Abstract
Context:
Sputum smear-negative and sputum-scarce pulmonary tuberculosis (PTB) is a diagnostic challenge. Xpert® Mycobacterium tuberculosis/rifampin (MTB/RIF) provides a rapid diagnosis on high-quality biological specimen obtained by bronchoscopy.
Aims:
The aim of this study is to evaluate Xpert® MTB/RIF on bronchoalveolar lavage (BAL) fluid in sputum smear-negative and sputum-scarce PTB patients.
Settings:
Tertiary care hospital in India.
Design:
This was prospective observational study.
Materials and Methods:
Between January 2015 and November 2016, we prospectively recruited sputum-smear negative and sputum-scarce patients under evaluation for PTB and performed BAL. Sensitivity, specificity, positive, and negative predictive values were calculated for the diagnosis of PTB on BAL fluid for acid-fast bacilli smear and Xpert® MTB/RIF using liquid culture as the reference standard and compared to the final diagnosis based on composite reference standard. Sensitivity, specificity, and predictive values were calculated with 95% class intervals. McNemar's test was used for comparison of sensitivities.
Results:
Of the 60 patients included, 52 (88.3%) had a final diagnosis of PTB and 16 (26.7%) were culture confirmed. Xpert® MTB/RIF had a sensitivity and specificity of 81% (54%–96%) and 73% (56%–85%) in culture confirmed cases; 46% (32%–60%) and 100% (63%–100%) for the final diagnosis; 32% (17%–51%) and 100% (54%–100%) in culture negative cases, respectively. Culture had a sensitivity of 32% (20%–47%) for the final diagnosis.
Conclusions:
In sputum smear-negative and sputum-scarce patients with clinico-radiological features of PTB Xpert® MTB/RIF has good sensitivity for diagnosis on BAL fluid. It is useful even when cultures are negative.
KEY WORDS: Bronchoalveolar lavage, bronchoscopy, diagnosis, pulmonary, tuberculosis, Xpert
INTRODUCTION
Tuberculosis (TB) remains a major global health problem causing significant morbidity and mortality. In 2015, there have been 10.4 million new TB cases (incidence) and 1.4 million TB deaths and an additional 0.4 million HIV associated TB deaths. One-fourth of the global incident TB cases occur in India making India the country with the highest TB burden. In 2015, out of the estimated global annual incidence of 10.4 million TB cases, 2.8 million were estimated to have occurred in India.[1]
TB deaths are unacceptable because the widely available anti-tubercular drugs have cure rates of more than 90%. The main challenge with TB is obtaining a rapid and accurate diagnosis to initiate early treatment. Patient with active pulmonary tuberculosis (PTB) may fail to produce sputum or when it is available acid-fast bacilli (AFB) may be negative in 40%–60% of cases.[2] Sputum-smear negative pulmonary TB is still infectious and remain a diagnostic challenge. The key to this challenge is having a more accurate and rapid diagnostic modality and obtaining a high-quality biological specimen. Xpert® Mycobacterium tuberculosis/rifampin (MTB/RIF) assay and bronchoscopy are useful in this direction.[3]
Xpert® MTB/RIF assay (Xpert, Cepheid, Sunnyvale, CA, USA) is a hemi-nested real-time PCR test that simultaneously identifies MTB/RIF resistance. Its diagnostic accuracy is comparable to culture in sputum samples and provides results within 2 h.[3]
Fiber-optic bronchoscopy is helpful in early detection and confirmation of sputum smear-negative and sputum-scarce PTB by providing high-quality biological samples like bronchoalveolar lavage (BAL) fluid.[4] There are no formal recommendations on utilizing Xpert® MTB/RIF on BAL fluid due to limited literature. The limited literature is more evident in India, a high TB burden country.
Our study evaluated Xpert® MTB/RIF on BAL samples in a tertiary care setting with a high incidence of TB, with the availability of bronchoscopy facilities, in patients with high probability of TB after an evaluation for an alternate diagnosis. The prospective design ensured a final diagnosis of PTB. The final results clearly depend on the epidemiological setting, the TB incidence and the evaluation of patients before recruitment.
MATERIALS AND METHODS
Study population
From January 2015 to November 2016, we conducted a prospective observational study at a tertiary care hospital in India. Any patient 18 years or older under evaluation for clinico-radiological features of PTB with a history of a cough >2 weeks and a chest X-ray with pulmonary parenchymal involvement was recruited after ensuring two consecutive sputum negative smears or inability to produce adequate sputum. Prior to enrolment patients had undergone evaluation for alternate diagnosis, further imaging studies, had received non-TB antibiotics at the discretion of the treating physician. Patients who had received more than 2 weeks of anti-tubercular therapy (ATT) in the past 90 days, unfit or unwilling for bronchoscopy, unwilling for follow-up were excluded from the study.
The study was approved by the Ethics Committee. At the time of enrolment written informed consent was obtained, and participant information sheet was provided in both Hindi and English.
Patient evaluation
Baseline demographic, clinical, radiological, and laboratory information were recorded in standardised pro formas. HIV testing was done after providing pretest counseling.
Bronchoscopy
Patients underwent flexible fiber-optic bronchoscopy which was performed using a bronchoscope of 6.2 mm insertion tube diameter (Pentax model EB-1970K). The visible part of the bronchial tree was inspected, and bronchoalveolar lavage was done from a single or multiple segments of the lung based on the radiographic findings and the discretion of the investigators. After instilling aliquots of 50 ml each BAL samples were collected in a trap bottle 3 times and then transferred and to a Falcon tube.
Bronchoalveolar lavage samples
A volume of one ml of BAL sample was transferred to the G4 version of Xpert® MTB/RIF (Cepheid, USA) cartridges without initial decontamination or centrifugation. The remaining BAL fluid was processed by the standard decontamination protocol, using NALC-NaOH method and centrifuged. AFB smear was done according to the standard protocol for Ziehl-Neelson staining.[5] The centrifuged sample after decontamination was inoculated for liquid culture in BACTEC mycobacterium growth indicator tube (MGIT) 960 system (BD Diagnostics, USA). Isolates were identified as MTB by immunochromatographic test kit (SD MPT64TB Ag kit). Any diagnostic sample that was detected as non-tuberculous mycobacterium (NTM) by culture method was considered as “non-TB.”
Final diagnosis
A final diagnosis of PTB was based on composite reference standard (CRS) which included two criteria-Culture confirmed PTB and Probable PTB. “Culture confirmed PTB” were cases with MTB culture positive on MGIT. “Probable PTB” were cases without MTB on culture or alternate diagnosis, showing resolution in the clinical and radiological features of PTB to ATT. The response to ATT was monitored during follow up of patients every 2 months for a total of 6 months. Rest of the cases either with an alternate diagnosis or showing no improvement with ATT were considered “Non-TB”
Analysis
Data collection was completed with Microsoft excel 2010. Statistical analysis was performed using MedCalc for Windows, version 17.0 (MedCalc Software, Ostend, Belgium). Descriptive statistics were performed. Sensitivity, specificity and predictive values were calculated with 95% class intervals (CIs). McNemar's test was used for comparison of sensitivities. Odds ratio was calculated with 95% CI for factors associated with Xpert positivity. A two-tailed P < 0.05 was considered statistically significant.
RESULTS
Of the 86 patients who were initially screened 60 were enrolled. The baseline demographic, clinical and radiological characteristic of the 60 enrolled patients is shown in Table 1. Following BAL they yielded 60 valid Xpert® MTB/RIF results as shown in Figure 1 of which 16 were culture confirmed PTB. Five cases had an alternate diagnosis established by bronchoscopy and labeled non-TB. Rest 39 were started on ATT and followed up. There were no drop outs from the study. Clinical and radiological resolution was seen in 36 to ATT and diagnosed as probable PTB. Three did not show response to ATT and labeled non-TB. Of the 60 patients, 52 (86.7%) had a final diagnosis of TB [Table 2].
Table 1.
Baseline characteristics of patients evaluated for sputum-smear negative and sputum-scarce pulmonary tuberculosis (n=60)

Figure 1.
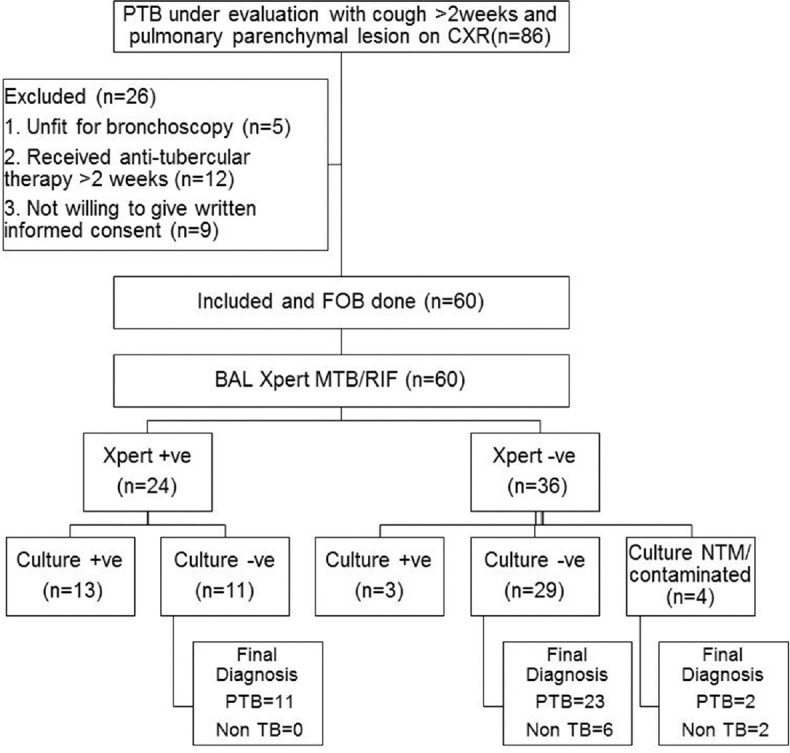
Flow diagram of the patients included in the study. PTB: Pulmonary tuberculosis; PTB: Pulmonary tuberculosis; CXR: Chest X-ray; FOB: Fiberoptic bronchoscopy; BAL: Bronchoalveolar lavage; TB: Tuberculosis; NTM: Nontuberculous mycobacteria
Table 2.
Final diagnosis of the patients evaluated for pulmonary tuberculosis (n=60)
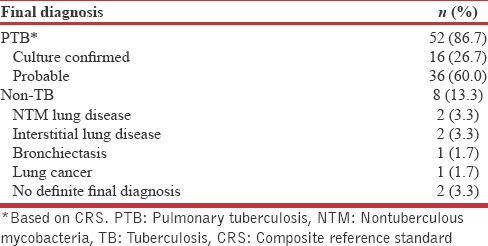
Performances of diagnostic tests
Using culture as the gold standard, Xpert had a sensitivity of 81.3% (54.4%–96.0%) which was significantly higher (P = 0.007) to that of AFB smear 18.8% (4%–45.7%). Using CRS for the final diagnosis, Xpert had a sensitivity of 46.2% (32.2%–60.5%) which was significantly higher (P > 0.001) to that of AFB smear 11.5% (4.4%–23.4%) [Table 3].
Table 3.
Performance values of the diagnostic tests on bronchoalveolar lavage fluid in patients with sputum-smear negative/scarce pulmonary tuberculosis (n=60)
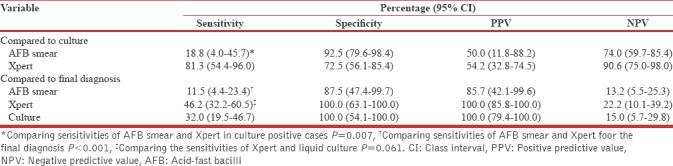
Gain in early pulmonary tuberculosis diagnosis
Xpert MTB/RIF had a gain of 10/16 (62.5%) above AFB smear in early diagnosis of culture confirmed PTB and a gain of 18/52 (34.6%) in a final diagnosis of PTB [Table 4].
Table 4.
Gain in early pulmonary tuberculosis diagnosis (n=60)
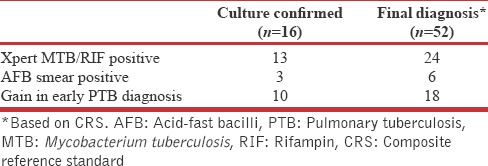
Xpert compared to culture
For the final diagnosis Xpert had a sensitivity of 46.2% (32.2%–60.5%) and was higher compared to culture 32% (19.5%–46.7% P = 0.061). Additional eleven cases would be detected by Xpert and an increment of three cases would be expected with cultures if Xpert had been used as a part of CRS. The sensitivity of Xpert in culture negative cases was 32.4% (17.4%–50.5%) [Table 5].
Table 5.
Xpert in the diagnosis of pulmonary tuberculosis

Factors associated with Xpert positivity
The presence of pneumonia was associated with significantly higher Xpert positivity (odds ratio = 4.0; CI: 1.3–12.0; P = 0.013).
Rifampicin sensitivity
All Xpert positive cases (n = 24) showed RIF sensitivity (100%) on Xpert MTB/RIF.
Past pulmonary tuberculosis
We had recruited 11 patients with history of past PTB of which 9 had a final diagnosis of active PTB and 2 had an alternate diagnosis. Xpert was positive in 6 of them and negative in 5. MTB cultures were negative in all 11.
DISCUSSION
In this study, we evaluated Xpert® MTB/RIF on BAL fluid in sputum smear-negative and sputum-scarce PTB patients in a high incidence setting and followed up the patients to ensure a final diagnosis based on CRS. Using culture as the reference standard Xpert on BAL fluid showed a high sensitivity (81.3%) conforming to the previous literature (80%–92.3%).[6,7,8,9,10,11,12] Furthermore, our study confirmed the gain in the early diagnosis of sputum smear-negative and sputum-scarce PTB by Xpert detecting more number of cases compared to smear microscopy.[6,8,12] This is due to the higher sensitivity of Xpert in comparison to AFB smear. The higher sensitivity is explained by the limit of detection of Xpert being 131 colony-forming unit (cfu)/ml compared to the limit of detection of AFB smear which is 10,000 cfu/ml.[13]
Using culture as the reference standard Xpert showed a very low specificity of 72.5% compared to other studies (91%–100%).[6,7,8,9,10,11,12] This is due to the higher Xpert positive culture negative cases (n = 11) observed as false positive cases when liquid culture is used as a gold standard. Xpert amplifies DNA from dead bacilli and reports a positive while cultures are negative.[14,15]
In a study Barnard et al. had raised caution in the interpretation of Xpert positive results which were negative for culture.[12] Of the nine Xpert positive culture negative cases only three cases had a final diagnosis of PTB. The remaining six cases were having either previous TB or dual pathology and hence an alternate diagnosis.
Our study recruited patients who were having clinical and radiological features consistent with active PTB, unlike the previously mentioned study in which patients having any two symptoms of PTB and one radiological feature were enrolled. In our study pre-enrollment, the patients underwent evaluation for possible alternate diagnosis. Patients who were Xpert positive and culture negative (n = 11) were treated with ATT on clinical and radiological features after which these patients showed resolution. We assume that these cases were, in fact, active PTB based on the clinical and radiological response to ATT.
One reason for Xpert positive culture negative results could have been the inclusion of cases which had received < 14 days of ATT which could have resulted in negative cultures. Furthermore in our clinical setting, patients with clinical and radiological features of pulmonary infection receive non-TB antibiotics when symptoms are present <2 weeks. Beta-lactams are reported to have early antitubercular activity comparable to the conventional ATT drugs other than isoniazid.[16] The effect of beta-lactams on respiratory samples is unknown. We hypothesize these reasons for Xpert positive culture negative results.
In sputum positive PTB, the time to sputum culture conversion is 39 days (interquartile range, 25–55). 10%–20% of the patients are expected to become sputum negative by day 14. The sputum smears and culture conversion rates depend on a number of factors including initial bacterial load.[17] In sputum smear-negative cases due to a lower bacterial load an earlier conversion might be expected. Data on using BAL fluid in sputum negative cases are lacking. Sputum negative cases are infective, and as a practical concern, we had to start ATT immediately in patients with both characteristic clinical and radiological features of PTB before doing BAL. Hence, we recruited cases receiving ATT < 14 days.
Clinical and radiological evaluation before bronchoscopy including a course of broad-spectrum antibiotics (Amoxicillin-clavulanate for 7 days) ensured a high pretest probability, and hence, these cases were likely to be active TB. However, the higher incidence of Xpert positivity in past PTB (6/11 = 54.5%) compared to cases with no history of PTB (18/49 = 36.7%) is a limitation of this study.
Due to the ability to detect nucleic acid of bacilli Xpert was more sensitive (P = 0.061) than culture for a final diagnosis of PTB and with clinical interpretation led to the diagnosis of additional 11 PTB cases compared to culture alone. This translated into a sensitivity of Xpert in culture-negative cases being 32.4%. Liquid Culture (MGIT) has a limit of detection of 10 cfu/ml and is higher than that of Xpert.[18] However, this is often not observed. More ever, in our view, Xpert is more valuable as a diagnostic tool if the clinical and radiological features of active PTB are present pretest.
We assessed Xpert first using MGIT culture as the gold standard. Culture is not 100% sensitive for the diagnosis of PTB on BAL and can miss a number of cases. Hence, it is an imperfect reference standard. This is especially true in our patients who have received varying periods of ATT or had a lower bacterial load. CRS has clearly shown to be more sensitive in detecting TB, especially EPTB. CRS has become a tool for evaluation of newer diagnostic tests for TB.[19,20,21]
Due to the high incidence of empirical ATT in our setting, an impact on time to diagnosis of PTB and initiation of ATT was not expected. In this study, FOB and BAL have contributed to an alternate diagnosis in five of the patients including two NTM on BAL culture and lung cancer which could have gone unnoticed with empirical ATT. Hence this has contributed to an early alternate diagnosis and specific treatment.
The study was limited by small sample size to obtain a significant difference between Xpert and culture. Furthermore, bronchoscopy may not be feasible in a resource-limited setting with limited expertise. The cost-effectiveness of our approach needs to be considered, and it may not be suitable under national program.
CONCLUSIONS
In sputum-smear negative and sputum-scarce patients with clinico-radiological features of active PTB Xpert® MTB/RIF has good sensitivity for diagnosis on BAL fluid. Xpert has higher sensitivity compared to AFB smear for the diagnosis of sputum smear negative and sputum scarce PTB on bronchoalveolar lavage fluid. Xpert is useful for diagnosis even when BAL cultures are negative.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to thank all the patients and their relatives for their participation in the study.
REFERENCES
- 1.World Health Organization. WHO/HTM/TB/2016.13. Geneva, Switzerland: World Health Organization; 2016. Global Tuberculosis Report, 2016. [Google Scholar]
- 2.Harries AD, Mphasa NB, Mundy C, Banerjee A, Kwanjana JH, Salaniponi FM, et al. Screening tuberculosis suspects using two sputum smears. Int J Tuberc Lung Dis. 2000;4:36–40. [PubMed] [Google Scholar]
- 3.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan A, Sharma SK. Fibreoptic bronchoscopy in the diagnosis of sputum smear-negative pulmonary tuberculosis: Current status. Indian J Chest Dis Allied Sci. 2008;50:67–78. [PubMed] [Google Scholar]
- 5.Revised National Tuberculosis Control Programme Laboratory Network Guidelines for Quality Assurance of Smear Microscopy for Diagnosing Tuberculosis. New Delhi: India; 2005. [Last accessed on 2017 Jul 01]. Central TB Division. Available from: http://www.tbcindia.nic.in/pdfs/RNTCP%20Lab%20Network%20Guidelines . [Google Scholar]
- 6.Lee HY, Seong MW, Park SS, Hwang SS, Lee J, Park YS, et al. Diagnostic accuracy of Xpert ® MTB/RIF on bronchoscopy specimens in patients with suspected pulmonary tuberculosis. Int J Tuberc Lung Dis. 2013;17:917–21. doi: 10.5588/ijtld.12.0885. [DOI] [PubMed] [Google Scholar]
- 7.Theron G, Peter J, Meldau R, Khalfey H, Gina P, Matinyena B, et al. Accuracy and impact of Xpert MTB/RIF for the diagnosis of smear-negative or sputum-scarce tuberculosis using bronchoalveolar lavage fluid. Thorax. 2013;68:1043–51. doi: 10.1136/thoraxjnl-2013-203485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Palud P, Cattoir V, Malbruny B, Magnier R, Campbell K, Oulkhouir Y, et al. Retrospective observational study of diagnostic accuracy of the Xpert ® MTB/RIF assay on fiberoptic bronchoscopy sampling for early diagnosis of smear-negative or sputum-scarce patients with suspected tuberculosis. BMC Pulm Med. 2014;14:137. doi: 10.1186/1471-2466-14-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin QQ, Jiao WW, Han R, Jiao AX, Sun L, Tian JL, et al. Rapid diagnosis of childhood pulmonary tuberculosis by Xpert MTB/RIF assay using bronchoalveolar lavage fluid. Biomed Res Int. 2014;2014:310194. doi: 10.1155/2014/310194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalil KF, Butt T. Diagnostic yield of bronchoalveolar lavage gene Xpert in smear-negative and sputum-scarce pulmonary tuberculosis. J Coll Physicians Surg Pak. 2015;25:115–8. [PubMed] [Google Scholar]
- 11.Walters E, Goussard P, Bosch C, Hesseling AC, Gie RP. GeneXpert MTB/RIF on bronchoalveolar lavage samples in children with suspected complicated intrathoracic tuberculosis: A pilot study. Pediatr Pulmonol. 2014;49:1133–7. doi: 10.1002/ppul.22970. [DOI] [PubMed] [Google Scholar]
- 12.Barnard DA, Irusen EM, Bruwer JW, Plekker D, Whitelaw AC, Deetlefs JD, et al. The utility of Xpert MTB/RIF performed on bronchial washings obtained in patients with suspected pulmonary tuberculosis in a high prevalence setting. BMC Pulm Med. 2015;15:103. doi: 10.1186/s12890-015-0086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess. 2007;11:1–96. doi: 10.3310/hta11030. [DOI] [PubMed] [Google Scholar]
- 14.Metcalfe JZ, Makumbirofa S, Makamure B, Mutetwa R, Peñaloza RA, Sandy C, et al. Suboptimal specificity of Xpert MTB/RIF among treatment-experienced patients. Eur Respir J. 2015;45:1504–6. doi: 10.1183/09031936.00214114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyles TH, Hughes J, Cox V, Burton R, Meintjes G, Mendelson M, et al. False-positive Xpert ® MTB/RIF assays in previously treated patients: Need for caution in interpreting results. Int J Tuberc Lung Dis. 2014;18:876–8. doi: 10.5588/ijtld.13.0853. [DOI] [PubMed] [Google Scholar]
- 16.Pagliotto AD, Caleffi-Ferracioli KR, Lopes MA, Baldin VP, Leite CQ, Pavan FR, et al. Anti-Mycobacterium tuberculosis activity of antituberculosis drugs and amoxicillin/clavulanate combination. J Microbiol Immunol Infect. 2016;49:980–3. doi: 10.1016/j.jmii.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Kanda R, Nagao T, Tho NV, Ogawa E, Murakami Y, Osawa M, et al. Factors affecting time to sputum culture conversion in adults with pulmonary tuberculosis: A Historical cohort study without censored cases. PLoS One. 2015;10:e0142607. doi: 10.1371/journal.pone.0142607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Zyl-Smit RN, Binder A, Meldau R, Mishra H, Semple PL, Theron G, et al. Comparison of quantitative techniques including Xpert MTB/RIF to evaluate mycobacterial burden. PLoS One. 2011;6:e28815. doi: 10.1371/journal.pone.0028815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigues C, et al. Xpert MTB/RIF: A new pillar in diagnosis of extrapulmonary tuberculosis? J Clin Microbiol. 2011;49:2540–5. doi: 10.1128/JCM.02319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh UB, Pandey P, Mehta G, Bhatnagar AK, Mohan A, Goyal V, et al. Genotypic, phenotypic and clinical validation of GeneXpert in extra-pulmonary and pulmonary tuberculosis in India. PLoS One. 2016;11:e0149258. doi: 10.1371/journal.pone.0149258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deshmukh M, Nikam C, Ragte T, Shetty A, Rodrigues C. Is a composite reference standard (CRS) an alternative to culture in assessment and validation of a single tube nested in-house PCR for TB diagnosis? Egypt J Chest Dis Tuberc. 2013;62:805–15. [Google Scholar]


