Abstract
Estradiol is abundant in the zebra finch auditory forebrain and has the capacity to modulate neural responses to auditory stimuli with specificity due to both hemisphere and sex. Arrhythmic song induces greater ZENK expression than rhythmic song in the caudomedial nidopallium (NCM), caudomedial mesopallium (CMM), and nucleus taeniae (Tn) of adult zebra finches. The increases in the auditory regions, NCM and CMM, may result from detection of errors in the arrhythmic song relative to the learned template. In the present study, zebra finches were treated with estradiol, the aromatase inhibitor fadrozole, or a control, and exposed to rhythmic or arrhythmic song to assess the effect of estradiol availability on neural responses to auditory rhythms. ZENK mRNA was significantly greater in the left hemisphere within NCM, CMM, and Tn. Main effects of sex were detected in both auditory regions, with increased ZENK in males in NCM and in females in CMM. In CMM, an effect of hormone treatment also existed. While no pairwise comparison was statistically significant, the pattern suggested greater ZENK expression in control compared to both fadrozole- and estradiol-treated birds. In NCM, an interaction between sex and hormone treatment suggested that the sex effect was restricted to control animals. An additional interaction in NCM among sex, stimulus rhythmicity, and hemisphere indicated that the strongest effect of laterality was present in males exposed to arrhythmic song. The hormone effects suggest that an optimal level of estradiol may exist for processing rhythmicity of auditory stimuli. The overall pattern for left lateralization parallels the left lateralization of language processing in humans and may suggest that this hemisphere is specialized for processing conspecific vocalizations. The reversed sex differences in NCM and CMM suggest that males and females differentially rely on components of the auditory forebrain for processing conspecific song.
Introduction
Estradiol (E2) influences perceptual systems in a large range of animals. The hormone may act at both peripheral and central levels. For example, estrogen receptor α is present in the retina [1–3], and estrogen receptors α and β are present in many sensory organs, including the olfactory epithelium [4], dorsal root ganglion, as well as the cochlea [5–7] in diverse species. Women with high levels of E2 show attenuation of auditory event-related potentials in the cortex [8], and auditory evoked responses have reduced latencies in the left hemisphere during high E2 phases of the menstrual cycle [9]. Auditory brainstem responses are also influenced by E2 levels in rats [10], rhesus monkeys [11], and humans [12].
In zebra finches, the caudomedial nidopallium (NCM), a secondary auditory cortical region, and nucleus taeniae (Tn), a region of the avian brain that appears analogous to the mammalian medial amygdala [13], are sites of substantial E2 synthesis and activity [14, 15]. Both areas have abundant expression of aromatase [16–18], the enzyme responsible for the metabolism of E2 from testosterone. NCM, Tn, and to a lesser degree the caudomedial mesopallium (CMM), another secondary auditory region, express both estrogen receptor α [19, 20] and the membrane bound receptor G-protein coupled receptor 30 (GPR30) [21]. Microdialysis in NCM of awake behaving zebra finches reveals a significant increase in E2 concentration during exposure to conspecific song in both males [22] and females [23].
Effects of E2 on auditory discrimination in songbirds have been assessed through multiple methodologies. Electrophysiological recordings within the zebra finch NCM with simultaneous retrodialysis of E2 showed consistently increased neural responses to conspecific songs [23–25]. The pattern is complicated, however, by increased responses to white noise detected in some studies but not others [23–25], and retrodialysis of fadrozole (a potent aromatase inhibitor) reducing overall responses to sound in one study, but not another [23, 24] [26]. Thus, while E2 within NCM seems to increase responses to conspecific song, its exact influence on auditory selectivity in this region is unclear. In seasonally breeding white-throated sparrows, no differences exist in expression of the immediate early gene ZENK in the NCM or CMM of non-breeding individuals between exposure to conspecific song or tones. However, with systemic E2 replacement higher ZENK is seen in birds exposed to song [27]. These effects are not due to an increase in ZENK in response to conspecific song, but instead to a decrease in response to tones [27]. Similar patterns of ZENK expression are seen in brain regions within the social behavior network [28] including Tn, where birds in the breeding season have greater ZENK expression in response to song than to tones or silence, and exogenous E2 induces this difference in non-breeding birds [28]. Together these studies suggest a strong role for E2 in auditory processing in songbirds.
Evidence of lateralization of processing of ecologically relevant auditory stimuli has been seen in both humans and songbirds. Linguistic perception appears consistently left lateralized in humans (reviewed in [29]). While many studies have detected lateral differences in conspecific song processing in zebra finches, the data are less consistent than those seen in humans. Depending on the housing conditions, auditory stimuli employed, and technique used to assess neural activity, right or left lateralization of function have been detected [30–34]. Thus, while the function of and conditions leading to lateral response specificity in zebra finches remain somewhat unclear, the data do suggest some lateral specialization of the processing of ecologically relevant auditory stimuli.
A growing body of evidence links rhythmic ability to linguistic skills in humans. In young adults, a positive correlation exists between the ability to process rhythmic sequences and language and literacy skills [37]. In addition, deficits in rhythm processing have been detected in a range of speech and language disorders such as dyslexia [38], stuttering [39], and specific language impairment [40, 41]. Elucidating the factors that influence neural processing of rhythm should aid in understanding these and other disorders involving rhythmic processing deficits. As a vocal learning species with a naturally rhythmic song [42], the zebra finch is a strong model to investigate rhythm processing.
Previous research from our group has investigated the effect of the rhythmicity of song on ZENK responses in NCM, CMM and Tn [43]. In all three regions, ZENK expression was increased in adult birds exposed to song modified to disrupt the natural temporal structure (arrhythmic) compared to song with the timing unaltered (rhythmic) [43]. Considering data from humans [44–46], these results were interpreted as the increased activity in NCM and CMM potentially indicating errors in arrhythmic song relative to the more natural vocalizations. Increased activity in Tn may reflect a perception of the singer as a poor potential mate [47, 48].
In order to further understand the mechanisms underlying auditory rhythm discrimination, here we investigated potential impacts of E2 on neural ZENK responses to rhythmic and arrhythmic song stimuli by both increasing and decreasing E2 availability. We tested both male and female zebra finches in order to determine whether E2 influences increased ZENK responses to rhythmic and arrhythmic songs previously detected in females compared to males in CMM [43]. We also analyzed the right and left hemispheres of each bird independently in order to determine whether any lateralization of function was present with this auditory perception task. We hypothesized that E2 facilitates auditory rhythm discrimination in zebra finches. Based on this hypothesis and our previous results, we predicted that with no hormone manipulation, greater ZENK expression would be detected in birds exposed to arrhythmic compared to rhythmic song. Furthermore, we expected that this effect would be attenuated by fadrozole treatment and exaggerated by E2 treatment.
Methods
Subjects
Zebra finches (54 male and 54 female, 9 birds in each combination of sex, hormone treatment, and song stimulus type; final group sizes presented in Table 1) hatched and were raised in walk-in aviaries (2.5′×6′×5′), housing 5-7 pairs of adult birds and their offspring. Birds were kept on a 12:12 light:dark cycle, and given ad libitum access to water, seed (Kaytee Finch Feed; Chilton, WI, USA), gravel, and cuttlebone. The birds received weekly dietary supplementation with spinach, oranges, bread, and hard boiled chicken eggs. When birds were a minimum of 90 days of age, they were transferred to walk-in single sex aviaries of the same dimensions housing 30-60 birds and allowed to acclimate to their new housing conditions for at least 10 days. These aviaries maintained auditory and visual contact with birds in the opposite sex aviary as well as mixed sex aviaries. Birds remained in the single sex aviaries until the start of hormone manipulation. All birds were between 0.59 and 1.77 years old with an average age of 1.2 years at the time of song exposure. All procedures were approved by the Institutional Animal Care and Use Committee of Michigan State University.
Table 1.
ZENK+ cells/mm2 means (standard error) for the caudomedial nidopallium (NCM), caudomedial mesopallium (CMM), and nucleus taeniae (Tn). The sample size is indicated on the second row of each cell.
| Fadrozole | Control | Estradiol | ||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |||
| CMM | Right | Rhythmic | 433.5 (60.6) 7 |
431.9 (32.4) 9 |
486.0 (59.2) 7 |
520.0 (48.1) 9 |
382.9 (39.0) 9 |
502.2 (43.4) 9 |
| Arrhythmic | 344.5 (61.4) 7 |
430.3 (33.5) 9 |
437.9 (41.6) 9 |
552.3 (49.2) 9 |
334.0 (59.8) 7 |
450.4 (53.5) 9 |
||
| Left | Rhythmic | 486.7 (59.7) 7 |
519.1 (53.4) 9 |
564.4 (90.8) 7 |
613.3 (63.8) 9 |
416.7 (46.6) 9 |
559.9 (60.5) 9 |
|
| Arrhythmic | 393.6 (71.0) 7 |
588.2 (67.3) 9 |
505.5 (60.0) 9 |
570.3 (38.3) 9 |
393.6 (74.7) 7 |
500.5 (58.9) 9 |
||
| NCM | Right | Rhythmic | 285.7 (56.4) 7 |
315.1 (61.0) 9 |
499.7 (79.9) 7 |
259.0 (31.6) 9 |
321.3 (69.9) 9 |
264.4 (38.2) 9 |
| Arrhythmic | 256.7 (62.6) 7 |
306.8 (54.5) 9 |
410.6 (66.4) 9 |
217.9 (37.9) 9 |
287.4 (101.8) 7 |
341.4 (33.8) 9 |
||
| Left | Rhythmic | 302.6 (45.1) 7 |
357.5 (65.5) 9 |
431.0 (52.8) 7 |
259.3 (25.6) 9 |
394.5 (102.9) 9 |
339.3 (85.3) 9 |
|
| Arrhythmic | 307.5 (71.6) 7 |
279.1 (52.0) 9 |
532.2 (120.2) 9 |
266.2 (42.1) 9 |
420.3 (92.34) 7 |
352.7 (41.7) 9 |
||
| Tn | Right | Rhythmic | 248.8 (32.2) 7 |
348.7 (47.4) 9 |
416.3 (80.2) 7 |
315.1 (43.3) 9 |
199.2 (41.4) 9 |
328.5 (62.6) 9 |
| Arrhythmic | 225.4 (52.2) 7 |
330.5 (54.4) 9 |
280.7 (63.1) 9 |
215.5 (32.5) 9 |
192.1 (62.6) 7 |
300.8 (57.6) 9 |
||
| Left | Rhythmic | 258.6 (32.1) 7 |
394.6 (49.7) 9 |
474.1 (70.7) 7 |
476.1 (43.1) 9 |
289.3 (53.1) 9 |
364.0 (70.8) 9 |
|
| Arrhythmic | 378.1 (98.0) 7 |
349.6 (75.6) 9 |
347.7 (57.7) 9 |
332.4 (54.1) 9 |
264.8 (52.3) 7 |
369.7 (59.4) 9 |
||
Hormone Manipulation
Three groups were created: circulating E2 was (1) increased via implants and (2) decreased using fadrozole injections; (3) control birds were treated with appropriate vehicles. Since fadrozole is water soluble and is commonly administered via injections in a saline vehicle, and E2 is lipid soluble and most easily administered via long-term treatment in Silastic capsules [49], we gave each group both an implant and daily injections in order to ensure all treatment groups experienced the same manipulations. As in [50], E2 capsules were created by packing 2mm of 17β-E2 (Steraloids, Newport, RI) into a 5mm length of Silastic tubing and sealing the ends with silicone. Blank pellets were sealed without packing with hormone. Birds were fully anesthetized with isoflurane and a blank or E2 pellet was implanted subcutaneously over the left breast muscle, and the incision was sealed with collodion. All birds were then injected with 0.05cc 0.1mg/ml Eloxiject (Henry Schein Animal Health, Dublin, OH) intraperitoneally for analgesia.
Birds implanted with E2 pellets were injected with 10μl saline into the right breast muscle. Those administered blank pellets were injected with 10μl saline (control group) or 10μl fadrozole (10μg/μl; Sigma-Aldrich, St Louis, MO) (fadrozole group) into the right breast muscle. The initial injection was administered immediately following the implant surgery while birds were anesthetized. Birds were then housed in small cages with one or two other birds of the same treatment group and sex, in a room where auditory and visual contact with the birds in the aviaries was maintained. On each of the following 6 days, each bird was given the same injection of saline or fadrozole into the right breast muscle as initially received. The effect of fadrozole on aromatase activity was not directly assessed in this study, but previous research has indicated that this schedule and dose of fadrozole treatment results in a 2/3 reduction in aromatase activity in the telencephalon of adult male and female zebra finches 24 hours following the last injection [26].
Stimulus Exposure
One day following the final injection of saline or fadrozole, birds were exposed to auditory stimuli in order to evaluate induced ZENK expression. Rhythmic and arrhythmic zebra finch songs were utilized from our previous studies on un-manipulated adult [43] and juvenile [51] zebra finches. All songs used in this experiment were novel to all subjects. Rhythmic stimuli were natural zebra finch songs with no alteration to the timing of the syllables. Arrhythmic stimuli were created by using the same songs utilized as rhythmic stimuli and modifying the duration of the inter syllable intervals in order to disrupt the natural rhythmic structure of the songs. Nine songs of each type were generated, and three subsets of the songs each containing three songs were assembled. Birds were placed, one at a time, into an acoustic isolation chamber (252-Mini Sound Shelter, IAC Acoustics, Bronx, New York, USA), a novel environment, and allowed to habituate for one hour. Each bird was then exposed to one of the subsets of three songs, with all songs presented at approximately 70dB. One of the three songs was repeated for thirty seconds, followed by thirty seconds of silence. This pattern was repeated for thirty minutes, with the order of the songs randomly determined. Immediately following the presentation of the stimuli, the birds were euthanized by rapid decapitation, and the brains were flash frozen in methyl butane in order to capture peak expression of ZENK mRNA [52]. Blood was collected from the neck following decapitation for analysis of E2 levels. Blood samples were centrifuged for 10 minutes at 10,000rpm at 4°C and plasma was separated and stored at -80°C until processed for radioimmunoassay. Retention of implants was confirmed following euthanasia.
All song exposures were recorded using a Canon Vixia HF R300 camcorder (Canon USA, Melville, NY). Recordings were reviewed to ensure birds did not sing and no extraneous background noise existed that might influence their auditory responses.
Radioimmunoassay
To obtain a general estimate of effectiveness of the hormone manipulations, a single radioimmunoassay was conducted in a manner adapted from Svec and Wade [50]. Parallelism and accurate detection of known quantities of E2 were first demonstrated with recently collected zebra finch plasma (data not shown). Samples were analyzed in three individuals from each combination of conditions (two sexes, three hormone manipulations, and two types of auditory stimuli). To provide an estimate of recovery following extraction, 2000 dpm of 3H-E2 (70 Ci/mmol; NET317250UC; PerkinElmer, Waltham, MA) was incubated at 4°C overnight with 100μl of plasma from control and fadrozole treated birds and 40μl of plasma from E2 treated birds. Steroids were then extracted twice with diethyl ether, and samples were dried under nitrogen. They were resuspended in 5μl of 100% ethanol, then combined with 500μl phosphate buffered saline (PBS) with gelatin and stored overnight at 4°C. A competitive binding assay was completed in duplicate samples, with a serially diluted standard curve (0.98–250 pg E2) in triplicate, by adding an E2 antibody (7010-2650; Bio-Rad AbD Serotec Inc., Raleigh, NC) with 3H-E2 and incubating overnight at 4°C. Six aliquots of a sample containing a known concentration of E2 were used to determine intra-assay precision, and water blanks were added as controls (n = 4). The next day, dextran-coated charcoal (0.025% dextran and 0.25% charcoal in PBS) was added and centrifuged at 3100rpm for 25min at 4°C in order to remove unbound tracer. The remaining sample was combined with scintillation fluid (UltimaGold; PerkinElmer, Waltham, MA) and analyzed with a scintillation counter (Tri-Carb 2910 TR; PerkinElmer, Waltham, MA). E2 levels were calculated by standardizing samples for individual recovery (ranged from 50-82%) and the volume assayed and compared to the standard curve. The intra-assay coefficient of variation was 12.7%.
Tissue Processing
Brains were sectioned coronally into 6 series at 20μm and thaw mounted onto SuperFrost Plus slides (Fisher Scientific, Hampton, NH). All series were stored at -80°C until further processing. One series from each animal was stained with thionin to facilitate identification of neuroanatomy.
ZENK mRNA expression in individual cells was visualized by in situ hybridization. Bacteria containing a pBlueScript SK+ plasmid with a clone of the zebra finch zenk gene were obtained from the Songbird ESTIMA clone collection [53]. Bacteria were grown overnight on lysogeny broth (LB) agar plates containing 100μg/ml ampicillin. Individual colonies were selected and allowed to grow overnight in LB with 100μg/ml ampicillin. DNA was isolated using the Wizard Plus SV Minipreps DNA Purification System (Promega, Madison, WI), and the sequence of the insert was confirmed in both directions. Bacteria were then regrown in LB with 100μg/ml ampicillin, and DNA was isolated using the NucleoBond Xtra Maxi kit (Macherey-Nagel, Bethlehem, PA). The DNA was linearized with SalI (T3) and EcoRI (T7) restriction enzymes, and stored at -20°C. Antisense (T3) and sense (T7) probes were transcribed per manufacturer instructions for the Digoxigenin RNA Labeling Kit (Roche Diagnostics, Indianapolis, IN, USA). The probes were purified by filtering through a column made with G50 Sephadex beads and stored at -80°C overnight.
Due to the large number of brain samples, tissue was processed in four runs with all groups represented in every run. One series of slides from each animal was processed using in situ hybridization for ZENK with antisense probes, and a second series from a minimum of two animals was used in each run for a control to confirm the absence of labeling with sense probes (not shown). Tissue was warmed to room temperature, then fixed in 4% paraformaldehyde for 10 minutes. Slides were rinsed 3×3 minutes in 0.1M PBS, and incubated in 0.25% acetic anhydride in triethanolamine-hydrochloride for 10 minutes. They were then washed 3×5 minutes in PBS and allowed to equilibrate in hybridization buffer for one hour. Slides were incubated at 56°C overnight in 250ng/μl anti-sense or sense probe in hybridization buffer, and the next day rinsed 2×5min in 2X SSC at 60°C. The slides were then incubated in 20μg/ml RNase in 2X SSC at 37°C for 30 minutes, rinsed for 15 minutes in 0.2X SSC at 37°C, then 3×5min in 0.2X SSC at 60°C. The brain sections were allowed to return to room temperature, then rinsed 3×5min in maleic acid buffer with 0.1% Tween-20 (MABT). The slides were incubated in 0.9% H2O2 in MABT for 30 min and rinsed 3×5min in MABT. They were incubated in 5% normal sheep serum in MABT for 30 min, then rinsed 3×5min in MABT. Next, the slides were incubated in 0.5μl/ml anti-digoxigenin-AP Fab fragments (Roche Diagnostics, Indianapolis, IN) in MABT for 2 hours, then rinsed 2×3min in MABT. Slides were rinsed 3×5min in detection buffer to equilibrate. The color reaction was performed by incubating slides in 4.5μl/ml NBT and 3.5μl/ml BCIP (Roche Diagnostics) in detection buffer for 1 hour 55min. This produced a blue reaction product in specific cells in sections treated with the antisense probe and no labeling in those exposed to the sense probe. Slides were rinsed 3×5min in TE buffer to stop the color reaction, then dehydrated and coverslipped with VectaMount (Vector Laboratories Inc., Burlingame, CA, USA). A qualitative assessment of the overall staining revealed numerous brain regions with minimal or no ZENK labeling, including HVC and the robust nucleus of the arcopallium (RA), indicating the specificity of the response in auditory and social behavior network regions.
An investigator blind to the stimulus exposure condition, sex, and age of the birds conducted analysis of all slides using ImageJ software (National Institutes of Health, Bethesda MD). All regions of interest were analyzed bilaterally in two adjacent sections. Boxes were placed within NCM, CMM and Tn as described and depicted in [43], and all cells containing a blue filled cytoplasm with a clear hole of a nucleus that were the appropriate size and shape were counted. For NCM, a 0.525 mm*0.393 mm box was placed with the medial corner under the hippocampus at the point where the ventricle begins to curve ventrally to run parallel with the midline. For CMM, a 0.496 mm*0.205 mm box was placed the ventricle just lateral to where it curves ventrally toward the midline between A 1.6 and A 1.2 from a songbird brain atlas [54]. For Tn, a 0.238 mm*0.244 mm box was placed near the ventral edge of the telencephalic lobe where a corner is formed by the ventral and medal edges of the lobe. The density of ZENK expressing cells (labeled nuclei per unit area) was calculated for each brain region and average density values were calculated for each region of interest in each hemisphere.
Data Analysis
A few animals were excluded from analysis of a particular brain region due to damage to individual tissue sections. Final sample sizes are indicated in Table 1. Separate mixed model ANOVAs were conducted for each brain region with hemisphere as a within subjects factor and sex, hormone manipulation, and stimulus type (rhythmic or arrhythmic) as between subjects factors. A main effect of hormone in CMM was followed with a post hoc Scheffe test to determine which pairs of hormone conditions differed. Two-way and three-way interactions detected within NCM were each probed with subsequent ANOVAs within groups (see Results for details). Bonferroni corrections were used to adjust for multiple comparisons, with adjusted α-levels indicated with each result. A one-way ANOVA was conducted on plasma estradiol levels with hormone manipulation as a factor. As only a subset of the birds in each group was evaluated, the data were collapsed across sex and stimulus type; we assumed that these factors would not influence estimates of the effectiveness of E2 treatments. All statistics were calculated using SPSS (IBM, Armonk, NY).
Results
Hormone manipulation
Two samples were eliminated from analysis due to technical mistakes. Five samples, two from fadrozole-treated and three from the control birds, were below the limit of detectability for this assay. To be conservative they were assigned the lowest detectable value for the purpose of statistical analysis. A significant effect of hormone treatment on circulating E2 levels was detected (F2,31=150.10, p<0.001; Figure 1). A post hoc Scheffe test indicated a greater concentration of the hormone in the E2-treated compared to both the control and fadrozole-treated groups (both p<0.05). However, the values were not significantly different in the control and fadrozole-treated groups (p>0.05).
Figure 1.
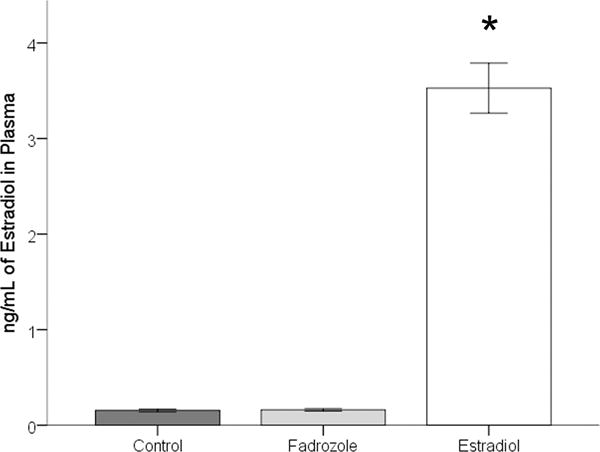
Concentration of estradiol detected in plasma. A significant increase in the concentration detected in estradiol-treated compared to control or fadrozole-treated birds is indicated by *. No difference was detected between control and fadrozole treated birds.
CMM
A significant main effect of hemisphere was detected, with a greater density of ZENK expressing cells in the left hemisphere than the right (F1,88=35.917, p<0.001; Figure 2). A main effect of sex was detected (F1,88=8.557, p=0.004; Figure 2), such that females had a greater density of ZENK expressing cells than males. The hormone manipulation also produced a significant effect on ZENK expression (F2,88=3.468, p=0.036; Figure 2). While post hoc comparisons indicated no significant differences between any of the three groups, on average, the density of ZENK+ cells was highest in the control birds compared to those with hormone manipulations. No main effect of rhythm stimulus type was detected (F1,88=1.308, p=0.256), and no significant interactions were detected between any of the variables (all F<1.338, p>0.267). Data for individual groups are presented in Table 1.
Figure 2.
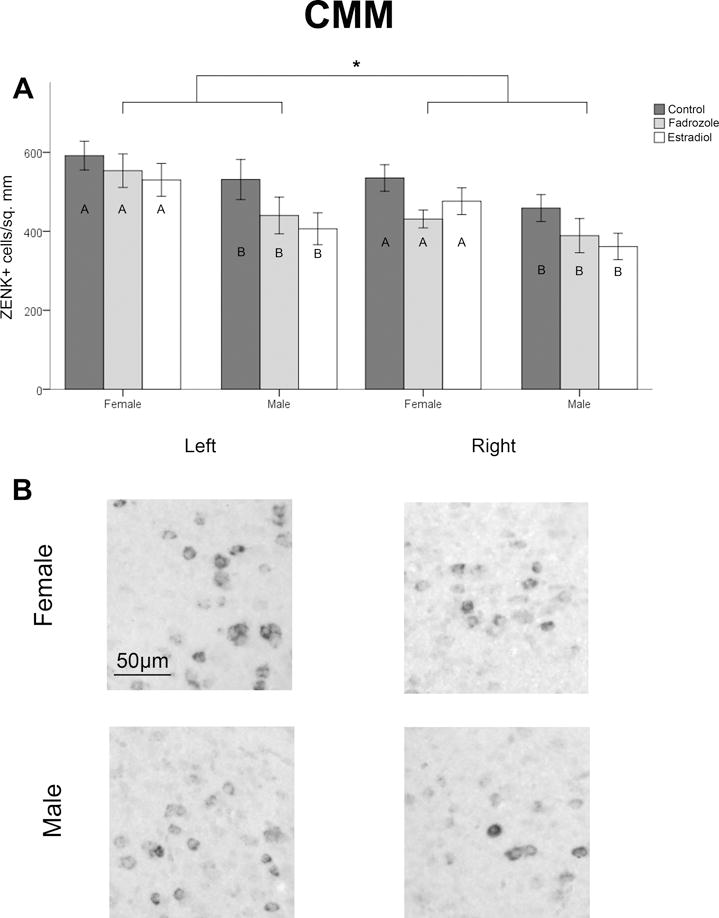
Density of cells expressing ZENK in CMM. Panel A depicts this measure across sexes and hormone conditions (mean ± SEM). Data are collapsed across stimulus type (rhythmic and arrhythmic), as no significant effects of this variable were detected. A main effect of hemisphere, with greater density of ZENK expression on the left, is indicated by *. A main effect of sex (female > male) is indicated by different letters within the bars. The photographs in panel B depict representative examples of ZENK expression in birds exposed to arrhythmic song in the left and right hemisphere of a female, and the left and right hemisphere of a male.
NCM
As seen in CMM, a significant main effect of hemisphere was detected with a greater density of ZENK expressing cells on the left compared to the right (F1,88=8.586, p=0.004; Figure 3). An increase in these cells was also found in males compared to females (F1,88=4.253, p=0.042; Figure 3). No main effects of stimulus rhythmicity (F1,88=0.014, p=0.908) or hormone manipulation (F2,88=0.886, p=0.416) were detected.
Figure 3.
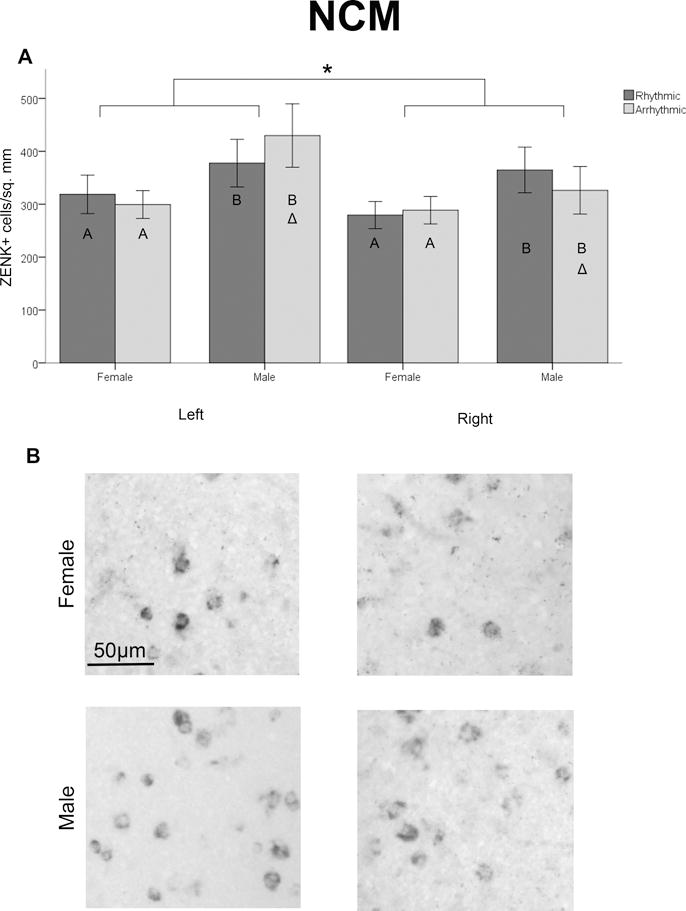
ZENK expressing cells in NCM. Panel A depicts the density of cells containing ZENK mRNA across the sexes and auditory stimuli (mean ± SEM). A main effect of hemisphere, with greater expression on the left, is indicated by *. Different letters within the bars indicate a main effect of sex, with greater ZENK expression in males compared to females. A three way interaction was detected, greater ZENK density was seen on the left than the right in arrhythmic song exposed males, indicated by Δ. Panel B shows pictures of representative samples of ZENK expression in control males and females exposed to rhythmic song.
Two interactions were detected among variables, which are described immediately below. None of the other possible interactions among hemisphere, sex, rhythm type, and treatment were statistically significant (all F<2.578, p>0.081). Data for the individual groups are presented in Table 1.
First, there was an interaction between sex and hormone treatment (F2,88=4.196, p=0.018; Figure 4). This result was probed in two ways. We conducted separate one-way ANOVAs within each sex with hormone condition as a factor, and no effect of hormone treatment was detected in males or females (F2,43=2.915, p=0.065; F2,51=1.541, p=0.224 respectively; α=0.025). We also used independent t-tests to analyze the effect of sex within each of the hormone manipulations. Here, within control birds, a greater density of ZENK expressing cells was present in males compared to females (t30=3.728, p=0.001; α=0.017), whereas no effect of sex was detected in the fadrozole- (t30=0.462, p=0.648; α=0.017) or E2- treated (t30=0.465, p=0.645; α=0.017) animals.
Figure 4.
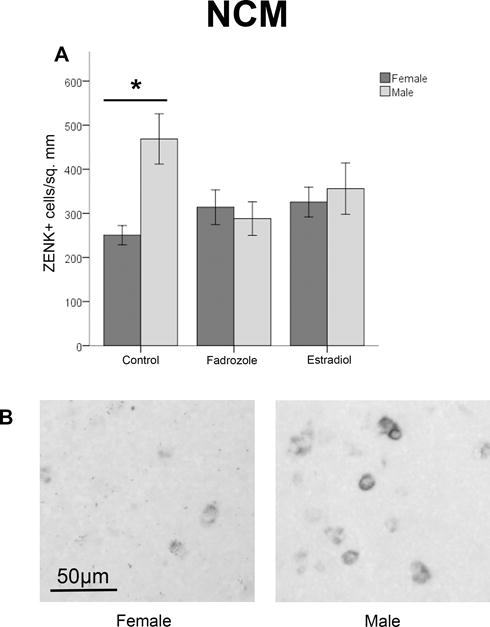
ZENK expression in the NCM of males and females across hormone manipulations. Panel A depicts the density of cells (mean ± SEM) across the three treatment groups. An interaction was detected; expression was greater in males than females only in the control animals, indicated by *. Panel B shows representative images from a control female and male.
Second, there was a significant three-way interaction between hemisphere, sex and rhythm stimulus type (F1,88=5.164, p=0.025; Figure 3). This result was further investigated with two-way, mixed-model ANOVAs (hemisphere × sex) within each auditory stimulus. A sex × hemisphere interaction was detected in birds exposed to arrhythmic (F1,48=5.804, p=0.020), but not rhythmic (F1,48=0.475, p=0.494) song. Therefore, within birds exposed to the arrhythmic song only, paired t-tests were used to assess the effects of hemisphere within each of the two sexes. The only significant effect detected was an increased density of cells expressing ZENK mRNA in the left compared to the right hemisphere of arrhythmic song exposed males (t22=2.917, p=0.008, α=0.0125; Figure 3).
Tn
Similar to both auditory regions, there was a significant main effect of hemisphere, with a greater density of ZENK expressing cells in the left compared to the right hemisphere (F1,88=18.936, p<0.001). There were no main effects of sex (F1,88=2.545, p=0.114), rhythm type (F1,88=2.324, p=0.131) or hormone manipulation (F2,88=1.963, p=0.147). There were also no significant interactions between any combination of hemisphere, sex, rhythm type, and hormone (all F<2.606, p>0.079). Data are presented in Table 1.
Discussion
Summary of Specific Effects
Across NCM, CMM and Tn, a greater density of cells expressing ZENK mRNA was detected in the left hemisphere. Sex differences in ZENK expression were detected in both auditory regions, although in opposite directions, with a higher density of cells in males in NCM and in females in CMM. An effect of hormone manipulation was also seen in CMM, and while no pairwise comparisons were statistically significant, the pattern suggested greater ZENK expression in the control birds compared to both the E2- and fadrozole-treated groups, across both sexes and song stimuli.
Statistically significant interactions were detected just in NCM. In the control group only, the density of cells expressing ZENK mRNA was greater in males than females. ZENK expressing cells were also increased in the left compared to the right hemisphere in males exposed to arrhythmic, but not rhythmic, song. Thus, these groups contributed substantially to the main effect of hemisphere. In the present study, effects of rhythmicity of the song stimulus were limited, but may have been reduced by particular methodological differences from our earlier studies [43, 51].
Methodological Issues
The lack of effects due to song stimulus type differs from our previous finding of an increased density of ZENK+ cells in response to arrhythmic compared to rhythmic song across all three brain areas investigated [43]. Several methodological aspects may relate to the differences between studies. For example, birds in the present study were housed with only one or two conspecifics, whereas they were kept in large group aviaries in our earlier work. It is possible that the current conditions were more stressful. Keeping zebra finches individually housed while maintaining auditory and visual contact with conspecifics can cause changes in ZENK expression in the social behavior network, which includes Tn [55]. The experience of undergoing anesthesia, a hormone implant, and a week of daily injections likely also induced some stress compared to our earlier study on adults. Future studies could assess cortisol levels to assess the stress effects of the manipulations.
Perhaps the most important difference between our studies, however, involved the quantification of ZENK protein in the earlier work [43] versus mRNA in the present study. This change was made because the antibody used in the previous study was no longer available. The relationship between mRNA and protein expression can vary widely between genes and tissue types [56, 57].
Previous studies assessing the timeline of peak ZENK mRNA expression in the zebra finch brain have not compared across sexes, hemispheres, or brain regions. Thus without an unstimulated control group for comparison, the possibility remains that sex and laterality differences detected in the present study were influenced by auditory exposure prior to our testing, due to possible variability in the ZENK expression timeline.
Our results also differ from studies indicating E2′s capacity to improve auditory discrimination to types of stimuli not used in the present experiment, such as conspecific vs. heterospecific song, tones, reverse song, or white noise [23, 24, 27, 28, 58]. It is unclear whether E2 can also modulate responses to auditory rhythms, as the anticipated difference between our stimulus types was not detected. Further work is needed to resolve this issue. NCM is a large, heterogeneous brain region. In white-throated sparrows, E2 enhances the ZENK response to song compared to tones in the rostral and medial portions of NCM, but not in other regions of the nucleus [58]. The white-throated sparrow brains were sectioned in the sagittal plane [58], whereas the brains in the present study were sectioned coronally in order to maintain consistency with our earlier work [43, 51]. While the area of NCM analyzed here is likely in the rostral region described in the sparrow study, it is difficult to make exact comparisons across different planes of section. It is possible that regions other than where we sampled might show an effect of E2.
It is also possible that the hormone manipulations did not provide sufficiently large differences in brain E2 availability from the control group. However, we think this is unlikely. While our assay was not sensitive enough to detect a significant reduction in circulating E2 in fadrozole compared to the control birds, the same fadrozole administration over only six days substantially reduces aromatase in the forebrain as a whole [26], where NCM, CMM and Tn are located. The pattern of ZENK expression across manipulations also indicates that fadrozole was effective in the brain, as the sex difference detected in NCM in control animals was eliminated by this drug.
A final methodological consideration is the duration of the hormone manipulations. E2 is rapidly synthesized in the NCM of zebra finches in response to conspecific song [22, 23]. In addition, the effects of E2 on auditory responses can appear rapidly and are likely mediated through a non-classical membrane bound receptor such as GPER1 (previously known as GPR30) [14]. Continuous exposure to E2 for one week may have overwhelmed these rapid E2 responses, washing out any modulation of auditory response that increased E2 may facilitate. We chose to use a longer term E2 modulation because it provided a minimally invasive way to influence E2 levels that had the possibility of impacting modulation through multiple E2 receptor types [26]. Future studies using infusion of fadrozole or E2 into NCM could allow for assessment of rapid E2 effects on rhythm perception.
Lateralization
The finding in the present study of a higher density of cells expressing ZENK mRNA in the left hemisphere across brain regions adds to a complex literature on lateralization of auditory perception. In humans, left lateralization of linguistic functions appears consistent across production and perception (reviewed in [29]). These results parallel the left lateralization following perception of conspecific vocalizations in the present study. However, the literature in songbirds is less consistent than that from humans. Similar to the present experiment, increased ZENK expression was detected in the left NCM of juvenile zebra finches exposed to novel conspecific song [30]. However, the same study did not find lateral differences in adults. Our previous work on juvenile zebra finches, using the same auditory stimuli as the current study [51], did not initially assess hemisphere as a factor, but further analysis of the data reveals no significant differences in the density of cells expressing ZENK protein between the left and right sides of the brain. Electrophysiological studies have also provided mixed results. Increased response strength in the left hemisphere has been detected among fast-learning birds trained in a GO/NoGO paradigm [31]. Zebra finches exposed to four or nine days of a heterospecific acoustic environment also showed left lateralization of electrophysiological responses in NCM [32], but those housed under typical conditions with conspecific song exposure showed right lateralization of NCM activity [32, 33]. An fMRI study indicated greater differences in response strength between bird’s own song, novel conspecific song, tutor song and tones in the right hemisphere of the zebra finch brain [34]. In contrast, in another fMRI study, the data suggest greater activation of the left hemisphere (although lateralization was not specifically assessed) [35]. This study also found greater influence of aromatase inhibition on neural activity in the left hemisphere [35]. Given the inconsistencies that can be detected across different measures of neural activity, and the fact that it is possible for neurons to fire without inducing ZENK expression (reviewed in [36]), further work is necessary to draw conclusions about the functional significance of lateral differences in gene expression detected in the present study.
CMM
The sex difference in CMM, with greater ZENK expression in females compared to males, parallels results from our previous study with adult zebra finches without hormone manipulations [43]. The quality of songs is an indicator to females of the fitness of males [59]. Thus, the consistent increase in activity in females compared to males in CMM may relate to females’ use of CMM for evaluation of songs of potential mates, a function typically not employed by males.
The trend for increased ZENK expression in control animals compared to those treated with either E2 or fadrozole suggests that an optimal level of the hormone may exist. This pattern of results is consistent with a study that found a trend in NCM for a reduced electrophysiological response to novel conspecific song in fadrozole- and E2-treated birds compared to saline-treated controls [60]. Together these results suggest that too much or too little available E2 within the auditory forebrain may inhibit optimal neural responses to auditory stimuli. Fadrozole can reduce both the aromatase activity within the zebra finch brain [26] and the total amount of E2 present in the telencephalon [61]. While our assay did not detect differences in plasma E2 levels between the control and fadrozole treated birds, those receiving the estrogen synthesis inhibitor likely experienced less E2 availability in the auditory brain due to decreased local synthesis as well as a possible reduction in the E2 released within NCM during song exposure [22, 23].
NCM
In contrast to the effect seen in CMM, a greater density of cells expressing ZENK mRNA was seen in males compared to females in NCM. While this effect was not detected in our previous study [43], a similar pattern of activity was detected in the NCM of zebra finches using electrophysiology, with males showing an enhanced response to novel conspecific songs compared to females [60]. The interaction between hormone manipulation and sex in the present study indicates that this sex difference is limited to the control animals. Similar to the present data from CMM, this result suggests that there may be an optimal level of circulating E2, and an increase or decrease can diminish sex differences in auditory processing. In addition, the opposite directions of sex differences between NCM and CMM may indicate that these areas are functionally specialized. Specifically, NCM may be more involved in processing by males for nest site defense, whereas CMM may aid in processing of song for value as a potential mate.
The interaction between sex, hemisphere, and rhythmicity of the song stimuli in NCM could present a challenge for interpretation. A significant increase in ZENK expression in the left relative to the right hemisphere was detected only in males exposed to arrhythmic song; it is difficult to speculate on potential reasons for such a specific effect. However, a trend for a greater density of ZENK+ cells in the left hemisphere existed in all other combinations of sex and rhythm type. Thus, while data from these males was largely responsible for the main effect of lateralization in NCM, it is unlikely that this interaction represents a functional difference across groups.
Tn
The lack of effect of stimulus rhythmicity detected in Tn contrasts with the results previously seen in adults that did not receive hormone manipulations [43], but both studies are consistent in finding no sex differences in ZENK expression in Tn. It would be parsimonious to suggest that the hormone manipulations in the present study eliminated the increased response to arrhythmic song [43], however the control birds also responded similarly to the two types of auditory stimuli (Table 1). Thus, other differences from our previous study likely influenced the results.
Conclusions
Overall the results of the present study suggest a strong left lateralization of neural activity in response to conspecific songs across brain regions, regardless of E2 levels or rhythm type. This effect parallels the lateralization of language seen in humans. In addition, the data suggest the possibility of an optimal level of E2 at which these neural responses are strongest. Sex differences were also opposite between auditory regions, with greater activity in NCM in males and in CMM in females. Further studies are needed to clarify potential relationships between E2 and rhythm perception, as well as the functional significance of sex differences and lateral differences in gene expression.
Acknowledgments
The authors thank Kelsey Stevenson and Rachel Smeenge for analysis of the videos and technical assistance, Katherine Jones for creation of the song stimuli, and Camilla Peabody for conducting the radioimmunoassay, and Claudio Mello for providing the ZENK clone. This work was partially funded by National Institutes of Health R01-MH096705 and Michigan State University’s program for Research in Autism, Intellectual and Neurodevelopmental Disabilities (RAIND).
Footnotes
The authors of the manuscript have no conflicts of interest to declare.
References
- 1.Wickham LA, et al. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol Scand. 2000;78(2):146–53. doi: 10.1034/j.1600-0420.2000.078002146.x. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi K, et al. Estrogen receptor expression in bovine and rat retinas. Invest Ophthalmol Vis Sci. 1998;39(11):2105–10. [PubMed] [Google Scholar]
- 3.Begay V, et al. Detection of estrogen receptor mRNA in trout pineal and retina: estradiol-17 beta modulates melatonin production by cultured pineal photoreceptor cells. Gen Comp Endocrinol. 1994;93(1):61–9. doi: 10.1006/gcen.1994.1008. [DOI] [PubMed] [Google Scholar]
- 4.Barni T, et al. Sex steroids and odorants modulate gonadotropin-releasing hormone secretion in primary cultures of human olfactory cells. J Clin Endocrinol Metab. 1999;84(11):4266–73. doi: 10.1210/jcem.84.11.6150. [DOI] [PubMed] [Google Scholar]
- 5.Stenberg AE, et al. Mapping of estrogen receptors alpha and beta in the inner ear of mouse and rat. Hear Res. 1999;136(1-2):29–34. doi: 10.1016/s0378-5955(99)00098-2. [DOI] [PubMed] [Google Scholar]
- 6.Noirot IC, et al. Presence of aromatase and estrogen receptor alpha in the inner ear of zebra finches. Hear Res. 2009;252(1-2):49–55. doi: 10.1016/j.heares.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Maruska KP, Fernald RD. Steroid receptor expression in the fish inner ear varies with sex, social status, and reproductive state. BMC Neurosci. 2010;11:58. doi: 10.1186/1471-2202-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walpurger V, et al. Effects of the menstrual cycle on auditory event-related potentials. Horm Behav. 2004;46(5):600–6. doi: 10.1016/j.yhbeh.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Tillman GD. Estradiol levels during the menstrual cycle differentially affect latencies to right and left hemispheres during dichotic listening: an ERP study. Psychoneuroendocrinology. 2010;35(2):249–61. doi: 10.1016/j.psyneuen.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Coleman JR, et al. Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hear Res. 1994;80(2):209–15. doi: 10.1016/0378-5955(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 11.Golub MS, Germann SL, Hogrefe CE. Endocrine disruption and cognitive function in adolescent female rhesus monkeys. Neurotoxicol Teratol. 2004;26(6):799–809. doi: 10.1016/j.ntt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Caruso S, et al. Auditory brainstem response in premenopausal women taking oral contraceptives. Hum Reprod. 2003;18(1):85–9. doi: 10.1093/humrep/deg003. [DOI] [PubMed] [Google Scholar]
- 13.Kuenzel WJ, et al. The avian subpallium: new insights into structural and functional subdivisions occupying the lateral subpallial wall and their embryological origins. Brain Res. 2011;1424:67–101. doi: 10.1016/j.brainres.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remage-Healey L, Jeon SD, Joshi NR. Recent evidence for rapid synthesis and action of oestrogens during auditory processing in a songbird. J Neuroendocrinol. 2013;25(11):1024–31. doi: 10.1111/jne.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heimovics SA, et al. Rapid and widespread effects of 17beta-estradiol on intracellular signaling in the male songbird brain: a seasonal comparison. Endocrinology. 2012;153(3):1364–76. doi: 10.1210/en.2011-1525. [DOI] [PubMed] [Google Scholar]
- 16.Shen P, et al. Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Brain Res Mol Brain Res. 1994;24(1-4):227–37. doi: 10.1016/0169-328x(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 17.Balthazart J, et al. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): implications for the neural action of steroids and nuclear definition in the avian hypothalamus. J Neurobiol. 1996;31(2):129–48. doi: 10.1002/(SICI)1097-4695(199610)31:2<129::AID-NEU1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 18.Saldanha CJ, et al. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423(4):619–30. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.Gahr M, Flugge G, Guttinger HR. Immunocytochemical localization of estrogen-binding neurons in the songbird brain. Brain Res. 1987;402(1):173–7. doi: 10.1016/0006-8993(87)91063-8. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs EC, Arnold AP, Campagnoni AT. Zebra finch estrogen receptor cDNA: cloning and mRNA expression. J Steroid Biochem Mol Biol. 1996;59(2):135–45. doi: 10.1016/s0960-0760(96)00096-9. [DOI] [PubMed] [Google Scholar]
- 21.Acharya KD, Veney SL. Characterization of the G-protein-coupled membrane-bound estrogen receptor GPR30 in the zebra finch brain reveals a sex difference in gene and protein expression. Dev Neurobiol. 2012;72(11):1433–46. doi: 10.1002/dneu.22004. [DOI] [PubMed] [Google Scholar]
- 22.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11(11):1327–34. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remage-Healey L, et al. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107(6):1621–31. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remage-Healey L, et al. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107(8):3852–7. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remage-Healey L, Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci. 2012;32(24):8231–41. doi: 10.1523/JNEUROSCI.1114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wade J, et al. Fadrozole: a potent and specific inhibitor of aromatase in the zebra finch brain. Gen Comp Endocrinol. 1994;94(1):53–61. doi: 10.1006/gcen.1994.1059. [DOI] [PubMed] [Google Scholar]
- 27.Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23(6):1523–9. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- 28.Maney DL, et al. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511(2):173–86. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- 29.Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 30.Moorman S, et al. Human-like brain hemispheric dominance in birdsong learning. Proc Natl Acad Sci U S A. 2012;109(31):12782–7. doi: 10.1073/pnas.1207207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell BA, Phan ML, Vicario DS. Neural responses in songbird forebrain reflect learning rates, acquired salience, and stimulus novelty after auditory discrimination training. J Neurophysiol. 2015;113(5):1480–92. doi: 10.1152/jn.00611.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang LM, Vicario DS. Exposure to a novel stimulus environment alters patterns of lateralization in avian auditory cortex. Neuroscience. 2015;285:107–18. doi: 10.1016/j.neuroscience.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phan ML, Vicario DS. Hemispheric differences in processing of vocalizations depend on early experience. Proc Natl Acad Sci U S A. 2010;107(5):2301–6. doi: 10.1073/pnas.0900091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voss HU, et al. Functional MRI of the zebra finch brain during song stimulation suggests a lateralized response topography. Proc Natl Acad Sci U S A. 2007;104(25):10667–72. doi: 10.1073/pnas.0611515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Groof G, et al. Topography and Lateralized Effect of Acute Aromatase Inhibition on Auditory Processing in a Seasonal Songbird. Journal of Neuroscience. 2017;37(16):4243–4254. doi: 10.1523/JNEUROSCI.1961-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ball GF, Gentner TQ. They’re playing our song: gene expression and birdsong perception. Neuron. 1998;21(2):271–274. doi: 10.1016/s0896-6273(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 37.Grube M, Cooper FE, Griffiths TD. Auditory temporal-regularity processing correlates with language and literacy skill in early adulthood. Cogn Neurosci. 2013;4(3-4):225–30. doi: 10.1080/17588928.2013.825236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muneaux M, et al. Deficits in beat perception and dyslexia: evidence from French. Neuroreport. 2004;15(8):1255–9. doi: 10.1097/01.wnr.0000127459.31232.c4. [DOI] [PubMed] [Google Scholar]
- 39.Wieland EA, et al. Evidence for a rhythm perception deficit in children who stutter. Brain Lang. 2015;144:26–34. doi: 10.1016/j.bandl.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corriveau K, Pasquini E, Goswami U. Basic auditory processing skills and specific language impairment: a new look at an old hypothesis. J Speech Lang Hear Res. 2007;50(3):647–66. doi: 10.1044/1092-4388(2007/046). [DOI] [PubMed] [Google Scholar]
- 41.Corriveau KH, Goswami U. Rhythmic motor entrainment in children with speech and language impairments: tapping to the beat. Cortex. 2009;45(1):119–30. doi: 10.1016/j.cortex.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Norton P, Scharff C. “Bird Song Metronomics”: Isochronous Organization of Zebra Finch Song Rhythm. Frontiers in Neuroscience. 2016;10(309) doi: 10.3389/fnins.2016.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lampen J, et al. Arrhythmic song exposure increases ZENK expression in auditory cortical areas and nucleus taeniae of the adult zebra Finch. PLoS One. 2014;9(9):e108841. doi: 10.1371/journal.pone.0108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang. 2006;96(3):280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tourville JA, Reilly KJ, Guenther FH. Neural mechanisms underlying auditory feedback control of speech. Neuroimage. 2008;39(3):1429–43. doi: 10.1016/j.neuroimage.2007.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geiser E, Notter M, Gabrieli JD. A corticostriatal neural system enhances auditory perception through temporal context processing. J Neurosci. 2012;32(18):6177–82. doi: 10.1523/JNEUROSCI.5153-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Svec LA, Licht KM, Wade J. Pair bonding in the female zebra finch: a potential role for the nucleus taeniae. Neuroscience. 2009;160(2):275–83. doi: 10.1016/j.neuroscience.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujii TG, Ikebuchi M, Okanoya K. Auditory Responses to Vocal Sounds in the Songbird Nucleus Taeniae of the Amygdala and the Adjacent Arcopallium. Brain Behav Evol. 2016;87(4):275–89. doi: 10.1159/000447233. [DOI] [PubMed] [Google Scholar]
- 49.Dziuk PJ, Cook B. Passage of steroids through silicone rubber. Endocrinology. 1966;78(1):208–11. doi: 10.1210/endo-78-1-208. [DOI] [PubMed] [Google Scholar]
- 50.Svec LA, Wade J. Estradiol induces region-specific inhibition of ZENK but does not affect the behavioral preference for tutored song in adult female zebra finches. Behav Brain Res. 2009;199(2):298–306. doi: 10.1016/j.bbr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lampen J, et al. Neural Responses to Rhythmicity in Juvenile Male and Female Zebra Finches. doi: 10.1016/j.beproc.2017.12.003. Behav Processes, Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994;14(11 Pt 1):6652–66. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Replogle K, et al. The Songbird Neurogenomics (SoNG) Initiative: community-based tools and strategies for study of brain gene function and evolution. BMC Genomics. 2008;9:131. doi: 10.1186/1471-2164-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stokes TM, Leonard CM, Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol. 1974;156(3):337–74. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- 55.Elie JE, et al. Housing conditions and sacrifice protocol affect neural activity and vocal behavior in a songbird species, the zebra finch (Taeniopygia guttata) C R Biol. 2015;338(12):825–37. doi: 10.1016/j.crvi.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Maier T, Guell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583(24):3966–73. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 57.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13(4):227–32. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanford SE, Lange HS, Maney DL. Topography of estradiol-modulated genomic responses in the songbird auditory forebrain. Dev Neurobiol. 2010;70(2):73–86. doi: 10.1002/dneu.20757. [DOI] [PubMed] [Google Scholar]
- 59.Nowicki S, Searcy WA. Song function and the evolution of female preferences: why birds sing, why brains matter. Ann N Y Acad Sci. 2004;1016:704–23. doi: 10.1196/annals.1298.012. [DOI] [PubMed] [Google Scholar]
- 60.Yoder KM, et al. He hears, she hears: are there sex differences in auditory processing? Dev Neurobiol. 2015;75(3):302–14. doi: 10.1002/dneu.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soma KK, et al. Dehydroepiandrosterone metabolism by 3beta-hydroxysteroid dehydrogenase/Delta5-Delta4 isomerase in adult zebra finch brain: sex difference and rapid effect of stress. Endocrinology. 2004;145(4):1668–77. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]


