent research groups, initially focusing on mesothelioma, CM, and UM.20, 21, 22 Shortly afterward, RCC was included this group.23 The molecular mechanisms and cellular pathway responsible for leading to specific tumor types, and the difference in disease outcomes remain unclear.14 The full spectrum of this syndrome is still being characterized through discovery of new associated tumors, including basal cell carcinoma, lung cancer, breast/ovarian cancer, meningioma, neuroendocrine tumors, and some types of sarcoma.14, 24, 25
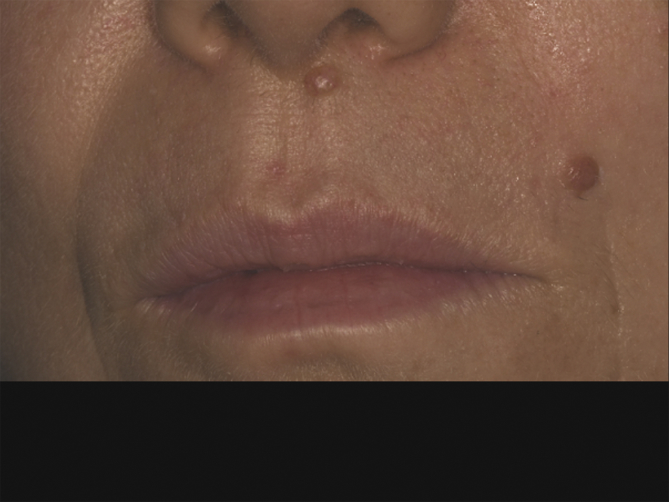
Wiesner et al. recognized that germline BAP1 mutation was associated with a benign atypical skin melanocytic tumor.22 They noted that this melanocytic lesion was histologically between benign Spitz nevus and malignant melanoma, so they named this lesion Melanocytic BAP1-mutated Atypical Intradermal Tumor (MBAIT), or atypical Spitz tumor (AST).22 Clinically, this tumor presents with skin-colored dome-shape papule or nodule of approximately 0.2–1.0 cm in diameter (Fig. 2). Histopathologically, MBAITs are mostly intradermal, showing absence of mitotic figures and Kamino bodies (common in Spitz).22 Unlike Spitz nevus, MBAIT nearly always demonstrate B-Raf Proto-Oncogene (BRAF) mutation.26 This lesion usually appears during the first two decades of life and increases in number with age.22 Consequently, MBAITs can highlight early detection of this syndrome, several years before development of the characteristic malignancies of the BAP1-TPDS.26 The incidence of MBAITs in BAP1-TPDS patients is unclear, because they were not assessed in several published cases and series.24 Instead of the term MBAITs, some employ the term “BAPomas” to describe this precursor skin lesion that occurs within families having germline BAP1 mutation.26 Due to the lack of long-term follow-up data, there is unknown malignant potential for this skin lesion.24
Fig. 2.
Two inconspicuous skin-tone dome-shaped cutaneous lesions suspected to be Melanocytic BAP1-mutated Atypical Intradermal Tumor (MBAIT) (Bapoma) in a 36-year-old woman with uveal melanoma (UM), germline BRCA1-associated protein-1 (BAP1) mutation and family history of skin melanoma.
Rai et al. reported 174 patients with germline BAP1 mutation and found that 130 (75%) developed at least one of the five main tumors, including UM (31%), MMe (22%), MBAIT (18%), CM (13%), and RCC (10%).24 They noted that 90% of cases showed positive family history for at least two of these tumors in their first or second-degree relatives.24 Affected individuals may have more than one type of primary cancer.20, 24, 25 By combining family histories, molecular genetics, and genealogical approaches, Carbone et al. uncovered a BAP1 cancer syndrome in a kindred whose members descended from a couple born in Germany in the early 1700s who immigrated to North America. Their descendants spread throughout the continent with mutation carriers demonstrating multiple malignancies (Table 1). In a core of 72 individuals (related to the four last generations), 22 (31%) developed one or more than one common BAP1-TPDS tumors including UM (8.5%), MMe (17%), CM (3%), and RCC (3%).27 Of course, this family pedigree is still in progress as new information is added to the pedigree as it is acquired. As more branches of the family are identified, they will be offered testing for BAP1.
Table 1.
BAP1 tumor predisposition syndrome (BAP1-TPDS). Frequency of related malignancies.
| Common BAP1-TPDS tumors | Study #1 Rai et al24 |
Study #2 Carbone et al27 |
|---|---|---|
| Uveal melanoma (UM) | 54/174 (31%) | 6/72 (8.5%) |
| Mesothelioma | 39/174 (22%) | 12/72 (17%) |
| Cutaneous melanoma (CM) | 23/174 (13%) | 2/72 (3%) |
| Renal cell carcinoma (RCC) | 18/174 (10%) | 2/72 (3%) |
| Atypical Spitz tumor (MBAIT) Other tumorsa | 32/174 (18%) | Unknown |
| Breast cancer | 9/95 (9.5%) | 3/37 (8.2%) |
| Basal cell carcinoma | 11/174 (6.3%) | 3/72 (4.2%) |
| Lung cancer | 6/174 (3.5%) | 2/72 (3%) |
| Ovarian carcinoma | 3/95 (3%) | 0/37 (0%) |
| Prostate cancer | 2/67 (3%) | 2/35 (6%) |
| Sarcoma | 4/174 (2.3%) | 2/72 (3%) |
| Cholangiocarcinoma | 4/174 (2.3%) | 0/72 (0%) |
| Meningioma | 3/174 (2%) | 0/72 (0%) |
| Neuroendocrine cancer | 2/174 (1.2%) | 0/72 (0%) |
| Colorectal cancer | 2/174 (1.2%) | 2/72 (3%) |
| Patients with ≥1 BAP1-TPDS common tumorsb | 134/174 (77%) | 22/72 (31%) |
| Patients with ≥2 BAP1-TPDS common tumorsb | 16/174 (9%) | 3/72 (4.2%) |
UM is the most commonly reported tumor in BAP1-TPDS cases.24 Median age of onset in a person with BAP1-TPDS is younger than the general population of UM (51 years vs 62 years).24 The UM in BAP1-TPDS is more aggressive with higher risk of metastasis and reduced overall survival.13, 28
MMe is the second most common cancer identified in BAP1-TPDS.24 The median age of onset in BAP1-TPDS cases is significantly earlier than sporadic MMe (55 years vs 72 years).29 The survival rate in persons with BAP1-related MMe may be significantly longer compared to sporadic MMe as several reports have documented that patients with germline BAP1 mutation showed a sevenfold longer overall survival compared to those with sporadic MMe.29, 30
CM in BAP1-TPDS cases can be present as single or multiple primary cutaneous lesions.31 The median age of onset of CM in BAP1-TPDS patients is earlier than the general population (46 vs 58 years).24 In comparison with the general population, CM association with BAP1-TPDS appears more aggressive, but the data are somewhat inconsistent.31
RCC in BAP cancer syndrome has a more aggressive nature.14, 32 Median age of RCC diagnosis is younger than the general population (46 vs 64 years), and the length of survival in BAP1-related RCC is substantially less (31.2 months vs 78.2 months).32
Genotype-phenotype correlation
Several different alterations in the BAP1 gene have been described, including large deletions of exons leading to loss of the N-terminal region, focal deletions, frameshift mutations due to insertions or deletions, splice site mutations, and base substitutions leading to non-sense and missense mutations.16, 33 In various tumors, including RCC, mesothelioma, metastasizing UM, and non-small cell lung cancer, the BAP1 gene is commonly lost by chromosomal deletion, and more than 70% of reported germline BAP1 mutations are truncation.11, 24, 33 By virtue of the complex function of BAP1, it is reasonable to suppose that the type of mutation and the gene regions in which they occur will lead to different functional consequences. For instance, truncating mutations frequently result in loss of the nuclear localization signal (NLS) and/or the C-terminal protein-binding domain (Fig. 1), while missense mutations affect the ubiquitin hydrolase function of BAP1.34 In fact, for reasons that are unclear, all four main cancers of BAP1-TPDS have been observed with all classes of mutations.24 Therefore, available data suggest no distinct genotype-phenotype correlation between location or type of the mutations and the type of cancers in patients.24
BAP1 gene mutation penetrance and prevalence
Like other tumor suppressor genes, e.g. Phosphatase and tensin homolog (PTEN) gene in Cowden syndrome and P53 gene in Li-Fraumeni syndrome, the germline mutation in the BAP1 gene is inherited in AD pattern.35 A patient inherits a non-functional BAP1 allele, and the remaining allele is inactivated later in life (two-hit hypothesis).14 This cancer syndrome follows an AD inheritance pattern, and each child of an individual with BAP1-TPDS has a 50% chance of inheriting the BAP1 pathogenic variant; however, penetrance appears to be incomplete, and the types of BAP1-related tumors can vary among different members of the same family.35 Therefore, it seems challenging to predict the incidence of BAP1-TPDS-related cancers in next generation.12
Newer evidence suggests that penetrance of BAP1 mutation is fairly high, and more than 80% of gene carriers are ultimately affected by at least one type of cancer.24 Most affected patients (90%) have at least two of the main tumors (UM, MMe, CM, or RCC) in their parents or second-degree relatives, and seldom could be found a carrier with negative family history for BAP1-TPDS between first and/or second-degree relatives.24 The proportion of BAP1-TPDS caused by a de novo pathogenic variant is unknown.24 Based on these findings, families which carry BAP1-TPDS should receive counseling regarding cancer risk management and offered testing for at-risk family members.14, 24
Uveal melanoma and BAP1 mutation
Somatic chromosomal alteration and genomic instability
In UM, the extent of genomic instability and chromosomal aberrations is relatively low compared to other cancer types such as breast cancer or CM.12 Also, the mutational load in UM tumors is low.12 Therefore, recurrent mutations in genes and chromosomal abnormalities in UM are likely to be specific for tumor progression rather than random event.12, 36 Cytogenetic abnormalities of UM are characterized by monosomy 3, 8p and 8q abnormalities, structural abnormality of chromosome 6, and deletion in chromosome 1.36, 37, 38, 39, 40 The association between deletion in chromosome 3 (monosomy 3) and metastatic death in UM was first described by Prescher et al40 and later confirmed by our team in Philadelphia in a large cohort of 1059 patients.37, 38 In regard to tumor size (small/medium/large), we found an association with single-chromosomal abnormalities with loss of disomy chromosome 3 (35%/52%/65%, respectively), loss of disomy 6 (15%/34%/51%), and loss of disomy 8 (19%/41%/69%) as well.37 This finding indicates that greater tumor size is correlated with greater single-chromosome mutational profile. Also, with regard to individual chromosomal mutational risk (compared with normal disomy), the greatest prognostic impact was found with 8p loss [Hazard ration (HR) 21.51, P < 0.001], 8q gain (HR 9.77, P < 0.001), and complete monosomy 3 (HR 6.68, P < 0.001).37, 38
An alternative test, gene expression profiling (GEP), revealed 3 distinct classes of UM tumors including class 1A and class 1B with low risk and class 2 with high risk for metastasis.41 The GEP of class 1A and 1B tumors resembles normal uveal melanocytes and low-grade uveal melanocytic tumors with 2 and 21% 5-years metastatic risk respectively, whereas the GEP of class 2 tumors is associated with a 72% 5-year metastatic risk, has correlated with chromosome 3 alterations, and has shown reduced expression of melanocytic genes. It resembles genetic characteristic of primitive neural/ectodermal cells.41
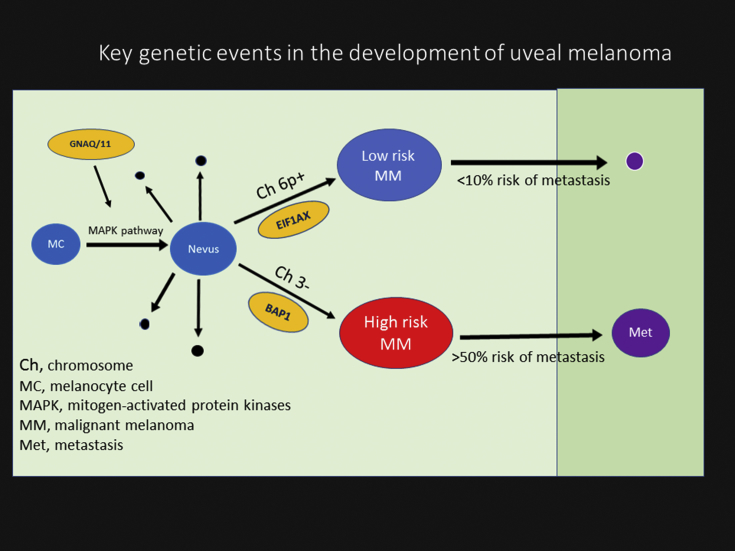
Several gene mutations in UM have been described, and these do not resemble other melanoma subtypes such as CM.12 The most commonly mutated genes are GNAQ, GNA11, EIF1AX, SF3B1, and BAP1.12, 36, 40 Activating mutations in GNAQ/GNA11 are the first described mutations in UM, and about 85% of all UMs carry a mutation in either of these genes.11, 12 These mutations have been found to up-regulate the mitogen-activated protein kinase (MAPK) pathway which is speculated to be the first event in malignant transformation of melanocytic cells.12 GNAQ/GNA11 mutations do not have known prognostic value, and they can be found in benign nevi and occur in similar frequencies in metastatic and non-metastatic tumors.11, 12 Mutations in other driver genes are likely to arise later in tumor development and have greater importance for patient outcome (Fig. 3).12, 36, 39 BAP1 mutation is associated with monosomy 3 or class 2 GEP tumors and impart poor survival.12, 14, 36 The frequency of somatic BAP1 mutation in primary UM has been estimated to be approximately 40% which closely resembles chromosome 3 status and strongly correlates with metastatic disease in UM.12, 21, 40 More than 80% of UM metastatic lesions demonstrate somatic mutations of BAP1. BAP1 is regarded as a key tumor-suppressor gene in monosomy 3-related UM.12 Mutations in this gene have not been detected in disomy 3 of UM,40 but other mutations including those in EIF1AX (48%) and/or SF3B1 (29%) genes have been identified.42 EIF1AX-mutated tumors show strong correlation with class 1 GEP tumors and improvement in patient survival, whereas SF3B1 mutated tumors seems to fall in between and are associated with late-onset metastatic disease.12
Fig. 3.
A schematic view of main molecular events in uveal melanoma (UM) progression. The earliest event is an activating mutation in GNAQ or GNA11, probably in a normal uveal melanocyte. This triggers inappropriate cell cycle through activation of the mitogen-activated protein kinase (MAPK) and perhaps other pathways. Usually the mutant cell clone does not progress into melanoma and is eliminated by apoptosis (black spheres). Rarely nevus will transform to low-risk or high-risk UM. Low-risk melanoma (blue sphere) demonstrates low risk for metastasis and often exhibits chromosome 6p gain and EIFA1X gene mutation and carries <10% risk for metastasis (small purple sphere). In contrast, melanomas classified as high risk (red sphere) often show chromosome 3 monosomy and mutation in BRCA1-associated protein-1 (BAP1) gene with >50% risk for metastasis (large purple sphere).
Familial uveal melanoma
UM is the most commonly intraocular tumor in adults. While this tumor is a relatively rare cancer in the general population, there is some evidence that supports the role of hereditary in familial form of UM.4 About 1% of all UM cases present to the form of familial.5, 6 FUM is defined as two or more family members with involvement with UM. Previous studies support the role of inheritance in formation of FUM.5, 6 Few genes have previously been implicated in predisposition for FUM, but BAP1 gene seems to have the strongest association.12, 14, 36 In the presence of BAP1 germline mutations, the risk for UM occurrence in carriers is estimated at up to 29%.24
The prevalence of germline BAP1 mutations rate among the unselected population of UM cases is about 2–3%.13, 43 It is predicted that germline BAP1 mutations are present in about 22% of FUM families overall.6 Turunen et al. found a frequency of 25% in cohort of eight families.44 Undoubtedly, family history of other cancers exerts significant effect on the likelihood of finding a BAP1 germline mutations. In families with FUM and no other history of BAP1-associated cancers, the chance for finding BAP1 germline mutations is as low as 8%. Conversely, with additional family history of CM, MMe, and RCC, the chance of BAP1 germline mutations can be approximately 50%.6
FUM families seems to have a higher cancer burden overall. For instance, the rate of second primary cancers in the probands is about 3-fold (31% vs 10%) higher than unselected UM.6 Rai et al. showed that FUM families without BAP1 mutation have lower rate of RCC and MMe compared to those with BAP1 mutations.6 Despite AD pattern inheritance of BAP1 gene mutations,14 FUM is indeed uncommon,4, 5 and it rarely involve more than 2–3 family members.4 The possible explanation for this reduced penetrance is probably due to two reasons: loss of the other copy of BAP1 is needed for the germline mutation to become manifested14 and BAP1 inactivation appears to be a relatively late event in UM progression and needs initial triggering events such as mutation in GNAQ/11.11, 12 While BAP1 is the most frequent known genetic cause of FUM, evidence shows less than 25% of families are positive for germline BAP1 mutations.6, 44 So it is likely that other genes exist, and future research on FUM families will probably identify novel genes and cancer predisposition syndrome.
Clinical characteristic of uveal melanoma in germline BAP1 mutations
Patients with germline BAP1 mutations and UM are implicated in this hereditary cancer syndrome (BAP1-TPDS). They exhibit more likelihood of family history of other cancers as well as UM. In comparison to unselected UM, there is increased frequency for family history of ocular melanoma (25% vs 1.9%).13 These patients tend to manifest tumors at a younger age (51 vs 62 years) and also exhibit larger tumor diameter (mean, 15.9 vs 12.3 mm) than those in the control group. Ciliary body involvement is more often noted in patients with BAP1 mutations (75% vs 21.6%). No difference is identified for some distances such as the distance between the tumor and optic disc or fovea, iris involvement, or extraocular extension.13 Metastatic disease developed more frequently in BAP1 germline mutations group compared to the control group (71% vs 18%). Generally, BAP1 germline mutation is associated with a 4-fold increased risk of metastasis and poor survival in patients.13 Affected cases usually show two clinical factors that correlate with poor prognosis including larger tumor diameter and higher rate of ciliary body involvement.37 Therefore, both somatic mutation of BAP1 in primary UM tumors and germline mutations increase the risk of metastasis and impart low survival rate.13, 40, 45, 46 In UM patients with germline BAP1 gene mutation, the mean survival is 4.74 years in comparison with 9.97 years in patients with normal BAP1 protein expression.46
Management and surveillance of the patients
Patients at high risk for harboring germline BAP1 mutations are offered BAP1 sequencing and genetic counseling. Physicians involved in the care of patients with UM should be aware of this cancer syndrome and the indications for BAP1 testing and genetic counseling. Genetic assessment and testing for BAP1 mutations should be taken into account for patients with two or more of primary tumors (UM, RCC, MMe, and CM) in themselves or first-degree relatives.24, 47 Of course, skin melanoma remains a bit overwhelming because of its relatively high prevalence in the general population.24
Germline BAP1 mutation test should be advised when a patient is diagnosed with UM at an early age (younger than 30 years) or one of the following is present in the patients or first relatives: (1) history of two UM cases or more in a family, (2) patients with UM and history of at least one other primary tumor (CM, RCC, and MMe) in themselves, (3) patients with UM and history of at least 2 other primary tumors in first- or second-degree relatives (there is controversy about exclusion of families with only multiple CM cases, given its frequency in the general population).24, 48 Also, germline BAP1 mutations should be suspected when a patient has multifocal UM.48 Although evidence-based management recommendations have not been established in these families, the cancer risk should not be overlooked.47 Once the germline BAP1 pathogenic variant has been identified in a family, all family members should be informed about the details of this syndrome.
Regular examinations, particularly for eye and skin tumors are necessary to facilitate early diagnosis and best prognosis.24 Yearly dilated eye examination and ophthalmic imaging by an ocular oncologist is suggested. The recommended age to begin screening is debatable. If a family member has been diagnosed at an early age for a specific cancer, screening for that cancer should begin for other members of that family approximately 5 years before the age of diagnosis.14, 24 In children, provider-based screening should be initiated around the time of puberty. Annual screening for MMe and RCC should be considered for carriers of BAP1 germline mutation.49, 50 Prenatal testing for BAP1-TPDS and preimplantation genetic diagnosis are also considered.47
If a UM is found in BAP1 mutation carriers, it should be managed as a high-risk tumor, and the ocular oncologist should monitor for systemic metastasis, including magnetic resonance imaging (MRI) of the abdomen and liver every 3–6 months and chest X-ray every 6–12 months.24, 47 Despite excellent local therapies for treating UM, there are no consistently effective neoadjuvant therapies for UM metastasis.47 The discovery of GNAQ/11 and BAP1 mutations in UM provides an opportunity for targeted therapy of metastatic disease. A recent publication on neoadjuvant sunitinib in high-risk patients revealed those <60 years old showed significant reduction in metastatic disease compared to those without neoadjuvant medication.51
Therapeutic targeting of BAP1 mutation poses a different challenge. First, the goal of therapy is to restore one or more functions of BAP1 that was lost following inactivation. This is technically more difficult than inhibiting an overactive oncogene. Second, it remains unclear which function of BAP1 is responsible for its anticancer role.36 With BAP1 protein deficiency, ubiquitinated histone 2A levels will increase in tumor cells. Histone deacetylase (HDAC) inhibitors such as valproic acid, trichostatin A, and suberoylanilide hydroxamic acid have been shown to reverse this condition in tumor cells with increased melanocytic differentiation and changing the cellular attributes to more differentiated low risk expression profile.14, 36
About 80% of UM have oncogenic mutations for GNAQ or GNA11 genes, and it appears to be early or perhaps an initiating event for malignant transformation because these mutations can be found in nevus as well and do not correlate with survival.11, 12 On the contrary, BAP1 inactivation mutation is a late event in tumor progression, beyond which metastasis and death await.12, 36 One strategy is to inhibit downstream signaling molecules that are activated by GNAQ/11 mutations. These include mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (MEK) that is shown to be upregulated in GNAQ/GNA11 mutated tumors.36, 47 A randomized phase 2 trial of Selumetinib, a selective MEK inhibitor, produced some mildly promising preliminary outcomes for UM.52
The ability of BAP1 gene mutation to cause multiple tumor types and high penetrance in carriers suggests that this gene has an important role for influencing cancer cell growth. This is the only high penetrance gene for hereditary UM identified so far. Clinician should be aware of the implications of germline BAP1 mutation and advise genetic testing and assessment for BAP1 germline mutation in suspected patients and families. Molecular prognostic testing also allows high-risk patients to be entered into clinical trials to assess the efficacy of adjuvant therapy, with the goal of delaying or preventing the outgrowth of micrometastatic disease. Improved prognostic and detection techniques in recent years have not been translated into improved outcomes for patients with UM. With progress in understanding the molecular landscape of UM and the development of treatments targeted to the pathways involving GNAQ/GNA11, BAP1, EIF1AX, SF3B1 mutations and epigenetic mechanisms, it is possible to improve the outcome of this malignant cancer. Greater understanding of the normal function of BAP1 in the melanocyte lineage and the adverse effects of BAP1 loss are needed to engineer agents that specifically target this mutation.
Footnotes
Support provided by Eye Tumor Research Foundation, Philadelphia, PA (CLS). No conflicting relationship exists for any author.
There is no conflict of interest for authors.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Shields C.L., Kels J.G., Shields J.A. Melanoma of the eye: revealing hidden secrets, one at a time. Clin Dermatol. 2015;33(2):183–196. doi: 10.1016/j.clindermatol.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Kaliki S., Shields C.L. Uveal melanoma: relatively rare but deadly cancer. Eye (Lond) 2017;31(2):241–257. doi: 10.1038/eye.2016.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shields C.L., Kaliki S., Furuta M., Mashayekhi A., Shields J.A. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8033 cases. Retina. 2012;32(7):1363–1372. doi: 10.1097/IAE.0b013e31824d09a8. [DOI] [PubMed] [Google Scholar]
- 4.Singh A.D., Shields C.L., De Potter P. Familial uveal melanoma. Clinical observations on 56 patients. Arch Ophthalmol. 1996;114(4):392–399. doi: 10.1001/archopht.1996.01100130388005. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Rahman M.H., Pilarski R., Ezzat S., Sexton J., Davidorf F.H. Cancer family history characterization in an unselected cohort of 121 patients with uveal melanoma. Fam Cancer. 2010;9(3):431–438. doi: 10.1007/s10689-010-9328-7. [DOI] [PubMed] [Google Scholar]
- 6.Rai K., Pilarski R., Boru G. Germline BAP1 alterations in familial uveal melanoma. Genes Chromosomes Cancer. 2017;56(2):168–174. doi: 10.1002/gcc.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henkind P., Roth M.S. Breast carcinoma and concurrent uveal melanoma. Am J Ophthalmol. 1971;71(1 Pt 2):198–203. doi: 10.1016/0002-9394(71)90390-4. [DOI] [PubMed] [Google Scholar]
- 8.Rednam K.R., Jampol L.M., Levine R., Goldberg M.F. Uveal melanoma in association with multiple malignancies. A case report and review. Retina. 1981;1(2):100–106. doi: 10.1097/00006982-198101020-00005. [DOI] [PubMed] [Google Scholar]
- 9.van Hees C.L., Jager M.J., Bleeker J.C., Kemme H., Bergman W. Occurrence of cutaneous and uveal melanoma in patients with uveal melanoma and their first-degree relatives. Melanoma Res. 1998;8(2):175–180. doi: 10.1097/00008390-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Diener-West M., Reynolds S.M., Agugliaro D.J., Collaborative Ocular Melanoma Study Group Second primary cancers after enrollment in the COMS trials for treatment of choroidal melanoma: COMS report no. 25. Arch Ophthalmol. 2005;123(5):601–604. doi: 10.1001/archopht.123.5.601. [DOI] [PubMed] [Google Scholar]
- 11.Herlyn M., Nathanson K.L. Taking the guesswork out of uveal melanoma. N Engl J Med. 2010;363(23):2256–2257. doi: 10.1056/NEJMe1010681. [DOI] [PubMed] [Google Scholar]
- 12.Helgadottir H., Höiom V. The genetics of uveal melanoma: current insights. Appl Clin Genet. 2016;9:147–155. doi: 10.2147/TACG.S69210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta M.P., Lane A.M., DeAngelis M.M. Clinical characteristics of uveal melanoma in patients with germline BAP1 mutations. JAMA Ophthalmol. 2015;133(8):881–887. doi: 10.1001/jamaophthalmol.2015.1119. [DOI] [PubMed] [Google Scholar]
- 14.Wang A., Papneja A., Hyrcza M., Al-Habeeb A., Ghazarian D. Gene of the month: BAP1. J Clin Pathol. 2016;69(9):750–753. doi: 10.1136/jclinpath-2016-203866. [DOI] [PubMed] [Google Scholar]
- 15.Yu H., Mashtalir N., Daou S. The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Mol Cell Biol. 2010;30(21):5071–5085. doi: 10.1128/MCB.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bononi A., Giorgi C., Patergnani S. BAP1 regulates IP3R3-mediated Ca2+ flux to mitochondria suppressing cell transformation. Nature. 2017; 22;546(7659):549–553. doi: 10.1038/nature22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen D.E., Proctor M., Marquis S.T. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 ring finger and enhances brca1-mediated cell growth suppression. Oncogene. 1998;16(9):1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 18.Carbone M., Emri S., Dogan A.U. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer. 2007;7(2):147–154. doi: 10.1038/nrc2068. [DOI] [PubMed] [Google Scholar]
- 19.Roushdy-Hammady I., Siegel J., Emri S., Testa J.R., Carbone M. Genetic-susceptibility factor and malignant mesothelioma in the cappadocian region of Turkey. Lancet. 2001;357(9254):444–445. doi: 10.1016/S0140-6736(00)04013-7. [DOI] [PubMed] [Google Scholar]
- 20.Testa J.R., Cheung M., Pei J. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43(10):1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harbour J.W., Onken M.D., Roberson E.D. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330(6009):1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiesner T., Obenauf A.C., Murali R. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43(10):1018–1021. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peña-Llopis S., Vega-Rubín-de-Celis S., Liao A. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44(7):751–759. doi: 10.1038/ng.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rai K., Pilarski R., Cebulla C.M., Abdel-Rahman M.H. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin Genet. 2016;89(3):285–294. doi: 10.1111/cge.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carbone M., Ferris L.K., Baumann F. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med. 2012;10:179. doi: 10.1186/1479-5876-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Shea S.J., Robles-Espinoza C.D., McLellan L. A population-based analysis of germline BAP1 mutations in melanoma. Hum Mol Genet. 2017;26(4):717–728. doi: 10.1093/hmg/ddw403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carbone M., Flores E.G., Emi M. Combined genetic and genealogic studies uncover a large BAP1 cancer syndrome kindred tracing back nine generations to a common ancestor from the 1700s. PLoS Genet. 2015;11(12) doi: 10.1371/journal.pgen.1005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Njauw C.N., Kim I., Piris A. Germline BAP1 inactivation is preferentially associated with metastatic ocular melanoma and cutaneous-ocular melanoma families. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farzin M., Toon C.W., Clarkson A. Loss of expression of BAP1 predicts longer survival in mesothelioma. Pathology. 2015;47(4):302–307. doi: 10.1097/PAT.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 30.Ohar J.A., Cheung M., Talarchek J. Germline BAP1 mutational landscape of asbestos-exposed malignant mesothelioma patients with family history of cancer. Cancer Res. 2016;76(2):206–215. doi: 10.1158/0008-5472.CAN-15-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar R., Taylor M., Miao B. BAP1 has a survival role in cutaneous melanoma. J Investig Dermatol. 2015;135(4):1089–1097. doi: 10.1038/jid.2014.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hakimi A.A., Ostrovnaya I., Reva B. Adverse outcomes in clear cell renal cell carcinoma with mutations of 3p21 epigenetic regulators BAP1 and SETD2: a report by MSKCC and the KIRC TCGA research network. Clin Cancer Res. 2013;19(12):3259–3267. doi: 10.1158/1078-0432.CCR-12-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murali R., Wiesner T., Scolyer R.A. Tumors associated with BAP1 mutations. Pathology. 2013;45(2):116–126. doi: 10.1097/PAT.0b013e32835d0efb. [DOI] [PubMed] [Google Scholar]
- 34.Battaglia A. The importance of multidisciplinary approach in early detection of BAP1 tumor predisposition syndrome: clinical management and risk assessment. Clin Med Insights Oncol. 2014;8:37–47. doi: 10.4137/CMO.S15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carbone M., Yang H., Pass H.I., Krausz T., Testa J.R., Gaudino G. BAP1 and cancer. Nat Rev Cancer. 2013;13(3):153–159. doi: 10.1038/nrc3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harbour J.W. The genetics of uveal melanoma: an emerging framework for targeted therapy. Pigment Cell Melanoma Res. 2012;25(2):171–181. doi: 10.1111/j.1755-148X.2012.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shields C.L., Say E.A.T., Hasanreisoglu M. Personalized uveal melanoma prognosis based on cytogenetic profile in 1059 cases over an 8-year period. The 2017 Harry S. Gradle lecture. Ophthalmology. 2017;124(10):1523–1531. doi: 10.1016/j.ophtha.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Shields C.L., Say E.A.T., Hasanreisoglu M. Cytogenetic abnormalities in uveal melanoma based on tumor features and size in 1059 patients: the 2016 W. Richard Green lecture. Ophthalmology. 2017;124(5):609–618. doi: 10.1016/j.ophtha.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 39.Chattopadhyay C., Kim D.W., Gombos D.S. Uveal melanoma: from diagnosis to treatment and the science in between. Cancer. 2016;122(15):2299–2312. doi: 10.1002/cncr.29727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van de Nes J.A., Nelles J., Kreis S. Comparing the prognostic value of BAP1 mutation pattern, chromosome 3 status, and BAP1 immunohistochemistry in uveal melanoma. Am J Surg Pathol. 2016;40(6):796–805. doi: 10.1097/PAS.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 41.Field M.G., Harbour J.W. Recent developments in prognostic and predictive testing in uveal melanoma. Curr Opin Ophthalmol. 2014;25(3):234–239. doi: 10.1097/ICU.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin M., Masshofer L., Temming P. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat Genet. 2013;45(8):933–936. doi: 10.1038/ng.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aoude L.G., Vajdic C.M., Kricker A., Armstrong B., Hayward N.K. Prevalence of germline BAP1 mutation in a population-based sample of uveal melanoma cases. Pigment Cell Melanoma Res. 2013;26(2):278–279. doi: 10.1111/pcmr.12046. [DOI] [PubMed] [Google Scholar]
- 44.Turunen J.A., Markkinen S., Wilska R. BAP1 germline mutations in Finnish patients with uveal melanoma. Ophthalmology. 2016;123(5):1112–1117. doi: 10.1016/j.ophtha.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Griewank K.G., van de Nes J., Schilling B. Genetic and clinico-pathologic analysis of metastatic uveal melanoma. Mod Pathol. 2014;27(2):175–183. doi: 10.1038/modpathol.2013.138. [DOI] [PubMed] [Google Scholar]
- 46.Kalirai H., Dodson A., Faqir S., Damato B.E., Coupland S.E. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing. Br J Cancer. 2014;111(7):1373–1380. doi: 10.1038/bjc.2014.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harbour J.W., Chao D.L. A molecular revolution in uveal melanoma: implications for patient care and targeted therapy. Ophthalmology. 2014;121(6):1281–1288. doi: 10.1016/j.ophtha.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao R., Pointdujour-Lim R., Ganguly A., Shields C.L. Multifocal choroidal melanoma in a patient with germ line BRCA1-associated protein 1 mutation. Retin Cases Brief Rep. 2018;12(1):1–4. doi: 10.1097/ICB.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt L.S., Linehan W.M. Genetic predisposition to kidney cancer. Semin Oncol. 2016;43(5):566–574. doi: 10.1053/j.seminoncol.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Righi L., Duregon E., Vatrano S. BRCA1-associated protein 1 (BAP1) immunohistochemical expression as a diagnostic tool in malignant pleural mesothelioma classification: a large retrospective study. J Thorac Oncol. 2016;11(11):2006–2017. doi: 10.1016/j.jtho.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 51.Valsecchi M.E., Orloff M., Sato R. Adjuvant sunitinib in high-risk patients with uveal melanoma: comparison with institutional controls. Ophthalmology. 2018;125(2):210–217. doi: 10.1016/j.ophtha.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Carvajal R.D., Sosman J.A., Quevedo J.F. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA. 2014;311(23):2397–2405. doi: 10.1001/jama.2014.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]