Abstract
Purpose: The study purpose was to pilot test a web-based human papillomavirus (HPV) vaccination intervention among young gay and bisexual men (YGBM).
Methods: In 2016, we recruited 150 unvaccinated YGBM aged 18–25 years from the United States. We randomized participants to the Outsmart HPV intervention or a control group.
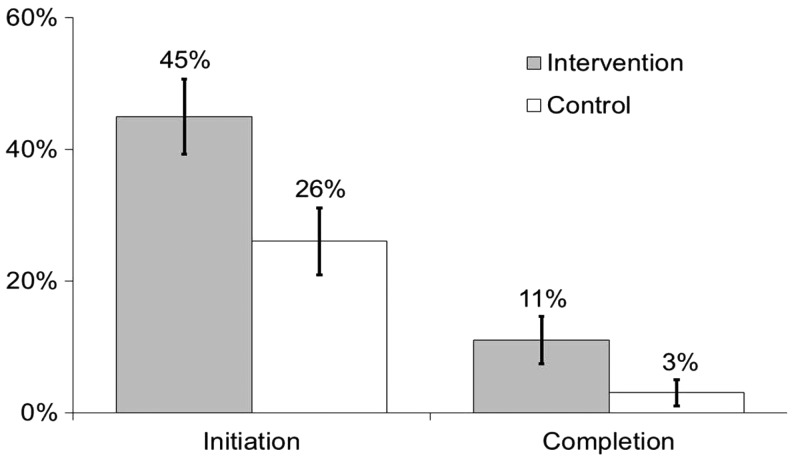
Results: HPV vaccine initiation was higher among the intervention group than the control group (45% vs. 26%; odds ratio [OR] = 2.34, 95% confidence interval [CI]: 1.18–4.67). We observed a trend toward higher HPV vaccine completion among the intervention group (11% vs. 3%; OR = 4.24, 95% CI: 0.87–20.66).
Conclusions: Outsmart HPV is a promising strategy for increasing HPV vaccination among YGBM.
Keywords: : HPV, HPV vaccine, intervention, sexual minority men
Introduction
Men who have sex with men, including those who self-identify as gay or bisexual, have high rates of human papillomavirus (HPV) infection and HPV-related disease, such as anal cancer.1 The Advisory Committee on Immunization Practices recommends routine HPV vaccination for males aged 11 or 12 years and catch-up vaccination for older males.2 Catch-up vaccination is recommended through age 26 for men who have sex with men, including those who self-identify as gay or bisexual or who intend to have sex with men.2 However, fewer than 20% of young gay and bisexual men (YGBM) within the recommended age range for HPV vaccination have received any vaccine doses, and fewer than 10% have completed the vaccine series.3,4 These vaccine coverage estimates are comparable to recent data for young adult males in the United States, based on a sample of the general population that would mostly include individuals who self-identify as heterosexual.5 In response to the low HPV vaccine coverage among YGBM, we developed and pilot tested Outsmart HPV, which, to our knowledge, is the first HPV vaccination intervention specifically for YGBM.
Materials and Methods
Participants and procedures
We recruited YGBM during July and September 2016 using paid Facebook advertisements that linked to the project website.6 The project website was mobile friendly and accessible by desktop/laptop, tablet computer, or smartphone. On the website, potential participants first completed an eligibility screener. To be eligible for this study, potential participants had to indicate their current gender as male, be aged 18–25 years, reside in the United States, self-identify as gay or bisexual, and not have received any HPV vaccine doses. We used age 25, instead of age 26, as the study's upper age limit so that men did not “age out” of the recommended HPV vaccination age range during the study.2 Eligible participants viewed the online consent form and had the opportunity to provide informed consent on the project website by clicking that they either consented to participate or did not consent to participate. Participants who provided consent created a project website account and completed the pretest survey (Time 1, “T1”). Participants were then randomized using a 1:1 allocation ratio to receive either intervention or control group materials (described in the Study Materials section). Recruitment continued until 150 participants (76 intervention and 74 control) were randomized.
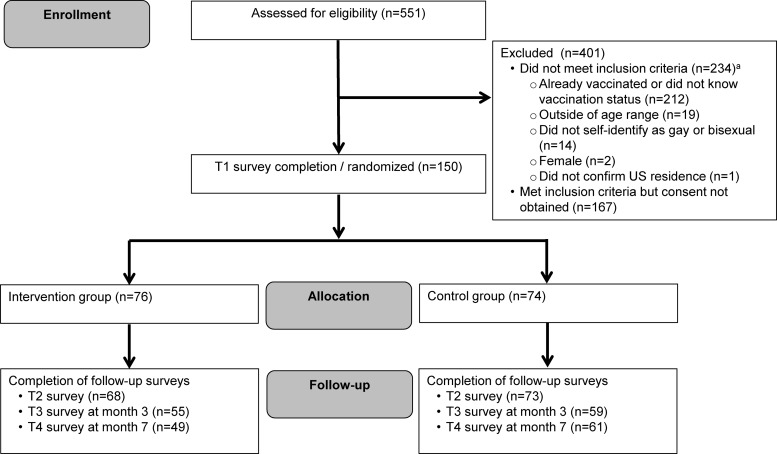
Immediately after viewing their materials, participants completed a post-test survey (T2). Additional follow-up surveys occurred 3 (T3) and 7 months (T4) later. Retention for the 7-month follow-up period was 73% (Fig. 1). Participants could earn up to $95 in gift cards for completing study surveys. The Institutional Review Board at The Ohio State University approved this study.
FIG. 1.
CONSORT flow diagram for the Outsmart HPV intervention. aIt was possible for potential participants not to meet multiple aspects of the study's inclusion criteria.
Study materials
We describe intervention and control group materials in detail elsewhere7 and briefly in the following sections. The project website delivered materials to both groups. After completing the T2 survey, participants could print or save a PDF summary of their materials. Participants could also log back into the project website and review their materials throughout the study, except when completing surveys.
Intervention condition
The Outsmart HPV intervention was based primarily on the protection-motivation theory8 and consisted of two components: (a) population-targeted, individually tailored content about HPV and HPV vaccine; and (b) monthly HPV vaccination reminders sent via email and/or text message. The first component presented content in four sequential sections:
1. “Learn about HPV” provided targeted information about HPV and HPV-related disease among gay and bisexual men. This included epidemiological information about these topics, such as the prevalence and transmission of HPV and HPV-related disease.
2. “Learn about the Vaccine” provided information about HPV vaccine recommendations for YGBM and vaccine effectiveness, as well as individually tailored testimonials (based on men's relationship status and if they had a regular healthcare provider) that illustrated reasons why men may decide to get vaccinated.
3. “Get Answers” provided information to address potential barriers and concerns about HPV and HPV vaccine using a question and answer format. All intervention group participants could view all questions and answers, but the order of information was tailored based on T1 survey responses. For example, information addressing the potential barriers and concerns endorsed by a participant on the T1 survey (e.g., reasons for not getting vaccinated thus far) was highlighted by appearing at the top of this section.
4. “Get Vaccinated” provided resources for accessing the HPV vaccine (e.g., finding a healthcare provider and potential transportation options), information about vaccine cost and health insurance, and skills-building strategies for talking with a provider about the vaccine. The website then prompted participants to create a customized “vaccination plan” that included tailored next steps for obtaining the HPV vaccine.
Control condition
Participants in the control group received standard information about HPV and the HPV vaccine, which was modeled closely after the Vaccine Information Statement (VIS) for HPV vaccine.9 The Centers for Disease Control and Prevention created the VIS to provide information about HPV and the HPV vaccine that is easy to read and understand. The VIS includes information about HPV and HPV-related disease, the health benefits of HPV vaccination, current HPV vaccine recommendations, and the potential risks associated with HPV vaccination. We formatted the VIS content to match the color and font scheme of the project website. We chose to model the control group content after the VIS because healthcare providers are required to give a VIS to patients before vaccination.
Measures
We used self-reported HPV vaccination data to examine two vaccination outcomes (yes or no for each): HPV vaccine initiation (receipt of one or more doses) and completion (receipt of all three doses recommended for our study's age range2). As the Outsmart HPV intervention provided skills-building strategies for talking with a healthcare provider about the vaccine (given the importance of provider recommendation for vaccination among YGBm3), we also examined whether participants indicated that they had talked with a healthcare provider about the HPV vaccine since study entry (yes or no). All outcomes were based on data from the T3 and T4 surveys. We categorized participants who did not complete follow-up surveys as “no” for all outcomes. The T1 survey assessed a range of demographic and health-related characteristics (Table 1) using items based on those from our past work.3 We assessed participants' electronic health (e-health) literacy using a four-item scale with Likert-type responses that were coded so higher values indicated greater e-health literacy (possible range 1–5).10,11
Table 1.
Participant Characteristics by Study Group
| Intervention (n = 76), n (%) | Control (n = 74), n (%) | |
|---|---|---|
| Demographics | ||
| Sexual identity | ||
| Bisexual | 14 (18) | 12 (16) |
| Gay | 62 (82) | 62 (84) |
| Age (years) | ||
| 18–21 | 31 (41) | 31 (42) |
| 22–25 | 45 (59) | 43 (58) |
| Race/ethnicity | ||
| White, non-Hispanic | 44 (58) | 41 (55) |
| African American, non-Hispanic | 8 (11) | 12 (16) |
| Other race, non-Hispanic | 5 (7) | 5 (7) |
| Hispanic | 19 (25) | 16 (22) |
| Relationship status | ||
| Othera | 58 (76) | 62 (84) |
| In partnership, married, or civil union | 18 (24) | 12 (16) |
| Education level | ||
| Some college or less | 49 (64) | 45 (61) |
| College degree or more | 27 (36) | 29 (39) |
| Household income | ||
| Less than $50,000 | 50 (66) | 64 (86) |
| $50,000 or more | 26 (34) | 10 (14) |
| Healthcare | ||
| Health insurance | ||
| None | 11 (14) | 16 (22) |
| On parents' insurance | 39 (51) | 28 (38) |
| Insures self | 26 (34) | 30 (41) |
| Has a regular healthcare provider | ||
| No | 38 (50) | 35 (47) |
| Yes | 38 (50) | 39 (53) |
| Had a routine medical check-up in the last year | ||
| No | 40 (53) | 40 (54) |
| Yes | 36 (47) | 34 (46) |
| Disclosed sexual orientation to a healthcare provider | ||
| No | 51 (67) | 46 (62) |
| Yes | 25 (33) | 28 (38) |
| Ever perceived discrimination from healthcare provider | ||
| No | 63 (83) | 65 (88) |
| Yes | 13 (17) | 9 (12) |
| Health literacy | ||
| Electronic health literacy,b mean (SD) | 3.99 (0.76) | 3.88 (0.91) |
| Sexual health | ||
| Age at sexual debut | ||
| Younger than 18 years | 38 (50) | 39 (53) |
| 18 years or older | 38 (50) | 35 (47) |
| Lifetime number of sexual partners | ||
| 11 or fewer | 40 (53) | 36 (49) |
| 12 or more | 36 (47) | 38 (51) |
| HIV status | ||
| Negative | 70 (92) | 72 (97) |
| Positive | 6 (8) | 2 (3) |
| Ever have an STI | ||
| No | 60 (79) | 59 (80) |
| Yes | 16 (21) | 15 (20) |
Percentages may not total 100 due to rounding.
Never married or were divorced, separated, or widowed.
Four-item scale; possible range 1–5 with higher values indicating greater electronic health (e-health) literacy.
HIV, human immunodeficiency virus; SD, standard deviation; STI, sexually transmitted infection.
Data analysis
We first used descriptive statistics to examine demographic and health-related characteristics, but, as recently suggested,12 did not use statistical tests to compare study groups on these baseline characteristics. We then used logistic regression models to compare study groups on all outcomes and produce odds ratios (ORs) and 95% confidence intervals (CIs). All analyses were intent-to-treat and used two-tailed statistical tests with a critical alpha of 0.05. We conducted analyses using Stata version 14.0 (StataCorp LP, College Station, TX).
Results
Table 1 provides data on baseline demographic and health-related characteristics. The majority (83%) of participants self-identified as gay. Most participants were aged 22–25 (59%); non-Hispanic White (57%); and never married or were divorced, separated, or widowed (80%); and did not have a college degree (63%). Participants were from 31 states and the District of Columbia. Most participants had health insurance through a parent (45%) or were self-insured (37%). Approximately half of participants had a regular healthcare provider (51%) and reported having a routine medical checkup in the last year (47%). Fifteen percent of participants reported perceived discrimination from a healthcare provider in the past. The descriptive statistics were highly similar between the intervention and control groups for almost all baseline characteristics.
Overall, 35% of participants (53/150) reported initiating the HPV vaccine series, and 7% (10/150) reported completing the series. HPV vaccine initiation was more common among participants in the intervention group compared to those in the control group (45% vs. 26%; OR = 2.34, 95% CI: 1.18–4.67; p = 0.02) (Fig. 2). We also observed a trend toward HPV vaccine completion being higher among the intervention group than the control group (11% vs. 3%; OR = 4.24, 95% CI: 0.87–20.66; p = 0.07). Finally, reports of talking with a healthcare provider about the HPV vaccine were more common among participants in the intervention group compared to those in the control group (46% vs. 30%; OR = 2.02, 95% CI: 1.03–3.95; p = 0.04).
FIG. 2.
Human papillomavirus vaccine initiation (i.e., receipt of any doses) and completion (i.e., receipt of all three doses) among young gay and bisexual men by study group. Bars indicate standard errors.
Discussion
The results of this pilot study suggest that Outsmart HPV is an effective intervention and a promising strategy for increasing HPV vaccination among YGBM. Indeed, the increase in HPV vaccine initiation among participants who received Outsmart HPV is comparable to some of the larger increases in HPV vaccination reported by past interventions.13,14 The intervention's effect on HPV vaccine completion was not as pronounced, although this may be attributable to many individuals who initiate the HPV vaccine series taking longer than our follow-up period of 7 months to complete the series.15 For individuals initiating the HPV vaccine series during our study's age range, the recommended series still consists of three doses over 6 months.2 Given the promising results of our pilot study, an important next step is to further establish the efficacy of Outsmart HPV in a larger randomized trial, as well as determine the mechanism by which the intervention affects vaccination. Our recent work showed that the intervention positively affected several theoretical constructs,7 but a larger randomized trial is needed to determine if these constructs act as mediators.
Both the intervention delivery method and approach were key strengths of Outsmart HPV and likely contributed to its success. Gay and bisexual men rely extensively on technology for health information, and our results provide further evidence that web-based interventions improve health behaviors among YGBM.16 These results also provide further evidence that multicomponent interventions may be more effective at increasing vaccination than single-component approaches.13 Importantly, Outsmart HPV content included resources to help YGBM navigate the logistics of obtaining the HPV vaccine (e.g., cost and health insurance) and provided skills training for talking with a provider about the vaccine. The latter likely helps explain the observed difference between study groups for talking with a healthcare provider about the HPV vaccine. Such content can address several of the barriers to HPV vaccination reported by YGBM in past work.3,17
Limitations
The study limitations include a modest sample size and self-reported HPV vaccination data. However, self-reported HPV vaccination data among young adults result in only a 2% net bias compared to medical records.18 We also did not collect data on the type of healthcare provider or clinic where participants received the HPV vaccine, or whether participants were transgender individuals. We recruited all participants through Facebook, which could limit the generalizability of the findings, although participants in our study were demographically similar to YGBM from other national studies.6
Conclusions
To our knowledge, Outsmart HPV is the first HPV vaccination intervention developed specifically for YGBM. The findings from this pilot study demonstrate the potential of this intervention as a technology-based strategy for reaching, and increasing HPV vaccination among, YGBM. Future efforts are needed to further establish the efficacy of Outsmart HPV in a larger randomized trial and to determine the mechanism by which the intervention affects vaccination behaviors.
Acknowledgments
This study was supported by the National Cancer Institute of the National Institutes of Health under Award Number R21CA194831. This clinical trial is registered at ClinicalTrials.gov: identifier NCT02835755. We thank the Center for Health Communications Research at the University of Michigan for their contributions during the development and implementation of this intervention.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The study sponsor played no role in study design; the collection, analysis, and interpretation of data; the writing of the article; or the decision to submit the article for publication.
Author Disclosure Statement
P.L.R. has received research grants from Merck Sharp & Dohme Corp. and Cervical Cancer-Free America, through an unrestricted educational grant from GlaxoSmithKline. E.D.P. has received research grants from Merck Sharp & Dohme Corp. These funds were not used to support this research study. None of the other authors have disclosures to report.
References
- 1.Machalek DA, Poynten M, Jin F, et al. : Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: A systematic review and meta-analysis. Lancet Oncol 2012;13:487–500 [DOI] [PubMed] [Google Scholar]
- 2.Meites E, Kempe A, Markowitz LE: Use of a 2-dose schedule for human papillomavirus vaccination—Updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016;65:1405–1408 [DOI] [PubMed] [Google Scholar]
- 3.Reiter PL, McRee AL, Katz ML, Paskett ED: Human papillomavirus vaccination among young adult gay and bisexual men in the United States. Am J Public Health 2015;105:96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agénor M, Peitzmeier SM, Gordon AR, et al. : Sexual orientation identity disparities in human papillomavirus vaccination initiation and completion among young adult US women and men. Cancer Causes Control 2016;27:1187–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis RM, Markowitz LE: Human papillomavirus vaccination coverage among females and males, National Health and Nutrition Examination Survey, United States, 2007–2016. Vaccine 2018;36:2567–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiter PL, Katz ML, Bauermeister JA, et al. : Recruiting young gay and bisexual men for a human papillomavirus vaccination intervention through social media: The effects of advertisement content. JMIR Public Health Surveill 2017;3:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McRee AL, Shoben A, Bauermeister JA, et al. : Outsmart HPV: Acceptability and short-term effects of a web-based HPV vaccination intervention for young adult gay and bisexual men. Vaccine 2018. pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers RW: Cognitive and physiological processes in fear appeals and attitude change: A revised theory of protection motivation. In: Social Psychophysiology: A Source Book. Edited by Cacioppo JT, Petty RE. New York: Guilford Press, 1983, pp 153–176 [Google Scholar]
- 9.Centers for Disease Control and Prevention: Vaccine information statements (VISs): HPV (Human Papillomavirus) VIS. 2016. Available at https://www.cdc.gov/vaccines/hcp/vis/current-vis.html Accessed May18, 2018
- 10.Horvath KJ, Bauermeister JA: eHealth literacy and intervention tailoring impacts the acceptability of a HIV/STI testing intervention and sexual decision making among young gay and bisexual men. AIDS Educ Prev 2017;29:14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norman CD, Skinner HA: eHEALS: The eHealth literacy scale. J Med Internet Res 2006;8:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer MR, Waterlander WE, Kuijper LD, et al. : Testing for baseline differences in randomized controlled trials: An unhealthy research behavior that is hard to eradicate. Int J Behav Nutr Phys Act 2015;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smulian EA, Mitchell KR, Stokley S: Interventions to increase HPV vaccination coverage: A systematic review. Hum Vaccin Immunother 2016;12:1566–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walling EB, Benzoni N, Dornfeld J, et al. : Interventions to improve HPV vaccine uptake: A systematic review. Pediatrics 2016;138:pii: [DOI] [PubMed] [Google Scholar]
- 15.Widdice LE, Bernstein DI, Leonard AC, et al. : Adherence to the HPV vaccine dosing intervals and factors associated with completion of 3 doses. Pediatrics 2011;127:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grov C, Breslow AS, Newcomb ME, et al. : Gay and bisexual men's use of the internet: Research from the 1990s through 2013. J Sex Res 2014;51:390–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerend MA, Madkins K, Phillips G 2nd, Mustanski B: Predictors of human papillomavirus vaccination among young men who have sex with men. Sex Transm Dis 2016;43:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolnick SJ, Parker ED, Nordin JD, et al. : Self-report compared to electronic medical record across eight adult vaccines: Do results vary by demographic factors? Vaccine 2013;31:3928–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]