Abstract
Background
Understanding the relative risks of maintenance treatment versus discontinuation of antipsychotics following remission in first episode psychosis (FEP) is an important area of practice.
Method
A systematic review and meta-analysis. Prospective experimental studies including a parallel control group were identified to compare maintenance antipsychotic treatment with total discontinuation or medication discontinuation strategies following remission in FEP.
Results
Seven studies were included. Relapse rates were higher in the discontinuation group (53%; 95% CIs: 39%, 68%; N = 290) compared with maintenance treatment group (19%; 95% CIs: 0.05%, 37%; N = 230). In subgroup analyses, risk difference of relapse was lower in studies with a longer follow-up period, a targeted discontinuation strategy, a higher relapse threshold, a larger sample size, and samples with patients excluded for drug or alcohol dependency. Insufficient studies included psychosocial functioning outcomes for a meta-analysis.
Conclusions
There is a higher risk of relapse for those who undergo total or targeted discontinuation strategies compared with maintenance antipsychotics in FEP samples. The effect size is moderate and the risk difference is lower in trials of targeted discontinuation strategies.
Declaration of interest
A.T. has received honoraria and support from Janssen-Cilag and Otsuka Pharmaceuticals for meetings and has been has been an investigator on unrestricted investigator-initiated trials funded by AstraZeneca and Janssen-Cilag. He has also previously held a Pfizer Neurosciences Research Grant. S.M. has received sponsorship from Otsuka and Lundbeck to attend an academic congress and owns shares in GlaxoSmithKline and AstraZeneca. J.H. has attended meetings supported by Sunovion Pharmaceuticals.
Antipsychotic medication in early psychosis
Since early intervention services became established for psychosis in the 1990s, there has been particular research interest in the treatment of first episode psychosis (FEP). Studies indicate that when antipsychotic medication is part of the treatment approach, 85–90% of FEP patients will experience symptom remission and 50% of patients will achieve functional recovery by 1 year.1 Nevertheless, longitudinal studies demonstrate that relapse following remission in FEP is common.2 Relapse has considerable negative consequences including functional disability and financial burden.3 Additionally, it may lead to biological alterations, increasing the risk of future treatment refractoriness.4,5 The majority of current guidelines that consider FEP recommend continuing antipsychotic medication for a period of time following remission in FEP to prevent relapse, e.g. the Australian Clinical Guidelines for Early Psychosis states that antipsychotic medication may be continued for 12 months or more and the National Institute for Health and Care Excellence guideline, Psychosis and Schizophrenia in Adults: Treatment and Management, states ‘Inform the service user that there is a high risk of relapse if they stop medication in the next 1–2 years’.6,7 The studies on which these guidelines are based often pre-date early intervention teams (who deliver a wide range of psychosocial interventions post-remission, including specific relapse prevention programmes) and commonly compared strict placebo regimes with maintenance. Recent trials have considered alternative discontinuation strategies, such as graded reductions and intermittent treatment strategies if breakthrough symptoms occur, which may show different outcomes.8,9 These trials may represent more common practice than the trials where the discontinuation arm receives only placebo and essentially stops medication. In fact, it is now considered ethically challenging not have a protocol that includes ‘rescue’ medication in a contemporary discontinuation trial.8 One recent discontinuation trial has suggested a worse long-term functional outcome in those who continue medication as opposed to a graded reduction group.8 However, these trials are clearly different from the previous placebo-controlled trials and as such have inherent biases, especially if clinicians are not blinded to study group. Possibly influenced by these new trials and their paradoxical findings, as well as other concerns such as long-term metabolic side effects of antipsychotics and long-term use being associated with the potential loss of grey and white matter;10 some clinicians' views on medication discontinuation following relapse during FEP appear to diverge from current guidelines.11 It is also clear that patients commonly stop or wish to stop medication following remission, and clinicians are commonly asked explicitly about relapse risk.11,12
Current literature
To date, there are two systematic reviews relating to FEP patients that specifically examine discontinuation versus maintenance medication trials. Zipursky et al13 compared the outcomes of non-affective FEP patients who remained on medication following remission with those who discontinued their medication. Six studies were identified, four of which were randomised controlled trials. Symptom recurrence rates (not the more commonly reported relapse rates) were particularly high in those who discontinued medication, with a 1-year weighted mean recurrence rate of 77% in discontinuation groups v. 3% in the maintenance groups.13 In a subgroup analysis of their Cochrane review on discontinuation versus maintenance studies in patients with schizophrenia, Leucht et al14,15 reported a smaller difference with regard to relapse (not recurrence) in first episode schizophrenia patients. They reported that relapse rates at 7–12 months were lower for patients continuing medication compared with those receiving placebo (maintenance 26% v. placebo 61%; risk ratio 0.47; 95% CIs 0.38–0.58), similar to the rate for multiple episode patients (27 v. 65%). De Hert et al (2015)16 reported that relapse rates were consistently higher in first episode patients who had received either placebo or intermittent treatment versus placebo in their study of schizophrenia patients, but do not specifically quote overall rates or a rate difference. Similarly, a recent review by Karson et al (2016)17 includes all FEP patients but contains both randomised and non-randomised studies and does not perform a meta-analysis of relapse rates.
However, the results from these reviews, which clearly favour medication continuation, have not resolved the debate among researchers and clinicians regarding discontinuation approaches.11,18,19 This may be because there are unanswered questions from these reviews. First, it is not clear whether relapse rates following discontinuation are similar across the broader FEP population (which include both affective and non-affective psychosis). This is important because the majority of early intervention services do not only treat patients who have a schizophrenia spectrum diagnosis.20 Second, extant reviews only include studies comparing maintenance medication to complete medication discontinuation. In most clinical services, patients more commonly reduce their medication slowly over many months (because abrupt withdrawal may potentially precipitate relapse21) or opt for strategies that include rescue medication or intermittent treatment if possible prodromal symptoms of relapse are identified. For example, the recent discontinuation trial of Wunderink et al,8,22 which used such an approach, and the trials of Gaebel et al,9,23 which compare different discontinuation strategies, are not included in these reviews. Third, the possible influence of additional psychosocial interventions (which are widespread in early intervention services) on the prevention of relapse has not been explored in these reviews. Finally, the differential effects of maintenance versus discontinuation medication on other important outcomes such as quality of life and vocational or social functioning, which have been questioned in recent discontinuation trials,8 have not been explored.
Aims of the study
With these issues in mind, the aims of the current systematic review and meta-analysis were: (a) to compare relapse (and hospital admission) rates, using the risk difference, between FEP patients who continued antipsychotic medication with those who had a specified medication discontinuation strategy; (b) to test whether study factors likely to affect relapse rates, such as relapse threshold and type of discontinuation strategy, explain any heterogeneity in risk difference estimates; and (c) to examine differences in psychosocial outcomes between groups receiving medication continuation versus discontinuation strategies.
Method
We used Cochrane Collaboration systematic review methodology to inform the methods of the review24 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines25 to inform the reporting (PRISMA checklist included as Supplementary Table 3 available at https://doi.org/10.1192/bjo.2018.17).
Databases and search strategy
MEDLINE, Embase, PsycINFO and all Cochrane library bibliographic databases were searched from inception to 13 October 2016. We used MeSH terms and the ‘explode’ facility in the bibliographic databases as well as free text keywords. In addition, we searched the references of all included studies and relevant reviews. We contacted experts in the area to enquire about unpublished/grey studies.
The MeSH terms used were: antipsychotic agents, psychotic disorders, schizophrenia, age of onset, time factors and recurrence. The keywords in the search were: [early OR recent OR first OR index OR primary] AND [psychotic OR psychos* OR schizophren* OR schizoaffective OR schizo affective] AND [antipsychotic* OR anti-psychotic* OR neuroleptic*] AND [discontinu* OR cessation OR withdrawal OR stopping OR maintenance] AND [remission OR recurrence].
Eligibility criteria
The following list details the inclusion criteria used for study selection:
Study design: randomised controlled trials.
Comparison groups: maintenance (continued) treatment with antipsychotic medications versus a discontinuation intervention. This could include strategies to discontinue antipsychotic medication completely or an intermittent treatment strategy approach (treating breakthrough or prodromal symptoms of relapse), so long as the majority of the sample discontinued their medication. We included those with and without an active or additional psychosocial component to the discontinuation, e.g. a relapse prevention strategy.
Participants: at least 75% of patients were defined as having a first episode of a psychotic disorder, or first episode of schizophrenia or being within 3 years of the first episode of a psychotic disorder.
A clear definition and duration of remission of psychotic symptoms. The participants were found to meet this definition before participation in the study.
Psychotic relapse rates reported with a minimum follow-up period of 6 months.
Studies were excluded if:
they were non-English language papers (where no translation was available);
the studies sampled people with organic disorders;
they did not include a parallel control group;
less than 50% of the patients in the discontinuation group were able to fully discontinue medication.
Abstract screening for eligibility
One author (A.R.) screened all titles and abstracts to identify potentially relevant articles for full-text retrieval. Two authors (A.R. and L.V.) independently assessed whether these full-text articles met the inclusion/exclusion criteria. A third researcher (A.T.) resolved any disagreements by consensus.
Data extraction
Data extraction was conducted independently by two researchers (A.R. and L.V.). Disagreements were resolved through discussion with a third researcher (A.T.). We recorded the following parameters for each study: setting, demographics, inclusion and exclusion criteria of participants, criteria used to define remission and relapse, number of participants in the maintenance and discontinuation group, the type and exact nature of discontinuation strategy, information about any other active treatment components, raw dichotomous and/or Kaplan–Meier rates of psychosis relapse (and admission to hospital) at follow-up time points and study end, functioning ratings at follow-up time points and study end, and medication adherence ratings.
Quality assessment
Three authors (A.R., S.A.S. and J.H.) conducted a quality assessment based on the Cochrane Collaboration risk of bias tool.24 Each entry was assigned a judgement of ‘low risk,’ ‘high risk’ or ‘unclear risk’. Disagreements between raters were resolved by consensus by a fourth researcher (A.T.). We summed risk of bias scores (i.e. low is 0, unclear or high is 1) for each bias judgement (e.g. allocation concealment) to calculate a total risk of bias score (out of 9) for each study. We categorised studies into low (score of 0–6 = 0) v. high (score ≥7 = 1) risk of bias for inclusion in the sub-analyses and meta-regression.26
Outcome parameters for meta-analysis
Our primary outcome was relapse, variously described in the studies (see Table 1). Most studies reported raw relapse rates, some studies reported relapse rates derived from survival curves and some studies reported both. Kaplan–Meier estimates may derive slightly higher relapse rates than raw figures30,32 as Kaplan–Meier calculations exclude censored data (i.e. those who have left the trial). In contrast, raw figures are based on the proportion of all participants entering the trial.33 Nevertheless, previous meta-analyses have combined the raw figures and those based on Kaplan–Meier estimates32 and this was the approach taken in this study. However, as a sensitivity analysis, we examined whether relapse rate calculation (i.e. raw proportion versus Kaplan–Meier proportion) had an impact on pooled risk difference. Our secondary outcome was hospital admission.
Table 1.
Randomised controlled studies comparing medication discontinuation with maintenance treatment for the prevention of relapse in first episode schizophrenia
| Study (location) |
Number of participants |
Diagnosis | Study design |
Remission duration |
Intervention group |
Control group |
Relapse definition | Follow-up duration after discontinuation |
Relapse rate |
Hospital admission rate |
|---|---|---|---|---|---|---|---|---|---|---|
| Kane et al, 198227 (USA) | 28; MT = 11 PL = 17 |
S (RDC), unspecified psychosis, other psychiatric disorder, manic disorder with schizotypal features | RCT (double-blind) | At least 4 weeks stable remission | Placebo | Fluphenazine hydrochloride (5–20 mg/day), fluphenazine decanoate i.m. (12.5–50 mg every 2 weeks) | Substantial clinical deterioration with a potential for marked social impairment | 1 year | 41 v. 0% | |
| Crow et al, 198628 (UK) | 120; MT = 54 PL = 66 |
Schizophrenic illness (PSE) | RCT (double-blind) | 30 days discharge status | Placebo | Flupenthixol i.m. (40 mg/month), chlorpromazine (200 mg), haloperidol (3 mg), pimozide (4 mg), trifluoperazine (5 mg) | Readmission to psychiatric care for any reason, readmission considered necessary by the clinicians responsible but for some reason not possible (e.g. lack of beds, refusal of patient), active antipsychotic medication considered by the clinician to have become essential because of features of imminent relapse | 2 years | 62 v. 46% (raw score) | |
| McCreadie et al, 198929 (UK) | 15; MT = 8 PL = 7 |
S (clinical) | RCT (double-blind) | 1 year relapse free on treatment | Placebo | Pimozide or flupenthixol i.m. | Hospital admission | 1 year | 57 v. 0% | |
| Gaebel et al, 200223 (Germany) | 115; MT = 36 PI = 39 CI = 40; patients who could be discontinued: PI = 31 CI = 32 |
S (ICD-9 (1978) and RDC) | RCT (open) | Stable clinical condition for at least 3 months | Targeted discontinuation:
|
Standard treatment (at least 100 mg chlorpromazine equivalents per day) | Psychotic deterioration of maximum intensity usually demanding hospital admission and minimum change score on the BPRS psychotic factor ≥10, GAS ≤ 20 and CGI ≥ 6 | 2 years | MT: 38% PI: 42% CI: 67% |
31 v. 38% |
| Chen et al, 201030 (Hong Kong) | 178; MT = 89 PL = 89 |
S, SCP, SCA, brief psychotic disorder NOS (DSM-IV, 1994) | RCT (double-blind) | 1 year relapse free on treatment | Placebo | 400 mg quetiapine | Reappearance of definite psychotic symptoms (beyond thresholds on PANNS subscales 3–5) and CGI severity ≥3 | 1 year | 63 v. 30% (raw score) | 16 v. 6% |
| Boonstra et al, 201131 (Netherlands) | 20; MT = 9 DS = 11 |
S, SCP, SCA (SCID-IV) | RCT (open) | 1 year in remission | Medication discontinuation | Medication continuation for a minimum of 6 months | ≥4 any PANSS core item and 20% increase in total PANSS or admission to hospital | 2 years | 91 v. 45% (Kaplan–Meier) | 36 v. 12% |
| Gaebel et al, 20119 (Germany) | 44; MT = 23 DS = 21 |
S, SCP (clinical ICD-10, 1992) | RCT (open) | 1 year relapse free on treatment | Targeted discontinuation: intermittent treatment | Risperidone or haloperidol continuation (2–8 mg) | PANSS positive change >10, CGI change ≥6 and GAF decrease >20 | 1 year | 19 v. 0% | 19 v. 0% |
MT, maintenance treatment; PL, placebo; S, schizophrenia; RDC, research diagnostic criteria; RCT, randomised controlled trial; PSE, present state examination; i.m., intramuscular; PI, prodrome based intervention; CI, crisis intervention; BPRS, brief psychiatric rating scale; GAS, global assessment scale; CGI, clinical global impression; SCP, schizophreniform psychosis; SCA, schizoaffective psychosis; NOS, not otherwise specified; DS, discontinuation strategy; PANSS, positive and negative syndrome scale; SCID-IV, Structured Clinical Interview for DSM-IV.
Narrative synthesis
We narratively combined findings on psychosocial outcomes (i.e. quality of life/subjective well-being, social functioning and employment) because results presented in the studies were not suitable for quantitative synthesis (i.e. the data could not be combined or there were insufficient studies in respective domains of interest).
Statistical analyses
Meta-analysis
Analyses were conducted in Stata version 13. We combined outcome data from all eligible studies, using the risk difference and s.e. We selected the risk difference as the most appropriate effect size34 because some studies lacked observed events in the control arm of the trial.27,29 The risk difference and s.e. were calculated using equations described in Leucht et al.32 Pooled risk differences for relapse (and hospital admission) were computed across studies using the metan command. A random effects model was chosen a priori as we were using real world data, which is likely to have variable population parameters.35 Heterogeneity among studies was assessed with the I2 statistic (reported with a P-value). We also calculated pooled relapse rates using the metaprop command to give an indication of the actual relapse rates in both groups. Although metaprop results will vary slightly from those produced by the metan command (due to extreme outcome proportions in some of the included studies), the Freeman–Tukey double arcsine transformation ensures that all CIs are at admissible rates and that all studies are retained in the analysis.36 This means that that the two analyses may not produce identical risk difference outcomes to relapse rate differences.
Significant heterogeneity in risk difference estimates across studies was addressed with subgroup and meta-regression analysis. We examined study-level characteristics (or factors), which we identified a priori as having the potential to influence relapse rates. These included: (a) specific trial discontinuation strategy (i.e. total medication discontinuation [e.g. placebo] versus graded discontinuation or intermittent treatment strategies), (b) follow-up period (i.e. ≤1 year v. >1 year, as a proxy for short-term and longer-term studies), (c) relapse threshold (defined as moderate versus high threshold, using the number of measurements of relapse criteria used in the studies as previously defined by37), and (d) documented psychosocial interventions (such as input from an early intervention team) in the treatment-as-usual group. We also considered aspects of study design; these were: (a) sample size (n < 40 v. n ≥ 40; a proxy for small and larger studies), (b) trial type (open versus blinded), (c) low versus high risk of bias, and (d) exclusion of patients with drug or alcohol dependency.
First, we used subgroup analysis to calculate individual pooled risk differences for each group according to study factor (e.g. a pooled risk difference for small and large studies, respectively). We present these subgroup risk differences graphically. The subgroup analysis provides two statistical results. For example, when examining the effect of trial type, we can ascertain whether the risk difference of relapse due to maintenance versus discontinuation medication is significant in group 1 (e.g. studies using open trials) and group 2 (e.g. studies with blinded trials), respectively. Second, we extended the subgroup analysis by using meta-regression to statistically test whether each study factor was significantly related to variations in risk difference across studies (i.e. whether study characteristic significantly influenced the magnitude of the risk difference).
Results
Included studies
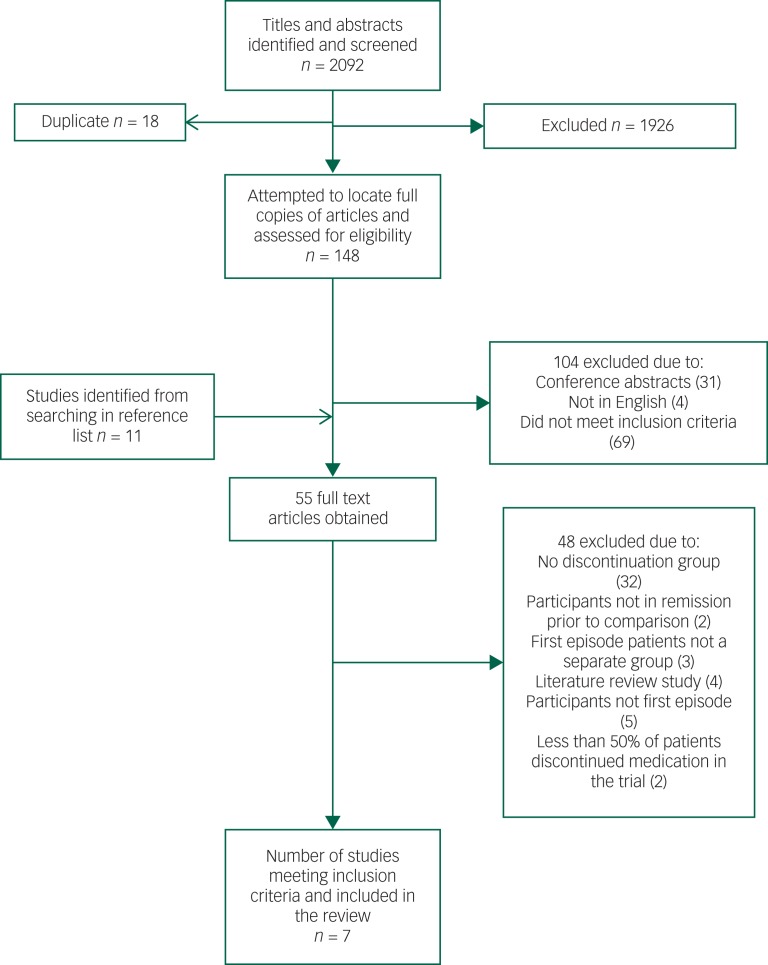
We identified seven studies for inclusion in the review, which in total included 520 participants. See Fig. 1 for the PRISMA flowchart outlining the search and selection strategy. Table 1 outlines the main characteristics of the studies included in the meta-analysis. Studies had been published between 1982 and 2013. One study was conducted in the Netherlands,31 two in Germany,9,23 two in the UK,28,29 one in the USA27 and one in Hong Kong.30 Four studies had a 1-year follow-up23,27,29,30 and three of the studies had a 2-year follow-up period.9,28,31
Fig. 1.
PRISMA flowchart outlining the search and selection strategy.
All seven studies had randomised designs: five studies compared maintenance with complete discontinuation of medication,27,28,29,30,31 and two compared maintenance with intermittent, targeted treatment and intermittent or rescue treatment if prodromal symptoms occurred during the reduction.9,23 Of the five complete discontinuation studies, four were double-blind studies with placebo medication in the intervention group.27–30 The other study was an open randomised trial with gradual withdrawal (6–12 weeks) of antipsychotics.31
Intermittent or targeted treatment approaches varied slightly across studies. Gaebel et al23 compared prodrome- and crisis-based intermittent treatment approaches with maintenance medication. Both intermittent interventions used a step-by-step discontinuation (50% every 2 weeks) of antipsychotic treatment following clinical stabilisation. In the prodrome-based approach, treatment was reintroduced if prodromal symptoms occurred. In the crisis-based approach, treatment was reintroduced if a ‘crisis’ (i.e. full relapse) occurred. As we could only use data from the same patient once in each meta-analyses,38 we elected to combine data from the prodrome-based intervention. In a separate study,9 the same research group used a similar prodrome-based targeted intermittent treatment in which the antipsychotic treatment was completely removed over a period of 3 months. Drug treatment was restarted if there were prodromal symptoms or early warning signs of an impending relapse.
A planned subgroup analysis of relapse rates by the psychosocial treatment offered in the treatment-as-usual group was not possible due to insufficient information. The reporting of the additional (psychosocial and other pharmacological) treatments in the trials was variable and it was not clear if any of the trials had specific non-pharmacological relapse prevention strategies.
Excluded studies
The studies of Wunderink et al8,22 were not included in the final review because they met an exclusion criteria (‘less than 50% of the patients in the discontinuation group were able to fully discontinue medication’) and were thus deemed to be closer to dose reduction studies than medication discontinuation studies. Given the importance of these two studies (due to the long-term follow-up), we performed a supplementary meta-analysis incorporating their relapse results (Supplementary Figure 1 and Table 2). The overall results with regard to relapse rates remain similar in this additional analysis.
Quality assessment
Using the Cochrane guidelines to assess risk of bias, none of the studies showed a low risk of bias in all seven domains (see Supplementary Table 1 for risk of bias allocations for each study). The mean risk of bias score for the studies was 6 out of 9.
Meta-analysis results
Relapse rates in maintenance medication versus discontinuation strategy
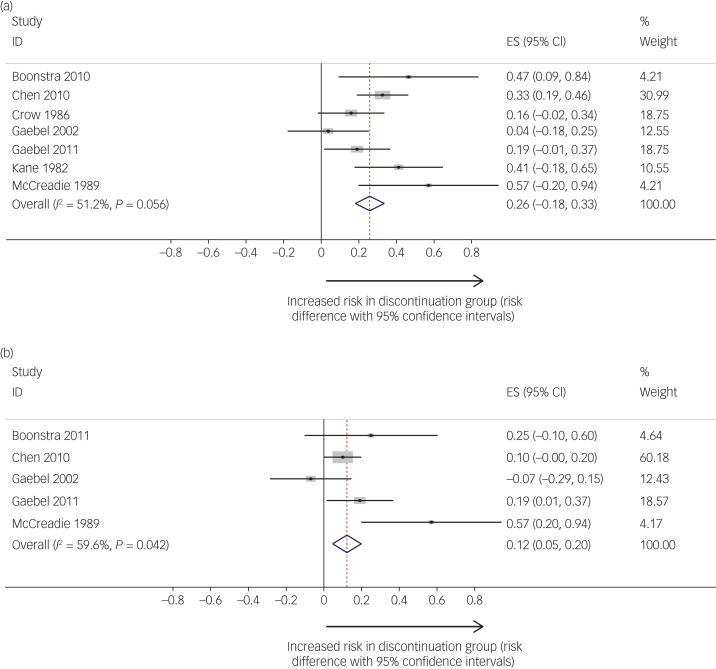
Pooled relapse rates did not vary as a function of calculation method (Kaplan–Meier versus raw figures), thus we included both types of estimate in our analyses. Seven studies compared relapse rates between maintenance and discontinuation groups.9,23,27–31 There was a significant pooled risk difference in relapse rates between maintenance and discontinuation groups (Fig. 2a), with higher relapse rates in the discontinuation groups. The pooled risk difference was 0.26 (95% CIs = 0.18, 0.34; I2 = 51.2%; P = 0.056). The pooled relapse rate for the discontinuation groups was 53% (95% CIs = 39%, 68%) and 19% (95% CIs = 0.05%, 37%) for the maintenance groups.
Fig. 2.
(a) Studies reporting risk difference (comparing maintenance to discontinuation groups) for relapse. (b) Studies reporting risk difference (comparing maintenance to discontinuation groups) for hospital admission. Relapse studies included in analysis: Boonstra et al (2011),31 Chen et al (2010),30 Crow et al (1986),28 Gaebel et al (2002, 2011),23,9 Kane et al (1982)27 and McCreadie et al (1989).29 Hospitalisation studies included in analysis: Boonstra et al (2011),31 Chen et al (2010),30 Gaebel et al (2002, 2011)23,9 and McCreadie et al (1989).35 ES, Effect size
Rates of hospital admission in maintenance medication versus discontinuation strategy
Five studies examined hospital admission as an outcome.9,23,27,29–31 There were significantly higher rates of readmission in the medication discontinuation group compared with the maintenance group (Fig. 2b); the risk difference was 0.12 (95% CIs = 0.05, 0.20; P = 0.002; I2 = 59.6%; P = 0.042). The pooled hospital admission rate for the discontinuation groups was 22% (95% CIs = 12%, 34%) and 11% (95% CIs = 0%, 32%) for the maintenance groups.
Subgroup analysis of relapse rates according to study characteristics
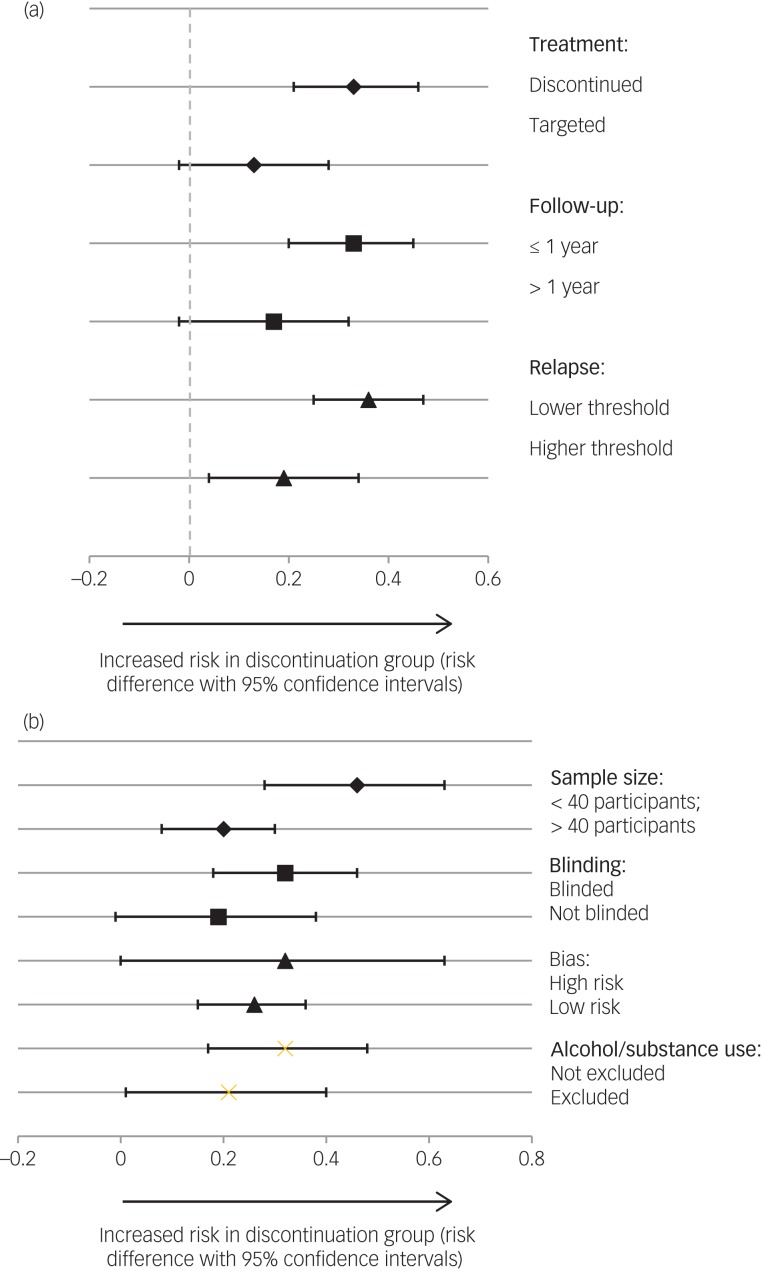
The pooled risk difference was higher with total discontinuation, e.g. placebo, compared with targeted discontinuation/intermittent treatment groups. For total discontinuation the risk difference was 0.33 (95% CIs = 0.21, 0.46; P < 0.001) and the risk difference for targeted discontinuation/intermittent treatment was 0.13 (95% CIs = −0.02, 0.28; P = 0.09) (see Fig. 3a). The pooled risk difference for studies with a longer follow-up was lower than for studies with a shorter follow-up (longer follow-up risk difference of 0.17; 95% CIs = −0.02, 0.36; P = 0.08; shorter follow-up risk difference of 0.33; 95% CIs = 0.20, 0.45; P < 0.001). The pooled risk difference was higher for studies with lower relapse thresholds as defined by Gleeson et al35 (risk difference of 0.36; 95% CIs = 0.25, 0.47; P < 0.001) than those with higher thresholds (risk difference of 0.19; 95% CIs = 0.04, 0.34; P = 0.015).
Fig. 3.
(a) Subgroup analysis of risk difference of relapse rates according to study characteristics (treatment strategy, follow-up period and relapse threshold). Discontinuation studies: Boonstra et al (2011) ,31 Chen et al (2010),30 Crow et al (1986),28 Kane et al (1982)27 and McCreadie et al (1989).29 Targeted discontinuation studies: Gaebel et al (2002, 2011).23,9 Follow-up ≤1 year studies: Chen et al (2010),30 Gaebel et al (2002),23 Kane et al (1982)27 and McCreadie et al (1989).29 Follow-up >1 year studies: Boonstra et al (2011),31 Crow et al (1986)28 and Gaebel et al (2002).23 Relapse studies with lower threshold: Boonstra et al (2011),3,1 Chen et al (2010)30 and Kane et al (1982).27 Relapse studies with higher threshold: Crow et al (1986),28 Gaebel et al (2002, 2011)23,9 and McCreadie et al (1989).29 (b) Subgroup analysis of risk difference of relapse rates according to study characteristics (sample size, blinding and risk of bias). Studies with sample size of <40 participants: Boonstra et al (2011),3,1Kane et al (1982)27 and McCreadie et al (1989).29 Studies with sample size of >40 participants: Chen et al (2010),30 Crow et al (1986)28 and Gaebel et al (2002, 2011).23,9 Blinded studies: Chen et al (2010),30 Crow et al (1986),28 Kane et al (1982)27 and McCreadie et al (1989).29 Open studies: Boonstra et al (2011)31 and Gaebel et al (2002, 2011).23,9 High risk of bias: Crow et al (1986),28 Kane et al (1982)27 and McCreadie et al (1989).29 Low risk of bias: Boonstra et al (2011),31 Chen et al (2010)30 and Gaebel et al (2002, 2011).23,9
Subgroup analysis of relapse rates according to study design features
Smaller studies had a higher pooled risk difference (risk difference of 0.46; 95% CIs = 0.28, 0.63; P < 0.001) than larger studies (risk difference of 0.20; 95% CIs = 0.08, 0.31; P = 0.001) (see Fig. 3b). Blinded studies had a higher pooled risk difference (risk difference of 0.32; 95% CIs = 0.18, 0.46; P < 0.001) than open studies (risk difference of 0.19; 95% CIs = −0.01, 0.38; P = 0.06). Pooled risk differences were higher for studies with a higher risk of bias score (higher risk-of-bias risk difference of 0.30; 95% CIs = 0.11, 0.58; P = 0.001; lower risk-of-bias risk difference of 0.25; 95% CIs = 0.09, 0.42; P < 0.002). Studies not excluding patients with drug or alcohol dependency had a higher pooled risk difference (risk difference of 0.32; 95% CIs = 0.17, 0.48; P < 0.001) than those excluding patients with drug or alcohol dependency (risk difference of 0.21; 95% CIs = 0.01, 0.40; P = 0.04).
Meta-regression according to study factors
Independent meta-regression analyses were conducted by entering each study factor individually to test if heterogeneity was significantly predicted by study factor (Table 2). There were no significant effects of study factor, although the effect of sample size in the trials was at a trend level (P = 0.07). Because none of the study factors were significantly associated with effect size at the individual level, multiple meta-regression analyses were not conducted.39
Table 2.
Univariate meta-regression results indicating impact of individual study characteristics on risk difference estimates
| Predictors | B | s.e. (B) | P-value |
|---|---|---|---|
| Intervention: targeted v. total discontinuationa | 0.21 | 0.11 | 0.11 |
| Follow-up period: ≤1 year v. >1 yearb | −0.17 | 0.11 | 0.21 |
| Relapse threshold: low v. highc | −0.18 | 0.09 | 0.10 |
| Trial type: blinded v. opend | −0.14 | 0.13 | 0.31 |
| Sample size: >40 v. ≥40e | 0.26 | 0.11 | 0.07 |
| Risk of bias score <6 v. ≥6f | 0.04 | 0.15 | 0.77 |
| Exclusion of patients with drug or alcohol dependence: yes v. nog | 0.12 | 0.13 | 0.40 |
a. Intervention: 0 = targeted; 1 = total.
b. Follow-up period: ≤1 year = 0; >1 year = 1.
c. Relapse threshold: low = 0; high = 1.
d. Trial type: blinded = 0; open = 1.
e. Sample size: >40 = 0; <40 = 1.
f. Risk of bias score: <7 = 0; ≥7 = 1.
Narrative synthesis
Psychosocial outcomes
Of the seven studies initially included in the review, only two9,30 provided information on psychosocial outcomes. These outcomes included: employment status, social functioning measures and quality of life/subjective well-being.
Employment
One study examined differences in employment outcome as a function of treatment regime. Chen et al30 found little difference between the number of patients who had lost employment at follow-up in the maintenance (27%) and placebo (32%) groups (x2 = 1.52, P = 0.68).
Social functioning
Gaebel et al9 investigated social functioning as a secondary outcome. They found that patients in the maintenance treatment group scored significantly higher than those in the intermittent treatment group on social functioning according to the Global Assessment of Functioning (GAF) after adjusting for social functioning at baseline (maintenance treatment: mean = 79.4 v. intermittent treatment: mean = 62.1; P < 0.001).
Quality of life/subjective well-being
Gaebel et al9 examined quality of life outcomes and found no significant effect of treatment regimen.
Discussion
Summary of results
Of the seven studies included in this review, there was a greater risk of relapse in the discontinuation groups than in the maintenance treatment groups (risk difference of 0.26; relapse risk of 19% in the maintenance group and 53% in the discontinuation group). The heterogeneity between studies was at trend level for significance. The risk difference was lower (0.12) when admission to hospital was the outcome.
Subgroup analysis suggested that the pooled risk difference of relapse between maintenance versus discontinuation studies was lower in studies with: a longer follow-up period, a targeted or intermittent discontinuation strategy as opposed to placebo only in the discontinuation group, a higher relapse threshold, a lower risk of bias and a larger sample size. When the statistical significance of these differences was tested in the meta-regression analysis (Table 2), however, group differences did not reach significance. This may have been partly due to a lack of power.
The narrative review of functional outcomes highlighted few differences in quality of life or functional outcomes between maintenance and discontinuation groups, although there were only two studies with appropriate data and no specific conclusions could be made. It is worth noting that in a recent long-term follow-up study8 (excluded from the current review due to less than 50% of participants fully discontinuing medication) FEP patients in the discontinuation strategy group experienced twice the functional recovery rate of those in the maintenance group. The current review highlights the importance of including these outcomes to further explore this question, especially as recovery in terms of functional recovery is of particular importance to patients.40
Comparison with previous studies
Our pooled relapse rates are lower (19% for maintenance group and 53% for discontinuation group) than the two extant meta-analyses. There are a number of possible reasons for this discrepancy. The review of Leucht et al15 was limited to patients with schizophrenia, included three older studies41–43 where the primary sample was not first episode schizophrenia patients and only those who received full medication discontinuation, e.g. placebo as opposed to including other discontinuation strategies (graded discontinuation and targeted or intermittent strategies). In our analysis, the risk difference for studies that employed discontinuation strategies was lower (risk difference of 0.13). The review of Zipursky et al13 used recurrence as their primary outcome as opposed to relapse in this study. We found in subgroup analyses that the risk difference was lower for studies that set a higher threshold for relapse and for those that had hospital admission as an outcome. They excluded studies with a minimum of 6 months of remission before discontinuation and it thus excluded some of the larger studies (e.g.30) included in our review (and the only study in an early intervention service). Furthermore, a number of studies with particularly high relapse rates included in their review44,45 did not meet our inclusion criteria due to not having a comparison group. The strategy of slowly reducing medication and monitoring for relapse (and providing prodrome-based interventions) is the standard practice in early intervention services worldwide if patients' medications are being discontinued.7 The older studies in the review did not generally have rescue medication protocols. Our results suggest that the relapse risk in these trials might be lower than in trials where placebo is the comparison group, and the risk difference of the two included studies was 0.13. This could be a function of the mechanism of discontinuation (i.e. no potential for rebound psychosis), the fact that people may still be exposed to small doses of antipsychotics if required, the discontinuation may be achieved over a longer timescale and there is access to rescue medication. The De Hert et al16 meta-analysis of patients with schizophrenia suggests that trials with intermittent treatment strategies had three times the odds of relapse compared with continuous treatment. Interestingly, the availability of rescue medication was the only study characteristic explaining systematic differences in the odds ratio for relapse between placebo versus continuous treatment across studies in their review.16 It should be noted that this result in our study should be interpreted with caution due to the low number of studies in this subgroup analysis and that the same research group that included an intermittent or prodrome-based treatment approach performed both studies.
The smaller risk difference in studies with longer follow-up periods also needs further consideration, although the number of participants in the subgroup analysis was small. Whether this is a potential artefact of the quality of the studies (including dropout rates), an influence of potential rebound psychosis after stopping medication or that the benefits of maintenance medication for preventing relapse are indeed more apparent in the short term need more research. As only one study30 was explicitly performed in an early intervention service and had a stated inclusion of FEP patients, it was not possible to examine if the results would be different in an FEP sample that includes other diagnoses with potentially better prognoses, such as brief psychotic disorder compared with a non-affective psychosis sample, or where specific early intervention psychosocial interventions were consistently employed. It is encouraging that studies are currently underway in this specific population (e.g.46,47).
Strengths and limitations
We have conducted a comprehensive review of the literature, using a methodologically robust strategy. However, it is important to highlight the limitations of the review. First, the number of studies in the review was relatively small (seven) with a relatively small number of participants to include in the meta-regression analysis, which will have limited power. This may explain why some of the hypothesised subgroup differences were not significant. A benchmark of ten studies is often suggested for the conduct of a meta-regression,38 however, numerous reviews have been conducted with fewer studies.46 The problems associated with using a smaller number of studies are particularly related to multivariate meta-regression,48 which we did not conduct in this study. There is a striking deficiency in the evidence base given how important the question of medication discontinuation after remission is to clinicians and patients.11 It is also clear that these trials have a number of methodological issues and there were some differences in results when features of trial quality were considered. Second, given the small number of trials reporting data on functional outcomes, we were unable to incorporate these outcomes in our meta-analyses. As these factors are particularly important to patients,40 this is a considerable omission in the literature and future trials should endeavour to incorporate these outcomes. Third, we combined intermittent treatment (or the use of treatment when prodromal symptoms occurred) approaches with trials where a placebo was used. This was done to represent the range of current discontinuation trials and include strategies that are common in clinical practice. Analysis indicated that heterogeneity between the included studies was not significant, although we accept that some may argue these trials are substantially different. We used relapse as our primary outcome as this is most commonly and consistently reported. However, we accept that for new trials in this area it will be ethically difficult not to provide interventions to individuals if there are signs of relapse, and recurrence may become a more accepted outcome (despite the variable significance). Definitions of relapse that are based on hospital admission are in our opinion too stringent and we would suggest studies use standard rating scale thresholds to assess relapse. Alternatively, an objective measure of recurrence that also includes functional impact and/or distress would give a more clinically significant outcome. We were unable to compare specific diagnostic groups, although all but one of the studies recruited patients with first episode schizophrenia. There was only one included study of a broader FEP group30 and three studies specifically excluded patients with drug or alcohol dependency, which may limit the generalisability to standard clinical settings. Finally, few of the studies had reliable measures of concordance for those on maintenance (or discontinuation) therapy, which we know can be variable in patients with psychotic disorders.
Clinical implications
This systematic review has demonstrated a substantial risk difference between those maintained on antipsychotic medication versus those who discontinue their medication following remission of a first episode of a psychotic disorder. However, this risk difference appears a little lower than found in previous studies and meta-analyses13,14 and lower for studies with intermittent treatment strategies. Early intervention services in the UK and worldwide generally practice graded medication discontinuation with ongoing relapse prevention interventions in remitted FEP patients,49 so the results of this review may represent current clinical practice more than previous reviews. It is also striking that the level of quality evidence with which to inform clinicians and advise patients is relatively weak, often does not include important outcomes such as social functioning and quality of life and is mostly short term; this could be part of the reason that controversy has remained in this area.19,49,50 Although there is some research suggesting that certain patients (such as those without a diagnosis of schizophrenia and a short duration of untreated psychosis) may be more likely to remain in remission following the first episode,2,51 it is not consistent and there is limited research into who might be more likely to experience symptom exacerbation following successful antipsychotic discontinuation.52 Well-designed, long-term trials – particularly those targeting potential ‘good prognosis’ patients, detailing the background psychosocial or relapse prevention strategies and those including a wide range of outcomes – would be especially helpful to the field.
Acknowledgements
The research was partially supported by an unrestricted research development grant from Coventry and Warwickshire National Health Service Partnership Trust.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1192/bjo.2018.17.
click here to view supplementary material
References
- 1.Robinson DG, Woerner MG, McMeniman M, Mendelowitz A, Bilder RM. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry 2004; 161: 473–9. [DOI] [PubMed] [Google Scholar]
- 2.Robinson D, Woerner M, Alvir J, Bilder R, Goldman R, Geisler S. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999; 56: 241–7. [DOI] [PubMed] [Google Scholar]
- 3.Kane J. Treatment strategies to prevent relapse and encourage remission. J Clin Psychiatry 2007; 68(suppl 14): 27–30. [PubMed] [Google Scholar]
- 4.Emsley R, Chiliza B, Asmal L, Harvey B. The nature of relapse in schizophrenia. BMC Psychiatry 2013; 13: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieberman JA, Alvir JM, Koreen A, Geisler S, Chakos M, Sheitman BB, et al. Psychobiologic correlates of treatment response in schizophrenia. Neuropsychopharmacol 1996; 14: 13S–21S. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence. Psychosis and Schizophrenia in Adults: Treatment and Management. National Institute for Health and Care Excellence, 2014. [Google Scholar]
- 7.Early Psychosis Guidelines Writing Group and EPPIC National Support Program. Australian Clinical Guidelines for Early Psychosis (2nd edn, update). Orygen, The National Centre of Excellence in Youth Mental Health, 2016. [Google Scholar]
- 8.Wunderink L, Nieboer RM, Wiersma D, Sytema S, Nienhuis FJ. Recovery in remitted first-episode psychosis at 7 years of follow-up of an early dose reduction/discontinuation or maintenance treatment strategy: long-term follow-up of a 2-year randomized clinical trial. JAMA Psychiatry 2013; 70: 913–20. [DOI] [PubMed] [Google Scholar]
- 9.Gaebel W, Riesbeck M, Wolwer W, Klimke A, Eickhoff M, Von W. Relapse prevention in first-episode schizophrenia-maintenance vs intermittent drug treatment with prodrome-based early intervention: results of a randomized controlled trial within the German Research Network on Schizophrenia. J Clin Psychiatry 2011; 72: 205–18. [DOI] [PubMed] [Google Scholar]
- 10.Ho B-C, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 2011; 68: 128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson A, Singh S, Birchwood M. Views of early psychosis clinicians on discontinuation of antipsychotic medication following symptom remission in first episode psychosis. Early Interv Psychiatry 2016; 10: 355–61. [DOI] [PubMed] [Google Scholar]
- 12.Hogman G, Sandamas G. A Question of Choice. National Schizophrenia Fellowship, 2001. [Google Scholar]
- 13.Zipursky RB, Menezes NM, Streiner DL. Risk of symptom recurrence with medication discontinuation in first-episode psychosis: a systematic review. Schizophr Res 2014; 152: 408–14. [DOI] [PubMed] [Google Scholar]
- 14.Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Davis JM. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev 2012; 5: Cd008016. [DOI] [PubMed] [Google Scholar]
- 15.Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Salanti G. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 2012; 379: 2063–71. [DOI] [PubMed] [Google Scholar]
- 16.De Hert M, Sermon J, Geerts P, Vansteelandt K, Peuskens J, Detraux J. The use of continuous treatment versus placebo or intermittent treatment strategies in stabilized patients with schizophrenia: a systematic review and meta-analysis of randomized controlled trials with first- and second-generation antipsychotics. CNS Drugs 2015; 29: 637–58. [DOI] [PubMed] [Google Scholar]
- 17.Karson C, Duffy RA, Eramo A, Nylander AG, Offord SJ. Long-term outcomes of antipsychotic treatment in patients with first-episode schizophrenia: a systematic review. Neuropsychiatr Dis Treat 2016; 12: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGorry P, Alvarez-Jimenez M, Killackey E. Antipsychotic medication during the critical period following remission from first-episode psychosis: less is more. JAMA Psychiatry 2013; 70: 898–900. [DOI] [PubMed] [Google Scholar]
- 19.Murray RM, Quattrone D, Natesan S, van Os J, Nordentoft M, Howes O, et al. Should psychiatrists be more cautious about the long-term prophylactic use of antipsychotics? Brit J Psychiatry 2016; 209: 361–5. [DOI] [PubMed] [Google Scholar]
- 20.Henry LP, Amminger GP, Harris MG, Yuen HP, Harrigan SM, Prosser AL, et al. The EPPIC follow-up study of first-episode psychosis: longer-term clinical and functional outcome 7 years after index admission. J Clin Psychiatry 2010; 71: 716–28. [DOI] [PubMed] [Google Scholar]
- 21.Moncrieff J. Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse. Acta Psychiatr Scand 2006; 114: 3–13. [DOI] [PubMed] [Google Scholar]
- 22.Wunderink L, Nienhuis F, Sytema S, Slooff C, Knegtering R, Wiersma D. Guided discontinuation versus maintenance treatment in remitted first-episode psychosis: relapse rates and functional outcome. J Clin Psychiatry 2007; 68: 654–61. [DOI] [PubMed] [Google Scholar]
- 23.Gaebel W, Janner M, Frommann N, Pietzcker A, Kopcke W, Linden M, et al. First vs multiple episode schizophrenia: two-year outcome of intermittent and maintenance medication strategies. Schizophr Res 2002; 53: 145–59. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009; 3: e123–30. [PMC free article] [PubMed] [Google Scholar]
- 26.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil 2004; 85: 1694–704. [DOI] [PubMed] [Google Scholar]
- 27.Kane J, Rifkin A, Quitkin F, Nayak D, Ramos-Lorenzi J. Fluphenazine vs placebo in patients with remitted, acute first-episode schizophrenia. Arch Gen Psychiatry 1982; 39: 70–73. [DOI] [PubMed] [Google Scholar]
- 28.Crow T, MacMillan J, Johnson A, Johnstone E. A randomised controlled trial of prophylactic neuroleptic treatment. Br J Psychiatry 1986; 148: 120–7. [DOI] [PubMed] [Google Scholar]
- 29.McCreadie RG, Wiles D, Grant S, Crockett GT, Mahmood Z, Livingston MG, et al. The Scottish first episode schizophrenia study. VII. Two-year follow-up. Scottish Schizophrenia Research Group. Acta Psychiatr Scand 1989; 80: 597–602. [DOI] [PubMed] [Google Scholar]
- 30.Chen E, Hui C, Lam M, Chiu C, Law C, Chung D. Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial. BMJ 2010; 341: c4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boonstra G, Burger H, Grobbee D, Kahn R. Antipsychotic prophylaxis is needed after remission from a first psychotic episode in schizophrenia patients: results from an aborted randomised trial. Int J Psychiatry Clin Pract 2011; 15: 128–34. [DOI] [PubMed] [Google Scholar]
- 32.Leucht S, Barnes TR, Kissling W, Engel RR, Correll C, Kane JM. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. Am J Psychiatry 2003; 160: 1209–22. [DOI] [PubMed] [Google Scholar]
- 33.Rich JT, Neely JG, Paniello RC, Voelker CC, Nussenbaum B, Wang EW. A practical guide to understanding Kaplan-Meier curves. Otolaryngol Head Neck Surg 2010; 143: 331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterne JA, Bradburn MJ, Egger M. Meta–Analysis in Stata™ Systematic Reviews in Health Care: Meta-Analysis in Context (2nd edn). BMJ Books, 2001, pp. 347–69. [Google Scholar]
- 35.Field AP, Gillett R. How to do a meta-analysis. Br J Math Stat Psychol 2010; 63: 665–94. [DOI] [PubMed] [Google Scholar]
- 36.Nyaga V, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gleeson JF, Alvarez-Jimenez M, Cotton SM, Parker AG, Hetrick S. A systematic review of relapse measurement in randomized controlled trials of relapse prevention in first-episode psychosis. Schizophr Res 2010; 119: 79–88. [DOI] [PubMed] [Google Scholar]
- 38.Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
- 39.Winsper C, Ganapathy R, Marwaha S, Large M, Birchwood M, Singh SP. A systematic review and meta-regression analysis of aggression during the first episode of psychosis. Acta Psychiatr Scand 2013; 128: 413–21. [DOI] [PubMed] [Google Scholar]
- 40.Slade M, Amering M, Farkas M, Hamilton B, O'Hagan M, Panther G, et al. Uses and abuses of recovery: implementing recovery-oriented practices in mental health systems. World Psychiatry 2014; 13: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rifkin A, Quitkin F, Rabiner CJ, Klein DF. Fluphenazine decanoate, fluphenazine hydrochloride given orally, and placebo in remitted schizophrenics. I. Relapse rates after one year. Arch Gen Psychiatry 1977; 34: 43–7. [DOI] [PubMed] [Google Scholar]
- 42.Pietzcker A, Gaebel W, Köpcke W, Linden M, Müller P, Müller-Spahn F, et al. Intermittent versus maintenance neuroleptic long-term treatment in Schizophrenia—2-year results of a German multicenter study. J Psychiatr Res 1993; 27: 321–39. [Google Scholar]
- 43.Hogarty GE, Goldberg SC. Drug and sociotherapy in the aftercare of schizophrenic patients; one year relapse rates. Arch Gen Psychiatry 1973; 28: 54–64. [DOI] [PubMed] [Google Scholar]
- 44.Emsley R, Oosthuizen P, Koen L, Niehaus D, Martinez L. Symptom recurrence following intermittent treatment in first episode schizophrenia successfully treated for two years. J Clin Psychiatry 2012; 73: e541–7. [DOI] [PubMed] [Google Scholar]
- 45.Gitlin M, Nuechterlein K, Subotnik K, Ventura J, Mintz J, Fogelson D. Clinical outcome following neuroleptic discontinuation in patients with remitted recent-onset schizophrenia. Am J Psychiatry 2001; 158: 1835–42. [DOI] [PubMed] [Google Scholar]
- 46.Sturup AE, Jensen HD, Dolmer S, Birk M, Albert N, Nielsen M, et al. TAILOR – tapered discontinuation versus maintenance therapy of antipsychotic medication in patients with newly diagnosed schizophrenia or persistent delusional disorder in remission of psychotic symptoms: study protocol for a randomized clinical trial. Trials 2017; 18: 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Killackey E. Dose reduction, relapse and functional recovery in first episode psychosis. Aust N Z J Psychiatry 2017; 51: 638–9. [DOI] [PubMed] [Google Scholar]
- 48.Sterne JAC, Egger M, Smith GD. Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001; 323: 101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvarez-Jimenez M, O'Donoghue B, Thompson A, Gleeson JF, Bendall S, Gonzalez-Blanch C, et al. Beyond clinical remission in first Episode psychosis: Thoughts on antipsychotic maintenance vs. guided discontinuation in the functional recovery era. CNS Drugs 2016; 30: 357–68. [DOI] [PubMed] [Google Scholar]
- 50.Gardos G, Cole JO. Maintenance antipsychotic therapy: is the cure worse than the disease? Am J Psychiatry 1976; 133: 32–6. [DOI] [PubMed] [Google Scholar]
- 51.Alvarez-Jimenez M, Gleeson JF, Henry LP, Harrigan SM, Harris MG, Amminger GP, et al. Prediction of a single psychotic episode: a 7.5-year, prospective study in first-episode psychosis. Schizophr Res 2011; 125: 236–46. [DOI] [PubMed] [Google Scholar]
- 52.Gaebel W, Riesbeck M, Wölwer W, Klimke A, Eickhoff M, von Wilmsdorff M, et al. Predictors for symptom re-exacerbation after targeted stepwise drug discontinuation in first-episode schizophrenia: results of the first-episode study within the German research network on schizophrenia. Schizophr Res 2016; 170: 168–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1192/bjo.2018.17.
click here to view supplementary material