Abstract
This secondary analysis compares health behavior outcomes for two groups of HIV+ substance users randomized in a 3-arm trial [1] to receive Patient Navigation with (PN+CM) or without (PN) contingent financial incentives (CM). Mean age of participants was 45 years; the majority was male (67%), African American (78%), unemployed (35%), or disabled (50%). Behaviors incentivized for PN+CM were (1) attendance at HIV care visits and (2) verification of an active HIV medication prescription. Incentives were associated with shorter time to treatment initiation and higher rates of behaviors during the 6-month intervention with exception of month 6 HIV care visits. Median HIV care visits were 3 (IQR 2–4) for PN+CM versus 1.5 (IQR 0–3) for PN (Wilcoxon p < 0.001); median validated medication checks were 4 (IQR 2–6) for PN+CM versus 1 (IQR 0–3) for PN (Wilcoxon p < 0.001). Viral suppression rates at end of treatment were not significantly different for the two groups but were directly related to the number of behaviors completed for both care visits (χ2(1) = 7.69, p = 0.006) and validated medication (χ2(1) = 8.49, p = 0.004). Results support use of incentives to increase performance of key healthcare behaviors. Adjustments to the incentive program may be needed to achieve greater rates of sustained health behavior change that result in improved viral load outcomes.
Keywords: : HIV healthcare, substance users, patient navigation, contingency management, medication adherence, viral suppression
Introduction
Major advances have been made in treatment for HIV, but many persons living with HIV (PLWH) are not able to receive the optimal benefits.1–3 A particularly vulnerable group includes those with substance use disorders (SUDs) in addition to HIV. These individuals often have lower rates of treatment initiation, retention, and antiretroviral therapy (ART) use.4–8 However, when this population receives support and interventions to improve adherence to treatment, it is possible to achieve similar outcomes to PLWH who do not have substance problems.9,10 These findings underscore the importance of identifying focused engagement in care interventions that are effective for PLWH in general and for vulnerable populations with SUDs in particular.
One promising approach to improved healthcare engagement for substance users living with HIV is the use of interpersonal strength-based support interventions such as case management or patient navigation (PN).11 The ARTAS study12 was a controlled trial that enrolled persons recently diagnosed with HIV (N = 316) contacted at various community settings where HIV testing was performed. Participants were randomly assigned to case management versus usual care referral to treatment. Case management involved up to five visits scheduled over 90 days in which linkage to care was encouraged and supported. The case management intervention successfully linked 78% of participants into care compared with 60% for the usual care referral group (p = 0.0039). Although results are mixed, other studies with less rigorous designs have also supported the promise of a supportive interpersonal intervention approach for HIV populations.11
Financial incentives have also shown promise as an intervention component to support HIV care linkage and follow-through.13 In one controlled study of persons with HIV (N = 90) who were failing treatment due to medication nonadherence, Javanbakht et al.14 found higher rates of clinically meaningful (10-fold or more) reduction in viral load among those randomized to a combined intervention using case management plus contingent financial incentives (45–55% with viral load reduction) compared to usual care (26–33% with reduction). Solomon et al.15 targeted linkage to and retention in care among HIV-infected drug users (N = 120) in Chennai, India. Those in the incentive arm were more likely to visit the HIV center (82% vs. 55%; p = 0.002), to initiate HIV treatment (45% vs. 27% p = 0.04) and to attend more HIV care visits (median = 8 vs. 3.5 sessions; p = 0.005). However, no differences in viral suppression were found.
Finally, a study by El-Sadr et al.16 randomized incentive versus usual care interventions at the clinic level in US cities (New York; Washington, DC). Unlike Solomon, they found no effect of financial incentives ($125 for meeting with a care provider) on rates of care linkage among newly diagnosed individuals contacted at HIV testing sites. However, financial incentives ($70 gift cards provided quarterly) for suppressed viral loads significantly increased rates of viral suppression and of care continuity among patients already enrolled at HIV care clinics. These studies provide support for continued exploration of financial incentives targeting linkage to and retention in care, as well as improving viral suppression outcomes.
A recently completed large multi-site study conducted within the National Drug Abuse Treatment Clinical Trials Network (CTN 0049/Project HOPE: Hospital as Opportunity for Patient Engagement) provided an opportunity to examine the potential value of adding contingent financial incentives (contingency management = CM) to a PN behavioral intervention platform whose goal was improving HIV outcomes among substance users with uncontrolled HIV. The main outcome article from the HOPE study17 showed no difference among study arms at the primary 12-month end-point, 6 months after the intervention ended. A secondary analysis, however, showed that immediately after conclusion of the 6-month long interventions, rates of viral load suppression among survivors were 38.2%, 43.1%, and 50.4% in usual care, PN only, and PN with incentives (PN+CM), respectively, with PN+CM rates being significantly (p = 0.03) higher compared to the usual care control. (Suppression rates in intent-to-treat sample = 35.2%, 39.1%; 46.2%; PN+CM vs. usual care p = 0.04).
Two previous articles have reported that rates of attendance at PN sessions were significantly higher in PN+CM than in PN only participants,17,18 while one article showed that viral load suppression was directly related to the number of PN visits attended.18 Finally, the primary outcome article,17 using data from the 6-month outcome assessment, shows that PN+CM participants self-reported more HIV care visits and more use of HIV medications compared to PN only participants. However, viral load suppression rates did not differ significantly at 6 months for PN versus PN+CM.
The present secondary post hoc analysis expands on these findings by analyzing data from the detailed PN database that documented performance of all target behaviors during the 6-month intervention to compare outcomes for PN+CM versus PN. The article focuses on two healthcare behaviors likely to mediate final viral outcomes: (1) number of visits with an HIV specialist doctor (or other qualified healthcare provider) who could write antiretroviral medication prescriptions and (2) validated possession of an active antiretroviral medication prescription. This article compares rates of these two healthcare behaviors during the 6-month intervention for PN+CM versus PN participants and examines the relationship between health behaviors and viral load suppression at the end of intervention (6-month outcome) time point. Findings will help to better understand the potential utility associated with incorporating incentives within the supportive PN intervention.
Methods
The HOPE study enrolled and randomized 801 persons with HIV and substance use recruited from 11 hospitals across the United States. The study was approved by local IRBs at each participating institution. Eligibility criteria included having a detectable HIV viral load (from hospital records) and evidence (by self-report and/or medical records) of any opioid, stimulant (cocaine, amphetamines, and ecstasy), or heavy alcohol use within the past year. Mean age of participants was 45 years; the majority was male (67%), African American (78%), unemployed (35%), or disabled (50%). Fifty nine percent were heavy alcohol users, while 77% used one or more drugs.
Participants were randomized within a 3-arm design; one randomized group received standard of care which typically included referral to HIV and substance use services; the remaining participants were randomized to one of two patient navigation interventions delivered with (PN + CM; N = 271) or without (PN only; N = 266) a multi-target incentive program. PN and PN+CM are the groups that are included in the present post hoc secondary analysis with data derived from a detailed PN monitoring system that tracked and recorded all target behaviors for both PN groups.18
The participants included in this analysis, hospitalized for a variety of medical problems, had an average hospital stay of 6.5 days with considerable variability (SD 6.6 days). Eligible patients were contacted by research staff on average 4.5 (SD 4.4) days after hospital entry to complete informed consent, baseline assessment, and randomization procedures. Hospital discharge occurred on average 1.9 days after randomization, again with considerable variability (SD 6.3 days).
Participants in both PN conditions were eligible to receive the same 11 session navigation intervention over 6 months with the first full session ideally occurring in the hospital. During PN sessions, navigators used motivational interviewing techniques to assist participants to draw on their own capabilities and resources while specifically encouraging them to engage in HIV care, initiate or reinstate ART, and take steps to reduce or stop their substance use, potentially including entry into SUD treatment. Session schedules were flexible in both timing and location, with the intent that they be more frequent during early months of the intervention and less frequent in later months.
PN+CM participants could earn up to a total of $1160 during the 6-month intervention by meeting target goals on seven different behaviors related to HIV treatment engagement and substance use abatement. Behavior targets included attending PN sessions, attending HIV care visits, providing evidence of an active antiretroviral medication prescription, entering substance abuse treatment, and providing drug negative urine samples at PN visits. Incentive payments were also available for meeting viral load suppression criteria. Details of the multi-target CM plan and rationale have been described.19
The present article focuses on the two healthcare-related targets assumed to most closely mediate ultimate viral load outcomes: (1) attendance at HIV care visits ($180) and (2) possession of an HIV medication prescription, as validated at PN sessions ($170). In addition, payments totaling $50 were available during the intervention for keeping an appointment at the clinical laboratory where a blood sample was drawn for viral load assessment. The number and timing of these visits was left to the discretion of the care providers who would be using the information to adjust clinical care. Although necessary for clinical care, these visits were not included in the secondary analysis reported in this study. Incentives ($150) were also available for meeting viral load outcome criteria, as explained below. The remainder of available earnings was for PN visits ($220), paperwork completion ($80), and substance abuse-related targets ($310).
Target behavior definitions and methods: HIV care visits
Making and keeping appointments with an HIV care provider was a key priority of the navigation intervention. Navigators were expected to be present with participants at each HIV care medical visit and to devote the PN sessions before and after to preparation and debriefing, respectively, of the medical visit. Preparation included review of visit expectations and written questions for the provider. Debriefing included review of participant's current health status and follow-up plans. Participants in PN+CM could earn up to $180 (15.5% of total earnings) for making and keeping up to four appointments with an HIV doctor or other type of care provider. HIV care visit incentives were available on an escalating schedule of $30, $40, $50, and $60 for each successive appointment attended. The escalating schedule was used to bolster motivation for making and keeping care appointments at more distal intervention time points. While variability was anticipated in HIV care expectations across sites and clinicians, four visits were selected as a reasonable expectation for clinical monitoring spaced throughout a 6-month intervention.
Target behavior definitions and methods: validated medication checks
“Validated possession of an active medication prescription” was the medication adherence target selected for receipt of contingent incentives. Medication checks were conducted at each PN visit for both PN and PN+CM participants to ascertain whether an active prescription was on hand. Typically, participants showed their pill bottles with labels indicating a currently active prescription. Alternatively, the PN could validate from the pharmacy that a currently active HIV medication prescription had been recently filled. If either of these criteria was met, the check was designated as “validated,” and PN+CM participants were eligible for incentive payments. In addition, once an initial prescription had been validated, navigators reviewed their self-reported pill taking with participants and discussed the importance of taking their medication regularly as prescribed. Up to seven payments were available to PN+CM for a total of $170 in earnings (14.7% of total possible earnings) for validated medication checks. The escalating payment schedule started at $20 for the first two checks then increased in $2 increments to $30 at the seventh validated check. The number of available incentive payments was fewer than the number of PN visits (N = 11) based on the assumption that several PN sessions would be required before a care visit could be arranged and a prescription obtained and filled.
Viral load suppression bonuses
PN+CM participants could earn two bonus payments for meeting prespecified viral load suppression criteria. A $50 incentive payment was available between study weeks 6 and 20 (or if not previously earned, at the 6-month follow-up for those without viral suppression at 6 months) if the viral load had dropped 1 full log unit or more from the participant's baseline level or met the criterion for suppression defined as a reading of ≤200 copies/mL. A second payment of $100 could be earned at the end of the intervention based on research data collected at the 6-month assessment if levels met criterion for viral suppression. The relationship between viral suppression at the end of treatment (6-month) time point and performance of the two healthcare target behaviors during the intervention was examined.
Incentive tracking and dispersal
Total earnings were calculated at each PN visit for PN+CM. Participants could choose to receive earnings immediately or hold them in an account for receipt at a later time. Payment was made in cash (four sites) or debit card deposit (one site), by gift cards to local retail establishments (four sites) or by a combination (two sites; one offering patient choice). Within sites, payment method was the same for research reimbursements to all participants and incentive payments to PN+CM.
Outcome measures and analysis
Data analysis focuses primarily on the two key healthcare behaviors, HIV care visits and validated medication checks, with a parallel analysis plan followed for each of these two target behaviors. Five analyses were conducted for each target to provide a comprehensive perspective on outcomes: (1) Individual participant distribution of target behavior performance (total number of care visits and of validated medication checks) was compared for the two groups using chi square; medians were compared using the Wilcoxon test due to the non-normal distribution of these frequencies; we report the normal-approximation to the Wilcoxon test statistic due to the relatively large sample.20 (2) Latency (days) from randomization to the first PN visit and from the first PN visit to the first instance of each target behavior was compared for PN+CM versus PN participants; t-tests were used for mean and Wilcoxon test for median comparisons. (3) Maintenance of target behaviors during the intervention was examined by comparing for PN+CM versus PN the percent of participants performing the target behavior at least once during each intervention month. A Generalized Estimating Equations (GEE) model with binary distribution and logistic link function was used to determine group effects, time effects, and group X time interactions. (4) To determine whether incentives were associated with the target behaviors independent of PN visit frequency, participants in both treatment groups were classified as having attended a low (0–5), medium (6–8), or high (9–11) number of sessions with their navigator. The number of target behaviors performed was examined using GEE with Poisson distribution to test for group (PN+CM vs. PN) associations, PN attendance frequency associations, and group X PN attendance interactions. Additional planned comparisons using t-tests were conducted between mean number of care visits and of validated medication checks for PN versus PN+CM within each attendance subgroup. (5) The association between target behavior performance and viral load suppression outcomes was examined for PN and PN+CM participants separately and for the two groups combined; percent virally suppressed at 6 months (end of treatment) was calculated for participants who had 0, 1–2, and 3–4 HIV care visits and for those who had 0–1, 2–3, 4–5, and 6–7 validated medication checks. Categories were defined based on observation of distributions; differences across categories in viral suppression rates were tested with chi square.
Results
Target behavior performance distributions
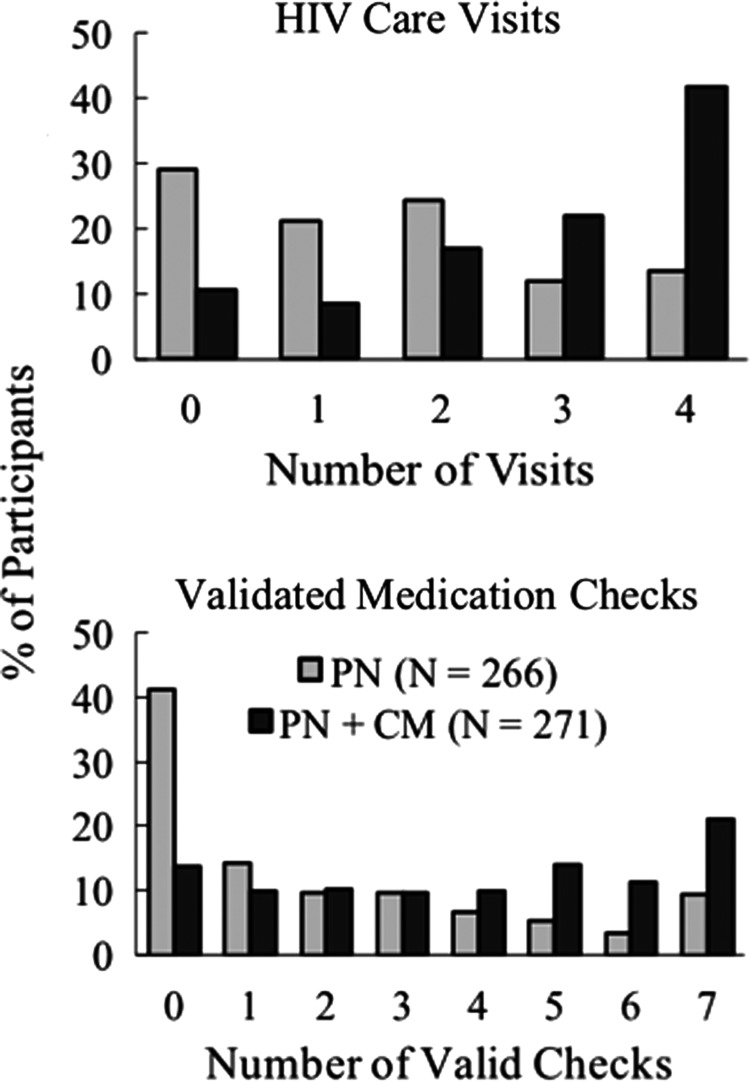
As shown in Fig. 1, distributions of target behavior performance differed substantially for PN versus PN+CM on both HIV care visits and validated medication checks. Median number of care visits was 3 (IQR 2–4) for PN+CM versus 1.5 (IQR 0–3) for PN (Wilcoxon test <0.0001). Zero contact with an HIV care provider was seen for 11% of PN+CM versus 29% of PN. Conversely, the percentage attending three or four care visits was 64% for PN+CM versus 26% for PN; 42% of PN+CM versus 14% PN attended the maximum number (4) of incentivized care visits.
FIG. 1.
shows the percentage of participants in PN (N = 266) and PN+CM (N = 271) who achieved 0–4 HIV care visits (top panel) and had 0–7 validated medication checks (bottom panel) during the 6-month intervention. Medications were validated by the participant showing a pill bottle or providing other verification that s/he was in possession of an active prescription. Incentives with escalating value were available independently for both targets with a total of $180 available for HIV care visits and $170 for validated medication checks. PN, patient navigation; CM, contingent financial incentives.
Median number of validated medication checks was 4 (IQR 2–6) for PN+CM versus 1 (IQR 0–3) for PN (Wilcoxon test <0.0001). Among PN+CM, 14% versus 41% of PN had zero validated medication checks, while the percentage with five to seven validated checks was 46% for PN+CM versus 18% for PN.
Target behavior initiation
Table 1 shows that time from randomization to the first PN visit, as well as time from the first PN visit to performance of the two key healthcare behaviors, was significantly shorter for PN+CM than for PN. For example, median time from randomization to first PN meeting for PN+CM (1 day) was significantly shorter than median time for PN (3 days, Wilcoxon p = 0.004); mean differences indicate substantial variability and outliers with average time to the first meeting being half as long for PN+CM than for PN (6.2 vs. 13.1 days, respectively). Similarly, median time from the first PN meeting to the first HIV care visit was 15 (IQR 7–34) days for PN+CM versus 25 (IQR 7–64) days for PN (p < 0.009).
Table 1.
Time to Initiate PN Sessions and Healthcare Behaviors (Means and Medians)
| PN | PN+CM | Statistics | |
|---|---|---|---|
| Mean (SD) days | t-test | ||
| Randomization to first PN visita | 13.1 (26.3) | 6.2 (14.1) | 0.0002 |
| First PN visit to first HIV care visitb | 40.1 (44.5) | 25.1 (30.5) | <0.0001 |
| First PN visit to first valid med checkc | 51.0 (49.5) | 34.5 (36.7) | 0.0002 |
| Median (IQR) days | Wilcoxon | ||
|---|---|---|---|
| Randomization to first PN visita | 3 (1–11) | 1 (1–6) | 0.0039 |
| First PN visit to first HIV care visitb | 25 (7–64) | 15 (7–34) | 0.0089 |
| First PN visit to first valid med checkc | 34 (11–83) | 21 (11–45) | 0.0092 |
Among those with any PN visit. N = 257 PN; N = 266 PN+CM.
Among those with any care visit. N = 189 PN; N = 242 PN+CM.
Among those with any validated medication check. N = 156 PN; N = 234 PN+CM.
PN, patient navigation; CM, financial incentives.
Target behavior persistence
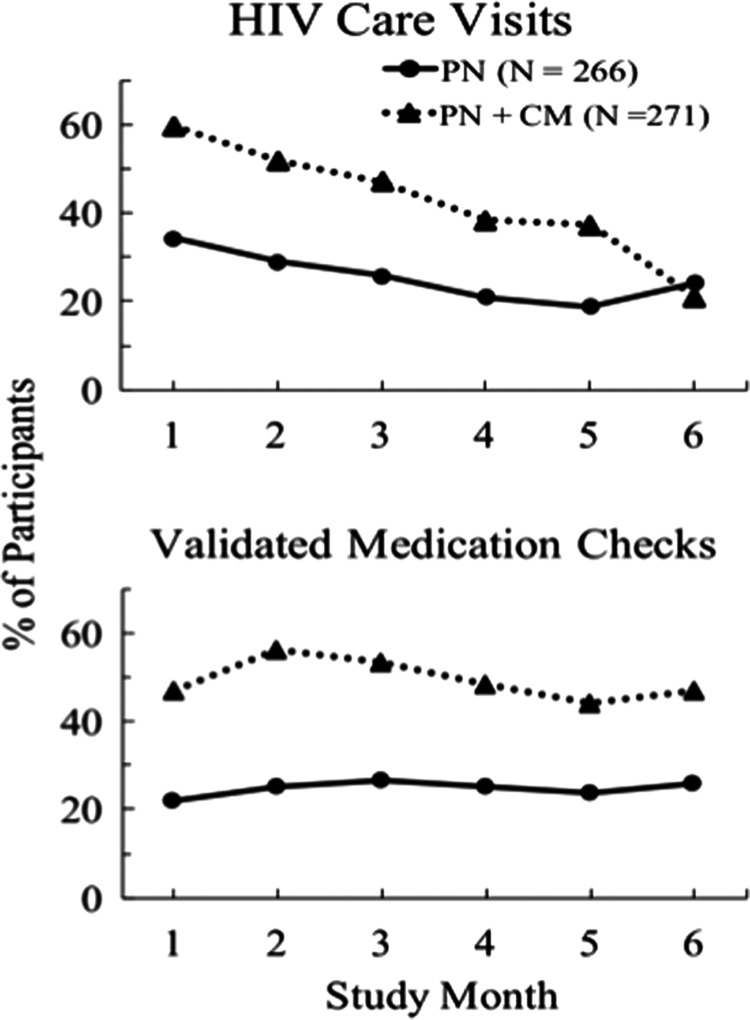
Figure 2 shows the percent of participants in each month completing at least one HIV care visit (top panel) or having at least one validated medication check (bottom panel). Care visit analysis showed significant group (χ2−(1) = 48.13, p < 0.001) and time (χ2(1) = 82.26, p < 0.001) associations and a significant interaction (χ2(1) = 10.02, p = 0.002). Percent with a care visit declined over time for both groups, but with the exception of month 6, PN+CM had a consistently higher percent of participants engaging in a care visit in any given month (35–60%) compared to PN (17–34% with a care visit).
FIG. 2.
shows the percent of participants in each intervention month who attended at least one HIV care visit (top panel) and the percent that had at least one validated medication check (bottom panel during each month of the 6-month intervention). Data are shown for PN (N = 266) and PN+CM (N = 271) participants.
Analysis of medication checks showed a significant group effect (χ2(1) = 43.68, p < 0.001) but no time association or group X time interaction. PN+CM had a consistently higher percent of participants with a validated medication check in any given month (44–56%) compared to PN (22–27%). In both groups, the percent with a validated medication check was higher in months 2 and 3 than in month 1, reflecting the delay in obtaining an initial prescription after the first HIV care visit.
Incentive associations controlling for PN contact
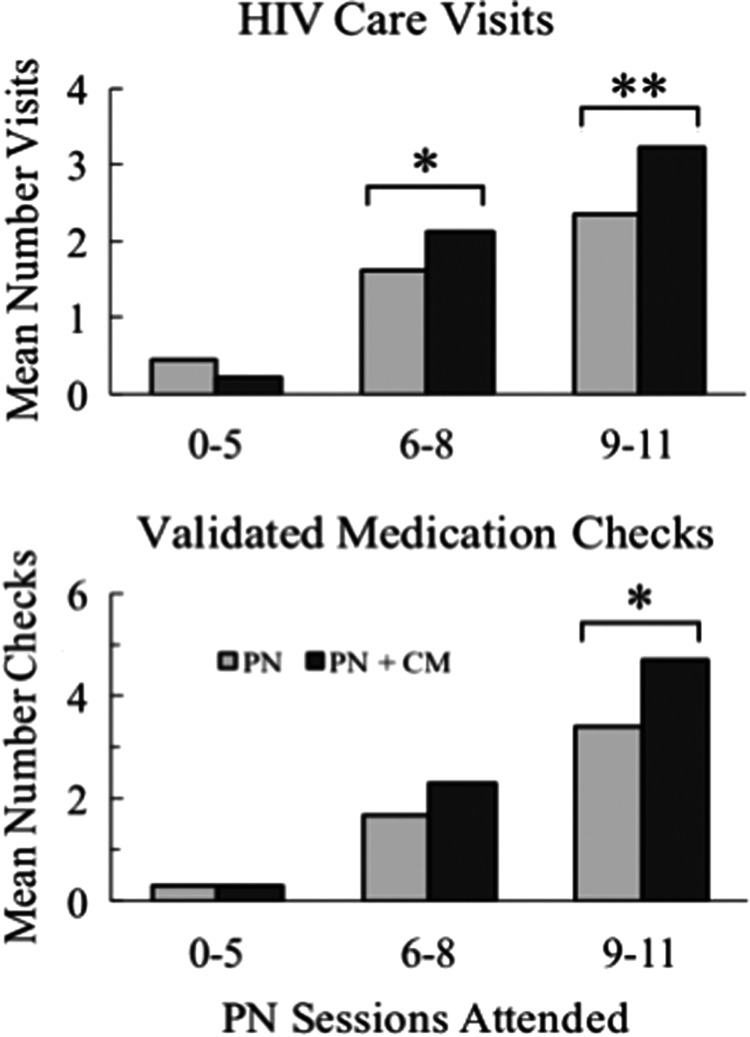
In Fig. 3, mean number of care visits (top panel) and of validated medication checks (bottom panel) is compared for PN+CM versus PN within each of three participant subgroups that had low (0–5), medium (6–8), or high (9–11) numbers of PN sessions attended. The GEE model showed significant PN attendance frequency association for both the number of care visits and validated medication checks (χ2(2) = 121.8, p < 0.001 and χ2(2) = 116.3, p < 0.001, respectively) but nonsignificant group association and group by PN attendance interactions. Planned comparisons within PN attendance categories, however, indicated that incentives were associated with an increased mean number of care visits attended for both the medium (2.1 vs. 1.6; p = 0.035) and high (3.2 vs. 2.4; p < 0.001) rate PN session attendance subgroups. Mean number of validated medication checks were significantly associated with incentives for the high rate PN attendance group (4.7 vs. 3.4; p < 0.001) while missing significance for the medium attendance subgroup (2.3 vs. 1.7; p = 0.095).
FIG. 3.
shows mean number of HIV care visits attended (top panel) and validated medication checks (bottom panel) for PN and PN+CM participants categorized into low (0–5), medium (6–8), and high (9–11) total number of PN sessions attended out of a possible 11 sessions. Bracketed bars with asterisks indicate significant differences between PN and PN+CM means. Sample sizes for PN session attendance categories are low (N = 68), medium (N = 94), and high (N = 104); sample sizes for PN+CM are low (N = 27), medium (N = 42), and high (N = 202).
Viral suppression associations
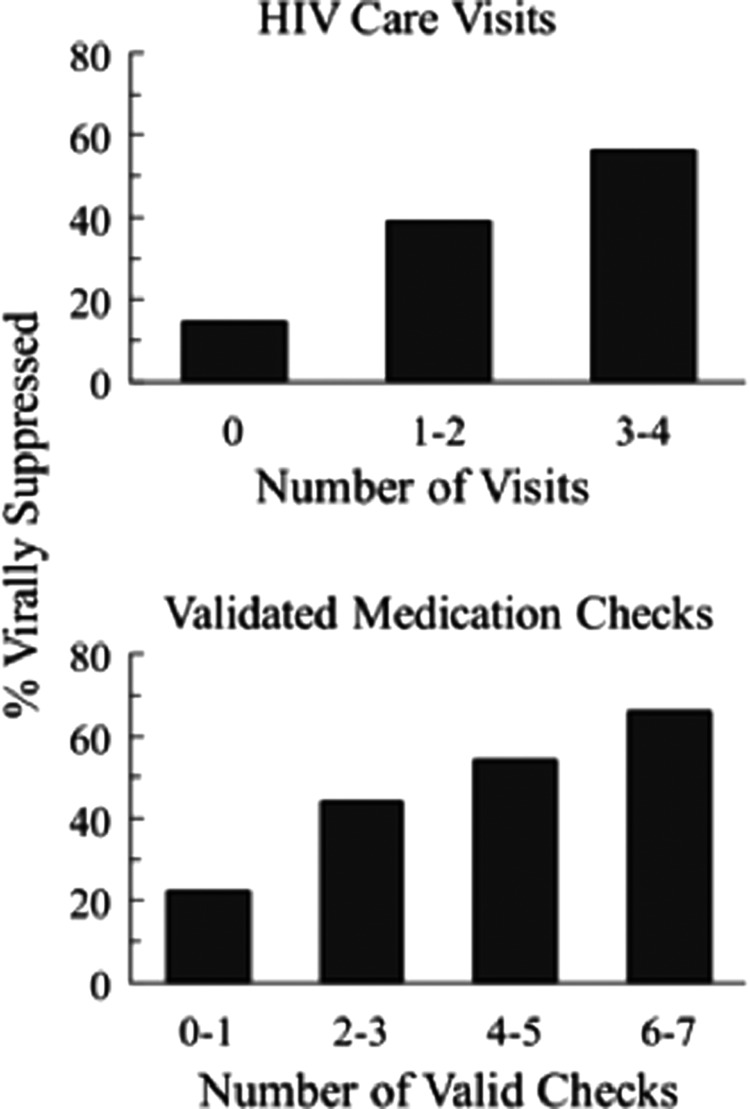
As previously noted, viral suppression rates at 6 months were not significantly different for PN (39%) versus PN+CM (46%). Nevertheless, there was a significant association between target behavior performance and viral suppression. Figure 4 shows the percent of all participants (groups collapsed) with viral load suppression at the end of treatment (6-month) follow-up time point as a function of the number of HIV care visits completed (top panel) and number of validated medication checks (bottom panel). Tables 2 and 3 show viral load suppression data separately for PN and PN+CM treatment groups to illustrate that similar patterns were seen within each group. In data collapsed across groups, there was a direct and significant relationship between viral suppression rates and the number of target behaviors completed for both HIV care visits (χ2(1) = 7.69, p = 0.006) and validated medication checks (χ2−(1) = 8.49, p = 0.004). Among those with 0 care visits, (N = 90), 14.4% had viral suppression. In contrast, among those with three to four care visits (N = 238), over half (56%) achieved viral suppression at end of treatment. Similarly, suppression rate at end of treatment among those with 0 validated medication checks (N = 128) was 18%, while rate of suppression was 54% among those with four-to-five validated checks (N = 95) and 66% among those with the highest number (6–7) of validated medication checks (N = 122).
FIG. 4.
shows the percent of all participants collapsed across PN and PN+CM (N = 508 due to missing viral load data) with suppressed viral load (≤200 copies/mL) at the 6-month assessment as a function of HIV care visits attended (top panel) and number of validated medication checks (bottom panel). Sample sizes for HIV care visits are 0 (N = 90), 1–2 (N = 180), and 3–4 (N = 238). Sample sizes for validated medication check categories are 0–1 (N = 188), 2–3 (N = 103), 4–5 (N = 95), and 6–7 (N = 122). Data for PN and PN+CM groups separately are shown in Tables 2 and 3.
Table 2.
Viral Suppression Outcomes at 6 Months Within Treatment Groups By Number of HIV Care Visits
| PN | PN+CM | |||
|---|---|---|---|---|
| Number HIV care visits | N in categorya | % virally suppressed | N in categorya | % virally suppressed |
| 0 | 66 | 18 | 24 | 4 |
| 1 | 52 | 44 | 21 | 19 |
| 2 | 63 | 38 | 44 | 43 |
| 3 | 31 | 55 | 59 | 56 |
| 4 | 36 | 58 | 112 | 56 |
| Total | 248 | 260 | ||
The association of treatment and viral suppression after stratifying for number of HIV care visits was nonsignificant (Cochran–Mantel–Haenszel Statistics = 1.1618, df = 1, p = 0.2811).
Includes participants for whom viral load data were available at 6 months with those deceased at 6 months (PN = 23; PN+CM = 22) counted as treatment failures.
Table 3.
Viral Suppression Outcomes at 6 Months Within Treatment Groups by Number of Validated Medication Checks
| PN | PN+CM | |||
|---|---|---|---|---|
| Number valid medication checks | N in categorya | % virally suppressed | N in categorya | % virally suppressed |
| 0–1 | 131 | 24 | 57 | 16 |
| 2–3 | 51 | 45 | 52 | 42 |
| 4–5 | 32 | 50 | 63 | 56 |
| 6–7 | 34 | 76 | 88 | 61 |
| Total | 248 | 260 | ||
The association of treatment and viral suppression after stratifying for number of valid medication checks was nonsignificant (Cochran–Mantel–Haenszel Statistics = 1.8474, df = 1, p = 0.1741).
Includes participants for whom viral load data were available at 6 months with those deceased at 6 months (PN = 23; PN+CM = 22) counted as treatment failures.
Discussion
Contingent financial incentives added to a PN intervention were associated with better engagement in the navigation intervention, including earlier initiation and higher sustained rates of key health-related behaviors deemed necessary to achieve a final goal of viral load suppression. In addition to higher rates of initiation, it was notable that incentives were associated with a shorter average time both to the initial HIV care visit and to first verified pick up of HIV medication among those who ever initiated these behaviors (Table 1). These robust results suggest value of incentives as a tool to enhance linkage to care, as well as speeding up or “kick starting” early steps in the care process within a navigation intervention.
It is encouraging that the PN intervention with versus without incentives was associated with higher rates of medical care visits. The PN intervention emphasized these visits as a key part of the navigation process, including preparation for each medical care visit and navigators accompanying participants to the visits. Given this emphasis, navigation itself may have been sufficient to promote high rates of attendance. Our data testify to the potential advantage of including incentives as part of a PN intervention to support performance of key healthcare behaviors that are the target of the PN intervention.
The gradual decline over months in percent of participants with an HIV care visit noted in Fig. 2 may reflect a natural clinical course in which checkups are scheduled at more widely spaced intervals over time. The sharp drop in PN+CM visits at month 6 is of potential concern for sustained postintervention care. This drop could reflect completion by PN+CM before month 6 of their limit of four incentivized visits. Thus, it may be advisable in future CM programs to provide additional earning opportunities for care visits (e.g., monthly opportunities throughout) to accommodate a wider range of usual care practice and to continue these incentives over a longer period of time. Whatever the reason for this drop, it is encouraging that the decline in care visits was not associated with a similar decline in rates of verified medication possession checks in month 6, the last study intervention month (Fig. 2).
Visits to the medical care provider are a necessary first step in obtaining effective HIV medications, but adherence to the prescribed medication is arguably the behavior most directly linked to viral load outcome and, therefore, of central interest as a target behavior in any behavioral support program. In this analysis, incentives were strongly associated with improved overall performance on the medication validation target (Figs. 1 and 2). As illustrated in Fig. 1, the percent of participants with high rates of performance on this key healthcare target was 2.5 times higher for the combined intervention with incentives than for PN alone (46% vs. 18%), while the percent with poor performance was nearly thrice higher for PN alone than for the combined intervention (14% vs. 41%).
Our secondary analysis suggests that the medication check procedure used here may be an adequate strategy for many HIV patients to support adherence. However, absolute rates of validated medication possession could be improved and closer monitoring may be beneficial for some patients. Thus, additional adherence-focused intervention will likely be needed to further improve performance on this critical mediating behavior with attention as well to other potential barriers to medication adherence such as lapses in insurance or in publicly funded drug assistance programs.21 Previous studies have shown that incentives can improve adherence to HIV medication when adherence is tracked daily using MEMS caps.22–24 In the future, it may be useful to take advantage of new electronic technology that can be used to directly track and provide both reminders and reinforcement for medication adherence.25,26
While attendance at navigation sessions was not a central focus of the present secondary analysis, we have added to previous observations that documented significantly more sessions attended by PN + CM (median = 11) versus PN (median = 7).17,18 Specifically, our data showed that incentives were associated with a substantial reduction in the average latency from randomization to the first navigation visit (Table 1). These data further support the suggestion that incentives targeting PN attendance are an especially useful feature of the CM program, resulting in earlier and more sustained engagement with the navigator and hence greater opportunity to take advantage of services they provide.
Because incentives were associated with increased contact with the navigators, it was not possible to directly differentiate the association of incentives per se with healthcare behaviors versus increased exposure to navigation interventions; to do this, a study design would be needed that includes a group receiving incentives for navigation visits only. It was nevertheless possible to partially disentangle these two components by comparing PN+CM versus PN outcomes within subgroups that had differing amounts of exposure to the navigators. This analysis (Fig. 3) clearly illustrates the strong association between PN session attendance and healthcare behavior outcomes, but also shows that incentives were associated with higher rates of healthcare behaviors even among those who had greater levels of engagement with the navigation intervention, as seen by attendance at 6 or more sessions. These data support the suggestion that incentives had an additive interaction with the PN intervention, further promoting adherence to key healthcare behaviors within the context of a combined intervention.
This study adds to the findings of previous studies that have incorporated financial incentives into an HIV healthcare intervention. Our data are most consistent with that of Solomon et al.15 in showing improved linkage to and participation in care without a significant impact on viral load outcomes. Two other studies14,16 found improved viral load outcomes with incentives among participants already enrolled in an HIV treatment program, accompanied by observation of better care continuity16 or increased medication adherence self-efficacy.14 As discussed in recent reviews,27,28 it is likely that differences in populations, settings, other services offered, and details of the incentive program, including incentive magnitude,29,30 could account for cross-study discrepancies. It remains for future research to determine the optimal components and parameters of behavioral interventions possibly including commitment contracting31 that can support the desired health outcome of sustained viral suppression.
It is notable that rates of performance of the two key healthcare behaviors targeted in this study were strongly associated with viral load outcomes independent of the intervention received (Fig. 4) and that impressive rates of suppression were noted within good performing subgroups. Thus, for example, 56% of those with repeated HIV care visits and two-thirds of those with the highest rate of validated medication checks during the intervention were virally suppressed at the end of treatment. Unfortunately, because of the multi-target incentive package offered, it is not possible to discern from the present design whether there was any added benefit to providing incentives directly contingent on achieving viral load suppression, but this strategy has been shown effective elsewhere.16 The association between behavioral outcomes and viral suppression suggests that the combined intervention is on the right track and that clinically meaningful improvements in the final physiologic outcome could be achieved by improving the behavioral support intervention in ways that further enhance the number of patients who initiate and sustain compliance with these key healthcare behaviors.
This study has limitations. Both the PN and CM components were complex interventions with single parameters chosen for study. Different results might be obtained if other navigator components and/or CM parameters had been used. Because this is a secondary post hoc analysis, results should be interpreted with caution. Because there was not a statistically significant difference between the two arms in viral load suppression at the 6-month time point, it remains uncertain whether further increasing medication prescription adherence and medical visits in this population would suffice to further improve viral load outcomes. The role of ongoing substance use was not examined in this article, but will be the topic of a future analysis. Finally, because participants were located in a hospital setting and selected for a randomized trial, they may not be representative of the larger population of people living with HIV.
In conclusion, contingent financial incentives added to a PN intervention were associated with better outcomes on key healthcare behaviors compared to outcomes seen for PN alone. Incentives appeared to promote early initiation and engagement in care, as well as sustaining higher rates of healthcare behaviors, throughout the 6-month intervention. The linear association between performance of healthcare behaviors during treatment and end-of-treatment viral load suppression outcomes supports the assertion that these are important mediating behaviors. Overall, findings support the utility of adding financial incentives to a PN platform with potential for improving HIV outcomes in difficult to treat populations, including out-of-treatment substance users.
Acknowledgments
Data sets analyzed for the current study are from CTN 0049, Project HOPE (clinicaltrials.gov Identifier: NCT01612169) and are available at the National Drug Abuse Treatment Clinical Trials Network DataShare website: http://datashare.nida.nih.gov. The HOPE study protocol was approved by institutional review boards at all participating institutions. The project HOPE clinical trial and subsequent article preparation activities were funded under NIDA Drug Abuse Treatment Clinical Trials Network cooperative agreements UG1DA013034, UG1DA015815, and UG1DA013720. D.J.F. and R.D. analyzed the data; all authors provided editorial input and approved the final article. Figures were created by Erin Martin at the Behavioral Pharmacology Research Unit. The CTN publications committee provided valuable presubmission feedback on the article.
Author Disclosure Statement
Drs. Stitzer, Metsch, Feaster, Gooden, del Rio, and Sorensen have received grants from the National Institute on Drug Abuse, National Institutes of Health (NIH). The authors declare that they have no other competing interests or conflicts of interest.
References
- 1.Aidala AA, Wilson MG, Shubert V, et al. Housing status, medical care, and health outcomes among people living with HIV/AIDS: A systematic review. Am J Public Health 2016;106:e1–e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Genberg BL, Lee Y, Rogers WH, Wilson IB. Four types of barriers to adherence of antiretroviral therapy are associated with decreased adherence over time. AIDS Behav 2015;19:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palumbo R. Discussing the Effects of Poor Health Literacy on Patients Facing HIV: A Narrative Literature Review. Int J Health Policy Manag 2015;4:417–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend 2010;112:178–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metsch LR, Bell C, Pereyra M, et al. Hospitalized HIV-infected patients in the era of highly active antiretroviral therapy. Am J Public Health 2009;99:1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proeschold-Bell RJ, Heine A, Pence BW, McAdam K, Quinlivan EB. A cross-site, comparative effectiveness study of an integrated HIV and substance use treatment program. AIDS Patient Care STDS 2010;24:651–658 [DOI] [PubMed] [Google Scholar]
- 7.Tobias CR, Cunningham W, Cabral HD, et al. Living with HIV but without medical care: Barriers to engagement. AIDS Patient Care STDS 2007;21:426–434 [DOI] [PubMed] [Google Scholar]
- 8.Ulett KB, Willig JH, Lin H, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS 2009;23:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malta M, Magnanini MM, Strathdee SA, Bastos FI. Adherence to antiretroviral therapy among HIV-infected drug users: A meta-analysis. AIDS Behav 2010;14:731–747 [DOI] [PubMed] [Google Scholar]
- 10.Wood E, Hogg RS, Lima VD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA 2008;300:550–554 [DOI] [PubMed] [Google Scholar]
- 11.Liau A, Crepaz N, Lyles CM, et al. Interventions to promote linkage to and utilization of HIV medical care among HIV-diagnosed persons: A qualitative systematic review, 1996–2011. AIDS Behav 2013;17:1941–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS 2005;19:423–431 [DOI] [PubMed] [Google Scholar]
- 13.Bassett IV, Wilson D, Taaffe J, Freedberg KA. Financial incentives to improve progression through the HIV treatment cascade. Curr Opin HIV AIDS 2015;10:451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javanbakht M, Prosser P, Grimes T, Weinstein M, Farthing C. Efficacy of an individualized adherence support program with contingent reinforcement among nonadherent HIV-positive patients: Results from a randomized trial. J Int Assoc Physicians AIDS Care 2006;5:143–150 [DOI] [PubMed] [Google Scholar]
- 15.Solomon SS, Srikrishnan AK, Vasudevan CK, et al. Voucher incentives improve linkage to and retention in care among HIV-infected drug users in Chennai, India. Clin Infect Dis 2014;59:589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Sadr WM, Donnell D, Beauchamp G, et al. Financial incentives for linkage to care and viral suppression among HIV-positive patients: A randomized clinical trial (HPTN 065). JAMA Int Med 2017;177:1083–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metsch LR, Feaster DJ, Gooden L, et al. Effect of patient navigation with or without financial incentives on viral Suppression among hospitalized patients with HIV infection and substance use: A randomized clinical trial. JAMA 2016;316:156–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stitzer M, Matheson T, Cunningham C, et al. Enhancing patient navigation to improve intervention session attendance and viral load suppression of persons with HIV and substance use: A secondary post hoc analysis of the Project HOPE study. Addict Sci Clin Pract 2017;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stitzer M, Calsyn D, Matheson T, Sorensen J, Gooden L, Metsch L. Development of a multi-target contingency management intervention for HIV positive substance users. J Subst Abuse Treat 2017;72:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellera CA, Julien M, Hanley JA. Normal approximations to the distributions of the Wilcoxon statistics: Accurate to what N? Graphical insights. J Statist Educ 2010;18:1–17 [Google Scholar]
- 21.Wohl DA, Kuwahara RK, Javadi K, et al. Financial barriers and lapses in treatment and care of HIV-infected adults in a southern state in the United States. AIDS Patient Care STDS 2017;31:463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigsby MO, Rosen MI, Beauvais JE, et al. Cue-dose training with monetary reinforcement: Pilot study of an antiretroviral adherence intervention. J Gener Int Med 2000;15:841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen MI, Dieckhaus K, McMahon TJ, et al. Improved adherence with contingency management. AIDS Patient Care STDs 2007;21:30–40 [DOI] [PubMed] [Google Scholar]
- 24.Sorensen JL, Haug NA, Delucchi KL, et al. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: A randomized trial. Drug Alcohol Depend 2007;88:54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimmel SE, Troxel AB, Loewenstein G, et al. Randomized trial of lottery-based incentives to improve warfarin adherence. Am Heart J 2012;164:268–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volpp KG, Loewenstein G, Troxel AB, et al. A test of financial incentives to improve warfarin adherence. BMC Health Serv Res 2008;8:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galárraga O, Genberg BL, Martin RA, Laws MB, Wilson IB. Conditional economic incentives to improve HIV treatment adherence: Literature review and theoretical considerations. AIDS Behav 2013;17:2283–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettifor A, MacPhail C, Nguyen N, Rosenberg M. Can money prevent the spread of HIV? A review of cash payments for HIV prevention. AIDS Behav 2012;16:1729–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006;101:192–203 [DOI] [PubMed] [Google Scholar]
- 30.Thornton RL. The demand for, and impact of, learning HIV status. Am Econ Rev 2008;98:1829–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsan M, Beshears J, Armstrong WS, et al. A commitment contract to achieve virologic suppression in poorly adherent patients with HIV/AIDS. AIDS 2017;31:1765–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]