Neurons in the human brain communicate with each other by releasing chemical messengers called neurotransmitters (electrical synapses are present, but in the distinct minority). The utility cycle of all neurotransmitter molecules is similar: they are synthesized and packaged into vesicles in the presynaptic cell; they are released from the presynaptic cell and bind to receptors on one or more postsynaptic cells, and once released into the synaptic cleft, they are rapidly removed or degraded. The total number of neurotransmitters is not known, but is likely to be well over 100. Despite this diversity, these agents can be classified into three broad categories: small-molecule neurotransmitters, neuropeptides, and unconventional transmitters. In general, small-molecule neurotransmitters mediate rapid reactions, whereas neuropeptides tend to modulate slower, ongoing brain functions. Abnormal transmitter functions may cause a wide range of neurological and psychiatric disorders; as a result altering the actions of neurotransmitters by pharmacological or other means is central to many modern therapeutic strategies.
Neurotransmitters are chemical signals released from presynaptic nerve terminals into the synaptic cleft. The subsequent binding of neurotransmitters to specific receptors on postsynaptic neurons (or other cell classes) briefly changes the electrical properties of the target cells. Over the years, a number of formal criteria have emerged that definitively identify a substance as a neurotransmitter. Identifying the neurotransmitter active at any particular synapse remains a difficult undertaking, and for many synapses (particularly in the brain), the nature of the neurotransmitter is not yet known. Substances that have not met all the criteria are referred to as putative neurotransmitters.
The special characteristics of neurotransmitters are made clearer by comparing their actions to another type of chemical signal, the hormones secreted by the endocrine system. Hormones typically influence target cells far removed from the hormone-secreting cell. This “action at a distance” is achieved by the release of hormones into the bloodstream. In contrast, the distance over which neurotransmitters act is always much less. At many synapses, transmitters bind only to receptors on the postsynaptic cell that directly underlies the presynaptic terminal, in such cases, the transmitters act over distances less than a micrometer. At other synapses, neurotransmitters diffuse locally to alter the electrical properties of multiple postsynaptic (and sometimes presynaptic) cells in the vicinity of the presynaptic release sites. While the distinction between neurotransmitters and hormones is generally clear-cut, a substance can act as a neurotransmitter in one region of the brain while serving as a hormone elsewhere. For example, vasopressin and oxytocin, two peptide hormones that are released into the circulation from the posterior pituitary, also function as neurotransmitters at a number of central synapses. A number of other peptides also serve as both hormones and neurotransmitters.
By the 1950s, the list of neurotransmitters had expanded to include three amines – epinephrine, dopamine, and serotonin – in addition to acetylcholine (Ach). Over the following decade, four amino acids – glutamate, aspartate, ã-aminobutyric acid (GABA), and glycine – were also shown to be neurotransmitters. Subsequently, other small molecules, including nor-epinephrine and histamine, were identified a transmitters, and considerable evidence now suggests that several purines (such as ATP, adenosine, and AMP) should be added to the list. The most recent class of molecules now know to be transmitters are a large number of polypeptides; since the 1970s, more than 100 such molecules have been shown to meet at least some of the criteria for a neurotransmitter. For purposes of discussion, it is useful to separate this variety of agents into two board categories based on size. Neuropeptides are relatively large transmitter molecules composed of 3 to 36 amino acids. Individual amino acids, such as glutamate and GABA, as well as acetylcholine, serotonin, and histamine, are much smaller than neuropeptides and are therefore called small-molecule neurotransmitters. Within the category of small-molecule neurotransmitters, the biogenic amines (dopamine, nor-epinephrine, epinephrine, serotonin, and histamine) are often discussed separately because their chemical properties and postsynaptic actions are distinct from the other neurotransmitters in this group.
Until recently, it was believed that a given neurone produced only a single type of neurotransmitter. There is now convincing evidence, however, that many neurones contain and release two or more different neurotransmitters. There are numerous examples of different peptides in the same terminal, as well as cases in which two small-molecule neurotransmitters are found within the same neurone, or in which a peptide neurotransmitter is found along with a small-molecule neurotransmitter. When more than one transmitter is present within a nerve terminal, the molecules are called co-transmitters. Because each class of transmitter is usually packaged in a separate population of synaptic vesicles, co-transmitters often are segregated within a presynaptic terminal (although there are also instances in which two or more co-transmitters are present in the same synaptic vesicle). The presence of co-transmitters lends considerable versatility to synaptic transmission. In particular, if a presynaptic terminal packages co-transmitters in different types of vesicles, then these transmitters need not be released simultaneously. In fact, co-transmitters release varies with the frequency of presynaptic stimulation: empirically, low-frequency stimulation often releases only small neurotransmitters, whereas high-frequency stimulation is required to release neuropeptides from the same presynaptic terminals. In this way, the presence of co-transmitters allows the chemical signaling properties of a synapse to changes according to the level of presynaptic activity.
The differential release of co-transmitters is probably based on the distribution of Ca++ and vesicles in presynaptic terminals. Typically, a presynaptic terminal packages small-molecule co-transmitters into relatively small synaptic vesicles (often with a clear core), some of which are docked at the plasma membrane, in contrast, peptide co-transmitters are contained within large dense-core synaptic vesicles that are farther away from the plasma membrane. At low firing frequencies, the concentration of Ca++ may increase only in the vicinity of presynaptic Ca++ channels, limiting release to small-molecule transmitters because of the selective fusion of small vesicles located immediately adjacent to the channels. High-frequency stimulation increases the Ca++ concentration more evenly throughout the presynaptic terminal, thereby inducing the release of neuropeptides from the larger, more distant vesicles.
Effective synaptic transmission requires close control of the concentration of neurotransmitters within the synaptic cleft. Neurones have therefore developed a sophisticated ability to regulate the synthesis, packaging, release, and degradation (or removal) of neurotransmitters. In general, each of these processes is specific for a particular transmitter and requires a number of enzymes that are found only in neurons that use the transmitter at their synapses. As a rule, the synthesis of small-molecule neurotransmitters occurs locally within presynaptic terminals. The enzymes needed for transmitter syntheses are transported to the nerve terminal cytoplasm at a rate of 0.5 to 5 millimetres a day, by a mechanism known as slow axonal transport. The precursor molecules used by these enzymes are usually taken into the nerve terminal by transport proteins found in the plasma membrane of the terminal. The synthetic enzymes generate a cytoplasmic pool of free neurotransmitter that must then be loaded into synaptic vesicles by vesicular membrane transport proteins.
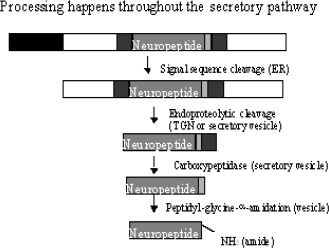
The mechanisms responsible for the synthesis and packaging of peptide transmitters are fundamentally different from those of the small-molecule neurotransmitters. Peptide-secreting neurones, like other cells, carry out gene transcription in their cell bodies. Transcription often results in the synthesis of polypeptides that are much larger than the final, “mature” peptide. Processing these polypeptides, called pre-propeptides (or pre-proproteins), takes place by a sequence of reactions in a number of intracellular organelles. Pre-propeptides are synthesized in the rough endoplasmic reticulum, where the signal sequence of amino acids – that is, the sequence indicating, that the peptide is to be secreted – is removed. The remaining polypeptide, called a propeptide (or proprotein), then traverses the Golgi apparatus and is packaged into vesicles in the trans-Golgi network. The final stages of peptide neurotransmitter processing occur after packaging into vesicles, and involve proteolytic cleavage, modification of the ends of the peptide, glycosylation, phosphorylation, and disulphide bond formation.
In general, neuropeptide synthesis is much like the synthesis of proteins secreted from non-neuronal cells (e.g. hepatic enzymes). A major difference, however, is that the neuronal axon often presents a very long distance between the site of a peptide’s synthesis and its secretion. The peptide-filled vesicles must therefore be transported along the axon to the synaptic terminal. The mechanism responsible for such movement, known as fast axonal transport, carries vesicles at rates up to 400 mm/day along cytoskeletal elements called microtubules. Microtubules are long, cylindrical filaments, 25 nm in diameter that is present throughout neurones and other cells. Peptide-containing vesicles are moved along these microtubule “tracks” by ATP-requiring “motor” proteins such as kinesin. Following their synthesis, neurotransmitters are stored within synaptic vesicles. The nature of these vesicles varies for different transmitters. Some of the small-molecule neurotransmitters – acetylcholine and the amino acid transmitters – are packaged in small vesicles 40-60 nm in diameter, the centers of which appear clear in electron micrographs, accordingly, these vesicles are referred to as small clear-core vesicles. Neurotransmitters are concentrated in synaptic vesicles by transporter proteins in an energy-requiring mechanism. Neuropeptides, in contrast, are packaged into larger synaptic vesicles that range from 90 to 250 nm in diameter and, with appropriate fixation, appear electron-dense in electron micrographs – hence, these are referred to as large dense-core vesicles. The biogenic amine neurotransmitters are packaged into at least two types of vesicles that are different from either the small clear-core vesicles or the large dense-core vesicles. Vesicles containing biogenic amines can either be small (40-60 nm diameter) dense-core vesicles, or larger (60-120 nm diameter) irregularly shaped, dense-core vesicles, depending on the particular class of neurone.
Once filled with transmitter molecules, vesicles associate with the presynaptic membrane and fuse with it in response to Ca2+ influx. The mechanisms of exocytotic release are similar for all transmitters, although there are differences in the speed of this process. In general, small-molecule transmitters are secreted more rapidly than peptides. For example, while secretion of ACh from motor neurones requires only a fraction of a millisecond, many neuroendocrine cells, such as those in the hypothalamus, require high-frequency bursts of action potentials for many second to release peptide hormones from their nerve terminals. These differences in the rate of transmitter release make neurotransmission relatively rapid at synapses employing small-molecule transmitters and slower at synapses that use peptides. Such differences in the rate of release probably arise from spatial differences in vesicle localization and presynaptic Ca++ signaling. Thus, the small clear-core vesicles used to store small-molecule transmitters are often docked at active zones (specialized regions of the presynaptic membrane), whereas the large dense-core vesicles used to store peptides are not. Biogenic amines are packaged into small vesicles that dock at active zones in some neurones, while in others they are packaged and released much like peptides.
When the transmitter has been secreted into the synaptic cleft, it binds to specific receptors on the postsynaptic cell to engage in another cycle of neurotransmitter release, binding, and signal generation. The mechanisms by which neurotransmitters are removed vary, but always involve diffusion in combination with reuptake into nerve terminals or surrounding glial cells, degradation by specific enzymes, or in some cases a combination of these. For most of the small-molecule neurotransmitters there are transporters that remove the transmitters (or their metabolites) from the synaptic cleft, ultimately delivering these molecules back to the presynaptic terminal. Not surprisingly, the particulars of the processes of synthesis, packaging, release and removal differ for each neurotransmitter.
17.1 Classical neuro-transmitters
17.1.1 Acetylcholine (ACh)
ACh is the neurotransmitter at neuromuscular junctions, at synapses in sympathetic and parasympathetic ganglia of the peripheral autonomic nervous system, and at many sites within the central nervous system. Two major cholinergic neuronal groups are the basal forebrain nuclear complex and the cholinergic nuclei of the brain-stem tegmentum. ACh may also act in some pain and chemosensory pathways. Whereas a great deal is known about the function of cholinergic transmission at the neuromuscular junction and at ganglionic synapses, the role of ACh in the central nervous system is not as well understood.
ACh is synthesized in nerve terminals from acetyl coenzyme A (acetyl CoA) and choline, in a reaction catalyzed by choline acetyltransferase (CAT). In contrast to most other small-molecule neurotransmitters, the postsynaptic actions of ACh are not terminated by reuptake, but by a powerful hydrolytic enzyme, acetylcholine-esterase (AChE). This enzyme is concentrated in the synaptic cleft, ensuring a rapid decrease in ACh concentration after its release from the presynaptic terminal. AChE has a very high catalytic activity (5000 molecules of ACh per AChE molecule per second) and hydrolyzes ACh into acetate and choline. Cholinergic nerve terminals contain a high-affinity, Na+-dependent transporter that takes up the choline produced by ACh hydrolysis. Among the many interesting drugs that interact with cholinergic enzymes are the organo-phosphates. These compounds include mustard gas (a chemical widely used in World War I), numerous insecticides, and sarin, the agent recently made notorious by a group of Japanese terrorists. Organo-phosphates can be lethal to humans (and insects) because they inhibit AChE, causing ACh to accumulate at cholinergic synapses. This build-up of ACh depolarizes the postsynaptic cell and renders it refractory to subsequent ACh release, causing neuromuscular paralysis.
17.1.2 Glutamate
Glutamate is generally conceded to be the most important transmitter for normal brain function. Nearly all excitatory neurons in the central nervous system are glutamatergic, and it is estimated that over half of all brain synapses release this agent. Glutamate plays an especially important role in clinical neurology because elevated concentrations of extracellular glutamate, released as a result of neural injury, are highly toxic to neurones. The most prevalent glutamate precursor in synaptic terminals is glutamine. Glutamine is released by glial cells and, within presynaptic terminals, is metabolized to glutamate by the mitochondrial enzyme glutaminase. Following its packaging and release, glutamate is removed from the synaptic cleft by high-affinity glutamate transporters present in both glial cells and presynaptic terminals. Glial cells contain the enzyme glutamine synthetase, which converts glutamate into glutamine. Glutamine is then transported out of the glial cells and into terminals. In this way, synaptic terminals work together with glial cells to maintain an adequate supply of the neurotransmitter. This synthetic pathway is referred to as the glutamate-glutamine cycle.
17.1.3 GABA and glycine
Most inhibitory neurons in the brain and spinal cord use either ?-aminobutyric acid (GABA) or glycine as a neurotransmitter. Remarkably, as many as one-third of the synapses in the brain appear to use GABA as their neurotransmitter. Unlike glutamate, GABA is not an essential metabolite, nor is it incorporated into protein. Thus, the presence of GABA in neurones and terminals is a good initial indication that the cells use GABA as a neurotransmitter. GABA is most commonly found in local-circuit interneurones, although the Purkinje cells of the cerebellum provide an example of a GABAergic projection neurone. GABA is synthesized from glutamate by the enzyme glutamic acid decarboxylase (GAD), which is found almost exclusively in GABAergic neurons. GAD requires a cofactor, pyridoxal phosphate, for activity. Because pyridoxal phosphate is derived from vitamin B6, a dietary deficiency of B6 can lead to diminished GABA synthesis. The significance of this fact became clear after a disastrous series of infant deaths was linked to the omission of vitamin B6 from infant formula. The lack of B6 resulted in a large reduction in the GABA content of the brain, the subsequent loss of synaptic inhibition caused seizures that in some cases were fatal. The mechanism of GABA removal is similar to that for glutamate; both neurones and glia contain high-affinity transporters for GABA. Most GABA is eventually converted to succinate, which is metabolized further in the tricarboxylic acid cycle that mediates cellular ATP synthesis. The enzymes required for this degradation, GABA aminotransferase and succinic semialdehyde dehydrogenase, are both mitochondrial enzymes. Inhibition of GABA breakdown causes a rise in tissue GABA content and an increase in the activity of inhibitory neurones. Because epileptic seizures can arise from a decrease in neuronal inhibition, a GABA aminotransferase inhibitor, sodium dipropylacetate, is widely used as an anticonvulsant. Drugs that act as agonists or as modulators on postsynaptic GABA receptors, such as barbiturates, are also used clinically to treat epilepsy, and are effective sedatives and anesthetics. The distribution of the neutral amino acid glycine in the central nervous system is more localized than that of GABA. Glycine inhibits the firing of spinal cord and brainstem motor neurones but has only a weak effect on neurones of the cerebral cortex. About half of the inhibitory synapses in the spinal cord use glycine, most of the others use GABA. Glycine is synthesized from serine by the mitochondrial isoform of serine hydroxymethyltransferase. Once released from the presynaptic cell, glycine is rapidly removed from the synaptic cleft by specific membrane transporters. Mutations in the genes that code for some of these enzymes result in hyperglycinaemia, a devastating neonatal disease characterized by lethargy, seizures, and mental retardation.
17.1.4 The biogenic amines
There are five established biogenic amine neurotransmitters: the three catecholamines – nor-epinephrine (nor-adrenaline), epinephrine (adrenaline) and dopamine – and histamine and serotonin. Some aspects of the synthesis and degradation of the amine neurotransmitters are still not well defined, but many of the properties of these processes fall somewhere between those of the other small-molecule neurotransmitters and those of the neuropeptides. Drugs that interfere with biogenic amine metabolism are especially important as treatments for a variety of clinical disorders. All the catecholamines are derived from a common precursor, the amino acid tyrosine. The first step in catecholamine synthesis is catalyzed by tyrosine hydroxylase and results in the synthesis of dihydroxy-phenylalanine (DOPA). Because tyrosine hydroxylase is rate-limiting for the synthesis of all three transmitters, its presence is a valuable criterion for identifying catecholaminergic neurons.
Dopamine is produced by the action of DOPA decarboxylase on DOPA. Although present in several brain regions, the major dopamine-containing area of the brain is the substantia nigra, which plays an essential role in the control of body movements. In Parkinson’s disease, the dopaminergic neurones of the substantia nigra degenerate, leading to a characteristic motor dysfunction. Because dopamine does not readily cross the blood-brain barrier, the disease can be treated by administering DOPA together with drugs that prevent catacholamine breakdown.
Norepinephrine synthesis requires dopamine ß-hydroxylase, which catalyzes the production of norepinephrine from dopamine. Neurones that synthesize norepinephrine are largely restricted to the locus coeruleus, a brainstem nucleus that projects diffusely to the midbrain and telencephalon. These neurones are especially important in modulating sleep and wakefulness.
Epinephrine is present at much lower levels in the brain than any of the other catecholamines. The enzyme that synthesizes epinephrine, phenyl-ethanolamine-N-methyltransferase, is present only in adrenaline-secreting neurones. Sensitive methods that identify epinephrine have confirmed the existence of epinephrine-containing neurones in the central nervous system and shown them to be located in two groups in the rostral medulla. The function of these epinephrine-containing neurones in the brain is not known. All three catecholamines are removed by reuptake into terminals, or into surrounding glial cells, by an Na+-dependent transporter. The two major enzymes involved in the catabolism of catecholamines are monoamine oxidase (MAO) and catechol O-methyltransferase (COMT), both of which are present within catecholaminergic nerve terminals and are the targets of numerous psychotropic drugs.
Histamine has long been known to be released from mast cells and platelets in response to allergic reactions or tissue damage. Only recently, however, has this amine been implicated as a neurotransmitter. Histamine is produced from the amino acid histidine by a histidine decarboxylase. High concentrations of histamine and histamine decarboxylase are found in the hypothalamus, from whence histaminergic neurones send sparse but widespread projections to almost all regions of the brain and spinal cord. Their function remains uncertain.
Serotonin, or 5-hydroxytryptamine (5-HT), is also synthesized from one of the common amino acids – in this case, tryptophan. An essential dietary requirement, tryptophan is taken up into neurones by a plasma membrane transporter and hydroxylated in a reaction catalyzed by the enzyme tryptophan-5-hydroxylase. As in the case of the catecholamines, this reaction is the rate-limiting step for 5-HT synthesis. Serotonin is located in discrete groups of neurons in the raphe regions of the pons and upper brain-stem, these cells send widespread projections to the telencephalon and diencephalon and have also been implicated in the regulation of sleep and wake-fullness.
17.1.5 ATP and other purines
All synaptic vesicles contain ATP, which is co-released with one or more “classical” neurotransmitters. There is now strong evidence that ATP acts as an excitatory neurotransmitter in the periphery. Postsynaptic actions of ATP have also been demonstrated in the central nervous system, specifically at dorsal horn neurons and in a subset of hippocampal neurons. Purines act on a large and diverse family of receptors, many of which have recently been cloned. Whether or not purines play a role in synaptic transmission depends on the presence and/or distribution of purinergic receptors near the sites of release. These receptors have been separated into two major families: the P1 receptors, activated predominantly by ATP and ADP, and the P2 receptors, activated predominantly by AMP and adenosine. These receptors can be either ion channels or G-protein-coupled receptors, and their activation may subtly shape postsynaptic responses to classical neurotransmitters. Alternatively, if purinergic receptors are located presynaptically, their activation could modulate neurotransmitter release. In any event, it seems likely that excitatory synaptic transmission mediated by purinergic receptors is widespread in the mammalian brain.
17.2 Peptide neurotransmitters (neuropeptides)
Many peptides are well known as hormones in endocrine cells, including neurons in the neuroendocrine regions of the brain such as the hypothalamus and pituitary. Advances in the ability to detect and isolate these molecules have now shown that peptides may also act as neurotransmitters, often being co-released with small-molecule neurotransmitters. The biological activity of the peptide neurotransmitters depends on the sequence of their amino acids. Propeptide precursors are often many times larger than their active peptide products and can given rise to more than one species of neuropeptide (Table 1).
Table 1.
The processing of neuropeptides
|
Since each of these peptide products can be separately contained in synaptic vesicles, transmission based on peptides often elicits complex postsynaptic responses. Peptide transmitters have been implicated in modulating emotions, and some, such as substance P and the opioid peptides, are involved in the perception of pain. Still other peptides, such as melanocyte-stimulating hormone, adrenocorticotropin, and ß-endorphin, regulate complex responses to stress, whereas neuronal vasopressin and oxytocin are implicated in learning and memory processes.
17.2.1 Substance P
Substance P is an 11-amino acid peptide present in high concentrations in the human hippocampus and neocortex. It is also released from C fibers, the small-diameter afferents in peripheral nerves that convey information about pain and temperature (as well as postganglionic autonomic signals). Substance P is a sensory neurotransmitter in the spinal cord, where its release can be inhibited by opioid peptides released from spinal cord interneurones, resulting in the suppression of pain. The protease responsible from the inactivation of Substance P is associated with synaptic membranes.
17.2.2 Opioid peptides
Morphine has long been known to be an especially effective analgesic. This and other opiate drugs affect the perception of pain by interacting with specific receptors expressed at a number of sites in the central and peripheral nervous systems. The endogenous opioid peptides were discovered during a search for endogenous compounds that mimicked the actions of morphine. It was hoped that such compounds would be analgesics, and that their understanding would shed light on addiction to morphine and other narcotics. The endogenous ligands of the opioid receptors have now been identified as a family of more than 20 opioid peptides grouped into three classes: the endorphins, the enkephalins, and the dynorphins, each class being liberated from an inactive pre-propeptide. These precursors are the product of three distinct genes: pre-pro-opiomelanocortin, pre-pro-enkephalin A, and pre-pro-dynorphin. Opioid precursor processing is tissue-specific due to the differential expression of the processing enzymes. The pro-opiomelanocortin precursor also contains the sequences for several non-opioid neuropeptides, such as the stress hormone adrenocorticotropic hormone (ACTH) and a-, ß-, and ?-melanocyte stimulating hormone (MSH). Opioid peptides are widely distributed throughout the brain. In general, these peptides tend to be depressants. When injected intracerebrally, they act as analgesics, and have been implicated in the mechanisms underlying acupuncture-induced analgesia. Unfortunately, the repeated administration of endorphins leads to tolerance and addiction. Although the results of opioid research have not yet provided a complete understanding of narcotic addiction, a solid basis of knowledge has been established that promises ultimately to solve this extraordinary social and medical problem.
17.2.3 Posterior pituitary peptides (vasopressin, oxytocin)
Vasopressin exerts a long-term facilitating effect on learning and memory processes, and also prevents and reverses retrograde amnesia. In contrast to vasopressin, oxytocin is an amnesic neuropeptide. Endogenous vasopressin and oxytocin in the dorsal septum or in the ventral hippocampus is of particular importance for learning and memory processes. The two nonapeptides are not only secreted from the neurohypophysis into the general circulation, but - probably upon some specific stimuli, and largely independently of their peripheral release - are also released intracerebrally (e.g. into septum). The experiments provide additional evidence for an involvement of endogenous vasopressin and oxytocin in the regulation of learning and memory processes. Based on electrophysiological findings, one might conclude that a neurotransmitter-like effect is associated with vasopressin in limbic brain structures. Vasopressin is capable of modulating long-term potentiation, which is believed to be an electrophysiological basis of memory processes, and to enhance the response to glutamate, which might be an indication that vasopressin acts as a neuromodulator of excitatory pathways in the limbic-midbrain. Interestingly, the coerulo-telencephalic noradrenaline system may mediate the effects of vasopressin on memory consolidation, providing a functional evidence for the interactions of classical transmitters with neuropeptides. Vasopressin and oxytocin are converted to highly selective memory molecules in the brain as [Cyt6]AVP4-9/5-9 and [Cyt6]AVP4-8/4-9. These peptide fragments are more effective than the parent nonapeptides on behavioral processes.
17.3 Unconventional transmitters (nitric oxide: new mechanisms of neurotrasnmitter action)
Some years ago nitric oxide (NO) was thought about primarily as a toxic gas relevant to air pollution. The discovery, in 1987, that NO is a signalling molecule that modulates vascular tone has sparked tremendous interest in the biological effects of this molecule, which is now recognized as a novel chemical messenger for a number of cell types, including neurones. Nitric oxide is certainly not a classical neurotransmitter in the central nervous system, it is a short-lived radical that interacts with surrounding neurones by diffusing across membranes, rather than being released by exocytosis and interacting with membrane-bound receptors. In this sense, NO represents a significant departure from all neurotransmitter mechanisms characterized to date. Nitric oxide is synthesized from L-arginine following stimulation of the enzyme nitric oxide synthase (NOS). Neuronal-type nitric oxide synthase (nNOS) is a widely distributed calmodulin-regulated enzyme and is coupled to a variety of neurotransmitter systems in the brain and in peripheral tissues. Once generated, NO can diffuse locally and interact with target molecules such as guanylyl cyclase, the enzyme catalyzing cGMP synthesis. NO and cGMP together comprise an especially wide-ranging signal transduction system that may also play an important role in neurological disease. An emerging hypothesis is that the balance between nitric oxide and superoxide generation is a critical factor in the etiology of some neurodegenerative diseases. Nitric oxide defies current classification schemes in that it can function both as a neurotransmitter and a second messenger. In any event, it demonstrates that, despite a century-long analysis of neurotransmitter mechanisms, this field still has some surprises in store.
17.4 Summary
The large number of neurotransmitters in the nervous system can be divided into three broad classes: small-molecule (classical) transmitters, neuropeptides and unconventional transmitters. Neurotransmitters are synthesized from defined precursors by regulated enzymatic pathways, packaged into one of a variety of vesicle types, and released into the synaptic cleft in a Ca++-dependent manner. Many synapses release more than one type of neurotransmitter, and multiple transmitters are sometimes packaged in the same synaptic vesicle. The postsynaptic effects of neurotransmitters are terminated by the transmitter back into cells, or by diffusion out of the synaptic cleft. Glutamate is the major excitatory neurotransmitter in the brain, whereas GABA and glycine are the major inhibitory neurotransmitters. The actions of these small-molecule neurotransmitters are typically faster than those of the neuropeptides. Thus, the small-molecule transmitters usually mediate synaptic transmission when a speedy response is essential, whereas the neuropeptide transmitters, as well as the biogenic amines and some other small-molecule neurotransmitters, can regulate or modulate ongoing activity in the brain or in peripheral target tissues. The enormous importance of drugs that influence transmitter actions in the treatment of neurological and psychiatric disorders guarantees that a steady stream of new information in this filed will be forthcoming.
Table 2.
Neuropeptides with verified (or putative) transmitter functions
| Vasopressin | Somatostatin |
| Oxytocin | Cortistatin |
| ACTH | CCK-8 |
| α-MSH | Coerulein |
| γ-Endorphin | NPY |
| α-Endorphin | Galanin |
| ß-Endorphin | Substance P |
| Endomorphin-2 | ANP |
| Dynorphine | BNP |
| Astressin | CNP |
| Orphanin/nociceptin | Angiotensin II |
| Nocistatin | CRF |
References
- 1.De Wied D. The neuropeptide concept. Prog Brain Res 1987;72: 93-108. [PubMed] [Google Scholar]
- 2.Kovács GL, De Wied D. Peptidergic modulation of learning and memory processes. Pharmacol. Rev 1994;46: 269-291. [PubMed] [Google Scholar]
- 3.Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia AS, McNamara JO. (Eds.) Neurosciences, Sinauer Pub., Sunderland, 1997. [Google Scholar]
- 4.Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR. Fundamental Neuroscience, Academic Press, San Diego, 1999. [Google Scholar]
- 5.Kokaia M, Holmberg K, Nanobashvili A, Xu ZQ, Kokaia Z, Lendahl U, Hilke S, Theodorsson E, Kahl U, Bartfai T, Lindvall O, Hokfelt T. Suppressed kindling epileptogenesis in mice with ectopic overexpression of galanin. Proc Natl Acad Sci U S A 2001;20: 14006-14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hokfelt T, Bartfai T, Bloom F. Neuropeptides: opportunities for drug discovery. Lancet 2003;2: 463-472. [DOI] [PubMed] [Google Scholar]
- 7.Kovács GL. Natriuretic peptides in alcohol withdrawal: central and peripheral mechanisms. Curr Med Chem 2003;10: 1241-1253. [DOI] [PubMed] [Google Scholar]


