Abstract
Background
Primary Sclerosing Cholangitis (PSC) is a rare chronic, cholestatic liver condition in which patients can experience a range of debilitating symptoms. Patient reported outcome measures (PROMs) could provide a valuable insight into the impact of PSC on patient quality of life and symptoms. A previous review has been conducted on the quality of life instruments used in liver transplant recipients. However, there has been no comprehensive review evaluating PROM use or measurement properties in PSC patients’ to-date. The aim of the systematic review was to: (a) To identify and categorise which PROMs are currently being used in research involving the PSC population (b) To investigate the measurement properties of PROMs used in PSC.
Methods
A systematic review of Medline, EMBASE and CINAHL, from inception to February 2018, was undertaken. The methodological quality of included studies was assessed using the Consensus-based Standards for selection of health Measurement Instruments (COSMIN) checklist.
Results
Thirty-seven studies were identified, which included 36 different PROMs. Seven PROMs were generic, 10 disease-specific, 17 symptom-specific measures and 2 measures on dietary intake. The most common PROMs were the Short form-36 (SF-36) (n = 15) and Chronic liver disease questionnaire (CLDQ) (n = 6). Only three studies evaluated measurement properties, two studies evaluated the National Institute of Diabetes Digestive and Kidney Diseases Liver Transplant (NIDDK-QA) and one study evaluated the PSC PRO; however, according to the COSMIN guidelines, methodological quality was poor for the NIDDK-QA studies and fair for the PSC PRO study.
Conclusion
A wide variety of PROMs have been used to assess health-related quality of life and symptom burden in patients with PSC; however only two measures (NIDDK-QA and PSC PRO) have been formally validated in this population. The newly developed PSC PRO requires further validation in PSC patients with diverse demographics, comorbidities and at different stages of disease; however this is a promising new measure with which to assess the impact of PSC on patient quality of life and symptoms.
Electronic supplementary material
The online version of this article (10.1186/s12955-018-0951-6) contains supplementary material, which is available to authorized users.
Keywords: Primary sclerosing cholangitis, Cholestasis, Patient reported outcome measures (PROMs), PROSPERO (Registration Number: CRD42016036544).
Background
Primary Sclerosing Cholangitis (PSC) is a chronic, cholestatic liver condition that results in inflammation and fibrosis that can involve the entire biliary tree [1]. PSC is a progressive disorder and can lead to cirrhosis, portal hypertension and liver failure [1].
Approximately 1 in 100,000 people in the general population is affected with PSC per year in Europe and the United States [2]. The disease occurs at any age, but is more prevalent in adults between the ages of 30–60 years and is more common in men than in women. Approximately 70–80% of patients with PSC have an associated inflammatory bowel disease (IBD) such as ulcerative colitis or Crohn’s disease [3]. Currently, there is no known licensed medication to prevent the progression of PSC, which if left untreated can result in increasing disability and even death [4]. In patients with end-stage PSC liver disease, the only therapeutic option currently available is a liver transplant [4].
Although overall disease progression can be slow, patients with PSC can experience a range of debilitating symptoms. In the early stage of the disease, symptoms include tiredness or fatigue. In more advanced cases, symptoms include pruritus, jaundice, abdominal pain, weight loss, fevers, hyperpigmentation, vitamin deficiencies and metabolic bone disease [5]; all of which can have a significant impact on health-related quality of life (HRQOL) [6, 7].
Increasingly in chronic diseases and terminal illness, it is recognised that maintaining HRQOL is an important consideration when the treatment is aimed at maintenance rather than a cure, or the treatment has a high level of toxicity [8]. Many of the current therapeutic interventions in PSC are aimed at managing symptoms. Measuring the impact of these interventions and preserving HRQOL is an important aspect of PSC care. This requires patient reported outcome measures (PROMs) that are sensitive enough to capture changes in HRQOL or symptoms over time.
Increasingly, PROMs use has demonstrated a positive contribution to clinical practice and research [9]. In clinical practice, aggregate level PROM data can help us to understand the burden of chronic medical conditions, identify health inequalities [10] and determine new areas for therapeutic interventions. They can also play a key role in benchmarking and audit. [11] At an individual patient level, PROMs can be used to monitor the response, adverse effects and benefits of treatments in routine practice, [12] facilitating communication between clinicians and patients regarding their HRQOL, symptom management and control [13–15].
A previous review investigating the quality of life (QOL) instruments used in liver transplant recipients has been conducted [16]. However, to date, no comprehensive review of PROMs used in PSC patients has been undertaken. There is a clear need to evaluate the measurement properties of the PROMs currently used in this population to determine the optimal measures for use in future research and routine care. Therefore the objectives of this systematic review were to: (a) identify and categorise PROMs currently used in research involving the PSC population; and (b) investigate their measurement properties, to help inform the selection of PROMs for use in future PSC research and routine practice.
Methods
The following guidelines were used, where applicable, to inform the conduct and reporting of this study: (i) the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [17] guidance (see Additional file 1 for the PRISMA checklist), (ii) COnsensus based Standards for the selection of health Measurement INstruments (COSMIN) guidance [18] and (iii) the updated method guidelines for systematic reviews in Cochrane collaboration back review group [19]. The study was registered with PROSPERO (Registration Number: CRD42016036544).
Search strategy
A systematic search was conducted on the following electronic databases: Medline, EMBASE and CINAHL from inception to 15 February 2018. The search terms “Primary sclerosing cholangitis” and “Patient reported outcome measures” were used, alongside synonyms and related terms (see Additional file 2 for the full search strategy). These terms were combined with the COSMIN search filters developed by VU University Medical Centre Amsterdam and University of Oxford (available on COSMIN website: http://www.cosmin.nl/). In addition, papers included in the full text review were subjected to a hand search of reference lists [20, 21].
Inclusion criteria
Studies were eligible if:
PROMs were included in the study meeting the FDA definition [22].
Study participants were patients with PSC.
In addition:
c) Studies that evaluated at least one measurement property (i.e. reliability, validity, responsiveness, interpretability) were included in the COSMIN quality review.
No restriction was placed on age or gender of participants or language, publication date or country of origin of the study.
Selection of studies
Two reviewers (FI/GT or GT/GK) independently screened studies according to their title and abstract to determine eligibility. Following this, the full text of potentially eligible studies was retrieved and screened independently by two independent reviewers (FI/GT or GT/GK). The protocol planned that discrepancies would be discussed with a third investigator (MG or DK or AS) to reach consensus; however, this was not required.
Data extraction
The two independent reviewers (GT plus FI, GK or AS) independently extracted the data from each study using a predefined form (including study design and patient level characteristics). Information regarding each PROM was extracted, including: constructs, therapeutic area, domains, number of items, scoring method, recall period, administration, completion time, data collection, cost/permission and measurement properties (reliability, validity, responsiveness, interpretability).
Content comparison of included PROMs
A summary of PROMs used in studies of PSC patients, including an overview of included domains and specific content was prepared. The PROMs were categorised according to their domains to facilitate comparison of the measures that have been used in PSC studies to-date.
Quality assessment
The COSMIN checklist [23] was used to assess the methodological quality of studies that reported on the measurement properties of PROMs used in the study. Two reviewers (FI/GT or GT/AW) independently completed the COSMIN checklist. The protocol planned that discrepancies would be discussed with a third reviewer; however, this was not required. Each measurement property was scored according to the quality of reporting by the publication, using a four-point rating scale: ‘excellent’, ‘good’, ‘fair’ and ‘poor’. The methodological quality of each study was rated by taking the lowest score (worst score counts method) per domain. For example, if any of the items of the domain reliability was scored ‘poor’, the overall score for regarding the methodological quality of reliability was rated as ‘poor’.
Evidence synthesis
Synthesis of measurement property evidence was performed using standardised criteria developed by Terwee 2011 [23]. The summary of the overall evidence of measurement properties of the PROMs was determined by the number of studies, the methodological quality of the studies, and consistency of the findings. Based on these factors the overall rating of a measurement property per PROM was ranked as “+” positive, “?” indeterminate or “-” negative and combined with an assessment of the overall level of supporting evidence (strong, moderate, limited, conflicting, unknown) as proposed by the Cochrane Back Review Group [24].
Results
Study selection
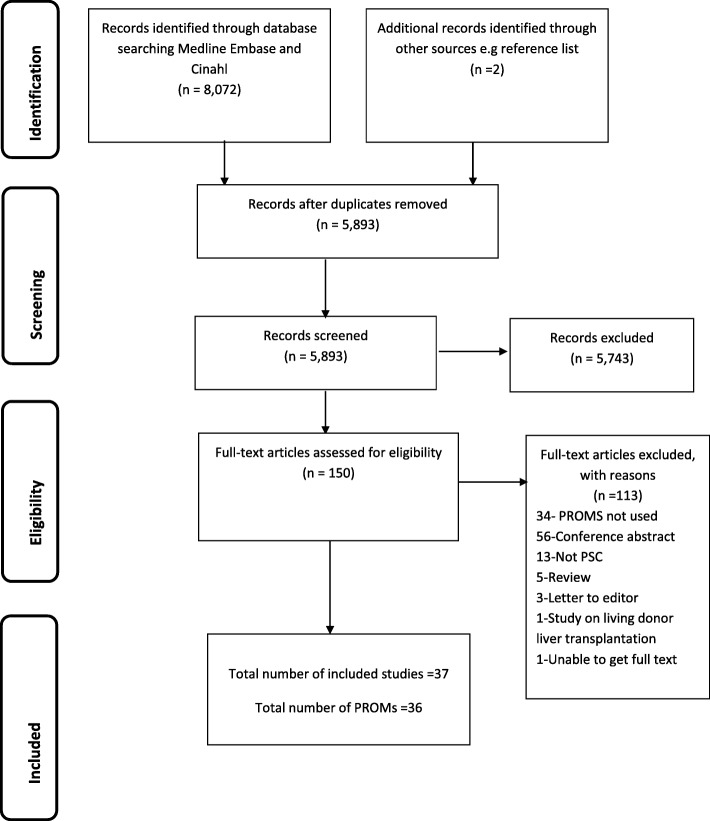
In total, 8074 studies were identified, 5893 remained after duplicate removal and 150 remained after reviewing titles and abstracts (Fig. 1). Following review of the 150 full texts, 37 studies, containing 36 different PROMs, were included.
Fig. 1.
PRISMA flowchart describing the identification, selection and inclusion of studies on PROM assessment in Primary sclerosing Cholangitis
Table 1 summarises the general characteristics of the included studies. The study designs included 17 cross-sectional studies, five randomised controlled trials (RCTs), four case-control studies, two validation study, two pilot study, two before and after study, one cost-effectiveness study, one case matched study, one longitudinal study, one cohort study and one retrospective case series study.
Table 1.
Characteristics of included studies
| Author (Year) (Reference) | Country | Study design | Sample size (PSC cases) | Mean age (SD) year | Gender (Male n %) | Disease stage | Mayo risk score / MELD Score | IBD (Yes/No (n (%)) | LT (Yes/No (n (%)) | PROM | Rationale for Assessment | PROM administration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gavaler (1991) [66] | USA | Cross- sectional study | 23 (23) | Quiescent group: 34.7 (6.2) Symptomatic group: 39.8 (1.6) | 15 (65%) | Symptomatic UC: Mild: 7 (40%) Moderate: 8 (47%) Severe: 2 (13%) |
NR | Yes (23 (100%)) | Yes (23 (100%)) | Study questionnaire: symptoms of UC | A | Postal & telephone |
| Gross (1999) [26] | USA | Before & after study | 157 (92) | Total sample: 50 (10) | 31 (34%) | NR | MRS: Mean 5.3 | NR | Yes (157 (100%)) | NIDDK-QA, pilot version NIDDKQA | A | Clinic |
| Kim (2000) [28] | USA | Validation study | 96 (17) | 45 (9.3) | 7 (41%) | PSC undergoing LT: 17 (100%) | MRS: mean (SD) = −0.1(1.0) | NR | PSC patients undergoing LT: 17 (100%) | NIDDK-QA, SF- 36 | D | Clinic |
| Bharucha (2000) [67] | USA | Pilot study | 20 (20) | 44 (11) | 12 (60%) | Early stage (1–2): 10 (50%), Late stage (3–4): 10 (50%) |
MRS: mean (SD) = 2.87 (0.95) | Yes (14 (70%)) | No | Grading system fatigue & pruritus | B | Unclear |
| Younossi (2000) [38] | USA | Cross-sectional study | 104 (29) | Total sample:: 55 (12) | Total sample 28 (97%) | NR | NR | NR | No | SF- 36, CLDQ | A | Unclear |
| Younossi (2001) [39] | USA | Cross-sectional study | 353 (45) | Total sample: 54 (11) | Total sample 38 (30%) | Total sample: Child-pugh class: no cirrhosis: 47 (13%) class A: 43 (12%) class B-27 (8%) class C-4 (1%) |
NR | NR | NR | SF-36, CLDQ | A | Clinic |
| Longworth (2003) [45] | England and Wales | Cost effectiveness study | 347 (70) | NR | 48 (69%) | NR | Of 41 patients MELD score median/IQR = 10/6–16 | NR | Yes (45) 64%)) | EuroQol EQ. 5D | C | Postal |
| Bjornsson (2004) [44] | England & Sweden | RCT | 93 (20) | NR | 13 (65%) | Cirrhosis: 5 (1%),Ludwig’s fibrosis score stage 1: 9 (44%), stage 2: 4(21%), stage 3:6(30%) | NR | Yes (16 (80%)) | No | PGWB, FIS, BDI, GSRS, Rome ll modular QA | A | Postal |
| Ter Borg (2004) [36] | Netherlands | RCT | 33 (11) | NR | 10 (91%) | NR | NR | NR | No | VAS, FFSS, MFI | B | NR |
| Ter Borg (2005) [48] | Netherlands | Cross-sectional study | 72 (27) | 45 (NR) | 19 (70%) | Cirrhosis: 15 (56%) | NR | Yes (2 (7%) | NR | VAS, FFSS, SF-36 | A | NR |
| Olsson (2005) [33] | Sweden, Norway, Denmark | RCT | 198 (198) | UDCA: 43.6(12.7) Placebo: 43.1 (11.2) | 139 (70%) | NR | NR | Yes (168 (85%)) | NR | SF- 36 | B | Unclear |
| Gorgun (2005) [21] | USA | Case matched study | 65 (65) | 43.37 (11.2) | 45 (69%) | NR | NR | Yes (65 (100%)) |
No | FPQ, CGQOL | A | |
| Mansour-Ghanaei (2006) [49] | Iran | RCT | 34 (6) | Total sample: 53.97 (11.93) | NR | NR | NR | NR | NR | VAS | B | Unclear |
| Mayo (2007) [50] | USA | RCT | 21 (4) | Total sample: 53.97 (11.93) | Total sample 5 (15%) | NR | aTotal sample MELD mean (range): 11(6–24) | NR | NR | VAS, IDS-SR30 | B | Unclear |
| Van os (2007) [52] | Netherlands | Cross-sectional study | 92(37) | 43.8(12.3) | 24 (65%) | Cirrhosis: 5 (13.5%) | NR) | NR | NR | BDI, SADS | A | Postal |
| Tillman (2009) [37] | Germany | Cross-sectional study | 511(13) | 42 (NR) | NR | NR | NR | NR | NR | SF- 36, FIS, WHOQOL-BREF, HADS | A | In clinic |
| Ananthakrishnan (2010) [47] | USA | Case-control study | 26 (26) | 40.7 (14.8) | 21 (80.8%) | NR | MELD score mean (range) 8 (6–20) | Yes (26(100%)) | No | SIBDQ, HBI, UCAI | A | Outpatient clinic |
| Aberg (2012) [30] | Finland | Cross-sectional study | 401 (56) | 53 (9) | 36 (64%) | NR | NR | NR | Yes (56 (100%)) | 15D, ad hoc questionnaire | A | Postal |
| Benito De Valle (2012) [29] | England & Sweden | Cross-sectional study | 182 (182) | 160 patients no LT: 50 (16) | 112 (70%) | Small duct disease: 17 (11%), Liver cirrhosis: 12 (8%), Decompensated liver disease: 9 (6%) | MRS mean (SD): 0.34 (1.10) | Yes (126 (79%)) | Yes (22 (12%)) | SF-36, CLDQ, FIS, HADS | A | Postal |
| Hagstrom (2012) [68] | Sweden | Cross-sectional study | 96 (96) | 47 (13) | 63 (66%) | Cases child pugh score of 10, significant fibrosis: 26 (27%), non-significant fibrosis: 70 (73%) | NR | Yes (73 (76%)) | Yes (12 (12.5%)) | LDH | A | Interview |
| Gulati (2013) [25] | USA | Cross-sectional study | 40 (24) | Total sample: 11.6 (4.5) | 17 (43%) | Total sample: Cirrhosis 22 (55%) | NR | Total sample: Yes (16 (65%)) | No | A | Unclear | |
| Block (2014) [69] | Norway & Sweden | Case-control study | 48 (48) | NR | 40 (83%) | NR | NR | 48 | Yes (IPAA: 11, IRA: 7) | OS | A | Scheduled follow up visit |
| Gotthardt (2014) [6] | Germany | Cross-sectional study | 113 (113) |
43.6 (14.2) | 81 (71.7%) | NR | MRS n: low/intermediate/ high =48 (42%) / 25 (22%) / 5 (4%) | Yes (71 (63%)) | NR | SF 36, PHQ-9 | A | Postal |
| Hov (2014) [70] | Norway | Case-control study | 240 (240) |
NR | 171 (71%) | NR | NR | Yes (183 (77%)) | Yes (94 (39%)) | Study questionnaire | A | Postal |
| Pavlides (2014) [34] | England | Retrospective case note review | 40 (PSC-IPAA = 21 & PSC-UC = 19) | NR | 31 (78%) | PSC-IPAA had dysplasia: 2 (5%) | NR | Yes (19 (47.5%)) | No | OS, CGQOL, FSFI, IIEF | A | Postal |
| Raszeja-Wyszomirska (2014) [35] | Poland | Cross-sectional study | 102 (102) | 36 (12) | 73 (72%) | Cirrhosis: 30 (29%) | NR | Yes (65 (64%)) | NR | SF 36, PBC-40, PBC-27 | A | Unclear |
| Cheung (2015) [32] | Canada | Cross-sectional study | 162 (99) | 46.1 (15.1) | 50 (51%) | Cirrhosis: 47 (48%), Decompensated liver disease: 16 (16%) | NR | Yes (74) | No | SF-36, PBC-40, PHQ-9, LDQOL, SIBDQ, 10 peered-reviewed QA on emotional and psychosocial | A | Postal or clinic |
| Dyson (2015) [20] | USA | Cross-sectional study | 40 (40) | 51 (13) | 31 (78%) | NR | NR | Yes (24 (60%)) | NR | FIS, ESS, HADs, COMPASS | A | Postal |
| Eaton (2015) [71] | Canada & USA | Case-control study | 1000 (1000) |
NR | 619 (72%) | NR | NR | Yes (741 ((74%)) | Yes (450 ((45%)) | HHQ | A | Postal or clinic |
| Haapamaki (2015) [31] | Finland | Cross-sectional study | 341 (341) |
43.3 (13.7) | 183 (54%) | ERC-score mean (SD): 5.9 (3.4) | NR | Yes (237 (69.5%)) | Yes (9 (2.6%)) | 15D, study questionnaire | A | ERC examination at the HUGH endoscopy unit |
| Kalaitzakis (2015) [27] | England and Sweden | Cross-sectional study | 163 (163) |
No LT: 50 (16) | No LT 122 (75%) |
No LT Small-duct disease: 15 (10%), Diver cirrhosis: 11 (8%), Decompensated liver disease: 8 (6%) | No LT MRS: mean (SD) = 0.11(1.42) | No LT Yes (116 (71%)) | Yes (19 (12%)) | SF 36, SF-6D, CLDQ, study questionnaire | A, C | Unclear |
| Raszeja-Wyszomirska (2015) [41] | Poland | Cross-sectional study | 33 (33) | 35.3 (13.38) | 11 (33%) | Cirrhosis: 6 (18%) | NR | Yes (22 (67%) | NR | SF 36, PBC-40, PBC-27 | A | NR |
| Carbone (2017) [46] | Italy | Longitudinal study | 227 (64) | 50(11) | 39 (66%) | NR | NR | NR | NR | EQ-5D | A | Clinic |
| Kempinska (2017) [40] | Poland | Cohort study | 275 (275) | Median 55, range 28–90 | 182 (66%) | NR | NR | NR | NR | SF 36, PBC-40, PBC-27 | A | NR |
| Kittanamongkolchai (2017) [51] | USA | Before and after study | 13 (5) | 46.4 (13.2) | 1 (20%) | NR | NR | NR | NR | Pruritus numerical rating scale | B | Physician administered |
| Tabibian (2017) [42] | USA | Pilot study | 16 (16) | 40 (NR) | 13 (81%) | All patients had stage 1–3 PSC | NR | 13 (81%) | NR | FFSS, 5-D itch scale, CLDQ, SF-36 | B | NR |
| Younossi (2017) [43] | USA | Validation study | 102 (102) | 44 (13) | 33 (32%) | Cirrhosis: 37 (39%) | NR | 67 (68%) | NR | PSC PRO, SF-36, CLDQ, PBC-40, 5-D Itch | D | ePRO website |
15D 15-dimensional health-related quality of life measure, 5-D Itch Five dimensions Itch, BDI Beck Depression Inventory, CGQOL Cleveland global quality of life questionnaire, CLDQ Chronic liver disease questionnaire, COMPASS Composite Autonomic Symptom Scale, ESS Epworth Sleepiness Scale, EQ. 5D EuroQol EQ. 5D, FFSS Fisk Fatigue Severity Scale, FIS Fatigue Impact Scale, FSFI Female Sexual Satisfaction Index, GSRS Gastrointestinal Symptom Rating Scale, HADS Hospital anxiety and depression scale, HBI Harvey-Bradshaw Index, HHQ Health Habits and History Questionnaires, IBD Inflammatory Bowel Disease, IDS-SR30 30-item Inventory of Depressive Symptomatology-self report, IIEF International index of erectile function, LDH Lifetime drinking history, LDQOL Liver Disease Quality of Life Questionnaire, LT Liver Transplant, MELD Model For End-Stage Liver Disease, MFI Multidimensional Fatigue Inventory, MRS Mayo Risk Score, NIDDK-QA National institute of diabetes and digestive and kidney disease liver transplant questionnaire, NR Not Reported, OS Oresland Scale, PBC-40 Primary Biliary Cirrhosis, PF Pouch Function Questionnaire, PGWB Psychological general well-being index, PHQ-9 Patient Health Questionnaire, PSC PRO Primary Sclerosing Cholangitis patient-reported outcome, RCT Randomised Controlled Trial, SADS Schedule for Affective Disorders and Schizophrenia, SD Standard Deviation, SF-36 Short form 36, SIBDQ Short Inflammatory Bowel Disease Questionnaire, UC Ulcerative Colitis, UCAI UC Activity Index, VAS Visual Analogue Scale, WHOQOL-BREF World Health Organization Quality of Life assessment instrument
aRationale for assessment: A; Burden (HRQOL /symptom) of disease, B: Effectiveness of treatment, C: Cost Effectiveness/Health Utilities, D:Validation of a Patient Reported Outcome Measure, (PROM)
Twenty seven of the 37 included studies used PROMs to examine the impact of PSC on patients and seven of these measured the effectiveness of treatments: one study evaluated the cost-effectiveness of liver transplantation, one study assessed health utilities and two were validation studies of the PROMs: the National Institute of Diabetes Digestive and Kidney Diseases Liver Transplant (NIDDK-QA) and the Primary Sclerosing Cholangitis Patient Reported Outcome (PSC PRO).
In total, 3742 patients with PSC were recruited to the included studies (sample size range n = 4–1000). All participants were adults, with the exception of one study [25] which included patients with the mean age of 11.6 years. Studies were heterogeneous in terms of population demographic characteristics. In the thirty-five studies that reported gender, the proportion of PSC patients who were males ranged from 15 to 97%. Five studies reported a relatively wide range of mean Mayo risk scores (− 0.1 to 2.87) for PSC patients, a score which estimates patient survival in PSC [6, 26–29]. Twenty-four studies described the proportion of IBD in PSC patients, ranging from 7 to 100%. In 12 studies, the percentage of PSC patients who had received a liver transplant ranged from 12 to 100%.
Characteristics of PROMs
Characteristics of the 36 included PROMs are presented in Table 2. The most frequently used PROM was the Short Form 36 health survey (SF-36) (n = 15), followed by the Chronic Liver Disease Questionnaire (CLDQ) (n = 6) and the Primary Biliary Cirrhosis (PBC)-40 (n = 5). All other PROMs were used in ≤3 studies (Table 1).
Table 2.
Characteristics of included PROMs
| PROM | Construct | Therapeutic area | Domains | Total No. of items | Scoring method | Recall period | Administration | Completion time | Data collectiona | Cost & permissionb |
|---|---|---|---|---|---|---|---|---|---|---|
| 15 D © | HRQOL | Generic | Mobility,Vision,Hearing, Breathing, Sleeping, Eating, Speech, Elimination, Usual Activities, Mental function,Discomfort, symptoms, Depression, Distress, Vitality, Sexual Activity | 15 | 1 to 5 levels | Present health status | Self-administered | 5–10 min | PP | A, B |
| 5-D Itch | Pruritus | Severity of symptoms | Duration, Degree, Direction, Disability, Distribution | 5 | 0–5 (0 being least problematic and 5 most problematic) | Last 2 weeks | Self-administered | < 5 min | PP | Unknown |
| BDI | Psychological functioning (incl. coping) | Psychology/ Behaviour | Cognitive-affective, Somatic | 21 | Higher score = greater depression | Last 2 weeks including today | Self-administered/ Interviewer-administered | 5–10 min | E, PP | B,D |
| CGQOL | HRQOL | Disease specific (IBD) | Unknown | 3 | 0–1.0 (1 being the best) | Unknown | Unknown | Unknown | PP | Unknown |
| CLDQ | HRQOL | Digestive System Diseases | Abdominal symptoms, Fatigue, Systemic symptoms, Activity, Emotional function, Worry | 29 | Higher score = better QoL | Last two weeks | Self-administered | 10 min | E, PP | B,D |
| COMPASS | Autonomic nervous system diseases | Signs and symptoms | Orthostatic intolerance, vasomotor, secretomotor, gastrointestinal, bladder and pupilometer | 31 | Higher score = higher autonomic symptom severity | In past year/ past 5 years | Self-administered | No information | PP | No information |
| EQ -5D | HRQOL | Generic | Mobility, Self-care, Usual activities, Pain/discomfort, Anxiety/depression | 5 + VAS (20 cm) | Higher score = better QoL | Today | Interviewer-administeredProxy-ratedSelf-administered | A few minutes | E, PP, IVR, T | B,D |
| ESS | Sleep disorder | Signs and symptoms | Sleep | 8 | Higher score = higher sleepiness | Over recent times | Self-administered | 2–3 min | E, PP | A,B |
| FFSS | HRQOL | Signs & symptoms | Fatigue | 9 | High score = higher fatigue | Past two weeks | Self-administered | < 5 min | E, PP | B,D |
| FIS | Symptoms of fatigue | Pathological Conditions, Signs and Symptoms | Cognitive functioning, Physical functioning, Psychosocial functioning | 40 | Lower score = less fatigue | Past four weeks | Self -administered | 10 min | PP | A,B |
| FSFI | Signs and symptoms | Female Urogenital Diseases & Pregnancy | Desire, Arousal, Lubrication, Orgasm, Global satisfaction, Pain | 19 | Higher score = better functioning | During the past 4 weeks | Self-administered | Information not found | E, PP | C |
| Grading system for fatigue & pruritus | Fatigue and Pruritus | Severity of symptoms | Unknown | Unknown | Pruritus, grades 0 -no, 1-mild, 2- sleep interference,3- substantial sleep disturbance Fatigue, grade 0- no; 1- present, but no interference with activity; 2-extra rest required & activity limited 3- patient unable to work a full day. |
Unknown | Unknown | Unknown | Unknown | Unknown |
| GSRS | Signs and symptoms | Signs & symptoms, Digestive system diseases | Abdominal pain syndrome, Reflux syndrome, Indigestion syndrome, Diarrhoea syndrome, Constipation syndrome | 15 | Lower score-better QoL | Last week | Self-administered | 10 min | PP | B,D |
| HADS | Signs and symptoms | Nervous System Diseases Mental Disorders | Anxiety, Depression | 14 | Lower score = better QoL | In the past week | Self-administered | 2–5 min | E, PP | C |
| HHHQ | Diet | Dietary habits | Patient demographics, Education, Medical surgical history and environmental exposure including dietary habits | 370 questions | Unknown | Unknown | Self-report | Unknown | Unknown | Unknown |
| IDS-SRS 30 | Signs and symptoms | Psychiatry/Psychology/Behaviour | Vegetative features, Cognitive changes, Mood disturbance, Endogenous symptoms, Anxiety symptoms | 30(28 initial version) | Higher score = higher severity | Past 7 days | Clinical-rated, interviewer-administered, self-administered | 10–15 min | E, IVR, PP | C |
| IIEF | HRQOL | Erectile Dysfunction | Erectile function, Orgasmic function, Sexual desire, Intercourse satisfaction, Overall satisfaction | 15 | Higher score = better QoL. Scores by dimension | Past 4 weeks | Self-administered | 15 min | PP | B,D |
| LDH | Alcohol consumption patterns | Intake assessment | Consumption levels (quantity), frequency of use, variability in consumption, types of beverages, drinking pattern, solitary versus social drinking, time of the day alcohol consumption | Unclear | Scored by hand or calculator | Unknown | Unknown | 20 min | Unknown | Cost nominal (copyright) |
| LDQOL 1.0 | HRQOL | Digestive System Diseases | - Generic core SF-36v2 - Disease-targeted scales: Liver disease-related symptoms, Effects of liver disease, Concentration/Memory, Health distress, Sleep, Loneliness, Hopelessness, Stigma of liver disease, Sexual functioning/problems |
72 | Higher score = Better HRQOL. | The past 4 weeks; Presently (for few items) | Self-administered | 18 (+/− 9) min | PP | D |
| MFI | Signs and symptoms | Pathological conditions, signs and symptoms | General fatigue, Physical fatigue, reduced activity, Reduced motivation, Mental fatigue | 20 | Lower score = better QoL | Lately | Self-administered | 5 min | PP | B |
| NIDDK-QA | HRQOL | Patients undergoing Liver transplant | Liver disease symptoms, physical functioning, health satisfaction & overall well-being (OWB) |
47 | Higher scores indicate better QOL | Unknown | Unknown | Unknown | Unknown | Unknown |
| OS | Functional outcome | IPAA or IRA | Bowel movements, urgency, evacuation difficulties, soiling or seepage, perianal/stomal soreness, protective pad, dietary restrictions and social handicap | Unclear | best 0, worst 15 | Unknown | Unknown | Unknown | Unknown | Unknown |
| PBC-27 | HRQOL | Disease specific | Symptoms, Dryness,Itch, Fatigue, Cognitive, Emotional and Social | 40 | Higher scores = greater symptoms impact & poorerHRQOL. | Last four weeks | Self-completion | < 5 min | PP | Unknown |
| PBC-40 | HRQOL | Disease specific | Other Symptoms domain, Itch, Fatigue, Cognitive, Social and Emotional | 27 | Higher scores = greater symptoms impact & poorerHRQOL. | Last four weeks | Self-completion | 5 min | PP | Free access |
| PedsQL 4.0 | HRQOL | Generic | Physical functioning, Emotional functioning, Social functioning,school functioning | 21 to 23 | Higher score = better QoL | Standard version: past one month. Acute version: past 7 days | Interviewer-administered Proxy-rated Self-administered |
5 min | PP | A,B |
| PGWB | HRQOL | Generic | Anxiety, Depression mood, Positive well-being, Self-control, General health, Vitality | 22 | Higher score = better QoL | Standard version = past month/ acute version = last week/ last four weeks | Self-administered/Interviewer-administered | 15 min | PP | |
| PHQ-9 | Depression | Severity of depression | Nine questions on symptoms | 10 | Depression severity:1–4: None; 5–9: Mild; 10–14: Moderate, 15–19: Moderately severe, 20 to 27: Severe | over past 2 weeks | Self-completion | 2 to 5 min | PP | Unknown |
| Pruritus numerical rating scale | Pruritus | Severity of symptoms | Unknown | Unknown | Numerical rating scale 0–10 (0 for having no symptoms and 10 for having the worst imaginable pruritus) | Unknown | Unknown | Unknown | Unknown | Unknown |
| PSC PRO | HRQOL | Disease specific | PSC symptoms, Physical function, Activities of Daily Living, Work Productivity, Role Function, Emotional Impact, Social/Leisure Impact, Q uality of Life, Total Impact of Symptoms | 42 | Module 1: 0–10 scale; Module 2 has 7 four item domains: 1–5 scale, summed within dmains and domain mean summed to give overall impact score | Module 1–24 h recall | Self-administered | 7–15 min | E, PP | Unknown |
| Rome ll modular questionnaire | Symptoms | Functional bowel disorder | Esophageal symptoms, Gastroduodenal symptoms, Bowel symptoms, Abdominal pain symptoms, Biliary symptoms and Anorectal symptoms | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| SADS | Signs and symptoms | Depression | Depressive mood and ideation, Endogenous (ie. Melancholic, vital or vegetative) features, Depressive syndrome, Suicidal ideation and behaviour | 30 | Unknown | Past week only | Unknown | Unknown | Unknown | Unknown |
| SF-36 | HRQOL | Generic | Physical Functioning, Role-Physical, Bodily Pain, General Health,Vitality, Social Functioning, Role-Emotional,Mental Health |
36 | 0 to 100, higher score = better health status | Standard version 4 weeks / Acute version 1 week | Self-administered/Interviewer-administered | 5–10 min | E, C, IVR, T, PP | B |
| SF-6D | Utilities & Health states | Generic- preference based measure | Physical functioning, role limitation, social functioning, pain, mental health, vitality | Unknown | 0.296-most severe problems 1.0-no problems | Unknown | Unknown | Unknown | Unknown | Unknown |
| SIBDQ | HRQOL | Digestive System Diseases | Bowel symptoms, systematic symptoms, Emotional function, Social function | 10 | 1 to 7, higher score = better QOL | Last two weeks | Self-administered/Interviewer-administered | 5 min | E, PP | D |
| VAS | Fatigue and Pruritus | Severity of symptoms | Fatigue, Energy, Pruritus | Pruritus: 10 cm line | Pruritus 0 -no pruritus / 10- worst pruritus imaginable | Right now | Self-administered | Vas: Fatigue < 2 min | PP | Free access |
| WHOQOL-BREF | HRQOL | Generic | Physical, Psychological, social relationship, Environment, + 2 overall QOL & general health status | 26 | Higher score = better QoL | Last 2 weeks | Interviewer-administered, self-administered | 5 min self-administration, 15–20 min interviewer-administration | PP | D |
15 D 15-dimensional health-related quality of life measure, 5-D Itch Five dimensions Itch, BDI: Beck Depression Inventory, CGQOL Cleveland global quality of life questionnaire, CLDQ Chronic liver disease questionnaire, COMPASS Composite Autonomic Symptom Scale, EQ. 5D EuroQol EQ. 5D, ESS Epworth Sleepiness Scale, FFSS Fisk Fatigue Severity Scale, FIS Fatigue Impact Scale, FSFI Female Sexual Satisfaction Index, GSRS Gastrointestinal Symptom Rating Scale, HADS Hospital anxiety and depression scale, HBI Harvey-Bradshaw Index, HHQ Health Habits and History Questionnaires, HRQOL Health-related quality of life, IBD Irritable Bowel Syndrome, IDS-SR30 30-item Inventory of Depressive Symptomatology-self report, IIEF International index of erectile function, LDH Lifetime drinking history, LDQOL Liver Disease Quality of Life Questionnaire, MFI Multidimensional Fatigue Inventory, NIDDK-QA National institute of diabetes and digestive and kidney disease liver transplant questionnaire, No. Number, OS Oresland Scale, PBC-40 Primary Biliary Cirrhosis, PF Pouch Function Questionnaire, PGWB Psychological general well-being index, PHQ-9 Patient Health Questionnaire, PSC PRO Primary Sclerosing Cholangitis patient-reported outcome, QoL Quality of Life, SADS Schedule for Affective Disorders and Schizophrenia, SF-36 Short form 36, SIBDQ Short Inflammatory Bowel Disease Questionnaire, UCAI UC Activity Index, VAS Visual Analogue Scale, WHOQOL-BREF World Health Organization Quality of Life assessment instrument
aPP: Paper & pen, E: E-version, IVR: Interactive Voice Response, T: Telephone, C: Computer
bA: Free access to academic/non-profitable research, B: Fees for commercial/pharmaceutical companies/academics, C: Free access to public domain, D: Contact author / licence / signature of a contract or agreement
There were seven generic measures including: the 15 Dimensional Health-Related Quality of Life Measure (15D ©) [30, 31]; SF-36® [6, 27–29, 32–43]; Short Form 6 health survey (SF-6D) [27]; Psychological General Well-being Index (PGWBI) [44]; Paediatric Quality of Life Inventory™ generic core scale (PedsQL™) [25]; EuroQOL (EQ. 5D) [37, 45, 46]; and the World Health Organization Quality of Life assessment instrument (WHOQOL-BREF) [37].
Ten disease-specific measures included: the Short form Liver Disease Quality of Life questionnaire (LDQOL 1.0) [32]; CLDQ [27, 29, 38, 39, 42, 43]; the NIDDK-QA [26, 28]; Rome II Modular Questionnaire; the Cleveland Global Quality of Life questionnaire (CGQOL) [34]; the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) [32, 47]; Oresland scale; PSC PRO; [43] PBC-27 [35, 40, 41]; and PBC-40 [32, 35, 40, 41, 43].
The 17 symptom-specific PROMs included: the FIS [29, 37, 44]; Gastrointestinal Symptom Rating Scale (GSRS) [44]; Fisk Fatigue Severity Scale (FFSS) [36, 42, 48]; Multidimensional Fatigue Inventory (MFI) [48]; VAS [48–50]; the 5-Dimension Itch; [42, 43] the Pruritus numerical rating scale; [51] the Hospital Anxiety and Depression Scale (HADS) [29]; Beck Depression Inventory (BDI) [44, 52]; Inventory of Depressive Symptomatology (IDS) [50]; Patient Health Questionnaire (PHQ-9) [6, 32]; Schedule for Affective Disorders and Schizophrenia (SADS) [52]; the Female Sexual Functioning Index (FSFI) [34]; International Index of Erectile Function (IIEF) [34]; Epworth Sleepiness Scale (ESS); [21] and Composite Autonomic Symptom Scale 31 (COMPASS 31) [21].
Two other measures included: the Lifetime Drinking History (LDH) and Health Habits and History Questionnaires (HHHQ), which focused on alcohol consumption and dietary intake.
Content comparison of included PROMs
The most frequent health domains (n = 6) included across the measures were: fatigue, pain, physical functioning, emotion, anxiety and general health.
Generic PROMs measured symptoms such as pain, physical functioning, emotion, mental health and depression. The disease- and symptom-specific PROMs targeted aspects surrounding gastro intestinal symptoms, such as abdominal pain, or gastroduodenal symptoms, sexual problems, somatic symptoms, depression, mood disturbance, and vegetative features (Additional file 3).
Quality assessment
Only three studies investigated measurement properties for PROMs, two studies evaluated the NIDDK-QA [26, 28] and one study evaluated the PSC PRO [43].
For NIDDK-QA, one validation study [28] included 76 Primary Biliary Cirrhosis (PBC) and 17 PSC patients. A second study examined health status and QOL in patients with cholestatic disease before and after a liver transplant. In this study the NIDDK-QA questionnaire was administered to 65 Primary Biliary Cirrhosis and 92 PSC patients [26]. The PSC PRO validation study included 102 patients with PSC who completed the PSC PRO and four other questionnaires (SF-36, CLDQ, PBC-40 and 5-D Itch Scale) using an ePRO website [43]. The results of the validation studies are presented in Table 3 and summarised below.
Table 3.
Results of measurement properties of NIDDK-QA
| PROM (Author, Year) | Total sample size | PSC sample size | Domains | Test retest reliability (Pearson Correlation) | Internal consistency (Cronbach’s Alpha) |
|---|---|---|---|---|---|
| NIDDK-QA (Kim, 2000) | 96 | 17 | Liver symptoms men women | 0.94 | Men = 0.94, women =0.87 |
| Physical function | 0.99 | 0.88 | |||
| Health satisfaction | 0.82 | NR | |||
| Overall well being | 0.83 | 0.91 | |||
| Time interval of 2 weeks | |||||
| NIDDK-QA (Gross, 1999) | 157 | 92 | Symptoms | NR | 0.81 & 0.85 |
| Functioning | NR | 0.82 & 0.88 | |||
| Index of General Affect (IGA) | NR | 0.91 & 0.93 | |||
| PSC PROM (Younossi, 2017) | 102 | Test retest n = 53 Internal consistency n = 155 | PSC Symptoms | 0.84 | 0.89 |
| Physical Function | 0.83 | 0.91 | |||
| Activities of Daily Living | 0.85 | 0.86 | |||
| Work Productivity | 0.7 | 0.93 | |||
| Role Function | 0.83 | 0.91 | |||
| Emotional Impact | 0.82 | 0.91 | |||
| Social/Leisure Impact | 0.8 | 0.93 | |||
| Quality of Life | 0.79 | 0.94 | |||
| Total Impact of Symptoms | 0.88 |
NIDDK-QA National institute of diabetes and digestive and kidney disease liver transplant questionnaire, PSC PRO Primary Sclerosing Cholangitis Patient Reported Outcome
Internal consistency
All the validation studies, appropriately calculated Cronbach’s alpha to estimate reliability and internal consistency. Reported Cronbach’s Alpha ranged from 0.87 to 0.94 for the NIDDK-QA and 0.86 to 0.94 for the PSC PRO which suggests good internal consistency. Criteria defined by the COSMIN tool meant that for the NIDDK-QA the measurement properties were evaluated as ‘poor’ in methodological quality in both studies primarily because of small sample sizes and a lack of information regarding the proportion of missing items and how missing items were managed. The PSC PRO was rated as ‘fair’ due to the lack of explicit reporting of missing items and sample size for unidemensionality analysis.
Reliability
Kim et al. (2000) [28] assessed test-retest reliability of the NIDDK-QA by administering the measure on two separate occasions approximately 2 weeks apart in 19 patients. Although Pearson’s correlation was high at 0.80 (range 0.82 to 0.94), this measurement property was evaluated as ‘poor’ methodological quality due to the small sample size. For the PSC PRO, 53 patients completed the PSC PRO a second time within 3 months and correlations between administrations was high (range 0.70–0.88). The reliability of the PSC PRO was rated as ‘fair’ due to this length of time between administrations.
Validity
Kim et al. (2000) [28] assessed concurrent validity, by investigating the correlation between the NIDDK-QA and SF-36. The authors postulated that observed correlations between theoretically related domains such as physical function and health satisfaction (r = 0.86 and 0.72 respectively) demonstrated concurrent validity of the tool. However, this measurement property was also evaluated with ‘poor’ methodological quality owing to the absence of details regarding the measurement properties of the comparator scale (SF-36) in this population, and issues with sample size and missing data.
Kim et al. (2000) [28] also measured discriminant validity and information on the significant differences in the item and domain level scores of NIDDK-QA reported. Again, this property was evaluated with ‘poor’ methodological quality, secondary to issues regarding sample size, proportion and handling of missing data.
For the PSC PRO, 26 PSC patients enrolled in cognitive interviews for assessment of content validity, which was rated as ‘excellent’ according to the COSMIN checklist. An external validation cohort of 102 patients completed the PSC PRO along with SF-36, CLDQ, PBC-40 and 5-D Itch Scale; all correlations were statistically significant. The structural validity measurement property was rated as ‘fair’ due to the sample size in relation to the number of items.
Evidence synthesis
Both NIDDK-QA studies reported limited information regarding internal consistency, reliability and validity (concurrent and discriminant). Using the COSMIN guidance these properties were rated as indeterminate due to the poor methodological ratings of both studies (Tables 4 and 5) (Additional file 4) [23]. The PSC PRO study [43] had higher methodological quality compared to the NIDDK-QA studies; however, as there was only one study the level of evidence is limited.
Table 4.
Methodological quality of each study per measurement property and PROM
| Author (Year) | PROM | Internal consistency | Test-retest reliability | Measurement error | Content validity | Structural validity | Hypothesis testing | Criterion validity | Cross structural validity |
|---|---|---|---|---|---|---|---|---|---|
| Discriminant validity | Concurrent validity | ||||||||
| Kim (2000) | NIDDK-QA | Poor | Poor | NR | NR | NR | Poor | Poor | NR |
| Gross (1999) | NIDDK-QA | Poor | NR | NR | NR | NR | NR | NR | NR |
| Younossi, (2017) | PSC PROM | Fair | Fair | NR | Excellent | Fair | NR | NR | NR |
NIDDK-QA National institute of diabetes and digestive and kidney disease liver transplant questionnaire; PSC PRO: Primary Sclerosing Cholangitis Patient Reported Outcome
Table 5.
Quality of measurement properties
| PROM | Internal consistency | Test-retest reliability | Measurement error | Content validity | Structural validity | Hypothesis testing | Criterion validity | Responsiveness |
|---|---|---|---|---|---|---|---|---|
| Discriminant validity | Concurrent validity | |||||||
| NIDDK-QA | ? | ? | NR | NR | NR | ? | ? | NR |
| PSC PROM | + | + | NR | + | + | NR | NR | NR |
Level of evidence (COSMIN): +++ or --- ‘Strong’ Consistent findings in multiple studies of good methodological quality, ++ or – ‘Moderate’ Consistent findings in multiple studies is fair, + or – ‘Limited’ One study of fair methodological quality, +/− ‘Conflicting’ Findings are conflicting,? ‘Unknown’ Studies of poor methodological quality. NIDDK-QA National institute of diabetes and digestive and kidney disease liver transplant questionnaire, PSC PRO Primary Sclerosing Cholangitis Patient Reported Outcome
Discussion
This review identified a total of 37 studies assessing 36 different PROMs used in patients with PSC; however, only one of these tools was specifically developed for the PSC population in accordance with FDA guidelines. The rationale for PROM utilization in the included studies varied. Most studies sought to measure the burden of the disease using constructs such as HRQOL and symptom severity; however, some studies examined the effectiveness of treatment, cost effectiveness and health utility. No studies researched the use of real-time monitoring of PROMs to directly inform PSC patient care in a routine clinical setting. Only three studies evaluated the measurement properties of PROMs in PSC patients: two studies evaluated the NIDDK-QA [26, 28] and one study evaluated the PSC PRO [43]. Currently, due to weakness in the methodological quality, there is limited evidence to support the use of these PROMs in the PSC population; however the PSC PRO is a promising new measure designed with patient input which requires further validation.
Clinicians or researchers wishing to use PROMs in PSC patients may consider use of both generic and disease specific measures. Choice of measurement selection should be informed through consideration on psychometric properties and patient input [53]. Generic measures such as the SF-36, although not formally validated in PSC patients, are widely used and allow comparison of the burden of PSC with other chronic disease, whilst the EQ-5D and SF-6D may be used to provide estimates of health utility to inform cost-effectiveness analysis [54]. Use of the PSC PRO will provide a more detailed assessment of symptoms and impact of symptoms relevant to PSC patients and help identify patients with varying disease severity [43, 55].
Although the PSC PRO has been developed with input from patients with and without IBD, questions focused on IBD symptoms appear fairly limited. This is important to note since 70–80% of PSC patients have co-existent IBD, most frequently ulcerative colitis [3]. This is a long term comorbidity and can occur even after a liver transplant [56]. The clinical course for patients with PSC and concomitant IBD can be different when compared to IBD or PSC alone [57]. PSC-IBD patients have higher incidence of rectal sparing, colorectal neoplasia, pouchitis following ileal pouch anal anastomosis (IPAA), pancolitis, and an overall poorer prognosis when compared to patients with IBD alone [57, 58]. Thus, PSC-IBD patients have additional symptoms and burdens that impact on activities of daily living with the consequential impact on HRQOL [59]. Additional use of an IBD measure such as the IBS-QOL may therefore be warranted [60].
Following further validation, the PSC PRO has potential for use in a number of ways to inform PSC patient care. The PRO may be used in clinical trials to assess the impact of new treatments or be used at the individual patient level in routine clinical practice to facilitate shared decision making and tailor care to individual patient needs. This approach has been highly successful in other settings such as cancer where routine monitoring using ePROs reduced emergency room admissions by 7%, hospital admissions by 4%, helped patients stay on treatment longer, improved patient quality of life by 31% and increased survival on average by 5 months at low cost [61, 62].
Strengths and limitations
This study is the first to undertake a systematic review of PROMs used in PSC, in accordance with the PRISMA [63] and COSMIN guidelines [64]. The use of COSMIN criteria has permitted a structured and comprehensive evaluation of the identified measures. However, the NIDDK QA studies evaluated in this review were carried out before the COSMIN guidance was available and at the time of publication the level and detail of reporting may have been deemed acceptable at that time. Another important consideration for research studies or clinical trials in rare diseases such as PSC are the small study populations. When guidelines such as COSMIN judge the quality of the methodology on sample sizes, it can make it more difficult to demonstrate sound methodological quality when there are only small numbers of patients available for recruitment and validation of PROs [65]. The use of international multi-centred studies may be one approach to overcome the small numbers available in studies that aim to evaluate and develop PROs for use in PSC in future studies.
Conclusion
In conclusion, a wide variety of PROMs are used to assess HRQOL and symptom burden in patients with PSC, but none have undergone comprehensive and extensive validation in this patient group. The PSC PRO is a promising new measure to assess symptoms and symptom impact in PSC patients; however further validation work is required. Collection of PROs in PSC patients can provide valuable information in a research setting and routine clinical practice to improve PSC patient care.
Additional files
PRISMA checklist. (DOCX 62 kb)
Medline search strategy, (DOCX 42 kb)
Content comparison. (DOCX 52 kb)
Cosmin checklist. (DOCX 22 kb)
Acknowledgments
Funding
This project was funded by the Metchley Park Medical Society. This paper presents independent research supported by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 15D
15 Dimensional health-related quality of life measure
- 5-D Itch
Five dimensional itch
- BDI
Beck depression inventory
- CGQOL
Cleveland global quality of life questionnaire
- CLDQ
Chronic liver disease questionnaire
- COMPASS 31
Composite autonomic symptom scale 31
- COSMIN
Consensus-based standards for selection of health measurement instruments
- EQ 5D
EuroQOL
- ESS
Epworth sleepiness scale
- FDA
Food and Drug Administration
- FFSS
Fisk fatigue severity scale
- FIS
Fatigue impact scale
- FSFI
Female sexual functioning index
- GSRS
Gastrointestinal symptom rating scale
- HADS
Hospital anxiety and depression scale
- HHHQ
Health habits and history questionnaires
- HRQOL
Health-related quality of life
- IBD
Inflammatory bowel disease
- IDS
Inventory of depressive symptomatology
- IIEF
International index of erectile function
- LDH
Lifetime drinking history
- LDQOL 1.0
Short form liver disease quality of life questionnaire
- MFI
Multidimensional fatigue inventory
- NIDDK-QA
National Institute of Diabetes Digestive and Kidney Diseases Liver Transplant
- PBC-27
Primary biliary cirrhosis
- PBC-40
Primary biliary cirrhosis
- PedsQL
Paediatric Quality of Life Inventory generic core scale
- PGWBI
Psychological General Well-being Index
- PHQ-9
Patient Health Questionnaire
- PROMs
Patient-reported outcome measures
- PSC PRO
Primary sclerosing cholangitis patient reported outcome
- PSC
Primary sclerosing cholangitis
- SADS
Schedule for affective disorders and schizophrenia
- SF-36
Short Form 36 health survey
- SF-6D
Short Form 6 health survey
- SIBDQ
Short inflammatory bowel disease questionnaire
- VAS
Visual analogue scale
- WHOQOL-BREF
World Health Organization Quality of Life assessment instrument
Authors’ contributions
FI, DK, AS, TP, LK, TK, JF and MC contributed to the study conception and design. FI and GK conducted the searches, FI, GT and GK completed the screening titles and abstracts; identifying eligible full text papers; data extraction and quality assessment. FI drafted the manuscript and GT, GK, DK, AS, TP, LK, TK, JF and MC provided feedback. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12955-018-0951-6) contains supplementary material, which is available to authorized users.
Contributor Information
Fatima Isa, Email: Fatima.isa@PHE.gov.uk.
Grace M. Turner, Email: G.Turner.1@bham.ac.uk
Geetinder Kaur, Email: G.Kaur.2@bham.ac.uk.
Derek Kyte, Email: D.G.Kyte@bham.ac.uk.
Anita Slade, Email: A.L.Slade@bham.ac.uk.
Tanya Pankhurst, Email: Tanya.Pankhurst@uhb.nhs.uk.
Larissa Kerecuk, Email: Larissa.Kerecuk@bch.nhs.uk.
Thomas Keeley, Email: Thomas.Keeley@PAREXEL.com.
James Ferguson, Email: James.Ferguson@uhb.nhs.uk.
Melanie Calvert, Email: M.Calvert@bham.ac.uk.
References
- 1.Williamson KD, Chapman RW. Primary sclerosing cholangitis: a clinical update. Br Med Bull. 2015;114(1):53–64. doi: 10.1093/bmb/ldv019. [DOI] [PubMed] [Google Scholar]
- 2.Primary Sclerosing Cholangitis [http://rarediseases.org/rare-diseases/primary-sclerosing-cholangitis/].
- 3.Ponsioen C. Diagnosis, prognosis, and Management of Primary Sclerosing Cholangitis. Gastroenterol Hepatol. 2013;9(7):453–465. [PMC free article] [PubMed] [Google Scholar]
- 4.Singh S, Talwalkar JA. Primary Sclerosing cholangitis: diagnosis, prognosis, and management. Clin Gastroenterol Hepatol. 2013;11(8):898–907. doi: 10.1016/j.cgh.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD: Pathogenesis of primary Sclerosing cholangitis and advances in diagnosis and management. Gastroenterology 2013, 145(3):10.1053/j.gastro.2013.1006.1052. [DOI] [PMC free article] [PubMed]
- 6.Gotthardt DN, Rupp C, Bruhin M, Schellberg D, Weiss KH, Stefan R, Donnerstag N, Stremmel W, Lowe B, Juenger J, et al. Pruritus is associated with severely impaired quality of life in patients with primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 2014;26(12):1374–1379. doi: 10.1097/MEG.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 7.De Valle MB, Rahman M, Lindkvist B, Bjornsson E, Chapman RW, Kalaitzakis E. Fatigue in patients with primary sclerosing cholangitis: an international survey study in two population-based patient cohorts. Gastroenterology. 2010;1:S320. [Google Scholar]
- 8.Phillips R, Gandhi M, Cheung YB, Findlay MP, Win KM, Hai HH, Yang JM, Lobo RR, Soo KC, Chow PKH. Summary scores captured changes in subjects' QoL as measured by the multiple scales of the EORTC QLQ-C30. J Clin Epidemiol. 2015;68(8):895–902. doi: 10.1016/j.jclinepi.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2(4):137–144. doi: 10.4103/2229-3485.86879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiegel BMR. Patient-reported outcomes in gastroenterology: clinical and research applications. J Neurogastroenterol Motility. 2013;19(2):137–148. doi: 10.5056/jnm.2013.19.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvert M, Thwaites R, Kyte D, Devlin N. Putting patient-reported outcomes on the 'Big data road Map'. J R Soc Med. 2015;108(8):299–303. doi: 10.1177/0141076815579896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167. doi: 10.1136/bmj.f167. [DOI] [PubMed] [Google Scholar]
- 13.Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, Selby PJ. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22(4):714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 14.Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288(23):3027–3034. doi: 10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]
- 15.Hilarius DL, Kloeg PH, Gundy CM, Aaronson NK. Use of health-related quality-of-life assessments in daily clinical oncology nursing practice: a community hospital-based intervention study. Cancer. 2008;113(3):628–637. doi: 10.1002/cncr.23623. [DOI] [PubMed] [Google Scholar]
- 16.Jay CL, Butt Z, Ladner DP, Skaro AI, Abecassis MM. A review of quality of life instruments used in liver transplantation. J Hepatol. 2009;51(5):949–959. doi: 10.1016/j.jhep.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. The BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, de Vet HCW. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010;19(4):539–549. doi: 10.1007/s11136-010-9606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, Bronfort G, van Tulder MW: 2015 updated method guideline for systematic reviews in the Cochrane back and neck group. Spine (Phila Pa 1976) 2015, 40(21):1660–1673. [DOI] [PubMed]
- 20.Dyson JK, Elsharkawy AM, Lamb CA, Al-Rifai A, Newton JL, Jones DE, Hudson M. Fatigue in primary sclerosing cholangitis is associated with sympathetic over-activity and increased cardiac output. Liver Int. 2015;35(5):1633–1641. doi: 10.1111/liv.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorgun E, Remzi FH, Manilich E, Preen M, Shen B, Fazio VW. Surgical outcome in patients with primary sclerosing cholangitis undergoing ileal pouch-anal anastomosis: a case-control study. Surgery. 2005;138(4):631–637. doi: 10.1016/j.surg.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 22.U. S. Department of Health, human services F. D. A. Center for Drug Evaluation Research, U. S. Department of Health, human services F. D. A . Center for Devices Radiological Health: guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. 2009. Center for Biologics Evaluation Research, U. S. Department of Health, human services F. D. A. [Google Scholar]
- 23.Terwee CB. Consensus-based standards for the selection of health measurement instruments checklist. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furlan AD, Pennick V, Bombardier C, van Tulder M: 2009 updated method guidelines for systematic reviews in the Cochrane back review group. Spine (Phila Pa 1976) 2009, 34(18):1929–1941. [DOI] [PubMed]
- 25.Gulati R, Radhakrishnan KR, Hupertz V, Wyllie R, Alkhouri N, Worley S, Feldstein AE. Health-related quality of life in children with autoimmune liver disease. J Pediatric Gastroenterol Nutri. 2013;57(4):444–450. doi: 10.1097/MPG.0b013e31829ef82c. [DOI] [PubMed] [Google Scholar]
- 26.Gross CR, Malinchoc M, Ray Kim W, Evans RW, Wiesner RH, Petz JL, Crippin JS, Klintmalm GB, Levy MF, Ricci P, et al. Quality of life before and after liver transplantation for cholestatic liver disease. Hepatology. 1999;29(2):356–364. doi: 10.1002/hep.510290229. [DOI] [PubMed] [Google Scholar]
- 27.Kalaitzakis E, De Valle MB, Rahman M, Lindkvist B, Bjornsson ES, Chapman RW, Kontodimopoulos N. Mapping chronic liver disease questionnaire (CLDQ) scores onto SF-6D utility values in patients with primary sclerosing cholangitis: Results from a population-based cohort study. Gastroenterology. 2014;1:S–738.
- 28.Kim WR, Lindor KD, Malinchoc M, Petz JL, Jorgensen R, Dickson ER. Reliability and validity of the NIDDK-QA instrument in the assessment of quality of life in ambulatory patients with cholestatic liver disease. Hepatology. 2000;32(5):924–929. doi: 10.1053/jhep.2000.19067. [DOI] [PubMed] [Google Scholar]
- 29.Benito de Valle M, Rahman M, Lindkvist B, Bjornsson E, Chapman R, Kalaitzakis E: Factors that reduce health-related quality of life in patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol 2012, 10(7):769–775.e762. [DOI] [PubMed]
- 30.Aberg F, Hockerstedt K, Roine RP, Sintonen H, Isoniemi H. Influence of liver-disease etiology on long-term quality of life and employment after liver transplantation. Clin Transpl. 2012;26(5):729–735. doi: 10.1111/j.1399-0012.2012.01597.x. [DOI] [PubMed] [Google Scholar]
- 31.Haapamaki J, Sintonen H, Barner-Rasmussen N, Farkkila M. Health-related quality of life among patients with primary sclerosing cholangitis. J Crohn's Colitis. 2014;8:S151–S152. doi: 10.1016/S1873-9946(14)60335-4. [DOI] [PubMed] [Google Scholar]
- 32.Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM: Factors that influence health-related quality of life in patients with primary Sclerosing cholangitis. Dig Dis Sci 2016;61(6):1692–9. [DOI] [PubMed]
- 33.Olsson R, Boberg KM, de Muckadell OS, Lindgren S, Hultcrantz R, Folvik G, Bell H, Gangsoy-Kristiansen M, Matre J, Rydning A, et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology. 2005;129(5):1464–1472. doi: 10.1053/j.gastro.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Pavlides M, Cleland J, Rahman M, Christian A, Doyle J, Gaunt R, Travis S, Mortensen N, Chapman R. Outcomes after ileal pouch anal anastomosis in patients with primary sclerosing cholangitis. J Crohn's Colitis. 2014;8(7):662–670. doi: 10.1016/j.crohns.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Raszeja-Wyszomirska J, Wunsch E, Krawczyk M, Rigopoulou EI, Bogdanos D and Milkiewicz P. Prospective evaluation of PBC-specific health-related quality of life questionnaires in patients with primary sclerosing cholangitis. Liver Int. 2015;35(6):1764–71. [DOI] [PubMed]
- 36.ter Borg PC, van Os E, van den Broek WW, Hansen BE, van Buuren HR. Fluvoxamine for fatigue in primary biliary cirrhosis and primary sclerosing cholangitis: a randomised controlled trial [ISRCTN88246634] BMC Gastroenterol. 2004;4:13. doi: 10.1186/1471-230X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tillmann HL, Wiese M, Braun Y, Wiegand J, Tenckhoff S, Mossner J, Manns MP, Weissenborn K. Quality of life in patients with various liver diseases: patients with HCV show greater mental impairment, while patients with PBC have greater physical impairment. J Viral Hepat. 2011;18(4):252–261. doi: 10.1111/j.1365-2893.2010.01292.x. [DOI] [PubMed] [Google Scholar]
- 38.Younossi ZM, Kiwi ML, Boparai N, Price LL, Guyatt G. Cholestatic liver diseases and health-related quality of life. Am J Gastroenterol. 2000;95(2):497–502. doi: 10.1111/j.1572-0241.2000.01774.x. [DOI] [PubMed] [Google Scholar]
- 39.Younossi ZM, Boparai N, Price LL, Kiwi ML, McCormick M, Guyatt G. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am J Gastroenterol. 2001;96(7):2199–2205. doi: 10.1111/j.1572-0241.2001.03956.x. [DOI] [PubMed] [Google Scholar]
- 40.Kempinska-Podhorodecka A, Milkiewicz M, Jabłonski D, Milkiewicz P, Wunsch E. ApaI polymorphism of vitamin D receptor affects health-related quality of life in patients with primary sclerosing cholangitis. PLoS One. 2017;12(4):e0176264. doi: 10.1371/journal.pone.0176264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raszeja-Wyszomirska J, Kucharski R, Zygmunt M, Safranow K, Miazgowski T. The impact of fragility fractures on health-related quality of life in patients with primary sclerosing cholangitis. Hepat Mon. 2015;15(4):e25539. doi: 10.5812/hepatmon.25539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabibian JH, Gossard A, El-Youssef M, Eaton JE, Petz J, Jorgensen R, Enders FB, Tabibian A, Lindor KD. Prospective clinical trial of rifaximin therapy for patients with primary sclerosing cholangitis. Am J Ther. 2017;24(1):e56–e63. doi: 10.1097/MJT.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Younossi ZM, Afendy A, Stepanova M, Racila A, Nader F, Gomel R, Safer R, Lenderking WR, Skalicky A, Kleinman L et al: Development and validation of a primary sclerosing cholangitis-specific patient-reported outcomes instrument: the PSC PRO. Hepatology (Baltimore, Md) 2017. 10.1002/hep.29664. [DOI] [PubMed]
- 44.Bjornsson E, Simren M, Olsson R, Chapman RW. Fatigue in patients with primary sclerosing cholangitis. Scand J Gastroenterol. 2004;39(10):961–968. doi: 10.1080/00365520410003434. [DOI] [PubMed] [Google Scholar]
- 45.Longworth L, Young T, Buxton MJ, Ratcliffe J, Neuberger J, Burroughs A, Bryan S, Team CP. Midterm cost-effectiveness of the liver transplantation program of England and Wales for three disease groups. Liver Transpl. 2003;9(12):1295–1307. doi: 10.1016/j.lts.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Carbone M, Cristoferi L, Cortesi PA, Rota M, Ciaccio A, Okolicsanyi S, Gemma M, Scalone L, Cesana G, Fabris L, et al. Optimising the clinical strategy for autoimmune liver diseases: principles of value-based medicine. Biochim Biophys Acta. 2018;1864(4 Pt B):1415–1422. doi: 10.1016/j.bbadis.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 47.Ananthakrishnan AN, Beaulieu DB, Naik AS, Zadvornova Y, Skaros S, Johnson K, Perera LP, Issa M, Binion DG, Saeian K. Does primary sclerosing cholangitis impact quality of life in patients with inflammatory bowel disease? Gastroenterology. 2009;1:A203. doi: 10.1002/ibd.21051. [DOI] [PubMed] [Google Scholar]
- 48.ter Borg PC, Fekkes D, Vrolijk JM, van Buuren HR. The relation between plasma tyrosine concentration and fatigue in primary biliary cirrhosis and primary sclerosing cholangitis. BMC Gastroenterol. 2005;5:11. doi: 10.1186/1471-230X-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mansour-Ghanaei F, Taheri A, Froutan H, Ghofrani H, Nasiri-Toosi M, Bagherzadeh AH, Farahvash MJ, Mirmomen S, Ebrahimi-Dariani N, Farhangi E, et al. Effect of oral naltrexone on pruritus in cholestatic patients. World J Gastroenterol. 2006;12(7):1125–1128. doi: 10.3748/wjg.v12.i7.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayo MJ, Handem I, Saldana S, Jacobe H, Getachew Y, Rush AJ. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology. 2007;45(3):666–674. doi: 10.1002/hep.21553. [DOI] [PubMed] [Google Scholar]
- 51.Kittanamongkolchai W, El-Zoghby ZM, Eileen Hay J, Wiesner RH, Kamath PS, LaRusso NF, Watt KD, Cramer CH, Leung N. Charcoal hemoperfusion in the treatment of medically refractory pruritus in cholestatic liver disease. Hepatol Int. 2017;11(4):384–389. doi: 10.1007/s12072-016-9775-9. [DOI] [PubMed] [Google Scholar]
- 52.van Os E, van den Broek WW, Mulder PGH, ter Borg PCJ, Bruijn JA, van Buuren HR. Depression in patients with primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol. 2007;46(6):1099–1103. doi: 10.1016/j.jhep.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 53.Haywood KL, Wilson R, Staniszewska S, Salek S. Using PROMs in healthcare: who should be in the driving seat-policy makers, health professionals, methodologists or patients? Patient. 2016;9(6):495–498. doi: 10.1007/s40271-016-0197-5. [DOI] [PubMed] [Google Scholar]
- 54.Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96:5–21. doi: 10.1093/bmb/ldq033. [DOI] [PubMed] [Google Scholar]
- 55.Martin LM, Sheridan MJ, Younossi ZM. The impact of liver disease on health-related quality of life: a review of the literature. Current Gastroenterol Rep. 2002;4(1):79–83. doi: 10.1007/s11894-002-0041-z. [DOI] [PubMed] [Google Scholar]
- 56.Joo M, Abreu-e-Lima P, Farraye F, Smith T, Swaroop P, Gardner L, Lauwers GY, Odze RD. Pathologic features of ulcerative colitis in patients with primary sclerosing cholangitis: a case-control study. Am J Surg Pathol. 2009;33(6):854–862. doi: 10.1097/PAS.0b013e318196d018. [DOI] [PubMed] [Google Scholar]
- 57.Loftus EV, Jr, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54(1):91–96. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Penna C, Dozois R, Tremaine W, Sandborn W, LaRusso N, Schleck C, Ilstrup D. Pouchitis after ileal pouch-anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut. 1996;38(2):234–239. doi: 10.1136/gut.38.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Achleitner U, Coenen M, Colombel J-F, Peyrin-Biroulet L, Sahakyan N, Cieza A. Identification of areas of functioning and disability addressed in inflammatory bowel disease-specific patient reported outcome measures. J Crohn's Colitis. 2012;6(5):507–517. doi: 10.1016/j.crohns.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Lee J, Lee EH, Moon SH. A systematic review of measurement properties of the instruments measuring health-related quality of life in patients with irritable bowel syndrome. Qual Life Res. 2016;25(12):2985–2995. doi: 10.1007/s11136-016-1421-4. [DOI] [PubMed] [Google Scholar]
- 61.Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, Rogak L, Bennett AV, Dueck AC, Atkinson TM, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terwee CB. Protocol for systematic reviews of measurement properties. 2011. [Google Scholar]
- 65.A decade of innovation in rare diseases [http://www.phrma.org/sites/default/files/pdf/PhRMA-Decade-of-Innovation-Rare-Diseases.pdf].
- 66.Gavaler J, Delemos B, Belle SH, Heyl AE, Tarter RE, Starzl TE, Gavaler C, Van Thiel DH. Ulcerative colitis disease activity as subjectively assessed by patient-completed questionnaires following orthotopic liver transplantation for sclerosing cholangitis. Dig Dis Sci. 1991;36(3):321–328. doi: 10.1007/BF01318204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bharucha AE, Jorgensen R, Lichtman SN, LaRusso NF, Lindor KD. A pilot study of pentoxifylline for the treatment of primary sclerosing cholangitis. Am J Gastroenterol. 2000;95(9):2338–2342. doi: 10.1111/j.1572-0241.2000.02324.x. [DOI] [PubMed] [Google Scholar]
- 68.Hagstrom H, Stal P, Stokkeland K, Bergquist A. Alcohol consumption in patients with primary sclerosing cholangitis. World J Gastroenterol. 2012;18(24):3105–3111. doi: 10.3748/wjg.v18.i24.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Block M, Jorgensen KK, Oresland T, Lindholm E, Grzyb K, Cvancarova M, Vatn MH, Boberg KM, Borjesson L. Colectomy for patients with ulcerative colitis and primary sclerosing cholangitis - what next? J Crohn's Colitis. 2014;8(5):421–430. doi: 10.1016/j.crohns.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Hov JR. Effects of coffee consumption, smoking, and hormones on risk for primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2014;12(6):1019–1028. doi: 10.1016/j.cgh.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 71.Eaton JE, Juran BD, Atkinson EJ, Schlicht EM, Xie X, de Andrade M, Lammert CS, Luketic VA, Odin JA, Koteish AA, et al. A comprehensive assessment of environmental exposures among 1000 north American patients with primary sclerosing cholangitis, with and without inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41(10):980–990. doi: 10.1111/apt.13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist. (DOCX 62 kb)
Medline search strategy, (DOCX 42 kb)
Content comparison. (DOCX 52 kb)
Cosmin checklist. (DOCX 22 kb)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.