Abstract
Aim: In this study, we tested the hypothesis that lipid peroxide-derived dicarboxylic acids (DCAs), by virtue of their ability to bind to calcium (Ca), might be involved in atherosclerotic calcification. We determined the ability of azelaic acid (AzA) to promote calcification in human aortic smooth muscle cells (HASMCs), identified AzA in human calcified atherosclerotic lesions, and compared its levels with control and noncalcified atherosclerotic lesions.
Results: HASMCs efficiently converted 9-oxononanoic acid (ONA), a lipid peroxide-derived monocarboxylic aldehyde, to AzA. In vitro incubations of AzA micelles with HASMC resulted in the formation of Ca deposits, which contained AzA. Liquid chromatography-mass spectrometry analysis of human control uninvolved artery, noncalcified, and calcified lesions showed significant increase of AzA in calcified lesions compared with noncalcified and control tissues. Calcified mouse atherosclerotic lesions also showed substantial presence of AzA in Ca complexes.
Innovation: This study identifies a DCA, AzA, as an integral part of the Ca complex. The study also demonstrates the conversion of a lipid peroxidation product, ONA, as a potential source of AzA, and establishes the presence of AzA in calcified materials isolated from human and mouse lesions.
Conclusion: The presence of AzA as a Ca sequestering agent in atherosclerotic lesions (i) might indicate participation of oxidized low-density lipoprotein (Ox-LDL) derived products in calcification, (ii) explain the potential correlation between calcification and overall plaque burden (as Ox-LDL has been suggested to be involved in atherogenesis), (iii) could contribute to plaque stabilization via its anti-inflammatory actions, and (iv) might explain why antioxidants failed to affect atherosclerosis in clinical studies. Antioxid. Redox Signal. 29, 471–483.
Keywords: : atherosclerosis, calcification, dicarboxylic acids, lipid peroxides, azelaic acid
Introduction
It is currently believed that atherosclerosis progresses as early fatty streak lesions progress to raised complex lesions, involving smooth muscle cell proliferation, which may eventually become calcified (14, 48, 52). Calcification is a major independent predictor of cardiovascular morbidity and mortality (11, 36). Smooth muscle cells express bone differentiation markers such as alkaline phosphatase, which is suggested to provide phosphate for the formation of calcium phosphate (12). Accordingly, it is widely believed that calcium (Ca) in lesions might be present as calcium phosphate. Calcification is mainly identified by staining techniques such as von Kossa or Alizarin Red S staining, or by physical imaging techniques such as computerized tomography (CT) scan (8, 24, 35, 42). Although these techniques detect mineralized Ca, they do not indicate the chemical form in which Ca is deposited.
Innovation.
Atherosclerosis has been suggested to involve the oxidation of low-density lipoprotein-associated polyunsaturated fatty acids. Many aldehydes are formed as a result of decomposition of lipid peroxides. However, their propensity to undergo further oxidation to carboxylic acid has been vastly ignored. This is the first study to demonstrate the ability of azelaic acid (AzA) to bind calcium and suggest that calcium azealate could contribute to atherosclerotic calcification. The presence of AzA in lesions might suggest why antioxidant trials might have failed to prevent atherosclerosis, as they would also inhibit the conversion of toxic aldehydes to carboxylic acids.
During the past three decades, the concept of oxidized low-density lipoprotein (Ox-LDL) has emerged as a key player in the development of atherosclerosis. Overwhelming in vitro and in vivo evidence supports the hypothesis (5, 38, 55). The failure of human antioxidant trials, however, raised concerns about the validity of the hypothesis, at least in humans. Numerous review articles have appeared on the topic, including the suggestion that oxidative stress might be beneficial under certain circumstances (29, 39, 40, 54).
The breakdown of peroxidized lipids into aldehydes, both free and core aldehydes, has been well documented (27, 32, 59). Linoleic acid is the major polyunsaturated fatty acid (PUFA) of LDL, and the oxidation of linoleic acid generates toxic and reactive aldehydes (e.g., 4-hydroxy nonenal [4-HNE] and 9-oxononanoic acid [ONA]), which have been shown to be highly proinflammatory (16, 22, 25, 43). However, aldehydes are also extremely oxidation labile and are readily converted into carboxylic acids by a variety of enzymatic and nonenzymatic processes (20, 37). The Ox-LDL particle is also associated with the increased presence of Lyso phosphatidylcholine (Lyso-PtdCho) that could carry aldehydes and their oxidation products into cells (41, 44).
Although 4-HNE has been well studied, aldehydes generated from the carboxylic acid end of fatty acid oxidation products, for example, ONA, have not been studied as extensively. Its oxidation product, azelaic acid (AzA), is a poorly studied dicarboxylic acid (DCA). We recently reported that animals fed AzA had decreased atherosclerotic lesions as compared with control atherosclerotic mice (29).
Calcium phosphate as the sole calcified material does not explain the association of calcification with lipid-rich domains nor the latter's intra- and extracellular presence or its correlation with overall plaque burden (11). In this study, we considered the possibility that AzA or other DCAs, derived from lipid peroxide-derived aldehydes, by virtue of their ability to bind Ca might explain the relationship of calcification with the etiology (Ox-LDL) of atherosclerosis and the association with lipid accumulation. As a medium chain DCA, it would also bind Ca with higher affinity than monocarboxylic acids (13, 18), and sequester Ca in lipid-rich domains. In addition, AzA has been noted to have anti-inflammatory properties and has been noted to decrease cytokine production by macrophages and inflammatory cells (3, 21). It also has been demonstrated to reduce oxidant production by leukocytes (15). Thus, AzA as a Ca binding lipid would explain the association of Ca with the lipid-rich plaque and the association of calcification with products derived from Ox-LDL, and would account for the correlation of calcification with the overall plaque burden. This study is the first to suggest a direct link between DCA products derived from oxidation of aldehydes and calcification.
Using in vitro cultured human aortic smooth muscle cells (HASMCs), we demonstrate the ability of ONA to get oxidized to AzA, and the ability of the latter to promote calcification in smooth muscle cells. We also unequivocally demonstrate the increased presence of AzA in calcified domains of atherosclerotic lesions by liquid chromatography-mass spectrometry (LC-MS) analysis, using both in vitro and in vivo (human and mice) samples.
Results
Decomposition of lipid peroxides by HASMCs
Lipid peroxides and their aldehyde breakdown products play major roles in the progression of atherosclerosis. To demonstrate an active role of HASMCs in the decomposition of lipid peroxides into aldehydes and to oxidize the latter into carboxylic acid, HASMCs were incubated with 100 μM of 13-hydroperoxyoctadecadienoic acid (HPODE), and increased AzA levels were observed by LC-MS analysis (Fig. 1A). HPODE (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars) degradation was also monitored by the loss of leukomethylene blue (LMB) reactivity and the decrease in optical density at 234 nm (loss of conjugated dienes). As seen in the figure, the peroxides were lost in <2 h in the presence of cells as compared with no cell incubations.
FIG. 1.
HASMC induced decomposition of HPODE and conversion of ONA to AzA is inhibited by α-tocopherol. HASMCs were grown to confluence and incubated with (A) HPODE and (B) ONA in the presence and absence of α-tocopherol for 6 h. After treatment, AzA was quantified by LC-MS analysis. *p < 0.05; **p < 0.01. AzA, azelaic acid; HASMC, human aortic smooth muscle cell; HPODE, hydroperoxyoctadecadienoic acid; LC-MS, liquid chromatography-mass spectrometry; ONA, 9-oxononanoic acid.
In addition, 14C-HPODE was prepared and used further to confirm the HPODE decomposition in the presence of HASMCs for 6 h in Hank's balanced salt solution (HBSS). Autoradiography data indicated that HPODE was converted to AzA in the medium in the presence of cells as compared with a no cell control, respectively (Supplementary Fig. S2A).
Conversion of ONA to AzA in the presence of α-tocopherol and HASMC
Although AzA is present in many food materials in unknown quantities, in the body, it is likely to be generated from fatty acids. Oxidized fatty acids decompose to generate a number of different short chain (e.g., malondialdehyde and hexanal) to medium chain aldehydes (e.g., 4-HNE and ONA). Oxidation of these aldehydes, enzymatically or nonenzymatically, is expected to yield carboxylic acids. The oxidation of ONA is expected to yield AzA. To evaluate whether ONA is converted to AzA by cells, ONA was prepared and incubated with HASMCs as described in the Materials and Methods section, both in the presence and absence of α-tocopherol. As shown in Figure 1B, LC-MS analysis showed a significant decrease in AzA at 6 h of incubation with HASMCs in the presence of α-tocopherol as compared with the absence of α-tocopherol, suggesting that antioxidants inhibit the conversion of ONA to AzA.
In addition, radioactive 14C-ONA was also synthesized and used in these experiments. Autoradiography data showed a significant increase in AzA as shown in the Supplementary Figure S2B in the absence of α-tocopherol, whereas there was no or less change in ONA concentration as compared with control cell incubations at all time intervals.
Loading of HASMC with AzA results in calcification
To assess Ca accumulation upon AzA supplementation, HASMCs were treated with mixed micelles of Lyso-PtdCho/AzA as indicated in the Materials and Methods section. At the concentrations used, little or no cytotoxicity was observed, as determined by microscopy and lactate dehydrogenase release. Percentage of cell viability was calculated using control untreated cells as 100% viability. As shown in Supplementary Figure S3, Lyso-PtdCho/AzA mixed micelles at the concentrations used have no obvious toxic effects on HASMCs as compared with Lyso-PtdCho alone. After incubation and washings, the cells were stained with von Kossa stain, which stains Ca deposits by a dark brown/black silver residue. As shown in Figure 2, there was minimal staining of control cells. Minimal staining was seen with Lyso-Ptdcho micelles alone. However, cells treated with the mixed micelles stained significantly darker, which suggests robust calcification. β-Glycerophosphate was used as a positive control. LC-MS analysis also confirmed the increased presence of AzA bound to Ca in HASMCs treated with Lyso-PtdCho/AzA mixed micelles, as shown in Figure 2F.
FIG. 2.
Calcification of HASMC induced by AzA micelle treatment. Cells were grown to confluence and incubated for 9 days under the following conditions: (A) untreated; (B) Na-AzA at 100 μmol/L; (C) Lyso-PtdCho micelles at 50 μmol/L; (D) Lyso-PtdCho/AzA micelles at 50 μmol/L/100 μmol/L; (E) 10 mmol/L of β-glycerophosphate. Cells were stained for Ca deposits using von Kossa staining (Ca deposits appear as dark brown spots). Image magnification 40 × . (F) AzA quantification in nmol of Ca deposit associated AzA. **p < 0.01. Ca, calcium; Na-AzA, sodium azelate; PtdCho, phosphatidylcholine.
Detection of AzA in tissues
Calcification involves generation of insoluble complexes. To identify whether the mineralized Ca could contain AzA as a Ca complex, we first removed the non-Ca-bound lipids by chloroform–methanol extraction, the soluble organic and aqueous phases were removed. This ensures that any non-Ca complexed AzA has been removed. The remaining insoluble material that contained protein and mineralized Ca complexes was subjected to acid hydrolysis with 10% acetic acid in methanol. The acid hydrolyzed material is expected to have Ca in the form of calcium chloride, and free AzA (no longer complexed with Ca). This was used to determine Ca and AzA concentrations. The tissue was processed as described in the Materials and Methods section.
Simply stated, this method first involved the removal of the non-Ca complex lipids. After these nonmineralized lipids were removed, the calcified material was subjected to acid hydrolysis, which hydrolyzed the complex forming calcium chloride and free AzA. This free AzA was then extracted and quantified via LC-MS. This ensures that any AzA analyzed was bound with Ca, as any free non-Ca complexed AzA would have been removed in the first lipid extraction.
Identification of calcification in aged apolipoprotein E-paraoxonase 1 double knockout mice
Apolipoprotein E (ApoE)-paraoxonase 1 (PON1) double knockout (DKO) mice of >2 years of age on a normal diet were used, as this DKO mouse model has been shown to have increased atherosclerotic lesions (49). Comparatively, aged wild type mice and younger DKO mice (4 months) were used as controls. A significant increase in body weight, liver weight, and plasma lipids was observed in the aged DKO mice as compared with controls (Supplementary Fig. S4). These mice also displayed extensive lesions upon dissection of the aortic arch, as indicated by the regions of white, opaque areas of the vessel (Fig. 3A). After confirming the presence of calcification by CT scan (Fig. 3B), histological examination of the aortic arch was performed and also displayed extensive calcification as determined by von Kossa (Fig. 3C) and Alizarin Red staining (Fig. 3D). Furthermore, extensive calcification of the aortic arch and aortic root was confirmed by X-ray imaging (Fig. 3E, F). Figure 3G–I represents the phosphate, Ca, and AzA levels from pooled samples (n = 9) in calcified and noncalcified lesions of mouse aortic samples. The results showed that AzA was found in significant amounts in calcified tissues as compared with the noncalcified region. The lesion area of all the aortas as compared with control animals is represented in Supplementary Figure S5.
FIG. 3.
Calcification in aged ApoE-PON1 double knockout mice. (A) Photograph of dissected aortic loop. (B) microCT scan of dissected aortic loop. (C) Ca stain of aortic loop cross sections (image magnification 10 × ) stained with (B) von Kossa stain and (D) Alizarin Red S stain. (E) Images of dissected mouse aorta. (F) X-ray scan image of dissected mouse aorta. (G) Ca quantification of mouse lesion calcified domains. (H) Phosphate quantification of mouse lesion calcified domains. (I) Fold increase in LC-MS signal for AzA in mouse calcified domains. Mean ± SEM, *p ≤ 0.05, ***p ≤ 0.001 (t-test). ApoE, apolipoprotein E; CT, computerized tomography; PON1, paraoxonase 1; SEM, standard error of the mean.
Identification of calcification in human atherosclerotic aortic plaques
To identify the presence of AzA in calcified human atherosclerotic plaques, we first needed to determine the presence of calcification in these human aortic samples by X-ray imaging (Fig. 4). Tissues were dissected to obtain nonlesion normal tissue, noncalcified atherosclerotic tissue, and calcified atherosclerotic lesions. Calcified domains were dissected from the noncalcified atherosclerotic plaques and reimaged to verify dissection (Fig. 4A). Control tissue was also analyzed to confirm that no calcification was present in the nondiseased tissue. The nondiseased and diseased pairs of aortic lesion tissue were acquired from the same individual; pathological reports of the tissues analyzed are shown in Supplementary Table S1. The tissues were used for the analysis of Ca, phosphate, and AzA levels. Figure 4B–D represents the Ca, phosphate, and AzA levels in normal tissue, calcified lesions, and noncalcified lesions of human aortic samples. Quantification of Ca, phosphate, and AzA of individual samples is represented in Supplementary Table S2. MicroCT X-ray analysis of samples is also represented in Figure 5. As seen in the figure, presence of Ca was increased in calcified lesions as compared with other two controls. This confirmed that the calcified lesions did indeed contain calcified deposits, whereas the normal and noncalcified tissues were not calcified.
FIG. 4.
Calcification in human aortic tissue samples (A) X-ray images showing calcified regions as dark regions. (B) Ca quantification of Ca deposit associated Ca. (C) Phosphate quantification in nmol of Ca deposit associated phosphate. (D) AzA quantification in nmol of Ca deposit associated AzA. Mean ± SEM, n = 9, **p ≤ 0.01, ***p ≤ 0.001 (one way analysis of variance Bonferroni multiple comparison test). ns, nonsignificant.
FIG. 5.
Human aortic tissue microCT images. (A, B) Representative cross sections of microCT images for human aortic tissue samples. (C) Densitometry of calcified domains. Mean ± SEM, *p ≤ 0.05, (t-test).
AzA quantification in tissue samples
Human and mouse tissues were analyzed in a validated LC-MS method for quantitative analysis of AzA using AzA-D14 as an internal standard (IS). The detected AzA presented in Supplementary Table S2 shows quantified AzA in nmol. The representative LC-electrospray ionization (ESI)-HRMS extracted ion chromatograms—AzA ([M–H]− 187.0976) and AzA-D14 (IS, ([M–H]− 201.1849)), ESI-MS, and MS/MS and proposed fragment ions are shown in Figure 6.
FIG. 6.
The representative LC-ESI-HRMS extracted ion chromatograms—AzA ([M–H]− 187.0976) and AzA-D14 (IS, ([M–H]− 201.1849)) (A), ESI-MS and MS/MS spectra (B) and proposed fragment ions (C). ESI, electrospray ionization; IS, internal standard; LC, liquid chromatography; MS, mass spectrometry.
Increased presence of Ca and AzA in calcified human atherosclerotic lesions
Ca was identified in the control, noncalcified, and calcified lesions. As seen in Figure 4, there was a several fold and significant increase in Ca in the calcified lesions, confirming the dissection and analytical techniques employed. The presence of AzA was established by LC-MS and we detected a significant increase in AzA concentrations in the calcified tissue as compared with the other two controls (Fig. 4). Although our focus was on AzA, oxidation of PUFA is expected to generate a plethora of DCAs, including a 12-carbon acid. We indeed observed several other minor mass ions in the isolated Ca complex, but have not established their chemical identities.
The amount of AzA, even if other DCAs (and even mono carboxylic acids) were present, did not fully match the levels of Ca. Combined with the unknown stoichiometry of the carboxylic acids with Ca, we kept in mind the possibility of the presence of free phosphates in the analysis. As shown in Figure 4, although phosphates were present in significantly higher amounts in the Ca complex than azelate, the results of our study suggest that in addition to calcium phosphates, calcium azelate also contributes to atherosclerotic calcification.
Discussion
In this study, we present a new paradigm that atherosclerotic calcification may also involve Ca binding DCA products derived from Ox-LDL.
Ca deposits are found more frequently and in greater amounts in elderly patients with atherosclerosis with more advanced lesions (7, 56). The extent of calcification has been correlated with plaque burden, suggesting that factors that contribute to atherosclerosis development may have a direct relationship with calcification.
The role of Ox-LDL and the effects of lipid peroxides and their aldehyde breakdown products on atherosclerosis have been studied extensively (46, 51). Aldehydes are chemically unstable intermediates and are readily oxidized to carboxylic acids, In addition to spontaneous oxidation, a variety of enzymes and processes are capable of converting aldehydes into carboxylic acids. In some cases (i.e., xanthine/aldehyde oxidases), the process itself generates free radicals (17, 28). We recently reported the oxidation of aldehydes by the myeloperoxidase/HOCl system (45). Of particular interest to us is aldehyde oxidase 1, which has been reported as an ATP binding cassette transporter-A1-interacting protein (50). Most carboxylic acids derived from the oxidation of aldehydes formed via the decomposition of lipid peroxides are simple, short chain molecules from the ω-end. These are very rapidly metabolized by simple β-oxidation pathways and are rarely used as an anabolic intermediate. The DCA could be from the middle of the molecule (e.g., malondialdehyde) or mostly from the carboxyl end (e.g., AzA). Obviously some type of esterase/phospholipase A2 is needed to release the carboxyl moiety.
In this study, we demonstrate that AzA may play a role in calcification. AzA is used extensively as an anti-inflammatory and antioxidant agent (19), but has not been implicated in atherosclerotic calcification. AzA has the ability to bind Ca forming a complex, and our in vitro studies have shown its ability to induce calcification of HASMC upon intracellular micelle delivery. To understand its involvement in vivo, we extracted calcified material from atherosclerotic plaques and were able to identify AzA upon acid hydrolysis of the insoluble calcified deposits. The LC-MS identification of AzA, both in mice and human lesions, in isolated mineralized Ca deposits, conclusively demonstrates that AzA was an integral part of the Ca deposits. Free AzA would have been removed by extraction with the organic solvent during lipid extraction, and any soluble Ca salts would have been partitioned into the aqueous phase. Thus, the presence of both Ca and AzA in the insoluble material and their release only upon acid hydrolysis suggest that they are present together. This is the first study to detect medium chain DCA in the Ca deposits of human atherosclerotic lesions.
Ox-LDL is intimately involved in atherosclerotic progression, as it is internalized by macrophages during the formation of early atherosclerotic fatty streak lesions. Ox-LDL also plays a role in subsequent development of advanced lesions by promoting smooth muscle cell (SMC) migration and affecting plaque vulnerability. Thus, the presence of oxidized lipids, and products derived from oxidized lipids, would be expected to correlate with the overall plaque burden. As this study implicates DCAs, including AzA in calcification, it would be no surprise that the extent of calcification is a representation of the overall plaque burden. Moreover, oxidized medium chain DCAs such as AzA are lipophilic, derived from lipids, and thus are expected to be present in the lipid-rich domain of the lesion, where they may bind to Ca and form insoluble complexes.
There has been a great deal of debate about the role of calcification in plaque rupture (1, 23, 31, 34). Calcification has been noted as both intra- and extracellular deposits (53), the latter predominantly is associated with extracellular lipids. Many researchers believe that coronary arterial calcification may represent an attempt to protect the weakened atherosclerotic plaque prone to rupture (1, 57). Calcified lesions and fibrotic lesions are much stiffer than cellular lesions and are unlikely to be associated with sites of plaque rupture (1, 57). In fact, a recent study showed that <15% of the ruptured human lesions showed evidence of associated calcification (58). It is speculated that the plaque rupture often occurs at the interface between a calcified and noncalcified atherosclerotic area of the lesion. Thus, calcification could be seen as a potential stabilizing force that may increase the biochemical stability of the plaque by imparting rigidity while at the same time decreasing the plaque's mechanical stability. Although the presence of calcium phosphate is not ruled out in this study, the finding that AzA, an anti-inflammatory molecule, is associated with Ca could suggest that it imparts other potential effects to the calcified domain besides affecting the stability of calcified plaques.
Although the current studies provide support to the hypothesis that AzA might contribute to aortic calcification, additional proof is needed to suggest that inhibition of AzA synthesis might lead to reduction of aortic calcification. Current limitations are as follows. (i) Laboratory mice require a longer period (>18 months) to develop calcification. At this point, technology does not exist to demonstrate the presence of AzA in vivo or to detect/quantify calcification in vivo in live mice. (ii) Although the current studies demonstrate that vitamin E or α-tocopherol could inhibit conversion of aldehydes to carboxylic acids, it is also known that α-tocopherol inhibits atherosclerosis in animal studies, which might lead to inconclusive evidence as both atherosclerosis and lipid peroxidation will be inhibited. (iii) Dosage of antioxidants or any specific inhibitors needed to inhibit this ONA to AzA conversion in vivo cannot be speculated at this point. Such studies might be of interest, but currently it is beyond the scope of this study. (iv) There are numerous enzymatic pathways (xanthine oxidase, various isoforms of aldehyde dehydrogenases, and aldehyde oxidases) that could oxidize ONA to AzA. It is nearly impossible to find common inhibitors that could be administered for a long time to prevent AzA formation in vivo.
Yet another limitation of this study is that the potential role of AzA in promoting calcification could only be deduced from data on current calcified deposits. In contrast, this study demonstrates the presence of AzA in association with mineralized Ca deposits. Whether the presence of AzA promotes calcification could only be surmised from its presence in the calcified regions of tissue samples; however, to establish the specific role of AzA in calcification is beyond the scope of this study as it requires longitudinal human studies.
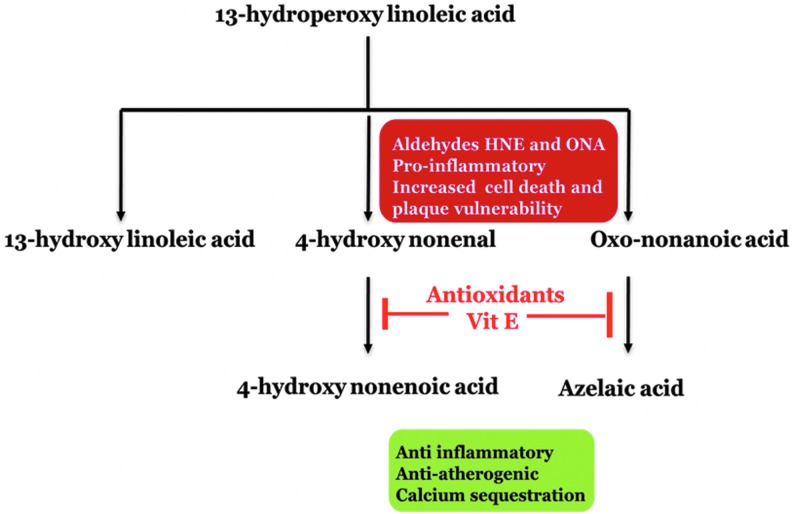
Our findings are of great interest and could explain the failure of antioxidant trials in treating human atherosclerosis. Antioxidants inhibit a variety of oxidative processes, including many enzymatic and nonenzymatic oxidative pathways. We have noted the inhibition of conversion of ONA to AzA in the presence of antioxidants (Fig. 1B). Thus, the formation of DCA from ONA may represent a protective mechanism to thwart plaque vulnerability. Furthermore, antioxidants under such circumstances might result in the accumulation of toxic and proinflammatory aldehydes and promote plaque vulnerability (Fig. 7). Combined with our findings (29) that AzA prevented atherosclerosis without affecting lipid levels and decreased CD68 positive macrophages in the lesions, this study suggests additional benefits in promoting the oxidation of toxic aldehydes to innocuous carboxylic acids.
FIG. 7.
Schematic representation of the hypothesis.
Materials and Methods
Materials and reagents
Reagents and chemicals were purchased from Sigma (St. Louis, MO). Declassified human atherosclerotic tissue samples were purchased from Collaborative human tissue network (CHTN) from Ohio State University. All studies were performed following approval provided by the Institutional Review Board (IRB) of the University of Central Florida.
Cell culture
HASMCs (purchased from ATCC) were cultured in Dulbecco's modified Eagle's medium/F12 supplemented with 10% fetal bovine serum.
Preparation of HPODE
13-HPODE(200 μmol/L) was prepared as described previously (47). Lipid peroxide generated in the reaction system was analyzed by LMB assay (4), and the amount of peroxide generated was quantitated. The oxidized fatty acid was extracted with butylated hydroxytoluene-free ether, dried under the stream of nitrogen, and stored at −20°C.
Similarly 200 μmol/L radioactive 14C-HPODE 1000 DPM/nmol was prepared and used for the experiments.
Synthesis of ONA
ONA was prepared from oleic acid via erythro-9,10- dihydroxysteric acid (26, 30). In brief, oleic acid was oxidized to dihydroxystearic acid using potassium permanganate and was oxidized further by sodium periodate to 9-ONA. After the reaction was completed, centrifugation was performed at 400 g for 5 min at 4°C to obtain clear supernatant. Supernatant was dried under the stream of nitrogen and purified by thin layer chromatography (TLC) using a solvent system of chloroform, tetrahydrofuran, and acetic acid (30/3.3/0.16) and confirmed by 2,4-dinitrophenylhydrazine staining. Quality of the ONA was further confirmed by LC-MS analysis. For cell culture studies, ONA was suspended in chloroform, dried under the stream of nitrogen, and reconstituted with ethanol, further diluted with phosphate-buffered saline (PBS) and used as needed. Similarly radioactive ONA (1000 dpm/nmol) was synthesized from 14C-oleic acid and used for the experiments. Standard AzA on TLC was confirmed by bromocresol green staining.
Treatment of cells with HPODE/ONA
HASMC were seeded at an initial density of 2 × 104. After attaining the confluency, cells were starved in serum-free medium for 3 h prior the treatments. Cells were treated with 100 nmol HPODE and 100 nmol of ONA for 4 h in HBSS in the presence and absence of 50 μmol/L α-tocopherol. After 4 h of incubation, intra- and extracellular levels of decomposition products were analyzed by LC-MS.
LC-MS analysis for in vitro cell culture experiments
Samples were analyzed by LC-MS as described in “Materials and Methods” section. Samples were extracted from cell lysate by 1 mL of methanol, pipetted well up and down about five times. The sample was centrifuged at 12000 rpm for 15 min and supernatant was collected and 5 μL of sample was injected for LC-MS analysis.
TLC and autoradiography 14C-HPODE/14C-ONA
After treatment of cells with HPODE and ONA in the presence and absence of α-tocopherol, the cell culture medium and cellular lipid extracts were dissolved in 20 μL of chloroform and loaded on Silica Gel G TLC plate (Sigma) with standards. Chloroform:tetrahydrofuran:acetic acid (90:10:0.5) was used as solvent system. After the separation, the TLC plate was left in the chemical hood for 10 min to let solvents evaporate. The dried TLC plate was covered with saran wrap and exposed to storage phosphor screen (Perkin Elmer; MultiSensitive Storage Screen) for up to 48 h. Autoradiography was performed by Cyclone Plus (Perkin Elmer Storage Phosphor System).
Micelle preparation
AzA (final concentration 100 μmol/L) was mixed with Lyso-PtdCho (final concentration 50 μmol/L), vortexed vigorously, and dried under a nitrogen stream. The dried mixture was resuspended in 10 mL of aqueous media, generating Lyso-PtdCho/AzA micelles, which were used for cell treatment every alternate day. A total of 10 mmol/L β-glycerophosphate was used as a positive control.
WST-1 cell viability assay
The effect of the Lyso-PtdCho and mixed micelles on cell viability was determined by WST-1 assay (Roche Applied Science, IN). HASMC cells (2.5 × 103 cells/100 μL) were seeded in a 96-well plate and incubated overnight at 37°C in the presence of 5% CO2. The medium was replaced with fresh medium containing 50 μmol/L Lyso-PtdCho, 50 μmol/L/100 μmol/L Lyso-PtdCho/AzA micelles. After incubation, 10 μL of WST-1 reagent (which is processed to formazan by cellular enzymes) was added to each well in 100 μL of cell culture medium. Cells were further incubated for 4 h at 37°C. Cell viability was determined by measuring absorbance at 450 nm (630 nm was used as reference wavelength) using a 96-well plate reader (Benchmark Plus Microplate Spectrophotometer System; Bio-Rad, Hercules, CA).
Induction of calcification in HASMCs by AzA
HASMCs were grown in standard growth medium supplemented with either Lyso-PtdCho (50 μmol/L) alone or with 50 μmol/L/100 μmol/L Lyso-PtdCho/AzA micelles, or with sodium azelate (100 μmol/L) for 9 days. As a positive control for calcification, HASMCs were treated with 10 mmol/L β-glycerophosphate. Calcification was detected by staining with Alizarin Red S and von Kossa staining.
Alizarin Red S staining
Tissue sections were washed with PBS twice, followed by two washes with deionized water, then stained with freshly prepared and filtered 2% Alizarin Red S (pH 4.2) reagent for 1 min with gentle agitation. Alizarin Red S reagent was removed followed by two washes with deionized water and PBS, then imaged using the light microscope with the Leica DFC295 camera (Leica Camera AG, Solms, Germany). Ca deposits were seen by their red color.
Von Kossa staining
After micelle treatment, cells were washed four times with PBS and fixed in 10% formalin for 30 min on ice. Formalin was aspirated and cells were washed three times with PBS, followed by four washes with deionized water, then incubated with 1% silver nitrate for 4 h (2 h under an ultraviolet light followed by 2 h under a bright fluorescent light). After staining, silver nitrate solution was aspirated and the cells were washed with deionized water once. To remove background and nonspecific deposits, cells were incubated with 5% sodium thiosulfate solution for 2 min, followed by washing twice with deionized water. Slides were mounted and imaged using the light microscope Leica DFC295 camera (Leica Camera AG, Solms, Germany). Ca deposits are observed as dark brown to black spots. Tissue sections were similarly stained.
Animals
ApoE-PON1 DKO mice were developed using PON1(−/−) and ApoE(−/−) mice of C57BL/6J background from Jackson Laboratories (Bar Harbor, ME). DKOs were developed by crossing ApoE(−/−) mice with PON1(−/−) mice for 12 generations, as described by Shih et al. (49). The mice were genotyped by polymerase chain reaction analysis of tail tip DNA, isolated according to the manufacturer's instructions using the Dneasy Blood and tissue DNA kit (Qiagen, Germantown, MD), followed by agarose gel electrophoresis. Specific primer sequences from Jackson Laboratory were used for synthesis and analysis of knockouts.
Twenty >2 years old mice weighing 30–35 g and 10 younger 4 months old mice weighing 18–22 g male ApoE-PON1 DKO mice were used. Ten 7-month-old C57BL6/J male mice were purchased and maintained. The animals were regularly monitored and a weekly record of body weight was maintained until used for experiments. All procedures were performed according to protocol approved by The Institutional Animal Care and Use Committee.
Collection of plasma and organs
After 2 years of age, mice were fasted overnight and anesthetized with 1–2% isoflurane. Fasting blood samples were collected into ethylenediaminetetraacetic acid tubes by heart puncture. Plasma was separated as described previously (40) and stored at −80°C.
Isolation and quantification of mice aortic lesions
Isolation of the aorta and quantification of aortic lesions were performed as described previously (6, 9, 10). Lesion areas were marked on photographs. The lesion area was quantified using ImageJ software (2). Calcified lesions in aortas were analyzed by X-ray using the Xtreme imaging system. For histological analysis, aortic roots were frozen immediately after imaging, cryosectioned, fixed, and used for Alizarin Red S staining and von Kossa staining to determine the presence of Ca, as mentioned previously. All stained sections were observed under light microscope using Leica DFC295 camera (Leica Camera AG).
X-ray analysis
Human and mouse aortic tissue samples were separated into calcified and noncalcified domains using the Bruker in vivo Xtreme imaging system (Billerica, MA) and analyzed using the Bruker Molecular Imaging Software. X-ray analysis was conducted using an exposure time of 1.20 s.
Isolation calcified and noncalcified regions of the human aortic tissues
After IRB approval, control and human calcified tissue samples were collected from CHTN. A total of 9–11 pairs of samples were used for this study. The same tissue provided uninvolved control, noncalcified, and calcified samples. Age, gender, pathology, and disease severities of subjects are mentioned in Supplementary Table S1. Calcified tissues were imaged by using the Xtreme imaging machine. Noncalcified and calcified tissues were separated and confirmed by X-ray analysis.
MicroCT analysis
To study the differences in the calcified and noncalcified human atherosclerotic plaques, μCT analysis was performed by using the Quantum FX μCT scanner (PerkinElmer, Waltham, MA) with the following settings: isotropic voxel size of 10 μm, 70 kV, 160 mA, 5 mm field of view, 3 min scan (FINE setting) per sample. Raw μCT images were processed using Quantum FX μCT software (PerkinElmer). For the analysis of calcified tissue, the volume enclosing the entire 6-cm aorta segment was selected. Images were segmented into calcified and noncalcified tissue on each volume of interest using a global threshold method. After calibration and identification of calcified tissue, the amount of Ca in the sample was quantified using the Quantum FX μCT Software.
Aortic tissue sample preparation for LC-MS analysis
Tissue (300 mg for human and 50 mg for mice) was weighed and lyophilized to complete dryness. Dried tissue samples of human (50 mg) and mice (10 mg) were taken for analysis. The sample extraction procedure involved two steps. First, lipids and material not associated with Ca domain were removed as follows: tissue samples were homogenized and extracted sequentially, with 6 mL of chloroform and then 6 mL of methanol. Samples were vortexed well and centrifuged at 2500 rpm for 20 min. The supernatants were removed. Second, for extraction of organic acids associated with Ca, the precipitation was incubated in methanol (6 mL × 2) containing 10% acetic acid (HAc) at 45°C for 45 min, vortexed well, centrifuged at 2500 rpm, and the supernatants were collected. Two extracts were combined and evaporated to dryness under the gentle stream of nitrogen. The residues were dissolved in 500 μL of methanol containing 0.1% formic acid. The samples were centrifuged at 12,000 rpm for 20 min and filtered through 0.22 μm polyvinyl difluoride syringe filters (Fisher Scientific; # 0992728A) for LC-MS/MS analysis. For Ca and phosphate detection, samples were incubated in water containing 10% HAc at 50°C for 1 h, after the removal of lipids and materials that are not associated with Ca.
LC-MS analysis
Samples were analyzed on Agilent 1200 series high-performance liquid chromatography (HPLC; Agilent Technologies, CA, USA). The HPLC system consisted of G1379B degasser, G1311A quaternary pump, HTC PAL autosampler, and G1316A column compartment coupled to 6520B hybrid quadrupole time-of-flight mass spectrometer (QTOF-MS) equipped with dual ESI source. Analytes were separated on PerkinElmer Brownlee SPP C18 (100 × 2.1 mm, L × ID, 2.7 μm PS; #N9308404) UHPLC column protected with C18 (5 × 2.1 mm, L × ID, 2.7 μm PS; # N9308513) guard column. Binary mobile phase gradient program was used to separate the molecules, pump-A: methanol and pump-B: water, both containing 0.1% (v/v) formic acid. The linear gradient program was as follows: 0–8 min, 90% B; 8–25 min, 10% B; 25–38 min, 10% B; 38–38 min, 90% B; 38.1–45 min 90% B. The column was operated at 45°C with a constant mobile phase flow rate of 0.3 mL/min.
Data were acquired in ESI negative mode for identification of organic acids associated with calcified domains. Mass spectral data were collected in the mass range of 20–1700 m/z, in auto MS/MS mode to obtain MS and MS/MS data simultaneously. The mass spectrometer was tuned at 4 GHz high-resolution mode at low 1700 m/z range with a manufacturer ESI-TOF calibration solution (Agilent Technologies; #G1969-85000). To ensure good mass accuracy, TOF reference mass solution (Agilent Technologies; #G1969-85001) was continuously introduced via the second nebulizer throughout the analysis. ESI source parameters were optimized and operated under the following conditions: capillary voltage, 3.5 kV; source temperature, 320°C; nitrogen (N2) used as a drying and nebulizer gas, 12 L/min and 50 psig, respectively.TOF parameters: fragmentor and skimmer voltages, 100 and 65 V, respectively. N2 used as a collision-induced dissociation gas for MS/MS studies, optimized collision energy set at 22 V. The MS data were collected and processed using MassHunter qualitative analysis version B.07.00 software.
The presence of AzA was confirmed by its high-resolution mass detected as deprotonated molecular ion at m/z 187.0987 eluted at 16.95 (±0.2). This was also confirmed further by analyzing authentic reference standard of AzA and MS/MS fragmentation spectral match with the reference standard.
Quantification of AzA
The developed LC-MS method was validated according to the FDA guidelines (Bioanalytical method validation, guidance for Industry) for quantitative analysis of AzA in three types of tissue samples (normal, diseased noncalcified, and calcified tissues). Limit of detection (LOD) and limit of quantifications (LOQs) were calculated in terms of signal-to-noise ratio (LOD, S/N ≥3 and LOQ, S/N ≥10) and were found to be 50 and 75 pmol, respectively. The linearity of AzA is examined in matrix spiked and in neat solvent spiked samples at the concentration range of 75 − 5000 pmol, using stable labeled isotope AzA-D14 as an IS at a constant concentration of 250 pmol. The calibration curves were graphed between the area under the curve ratio of AzA/AzA-D14 against the concentration of AzA. The matrix spiked and neat solvent calibration curves of AzA are shown to be linear, with regression coefficients (R2) being >0.995. Quality control samples were analyzed in triplicates (n = 3) at three different concentrations 75, 150, and 300 pmol to evaluate precision of the method in terms relative standard deviations (%), observed to be <13%.
Matrix effect of tissue samples
Matrix effect (ME, %) of the method was determined by comparing response ratio of analyte and IS (AzA/AzA-D14, area under curve) in standard solution against the matrix spiked standards (33). ME was calculated at three different concentration levels, 75 (LOQ), 150, and 300 pmol, in triplicates (n = 3), and found to be 10.13%, 8.63%, and 5.6%, respectively.
Extraction recovery of AzA from tissue samples
Extraction recovery (ER, %) of AzA was calculated by pre-extraction and postextraction spikes of calcium-azelate (Ca-AzA). Blank matrix ensured that AzA was absent in tissue extract/diluted matrix for matrix spiking experiments. Pre-extraction spike: Ca-AzA was added to the tissue samples and stored at −80°C overnight, thawed the next day, homogenized, and extracted as described in the sample preparation portion of the “Materials and Methods” section. Postextraction spike: Ca-AzA was added to the acidified methanol tissue extract and incubated at 45°C for 30 min. ER was carried out at three different concentrations, 75 (LOQ), 150, and 300 pmol in triplicates (n = 3) and found to be 71.7%, 76.7%, and 81.2%, respectively.
Ca and phosphate quantification
Samples were prepared as described in the Materials and Methods section: Ca and phosphate were quantified using Abcam (Cambridge, MA) colorimetric assay kits, Ca colorimetric assay (ab102505), and phosphate colorimetric assay (ab65622) following the instructions of supplier's protocol.
Statistical analysis
Values are presented as a mean ± standard deviation, and statistical analyses were performed by using student's t-test, with p < 0.05 as the level of significance. For multiple group comparisons, one way analysis of variance Bonferroni multiple comparison test was used with p < 0.05 as the level of significance.
Supplementary Material
Abbreviations Used
- 4-HNE
4-hydroxy nonenal
- ApoE
apolipoprotein E
- AzA
azelaic acid
- Ca
calcium
- CHTN
Collaborative human tissue network
- CT
computerized tomography
- DCA
dicarboxylic acid
- DKO
double knockout
- ER
extraction recovery
- ESI
electrospray ionization
- HAc
acetic acid
- HASMC
human aortic smooth muscle cell
- HBSS
Hank's balanced salt solution
- HPLC
high-performance liquid chromatography
- HPODE
hydroperoxyoctadecadienoic acid
- IRB
Institutional Review Board
- IS
internal standard
- LC-MS
liquid chromatography-mass spectrometry
- LMB
leukomethylene blue
- LOD
limit of detection
- LOQ
limit of quantification
- ME
matrix effect
- ns
nonsignificant
- ONA
9-oxononanoic acid
- Ox-LDL
oxidized LDL
- PBS
phosphate-buffered saline
- PON1
paraoxonase 1
- PtdCho
phosphatidylcholine
- PUFA
polyunstaturated fatty acid
- SEM
standard error of the mean
- TLC
thin layer chromatography
Acknowledgment
This study was supported by NIH R01HL123283-01 (S.P.) grant on “Role of aldehyde oxidation in atherosclerosis.”
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Abedin M, Tintut Y, and Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 24: 1161–1170, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Abramoff MD, Magelhaes PJ, and Ram SJ. Image processing with ImageJ. Biophotonics Int 11: 36–42, 2004 [Google Scholar]
- 3.Akamatsu H, Komura J, Asada Y, Miyachi Y, and Niwa Y. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch Dermatol Res 283: 162–166, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Auerbach BJ, Kiely JS, and Cornicelli JA. A spectrophotometric microtiter-based assay for the detection of hydroperoxy derivatives of linoleic acid. Anal Biochem 201: 375–380, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Berliner J. Introduction. Lipid oxidation products and atherosclerosis. Vasc Pharmacol 38: 187–191, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Bhaskaran S, Santanam N, Penumetcha M, and Parthasarathy S. Inhibition of atherosclerosis in low-density lipoprotein receptornegative mice by sesame oil. J Med Food 9: 487–490, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Budoff JM. and Gul KM. Expert review on coronary calcium. Vasc Health Risk Manag 4: 315–324, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardús A, Panizo S, Parisi E, Fernandez E, and Valdivielso JM. Differential effects of vitamin D analogs on vascular calcification. J Bone Miner Res 22: 860–866, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Chandrakala AN, Selvarajan K, Burge KY, Litvinov D, Sengupta B, and Parthasarathy S. Water-soluble components of sesame oil reduce inflammation and atherosclerosis. J Med Food 19: 1–9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandrakala AN, Selvarajan K, Litvinov D, and Parthasarathy S. Anti-atherosclerotic and anti-inflammatory actions of sesame oil. J Med Food 18: 11–20, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demer LL. and Tintut Y. Vascular calcification pathobiology of a multifaceted disease. Circulation 104: 1881–1883, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, and Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 21: 1998–2003, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Doyle IR, Ryall RL, and Marshall VR. Inclusion of proteins into calcium oxalate crystals precipitated from human urine: a highly selective phenomenon. Clin Chem 37: 1589–1594, 1991 [PubMed] [Google Scholar]
- 14.Eggen DA, Strong JP, and McGill HC. Coronary calcification: relationship to clinically significant coronary lesions and race, sex and topographic distribution. Circulation 32: 948–955, 1965 [DOI] [PubMed] [Google Scholar]
- 15.Elewski B. and Thiboutot D. A clinical overview of azelaic acid. Cutis 77: 12–16, 2006 [PubMed] [Google Scholar]
- 16.Esterbauer H, Koller E, Slee RG, and Koster JF. Possible involvement of the lipid-peroxidation product 4-hydroxynonenal in the formation of fluorescent chromolipids. Biochem J 239: 405–409, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fruebis J, Parthasarathy S, and Stienberg D. Evidence for a concerted reaction between lipid hydroperoxides and polypeptides. Proc Natl Acad Sci U S A 89: 10588–10592, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godfraind T, Sturbois X, and Verbeke N. Calcium incorporation by smooth muscle microsomes. Biochim Biophys Acta 455: 254–268, 1976 [DOI] [PubMed] [Google Scholar]
- 19.Gooderham M. Rosacea and its topical management. Skin Therapy Lett 14: 1–3, 2009 [PubMed] [Google Scholar]
- 20.Hayden MR, Tyagi SC, Kolb L, Sowers JR, and Khanna R. Vascular ossification—calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis—calcific uremic arteriolopathy: the emerging role of sodium thiosulfate. Cardiovasc Diabetol 4: 4, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iraji F, Sadeghinia A, Shahmoradi Z, Siadat AH, and Jooya A. Efficacy of topical azelaic acid gel in thetreatment of mild-moderate acne vulgaris. Indian J Dermatol Venereol Leprol 73: 94–96, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Jessup W, Jurgens G, Lang J, Esterbauer H, and Dean RT. Interaction of 4-hydroxynonenal-modified low-density lipoproteins with the fibroblast apolipoprotein B/E receptor. Biochem J 234: 245–248, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson RC, Leopold JA, and Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res 99: 1044–1059, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Jono S, Nishizawa Y, Shioi A, and Morii H. 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation 98: 1302–1306, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Jürgens G, Lang J, and Esterbauer H. Modification of human low-density lipoprotein by the lipid peroxidation product 4-hydroxynonenal. Biochim Biophys Acta 875: 103–114, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Kaneko T, Kaji K, and Matsuo M. Cytotoxicities of a linoleic acid hydroperoxide and its related aliphatic aldehydes toward cultured human umbilical vein endothelial cells. Chem Biol Interact 67: 295–304, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Kamido H, Eguchi H, Ikeda H, Imaizumi T, Yamana K, Hartvigsen K, Ravandi A, and Kuksis A. Core aldehydes of alkyl glycerophosphocholines in atheroma induce platelet aggregation and inhibit endothelium-dependent arterial relaxation. J Lipid Res 43: 158–166, 2002 [PubMed] [Google Scholar]
- 28.Li WG, Stoll LL, Rice JB, Xu SP, Miller FJ, Jr., Chatterjee P, Hu L, Oberley LW, Spector AA, and Weintraub NL. Activation of NAD(P)H oxidase by lipid hydroperoxides: mechanism of oxidant-mediated smooth muscle cytotoxicity. Free Radic Biol Med 34: 937–946, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Litvinov D, Selvarajan K, Garelnabi M, Brophy L, and Parthasarathy S. Anti-atherosclerotic actions of azelaic acid, an end product of linoleic acid peroxidation, in mice. Atherosclerosis 209: 449–454, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez MA, Vicente J, Kulasekaran S, Vellosillo T, Martínez M, Irigoyen ML, Cascón T, Bannenberg G, Hamberg M, and Castresana C. Antagonistic role of 9-lipoxygenase-derived oxylipins and ethylene in the control of oxidative stress, lipid peroxidation and plant defence. Plant J 67: 447–458, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Mackey RH, Venkitachalam L, and Sutton-Tyrrell K. Calcifications, arterial stiffness and atherosclerosis. Adv Cardiol 44: 234–244, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Marathe GK, Davies SS, Harrison KA, Silva AR, Murphy RC, Castro-Faria-Neto H, Prescott SM, Zimmerman GA, and McIntyre TM. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. J Biol Chem 274: 28395–28404, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Marchi I, Viette V, Badoud F, Fathi M, Saugy M, Rudaz S, and Veuthey J. Characterization and classification of matrix effects in biological samples analyses. J Chromatogr 1217: 4071–4078, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Mazzini MJ. and Schulze PC. Proatherogenic pathways leading to vascular calcification. Eur J Radiol 57: 384–389, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Mizobuchi M, Finch JL, Martin DR, and Slatopolsky E. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int 72: 709–715, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Nicoll R. and Henein MY. The predictive value of arterial and valvular calcification for mortality and cardiovascular events. IJC Heart Vessels 3: 1–5, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Toole TE, Zheng YT, Hellmann J, Conklin DJ, Barski O, and Bhatnagar A. Acrolein activates matrix metalloproteinases by increasing reactive oxygen species in macrophages. Toxicol Appl Pharmacol 236: 194–201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parthasarathy S. Modified Lipoproteins in the Pathogenesis of Atherosclerosis. Austin, TX: R. G. Landes Co, 1994 [Google Scholar]
- 39.Parthasarathy S, Merchant NK, Penumetcha M, and Santanm N. Oxidation and cardiovascular disease-potential role of oxidants in inducing antioxidant defense enzymes. J Nucl Cardiol 8: 379–389, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Parthasarathy S, Santanam N, Ramachandran S, and Meilhac O. Oxidants and antioxidants in atherogenesis: an appraisal. J Lipid Res 40: 2143–2157, 1999 [PubMed] [Google Scholar]
- 41.Parthasarathy S, Steinbrecher P, Barnett J, Witztum JL, and Steinberg D. Essential role of phospholipase A2 activity in endothelial cell induced modification of low density lipoprotein. Proc Natl Acad Sci U S A 82: 3000–3004, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi YF, Wang SH, Zhang BH, Bu DF, Shu TC, and Du JB. Changes in amount of ADM mRNA and RAMP2 mRNA in calcified vascular smooth muscle cells. Peptides 24: 287–294, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Quehenberger O, Koller E, Jürgens G, and Esterbauer H. Investigation of lipid peroxidation in human low density lipoprotein. Free Radic Res Commun 3: 233–242, 1987 [DOI] [PubMed] [Google Scholar]
- 44.Quinn MT, Parthasarathy S, and Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A 85: 2805–2809, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raghavamenon A, Garelnabi M, Babu S, Aldrich A, Litvinov D, and Parthasarathy S. Alpha-tocopherol is ineffective in preventing the decomposition of preformed lipid peroxides and may promote the accumulation of toxic aldehydes: a potential explanation for the failure of antioxidants to affect human atherosclerosis. Antioxid Redox Signal 11: 1237–1248, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Requena JR, Fu MX, Ahmed MU, Jenkins AJ, Lyons TJ, Baynes JW, and Thorpe SR. Quantification of malondialdehyde and 4- hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochem J 322: 317–325, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rong R, Ramachandran S, Penumetcha M, Khan N, and Parthasarathy S. Dietary oxidized fatty acids may enhance intestinal apolipoprotein A-I production. J Lipid Res 43: 557–564, 2002 [PubMed] [Google Scholar]
- 48.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Shih DM, Xia Y, Wang X, Milleri E, Castellani LW, Subbanagounder G, Cheroutre H, Faull KF, Berliner JA, Witztumi JL, and Lusis AJ. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem 275: 17527–17535, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Sigruener A, Buechler C, Orsó E, Hartmann A, Wild PJ, Terracciano L, Roncalli M, Bornstein SR, and Schmitz G. Human aldehyde oxidase 1 interacts with ATP-binding cassette transporter-1 and modulates its activity in hepatocytes. Horm Metab Res 39: 781–789, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Spiteller G. Linoleic acid peroxidation—the dominant lipid peroxidation process in low density lipoprotein—and its relationship to chronic diseases. Chem Phys Lipids 95: 105–162, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Stary H, Chandler A, Glagov S, Guyton J, Insull W, Jr., Rosenfeld M, Schaffer SA, Schwartz CJ, Wagner WD, and Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb 14: 840–856, 1994 [DOI] [PubMed] [Google Scholar]
- 53.Stary HC. Natural history of calcium deposits in atherosclerosis progression and regression. Z Kardiol 89: 28–35, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Steinberg D. Oxidative modification of LDL and atherogenesis. Circulation 95: 1062–1071, 1997 [DOI] [PubMed] [Google Scholar]
- 55.Steinberg D, Parthasarathy S, Crew TE, Khoo JC, and Witztum JL. Beyond cholesterol: modification of low-density lipoprotein that increase its atherogenecity. N Engl J Med 320: 915–924, 1989 [DOI] [PubMed] [Google Scholar]
- 56.Tamar SP, McClelland RL, Jorgensen NW, Bild D, Burke GL, Guerci AD. and Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction: the multi-ethnic study of atherosclerosis. JAMA 303: 1610–1616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, and Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A 103: 14678–14683, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Virmani R, Burke AP, Farb A, and Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol 47: c13–c18, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Young SG. and Parthasarathy S. Why are low-density lipoproteins atherogenic? West J Med 160: 153–164, 1994 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.