Abstract
Aim
Maturation abnormalities of the brain cells have been suggested in several neuropsychiatric disorders, including schizophrenia, bipolar disorder, autism spectrum disorders, and epilepsy. In this study, we examined the expression patterns of neuronal maturation markers in the brain of a mouse model of dementia with Lewy body-linked mutant β-synuclein (βS), especially in the hippocampus, to explore whether such brain abnormalities occur in neurodegenerative disorders as well.
Methods
Quantitative PCR (qPCR) and immunohistochemical analyses were performed using the hippocampus of 14-month-old P123H βS transgenic (Tg) mice to evaluate the expression of molecular markers for maturation of dentate granule cells.
Results
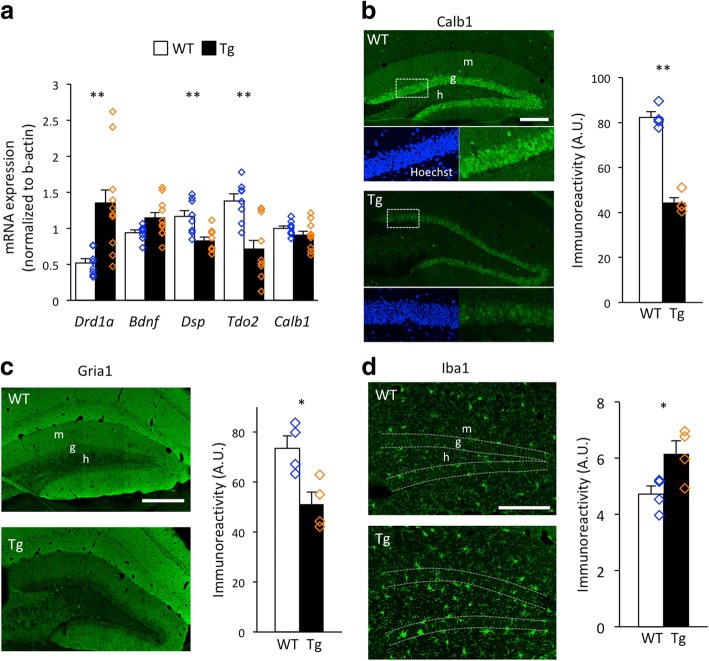
Based on qPCR results, expression of Tdo2 and Dsp (markers of mature granule cells) was decreased and that of Drd1a (a marker of immature granule cells) was increased in the hippocampus of P123H βS Tg mice compared to that in wild-type controls. Immunohistochemical analysis revealed decreased expression of mature granule cell markers Calb1 and Gria1, along with increased expression of the microglial marker Iba1, in the hippocampal dentate gyrus region of P123H βS Tg mice. P123H βS Tg mice exhibited immature-like neuronal molecular expression patterns and microgliosis in the hippocampus. Pseudo-immaturity of dentate granule cells, associated with neuroinflammation, may be a shared endophenotype in the brains of at least a subgroup of patients with neuropsychiatric disorders and neurodegenerative diseases.
Electronic supplementary material
The online version of this article (10.1186/s13041-018-0378-3) contains supplementary material, which is available to authorized users.
Keywords: β-Synuclein, Endophenotype, Hippocampus, Immature dentate gyrus, Neurodegenerative disorders
Main text
“Immature dentate gyrus (iDG)” phenotype is commonly found in several mouse models of neuropsychiatric disorders [1], including schizophrenia/intellectual disability [2], bipolar disorder [3], and epilepsy [4, 5]. In this phenotype, almost all granule cells in the adult hippocampal DG are arrested in a pseudo-immature state, in terms of molecular and electrophysiological characteristics. More specifically, molecular features of iDG include a decrease in expression of mature granule cell markers (e.g., tryptophan 2,3-dioxygenase [Tdo2], Desmoplakin [Dsp], and Calbindin 1 [Calb1] [6]) along with an increase in the expression of immature granule cell marker (dopamine receptor D1 [Drd1a]) [1]. iDG-like phenotype was also observed in patients with schizophrenia and bipolar disorder [7]. As for neurodegenerative disorders, recent studies revealed that Alzheimer’s disease mouse model showed a drastic decrease in the expression of Calb1 in adult DG, suggestive of iDG-like phenotype in the mouse model [8, 9]. In the present study, we investigated whether a mouse model of dementia with Lewy body-linked mutant β-synuclein (P123H βS) exhibits iDG-related molecular features. The P123H βS transgenic (Tg) mouse showed many behavioral abnormalities, including hyperlocomotor activity, impairment of nest building, and impaired spatial memory, in the middle stage (6–10 months of age), before the onset of motor dysfunction that became apparent in the later stage (12–18 months) [10, 11]. In the brain of these mice, neuritic pathologies such as βS accumulation and axonal swellings, and astrogliosis were observed in various regions, including hippocampus, during the middle to late stage [10]. However, maturation abnormalities in the brain have not been examined in these mice.
Whole hippocampus of the mouse (14 months of age) was dissected out and quantitative PCR (qPCR) analysis was conducted to examine mRNA expression levels of Drd1a, Bdnf, Tdo2, Dsp, and Calb1. The detailed method for qPCR is described in the Additional file 1. Expression of Drd1a was significantly increased and that of Bdnf showed an increasing trend in the hippocampus of P123H βS Tg mice compared to that in wild-type mice (Fig. 1a, Additional file 1: Table S1). Expression of Tdo2 and Dsp was significantly decreased in the P123H βS Tg mice while that of Calb1 was comparable between P123H βS Tg and wild-type mice. To assess Calb1 expression, focusing on the DG region, we conducted immunohistochemical analyses (see Additional file for the detailed method). The results showed a significant decrease in Calb1 expression in the DG granule cell layer of P123H βS Tg mice (Fig. 1b, Additional file 1: Figure S1). Notably, a patch-like reduction of Calb1 immunoreactivity was observed in the DG granule cell layer of P123H βS Tg mice, which may not have been due to apparent cell loss, since nuclear staining was observed uniformly throughout the DG granule cell layer. We also found a decrease in immunoreactivity for Gria1, whose expression increased with maturation of granule cells [12], but decreased in the DG of typical mouse models with iDG [12], and in that of P123H βS Tg mice (Fig. 1c). In addition, immunoreactivity for ionized calcium binding adaptor molecule 1 (Iba1) was increased in the DG of P123H βS Tg mice (Fig. 1d), suggesting that microglia are activated in the DG of these mice.
Fig. 1.
iDG-like molecular phenotypes in the hippocampus of P123H βS Tg mice. a Results of quantitative PCR. Bar graphs represent relative mRNA expression levels normalized to β-actin mRNA. Data obtained from two independent experiments were combined and shown as the mean ± SEM. (n = 8 for wild-type mice and n = 10 for P123H βS Tg mice). *P < 0.05, **P < 0.01 versus wild-type mice. b–d Representative images (left panels) and the semi-quantitative results (right panels) of immunofluorescence imaging of Calb1 (b), Gria1 (c), and Iba1 (d) in the DG of wild-type and P123H βS Tg mice. Higher magnification of the boxed area is shown below the corresponding panel (b). Data are shown as the mean ± SEM (n = 4 for each genotype). *P < 0.05, **P < 0.01 versus wild-type mice. Scale bars, 200 μm (b, d) and 500 μm (c). g, granule cell layer; h, hilus; m, molecular layer
We found a significant decrease in Calb1 expression in the DG granule cell layer by immunohistochemical analysis. However, the extent of this reduction was low relative to that found in other mouse models with iDG, such as Camk2a+/− mice, Shn2 KO mice, and mutant Snap25 knock-in mice, whose Calb1 expression in the DG granule cell layer was almost completely depleted [2–4]. Therefore, the present qPCR analysis of whole hippocampus samples might have failed to detect a decrease in Calb1 expression in P123H βS Tg mice, due to the presence of cells (other than granule cells) that express Calb1, such as pyramidal cells in the Ammon’s horn region and particular types of interneurons that exist throughout the brain. The discrepancy between mRNA and protein levels of Calb1 may also be accounted for by some post-transcriptional mechanisms and/or differences in their half lives [13].
Patch-like reduction of Calb1 expression in the DG granule cell layer was found in P123H βS Tg mice; a similar phenotype was observed in a mouse model of Alzheimer’s disease (line J20) [8, 9]. In those papers, it was suggested that Calb1 downregulation was induced by seizure activity in patients and mouse models [8, 9]. Patch-like reduction of Calb1 in the DG has been observed in epilepsy models [5]. Considering that epileptic seizures have been observed in P123H βS Tg mice (unpublished observation), seizure activity might have caused Calb1 downregulation in these Tg mice. Interestingly, patch-like reduction of Calb1 in the DG have also been found in adult mice treated with antidepressant fluoxetine [14] and electroconvulsive stimulation [15]. Assuming that typical models showing robust depletion of Calb1, such as Camk2a+/− and Shn2 KO mice [2, 3], display iDG phenotypes developmentally generated, there is the possibility that these weaker phenotypes are features of iDG that are induced by dematuration in adults.
In conclusion, P123H βS Tg mice exhibited iDG-like signatures and microgliosis in the DG. It would be of interest to determine the time of appearance of the iDG phenotype in these mice in relation to that of behavioral abnormalities.
Additional file
Materials and Methods. Table S1. Raw data of qPCR analysis (average Cq value). Figure S1. Immunohistochemical images used for quantitative analysis in this study. (DOCX 25253 kb)
Acknowledgements
We thank the members of Hashimoto lab for the animal husbandry.
Funding
This work was supported by the Grants-in-Aid for Scientific Research (JP25242078) from Japan Society for the Promotion of Science; and the Grant-in-Aid for Scientific Research on Innovative Areas (JP24111546, JP24111555, JP25116526, and JP16H06462) from the Ministry of Education, Culture, Sports, Science and Technology.
Availability of data and materials
All data are available in the Additional file 1.
Abbreviations
- Bdnf
Brain-derived neurotrophic factor
- Calb1
Calbindin
- Camk2a
Calcium/calmodulin-dependent protein kinase II alpha
- DG
Dentate gyrus
- Drd1a
Dopamine receptor D1
- Dsp
Desmoplakin
- Gria1
Glutamate receptor, ionotropic, AMPA 1
- Iba1
Ionized calcium binding adaptor molecule 1
- iDG
Immature dentate gyrus
- PBS
Phosphate-buffered saline
- PCR
Polymerase chain reaction
- Shn2
Schnurri-2
- Snap25
Synaptosomal-associated protein, 25 kDa
- Tdo2
Tryptophan 2,3-dioxygenase
- βS
β-synuclein
Authors’ contributions
HH, MF, MH, and TM conceived and designed the experiments. HH and TM wrote the article. MF prepared the samples; HH and JU performed molecular experiments and analyzed the data. All authors read and approved the final manuscript.
Ethics approval
The experimental procedures and housing conditions were approved by the Institutional Animal Care and Use Committee at Tokyo Metropolitan Institute of Medical Science (Setagaya-ku, Tokyo, Japan). Frozen or fixed mouse brain samples were prepared at Tokyo Metropolitan Institute of Medical Science and transported to Fujita Health University (Toyoake, Aichi, Japan) for analysis.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13041-018-0378-3) contains supplementary material, which is available to authorized users.
Contributor Information
Hideo Hagihara, Email: h-hagi@fujita-hu.ac.jp.
Masayo Fujita, Email: fujita-ms@igakuken.or.jp.
Juzoh Umemori, Email: juzoh.umemori@helsinki.fi.
Makoto Hashimoto, Email: hashimoto-mk@igakuken.or.jp.
Tsuyoshi Miyakawa, Email: miyakawa@fujita-hu.ac.jp.
References
- 1.Hagihara H, Takao K, Walton NM, Matsumoto M, Miyakawa T. Immature dentate gyrus: an endophenotype of neuropsychiatric disorders. Neural Plasticity. 2013;2013:318596. doi: 10.1155/2013/318596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takao K, Kobayashi K, Hagihara H, Ohira K, Shoji H, Hattori S, et al. Deficiency of schnurri-2, an MHC enhancer binding protein, induces mild chronic inflammation in the brain and confers molecular, neuronal, and behavioral phenotypes related to schizophrenia. Neuropsychopharmacology. 2013;38:1409–1425. doi: 10.1038/npp.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamasaki N, Maekawa M, Kobayashi K, Kajii Y, Maeda J, Soma M, et al. Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Molecular Brain. 2008;1:6. [DOI] [PMC free article] [PubMed]
- 4.Ohira K, Kobayashi K, Toyama K, Nakamura HK, Shoji H, Takao K, et al. Synaptosomal-associated protein 25 mutation induces immaturity of the dentate granule cells of adult mice. Molecular Brain. 2013;6:12. doi: 10.1186/1756-6606-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin R, Kobayashi K, Hagihara H, Kogan JH, Miyake S, Tajinda K, et al. The immature dentate gyrus represents a shared phenotype of mouse models of epilepsy and psychiatric disease. Bipolar Disorders. 2013;15:405–21. [DOI] [PMC free article] [PubMed]
- 6.Radic T, Frieß L, Vijikumar A, Jungenitz T, Deller T, Schwarzacher SW. Differential postnatal expression of neuronal maturation markers in the dentate gyrus of mice and rats. Front Neuroanat. 2017;11:104. [DOI] [PMC free article] [PubMed]
- 7.Walton NM, Zhou Y, Kogan JH, Shin R, Webster M, Gross AK, et al. Detection of an immature dentate gyrus feature in human schizophrenia/bipolar patients. Translational Psychiatry. 2012;2:e135. doi: 10.1038/tp.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You JC, Muralidharan K, Park JW, Petrof I, Pyfer MS, Corbett BF, et al. Epigenetic suppression of hippocampal calbindin-D28k by ΔFosB drives seizure-related cognitive deficits. Nature Medicine. 2017;23:1377–1383. doi: 10.1038/nm.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita M, Sugama S, Sekiyama K, Sekigawa A, Tsukui T, Nakai M, et al. A β-synuclein mutation linked to dementia produces neurodegeneration when expressed in mouse brain. Nature Communications. 2010;1:110. doi: 10.1038/ncomms1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita M, Hagino Y, Takamatsu Y, Shimizu Y, Takamatsu Y, Ikeda K, Hashimoto H. Early manifestation of depressive-like behavior in transgenic mice that express dementia with Lewy body-linked mutant β-synuclein. Neuropsychopharmacology Reports. 2018;38:95–97. [DOI] [PMC free article] [PubMed]
- 12.Hagihara H, Ohira K, Toyama K, Miyakawa T. Expression of the AMPA receptor subunits GluR1 and GluR2 is associated with granule cell maturation in the dentate gyrus. Front Neurosci. 2011;5:100. [DOI] [PMC free article] [PubMed]
- 13.Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biology. 2003;4:117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi K, Ikeda Y, Sakai A, Yamasaki N, Haneda E, Miyakawa T, Suzuki H. Reversal of hippocampal neuronal maturation by serotonergic antidepressants. PNAS. 2010;107:8434–8439. doi: 10.1073/pnas.0912690107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imoto Y, Segi-Nishida E, Suzuki H, Kobayashi K. Rapid and stable changes in maturation-related phenotypes of the adult hippocampal neurons by electroconvulsive treatment. Molecular Brain. 2017;10:8. doi: 10.1186/s13041-017-0288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods. Table S1. Raw data of qPCR analysis (average Cq value). Figure S1. Immunohistochemical images used for quantitative analysis in this study. (DOCX 25253 kb)
Data Availability Statement
All data are available in the Additional file 1.