Abstract
Introduction:
Community-acquired pneumonia (CAP) is a common cause of morbidity and mortality in India. There is a need to understand the risk factors associated with severity of CAP in our population. This study was part of the international global initiative for methicillin-resistant Staphylococcus aureus (MRSA) pneumonia study to evaluate MRSA.
Methods:
A total of 100 consecutive cases of pneumonia admitted to the Department of Pulmonary Medicine in a tertiary care hospital were recruited in the study during March–July 2015. The severity of pneumonia was assessed based on the CURB-65 score. Individuals with pneumonia and CURB-65 score >2 were compared with subjects with CURB-65 score ≤2. Individuals were also evaluated for the causative organism and its resistance pattern with specific reference to the presence of MRSA.
Results:
Mean age of patients was 54.03 years, 66% were men. Patients were managed either in the intensive care unit (42%) or wards/high dependency unit (58%), 22% needed noninvasive ventilation and 18% needed mechanical ventilation within 24 h of admission. On multivariate analysis, prior respiratory infection (within last 1 year), obesity (body mass index >30), and alcoholism, old age (>60 years) were independently associated risk factors for severe pneumonia. There were no cases of MRSA. In 34% of cases, organisms could be identified. Most common organisms were Klebsiella (8%), influenza (8%), and Pseudomonas (5%).
Conclusion:
Prior respiratory infection, obesity, alcoholism, and old age (>60 years) were observed to be important risk factors for severe CAP. Prospective studies should evaluate effect of weight reduction and cessation of alcohol consumption on recurrences of pneumonia in this population and on the severity of pneumonia.
KEY WORDS: Community-acquired pneumonia, CURB-65, global initiative for methicillin-resistant Staphylococcus aureus pneumonia, methicillin-resistant Staphylococcus aureus, Pneumonia
INTRODUCTION
Community-acquired pneumonia (CAP) is a common disease causing significant morbidity and mortality worldwide. The annual incidence of CAP varies from 5 to 11/1000 population with a higher incidence in the elderly. Around 20% of them require hospitalization incurring significant economic burden on the society.[1] The World Health Organization global burden of disease study estimated that lower respiratory tract infections, which include CAP, were 429.2 million episodes of illness worldwide.[2] There is a need to identify risk factors for severe pneumonia as there are sparse data in Indian population and mortality rates in severe pneumonia admitted to the intensive care unit (ICU) is high approaching 25%.[3]
METHODOLOGY
Study design
We conducted a prospective study in the Department of Pulmonology in an 1800 bedded tertiary care center during March–July 2015. Consecutive adult patients admitted with CAP were included in the study after obtaining informed consent. Patients were triaged based on CURB65 score. All patients with CURB65 score more than 2 were admitted to ICU/high-dependency unit (HDU), and rest were treated in wards.[4] All patients were treated with appropriate antibiotics as per guidelines. Sputum from cooperative patients and blind tracheal aspirate from patients on mechanical ventilation was sent for microbiological analysis. Single H1N1 throat swab on the day of admission was taken for patients with suspected viral pneumonia consistent with category C symptoms as per guidelines recommended by Ministry of health and family welfare.[5] We defined CAP as new or progressive pulmonary infiltrates on chest radiograph with at least two of the following four: fever, cough, purulent sputum production, or leukocytosis over 10,000/mm3.[4]
Data collection
All patients were interviewed with global initiative for methicillin-resistant Staphylococcus aureus pneumonia (GLIMP) questionnaire, which included demographic data, risk factors such as smoking, alcoholism, diabetes, dialysis, cirrhosis, obesity, use of medications such as steroids, chemotherapeutic agents, proton pump inhibitors, inhaled steroids, prior respiratory infection (<1 year), number of admissions, EMD visits, use of IV or oral antibiotics, previous influenza and pneumococcal immunization status, respiratory medical history such as chronic obstructive pulmonary disease (COPD), bronchiectasis, asthma, active lung cancer, ILD, OSA, cardiovascular disease including arrhythmias, myocardial infarction, heart failure, stroke, hypertension, and immunosuppressive conditions such as HIV, neutropenia, hematological malignancy, active solid organ malignancy, aplastic anemia and asplenia, and details of events during the first 24 h of admission such as need for ICU/HDU, need for noninvasive ventilation (NIV), or invasive mechanical ventilation and vasopressors. Prior microbiological history (<1 year) of infection or colonization with methicillin-resistant Staphylococcus aureus (MRSA), extended spectrum β-lactamase producing Gram-negative bacilli, and Pseudomonas aeruginosa were collected. Details of investigations sent within 24 h of admission such as blood culture, sputum Gram-stain and culture, Pneumococcal and Legionella urinary antigen test, viral nasopharyngeal swab, bronchoalveolar lavage and protected brush specimen, chlamydia pneumonia serology, pleural fluid culture, MRSA nasopharyngeal swab and lung biopsy. Data regarding isolation of pathogenic organisms and source of specimen were recorded, and antibiotics used to treat pneumonia within 24 h of admission were noted.
The Institutional Ethics Committee approved the study JSSMC/IEC/03/6756/2015–16. Informed consent from patient/legal representative was taken before inclusion in the study.
Analysis
Descriptive data are presented as frequencies (percentages) for discrete variables and as means (standard deviations) or medians (interquartile ranges) for continuous variables. Inferential statistics like Chi-square test was used. Univariate and multivariate logistic regression analysis was done for factors associated with severity of CAP. All statistical tests were two-tailed, and factors were considered statistically significant at P < 0.05. IBM SPSS version 22 (IBM, USA) and CDC Epi Info version 7 (Centres for disease control and prevention, USA) was used for analysis.
RESULTS
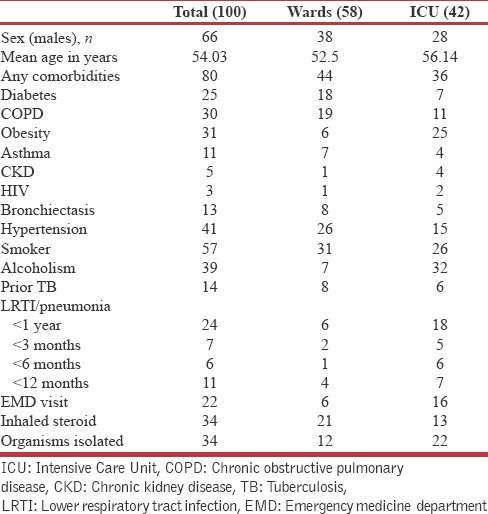
A total of 100 consecutive patients with community-acquired pneumonia were interviewed with GLIMP questionnaire. Flow of patients is depicted in Figure 1. Demographic and clinical variables are enumerated in Table 1. More than half the population were smokers, nearly one-third were obese (body mass index [BMI] >30) and one quarter was diabetic. Forty-two patients needed ICU care <24 h of admission of which 18% needed invasive mechanical ventilation, 16% needed vasopressors, and 18% needed NIV within 24 h of admission. About 18% of patients had previous influenza vaccination, and only 6% had the previous pneumococcal vaccination. In 94% of patients, sputum was sent for Gram-stain and culture within 24 h of admission and blood culture was sent in 12% of patients within 24 h of admission.
Figure 1.
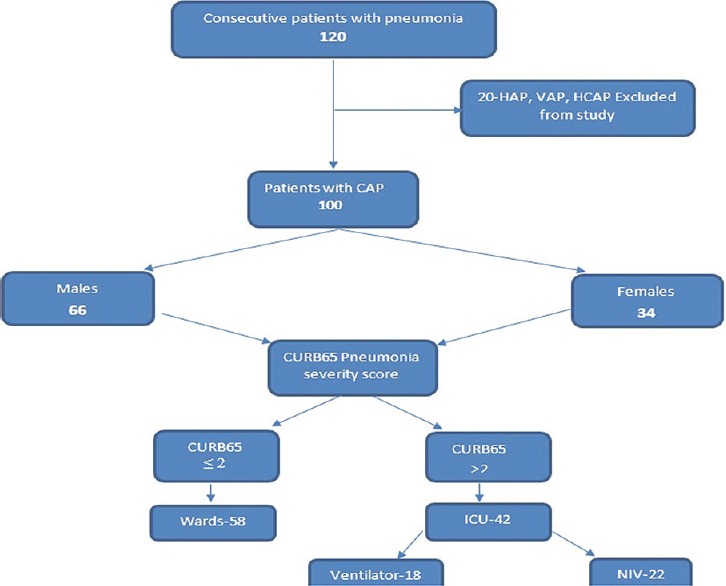
Flow chart depicting number of patients screened, included in the study, needing intensive care unit care, mechanical ventilation, noninvasive ventilation
Table 1.
Clinical Profile of patients with community-acquired pneumonia
We could isolate etiological organisms only in 34% patients. Klebsiella pneumonia (8/34) and influenza virus (H1N1) (8/34) were the most common organisms isolated [Table 2]. Higher isolation rates were found in patients admitted to the ICUs. There were no cases of MRSA in our study. We found that P. aeruginosa had high resistance to levofloxacin (100%), azithromycin (100%), amikacin (60%), and piperacillin-tazobactam (80%). Antibiotic resistance pattern is depicted in Figure 2.
Table 2.
Microbiological profile of patients with community acquired pneumonia
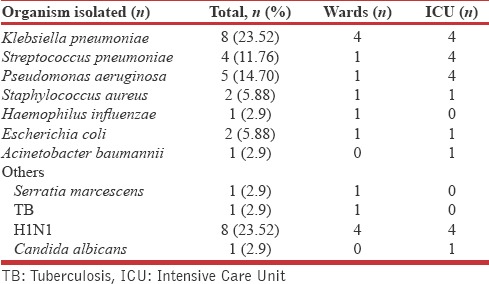
Figure 2.
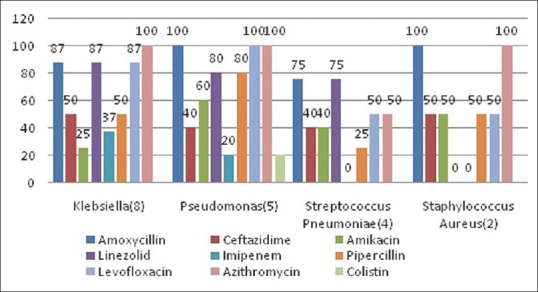
Antibiotic resistance pattern among patients with community acquired pneumonia
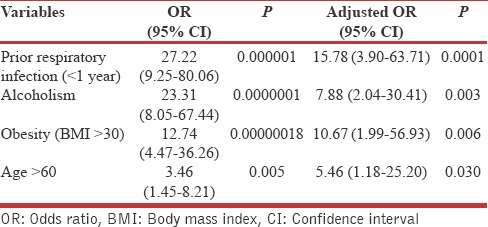
On univariate logistic regression analysis, we found prior respiratory infection (<1 year), obesity (BMI >30), alcoholism, age >60 years, dialysis, and active lung cancer were found to be risk factors for severe pneumonia [Table 3]. Dialysis and active lung cancer were excluded from multivariate analysis due to low cell values. On multivariate analysis, we found prior respiratory infection, obesity (BMI >30), alcoholism, age >60 years were independently associated with risk for severe pneumonia [Table 4].
Table 3.
Univariate logistic regression analysis of factors influencing severity of community acquired pneumonia
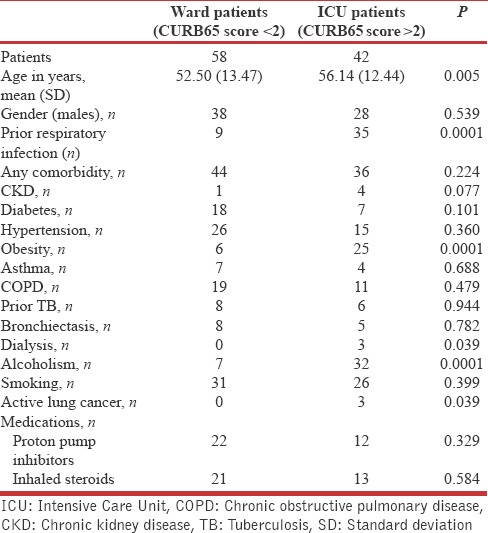
Table 4.
Multivariate logistic regression analysis of factors influencing severity of community acquired pneumonia
DISCUSSION
CAP is associated with significant mortality and morbidity all over the world, and it is the third leading cause of death in adults.[6,7] Hence, it is important to identify the risk factors for severity of CAP which helps in prognosticating the patients and better management so as to mitigate the negative outcomes of CAP. We found in our study that prior respiratory infection (within the last 1 year), obesity (BMI >30), alcoholism, old age (>60 years) were significant and independent risk factors associated with severity of pneumonia on multivariate analysis. Studies have observed various factors such as COPD,[8] smoking,[9,10] altered sensorium, obesity,[10] alcoholism,[11,12] prior respiratory infection (<1 year),[8] diabetes,[13] and increasing age[14] as risk factors for CAP.
Prior respiratory infection (<1 year) was associated with the development of CAP (odds ratios [OR]-15.78). A Spanish study observed subjects with a previous history of pneumonia had 2-fold higher risk for a recurrence of CAP (OR-2.73).[9] A German study found prior respiratory infection in the past 1 year was associated with higher risk for CAP (OR-3.6).[8] Jackson observed that prior admission for pneumonia was associated with higher risk for the subsequent CAP (OR-1.98).[15] The possible mechanism hypothesized is that previous pneumonias are associated with an impairment of ciliary function that takes time to recover, which predisposes the subject to recurrent pneumonias.[16] Prior viral infection like influenza also predisposes to bacterial infections such as streptococcal pneumonia.[17] Viral infections lead to bronchoconstriction, increased mucus production and stronger adhesion of bacteria like pneumococci to virus-infected cells. Viral infection also causes ciliary dysfunction and impairment of leukocyte function.[18,19] Obesity is associated with impaired T and B-cell function due to the metabolic effects of obesity such as hyperglycemia and insulin resistance.[20] A US study has shown that excessive weight gain (>40 lb) during adulthood doubled the risk of pneumonia and observed decreased risk of CAP with increasing activity.[10] Obese individuals tend to have decreased physical activity, and it is reported that moderate physical activity enhances natural killer cell activity.[21] Recent study done in Japan have shown obesity is associated with immune dysfunction which is reversible on weight reduction.[22] Thus, obesity is a modifiable risk factor for pneumonia, which can be reduced by lifestyle modifications.
Alcoholism is associated with two to nine times risk of developing pneumonia.[23] There are several reasons for increased susceptibility for pneumonia in alcoholics. Alcoholism can cause neutrophil and macrophage dysfunction and also causes impairment of ciliary and surfactant function.[24] Binge alcohol consumption can cause increased risk of aspiration due to suppression of cough reflex. Chronic alcoholics are prone for malnutrition and vitamin deficiencies which further predispose them for infections including pneumonia.[25] Alcoholism also predisposes the individuals to chronic diseases such as cirrhosis of the liver which causes immune dysfunction and malignancies of esophagus, pharynx, and larynx which increases the likelihood of development of pneumonia.[26] There are few studies that have shown a positive correlation between amount of alcohol consumption and risk for developing pneumonia. Kornum et al. in their study found consumption of >50 alcoholic drinks per week and binge alcohol drinkers had increased risk for pneumonia-related hospitalizations.[11] These studies suggest that alcoholism is a major avoidable risk factor for pneumonia in adults.
Advancing age is associated with impaired immune system function, and there is increased risk of infectious disease including pneumonia. Polymorphonuclear leukocytes in elderly have decreased chemotactic ability, and decreased uptake of microorganisms and antigen processing ability of macrophages is reduced in elderly.[27] Elderly patients are more prone for pneumonia for various reasons such as physical and cognitive impairment, aspiration, advanced chronic diseases, malnutrition, and hypoalbuminemia.[27,28,29] A Japanese study found age >65 years was an independent risk factor for severe CAP (OR=2.42) and also mortality.[13]
Microbiological confirmation was obtained in 34/100 cases which is lower compared to studies from Shimla 75.6%,[30] Ludhiana 47.7%,[31] and other parts of the world (Spain [42%], Singapore [68%], UK [62%]).[32,33] Low rate of isolation may be due to prior use of antibiotics before admission and since we did not attempt to isolate anaerobic organisms and atypical organisms such as Legionella, mycoplasma, and viruses other than H1N1. In our study, K. pneumonia (8) and H1N1 pneumonia (8) were most common cause for CAP. Among bacteria, Klebsiella was the most common organism in contrast to other studies where Streptococcus was the most common.[13,28,34,35] Possible explanations for this difference could be due to a higher number of patients with chronic respiratory disease (43%), diabetic patients (25%) and patients with repeated visits to EMD with respiratory infections (22%) in our study population. Pseudomonas was isolated from five subjects, and most of them [4/5 (80%)] had bronchiectasis. Klebsiella was isolated from eight subjects and most of them [7/8 (88%)] had COPD. Additional comorbidity of diabetes in COPD did not increase the frequency of isolation of Klebsiella; COPD alone [4/7 (57%)] and COPD with diabetes [3/5 (60%)]. A Singapore study observed K. pneumoniae to be the most common organism identified in patients with severe CAP admitted to ICU (9, 15%).[33] We isolated only one case of Acinetobacter, which was from a patient with COPD.
It is interesting to note that we did not have any MRSA pneumonia in our study. GLIMP study observed 3% global incidence of MRSA pneumonia among patients with CAP while the incidence in India was found to be 1.4%.[36] Other studies done in the US and Canada found the incidence of MRSA 2.4% and 14%, respectively.[37,38] Overall, most organisms causing CAP were found to be sensitive to carbapenems (61.7%) followed by amikacin (35.2%) in our study while a study done in Mangalore and Kerala found Ciprofloxacin (49%) and Amikacin (44.84%) to be most sensitive antibiotic, respectively.[35,39]
The main strength of our study is that we have used a validated questionnaire from a large multinational GLIMP study and have looked extensively into risk factors influencing the development of CAP. Limitations of our study included smaller sample size insufficient to evaluate all the risk factors. Few of the risk factors had low cell values, and therefore, we did not have sufficient power to evaluate these risk factors. We have not evaluated the dose-response relationship between alcohol consumption and pneumonia risk.
CONCLUSION
We observed that prior respiratory illnesses within the last year, alcoholism, obesity are independently associated with severe CAP. We identified alcoholism and obesity as important independent risk factors that are amenable to lifestyle modification. A prospective cohort study is needed to evaluate the benefits of cessation of alcohol and weight reduction in these patients on the frequency and severity of pneumonia. It is important to include Gram-negative coverage in empiric antibiotic therapy for CAP in a patient with the previous chronic respiratory disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors would like to acknowledge Global Initiative for MRSA pneumonia (GLIMP) principal investigator Dr. Stefano Aliberti and GLIMP team for permission to use the GLIMP questionnaire. We also acknowledge Principal, JSS Medical College and Director, JSS Medical College Hospital, Mysuru for permission to conduct the study.
REFERENCES
- 1.Brar NK, Niederman MS. Management of community-acquired pneumonia: A review and update. Ther Adv Respir Dis. 2011;5:61–78. doi: 10.1177/1753465810381518. [DOI] [PubMed] [Google Scholar]
- 2.WHO | The Global Burden of Disease: 2004 Update. [Last accessed on 2017 Jun 09]. Available from: http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/
- 3.Pachon J, Prados MD, Capote F, Cuello JA, Garnacho J, Verano A, et al. Severe community-acquired pneumonia. Etiology, prognosis, and treatment. Am Rev Respir Dis. 1990;142:369–73. doi: 10.1164/ajrccm/142.2.369. [DOI] [PubMed] [Google Scholar]
- 4.BTS Guidelines for the Management of Community Acquired Pneumonia in Adults: Update 2009 | British Thoracic Society | Better lung health for all. [Last accessed on 2017 Jun 27]. Available from: https://www.brit-thoracic.org.uk/standards-of-care/guidelines/bts-guidelines-for-the-management-of-community-acquiredpneumonia-in-adults-update-2009/
- 5.Mukherjee S. Management of Swine flu (H1N1 Flu) Outbreak and its Treatment Guidelines. [Last accessed on 2017 Jun 27]. Available from: http://www.caijournal.com/article.asp?issn=2225-6482;year=2015;volume=2;issue=3;spage=71;epage=78;aulast=Mukherjee .
- 6.Lopez AD, Murray CC. The global burden of disease, 1990-2020. Nat Med. 1998;4:1241–3. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 7.Suttorp N, Welte T, Marre R, Stenger S, Pletz M, Rupp J, et al. CAPNETZ. The competence network for community-acquired pneumonia (CAP) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2016;59:475–81. doi: 10.1007/s00103-016-2318-7. [DOI] [PubMed] [Google Scholar]
- 8.Schnoor M, Klante T, Beckmann M, Robra BP, Welte T, Raspe H, et al. Risk factors for community-acquired pneumonia in German adults: The impact of children in the household. Epidemiol Infect. 2007;135:1389–97. doi: 10.1017/S0950268807007832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almirall J, Bolíbar I, Balanzó X, González CA. Risk factors for community-acquired pneumonia in adults: A population-based case-control study. Eur Respir J. 1999;13:349–55. doi: 10.1183/09031936.99.13234999. [DOI] [PubMed] [Google Scholar]
- 10.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW, et al. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160:3082–8. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 11.Kornum JB, Due KM, Nørgaard M, Tjønneland A, Overvad K, Sørensen HT, et al. Alcohol drinking and risk of subsequent hospitalisation with pneumonia. Eur Respir J. 2012;39:149–55. doi: 10.1183/09031936.00000611. [DOI] [PubMed] [Google Scholar]
- 12.de Roux A, Cavalcanti M, Marcos MA, Garcia E, Ewig S, Mensa J, et al. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest. 2006;129:1219–25. doi: 10.1378/chest.129.5.1219. [DOI] [PubMed] [Google Scholar]
- 13.Ishiguro T, Takayanagi N, Yamaguchi S, Yamakawa H, Nakamoto K, Takaku Y, et al. Etiology and factors contributing to the severity and mortality of community-acquired pneumonia. Intern Med. 2013;52:317–24. doi: 10.2169/internalmedicine.52.8830. [DOI] [PubMed] [Google Scholar]
- 14.Koivula I, Sten M, Mäkelä PH. Risk factors for pneumonia in the elderly. Am J Med. 1994;96:313–20. doi: 10.1016/0002-9343(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 15.Jackson ML, Neuzil KM, Thompson WW, Shay DK, Yu O, Hanson CA, et al. The burden of community-acquired pneumonia in seniors: Results of a population-based study. Clin Infect Dis. 2004;39:1642–50. doi: 10.1086/425615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.RB Dowling. Bacterial toxins which perturb ciliary function and respiratory epithelium. [Last accessed on 2017 Jun 27];J Appl Microbiol (Wiley Online Library) 1998 84:S138–148. doi: 10.1046/j.1365-2672.1998.0840s1138s.x. Available from: http://www.onlinelibrary.wiley.com/doi/10.1046/j.1365-2672.1998.0840s1138S.x/abstract . [DOI] [PubMed] [Google Scholar]
- 17.Yoon YK, Yang KS, Sohn JW, Lee CK, Kim MJ. Impact of preceding respiratory viral infections on the clinical severity of patients with pneumococcal pneumonia. Influenza Other Respir Viruses. 2014;8:549–56. doi: 10.1111/irv.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hament JM, Kimpen JL, Fleer A, Wolfs TF. Respiratory viral infection predisposing for bacterial disease: A concise review. FEMS Immunol Med Microbiol. 1999;26:189–95. doi: 10.1111/j.1574-695X.1999.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 19.Tristram DA, Hicks W, Jr, Hard R. Respiratory syncytial virus and human bronchial epithelium. Arch Otolaryngol Head Neck Surg. 1998;124:777–83. doi: 10.1001/archotol.124.7.777. [DOI] [PubMed] [Google Scholar]
- 20.Stallone DD. The influence of obesity and its treatment on the immune system. Nutr Rev. 1994;52:37–50. [PubMed] [Google Scholar]
- 21.Nieman DC, Miller AR, Henson DA, Warren BJ, Gusewitch G, Johnson RL, et al. Effects of high- vs. moderate-intensity exercise on natural killer cell activity. Med Sci Sports Exerc. 1993;25:1126–34. [PubMed] [Google Scholar]
- 22.Tanaka S, Inoue S, Isoda F, Waseda M, Ishihara M, Yamakawa T, et al. Impaired immunity in obesity: Suppressed but reversible lymphocyte responsiveness. Int J Obes Relat Metab Disord. 1993;17:631–6. [PubMed] [Google Scholar]
- 23.Samokhvalov AV, Irving HM, Rehm J. Alcohol consumption as a risk factor for pneumonia: A systematic review and meta-analysis. Epidemiol Infect. 2010;138:1789–95. doi: 10.1017/S0950268810000774. [DOI] [PubMed] [Google Scholar]
- 24.Leber B, Mayrhauser U, Rybczynski M, Stadlbauer V. Innate immune dysfunction in acute and chronic liver disease. Wien Klin Wochenschr. 2009;121:732–44. doi: 10.1007/s00508-009-1288-2. [DOI] [PubMed] [Google Scholar]
- 25.Manari AP, Preedy VR, Peters TJ. Nutritional intake of hazardous drinkers and dependent alcoholics in the UK. Addict Biol. 2003;8:201–10. doi: 10.1080/1355621031000117437. [DOI] [PubMed] [Google Scholar]
- 26.Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, et al. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction. 2010;105:817–43. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandra RK. Nutritional regulation of immunity and risk of infection in old age. Immunology. 1989;67:141–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Riquelme R, Torres A, El-Ebiary M, de la Bellacasa JP, Estruch R, Mensa J, et al. Community-acquired pneumonia in the elderly: A multivariate analysis of risk and prognostic factors. Am J Respir Crit Care Med. 1996;154:1450–5. doi: 10.1164/ajrccm.154.5.8912763. [DOI] [PubMed] [Google Scholar]
- 29.Salive ME, Satterfield S, Ostfeld AM, Wallace RB, Havlik RJ. Disability and cognitive impairment are risk factors for pneumonia-related mortality in older adults. Public Health Rep. 1993;108:314–22. [PMC free article] [PubMed] [Google Scholar]
- 30.Bansal S, Kashyap S, Pal LS, Goel A. Clinical and bacteriological profile of community acquired pneumonia in Shimla, Himachal Pradesh. Indian J Chest Dis Allied Sci. 2004;46:17–22. [PubMed] [Google Scholar]
- 31.Oberoi A, Aggarwal A. Bacteriological Profile, Serology and Antibiotic Sensitivity Pattern of Micro-Organisms from Community Acquired Pneumonia. [Last accessed on 2017 Aug 18]. Available from: http://www.imsear.hellis.org/handle/123456789/171303 .
- 32.Howard LS, Sillis M, Pasteur MC, Kamath AV, Harrison BD. Microbiological profile of community-acquired pneumonia in adults over the last 20 years. J Infect. 2005;50:107–13. doi: 10.1016/j.jinf.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Lee KH, Hui KP, Tan WC, Lim TK. Severe community-acquired pneumonia in Singapore. Singapore Med J. 1996;37:374–7. [PubMed] [Google Scholar]
- 34.Almirall J, Bolíbar I, Vidal J, Sauca G, Coll P, Niklasson B, et al. Epidemiology of community-acquired pneumonia in adults: A population-based study. Eur Respir J. 2000;15:757–63. doi: 10.1034/j.1399-3003.2000.15d21.x. [DOI] [PubMed] [Google Scholar]
- 35.Menon RU, George AP, Menon UK. Etiology and anti-microbial sensitivity of organisms causing community acquired pneumonia: A Single hospital study. J Family Med Prim Care. 2013;2:244–9. doi: 10.4103/2249-4863.120728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aliberti S, Reyes LF, Faverio P, Sotgiu G, Dore S, Rodriguez AH, et al. Global initiative for Meticillin-resistant Staphylococcus aureus pneumonia (GLIMP): An international, observational cohort study. Lancet Infect Dis. 2016;16:1364–76. doi: 10.1016/S1473-3099(16)30267-5. [DOI] [PubMed] [Google Scholar]
- 37.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, Albrecht V, Limbago B, et al. Prevalence of Methicillin-resistant Staphylococcus aureus as an etiology of community-acquired pneumonia. Clin Infect Dis. 2012;54:1126–33. doi: 10.1093/cid/cis022. [DOI] [PubMed] [Google Scholar]
- 38.Tadros M, Williams V, Coleman BL, McGeer AJ, Haider S, Lee C, et al. Epidemiology and outcome of pneumonia caused by Methicillin-resistant Staphylococcus aureus (MRSA) in Canadian hospitals. PLoS One. 2013;8:e75171. doi: 10.1371/journal.pone.0075171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acharya VK, Padyana M, BU, RA, Acharya PR, Juneja DJ, et al. Microbiological profile and drug sensitivity pattern among community acquired pneumonia patients in tertiary care centre in Mangalore, coastal Karnataka, India. J Clin Diagn Res. 2014;8:MC04–6. doi: 10.7860/JCDR/2014/7426.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]


