Abstract
Purpose
Keratoconus is a progressive disease of the cornea which can lead to blindness as irregular astigmatism increases. Currently, a variety of methods are available for the treatment of keratoconus, and in certain cases, it may be difficult to choose the most appropriate option. This article reviews available treatment modalities for keratoconus to provide the practitioner with practical and useful information for selecting the most suitable option for each individual patient.
Methods
To review treatment methods for different stages of keratoconus, PubMed (United States National Library of Medicine) and Scopus (Elsevier BV) databases were searched using the keywords “keratoconus”, “contact lens”, “cross-linking”, “Intacs”, “keratoplasty”, “gene therapy”, and “irregular astigmatism”, and related articles were reviewed based on disease assessment parameters and treatment methods.
Results
Various methods are available for the treatment of keratoconus: eyeglasses and contact lenses in the early stages, cross-linking for stabilizing disease progression, intrastromal corneal ring segments (ICRS) for reducing refractive errors or flattening the cornea, and penetrating keratoplasty (PK) and deep anterior lamellar keratoplasty (DALK), conductive keratoplasty, gene therapy and more recently, bowman layer transplantation (BL transplantation) in advanced stages of the disease. To achieve optimum results, it is essential to choose the best option for each individual patient.
Conclusions
A commonality of the reviewed papers was the advancement of novel diagnostic and treatment methods in ophthalmology, which can delay the need for corneal grafting. A better understanding of keratoconus treatment options can help enhance visual rehabilitation and prevent blindness in keratoconus patients.
Keywords: Keratoconus, Contact lens, Cross-linking, Intacs, Keratoplasty, Keraflex, Gene therapy, Bowman layer transplantation
Introduction
Keratoconus is a bilateral progressive non-inflammatory disease which can present as corneal stromal thinning in either gender.1, 2 Reported prevalence rates of the disease range from 20 in 100,0003, 4 to 1 in 500,000.5 They vary in relation to environmental, genetic, and ethnic factors, and prevalence rates are different in different races.6
According to a study in the UK, the prevalence of keratoconus is 4.4–7.5 times higher in Asian populations compared to white populations.7, 8 Epidemiologic studies in Iran have reported rates between 0.75% and 3.5%, and they varied by the studied population in different areas and the imaging system used for this purpose.9, 10, 11 Keratoconus was first described by John Nottingham more than 150 years ago, but in the past decades, our understanding of the disease and its treatment options have dramatically changed.11 As technology enhances, corneal specialists are devising novel techniques for the effective treatment of keratoconus.
Multiple genes are involved in the development of keratoconus with a relatively high prevalence of positive family history.12, 13, 14 Although both genders are affected, men seem to be more commonly involved.8, 15, 16, 17 The etiology of keratoconus is still unknown. However, we know the disease is multifactorial and influenced by genetic, environmental, and biochemical factors.16, 18 The onset of symptoms can be during adolescence and young adulthood, and it may manifest as reduced vision, corneal astigmatism, increasing higher order aberrations, and fluctuating vision.7, 12
Subjects with genetic predisposition are also affected by environmental factors.19 In most cases, the disease is unilateral, although in one study it was stated that after 16 years, 50% of cases show signs in the fellow eye as well.20
In advanced stages of the disease, there is severe vision reduction due to high myopia, irregular astigmatism, and corneal scarring.18, 21 In 12–20% of cases, the disease may lead to corneal transplantation.2, 22, 23, 24
Various grading systems are available for grading keratoconus. The most important ones include the Amsler-Krumeich classification system which classifies cases based on the amount of myopia and astigmatism, corneal thickness or scarring, and central k-readings.25 Other systems include the Shabayek-Alió grading system which considers corneal higher order aberrations26 and the keratoconus severity score (KSS) system which classifies cases based on average corneal power and root mean square (RMS).27 Depending on the grading system, various treatment nomograms have been proposed; however, despite extensive studies on the management of keratoconus, there is still no standard protocol for the treatment of patients with different degrees of keratoconus. Nonetheless, the main goal of novel approaches introduced in recent years is to improve vision and prevent vision loss at advanced stages of the disease. This article will review various modalities available for the treatment of keratoconus with special focus on evidence-based and clinical applications to provide the practitioner with practical and useful information for selecting the most suitable option for each individual patient.
Methods
The literature review for this study was based on a search in PubMed (United States National Library of Medicine) and Scopus (Elsevier BV) databases using the following keywords: “keratoconus” and “contact lens” or “cross-linking” or “Intacs” or “keratoplasty” or “gene therapy” or “irregular astigmatism” for a time frame between 2003 and 2017. Retrieved articles were first reviewed by title and abstract, and then the full texts of relevant articles were examined and reviewed regarding parameters related to the diagnosis and treatment of the disease.
Spectacles and contact lenses
The management of keratoconus depends on the disease progression and its stage. Spectacles can provide acceptable vision for patients in very early stages, and they are especially appropriate for those who achieve 20/40 or better visual acuity. However, spectacles cannot correct irregular astigmatism, and in such cases, hard contact lenses can provide better vision for the patient.28 Current advances in contact lens design offer various fitting options for the correction of irregular astigmatism in keratoconus patients. In addition, novel imaging technologies such as corneal topography and anterior segment optical coherence tomography (OCT) can be helpful in fitting modern lenses.29 Different lenses can be fit depending on the type, location, and the size of the cone.30 Such developments in ophthalmology support improved comfort for contact lens wearers while corneal health is maintained.
Contact lenses can provide acceptable vision for most keratoconus patients, and a variety of lenses can be suggested depending on disease progression.31 In early stages, a Toric soft lens may be sufficient for correcting myopia and regular astigmatism. However, as the diseases progresses, such lenses are no longer capable of correcting the refractive error, and there is need for special lenses such as Rose K, hybrid lenses, piggy back, or scleral lenses.32
Studies indicate that various RGP lenses do not differ in terms of visual acuity results, but certain lenses such as Rose K can be more comfortable to the patient to wear over long hours and are more tolerable.32, 33 The new generation of lenses can be used for cases who cannot tolerate RGP lenses or lack proper centration due to the advanced stage of the disease.34, 35 These lenses offer patients more stable vision, but although they support better corneal oxygenation, the risk of hypoxia still exists, and the technique for placing and removing them can be difficult to handle.36, 37
Choosing the appropriate type of lens and proper fitting can help avoid the need for corneal transplantation in severe cases of keratoconus. Even after corneal grafting, patients may need special contact lenses to correct residual astigmatism. Smiddy et al. have shown that up to 70% of patients can successfully use contact lenses after corneal grafting.28 Patients may need to use contact lenses after cross-linking or ring implantation, and the fitting pattern can be different from that in untreated eyes.32
Corneal collagen cross-linking
Corneal collagen cross-linking (CXL) is a novel invasive method for modifying the stromal structure of the cornea which has recently been approved by the Food and Drug Administration (FDA) for the management of advanced cases of keratoconus. This method relies on the interaction between UVA at a wavelength of 370 nm and topical riboflavin (vitamin B) for 30 min. The main effect of CXL is that it prevents disease progression through the formation of chemical bonds among collagen fibrils. As the pioneers of the procedure, Wollensak et al. examined the cornea after CXL using a microcomputer-controlled biomaterial tester and observed 328.9% increased corneal rigidity.38 Various studies have concluded that this treatment approach can reduce corneal ectasia.8, 39, 40, 41 It is recommended for younger patients with high risk of progression as well as those who have a clear cornea with a minimum thickness of 400 microns (μm).42 Some of these studies have had 3 or more years of follow-up times,41, 43, 44, 45, 46, 47, 48, 49, 50 and they confirm the safety and efficacy of this method (Table 1).
Table 1.
Summary of studies using cross-linking alone or combined with other techniques for the treatment of keratoconus.
| Authors | Country | Design (eyes) | Follow-up (m) | Results |
|---|---|---|---|---|
| Caporossi et al (2006)41 | Italy | Case series (10) | 3 | Kmax decreased by 1.9 D; BCVA improved 1.66 lines. |
| Raiskup-wolf et al (2008)43 | Germany | Retrospective case series (241) | 6–72 | Kmax decreased by 2.57 D; BCVA improved to 58%. |
| Grewal et al. (2009)44 | India | Uncontrolled, prospective trial (102) | 12 | Stable BCVA, spherical equivalent and corneal curvature. |
| Coskunseven et al. (2009)45 | Turkey | Controlled (fellow eye) non-randomized (19/19) | 5–12 | Kmax decreased by 1.57 D; BCVA improved by 0.10. |
| Kanellopoulos (2009)46 | Greece | Prospective study (325) | 24–68 | The mean UCVA and BCVA improved in both groups, and mean reduction in spherical equivalent refraction and K were 2.50 ± 1.20 D and 2.75 ± 1.30 D, respectively, in PRK group. Mean reduction in spherical equivalent refraction and K were 3.20 ± 1.40 D and 3.50 ± 1.3 D respectively, in simultaneous group. UCVA and BCVA, a greater mean reduction in spherical equivalent refraction and keratometry, and less corneal haze were seen in PRK plus CXL group. |
| Richoz et al. (2013)47 | Switzerland | Retrospective, interventional cases series (66) | 12–62 | BCVA improved 1 line or more in 19 cases; mean K (max) reduced 2 D after CXL and keratoconus index reduced; the R (min) after CXL increased (P < 0.001). |
| Witting-Silva et al. (2014)48 | Australia | Prospective, randomized controlled trial (100) | 3, 6, 12, 24, and 36 | Kmax flattened by −0.72 ± 0.15 D, −0.96 ± 0.16 D, and −1.03 ± 0.19 D at 12, 24, and 36 months, respectively both UCVA and BCVA improved at 36 months |
| Kymionis GD et al. (2014)49 | Greece | Prospective case series (23) | 24–56 | The mean UCVA improved from 0.99 ± 0.57 logMAR preoperatively to 0.61 ± 0.36 logMAR at the last follow-up. The mean BCVA, from 0.27 ± 0.24 logMAR to 0.17 ± 0.14 logMAR; The mean steep and mean flat keratometry decreased from 53.39 ± 7.14 D and 47.17 ± 4.87 D. |
| Kontadakis GA et al. (2016)50 | Greece | Prospective, comparative interventional case series (60) | 36 | BCVA was 0.09 ± 0.10 logMAR in the tPRK-CXL group and 0.15 ± 0.12 logMAR in the CXL group. UCVA was 0.27 ± 0.25 logMAR in the tPRK-CXL group and 0.69 ± 0.58 logMAR in the CXL group. Improved in 2 lines. |
BCVA: Best corrected visual acuity, UCVA: Uncorrected visual acuity, Kmax: Maximum curvature, D: Diopter, logMAR: The Logarithm of the minimum angle of resolution, CXL: Corneal cross-linking, tPRK: Transepithelial photorefractive keratectomy.
The “Dresden Protocol” is the common protocol for standard CXL which has been found suitable for corneas that exhibit a minimum thickness of 400 μm.44, 51 However, as keratoconus progresses, corneal thickness tends to reduce to below 400 μm, and in these cases, the standard approach may lead to endothelial cell count loss; therefore, a hypo-osmolar riboflavin solution is recommended for thin corneas.45 Two other approaches for CXL are the epithelium-off and epithelium-on methods. The former approach is based on the Dresden protocol and can lead to corneal stromal opacity, inflammation, and pain.48, 50 The epithelium-on approach is a modified technique which is completed without epithelial debridement. Studies have reported inconsistent results with this method, and it should be avoided in cases with dry eye or very thin corneas that have reduced epithelial thickness.52, 53, 54
Since the conventional cross-linking protocol is time consuming, today the alternative method or the high-fluence accelerated CXL is used instead. With this method, the UV irradiation time is reduced by increasing the intensity of the irradiance.55 Several protocols are available, and the goal is to reduce the treatment time and minimize patient discomfort. Studies have shown that Kmean and Kmax are reduced with this method, the cornea achieves stabilization,55, 56 and similar to the standard method, disease progression is slowed or halted.55, 56, 57, 58, 59 Complications reported in association with cross-linking include haze,60 corneal infection,61 corneal edema,62 and rarely corneal melting.63 Nonetheless, success rates with this method are quite high.64, 65
CXL is performed only or in combination with photorefractive keratectomy (PRK), laser assisted in situ keratomileusis (LASIK), thermal keratoplasty, Intacs, and orthokeratology for better improvement of visual acuity (Table 1).49, 50, 66, 67, 68, 69, 70 McQuaid et al. conducted a 6-year follow-up study and observed that the achieved corneal flattening was more than 2.0 diopters (D) in 70% of the cases and visual acuity had improved. The authors also state that PRK alone can only reduce the corneal power by 10%, but its efficiency can be increased by 70% if done in conjunction with CXL.71 Further studies are needed to determine the efficacy and long-term stability of CXL and its potential issues in combined approaches.
Intrastromal corneal ring segment
Intrastromal corneal ring segments (ICRS) are made of polymethyl methacrylate (PMMA) and are implanted deep in the stroma to reduce the corneal curvature. These rings were first assessed for the treatment of myopia72, 73; ICRS manage myopia and keratoconus with different mechanisms. In myopia, they flatten the cornea which improves vision by refocusing light rays, while in keratoconus, ICRS reduce corneal distortion by flattening the steep area of the cornea and reshaping it.74 Burris et al. reported favorable outcomes in the treatment of keratoconus and stabilized cases of post-refractive surgery ectasia.74
Patient selection criteria for Intacs implantation include severe reduction of visual acuity, lack of functional vision with spectacle or lens correction, minimum age of 21 years, clear central corneas, minimum corneal thickness of 400 μm, and when penetrating keratoplasty (PK) is the only remaining option for regaining visual function.75 Visual recovery can take 3–12 months after surgery, and the 10-year follow-up study by Torquetti et al. indicated that visual acuity improves and the patient achieves stable visual results.76 In general, keratoconus patients in the moderate and severe stage of the disease who have no corneal scarring and cannot tolerate contact lenses are the best candidates for intracorneal ring segments. Rings are contraindicated for cases who have a Kmax>70 D, central corneal scarring, corneal opacity, or hydropsis.77, 78 Surgical success and vision improvement depend on a number of factors such as proper ring placement, accurate implantation depth, and the diameter of the optical zone. Improper positioning of the ring can lead to overcorrection or undercorrection.78 In a study by Hashemi et al. on 12 patients with post-LASIK ectasia, best corneal flattening effects were achieved when the ring was implanted at a depth equal to 60–80% of the corneal thickness; rings implanted deeper in the cornea may have no effect on improving visual acuity.79
Four types of ICRS are available for keratoconus: 1) Intacs (Addition technology Inc.) 2) Intacs SK (Addition technology Inc.), 3) Ferrara Rings (Ferrara ophthalmics) and 4) Keraring (Mediphacos).
Intacs and Ferrara rings are the more popular types of commercially available ICRS for the management of keratoconus, Intacs is available in 150 arc length segments with a hexagonal cross-section and various sizes ranging from 0.210 to 0.450 mm which are chosen based on the amount and type of refractive errors. These are implanted in the 7–8 mm corneal zone and can correct −8.00 D to +10.00 D.75 Intacs-SK (severe keratoconus) are used for high amounts of refractive errors and are implanted in the 6–7 mm zone. The cross-section of the Ferrara ring is triangular, and its internal and external diameters are 4.4 and 5.6 mm, respectively (Table 2).80 In a previous study, we compared the conventional single segment and SK rings in patients with inferior keratoconus. In both groups, uncorrected and corrected visual acuity (UCVA, BCVA) improved and keratometry reduced, and we concluded that both methods are effective for the treatment of keratoconus.81 Also, in another study, we studied visual outcomes after MyoRing (Dioptex, GmbH, Linz, Austria) implantation to compare 250 and 300 μm depths. Results were comparable in the two groups, and this method can be applied in keratoconus patients with thinner corneas.82
Table 2.
The characteristics of the most popular intracorneal ring segment implants.
| Characteristics | INTACS | Ferrara Ring | Keraring |
|---|---|---|---|
| Arc, length/degree | 150 | 160 | 90,120,160,210,240 |
| Cross section | Hexagonal | Triangular | Triangular |
| Thickness/mm | 0.25–0.45 (0.05 increments) | 0.20–0.35 (0.05 increments) | 0.15–0.30 (0.05 increments) |
| Internal diameter/mm | 6.77 | 4.40 | 5.0 |
| External diameter/mm | 8.10 | 5.60 | 6.0 |
For Intacs insertion, the canal can be created mechanically or with laser femtosecond at a depth of 70–75% of the minimum pachymetry at the insertion site.81, 82 Some studies have reported good visual results using the mechanical method,40, 81 but the mechanical technique can be associated with epithelial defects, shallow placement of the segments, stromal thinning, and corneal stromal edema, while the risk of such complications is reduced when femtosecond laser is used.83 In the study by Piñero et al., higher order aberrations, spherical aberrations, and coma were assessed and compared between these two methods, and a significant difference was observed between mechanical and femtosecond techniques.84
Today, Intacs is used in combination with other treatment options such as cross-linking for stabilizing disease progression. In this regard, combining Intacs with riboflavin increases its flattening effect.85 Combining with CXL is recommended for patients in the moderate to severe stages of the disease who have a minimum corneal thickness of 450 μm.66, 86, 87 Studies have shown that irregular astigmatism is reduced, and visual acuity can improve by 60% with a decrease in the corneal curvature.88, 89 Intacs must be implanted before or simultaneously with CXL, and the reverse, i.e. first CXL and then ring implantation because the corneal structure is changed, and it can have little effect in flattening the cornea.90, 91, 92 Further investigations seem to be necessary to study long-term effects of such combined procedures.
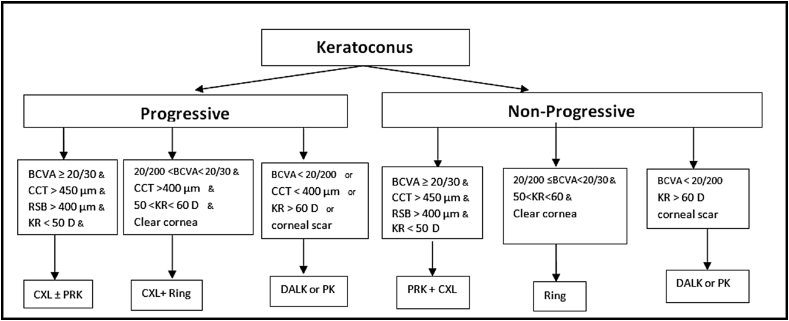
There are a variety of nomograms for the treatment of keratoconus which are mainly based on the grade of keratoconus, risk factors, the progressive nature of the disease, and contact lens tolerance or intolerance.42, 93, 94, 95 Nonetheless, based on their previous experiences, the authors of this article suggest the simpler and more practical approach, presented in Fig. 1, to select the most appropriate management that would help improve visual acuity in keratoconus patients.
Fig. 1.
Surgical algorithm for management of keratoconus. *Age<25 years should be considered as progressive keratoconus. ** Rigid gas permeable (RGP) lenses should be considered in any case if tolerant. *** Photorefractive keratectomy (PRK) is only recommended for age>25 years and in conjunction with corneal cross-linking (CXL). BCVA: Best corrected visual acuity, CCT: Central corneal thickness, RSB: Residual stromal bed, KR: Keratometry reading, D: Diopter, CXL: Cross-linking, PRK: Photorefractive keratectomy, DALK: Deep anterior lamellar keratoplasty, PK: Penetrating keratoplasty.
Based on various studies mentioned in previous sections, most study the efficacy and stability of CXL, either alone or in combination with other treatment modalities.96
Combined MyoRing and corneal cross-linking as a novel technique
Intracorneal continuous rings (ICCR) were first used in myopes by Binder in 1980 with no clinical results.97 As technology advanced, easier and safer methods were introduced for canal creation and ring insertion. A new design of this type is the Myoring (Dioptex, GmbH) which is suitable for the treatment of keratoconus, myopia, and post-LASIK ectasia.52, 53, 54 It is still unclear whether MyoRing for non-central keratoconus works as well as with central keratoconus, but several studies have shown that MyoRing is effective in the treatment of irregular astigmatism.98, 99, 100, 101
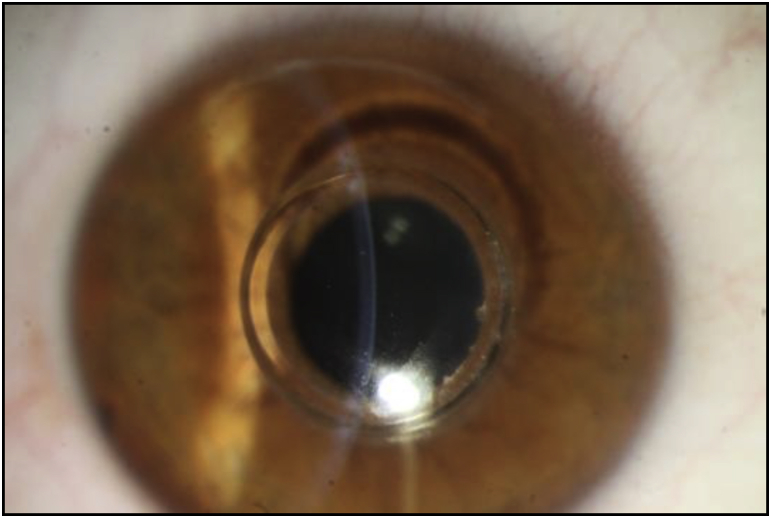
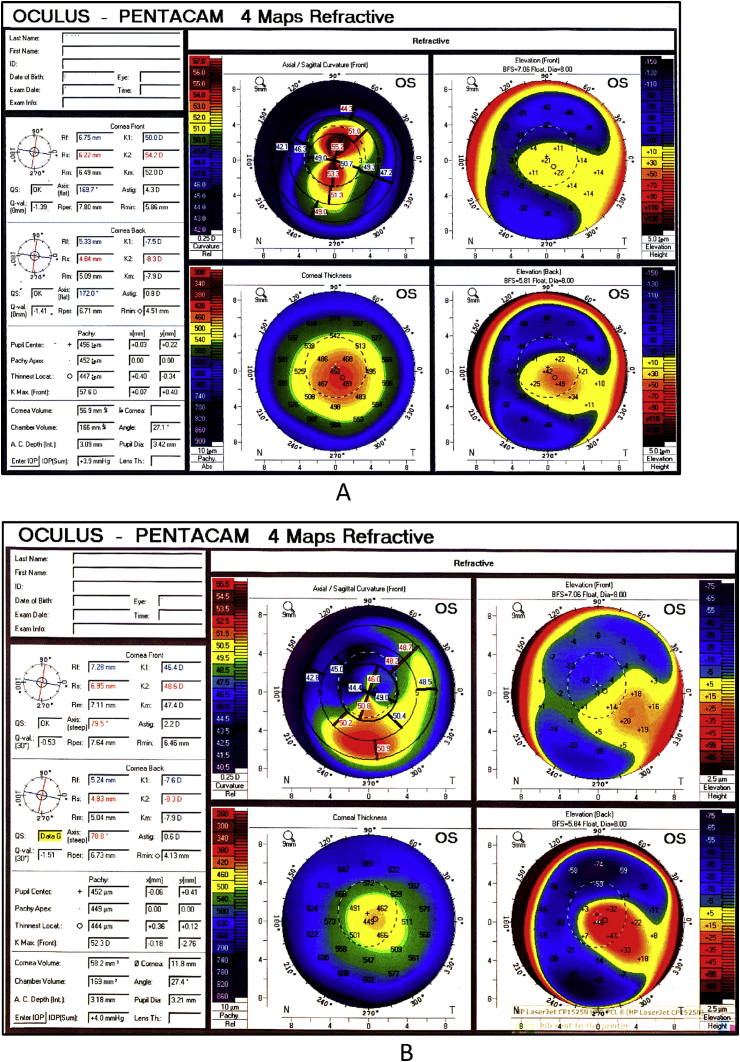
In a new approach, we combined MyoRing with accelerated CXL using Dextran-free riboflavin in patients with advanced keratoconus. After creating a corneal pocket with femtosecond laser, riboflavin was injected into it. At two years after the procedure, the central keratometry was significantly reduced. We believe that MyoRing conducted with femtosecond laser combined with accelerated CXL is safe and effective in the treatment of keratoconus and improves visual acuity (Fig. 2, Fig. 3).102
Fig. 2.
Slit-lamp image of a keratoconus eye implanted with MyoRing.
Fig. 3.
MyoRing combined with cross-linking done in a keratoconus patient. A) Preoperative: −6.00/-5.50–170, Visual acuity: 20/50; B) 12 month postoperative: −1.00/-3.00–165, Visual acuity: 20/30.
Although intracorneal ring segments have shown good results in terms of improved visual acuity in keratoconus patients, certain complications can be expected during or after surgery. Intraoperative complications are rare, and they are usually related to creating the corneal tunnel, insufficient tunnel depth, asymmetry or decentration, or bowman's layer perforation.80, 103, 104 Corneal neovascularization, keratitis, deposits around ring segment, corneal haze, halos, pain, corneal melting or edema, segment extrusion, visual fluctuation, and photophobia are rare postoperative complications.88, 104, 105, 106
The reported rate of postoperative complications ranges between 0.5% and 30%.107, 108 Creating the tunnel with femtosecond laser instead of manual dissection can reduce the rate of complications. Kanellopoulos et al. who studied Intacs implantation in 20 cases of moderate and severe keratoconus reported segment migration in 6 eyes and corneal neovascularization in one case at 6 months after the procedure.107 Ibrahim et al. reported one case of neovascularization at 18 months after surgery.108 Keratitis is a rare infection which has been reported in less than 2% of cases.109, 110, 111
Phakic intraocular lenses
Phakic intraocular lenses (PIOLs) are recommended for cases with high irregular astigmatism such as pellucid marginal degeneration, and keratoconus who are at least 21 years of age do not achieve sufficient correction with spectacles or contact lenses, and are not suitable candidates for refractive surgery or excimer laser procedures.112, 113 The lenses may be designed for implantation in the anterior or posterior chamber, and different types include the anterior chamber angle-fixated PIOL (Alcon AcrySof Cachet Phakic IOL), anterior chamber iris-fixated PIOL (Artisan/Verisyse™ and Artiflex/Veriflex™ PIOL), and posterior chamber PIOL [Visian Implantable Collamer Lens (ICL) STAAR Surgical; Epi.Lens, Acri.Tec (Carl Zeiss Meditec, Jena, Germany)].
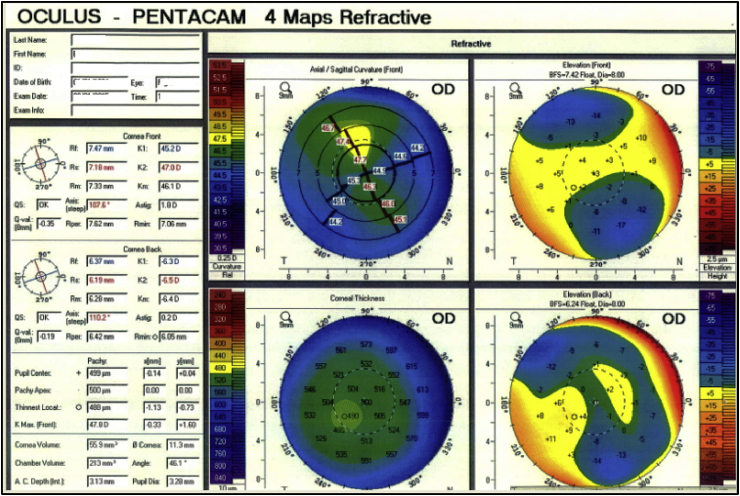
Toric PIOLs, which are specially designed for cases of astigmatism, are recommended for patients with progressive keratoconus, and the procedure can be combined and performed simultaneously with CXL or ICRS.114 A summary of various reports recommending the use of PIOLs.113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126 Based on these studies, PIOLs can be useful in reducing myopia and irregular astigmatism in different stages of keratoconus, especially in grades I and II of the disease and in cases of advanced keratoconus to correct the residual refractive error after deep anterior lamellar keratoplasty (DALK), and one-year visual outcomes in terms of UCVA and BCVA have been acceptable with no severe intraoperative or postoperative complications (Table 3).114, 115, 127, 128, 129, 130, 131, 132 Fig. 4 demonstrates a patient with high astigmatism and myopia with thin cornea for which we used PIOLs. For patients who have a cornea too thin for LASIK, PIOL implantation can be very effective. However, the use of intraocular lenses in patients with keratoconus is still under investigation, and further studies with more thorough follow-ups are necessary.
Table 3.
Different results of phakic intraocular lenses (PIOL) studies.
| Authors | Country | Design (eyes) | Follow-up(m) | Results |
|---|---|---|---|---|
| Kamiya K et al. (2008)114 | Japan | - (4) | – | Preoperatively, manifest refraction was −10.00–6.00 × 100 in case 1 and -8.00–2.75 × 100 in case 2. Postoperatively, the manifest refraction was +0.50–1.00 × 90 in case 1 and -0.25–1.25 × 100 in case 2. |
| Venter J. (2009)115 | UK | Retrospective study (18) | 12 | The implantation of Artisan in patients with keratoconus and myopia, 94% of eyes had a UCVA of 0.63 or better and 72% of eyes gained one or more lines of BCVA. |
| Güell JL et al. (2008)127 | Spain | Case series (399) Four groups |
1,3,60 | Group 4 had a mean preoperative cylinder of −3.24 ± 1.02 D, which decreased to −0.83 ± 0.74 D postoperatively. Implantation of iris-claw PIOLs is an effective procedure. |
| Al-Dreihi MG et al. (2013)128 | Syria | Case report | 12 | Artisan iris-fixated Toric phakic intraocular lens implanted for the correction of high astigmatism following deep anterior lamellar keratoplasty in a 23-year-old woman. UCVA and BCVA were 20/20 after one-year follow-up and refractive cylinder was −1.00 D and endothelial cell count was 1827 cells/mm2. |
| Alfonso JF et al. (2014)129 | Spain | Case series (35) | 12 | Mean sphere was −5.46 ± 4.17 (SD) and mean cylinder was −3.14 ± 1.19 D. Postoperatively, the mean decimal UCVA was 0.89 ± 0.16 and 43% of eyes gained 1 or more lines. More than 50% of eyes had a Snellen UCVA of 20/20. |
| Venter J (2015)130 | UK | Case series (103) Two groups |
60 | The mean BCVA improved from 0.51 ± 0.15 logMAR to 0.34 ± 0.16 logMAR (P < 0.001) in Group 1 and from 0.54 ± 0.17 logMAR to 0.46 ± 0.14 logMAR in Group 2 (P < 0.005). The iris-fixated PIOL was effective option for improving visual acuity in amblyopic eyes. |
| Qin Q et al. (2017)131 | China | Case series (9) | 18 | UCVA and BCVA improved after 6 month after TICL implantation following DALK surgery. The deviation of TICL axis was less than 10° and no severe complication after surgery was found. |
| Tiveron MC Jr et al. (2017)132 | Brazil | Retrospective case series (24) | 12 | UCVA was better in 88% of eyes after Toric Iris Claw phakic intraocular lens implantation following DALK surgery 92% of eyes had spherical equivalent ≤±1.00D with efficacy and safety 0.93 and 1.00, respectively after 12 month. Mean endothelial cell loss was 6.10% and no severe complication was found after surgery. |
BCVA: Best corrected visual acuity, UCVA: Uncorrected visual acuity, D: Diopter, logMAR: The logarithm of the minimum angle of resolution, PIOL: Phakic intraocular lens, TICL: Toric implantable collamer lens, DALK: Deep anterior lamellar keratoplasty.
Fig. 4.
Patient with superior steepening and high myopia, preoperative: −6.00/-2.25–20, Visual acuity: 20/30; A phakic intraocular lens was implanted for correction.
Side effects associated with these lenses include glare and halos,116, 117, 118 pupil ovalization,119 intraocular pressure elevation,120 lens deposits,121 induced astigmatism,122 and loss of corneal endothelium.119, 123, 124 PIOLs should not be used in patients with an endothelial cell count <2000/mm2.123, 124 Also, PIOL implantation can be followed by cataract formation on account of surgical trauma.125, 126 Today, the risk of complications is greatly reduced with improved lens designs and materials, and the advent of foldable intraocular lens designs which allow for small incision surgery.
Corneal transplantation
Patients with advanced keratoconus cannot achieve sufficient correction and vision with contact lenses, glasses, or even ICRS, and for such patients, keratoplasty (penetrating or lamellar) is used depending on the extent of corneal scarring. Approximately 12–20% of keratoconus patients will eventually need a corneal transplant.31 For over 70 years, the corneal grafting surgery has been conducted with different techniques for the treatment of keratoconus patients.133
PK provides good vision in the long-term. Visual rehabilitation is often slow after PK and is a factor of residual astigmatism after surgery and anisometropia. Pramanik et al. observed that 73% of severe keratoconus patients who had undergone PK had BCVA≥20/40 at 14 years after surgery, and the rate of keratoconus recurrence at 25 years was about 12%.23
Long follow-ups have shown rejection rates of 5.8–41% in the first two years.134, 135, 136 Most patients require spectacles after surgery, and in certain cases, contact lenses can offer 1–2 lines better vision compared to spectacles, but they do not achieve visual stability until one year after surgery.135, 137, 138, 139, 140, 141 In a 24-month follow-up study by Asena and Altınörs, 83% of the patients needed spectacles, and 17% needed to wear contact lenses.142
Despite these results, the development of new techniques such as femtosecond-assisted keratoplasty has improved visual outcomes of corneal grafting.143, 144 Studies have shown that cases with deep central corneal scarring benefit from PK better than DALK.145
In general, despite excellent results with PK, DALK may be performed for keratoconus patients with no risk of endothelial rejection to reduce the dose of steroids and the risk of secondary glaucoma.146 The term “deep lamellar keratoplasty” (DLK) was first applied by Archila who showed that injecting air into the stroma can facilitate the removal of the host tissue.147 In keratoconus, the technique is used to reach into stromal depths close to the Descemet's membrane (DM). This method allows the surgeon to use larger (9 mm) grafts which support better bonding between the donor and host corneas without placing the endothelium at risk.148 Parker et al. reported stromal rejection rates of 3–14.3% after DALK, and 3–31% may occur during the first three days after surgery.113
Studies have shown that the chances of glaucoma are 40% lower with DALK compared to PK, and this may be due to lesser need for steroids after DALK.149, 150 Another advantage of this method is the lack of endothelial rejection after DALK because there is no endothelial defense reaction. This is of special importance for young keratoconus patients and those who are at risk of rejection such as patients with atopic dermatitis.
The specific strategies outlined for standardizing the DALK procedure have helped reduce intraoperative and postoperative complications. This surgical method is now safer and more reproducible and is known as a standard procedure in the treatment of keratoconus.151 The different techniques of DALK include layer-by-layer manual dissection, Archila, Melles, Anwar's big-bubble, viscoelastic dissection, hydrodelamination, and femtosecond laser-assisted DALK.148
The layer-by-layer method is used in those with stromal scarring and is a time-consuming approach.152 In the Archila technique, air is injected into the cornea until it is opaque, then a deep incision down to the DM is made such that a layer of stroma is maintained to protect the DM.153 In the Melles technique, the aqueous in the anterior chamber is completely replaced with an air bubble. The difference between the refractive indices of air and the corneal tissue creates a mirror image which helps determine the depth of the incision and location of the DM.154
The big bubble technique, which was introduced by Anwar and Teichmann,155 constitutes the injection of air deep into the stroma, and the surface of DM appears smooth after removing the stroma. The air is injected slowly until a large bubble is formed, and then, the anterior two-thirds of the cornea is debulked using a crescent blade. With viscoelastic dissection, the anterior stroma is removed, and then air is injected into the anterior chamber, and finally, the posterior stroma is separated from the DM using an ophthalmic viscoelastic device (OVD).156 During the hydrodelamination technique, which was described by Sugita and Kondo,157 a saline solution is injected into the cornea to separate the stroma, and then lamellar dissection is done deep down to the DM.
Today, the use of femtosecond laser is of special importance in ophthalmology, and its use in femtosecond laser-assisted DALK allows the surgeon to make incisions at specific depths and precision levels of 0.1 mm158 without any damage to the surrounding tissues, and thus, better matching between the donor and host tissues which may lead to faster visual rehabilitation.158, 159 There are also various methods for stitching the donor cornea to the host bed using Mersilene and 10-0 nylon. Stitches can be used alone or in combination, and it is important that they contain a large percentage of the cornea to prevent their loosening in the future.160 The availability of different methods helps surgeons use the appropriate method under different circumstances.
Studies have shown that visual rehabilitation is better with DALK compared to PK, but refractive and topographic outcomes are comparable between the two methods. The reported rates of postoperative complications such as graft rejection, secondary glaucoma, complicated cataracts, and constant endothelial cell loss are higher with PK.135, 161, 162, 163, 164, 165, 166
Conductive keratoplasty and new microwave procedure
Conductive keratoplasty (CK) is a non-invasive treatment and tissue-saving technique. In this method, no laser or incisions are involved. Instead, radio wave (350 HZ) energy is applied to the corneal stroma at 8–32 points. As a result of the generated heat, tissue temperature may rise up to 65 °C which causes permanent collagen shrinkage, corneal steepening in flat regions, and correction of refractive errors through corneal remodeling. In this method, since no tissue is removed, it may also be effective for thin corneas.167, 168 This method was FDA approved in 2002 and can be used for the correction of low and moderate hyperopia (+0.75 to +3.00 D) and astigmatism less than 0.75 D.169, 170 In addition, this method is used for the treatment of presbyopia171, 172, 173, myopic over-correction or induced hyperopia and astigmatism after myopic LASIK or PRK59, 168, 174, 175 and residual astigmatism after cataract surgery, and results have shown to be safe and effective.176 However, it seems that CK alone may not be effective for high astigmatism and keratoconus, and it needs to be combined with other options, or the surgeon may need to apply more spots in flat areas.174, 177 Using topography-guided conductive keraoplasty (TGCK) in advanced keratoconus, Kato et al. observed stable vision improvement at 12 months after surgery in 70% of the eyes.178 This surgical technique is suggested for cases of non-progressive advanced keratoconus who cannot tolerate contact lenses.179 Alio et al. also used CK for corneal remodeling in keratoconus patients. In their method, the probe is placed in thin and non-ectatic areas of the cornea to achieve selective steepening in flat regions of the cornea. Postoperatively, significant changes were observed in patients' topography, and their visual acuity improved;168 however, unlike cross-linking, CK cannot stop disease progression and may be more effective when combined with cross-linking. Limited studies have been published in this field as case reports. Kymionis et al. compared combined CK-CXL versus CXL alone in a study of two keratoconus cases and showed little difference in terms of visual acuity.180 In 2012, Keraflex was introduced as a new method for keratoconus treatment. In this method, low energy microwaves are used for less than 1 second along with mitomycin and UV rays as accelerated CXL. This method, which is performed with the Vedra System, causes central corneal flattening while protecting the integrity and biomechanics of the cornea. As the tissue temperature rises, collagen shrinkage occurs at 150 μm in the stroma, and to prevent damage to the Bowman layer during treatment, the Vedra KXS cooling system is used. With this method, increased corneal sphericity and reduced keratometric values can improve contact lens tolerance for patients, and refractive status improves as a result of decreased higher order aberrations.181 The main goal of this method is to maintain the results and refractive stability with cross-linking, but it seems that corneal collagen cross-linking is effective for preventing regression181 and more studies are needed in this regard. Regression is one of the major complications of CK, either alone or combined with other methods, and it can be related to the degree of corneal irregularity and the degree of refractive error that causes unstable and unpredictable results168, 180, 182, 183 and it seems to occur more commonly in younger people.184 Also, in the first few days after surgery, patients complained of foreign body sensation and light sensitivity.169, 170, 175, 182, 185 In one case report, corneal iron ring deposits were reported in a 54-year-old woman treated for hyperopia using CK after one year of follow-up186 and one case of corneal perforation was reported in an elderly patient who received CK for the treatment of induced astigmatism two years after LASIK, which was probably on account of the previous surgery.187
Gene therapy
Studies have shown that although keratoconus is a multifactorial disease, the pathogenesis of the disease is very much affected by genetic factors and positive family history with a rate of 5% to 28%.188, 189, 190 According to one study, those with a family history of the disease are 15–67 times more likely to develop the disease.191 There may be a correlation between keratoconus and consanguineous marriages.6, 7, 8 Studies in twins and dizygotic and monozygotic comparisons and observing concordance in dizygotic twins indicate the genetic influence in the incidence of disease.17, 192, 193 Linkage analysis in families can help detect patterns showing which chromosome region is modified194 which exist at least in candidate genetic loci19195 and another linkage locus such as locus chr5q32-33, chr14q11.2, chr16q22.3-q23.1.196, 197 Genome studies can find highly complex genetic factors associated with keratoconus; ZNF469 was found in 23% of sporadic Keratoconus198 and seems to play an important role in disease progression. A large number of candidate genes associated with keratoconus pathogenesis have been identified, among which, visual system homeobox 1 (VSX1) and superoxide dismutase 1 (SOD1) are the most notable.195 In a study by Saee-Rad et al. In Iran, after RNA extraction from normal and keratoconus corneas, three SOD1, TGF-β1, and DUSP1 genes were studied. Of these, TGF-β1 and DUSP1 genes were found to have an effective role in the development of inflammatory processes and can be helpful for better understating of the pathogenesis of the disease.199 In gene therapy, the gene of interest is delivered into the target cell using a vector and genetic expression begins with protein synthesis.194 Gene therapy can be a very promising and effective way to change the course of the disease upon identifying pathogenic genes and changing the structure of cell proteins.
Bowman layer transplantation
Bowman layer transplantation (BL transplantation) is a novel surgical technique proposed by Digk et al., in 2014.200 In keratoconus, corneal ectasia occurs as the bowman layer destabilizes and undergoes layer fragmentation with disease progression. Therefore, novel treatment modalities aim to graft an isolated bowman layer in the mid-stroma in order to improve corneal stability and prevent disease progression and ectasia.201 In this method, a pocket is created in the mid-stroma where an isolated bowman layer as inlayed. The graft is prepared manually by dissecting a layer from the anterior stromal cornea of the donor, about 9–11 mm in diameter. This technique is suitable for very thin corneas to prevent corneal perforation. The prepared graft is rinsed with BSS and stained with tryptan blue, and then placed into the pocket through a sclera tunnel. The first steps of the procedure are similar to DALK, but overall, this method is a substitute to delay DALK and PK. It is recommended for cases who are contact lens intolerant or have a corneal scar, and after surgery, patients are able to wear lenses for longer durations. After the preliminary study, patients were followed up for 3 years, and results showed that mean keratometry reduced from 77.2 ± 6.2 to 69.2 ± 3.7 D and mean pachymetry increased from 332 ± 59 before surgery to 360 ± 50 μm after surgery. Mean visual acuity was also improved by 1–2 lines of BCVA.200, 202 Higher order aberrations, especially spherical aberration, had a appreciably decreased in both the anterior and posterior corneal surfaces.203 With this method, the risk of graft rejection and steroid overuse is reduced. In most patients disease progression is stable and under control, and functional vision is improved. Potential complications include DM perforation which is observed in about 6% of patients, and in such cases, PK or DALK may be used for the patient.200, 202
Today, several treatments are available for keratoconus. Glasses and contact lenses are used in early stages of keratoconus, CXL is applied to stabilize disease progression, ICRS flatten the cornea and are used to reduce refractive errors, and PK and DALK, conductive keratoplasty, gene therapy and recently BL are performed in advanced stages of the disease.
Although there is no single algorithm for the treatment of keratoconus, the general rule is the maximum use of CXL to prevent disease progression and concurrent or complementary treatments such as ring and intraocular lenses and minimizing the need for corneal grafting in mild to moderate cases to maintain vision in these patients. Contact lenses still have an important role as an effective option.
Combined approaches such as CXL and PRK are new horizons for mild cases of keratoconus. Today, modern technology is very useful in understanding the pathophysiology and the diagnosis of keratoconus. Although improved surgical techniques and the use of femtosecond laser is helpful towards visual rehabilitation, it seems patient selection is the most important factor for proper management and achieving optimum results. In the future, further advances in treatment methods for keratoconus will make it exciting for specialists to choose the most appropriate method for each patient and predict outcomes.
Footnotes
There is no financial interest for authors in any methods or materials mentioned in this article.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Kennedy R.H., Bourne W.M., Dyer J.A. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267–273. doi: 10.1016/0002-9394(86)90817-2. [DOI] [PubMed] [Google Scholar]
- 2.Rabinowitz Y.S. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 3.Jonas J.B., Nangia V., Matin A., Kulkarni M., Bhojwani K. Prevalence and associations of keratoconus in rural Maharashtra in central India: the central India eye and medical study. Am J Ophthalmol. 2009;148(5):760–765. doi: 10.1016/j.ajo.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Waked N., Fayad A., Fadlallah A., El Rami H. Keratoconus screening in a Lebanese students' population. J Fr Ophtalmol. 2012;35(1):23–29. doi: 10.1016/j.jfo.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Gorskova E., Sevost'ianov E. Epidemiology of keratoconus in the urals. Vestn Oftalmol. 1998;114(4):38. [PubMed] [Google Scholar]
- 6.Gordon-Shaag A., Millodot M., Essa M., Garth J., Ghara M., Shneor E. Is consanguinity a risk factor for keratoconus? Optom Vis Sci – Off Publ Am Acad Optometry. 2013;90(5):448–454. doi: 10.1097/OPX.0b013e31828da95c. [DOI] [PubMed] [Google Scholar]
- 7.Cozma I., Atherley C., James N. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asian and white patients. Eye (London, England) 2005;19(8):924–925. doi: 10.1038/sj.eye.6701677. [DOI] [PubMed] [Google Scholar]
- 8.Georgiou T., Funnell C.L., Cassels-Brown A., O'Conor R. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asians and white patients. Eye (London, England) 2004;18(4):379–383. doi: 10.1038/sj.eye.6700652. [DOI] [PubMed] [Google Scholar]
- 9.Hashemi H., Beiranvand A., Khabazkhoob M. Prevalence of keratoconus in a population-based study in Shahroud. Cornea. 2013;32(11):1441–1445. doi: 10.1097/ICO.0b013e3182a0d014. [DOI] [PubMed] [Google Scholar]
- 10.Hashemi H., Khabazkhoob M., Fotouhi A. Topographic keratoconus is not rare in an Iranian population: the Tehran eye study. Ophthal Epidemiol. 2013;20(6):385–391. doi: 10.3109/09286586.2013.848458. [DOI] [PubMed] [Google Scholar]
- 11.Hashemi H., Khabazkhoob M., Yazdani N. The prevalence of keratoconus in a young population in Mashhad, Iran. Ophthalmic Physiol Opt. 2014;34(5):519–527. doi: 10.1111/opo.12147. [DOI] [PubMed] [Google Scholar]
- 12.Chang H.Y., Chodosh J. The genetics of keratoconus. Semin Ophthalmol. 2013;28(5-6):275–280. doi: 10.3109/08820538.2013.825295. [DOI] [PubMed] [Google Scholar]
- 13.Hallermann W., Wilson E.J. Genetic aspects of keratoconus (author's transl) Klin Monbl Augenheilkd. 1977;170(6):906–908. [PubMed] [Google Scholar]
- 14.Shneor E., Millodot M., Blumberg S., Ortenberg I., Behrman S., Gordon-Shaag A. Characteristics of 244 patients with keratoconus seen in an optometric contact lens practice. Clin Exp Optom. 2013;96(2):219–224. doi: 10.1111/cxo.12005. [DOI] [PubMed] [Google Scholar]
- 15.Millodot M., Shneor E., Albou S., Atlani E., Gordon-Shaag A. Prevalence and associated factors of keratoconus in Jerusalem: a cross-sectional study. Ophthal Epidemiol. 2011;18(2):91–97. doi: 10.3109/09286586.2011.560747. [DOI] [PubMed] [Google Scholar]
- 16.Pearson A.R., Soneji B., Sarvananthan N., Sandford-Smith J.H. Does ethnic origin influence the incidence or severity of keratoconus? Eye (London, England) 2000;14(4):625–628. doi: 10.1038/eye.2000.154. [DOI] [PubMed] [Google Scholar]
- 17.Weed K.H., MacEwen C.J., McGhee C.N. The variable expression of keratoconus within monozygotic twins: dundee University Scottish Keratoconus Study (DUSKS) Contact Lens Anterior Eye J B.C.L.A. 2006;29(3):123–126. doi: 10.1016/j.clae.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Arnal E., Peris-Martínez C., Menezo J.L., Johnsen-Soriano S., Romero F.J. Oxidative stress in keratoconus? Invest Ophthalmol Vis Sci. 2011;52(12):8592–8597. doi: 10.1167/iovs.11-7732. [DOI] [PubMed] [Google Scholar]
- 19.Davidson A.E., Hayes S., Hardcastle A.J., Tuft S.J. The pathogenesis of keratoconus. Eye (London, England) 2014;28(2):189–195. doi: 10.1038/eye.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., Rabinowitz Y.S., Rasheed K., Yang H. Longitudinal study of the normal eyes in unilateral keratoconus patients. Ophthalmology. 2004;111(3):440–446. doi: 10.1016/j.ophtha.2003.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Krachmer J.H., Feder R.S., Belin M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28(4):293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 22.Lass J.H., Lembach R.G., Park S.B. Clinical management of keratoconus. A multicenter analysis. Ophthalmology. 1990;97(4):433–445. doi: 10.1016/s0161-6420(90)32569-1. [DOI] [PubMed] [Google Scholar]
- 23.Pramanik S., Musch D.C., Sutphin J.E., Farjo A.A. Extended long-term outcomes of penetrating keratoplasty for keratoconus. Ophthalmology. 2006;113(9):1633–1638. doi: 10.1016/j.ophtha.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 24.Wagner H., Barr J.T., Zadnik K. Collaborative longitudinal evaluation of keratoconus (CLEK) study: methods and findings to date. Cont Lens Anterior Eye. 2007;30(4):223–232. doi: 10.1016/j.clae.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii R., Kamiya K., Igarashi A., Shimizu K., Utsumi Y., Kumanomido T. Correlation of corneal elevation with severity of keratoconus by means of anterior and posterior topographic analysis. Cornea. 2012;31(3):253–258. doi: 10.1097/ICO.0B013E31823D1EE0. [DOI] [PubMed] [Google Scholar]
- 26.Alió J.L., Shabayek M.H. Corneal higher order aberrations: a method to grade keratoconus. J Refract Surg. 2006;22(6):539–545. doi: 10.3928/1081-597X-20060601-05. [DOI] [PubMed] [Google Scholar]
- 27.McMahon T.T., Szczotka-Flynn L., Barr J.T. A new method for grading the severity of keratoconus: the Keratoconus Severity Score (KSS) Cornea. 2006;25(7):794–800. doi: 10.1097/01.ico.0000226359.26678.d1. [DOI] [PubMed] [Google Scholar]
- 28.Smiddy W.E., Hamburg T.R., Kracher G.P., Stark W.J. Keratoconus. Contact lens or keratoplasty? Ophthalmology. 1988;95(4):487–492. [PubMed] [Google Scholar]
- 29.Bilgin L.K., Yilmaz S., Araz B., Yuksel S.B., Sezen T. 30 years of contact lens prescribing for keratoconic patients in Turkey. Cont Lens Anterior Eye. 2009;32(1):16–21. doi: 10.1016/j.clae.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Perry H.D., Buxton J.N., Fine B.S. Round and oval cones in keratoconus. Ophthalmology. 1980;87(9):905–909. doi: 10.1016/s0161-6420(80)35145-2. [DOI] [PubMed] [Google Scholar]
- 31.Jhanji V., Sharma N., Vajpayee R.B. Management of keratoconus: current scenario. Br J Ophthalmol. 2011;95(8):1044–1050. doi: 10.1136/bjo.2010.185868. [DOI] [PubMed] [Google Scholar]
- 32.Rathi V.M., Mandathara P.S., Dumpati S. Contact lens in keratoconus. Indian J Ophthalmol. 2013;61(8):410–415. doi: 10.4103/0301-4738.116066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez-Velazquez F.J. Kerasoft IC compared to Rose-K in the management of corneal ectasias. Cont Lens Anterior Eye J Br Contact Lens Assoc. 2012;35(4):175–179. doi: 10.1016/j.clae.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Abdalla Y.F., Elsahn A.F., Hammersmith K.M., Cohen E.J. SynergEyes lenses for keratoconus. Cornea. 2010;29(1):5–8. doi: 10.1097/ICO.0b013e3181a9d090. [DOI] [PubMed] [Google Scholar]
- 35.Barnett M., Mannis M.J. Contact lenses in the management of keratoconus. Cornea. 2011;30(12):1510–1516. doi: 10.1097/ICO.0b013e318211401f. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez-Velazquez F.J. Severe epithelial edema in Clearkone SynergEyes contact lens wear for keratoconus. Eye Contact Lens. 2011;37(6):381–385. doi: 10.1097/ICL.0b013e31822a33a6. [DOI] [PubMed] [Google Scholar]
- 37.Pilskalns B., Fink B.A., Hill R.M. Oxygen demands with hybrid contact lenses. Optom Vis Sci – Off Publ Am Acad Optometry. 2007;84(4):334–342. doi: 10.1097/OPX.0b013e3180421748. [DOI] [PubMed] [Google Scholar]
- 38.Wollensak G., Spoerl E., Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29(9):1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 39.Ashwin P.T., McDonnell P.J. Collagen cross-linkage: a comprehensive review and directions for future research. Br J Ophthalmol. 2010;94(8):965–970. doi: 10.1136/bjo.2009.164228. [DOI] [PubMed] [Google Scholar]
- 40.Carrasquillo K.G., Rand J., Talamo J.H. Intacs for keratoconus and post-LASIK ectasia: mechanical versus femtosecond laser-assisted channel creation. Cornea. 2007;26(8):956–962. doi: 10.1097/ICO.0b013e31811dfa66. [DOI] [PubMed] [Google Scholar]
- 41.Raiskup-Wolf F., Hoyer A., Spoerl E., Pillunat L.E. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34(5):796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 42.Shetty R., Kaweri L., Pahuja N. Current review and a simplified “five-point management algorithm” for keratoconus. Indian J Ophthalmol. 2015;63(1):46–53. doi: 10.4103/0301-4738.151468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caporossi A., Baiocchi S., Mazzotta C., Traversi C., Caporossi T. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen: preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006;32(5):837–845. doi: 10.1016/j.jcrs.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 44.Grewal D.S., Brar G.S., Jain R., Sood V., Singla M., Grewal S.P. Corneal collagen crosslinking using riboflavin and ultraviolet-A light for keratoconus: one-year analysis using Scheimpflug imaging. J Cataract Refract Surg. 2009;35(3):425–432. doi: 10.1016/j.jcrs.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 45.Coskunseven E., Jankov M.R., 2nd, Hafezi F. Contralateral eye study of corneal collagen cross-linking with riboflavin and UVA irradiation in patients with keratoconus. J Refract Surg. 2009;25(4):371–376. doi: 10.3928/1081597X-20090401-02. [DOI] [PubMed] [Google Scholar]
- 46.Kanellopoulos A.J. Comparison of sequential vs same-day simultaneous collagen cross-linking and topography-guided PRK for treatment of keratoconus. J Refract Surg. 2009;25(9):812–818. doi: 10.3928/1081597X-20090813-10. [DOI] [PubMed] [Google Scholar]
- 47.Richoz O., Mavrakanas N., Pajic B., Hafezi F. Corneal collagen cross-linking for ectasia after LASIK and photorefractive keratectomy: long-term results. Ophthalmology. 2013;120(7):1354–1359. doi: 10.1016/j.ophtha.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 48.Wittig-Silva C., Chan E., Islam F.M., Wu T., Whiting M., Snibson G.R. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. 2014;121(4):812–821. doi: 10.1016/j.ophtha.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 49.Kymionis G.D., Grentzelos M.A., Kankariya V.P. Long-term results of combined transepithelial phototherapeutic keratectomy and corneal collagen crosslinking for keratoconus: cretan protocol. J Cataract Refract Surg. 2014;40(9):1439–1445. doi: 10.1016/j.jcrs.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 50.Kontadakis G.A., Kankariya V.P., Tsoulnaras K., Pallikaris A.I., Plaka A., Kymionis G.D. Long-term comparison of simultaneous topography-guided photorefractive keratectomy followed by corneal cross-linking versus corneal cross-linking alone. Ophthalmology. 2016;123(5):974–983. doi: 10.1016/j.ophtha.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Wollensak G., Spoerl E., Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 52.Hafezi F., Mrochen M., Iseli H.P., Seiler T. Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. J Cataract Refract Surg. 2009;35(4):621–624. doi: 10.1016/j.jcrs.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 53.Kymionis G.D., Mikropoulos D.G., Portaliou D.M., Voudouragkaki I.C., Kozobolis V.P., Konstas A.G. An overview of corneal collagen cross-linking (CXL) Adv Ther. 2013;30(10):858–869. doi: 10.1007/s12325-013-0065-9. [DOI] [PubMed] [Google Scholar]
- 54.Mazzotta C., Balestrazzi A., Baiocchi S., Traversi C., Caporossi A. Stromal haze after combined riboflavin-UVA corneal collagen cross-linking in keratoconus: in vivo confocal microscopic evaluation. Clin Exp Ophthalmol. 2007;35(6):580–582. doi: 10.1111/j.1442-9071.2007.01536.x. [DOI] [PubMed] [Google Scholar]
- 55.Cinar Y., Cingu A.K., Turkcu F.M. Accelerated corneal collagen cross-linking for progressive keratoconus. Cutan Ocul Toxicol. 2014;33(2):168–171. doi: 10.3109/15569527.2013.816724. [DOI] [PubMed] [Google Scholar]
- 56.Kanellopoulos A.J. Long term results of a prospective randomized bilateral eye comparison trial of higher fluence, shorter duration ultraviolet A radiation, and riboflavin collagen cross linking for progressive keratoconus. Clin Ophthalmol (Auckland, NZ) 2012;6:97–101. doi: 10.2147/OPTH.S27170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sadoughi M.M., Einollahi B., Baradaran-Rafii A., Roshandel D., Hasani H., Nazeri M. Accelerated versus conventional corneal collagen cross-linking in patients with keratoconus: an intrapatient comparative study. Int J Ophthalmol. 2016 Dec 29:1–8. doi: 10.1007/s10792-016-0423-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 58.Hashemi H., Fotouhi A., Miraftab M. Short-term comparison of accelerated and standard methods of corneal collagen crosslinking. J Cataract Refract Surg. 2015;41(3):533–540. doi: 10.1016/j.jcrs.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 59.Hashemian H., Jabbarvand M., Khodaparast M., Ameli K. Evaluation of corneal changes after conventional versus accelerated corneal cross-linking: a randomized controlled trial. J Refract Surg. 2014;30(12):837–842. doi: 10.3928/1081597X-20141117-02. [DOI] [PubMed] [Google Scholar]
- 60.Mangioris G.F., Papadopoulou D.N., Balidis M.O., Poulas J.L., Papadopoulos N.T., Seiler T. Corneal infiltrates after corneal collagen cross-linking. J Refract Surg. 2010;26(8):609–611. doi: 10.3928/1081597X-20100326-01. [DOI] [PubMed] [Google Scholar]
- 61.Holopainen J.M., Krootila K. Transient corneal thinning in eyes undergoing corneal cross-linking. Am J Ophthalmol. 2011;152(4):533–536. doi: 10.1016/j.ajo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 62.Kodavoor S.K., Sarwate N.J., Ramamurhy D. Microbial keratitis following accelerated corneal collagen cross-linking. Oman J Ophthalmol. 2015;8(2):111–113. doi: 10.4103/0974-620X.159259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Labiris G., Kaloghianni E., Koukoula S., Zissimopoulos A., Kozobolis V.P. Corneal melting after collagen cross-linking for keratoconus: a case report. J Med Case Rep. 2011;5:152. doi: 10.1186/1752-1947-5-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ivarsen A., Hjortdal J. Collagen cross-linking for advanced progressive keratoconus. Cornea. 2013;32(7):903–906. doi: 10.1097/ICO.0b013e31828321dd. [DOI] [PubMed] [Google Scholar]
- 65.Sloot F., Soeters N., van der Valk R., Tahzib N.G. Effective corneal collagen crosslinking in advanced cases of progressive keratoconus. J Cataract Refract Surg. 2013;39(8):1141–1145. doi: 10.1016/j.jcrs.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 66.Kilic A., Kamburoglu G., Akinci A. Riboflavin injection into the corneal channel for combined collagen crosslinking and intrastromal corneal ring segment implantation. J Cataract Refract Surg. 2012;38(5):878–883. doi: 10.1016/j.jcrs.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 67.Raiskup F., Hoyer A., Spoerl E. Permanent corneal haze after riboflavin-UVA-induced cross-linking in keratoconus. J Refract Surg. 2009;25(9):S824–S828. doi: 10.3928/1081597X-20090813-12. [DOI] [PubMed] [Google Scholar]
- 68.Soeters N., Wisse R.P., Godefrooij D.A., Imhof S.M., Tahzib N.G. Transepithelial versus epithelium-off corneal cross-linking for the treatment of progressive keratoconus: a randomized controlled trial. Am J Ophthalmol. 2015;159(5):821–828. doi: 10.1016/j.ajo.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Spadea L., Mencucci R. Transepithelial corneal collagen cross-linking in ultrathin keratoconic corneas. Clin Ophthalmol. 2012;6:1785–1792. doi: 10.2147/OPTH.S37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stojanovic A., Zhou W., Utheim T.P. Corneal collagen cross-linking with and without epithelial removal: a contralateral study with 0.5% hypotonic riboflavin solution. Biomed Res Int. 2014;2014 doi: 10.1155/2014/619398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McQuaid R., Cummings A.B., Mrochen M. The theory and art of corneal cross-linking. Indian J Ophthalmol. 2013;61(8):416–419. doi: 10.4103/0301-4738.116069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nose W., Neves R.A., Burris T.E., Schanzlin D.J. Belfort Junior R. Intrastromal corneal ring: 12-month sighted myopic eyes. J Refract Surg. 1996;12(1):20–28. doi: 10.3928/1081-597X-19960101-08. [DOI] [PubMed] [Google Scholar]
- 73.Schanzlin D.J., Asbell P.A., Burris T.E., Durrie D.S. The intrastromal corneal ring segments. Phase II results for the correction of myopia. Ophthalmology. 1997;104(7):1067–1078. doi: 10.1016/s0161-6420(97)30183-3. [DOI] [PubMed] [Google Scholar]
- 74.Burris T.E., Ayer C.T., Evensen D.A., Davenport J.M. Effects of intrastromal corneal ring size and thickness on corneal flattening in human eyes. Refract Corneal Surg. 1991;7(1):46–50. [PubMed] [Google Scholar]
- 75.Chou B., Wachler B.S.B. Intacs for a keratocone: a promising new option? Rev Optometry. 2000;137(4) 97–97. [Google Scholar]
- 76.Torquetti L., Ferrara G., Almeida F. Intrastromal corneal ring segments implantation in patients with keratoconus: 10-year follow-up. J Refract Surg. 2014;30(1):22–26. doi: 10.3928/1081597X-20131217-02. [DOI] [PubMed] [Google Scholar]
- 77.Krachmer J.H.M.M., Holland E.J. Elsevier; Philadelphia: 2011. Cornea. [Google Scholar]
- 78.Alio J.L., Vega-Estrada A., Esperanza S., Barraquer R.I., Teus M.A., Murta J. Intrastromal corneal ring segments: how successful is the surgical treatment of keratoconus? Middle East Afr J Ophthalmol. 2014;21(1):3–9. doi: 10.4103/0974-9233.124076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hashemi H., Yazdani-Abyaneh A., Beheshtnejad A., Jabbarvand M., Kheirkhah A., Ghaffary S.R. Efficacy of intacs intrastromal corneal ring segment relative to depth of insertion evaluated with anterior segment optical coherence tomography. Middle East Afr J Ophthalmol. 2013;20(3):234–238. doi: 10.4103/0974-9233.114800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ertan A., Colin J. Intracorneal rings for keratoconus and keratectasia. J Cataract Refract Surg. 2007;33(7):1303–1314. doi: 10.1016/j.jcrs.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 81.Hashemian M.N., Zare M.A., Mohammadpour M., Rahimi F., Fallah M.R., Panah F.K. Outcomes of single segment implantation of conventional intacs versus intacs SK for keratoconus. J Ophthalmic Vis Res. 2014;9(3):305–309. doi: 10.4103/2008-322X.143359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jabbarvand M., Hashemi H., Mohammadpour M., Khojasteh H., Khodaparast M., Hashemian H. Implantation of a complete intrastromal corneal ring at 2 different stromal depths in keratoconus. Cornea. 2014;33(2):141–144. doi: 10.1097/ICO.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 83.Shabayek M.H., Alio J.L. Intrastromal corneal ring segment implantation by femtosecond laser for keratoconus correction. Ophthalmology. 2007;114(9):1643–1652. doi: 10.1016/j.ophtha.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 84.Pinero D.P., Alio J.L., El Kady B. Refractive and aberrometric outcomes of intracorneal ring segments for keratoconus: mechanical versus femtosecond-assisted procedures. Ophthalmology. 2009;116(9):1675–1687. doi: 10.1016/j.ophtha.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 85.Chan C.C., Sharma M., Wachler B.S. Effect of inferior-segment Intacs with and without C3-R on keratoconus. J Cataract Refract Surg. 2007;33(1):75–80. doi: 10.1016/j.jcrs.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 86.Koller T., Iseli H.P., Donitzky C., Ing D., Papadopoulos N., Seiler T. Topography-guided surface ablation for forme fruste keratoconus. Ophthalmology. 2006;113(12):2198–2202. doi: 10.1016/j.ophtha.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 87.Yeung S.N., Lichtinger A., Ku J.Y., Kim P., Low S.A., Rootman D.S. Intracorneal ring segment explantation after intracorneal ring segment implantation combined with same-day corneal collagen crosslinking in keratoconus. Cornea. 2013;32(12):1617–1620. doi: 10.1097/ICO.0b013e3182a738ba. [DOI] [PubMed] [Google Scholar]
- 88.Shetty R., Kurian M., Anand D., Mhaske P., Narayana K.M., Shetty B.K. Intacs in advanced keratoconus. Cornea. 2008;27(9):1022–1029. doi: 10.1097/ICO.0b013e318172fc54. [DOI] [PubMed] [Google Scholar]
- 89.Ganesh S., Shetty R., D'Souza S., Ramachandran S., Kurian M. Intrastromal corneal ring segments for management of keratoconus. Indian J Ophthalmol. 2013;61(8):451–455. doi: 10.4103/0301-4738.116065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Colin J., Touboul D., Bedi R. Refractive and keratometric outcomes of intacs continue to improve until 6 months. Cornea. 2011;30(9):1068–1069. doi: 10.1097/ICO.0b013e3181eeb45f. 1068; author reply. [DOI] [PubMed] [Google Scholar]
- 91.El-Raggal T.M. Effect of corneal collagen crosslinking on femtosecond laser channel creation for intrastromal corneal ring segment implantation in keratoconus. J Cataract Refract Surg. 2011;37(4):701–705. doi: 10.1016/j.jcrs.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 92.Legare M.E., Iovieno A., Yeung S.N. Intacs with or without same-day corneal collagen cross-linking to treat corneal ectasia. Can J Ophthalmol. 2013;48(3):173–178. doi: 10.1016/j.jcjo.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 93.Khan M.I., Injarie A., Muhtaseb M. Intrastromal corneal ring segments for advanced keratoconus and cases with high keratometric asymmetry. J Cataract Refract Surg. 2012;38(1):129–136. doi: 10.1016/j.jcrs.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 94.Rabinowitz Y.S. Intacs for keratoconus. Curr Opin Ophthalmol. 2007;18(4):279–283. doi: 10.1097/ICU.0b013e3281fc94a5. [DOI] [PubMed] [Google Scholar]
- 95.Shetty R., D'Souza S., Ramachandran S., Kurian M., Nuijts R.M. Decision making nomogram for intrastromal corneal ring segments in keratoconus. Indian J Ophthalmol. 2014;62(1):23. doi: 10.4103/0301-4738.126170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mohammadpour Mehrdad, Masoumi Ahmad, Mirghorbani Masoud, Shahraki Kianoosh, Hashemi Hassan. Updates on corneal collagen crosslinking: indications, techniques and clinical outcomes. J Curr Ophthalmol. 2017:1–13. doi: 10.1016/j.joco.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Binder P. Hydrogel implants for the correction of myopia. Curr Eye Res. 1982;2(7):435–441. doi: 10.3109/02713688208996346. [DOI] [PubMed] [Google Scholar]
- 98.Daxer A. Adjustable intracorneal ring in a lamellar pocket for keratoconus. J Refract Surg. 2010;26(3):217–221. doi: 10.3928/1081597X-20100224-08. [DOI] [PubMed] [Google Scholar]
- 99.Daxer A., Mahmoud H., Venkateswaran R.S. Intracorneal continuous ring implantation for keratoconus: one-year follow-up. J Cataract Refract Surg. 2010;36(8):1296–1302. doi: 10.1016/j.jcrs.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 100.Mahmood H., Venkateswaran R., Daxer A. Implantation of a complete corneal ring in an intrastromal pocket for keratoconus. J Refract Surg. 2011;27(1):63–68. doi: 10.3928/1081597X-20100212-11. [DOI] [PubMed] [Google Scholar]
- 101.Mojaled Nobari S., Villena C., Jadidi K. Predictability, stability and safety of MyoRing implantation in keratoconic eyes during one year follow-up. Iran J Ophthalmol. 2014;26(3):136–143. [Google Scholar]
- 102.Mohammadpour M., Hahemi H., Jabbarvand M. Technique of simultaneous femtosecond laser assisted Myoring implantation and accelerated intrastromal collagen cross-linking for management of progressive keratoconus: a novel technique. Cont Lens Anterior Eye. 2016;39(1):9–14. doi: 10.1016/j.clae.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 103.El-Husseiny M., Tsintarakis T., Eppig T., Langenbucher A., Seitz B. Intacsintracorneal ring segments in keratoconus. Ophthalmol Z Dtsch Ophthalmol Ges. 2013;110(9):823–826. doi: 10.1007/s00347-013-2821-2. 828-829. [DOI] [PubMed] [Google Scholar]
- 104.Pinero D.P., Alio J.L. Intracorneal ring segments in ectatic corneal disease – a review. Clin Exp Ophthalmol. 2010;38(2):154–167. doi: 10.1111/j.1442-9071.2010.02197.x. [DOI] [PubMed] [Google Scholar]
- 105.Cosar C.B., Sridhar M.S., Sener B. Late onset of deep corneal vascularization: a rare complication of intrastromal corneal ring segments for keratoconus. Eur J Ophthalmol. 2009;19(2):298–300. doi: 10.1177/112067210901900222. [DOI] [PubMed] [Google Scholar]
- 106.Zare M.A., Hashemi H., Salari M.R. Intracorneal ring segment implantation for the management of keratoconus: safety and efficacy. J Cataract Refract Surg. 2007;33(11):1886–1891. doi: 10.1016/j.jcrs.2007.06.055. [DOI] [PubMed] [Google Scholar]
- 107.Kanellopoulos A.J., Pe L.H., Perry H.D., Donnenfeld E.D. Modified intracorneal ring segment implantations (INTACS) for the management of moderate to advanced keratoconus: efficacy and complications. Cornea. 2006;25(1):29–33. doi: 10.1097/01.ico.0000167883.63266.60. [DOI] [PubMed] [Google Scholar]
- 108.Ibrahim T.A. After 5 years follow-up: do Intacs help in keratoconus. J Cataract Refract Surg. 2006;1:45–48. [Google Scholar]
- 109.Hashemi H., Ghaffari R., Mohammadi M., Moghimi S., Miraftaab M. Microbial keratitis after INTACS implantation with loose suture. J Refract Surg. 2008;24(5):551–552. doi: 10.3928/1081597X-20080501-17. [DOI] [PubMed] [Google Scholar]
- 110.Ibanez-Alperte J., Perez-Garcia D., Cristobal J.A., Mateo A.J., Rio B.J., Minguez E. Keratitis after implantation of intrastromal corneal rings with spontaneous extrusion of the segment. Case Rep Ophthalmol. 2010;1(2):42–46. doi: 10.1159/000320585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mulet M.E., Perez-Santonja J.J., Ferrer C., Alio J.L. Microbial keratitis after intrastromal corneal ring segment implantation. J Refract Surg. 2010;26(5):364–369. doi: 10.3928/1081597X-20090617-06. [DOI] [PubMed] [Google Scholar]
- 112.Pineda R., 2nd, Chauhan T. Phakic intraocular lenses and their special indications. J Ophthalmic Vis Res. 2016;11(4):422–428. doi: 10.4103/2008-322X.194140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leccisotti A., Fields S.V. Angle-supported phakic intraocular lenses in eyes with keratoconus and myopia. J Cataract Refract Surg. 2003;29(8):1530–1536. doi: 10.1016/s0886-3350(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 114.Kamiya K., Shimizu K., Ando W., Asato Y., Fujisawa T. Phakic toric Implantable Collamer Lens implantation for the correction of high myopic astigmatism in eyes with keratoconus. J Refract Surg. 2008;24(8):840–842. doi: 10.3928/1081597X-20081001-12. [DOI] [PubMed] [Google Scholar]
- 115.Venter J. Artisan phakic intraocular lens in patients with keratoconus. J Refract Surg. 2009;25(9):759–764. doi: 10.3928/1081597X-20090813-01. [DOI] [PubMed] [Google Scholar]
- 116.Maroccos R., Vaz F., Marinho A., Guell J., Lohmann C.P. Glare and halos after “phakic IOL”. Surgery for the correction of high myopia. Ophthalmol Z Dtsch Ophthalmol Ges. 2001;98(11):1055–1059. doi: 10.1007/s003470170024. [DOI] [PubMed] [Google Scholar]
- 117.Moshirfar M., Holz H.A., Davis D.K. Two-year follow-up of the Artisan/Verisyse iris-supported phakic intraocular lens for the correction of high myopia. J Cataract Refract Surg. 2007;33(8):1392–1397. doi: 10.1016/j.jcrs.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 118.Tahzib N.G., Bootsma S.J., Eggink F.A., Nuijts R.M. Functional outcome and patient satisfaction after Artisan phakic intraocular lens implantation for the correction of myopia. Am J Ophthalmol. 2006;142(1):31–39. doi: 10.1016/j.ajo.2006.01.088. [DOI] [PubMed] [Google Scholar]
- 119.Alio J.L., Abdelrahman A.M., Javaloy J., Iradier M.T., Ortuno V. Angle-supported anterior chamber phakic intraocular lens explantation causes and outcome. Ophthalmology. 2006;113(12):2213–2220. doi: 10.1016/j.ophtha.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 120.Ardjomand N.K.H., Vidic B., El-Shabrawi Y., Faulborn J. Pupillary block after phakic anterior chamber intraocular lens implantation. J Cataract Refract Surg. 2002;28(6):1080–1081. doi: 10.1016/s0886-3350(01)01114-2. [DOI] [PubMed] [Google Scholar]
- 121.Stulting R.D.J.M., Maloney R.K., Assil K.K., Arrowsmith P.N., Thompson V.M. Three-year results of Artisan/Verisyse phakic intraocular lens implantation. Results of the United States Food and drug administration clinical trial. Ophthalmology. 2008;115(3):464–472. doi: 10.1016/j.ophtha.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 122.Alio J.L., Mulet M.E., Shalaby A.M. Artisan phakic iris claw intraocular lens for high primary and secondary hyperopia. J Refract Surg. 2002;18(6):697–707. doi: 10.3928/1081-597X-20021101-06. [DOI] [PubMed] [Google Scholar]
- 123.Benedetti S., Casamenti V., Marcaccio L., Brogioni C., Assetto V. Correction of myopia of 7 to 24 diopters with the Artisan phakic intraocular lens: two-year follow-up. J Refract Surg. 2005;21(2):116–126. doi: 10.3928/1081-597X-20050301-05. [DOI] [PubMed] [Google Scholar]
- 124.Dick H.B., Budo C., Malecaze F. Foldable Artiflex phakic intraocular lens for the correction of myopia: two-year follow-up results of a prospective European multicenter study. Ophthalmology. 2009;116(4):671–677. doi: 10.1016/j.ophtha.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 125.Menezo J.L., Peris-Martinez C., Cisneros-Lanuza A.L., Martinez-Costa R. Rate of cataract formation in 343 highly myopic eyes after implantation of three types of phakic intraocular lenses. J Refract Surg. 2004;20(4):317–324. doi: 10.3928/1081-597X-20040701-03. [DOI] [PubMed] [Google Scholar]
- 126.Munoz G., Montes-Mico R., Belda J.I., Alio J.L. Cataract after minor trauma in a young patient with an iris-fixated intraocular lens for high myopia. Am J Ophthalmol. 2003;135(6):890–891. doi: 10.1016/s0002-9394(02)02157-8. [DOI] [PubMed] [Google Scholar]
- 127.Guell J.L., Morral M., Gris O., Gaytan J., Sisquella M., Manero F. Five-year follow-up of 399 phakic Artisan-Verisyse implantation for myopia, hyperopia, and/or astigmatism. Ophthalmology. 2008;115(6):1002–1012. doi: 10.1016/j.ophtha.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 128.Al-Dreihi M.G., Louka B.I., Anbari A.A. Artisan iris-fixated toric phakic intraocular lens for the correction of high astigmatism after deep anterior lamellar keratoplasty. Digit J Ophthalmol – DJO. 2013;19(2):39–41. doi: 10.5693/djo.02.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alfonso J.F., Lisa C., Alfonso-Bartolozzi B., Perez-Vives C., Montes-Mico R. Collagen copolymer toric phakic intraocular lens for myopic astigmatism: one-year follow-up. J Cataract Refract Surg. 2014;40(7):1155–1162. doi: 10.1016/j.jcrs.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 130.Venter J.A., Pelouskova M., Schallhorn S.C., Collins B.M. Visual acuity improvement in adult amblyopic eyes with an iris-fixated phakic intraocular lens: long-term results. J Cataract Refract Surg. 2015;41(3):541–547. doi: 10.1016/j.jcrs.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 131.Qin Q., Yang L., He Z., Huang Z. Clinical application of TICL implantation for ametropia following deep anterior lamellar keratoplasty for keratoconus: a CONSORT-compliant article. Medicine. 2017;96(8) doi: 10.1097/MD.0000000000006118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tiveron M.C., Jr., Alio Del Barrio J.L., Kara-Junior N. Outcomes of toric Iris-Claw phakic intraocular lens implantation after deep anterior lamellar keratoplasty for keratoconus. J Refract Surg. 2017;33(8):538–544. doi: 10.3928/1081597X-20170616-02. [DOI] [PubMed] [Google Scholar]
- 133.Castroviejo R. Keratoplasty for the treatment of keratoconus. Trans Am Ophthalmol Soc. 1948;46:127–153. [PMC free article] [PubMed] [Google Scholar]
- 134.Choi J.A., Lee M.A., Kim M.S. Long-term outcomes of penetrating keratoplasty in keratoconus: analysis of the factors associated with final visual acuities. Int J Ophthalmol. 2014;7(3):517–521. doi: 10.3980/j.issn.2222-3959.2014.03.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Javadi M.A., Motlagh B.F., Jafarinasab M.R. Outcomes of penetrating keratoplasty in keratoconus. Cornea. 2005;24(8):941–946. doi: 10.1097/01.ico.0000159730.45177.cd. [DOI] [PubMed] [Google Scholar]
- 136.Niziol L.M., Musch D.C., Gillespie B.W., Marcotte L.M., Sugar A. Long-term outcomes in patients who received a corneal graft for keratoconus between 1980 and 1986. Am J Ophthalmol. 2013;155(2):213–219. doi: 10.1016/j.ajo.2012.08.001. e213. [DOI] [PubMed] [Google Scholar]
- 137.Brahma A., Ennis F., Harper R., Ridgway A., Tullo A. Visual function after penetrating keratoplasty for keratoconus: a prospective longitudinal evaluation. Br J Ophthalmol. 2000;84(1):60–66. doi: 10.1136/bjo.84.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brierly S.C., Izquierdo L., Jr., Mannis M.J. Penetrating keratoplasty for keratoconus. Cornea. 2000;19(3):329–332. doi: 10.1097/00003226-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 139.Rocha G.A., Miziara P.O., Castro A.C., Rocha A.A. Visual rehabilitation using mini-scleral contact lenses after penetrating keratoplasty. Arq Bras Ofthalmol. 2017;80(1):17–20. doi: 10.5935/0004-2749.20170006. [DOI] [PubMed] [Google Scholar]
- 140.Severinsky B., Behrman S., Frucht-Pery J., Solomon A. Scleral contact lenses for visual rehabilitation after penetrating keratoplasty: long term outcomes. Cont Lens Anterior Eye J Br Contact Lens Assoc. 2014;37(3):196–202. doi: 10.1016/j.clae.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 141.Sutton G., Hodge C., McGhee C.N. Rapid visual recovery after penetrating keratoplasty for keratoconus. Clin Exp Ophthalmol. 2008;36(8):725–730. doi: 10.1111/j.1442-9071.2008.01900.x. [DOI] [PubMed] [Google Scholar]
- 142.Asena L., Altinors D.D. Visual rehabilitation after penetrating keratoplasty. Exp Clin Transpl official J Middle East Soc Organ Transplant. 2016;14(Suppl 3):130–134. [PubMed] [Google Scholar]
- 143.Mian S.I., Shtein R.M. Femtosecond laser-assisted corneal surgery. Curr Opin Ophthalmol. 2007;18(4):295–299. doi: 10.1097/ICU.0b013e3281a4776c. [DOI] [PubMed] [Google Scholar]
- 144.Soong H.K., Malta J.B. Femtosecond lasers in ophthalmology. Am J Ophthalmol. 2009;147(2):189–197. doi: 10.1016/j.ajo.2008.08.026. e182. [DOI] [PubMed] [Google Scholar]
- 145.Parker J.S., van Dijk K., Melles G.R. Treatment options for advanced keratoconus: a review. Surv Ophthalmol. 2015;60(5):459–480. doi: 10.1016/j.survophthal.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 146.Reinhart W.J., Musch D.C., Jacobs D.S., Lee W.B., Kaufman S.C., Shtein R.M. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty a report by the american academy of ophthalmology. Ophthalmology. 2011;118(1):209–218. doi: 10.1016/j.ophtha.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 147.Archila E.A. Deep lamellar keratoplasty dissection of host tissue with intrastromal air injection. Cornea. 1984;3(3):217–218. [PubMed] [Google Scholar]
- 148.Feizi S., Javadi M.A., Fekri Y. Use of deep anterior lamellar keratoplasty (DALK) for keratoconus: indications, techniques and outcomes. Expert Rev Ophthalmol. 2016;11(5):347–359. [Google Scholar]
- 149.Musa F.U., Patil S., Rafiq O., Galloway P., Ball J., Morrell A. Long-term risk of intraocular pressure elevation and glaucoma escalation after deep anterior lamellar keratoplasty. Clin Exp Ophthalmol. 2012;40(8):780–785. doi: 10.1111/j.1442-9071.2012.02796.x. [DOI] [PubMed] [Google Scholar]
- 150.Tan D.T., Anshu A., Parthasarathy A., Htoon H.M. Visual acuity outcomes after deep anterior lamellar keratoplasty: a case-control study. Br J Ophthalmol. 2010;94(10):1295–1299. doi: 10.1136/bjo.2009.167528. [DOI] [PubMed] [Google Scholar]
- 151.Cursiefen C., Schaub F., Bachmann B. Update: deep anterior lamellar keratoplasty (DALK) for keratoconus. When, how and why. Ophthalmologe. 2016;113(3):204–212. doi: 10.1007/s00347-015-0204-6. [DOI] [PubMed] [Google Scholar]
- 152.Tsubota K., Kaido M., Monden Y., Satake Y., Bissen-Miyajima H., Shimazaki J. A new surgical technique for deep lamellar keratoplasty with single running suture adjustment. Am J Ophthalmol. 1998;126(1):1–8. doi: 10.1016/s0002-9394(98)00067-1. [DOI] [PubMed] [Google Scholar]
- 153.Archila E.A. Deep lamellar keratoplasty dissection of host tissue with intrastromal air injection. Cornea. 1987;6(1):56. [PubMed] [Google Scholar]
- 154.Melles G.R., Rietveld F.J., Beekhuis W.H., Binder P.S. A technique to visualize corneal incision and lamellar dissection depth during surgery. Cornea. 1999;18(1):80–86. [PubMed] [Google Scholar]
- 155.Anwar M., Teichmann K.D. Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28(3):398–403. doi: 10.1016/s0886-3350(01)01181-6. [DOI] [PubMed] [Google Scholar]
- 156.Melles G.R., Lander F., Rietveld F.J., Remeijer L., Beekhuis W.H., Binder P.S. A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br J Ophthalmol. 1999;83(3):327–333. doi: 10.1136/bjo.83.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sugita J., Kondo J. Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol. 1997;81(3):184–188. doi: 10.1136/bjo.81.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shehadeh-Mashor R., Chan C.C., Bahar I., Lichtinger A., Yeung S.N., Rootman D.S. Comparison between femtosecond laser mushroom configuration and manual trephine straight-edge configuration deep anterior lamellar keratoplasty. Br J Ophthalmol. 2014;98(1):35–39. doi: 10.1136/bjophthalmol-2013-303737. [DOI] [PubMed] [Google Scholar]
- 159.Shousha M.A., Yoo S.H., Kymionis G.D. Long-term results of femtosecond laser-assisted sutureless anterior lamellar keratoplasty. Ophthalmology. 2011;118(2):315–323. doi: 10.1016/j.ophtha.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 160.Karimian F., Feizi S. Deep anterior lamellar keratoplasty: indications, surgical techniques and complications. Middle East Afr J Ophthalmol. 2010;17(1):28. doi: 10.4103/0974-9233.61214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Akdemir M.O., Kandemir B., Sayman I.B., Selvi C., Kamil Dogan O. Comparison of contrast sensitivity and visual acuity between deep anterior lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Int J Ophthalmol. 2012;5(6):737–741. doi: 10.3980/j.issn.2222-3959.2012.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bahar I., Kaiserman I., Srinivasan S., Ya-Ping J., Slomovic A.R., Rootman D.S. Comparison of three different techniques of corneal transplantation for keratoconus. Am J Ophthalmol. 2008;146(6):905–912. doi: 10.1016/j.ajo.2008.06.034. e901. [DOI] [PubMed] [Google Scholar]
- 163.Han D.C., Mehta J.S., Por Y.M., Htoon H.M., Tan D.T. Comparison of outcomes of lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2009;148(5):744–751. doi: 10.1016/j.ajo.2009.05.028. e741. [DOI] [PubMed] [Google Scholar]
- 164.Sogutlu Sari E., Kubaloglu A., Unal M. Penetrating keratoplasty versus deep anterior lamellar keratoplasty: comparison of optical and visual quality outcomes. Br J Ophthalmol. 2012;96(8):1063–1067. doi: 10.1136/bjophthalmol-2011-301349. [DOI] [PubMed] [Google Scholar]
- 165.Yuksel B., Kandemir B., Uzunel U.D., Celik O., Ceylan S., Kusbeci T. Comparison of visual and topographic outcomes of deep-anterior lamellar keratoplasty and penetrating keratoplasty in keratoconus. Int J Ophthalmol. 2017;10(3):385–390. doi: 10.18240/ijo.2017.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang Y.M., Wu S.Q., Yao Y.F. Long-term comparison of full-bed deep anterior lamellar keratoplasty and penetrating keratoplasty in treating keratoconus. J Zhejiang Univ Sci B. 2013;14(5):438–450. doi: 10.1631/jzus.B1200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Du T.T., Fan V.C., Asbell P.A. Conductive keratoplasty. Curr Opin Ophthalmol. 2007;18(4):334–337. doi: 10.1097/ICU.0b013e3281df2cf0. [DOI] [PubMed] [Google Scholar]
- 168.Alio J.L., Claramonte P.J., Caliz A., Ramzy M.I. Corneal modeling of keratoconus by conductive keratoplasty. J Cataract Refract Surg. 2005;31(1):190–197. doi: 10.1016/j.jcrs.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 169.Asbell P.A., Maloney R.K., Davidorf J., Hersh P., McDonald M., Manche E. Conductive keratoplasty for the correction of hyperopia. Trans Am Ophthalmol Soc. 2001;99:79–84. discussion 84–77. [PMC free article] [PubMed] [Google Scholar]
- 170.Pallikaris G., Naoumidi T.L., Panagopoulou S.I., Alegakis A.K., Astyrakakis N.I. Conductive keratoplasty for low to moderate hyperopia: 1-year results. J Refract Surg. 2003;19(5):496–506. doi: 10.3928/1081-597X-20030901-04. [DOI] [PubMed] [Google Scholar]
- 171.Stahl J.E. Conductive keratoplasty for presbyopia: 3-year results. J Refract Surg. 2007;23(9):905–910. doi: 10.3928/1081-597X-20071101-07. [DOI] [PubMed] [Google Scholar]
- 172.McDonald M.B., Durrie D., Asbell P., Maloney R., Nichamin L. Treatment of presbyopia with conductive keratoplasty: six-month results of the 1-year United States FDA clinical trial. Cornea. 2004;23(7):661–668. doi: 10.1097/01.ico.0000126321.13143.a0. [DOI] [PubMed] [Google Scholar]
- 173.Tomita M., Watabe M., Ito M., Tsuru T. Conductive keratoplasty for the treatment of presbyopia: comparative study between post- and non-LASIK eyes. Clin Ophthalmol (Auckland, N.Z.) 2011;5:231–237. doi: 10.2147/OPTH.S16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chang J.S., Lau S.Y. Conductive keratoplasty to treat hyperopic overcorrection after LASIK for myopia. J Refract Surg. 2011;27(1):49–55. doi: 10.3928/1081597X-20100212-10. [DOI] [PubMed] [Google Scholar]
- 175.Hersh P.S., Fry K.L., Chandrashekhar R., Fikaris D.S. Conductive keratoplasty to treat complications of LASIK and photorefractive keratectomy. Ophthalmology. 2005;112(11):1941–1947. doi: 10.1016/j.ophtha.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 176.Claramonte P.J., Alio J.L., Ramzy M.I. Conductive keratoplasty to correct residual hyperopia after cataract surgery. J Cataract Refract Surg. 2006;32(9):1445–1451. doi: 10.1016/j.jcrs.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 177.Habibollahi A., Hashemi H., Mehravaran S., Khabazkhoob M. Visual outcomes of conductive keratoplasty to treat hyperopia and astigmatism after laser in situ keratomileusis and photorefractive keratectomy. Middle East Afr J Ophthalmol. 2011;18(3):238–242. doi: 10.4103/0974-9233.84055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kato N., Toda I., Kawakita T., Sakai C., Tsubota K. Topography-guided conductive keratoplasty: treatment for advanced keratoconus. Am J Ophthalmol. 2010;150(4):481–489. doi: 10.1016/j.ajo.2010.05.014. e481. [DOI] [PubMed] [Google Scholar]
- 179.Lyra J.M., Trindade F.C., Lyra D., Bezerra A. Outcomes of radiofrequency in advanced keratoconus. J Cataract Refract Surg. 2007;33(7):1288–1295. doi: 10.1016/j.jcrs.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 180.Kymionis G.D., Kontadakis G.A., Naoumidi T.L., Kazakos D.C., Giapitzakis I., Pallikaris I.G. Conductive keratoplasty followed by collagen cross-linking with riboflavin-UV-A in patients with keratoconus. Cornea. 2010;29(2):239–243. doi: 10.1097/ICO.0b013e3181a818ab. [DOI] [PubMed] [Google Scholar]
- 181.Vega-Estrada A., Alio J.L., Plaza Puche A.B., Marshall J. Outcomes of a new microwave procedure followed by accelerated cross-linking for the treatment of keratoconus: a pilot study. J Refract Surg. 2012;28(11):787–793. doi: 10.3928/1081597X-20121011-07. [DOI] [PubMed] [Google Scholar]
- 182.Esquenazi S., He J., Kim D.B., Bazan N.G., Bui V., Bazan H.E. Wound-healing response and refractive regression after conductive keratoplasty. J Cataract Refract Surg. 2006;32(3):480–486. doi: 10.1016/j.jcrs.2005.12.077. [DOI] [PubMed] [Google Scholar]
- 183.Moshirfar M., Anderson E., Hsu M., Armenia J.M., Mifflin M.D. Comparing the rate of regression after conductive keratoplasty with or without prior laser-assisted in situ keratomileusis or photorefractive keratectomy. Middle East Afr J Ophthalmol. 2012;19(4):377–381. doi: 10.4103/0974-9233.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Elsheikh A., Wang D., Brown M., Rama P., Campanelli M., Pye D. Assessment of corneal biomechanical properties and their variation with age. Curr Eye Res. 2007;32(1):11–19. doi: 10.1080/02713680601077145. [DOI] [PubMed] [Google Scholar]
- 185.Pallikaris I.G., Naoumidi T.L., Astyrakakis N.I. Long-term results of conductive keratoplasty for low to moderate hyperopia. J Cataract Refract Surg. 2005;31(8):1520–1529. doi: 10.1016/j.jcrs.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 186.Kymionis G.D., Naoumidi T.L., Aslanides I.M., Pallikaris I.G. Corneal iron ring after conductive keratoplasty. Am J Ophthalmol. 2003;136(2):378–379. doi: 10.1016/s0002-9394(03)00224-1. [DOI] [PubMed] [Google Scholar]
- 187.Kymionis G.D., Titze P., Markomanolakis M.M., Aslanides I.M., Pallikaris I.G. Corneal perforation after conductive keratoplasty with previous refractive surgery. J Cataract Refract Surg. 2003;29(12):2452–2454. doi: 10.1016/s0886-3350(03)00347-x. [DOI] [PubMed] [Google Scholar]
- 188.Ihalainen A. Clinical and epidemiological features of keratoconus genetic and external factors in the pathogenesis of the disease. Acta Ophthalmol Suppl. 1986;178:1–64. [PubMed] [Google Scholar]
- 189.Rabinowitz Y.S. The genetics of keratoconus. Ophthalmol Clin North Am. 2003;16(4):607–620. doi: 10.1016/s0896-1549(03)00099-3. (vii) [DOI] [PubMed] [Google Scholar]
- 190.Weed K.H., MacEwen C.J., Giles T., Low J., McGhee C.N. The Dundee University Scottish Keratoconus study: demographics, corneal signs, associated diseases, and eye rubbing. Eye (London, England) 2008;22(4):534–541. doi: 10.1038/sj.eye.6702692. [DOI] [PubMed] [Google Scholar]
- 191.Wang Y., Rabinowitz Y.S., Rotter J.I., Yang H. Genetic epidemiological study of keratoconus: evidence for major gene determination. Am J Med Genet. 2000;93(5):403–409. [PubMed] [Google Scholar]
- 192.Bechara S.J., Waring G.O., 3rd, Insler M.S. Keratoconus in two pairs of identical twins. Cornea. 1996;15(1):90–93. [PubMed] [Google Scholar]
- 193.Zadnik K., Mannis M.J., Johnson C.A. An analysis of contrast sensitivity in identical twins with keratoconus. Cornea. 1984;3(2):99–103. [PubMed] [Google Scholar]
- 194.Farjadnia M., Naderan M., Mohammadpour M. Gene therapy in keratoconus. Oman J Ophthalmol. 2015;8(1):3–8. doi: 10.4103/0974-620X.149854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gordon-Shaag A., Millodot M., Shneor E., Liu Y. The genetic and environmental factors for keratoconus. BioMed Res Int. 2015;2015:795738. doi: 10.1155/2015/795738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bisceglia L., De Bonis P., Pizzicoli C. Linkage analysis in keratoconus: replication of locus 5q21.2 and identification of other suggestive Loci. Invest Ophthalmol Vis Sci. 2009;50(3):1081–1086. doi: 10.1167/iovs.08-2382. [DOI] [PubMed] [Google Scholar]
- 197.Tyynismaa H., Sistonen P., Tuupanen S. A locus for autosomal dominant keratoconus: linkage to 16q22.3-q23.1 in Finnish families. Invest Ophthalmol Vis Sci. 2002;43(10):3160–3164. [PubMed] [Google Scholar]
- 198.Vincent A.L., Jordan C.A., Cadzow M.J., Merriman T.R., McGhee C.N. Mutations in the zinc finger protein gene, ZNF469, contribute to the pathogenesis of keratoconus. Invest Ophthalmol Vis Sci. 2014;55(9):5629–5635. doi: 10.1167/iovs.14-14532. [DOI] [PubMed] [Google Scholar]
- 199.Saee-Rad S., Raoofian R., Mahbod M. Analysis of superoxide dismutase 1, dual-specificity phosphatase 1, and transforming growth factor, beta 1 genes expression in keratoconic and non-keratoconic corneas. Mol Vis. 2013;19:2501–2507. [PMC free article] [PubMed] [Google Scholar]
- 200.van Dijk K., Parker J., Tong C.M. Midstromal isolated Bowman layer graft for reduction of advanced keratoconus: a technique to postpone penetrating or deep anterior lamellar keratoplasty. JAMA Ophthalmol. 2014;132(4):495–501. doi: 10.1001/jamaophthalmol.2013.5841. [DOI] [PubMed] [Google Scholar]
- 201.Abou Shousha M., Perez V.L., Fraga Santini Canto A.P. The use of Bowman's layer vertical topographic thickness map in the diagnosis of keratoconus. Ophthalmology. 2014;121(5):988–993. doi: 10.1016/j.ophtha.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.van Dijk K., Liarakos V.S., Parker J. Bowman layer transplantation to reduce and stabilize progressive, advanced keratoconus. Ophthalmology. 2015;122(5):909–917. doi: 10.1016/j.ophtha.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 203.Luceri S., Parker J., Dapena I. Corneal densitometry and higher order aberrations after bowman layer transplantation: 1-year results. Cornea. 2016;35(7):959–966. doi: 10.1097/ICO.0000000000000860. [DOI] [PubMed] [Google Scholar]