Summary
The importance of inflammation and inflammatory pathways in atherosclerotic disease and acute coronary syndromes (ACS) is well established. The success of statin therapy rests not only on potently reducing levels of low-density lipoprotein cholesterol, but also on the many beneficial, pleiotropic effects statin therapy has on various inflammatory mechanisms in atherosclerotic disease, from reducing endothelial dysfunction to attenuating levels of serum C-reactive protein. Due to the growing awareness of the importance of inflammation in ACS, investigators have attempted to develop novel therapies against known markers of inflammation for several decades. Targeted pathways have ranged from inhibiting C5 cleavage with a high-affinity monoclonal antibody against C5 to inhibiting the activation of the p38 mitogen-activated protein kinase signaling cascades. In each of these instances, despite promising early preclinical and mechanistic studies and phase 2 trials suggesting a potential benefit in reducing post-MI complications or restenosis, these novel therapies have failed to show benefits during large, phase 3 clinical outcomes trials. This review discusses several examples of novel anti-inflammatory therapies that failed to show significant improvement on clinical outcomes when tested in large, randomized trials and highlights potential explanations for why targeted therapies against known markers of inflammation in ACS have failed to launch.
Key Words: acute coronary syndrome, anti-inflammatory, drug targets
Abbreviations and Acronyms: ACS, acute coronary syndromes; CABG, coronary artery bypass graft; CAD, coronary artery disease; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; IL, interleukin; LDL-C, low-density lipoprotein cholesterol; Lp-PLA2, lipoprotein-associated phospholipase A2; MAPK, mitogen-activated protein kinase; MI, myocardial infarction; NSTEMI, non–ST-segment myocardial infarction; PCI, percutaneous coronary intervention; PSGL, P-selectin glycoprotein ligand; sPLA2, secretory phospholipase A2; STEMI, ST-segment elevation myocardial infarction; SVG, saphenous vein grafts; TBR, tissue-to-background ratio
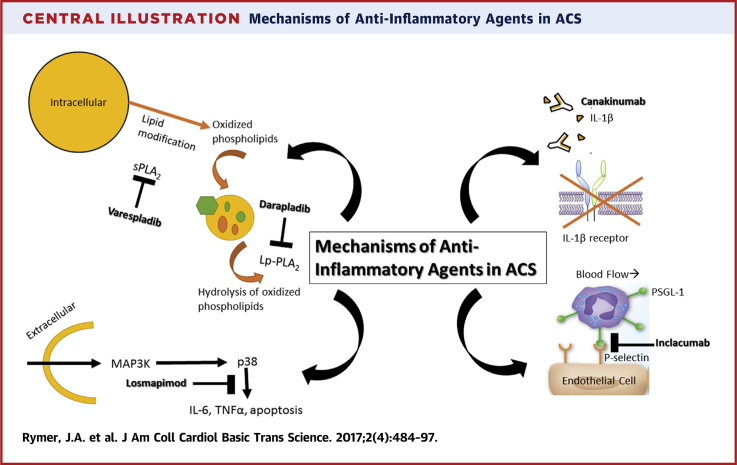
Central Illustration
Over the past 2 decades, there has been increasing interest in discovering novel therapeutic agents for reducing residual risk among patients with acute coronary syndromes (ACS), including ST-segment elevation myocardial infarction (STEMI), non–ST-segment myocardial infarction (NSTEMI), and unstable angina. Each year, roughly 1.1 million patients are hospitalized with an ACS event in the United States (1). Although the overall incidence of ACS appears to be declining, the direct and indirect costs associated with treating patients with ACS and its downstream sequelae, including congestive heart failure and repeat revascularization, remain a medical and economic burden 2, 3, 4.
Atherosclerotic disease leading to an ACS event is a complex process of atheroma formation and eventual plaque rupture. The 30-day rate of recurrent events post-ACS is estimated to be around 2% 5, 6. High-sensitivity C-reactive protein (hsCRP) (a marker of inflammation), can be elevated for several months after an ACS event, and is a marker of risk for subsequent development of heart failure and increased mortality (7). These observations led to a focus on inflammatory pathways as participants in the formation, proliferation, and rupture of atherosclerotic plaques (8). The intersection of ACS pathobiology, inflammation, and high rates of recurrent events post-ACS has generated interest in development of therapeutics that target inflammatory pathways to mitigate the development of post-ACS complications.
In this review, we examine the fate of work over the past decade to develop therapeutics that target various inflammatory pathways and their components (Central Illustration). We will focus on therapeutic agents directed at the complement pathway and cleavage of C5, secretory phospholipase A2 (sPLA2), lipoprotein-associated phospholipase A2 (Lp-PLA2), interleukin-1, and the mitogen-activated protein kinase (MAPK) signaling cascades (Table 1). We examine their development from animal models and other preclinical investigations through early and late-phase randomized clinical trials that tested the experimental drugs in ACS patients. We then offer potential explanations for why work targeting these inflammatory components and pathways in ACS has not translated into therapeutic successes and describe current, promising novel therapies that are still under investigation.
Central Illustration.
Mechanisms of Anti-Inflammatory Agents in ACS
ACS = acute coronary syndromes; IL = interleukin; Lp-PLA2 = lipoprotein-associated phospholipase A2; MAPK = mitogen-activated protein kinase; PSGL-1 = P-selectin glycoprotein ligand-1; sPLA2 = secretory phospholipase A2; TNF = tumor necrosis factor.
Table 1.
Inflammatory Targets With Mechanisms of Action and Pertinent Studies
| Inflammatory Target | Mechanism | Atherosclerotic Cardiovascular Disease |
Other Clinical Conditions |
||
|---|---|---|---|---|---|
| Therapeutic Agent | Pertinent Studies | Therapeutic Agent | Indication/Pertinent Studies | ||
| C5, complement cascade | Single-chain fragment of a humanized monoclonal antibody that binds to C5 preventing cleavage to C5a and C5b-9 | Pexelizumab | Phase 2: CARDINAL Program (COMPLY [15] and COMMA [16] in STEMI) Phase 3: APEX AMI (STEMI) (20), PRIMO-CABG 1 (CABG) (25), PRIMO-CABG II (CABG) (27) |
Eculizumab | Atypical hemolytic uremic syndrome (99), pregnant patients with paroxysmal nocturnal hemoglobinuria (100), macular degeneration (101) |
| sPLA2 | Nonspecific inhibitor of sPLA2 activity | Varespladib | Phase 2: FRANCIS (ACS) (37) Phase 3: VISTA-16 (ACS) (39) |
||
| Lp-PLA2 | Direct selective inhibitor of Lp-PLA2 | Darapladib | Chronic Stable Angina: STABILITY (stable CAD) (48) ACS: SOLID-TIMI (STEMI or NSTEMI) 52 (49) |
Rilapladib | Platelet aggregation inhibition (102) |
| IL-1β | Human monoclonal antibody targeted at IL-1β | Canakinumab | Phase 2 (patients with high cardiovascular risk) (61) Phase 3: CANTOS (post-ACS) |
Anakinra (IL1-RA) | Recurrent pericarditis (103) Rheumatoid arthritis (104) Severe hidradenitis suppurativa (105) |
| P-selectin | Highly specific P-selectin human recombinant monoclonal antibody | Inclacumab | Phase 2 (NSTEMI, CABG) 69, 72 | Crizanlizumab | Sickle cell pain (106) |
| P38 MAPK signaling cascade | selective inhibitor of the p38 MAPK signaling cascade | Losmapimod | Phase 1b: (NSTEMI) (85) Phase 3: (acute MI) (86) |
Doramapimod | Active Crohn’s disease (107) |
ACS = acute coronary syndromes; AMI = acute myocardial infarction; APEX AMI = Assessment of Pexelizumab in Acute Myocardial Infarction; CABG = coronary artery bypass graft; CAD = coronary artery disease; CANTOS = Canakinumab Anti-Inflammatory Thrombosis Outcomes Study; CARDINAL = Complement And ReDuction of INfarct size after Angioplasty or Lytics; COMMA = COMplement inhibition in Myocardial infarction treated with Angioplasty; COMPLY = COMPlement inhibition in myocardial infarction treated with thromboLYtics; FRANCIS = Fewer Recurrent Acute Coronary Events With Near-Term Cardiovascular Inflammation Suppression; IL = interleukin; Lp-PLA2 = lipoprotein-associated phospholipase A2; MAPK = mitogen-activated protein kinase; MI = myocardial infarction; NSTEMI = non–ST-segment myocardial infarction; PRIMO-CABG = Pexelizumab for Reduction of Infarction and Mortality in Coronary Artery Bypass Graft Surgery; SOLID-TIMI 52 = Stabilization of pLaques usIng Darapladib-Thrombolysis In Myocardial Infarction 52; sPLA2 = secretory phospholipase A2; STABILITY = Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy; STEMI = ST-segment elevation myocardial infarction; VISTA-16 = Vascular Inflammation Suppression to Treat Acute Coronary Syndrome for 16 weeks.
Case Studies of Anti-Inflammatory Agents for Cardiovascular Disease
Pexelizumab
Pexelizumab was an early example of an anti-inflammatory therapy for ACS, targeting the C5 component of the complement cascade. Cleavage of the C5 component of the complement pathway results in formation of C5a and C5b-9, the membrane attack complex (9). C5a is proinflammatory, and the membrane attack complex is associated with activation of endothelial cells and leukocytes. Pexelizumab is a single-chain fragment of a humanized monoclonal antibody that binds to C5 with high affinity, preventing its cleavage. Early in vitro and human studies suggested that myocardial cell necrosis during an ACS event triggered the release of subcellular membrane constituents, which resulted in the activation of the complement system (10). For example, in a rabbit model of ischemia induced by ligature to a large marginal branch of the circumflex artery, there was accumulation of the membrane attack complex (C5b-C9) in infarcted myocardium, and rapid activation of the complement pathway occurred during reperfusion (11). One group of rabbits (n = 17) underwent circumflex coronary occlusion for variable time periods (from 0.5 to 29 h) without subsequent reperfusion, whereas the other group (n = 23) underwent coronary occlusion (from 0.5 to 6 h) with subsequent reperfusion. Although C5b-C9 was detected in the infarcted myocardium 5 to 6 h after occlusion without reperfusion, it was detected much earlier (at 30 min) in the group that underwent reperfusion, suggesting that the presence of reperfusion rapidly activates the complement pathway.
In addition to the association of complement activation with infarction and reperfusion injury, in a rat model of myocardial infarction (MI) and reperfusion, infusion of monoclonal antibodies against the rat C5 component prior to ligating the left anterior descending artery for 30 min, followed by reperfusion, significantly reduced left ventricular polymorphonuclear leukocyte infiltration, apoptosis and necrosis, and overall infarct size (12). In another study of myocardial ischemia and reperfusion injury in rats, injection of C5 short hairpin ribonucleic acid 2 days before induction of ischemia inhibited C5 expression, significantly decreased the level of troponin T, and reduced the infarct size by 40% (13). Additionally, in a pig model, infusion of a monoclonal antibody to C5a (at the time of onset of ischemia) followed by 50 min of occlusion of the left anterior descending artery using an occluder catheter and 3 h of reperfusion resulted in significant reduction in infarct size and reperfusion injury (14).
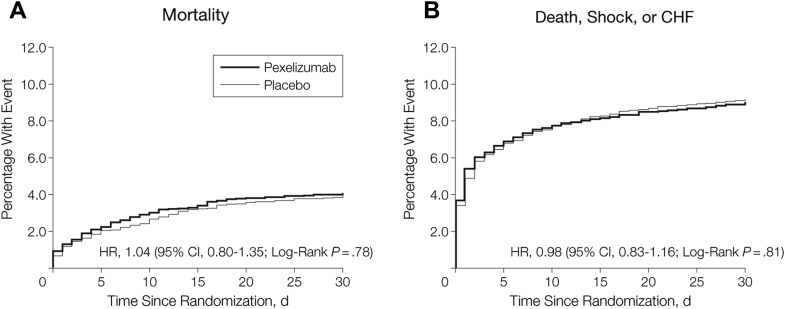
Based on these promising animal data and other early studies, a phase 2 program was launched. The CARDINAL (Complement And ReDuction of INfarct size after Angioplasty or Lytics) phase 2 program included 2 phase 2, parallel group, double-blind, placebo-controlled trials of pexelizumab in STEMI patients. These trials tested the effect of pexelizumab (bolus plus infusion administered <6 h after symptom onset) on infarct size (creatine kinase–MB area under the curve at 72 h) and clinical composite outcomes after fibrinolytic reperfusion therapy in the COMPLY (COMPlement inhibition in myocardial infarction treated with thromboLYtics) trial (15) or primary percutaneous coronary intervention (PCI) in the COMMA (COMplement inhibition in Myocardial infarction treated with Angioplasty) trial (16). The COMPLY investigators found no difference in infarct size or 90-day composite clinical outcomes for pexelizumab versus placebo among STEMI patients who received fibrinolytic therapy. Similarly, in the COMMA trial, among STEMI patients treated with primary PCI, there was no difference in infarct size or the 90-day primary composite of death, heart failure, shock, or stroke between patients treated with placebo versus pexelizumab. However, 90-day mortality was significantly lower among patients who received pexelizumab compared with placebo (1.8% vs. 5.9%; p = 0.014) (Figure 1). Interestingly, this mortality benefit was independent of infarct size, evidence of angiographic or electrocardiographic reperfusion, and extent of ST-segment elevation (17). In addition, the composite of worsening heart failure, shock, and disabling stroke was less frequent among patients treated with pexelizumab (8.5%) versus placebo (11.1%) (relative risk: 0.77; 95% confidence interval [CI]: 0.46 to 1.29). The COMMA investigators hypothesized that an undetected pathway, potentially related to reduced apoptosis, inflammatory cytokines, inducible nitric oxide synthase, and/or remodeling, could potentially explain the mortality benefit and lower rate of cardiogenic shock without a demonstrable reduction in infarct size 12, 18, 19.
Figure 1.
6-Month Kaplan-Meier Curves for Mortality by Randomized Treatment Assignment (COMMA, Phase 2)
COMMA = COMplement inhibition in Myocardial infarction treated with Angioplasty.
Reproduced with permission from Granger et al. (16).
Based on the favorable effects of pexelizumab on mortality among patients with STEMI undergoing primary PCI in the COMMA trial, a phase 3 trial, APEX AMI (Assessment of Pexelizumab in Acute Myocardial Infarction), was undertaken. APEX AMI randomized patients with STEMI = undergoing primary PCI <6 h after symptom onset to either placebo or pexelizumab (bolus administered before PCI, and infusion administered over the subsequent 24 h) (20). Despite the mortality benefit observed with pexelizumab treatment in the COMMA trial, there was no 30- or 90-day mortality benefit observed in the pexelizumab group compared with placebo in APEX AMI (Figure 2).
Figure 2.
30-Day Mortality and Composite of Death, Shock, or CHF by Treatment With Pexelizumab Versus Placebo (APEX-AMI, Phase 3)
(A) 30-day mortality; (B) composite of death, shock, or CHF. APEX AMI = Assessment of Pexelizumab in Acute Myocardial Infarction; CHF = congestive heart failure; CI = confidence interval; HR = hazard ratio.
Modified with permission from Armstrong et al. (20).
Pexelizumab was also explored as a treatment to reduce post–coronary artery bypass graft (CABG) complications. Among patients undergoing cardiopulmonary bypass for CABG surgery, an inflammatory response involving activation of the complement system has been described (21). Preclinical studies demonstrated that the interaction of blood with bioincompatible surfaces of the extracorporeal circuit, as well as ischemia and reperfusion while on cardiopulmonary bypass, activated the alternative complement pathway, including C3a and C5a, and various cytokine pathways 22, 23, 24. The PRIMO-CABG I (Pexelizumab for Reduction of Infarction and Mortality in Coronary Artery Bypass Graft Surgery I) trial, a phase 3, double-blind, placebo-controlled study, examined the outcomes of 3,099 CABG patients randomized to pexelizumab versus placebo (25). To be enrolled in PRIMO-CABG I, patients had to have 1 or more pre-defined risk factors, including female sex, recent or multiple MIs, urgent surgery, previous CABG surgery, a history of diabetes mellitus, and advanced age. The pexelizumab group had significantly lower 30-day death or MI (11.5%) than the placebo group (14.0%) (p = 0.030). A post-hoc analysis of PRIMO-CABG I demonstrated that pexelizumab significantly reduced the composite endpoint (death or MI through post-operative day 30) by 28% among patients with 2 or more risk factors (p = 0.004) and by 44% among patients with 3 or more risk factors (p < 0.001) (26).
As a result of the post-hoc analysis of the PRIMO-CABG I study, another phase 3 trial, PRIMO-CABG II, was designed to determine the effectiveness of pexelizumab in higher-risk CABG patients with or without valve surgery. PRIMO-CABG II was a randomized, double-blind, placebo-controlled trial of 4,254 patients undergoing CABG with or without concomitant valve surgery who had 2 or more pre-operative risk factors. The primary endpoint was MI or death through 30 days post-CABG (27). Randomization was stratified by type of surgery planned, and the bolus of pexelizumab was administered as soon as possible after induction of anesthesia. Despite the findings in the high-risk subset analysis from PRIMO-CABG I, there was no difference in death or MI at 30 days between the pexelizumab and placebo groups. When data from both PRIMO-CABG I and II were combined, the highest-risk subset of patients (2% or more predicted mortality using the Society of Thoracic Surgeons mortality model) had a significantly lower 30-day rate of death with pexelizumab treatment (5.7%) compared with placebo (8.1%) (p = 0.024).
A subsequent meta-analysis of all 7 randomized clinical trials of pexelizumab, which included over 15,000 patients with ischemic heart disease (7,019 with STEMI and 8,177 undergoing CABG surgery), showed no benefit of pexelizumab in STEMI (28). However, in the CABG subpopulation, there was a 26% reduction in mortality among patients treated with pexelizumab compared with placebo. Despite this, given the overall weak effect of pexelizumab compared with placebo, Alexion, the developer of pexelizumab, discontinued further investigations and development of the drug.
Varespladib
Within the phospholipase family, sPLA2 is 1 of the enzymes most closely associated with increased risk for cardiovascular disease (29). sPLA2 is present in human atherosclerotic lesions, and hydrolysis of low density lipoprotein cholesterol (LDL-C) by sPLA2 results in a particle that contributes to lipid accumulation in human macrophages (30). Hydrolysis of lipoprotein phospholipid by sPLA2 produces free fatty acids that stimulate oxidative stress and inflammation of the arterial walls (31). Moreover, the interaction of LDL-C and sPLA2 results in smaller LDL-C particles taken up by macrophages, which may serve to form foam cells (30). sPLA2 also interacts with tumor necrosis factor alpha (TNF-α) receptor signaling in atherogenesis (32). Based on these laboratory observations of the roles of sPLA2 in the inflammatory milieu of atherosclerosis, sPLA2 was an attractive therapeutic target to improve cardiovascular outcomes.
In addition to the increasing understanding of the potential roles sPLA2 plays in atherosclerotic disease and atherogenesis, clinical investigation of sPLA2 also supported its role as an inflammatory and prognostic marker among patients with stable and unstable coronary artery disease (CAD). In a study of patients undergoing coronary angiography alone or PCI if significant CAD was present, significantly elevated sPLA2 levels were observed among patients immediately after undergoing PCI, but not among patients who had undergone coronary angiography alone (33). Further, elevation of sPLA2 after PCI was an independent predictor of recurrent cardiovascular events 2 years after intervention. In a small study of patients with unstable angina, elevated levels of sPLA2 were highly predictive of recurrent cardiovascular events and recurrent revascularization (34). Highly elevated baseline sPLA2 levels drawn from patients with NSTEMI and patients with STEMI also strongly predicted risk of recurrent cardiovascular events and death (35).
Given the mounting evidence for sPLA2 in the pathogenesis of atherosclerotic disease, varespladib methyl, a nonspecific inhibitor of sPLA2 activity, was developed as a potential target to attenuate sPLA2 activity. Among stable CAD patients, varespladib significantly reduced sPLA2 levels in a dose-dependent manner (36). In a phase 2 randomized controlled trial in 625 ACS patients, varespladib (500 mg daily) and atorvastatin (80 mg) significantly reduced LDL-C, hsCRP, and sPLA2 levels at 8 and 24 weeks after the ACS event compared with placebo and atorvastatin (37). There were no differences in rates of major adverse cardiovascular events between treatment groups. The PLASMA II (Phospholipase Levels and Serological Markers of Atherosclerosis II) trial of 135 stable CAD patients also demonstrated that daily varespladib (500 mg) significantly reduced LDL-C, non–high-density lipoprotein cholesterol (HDL-C), and very low-density lipoprotein cholesterol concentrations compared with placebo (38).
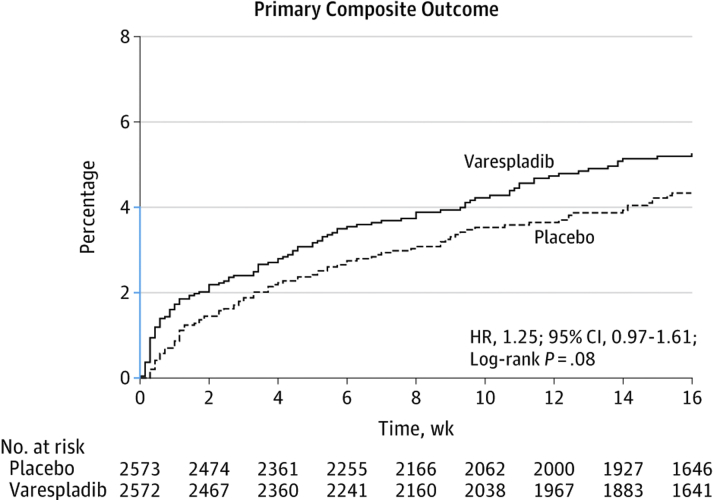
Based on these favorable effects on intermediate endpoints in phase 2 testing, the VISTA-16 (Vascular Inflammation Suppression to Treat Acute Coronary Syndrome for 16 weeks) trial examined the efficacy of varespladib versus placebo on post-ACS outcomes in 5,145 ACS patients (39). In a striking failure to translate, despite lowering levels of LDL-C and hsCRP, daily treatment with varespladib (500 mg) for 4 months after an ACS event was associated with higher rates of the composite primary outcome (cardiovascular mortality, nonfatal MI, nonfatal stroke, or unstable angina requiring hospitalization) (Figure 3). The trial was terminated early for potential harm.
Figure 3.
Kaplan-Meier Survival Curves for the Primary Composite Outcome in Patients Treated With Placebo Versus Varespladib (VISTA-16, Phase 3)
VISTA-16 = Vascular Inflammation Suppression to Treat Acute Coronary Syndrome for 16 weeks; other abbreviations as in Figure 2.
Modified with permission from Nicholls et al. (39).
Darapladib
Lipoprotein-associated phospholipase A2 (Lp-PLA2), another member of the phospholipase family, is also implicated for its potential role in the progression of atherosclerotic disease. Lp-PLA2 activity produces oxidized nonesterified fatty acid molecules that can be pro-inflammatory (40). Further, it is bound to apolipoprotein B–containing lipoproteins, and is highly expressed in the necrotic cores and nearby macrophages of thin-cap fibroatheromas and ruptured plaques (41). Concentrations of Lp-PLA2 are higher in unstable and ruptured atherosclerotic plaques than stable plaques (42). Deficiency of Lp-PLA2 activity is associated with lower risk for developing CAD (43), and higher levels of Lp-PLA2 are associated with increased risk of coronary events among elderly patients and patients with stable coronary disease 44, 45. Based on these observations, therapies to inhibit Lp-PLA2 in patients with stable coronary disease and ACS were developed.
Darapladib, a direct selective inhibitor of Lp-PLA2, is 1 such compound that inhibits Lp-PLA2 activity by 59% (46). In an early swine model of atherosclerosis using domestic pigs given streptozotocin and a hyperlipidemic diet to induce diabetes and hyperlipidemia, darapladib inhibited the development of atherosclerotic lesions and reduced the levels of Lp-PLA2, macrophage content, and the necrotic core area in plaques (47). The IBIS-2 (Integrated Biomarker and Imaging Study 2) was an early clinical study of the effects of 12 months of darapladib treatment on coronary atheroma deformability, necrotic core volume, and C-reactive protein (CRP) levels (46). Although there was no significant difference between the darapladib-treated and placebo group with regard to the primary outcome, atheroma deformability, there was a significant decrease in necrotic core volume in the darapladib group (−0.5 ± 13.9 mm3; p = 0.71). These early studies led to phase 3 testing of darapladib in both chronic stable CAD and ACS.
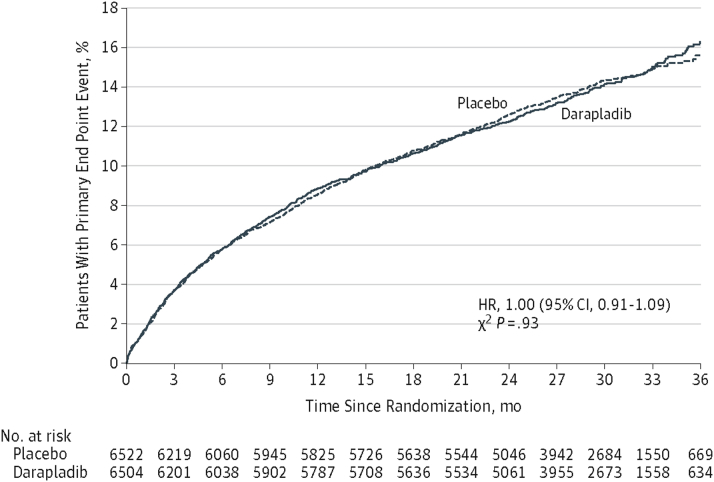
In the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial, 15,828 patients with chronic stable coronary disease were randomized to once daily darapladib (160 mg) or placebo. The primary endpoint was a composite of death, MI, or stroke. Compared with placebo, there was no difference in rates of the composite endpoint, all-cause mortality, or the individual components of the primary composite endpoint (48). The SOLID-TIMI 52 (Stabilization of pLaques usIng Darapladib-Thrombolysis In Myocardial Infarction 52) trial randomized over 13,000 patients within 30 days of either NSTEMI or STEMI to darapladib (160 mg) or placebo (49). As in the STABILITY trial in stable coronary disease, there was no difference in the primary composite endpoint (coronary heart disease death, MI, or urgent revascularization) (Figure 4) or in any of the individual components of the primary endpoint.
Figure 4.
Cumulative Incidence Curves for CHD Death, MI, or Urgent Coronary Revascularization for Myocardial Ischemia for Treatment With Darapladib Versus Placebo (SOLID-TIMI 52, Phase 3)
CHD = coronary heart disease; MI = myocardial infarction; SOLID-TIMI 52 = Stabilization of pLaques usIng Darapladib-Thrombolysis In Myocardial Infarction 52; other abbreviations as in Figure 2.
Reproduced with permission from O’Donoghue et al. (49).
In a substudy from the STABILITY trial, baseline levels of Lp-PLA2 activity were associated with composite cardiovascular events, rehospitalization for heart failure, and cardiovascular and all-cause mortality (50). However, although treatment with darapladib led to a persistent 65% reduction in Lp-PLA2 activity among patients treated with darapladib compared with placebo, there was no association between suppressed Lp-PLA2 activity and outcomes. Thus, Lp-PLA2 is a biomarker of risk for acute cardiovascular events and mortality, but has no role as a therapeutic target to improve cardiovascular outcomes.
Canakinumab
Since the 1980s, interleukin(IL)-1 has been implicated in induction of procoagulant activity and adhesion of monocytes and leukocytes to human vascular endothelial cells 51, 52. IL-1β and -1Ra, 2 of the 3 proteins encoded by IL-1, were detected in human atherosclerotic lesions, and dysregulation in IL-1Ra and IL-1β with subsequent imbalance in proinflammatory and anti-inflammatory cytokines has been hypothesized to promote atherosclerotic disease 51, 53, 54. In clinical studies, patients with unstable angina had significantly higher levels of IL-1Ra than patients with stable coronary disease, and elevated IL-1Ra levels preceded elevations in troponin and creatinine kinase in patients with STEMI (55). IL-1β has been detected in the luminal endothelium and macrophages of atherosclerotic lesions, but not in normal coronary arteries (56). In addition to cardiovascular disease, IL-1 and IL-1Ra activity is associated with a host of other inflammatory conditions, including type II diabetes, inflammatory bowel disease, and gout 57, 58, 59.
Canakinumab is a human monoclonal antibody targeted at IL-1β. It was used in rheumatoid arthritis and type II diabetes to suppress interaction of IL-1β with its receptors, resulting in a sustained and rapid suppression of IL-1β, and subsequent reductions in hsCRP and IL-6 (51). Using a mouse model of MI due to left anterior descending coronary artery ligation, a genetically engineered mouse antibody that binds to IL-1β with high affinity was administered at the time of surgical ligation (0.05 mg/kg dose vs. 0.5 mg/kg dose vs. 5 mg/kg dose vs. placebo) (60). Transthoracic echocardiograms at 7, 14, and 28 days after administration of the antibody revealed significantly less increase in left ventricular end-diastolic dimension and left ventricular end-systolic dimension at 4 weeks in the 2 higher-dose groups. A phase 2b trial of canakinumab in 556 patients with type II diabetes and high cardiovascular risk demonstrated significantly reduced levels of hsCRP, IL-6, and fibrinogen among patients who received monthly injections of canakinumab for 4 months compared with placebo (61). There was no effect of canakinumab on LDL-C, HDL-C, or non–HDL-C levels between groups. Currently, the CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) is randomizing post-ACS patients to canakinumab versus standard post-ACS medical therapy with a primary composite endpoint of nonfatal MI, nonfatal stroke, or cardiovascular death.
Inclacumab
P-selectin is a cell adhesion molecule that plays a critical role in leukocyte tethering and platelet rolling and adhesion on an activated vessel wall (62). Expression of P-selectin results in leukocyte recruitment and thrombus formation and growth. P-selectin works to mediate leukocyte, platelet, and endothelial interactions through the binding of P-selectin to the P-selectin glycoprotein ligand (PSGL)-1 located on the surface of leukocytes. In humans, higher levels of P-selectin were found among patients with unstable angina compared with patients with stable angina (63), and higher levels of P-selectin were associated with increased rates of cardiovascular mortality and sudden cardiac death in male dialysis patients (64). Thus, inhibition of P-selectin or PSGL-1 has the potential to reduce platelet adhesion, macrophage infiltration, and the development of atherosclerotic lesions 65, 66.
Animal models using atherosclerosis-prone apolipoprotein E knockout mice demonstrated that a single injection of either an anti–P-selectin monoclonal antibody or an anti–PSGL-1 monoclonal antibody significantly inhibited neointimal formation and macrophage content in the arterial wall when given 3 h before carotid artery wire injury (67). In a pig model of balloon-mediated injury to the left anterior descending and right coronary arteries, administration of a recombinant immunoglobulin to PSGL-1 15 min before balloon injury significantly reduced neointimal hyperplasia and levels of TNF-α, IL-1β, and macrophages at the site of the balloon injury compared with placebo (68). Further, in a porcine model of balloon injury and stent placement recombinant PSGL-1 was effective at reducing platelet-leukocyte reactions and rates of in-stent restenosis (69). Pigs underwent angioplasty injury to their coronary arteries, and 2 weeks later had stents implanted at the sites of injury 15 min after administration of recombinant PSGL-1 versus placebo. Pigs treated with recombinant PSGL-1 had 3-fold larger residual lumen 4 weeks later. Additionally, in a mouse model of P-selectin activity, P-selectin knockout mice demonstrated inhibited migration of smooth muscle cells into the atherosclerotic lesion compared with wild-type mice (70).
Given the promising results for P-selectin inhibition in animal models of vascular injury, inclacumab was developed as a highly specific human recombinant monoclonal antibody against P-selectin. In the phase 2 SELECT-ACS (Effects of the P-Selectin Antagonist Inclacumab on Myocardial Damage after Percutaneous Coronary Intervention for Non-ST-Elevation Myocardial Infarction) trial, 544 patients with NSTEMI who were scheduled to undergo coronary angiography were randomized to an infusion of placebo, a 5-mg/kg inclacumab infusion, or a 20-mg/kg inclacumab infusion, between 1 and 24 h before PCI (71). The primary endpoint was change in troponin I level from baseline at 16 and 24 h. There was no significant difference in change in placebo-adjusted geometric mean percent change in troponin I levels at 16 and 24 h from baseline in the group who received the 5-mg/kg inclacumab infusion compared with placebo. However, in the group that received the 20-mg/kg inclacumab infusion compared with placebo, there was a significant difference in placebo-adjusted geometric mean percent change in troponin I levels at 24 h (decrease by 23.8%; p = 0.05) but not at 16 h (decrease by 22.4%; p = 0.07). The dose-dependent effect of inclacumab on infarct size as assessed by change in troponin I levels at 24 h in SELECT-NSTEMI suggested that inhibition of P-selectin with inclacumab could be a viable treatment for patients with NSTEMI. Ultimately, a large phase 3 trial will be needed to determine if the use of inclacumab in patients with NSTEMI translates into an actual improvement in clinical outcomes, including rates of post-MI heart failure and mortality.
Based on several preclinical studies, inclacumab has also been studied in patients undergoing CABG. Failure of saphenous vein grafts (SVGs) remains an ongoing concern for patients undergoing CABG and is believed to be mediated by the binding of PSGL-1 to platelet P-selectin and platelet-leukocyte interactions. SELECT-CABG (Effects of the P-selectin Antagonist Inclacumab in Patients Undergoing Coronary Artery Bypass Graft Surgery) was a phase 2 trial of inclacumab for SVG preservation. In this trial, 384 patients undergoing CABG (with placement of at least 1 SVG) were randomized to receive a 20-mg/kg infusion of inclacumab or placebo (starting between 4 h and 6 weeks before CABG) and continuing at 4-week intervals for 32 weeks (72). At the conclusion of treatment, there was no significant difference in the per-protocol population in the percentage of patients with ≥1 SVG with a diameter stenosis >50% (26.4% placebo group vs. 22.3% inclacumab group; adjusted odds ratio: 0.80; 95% CI: 0.47 to 1.38; p = 0.43). Similarly, there was no significant difference in the percentage of patients with ≥1 SVG with a diameter stenosis >50% in the intention-to-treat population at 1 year. The results of the SELECT-CABG trial demonstrated little practical use for inclacumab in the post-CABG population, despite promising results in animal models of arterial injury, pre-clinical studies, and SELECT-ACS.
Losmapimod
The intracellular MAPK signaling cascades have been investigated for their potential role in the pathogenesis of atherosclerotic disease. The p38 MAPK family is a part of the group of stress-activated kinases that transduce extracellular signals that allow for cellular adaptation (73). However, after atherosclerotic plaque rupture, ischemia, or vascular injury, the p38 MAPKs are activated in endothelial cells, myocardium, and macrophages; regulate the transcription and translation of inflammatory molecules, such as TNF-α and interleukins 1, 6, and 8; and aid in the formation of reactive oxygen species 5, 73, 74, 75. Among patients with advanced heart failure and ischemic cardiomyopathy, p38 MAPK activation has been associated with fibrosis, apoptosis, and hypertrophy, which have been linked to cardiac remodeling (76).
Losmapimod is a selective inhibitor of the p38 MAPK signaling cascade, developed by GlaxoSmithKline (Brentford, United Kingdom). Various preclinical studies supported the investigation of losmapimod and p38 MAPK signaling in ACS. In a model of p38 MAPK activation, rabbit hearts were excised and mounted on a Langendorff perfusion apparatus, with exposure to 30 min of global ischemia, with either 60 (to test p38 MAPK levels) or 120 min (for myocardial necrosis) of reperfusion time (77). The hearts were then split into 4 groups: administration of normal saline, administration of p38 MAPK inhibitor 10 min before ischemia and during reperfusion, administration of p38 MAPK inhibitor at the time of reperfusion and throughout, or administration of p38 MAPK inhibitor 10 min after reperfusion began and throughout. Overall, the administration of the p38 MAPK inhibitor resulted in decreased myocardial apoptosis (14.7 ± 3.2% vs. 30.6 ± 3.5% placebo; p < 0.01). Importantly, administering the inhibitor before ischemia and during reperfusion demonstrated the greatest inhibitory effect on p38 MAPK activity, whereas administration 10 min after reperfusion was not beneficial. Similarly, in a rat model of 30 min of induced coronary artery ischemia, followed by 120 min of reperfusion, the most benefit for reduction of ventricular tachycardia/ventricular fibrillation incidence and preservation of ventricular function was when rats were given a p38 MAPK inhibitor either prior to or at the onset of ischemia (78). Other animal models have demonstrated slowed atherosclerotic progression, as well as reduction of post-infarction remodeling with use of p38 MAPK inhibitors 79, 80.
Initial human studies of p38 MAPK inhibitors for patients with ACS or receiving PCI established the basis for later randomized controlled clinical outcomes trials. The implantation of a stent during PCI results in cytokine production and inflammation, including elevated CRP levels, thought to be driven by the MAPK cascade (81). In a small randomized study (n = 92), patients with coronary disease on a statin were given either a selective MAPK inhibitor or placebo daily beginning 3 days before planned PCI and continuing for a total of 28 days (82). The patients receiving SB-681323 had significantly lower levels of hsCRP on days 5 (37% lower; p = 0.04) and 28 (40% lower; p = 0.003) when compared with placebo. In a study of the effect of losmapimod on vascular inflammation in the carotid arteries and aorta, 99 participants with known atherosclerotic disease on a statin were randomized to receive losmapimod 7.5 mg daily, 7.5 mg twice daily, or placebo (83). The primary endpoint was the change in baseline in the average tissue-to-background ratio (TBR) of the index vessel (the artery with the highest average maximum TBR at baseline) using FDG positron emission tomography/computed tomography. Although there was no significant difference in the primary endpoint (the change from baseline in average TBR across all segments in the index vessel in either of losmapimod groups vs. placebo), there was a significant reduction in average TBR in active segments of the index artery (losmapimod twice daily vs. placebo: ΔTBR: −0.10 [95% CI: −0.19 to −0.02]; p = 0.0125; losmapimod once daily vs. placebo: ΔTBR: −0.10 [95% CI: −0.18 to −0.02]; p = 0.0194). Finally, as oxidized LDL activates the p38 MAPK cascade and reduces nitric oxide production, a small study in hypercholesteremic patients (n = 56) examined the effects of losmapimod on compromised nitric oxide vasoregulation (84). Patients were randomized to losmapimod 7.5 mg twice daily or placebo for 28 days, and then underwent venous occlusion plethysmography of forearm blood flow after serial intra-arterial infusions of acetylcholine, sodium nitroprusside, and N(G)-monomethyl-L-arginine (L-NMMA). Responses to acetylcholine, sodium nitroprusside, and L-NMMA were significantly improved in hypercholesterolemic patients who received losmapimod compared with placebo.
Based on these preclinical studies and the mechanistic studies of losmapimod in humans, the phase 1b SOLSTICE (Study Of LoSmapimod Treatment on Inflammation and infarCt sizE) was performed (85). SOLSTICE randomized over 500 patients with NSTEMI to treatment for 12 weeks with either losmapimod (either 7.5 or 15 mg loading dose followed by 7.5 mg twice daily) or placebo within 18 h of presenting with an NSTEMI and at least 2 h prior to PCI to examine its mechanistic effects. The coprimary outcomes were inflammation (hsCRP concentration at 12 weeks) and infarct size (area under the curve for troponin I over 72 h or hospital discharge, whichever came first). Other outcomes included hsCRP concentrations at 14 weeks, IL-6 concentrations at 24 h and 12 weeks, infarct size by creatine kinase–MB area under the curve (AUC) and peak troponin I concentration over 72 h or discharge, B-type natriuretic peptide levels at 72 h and 12 weeks, cardiac magnetic resonance imaging infarct size and LVEF (primary substudy endpoint) at 3 to 5 days and 12 weeks, and left ventricular function and ventricular volumes at 3 to 5 days and 12 weeks. Although hsCRP levels were significantly lower in the combined losmapimod arms versus placebo at 72 h (hsCRP: geometric mean 64.1 mmol/l, 95% CI: 53.0 to 76.7 vs. 110.8 mmol/l, 95% CI: 83.1 to 147.7; p = 0.0009), there was no difference in hsCRP levels at 12 weeks. Peak troponin I levels were also similar between the pooled losmapimod arms and placebo. However, markers of improved ventricular function or remodeling suggested potential benefit. For example, BNP levels were similar at 72 h but significantly lower at 12 weeks among losmapimod-treated patients compared with placebo. In a cardiac magnetic resonance imaging substudy (n = 93), left ventricular ejection fraction (LVEF) was significantly lower among losmapimod-treated patients at 3 to 5 days and 12 weeks after NSTEMI presentation. Similarly, left ventricular end-diastolic and end-systolic dimensions were significantly lower at both time points in the losmapimod-treated group. Additionally, although SOLSTICE was not powered for clinical outcomes, there were numerically fewer major adverse cardiac events in the pooled losmapimod group versus placebo (hazard ratio: 0.82; 95% CI: 0.49 to 1.37).
At the completion of the SOLSTICE trial, there was felt to be enough mechanistic evidence on pathways of ventricular remodeling to examine the effect of losmapimod on clinical events in both patients with STEMI and patients with NSTEMI. The LATITUDE-TIMI 60 (Losmapimod to Inhibit p38 MAP Kinase as a Therapeutic Target and Modify Outcomes After an Acute Coronary Syndrome-Thrombolysis In Myocardial Infarction 60) trial was a multinational randomized, placebo-controlled, double-blind, parallel-group phase 3 trial that randomized 3,503 patients to either twice-daily losmapimod (7.5 mg) or placebo for 12 weeks (86). Participants received the study drug or placebo as early as possible during the hospitalization, but at least before coronary revascularization was performed. Patients were followed for an additional 12 weeks after the end of treatment. Although the losmapimod group had significantly lower levels of hsCRP and N-terminal pro–B-type natriuretic peptide at both 4 and 12 weeks after drug initiation compared with the placebo group, there was no difference in the primary composite endpoint of cardiovascular death, MI, or recurrent ischemia between treatment groups (hazard ratio: 1.16; 95% CI: 0.91 to 1.47; p = 0.24) (Figure 5).
Figure 5.
Cumulative Incidence Curves for Cardiovascular Death, MI, or Severe Recurrent Ischemia Leading to Urgent Revascularization Through 12 Weeks With Losmapimod Versus Placebo (LATITUDE-TIMI 60, Phase 3)
CI = confidence interval; LATITUDE-TIMI 60 = Losmapimod to Inhibit p38 MAP Kinase as a Therapeutic Target and Modify Outcomes After an Acute Coronary Syndrome-Thrombolysis In Myocardial Infarction 60; MI = myocardial infarction.
Reproduced with permission from O’Donoghue et al. (86).
Failure of Anti-Inflammatory Therapies in Cardiovascular Disease
Surrogate marker versus therapeutic agent
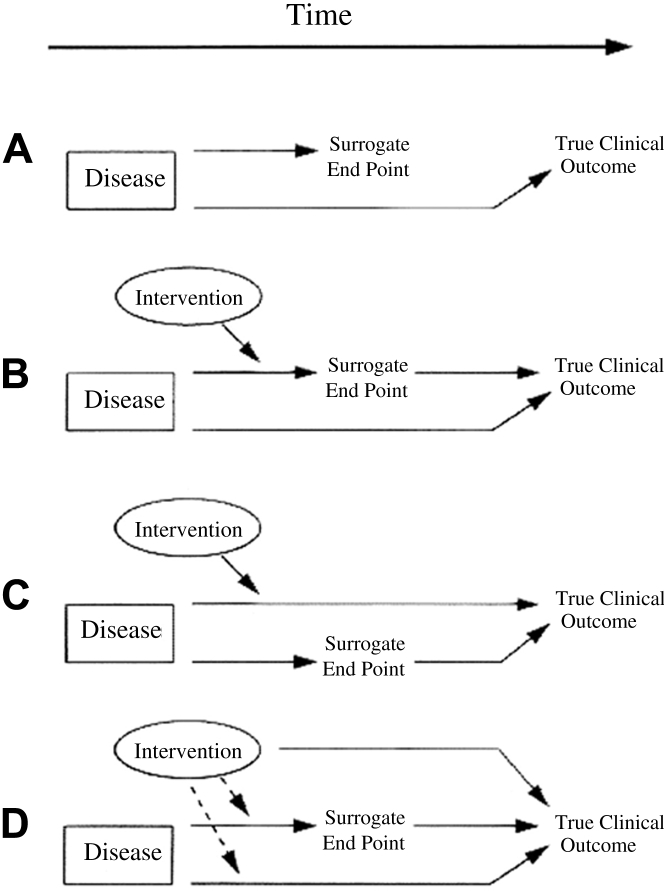
Although many inflammatory pathways have been targeted for a blockbuster effect on the pathogenesis of atherosclerosis, the novel therapeutic agents targeting these pathways have consistently failed to demonstrate significant benefit on clinical outcomes. There are several possible explanations for why targeting inflammation has failed to translate to effective therapeutics. First and foremost is the possibility that inflammatory molecules that have been targeted, although clearly correlated with the atherosclerosis and risk for acute coronary events, may be imperfect surrogates for clinical endpoints. Thus, although LP-PLA2 is highly expressed in the necrotic cores and nearby macrophages of thin-cap fibroatheromas and ruptured plaques (41) and a deficiency of LP-PLA2 has been associated with decreased cardiovascular risk (43), the use of darapladib, an inhibitor of LP-PLA2 activity, failed to show benefit on cardiovascular outcomes. Similarly, although the activities of LP-PLA2, C5, sPLA2, and IL-1 are associated with cardiovascular risk and poor cardiovascular outcomes, inhibition of the activity of these molecules does not translate to improved outcomes. One of the classic demonstrations of the importance of distinguishing between a “correlate” and a “surrogate” comes from the work done by David DeMets and Thomas Fleming 87, 88. They showed that a disease state may influence causally both a biomarker (e.g., increase the level or activity of an inflammatory molecule in atherosclerotic plaque) and the true clinical efficacy endpoint (e.g., occurrence of MI) without the biomarker actually lying in the pathway from disease to clinical endpoint. In this case, a preclinical or mechanistic study might be misleading by suggesting a causal relationship between the biomarker and clinical endpoint when only a correlation exists. Perhaps even more likely, in the case of the inflammatory molecules and pathways we reviewed, there may be multiple and redundant inflammatory pathways that influence the occurrence of clinical endpoints. If the surrogate that is targeted by a novel therapeutic lies in only 1 of these pathways, the others remain active in their influence on the clinical outcome of interest, and the estimation of the effect of the novel treatment could be over or underestimated. It is also possible that the surrogate targeted by a novel therapeutic lies in several pathways. In this case, the novel treatment for the surrogate may differentially affect the pathways: there may be a beneficial effect on 1 pathway, but undesirable or deleterious (and sometimes, unpredicted) consequences on the other pathways (Figure 6). The end result of each scenario would be a failure to translate, and may suggest that highly specific, targeted therapy to a single molecule may not be the ideal approach to modulating inflammation as a treatment for ischemic heart disease.
Figure 6.
Potential Reasons for Surrogate Endpoint Failure
(A) The surrogate endpoint is not in the causal pathway of the disease state. (B) There are several causal pathways of the disease state, but the intervention only impacts the pathway mediated by the surrogate endpoint. (C) There are several causal pathways of the disease state, but the pathway mediated by the surrogate endpoint is not impacted by the intervention. (D) The intervention acts on a pathway separate from the disease process.
Reproduced with permission from Fleming and DeMets (88).
Medications targeted at 1 pathway or molecule also may have substantial off-target effects. In the case of statins, these may have been beneficial effects on inflammation. Although cited for their LDL-C–lowering properties, statins likely have significant pleiotropic effects that were under-recognized at the time of their development that may explain some of their benefit (89). Not only do statins lower LDL-C, they improve endothelial dysfunction (90); reduce oxidized LDL, macrophages, and T cells in atherosclerotic plaques (91); and reduce levels of adhesion molecules, such as E-selectin, P-selectin, and ICAM-1 92, 93, as well as C-reactive protein (94). Direct targeting of any of these pathways individually likely would have been less successful. Conversely, the clinical failure of many of the agents reviewed here may be the result of unanticipated effects on pathways downstream or parallel to those directly targeted.
Translation of work in animal models to humans
We have shown several examples of promising anti-inflammatory therapies in animal models of myocardial ischemia, infarction, and reperfusion that did not translate to benefit in phase 3 clinical outcomes trials in humans. This may reflect that animal models of atherosclerosis and acute coronary events do not completely mimic these processes in humans. For example, to simulate ischemia and reperfusion in animal studies, ligation of coronary arteries or balloon occlusion must be performed. The extent to which artificial occlusion and ligation mimics an ischemic event in humans is not completely known. Small-animal models used for investigating interventions to inhibit luminal narrowing after arterial instrumentation have not closely simulated human stenotic lesions and the instrumentation of these lesions (95). Additional reasons mouse models fail to mimic human experiments were highlighted in a prior compelling review, and include the use of congenic strains with genetic homogeneity and lack of immunological exposures in experimental mice (96). Continued failure in large clinical trials of promising, new anti-inflammatory drugs that demonstrated efficacy in animal models is also likely contributed to by failure of even large-animal models of atherosclerotic disease to simulate atherosclerotic disease in humans and mechanical induction of ischemia to approximate ischemic and plaque rupture events in humans. Although porcine models of coronary disease most closely mimic human coronary anatomy and the histological characteristics of the proliferative response to deep coronary artery injury (97), pigs are highly prone to vasospasm during mechanical manipulation (98). Even large-animal models have not been predictive of outcomes in humans when using interventions to alter hemostasis 99, 100. Additionally, no suitable large-animal model exists of plaque rupture that closely approximates human plaque rupture (95).
Timing of administration of anti-inflammatory drugs
Perhaps 1 of the most compelling explanations for why the anti-inflammatory agents we discussed appeared beneficial in animal models but not in large, clinical outcomes trials is the timing of administration of these anti-inflammatory drugs. It may be that to have their greatest effect on clinical outcomes, these drugs must be present at the site of ischemic injury at the time of the ischemia-reperfusion insult. For example, in animal models of losmapimod treatment in ischemia-reperfusion, the greatest cardioprotective benefit came when the p38 MAPK inhibitor was administered before or at the onset of ischemia, with little to no benefit when administered at the onset of reperfusion 77, 78. However, the occurrence of acute coronary events in humans does not easily accommodate the administration of agents like losmapimod prior to the occlusion of a coronary artery (as in animal models of coronary ligation). Further, the design and logistics of human studies often do not allow for controlled, early administration at the time of reperfusion. In SOLSTICE, losmapimod could be administered any time within 18 h of presentation with NSTEMI (85). At 18 h after presentation with NSTEMI, ischemic injury and the resultant inflammatory process is likely well underway, which may explain the discrepancy in results between the preclinical and clinical studies. Similarly, although the recombinant immunoglobulin to PSGL-1 was administered 15 min prior to balloon injury in an animal model (66), the administration of inclacumab occurred periprocedurally in SELECT-ACS (69). Unfortunately, earlier administration of these novel agents prior to or at the onset of ischemia is impractical in clinical practice.
Conclusions
Over the past several decades, many novel therapies have targeted inflammatory pathways involved in ACS and atherosclerotic disease with the hope of improving outcomes post-ACS. Although many agents showed promising results in early animal models and preclinical studies, demonstrating reductions in levels of inflammatory markers and infarct size, these results failed to translate to therapeutic benefit when tested in large randomized clinical outcomes trials. There are many potential explanations for why these seemingly promising therapies failed in clinical testing, from incomplete understanding of the role of inflammation in CAD and ACS to imperfect animal models of disease and treatment. This does not mean that we should abandon investigation into modulation of inflammation to improve cardiovascular outcomes. Rather, these experiences should challenge us scientifically to more deeply understand the contribution of inflammation in ACS (correlate or mediator), while at the same time rethinking the animal model constructs we use in early drug development so that they as closely as possible mimic human clinical conditions.
Footnotes
Both authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Go A.S., Mozaffarian D., Roger V.L. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Z., Winget M. Economic burden of illness of acute coronary syndromes: medical and productivity costs. BMC Health Services Research. 2011;11:2–9. doi: 10.1186/1472-6963-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghushchyan V., Nair K.V., Page R.L. Indirect and direct costs of acute coronary syndromes with comorbid atrial fibrillation, heart failure, or both. Vasc Health Risk Manag. 2015;11:25–34. doi: 10.2147/VHRM.S72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh R.W., Sidney S., Chandra M., Sorel M., Selby J.V., Go A.S. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 5.Tobbia P., Brodie B.R., Witzenbichler B. Adverse event rates following primary PCI for STEMI at US and non-US hospitals: three-year analysis from the HORIZONS-AMI trial. EuroIntervention. 2013;8:1134–1142. doi: 10.4244/EIJV8I10A176. [DOI] [PubMed] [Google Scholar]
- 6.Brieger D., Fox K.A., Fitzgerald G. Predicting freedom from clinical events in non-ST-elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart. 2009;95:888. doi: 10.1136/hrt.2008.153387. [DOI] [PubMed] [Google Scholar]
- 7.Suleiman M., Khatib R., Agmon Y. Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction predictive role of C-reactive protein. J Am Coll Cardiol. 2006;47:962–968. doi: 10.1016/j.jacc.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 8.Kragholm K., Newby L.K., Melloni C. Emerging treatment options to improve cardiovascular outcomes in patients with acute coronary syndrome: focus on losmapimod. Drug Des Devel Ther. 2015;9:4279–4286. doi: 10.2147/DDDT.S69546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas T.C., Rollins S.A., Rother R.P. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol. 1996;33:1389–1401. doi: 10.1016/s0161-5890(96)00078-8. [DOI] [PubMed] [Google Scholar]
- 10.Pinckard R.N., Olson M.S., Giclas P.C., Terry R., Boyer J.T., O'Rourke R.A. Consumption of classical complement components by heart subcellular membranes in vitro and in patients after acute myocardial infarction. J Clin Invest. 1975;56:740–750. doi: 10.1172/JCI108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathey D., Schofer J., Schafer H.J. Early accumulation of the terminal complement-complex in the ischaemic myocardium after reperfusion. Eur Heart J. 1994;15:418–423. doi: 10.1093/oxfordjournals.eurheartj.a060516. [DOI] [PubMed] [Google Scholar]
- 12.Vakeva A.P., Agah A., Rollins S.A. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–2267. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- 13.Tang K., Cheng Y., Wu S., Liu L., Cheng L. Protective effect of C5 shRNA on myocardial ischemia-reperfusion injury in rats. Can J Physiol Pharmacol. 2012;90:1394–1402. doi: 10.1139/y2012-114. [DOI] [PubMed] [Google Scholar]
- 14.Amsterdam E.A., Stahl G.L., Pan H.L., Rendig S.V., Fletcher M.P., Longhurst J.C. Limitation of reperfusion injury by a monoclonal antibody to c5a during myocardial infarction in pigs. Am J Physiol. 1995;268:H448–H457. doi: 10.1152/ajpheart.1995.268.1.H448. [DOI] [PubMed] [Google Scholar]
- 15.Mahaffey K.W., Granger C.B., Nicolau J.C. Effect of pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to fibrinolysis in acute myocardial infarction: The COMPlement inhibition in myocardial infarction treated with thromboLYtics (COMPLY) trial. Circulation. 2003;108:1176–1183. doi: 10.1161/01.CIR.0000087404.53661.F8. [DOI] [PubMed] [Google Scholar]
- 16.Granger C.B., Mahaffey K.W., Weaver W.D. Effect of pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous intervention acute myocardial infarction: the COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial. Circulation. 2003;108:1184–1190. doi: 10.1161/01.CIR.0000087447.12918.85. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong P.W., Mahaffey K.W., Chang W.C. Concerning the mechanism of pexelizumab’s benefit in acute myocardial infarction. Am Heart J. 2006;151:787–790. doi: 10.1016/j.ahj.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Stahl G.L., Amsterdam E.A., Symons J.D. Role of thromboxane A2 in the cardiovascular response to intracoronary C5a. Circ Res. 1990;66:1103–1111. doi: 10.1161/01.res.66.4.1103. [DOI] [PubMed] [Google Scholar]
- 19.Topham P.S., Haydar S.A., Kuphal R. Complement-mediated injury reversibly disrupts glomerular epithelial cell actin microfilaments and focal adhesions. Kidney Int. 1999;55:1763–1775. doi: 10.1046/j.1523-1755.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong P.W., Granger C.B., Adams P.X., for the APEX AMI Investigators Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- 21.Fitch J.C., Rollins S., Matis L. Pharmacology and biological efficacy of a recombinant, humanized, single-chain antibody C5 complement inhibitor in patients undergoing coronary artery bypass graft surgery with cardiopulmonary bypass. Circulation. 1999;100:2499–2506. doi: 10.1161/01.cir.100.25.2499. [DOI] [PubMed] [Google Scholar]
- 22.Royston D. Systemic inflammatory responses to surgery with CPB. Perfusion. 1996;11:177–189. doi: 10.1177/026765919601100302. [DOI] [PubMed] [Google Scholar]
- 23.Wan S., LeClerc J.-L., Vincent J.-L. Inflammatory response to CPB. Chest. 1997;112:676–692. doi: 10.1378/chest.112.3.676. [DOI] [PubMed] [Google Scholar]
- 24.Hall R.I., Smith M.S., Rocker G. The systemic inflammatory response to CPB. Anesth Analg. 1997;85:766–782. doi: 10.1097/00000539-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Verrier E.D., Shernan S.K., Taylor K.M. Terminal complement blockade with pexelizumab during coronary artery bypass graft surgery requiring cardiopulmonary bypass: a randomized trial. JAMA. 2004;291:2319–2327. doi: 10.1001/jama.291.19.2319. [DOI] [PubMed] [Google Scholar]
- 26.Haverich A., Shernan S.K., Levy J.H. Inhibition of complement activation by pexelizumab reduced death and myocardial infarction in higher risk cardiac surgical patients. Ann Thorac Surg. 2006;82:486–492. doi: 10.1016/j.athoracsur.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 27.Smith P.K., Shernan S.K., Chen J.C. Effects of C5 complement inhibitor pexelizumab on outcome in high-risk coronary artery bypass grafting: combined results from the PRIMO-CABG I and II trials. J Thorac Cardiovasc Surg. 2011;142:89–98. doi: 10.1016/j.jtcvs.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 28.Testa L., Van Gaal W.J., Bhindi R. Pexelizumab in ischemic heart disease: a systematic review and meta-analysis on 15,196 patients. J Thorac Cardiovasc Surg. 2008;136:884–893. doi: 10.1016/j.jtcvs.2007.12.062. [DOI] [PubMed] [Google Scholar]
- 29.Nicholls S.J., Cavender M.A., Kastelein J.P. Inhibition of secretory phospholipase A(2) in patients with acute coronary syndromes: rationale and design of the Vascular Inflammation Suppression to Treat Acute Coronary Syndrome for 16 Weeks (VISTA-16) trial. Cardiovasc Drugs Ther. 2012;26:71–75. doi: 10.1007/s10557-011-6358-9. [DOI] [PubMed] [Google Scholar]
- 30.Karabina S.A., Brocheriou S., Naour G.L. Atherogenic properties of LDL particles modified by human group X secreted phospholipase A2 on human endothelial cell function. FASEB. 2006;20:2547–2549. doi: 10.1096/fj.06-6018fje. [DOI] [PubMed] [Google Scholar]
- 31.Gora S., Maouche S., Atout R. Phospholipolyzed LDL induces an inflammatory response in endothelial cells through endoplasmic reticulum stress signaling. FASEB. 2010;24:3284–3297. doi: 10.1096/fj.09-146852. [DOI] [PubMed] [Google Scholar]
- 32.Fuentes L., Hernandez M., Fernandez-Aviles F.J. Cooperation between secretory phospholipase A2 and TNF-receptor superfamily signaling: implications for the inflammatory response in atherogenesis. Circ Res. 2002;91:681–688. doi: 10.1161/01.res.0000038341.34243.64. [DOI] [PubMed] [Google Scholar]
- 33.Liu P.Y., Li Y.H., Wei-Chuan T. Prognostic value and the changes of plasma levels of secretory type II phospholipase A2 in patients with coronary artery disease undergoing percutaneous coronary intervention. Eur Heart J. 2003;24:1824–1832. doi: 10.1016/j.ehj.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Kugiyama K., Ota Y., Sugiyama S. Prognostic value of plasma levels of secretory type II phospholipase A2 in patients with unstable angina pectoris. Am J Cardiol. 2000;86:718–722. doi: 10.1016/s0002-9149(00)01069-9. [DOI] [PubMed] [Google Scholar]
- 35.Mallat Z., Steg G., Benessiano J. Circulating secretory phospholipase A2 activity predicts recurrent events in patients with severe acute coronary syndromes. J Am Coll Cardiol. 2005;46:1249–1257. doi: 10.1016/j.jacc.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 36.Rosenson R.S., Hislop C., McConnell D. Effects of 1-H-indole-3-glyoxamide (A-002) on concentration of secretory phospholipase A2 (PLASMA study): a phase II double-blind, randomised, placebo-controlled trial. Lancet. 2009;373:649–658. doi: 10.1016/S0140-6736(09)60403-7. [DOI] [PubMed] [Google Scholar]
- 37.Rosenson R.S., Hislop C., Elliott M. Effects of varespladib methyl on biomarkers and major cardiovascular events in acute coronary syndrome patients. J Am Coll Cardiol. 2010;56:1079–1088. doi: 10.1016/j.jacc.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Rosenson R.S., Elliott M., Stasiv Y. Randomized trial of an inhibitor of secretory phospholipase A2 on atherogenic lipoprotein subclasses in statin-treated patients with coronary heart disease. Eur Heart J. 2011;32:999–1005. doi: 10.1093/eurheartj/ehq374. [DOI] [PubMed] [Google Scholar]
- 39.Nicholls S.J., Kastelein J., Schwartz G.G. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311:252–262. doi: 10.1001/jama.2013.282836. [DOI] [PubMed] [Google Scholar]
- 40.MacPhee C.H., Moores K.E., Boyd H.F. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J. 1999;338:479–487. [PMC free article] [PubMed] [Google Scholar]
- 41.Kolodgie F.D., Burke A.P., Skorija K.S. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2523–2529. doi: 10.1161/01.ATV.0000244681.72738.bc. [DOI] [PubMed] [Google Scholar]
- 42.Häkkinen T., Luoma J.S., Hiltunen M.O. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:2909–2917. doi: 10.1161/01.atv.19.12.2909. [DOI] [PubMed] [Google Scholar]
- 43.Jang Y., Waterworth D., Lee J.-E. Carriage of the V279F null allele within the gene encoding Lp-PLA2 is protective from coronary artery disease in South Korean males. PloS One. 2011;6:e18208. doi: 10.1371/journal.pone.0018208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The Lp-PLA2 Studies Collaboration Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Angelantonio E., Gao P., Pennells L. Lipid-related markers and cardiovascular disease prediction. JAMA. 2012;307:2499–2506. doi: 10.1001/jama.2012.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serruys P.W., Garcia-Garcia H.M., Buszman P. Effects of the direct-lipoprotein associated phospholipase A2 inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118:1172–1182. doi: 10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- 47.Wilensky R.L., Shi Y., Mohler E.R. Inhibition of lipoprotein-associated Phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nature Medicine. 2008;14:1059–1066. doi: 10.1038/nm.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White H.D., Held C., Stewart R. Darapladib for preventing ischemic events in stable coronary disease. N Eng J Med. 2014;370:1702–1711. doi: 10.1056/NEJMoa1315878. [DOI] [PubMed] [Google Scholar]
- 49.O’Donoghue M.L., Braunwald E., White H.D. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA. 2014;312:1006–1015. doi: 10.1001/jama.2014.11061. [DOI] [PubMed] [Google Scholar]
- 50.Wallentin L., Held C., Armstrong P.W. Lipoprotein-associated phospholipase A2 activity is a marker of risk but not a useful target for treatment in patients with stable coronary heart disease. J Am Heart Assoc. 2016;5:e003407. doi: 10.1161/JAHA.116.003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ridker P.M., Thuren T., Zalewski A. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: Rationale and Design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Bevilacqua M.P., Pober J.S., Wheeler M.E. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985;76:2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dinarello C.A. Interleukin-1beta and the autoinflammatory diseases. N Eng J Med. 2009;360:2467–2470. doi: 10.1056/NEJMe0811014. [DOI] [PubMed] [Google Scholar]
- 54.Fearon W.F., Fearon D.T. Inflammation and cardiovascular disease: role of the interleukin-1 receptor antagonist. Circulation. 2008;117:2577–2579. doi: 10.1161/CIRCULATIONAHA.108.772491. [DOI] [PubMed] [Google Scholar]
- 55.Patti G., D’Ambrosio A., Dobrina A. Interleukin-1 receptor antagonist: a sensitive marker of instability in patients with coronary artery disease. J Thromb Thrombolysis. 2002;14:139–143. doi: 10.1023/a:1023284912712. [DOI] [PubMed] [Google Scholar]
- 56.Galea J., Armstrong J., Gadsdon P. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16:1000–1006. doi: 10.1161/01.atv.16.8.1000. [DOI] [PubMed] [Google Scholar]
- 57.Larsen C.M., Faulenbach M., Vaag A. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 58.Casini-Raggi V., Kam L., Chong Y.J. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995;154:2434–2440. [PubMed] [Google Scholar]
- 59.Martinon F., Petrilli V., Mayor A. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 60.Abbate A., Van Tassell B.W., Seropian I.M. Interleukin-1β modulation using a genetically engineered antibody prevents adverse cardiac remodeling following acute myocardial infarction in the mouse. Eur J Heart Fail. 2010;12:319–322. doi: 10.1093/eurjhf/hfq017. [DOI] [PubMed] [Google Scholar]
- 61.Ridker P.M., Howard C.P., Walter V. Effects of interleukin-1[beta] inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 62.Blann A.D., Nadar S.K., Lip G.Y.H. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24:2166–2179. doi: 10.1016/j.ehj.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 63.Tenaglia A.N., Buda J.A., Wilkins G.R. Levels of expression of P-selectin, E-selectin, and intercellular adhesion molecule-1 in coronary atherectomy specimens from patients with stable and unstable angina pectoris. Am J Cardiol. 1997;79:742–747. doi: 10.1016/s0002-9149(96)00861-2. [DOI] [PubMed] [Google Scholar]
- 64.Scialla J.J., Plantinga L.C., Kao W.H.L. Soluble P-selectin levels are associated with cardiovascular mortality and sudden cardiac death in male dialysis patients. Am J Nephrol. 2011;33:224–230. doi: 10.1159/000324517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wagner D.D., Burger P.C. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 66.Burger P.C., Wagner D.D. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 67.Phillips W.J., Barringhaus K.G., Sanders J.M. Single injection of P-selectin or P-selectin glycoprotein ligand-1 monoclonal antibody blocks neointima formation after arterial injury in apolipoprotein E-deficient mice. Circulation. 2003;107:2244–2249. doi: 10.1161/01.CIR.0000065604.56839.18. [DOI] [PubMed] [Google Scholar]
- 68.Wang K., Zhou Z., Zhou X. Prevention of intimal hyperplasia with recombinant soluble P-selectin glycoprotein ligand-immunoglobulin in the porcine coronary artery balloon injury model. J Am Coll Cardiol. 2001;38:577–582. doi: 10.1016/s0735-1097(01)01347-x. [DOI] [PubMed] [Google Scholar]
- 69.Tanguay J.F., Geoffrey P., Sirois M.G. Prevention of in-stent restenosis via reduction of thrombo-inflammatory reactions with recombinant P-selectin glycoprotein ligand-1. Thromb Haemost. 2004;91:1186–1193. doi: 10.1160/TH03-11-0701. [DOI] [PubMed] [Google Scholar]
- 70.Tardif J.C., Tanguay J.F., Wright S.R. Effects of the P-Selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non-ST-segment elevation myocardial infarction: results of the SELECT-ACS Trial. J Am Coll Cardiol. 2013;61:2048–2055. doi: 10.1016/j.jacc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Stahli B.E., Tardif J.C., Carrier M. Effects of P-selectin antagonist inclacumab in patients undergoing coronary artery bypass graft surgery: SELECT-CABG trial. J Am Coll Cardiol. 2016;67:344–346. doi: 10.1016/j.jacc.2015.10.071. [DOI] [PubMed] [Google Scholar]
- 72.Martin E.D., Bassi R., Marber M.S. p38 MAPK in cardioprotection—are we there yet? Br J Pharmacol. 2015;172:2101–2113. doi: 10.1111/bph.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fisk M., Gajendragadkar P.R., Mäki-Petäjä K.M., Wilkinson I.B., Cheriyan J. Therapeutic potential of p38 MAP kinase inhibition in the management of cardiovascular disease. Am J Cardiovasc Drugs. 2014;14:155–165. doi: 10.1007/s40256-014-0063-6. [DOI] [PubMed] [Google Scholar]
- 74.Cheriyan J., Webb A.J., Sarov-Blat L. Inhibition of p38 mitogen-activated protein kinase improves nitric oxide-mediated vasodilatation and reduces inflammation in hypercholesterolemia. Circulation. 2011;123:515–523. doi: 10.1161/CIRCULATIONAHA.110.971986. [DOI] [PubMed] [Google Scholar]
- 75.Denise Martin E., De Nicola G.F., Marber M.S. New therapeutic targets in cardiology: p38 alpha mitogen-activated protein kinase for ischemic heart disease. Circulation. 2012;126:357–368. doi: 10.1161/CIRCULATIONAHA.111.071886. [DOI] [PubMed] [Google Scholar]
- 76.Ma X.L., Kumar S., Gao F. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;13:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- 77.Surinkaew S., Kumphune S., Chattipakorn S. Inhibition of p38 MAPK during ischemia, but not reperfusion, effectively attenuates fatal arrhythmia in ischemia/reperfusion heart. J Cardiovasc Pharmacol. 2013;61:133–141. doi: 10.1097/FJC.0b013e318279b7b1. [DOI] [PubMed] [Google Scholar]
- 78.See F., Thomas W., Way K. p38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J Am Coll Cardiol. 2004;44:1679–1689. doi: 10.1016/j.jacc.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 79.Seeger F.H., Sedding D., Langheinrich A.C., Haendeler J., Zeiher A.M., Dimmeler S. Inhibition of the p38 MAP kinase in vivo improves number and functional activity of vasculogenic cells and reduces atherosclerotic disease progression. Basic Res Cardiol. 2010;105:389–397. doi: 10.1007/s00395-009-0072-9. [DOI] [PubMed] [Google Scholar]
- 80.Almagor M., Keren A., Banai S. Increased C-reactive protein level after coronary stent implantation in patients with stable coronary artery disease. Am Heart J. 2003;145:248–253. doi: 10.1067/mhj.2003.16. [DOI] [PubMed] [Google Scholar]
- 81.Sarov-Blat L., Morgan J.M., Fernandez P. Inhibition of p38 mitogen-activated protein kinase reduces inflammation after coronary vascular injury in humans. Arterioscler Thromb Vasc Biol. 2010;30:2256–2263. doi: 10.1161/ATVBAHA.110.209205. [DOI] [PubMed] [Google Scholar]
- 82.Elkhawad M., Rudd J.H., Sarov-Blat L. Effects of p38 mitogen-activated protein kinase inhibition on vascular and systemic inflammation in patients with atherosclerosis. J Am Coll Cardiol Img. 2012;5:911–922. doi: 10.1016/j.jcmg.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 83.Newby L.K., Marber M.S., Melloni C. Losmapimod, a novel p38 mitogen-activated protein kinase inhibitor, in non-ST-segment elevation myocardial infarction: results of a randomised phase 2 trial. Lancet. 2014;384:1187–1195. doi: 10.1016/S0140-6736(14)60417-7. [DOI] [PubMed] [Google Scholar]
- 84.O’Donoghue M.L., Glaser R., Cavender M.A. Effect of losmapimod on cardiovascular outcomes in patients hospitalized with acute myocardial infarction: a randomized clinical trial. JAMA. 2016;315:1591–1599. doi: 10.1001/jama.2016.3609. [DOI] [PubMed] [Google Scholar]
- 85.Fleming T. Surrogate endpoints and FDA’s accelerated approval process. Health Aff (Millwood) 2005;24:67–78. doi: 10.1377/hlthaff.24.1.67. [DOI] [PubMed] [Google Scholar]
- 86.Fleming T.R., DeMets D.L. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 87.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:39–43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 88.Eichstadt H.W., Eskotter H., Hoffman I. Improvement of myocardial perfusion by short-term fluvastatin therapy in coronary artery disease. Am J Cardiol. 1995;76:122A–125A. doi: 10.1016/s0002-9149(05)80033-5. [DOI] [PubMed] [Google Scholar]
- 89.Crisby M., Nordin-Fredriksson G., Shah P.K. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation. 2001;103:926–933. doi: 10.1161/01.cir.103.7.926. [DOI] [PubMed] [Google Scholar]
- 90.Hackman A., Abe Y., Insull W., Jr. Levels of soluble cell adhesion molecules in patients with dyslipidemia. Circulation. 1996;93:1334–1338. doi: 10.1161/01.cir.93.7.1334. [DOI] [PubMed] [Google Scholar]
- 91.Seljeflot I., Tonstad S., Hjermann I. Reduced expression of endothelial cell markers after 1 year treatment with simvastatin and atorvastatin in patients with coronary heart disease. Atherosclerosis. 2002;162:179–185. doi: 10.1016/s0021-9150(01)00696-7. [DOI] [PubMed] [Google Scholar]
- 92.Ridker P.M., Rifai N., Pfeffer M.A., for the Cholesterol and Recurrent Events (CARE) Investigators Long-term effects of pravastatin on plasma concentration of C-reactive protein. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 93.Conn P.M. Elsevier; London: 2013. Animal Models for the Study of Human Disease. [Google Scholar]
- 94.Libby P. Murine “model” monotheism: an iconoclast at the altar of mouse. Circ Res. 2015;117:921–925. doi: 10.1161/CIRCRESAHA.115.307523. [DOI] [PubMed] [Google Scholar]
- 95.Schwartz R.S., Murphy J.G., Edwards W.D. Restenosis after balloon angioplasty: a practical proliferative model in porcine coronary arteries. Circulation. 1990;82:2190–2200. doi: 10.1161/01.cir.82.6.2190. [DOI] [PubMed] [Google Scholar]
- 96.Bloor C., White F., Roth D. The pig as a model of myocardial ischemia and gradual coronary artery occlusion. In: Swindle M.M., editor. Swine as Models in Biomedical Research. Iowa State University Press; Ames, Iowa: 1992. pp. 163–175. [Google Scholar]
- 97.Buchwald A.B., Unterberg C., Nebendahl K. Low-molecular-weight heparin reduces neointimal proliferation after coronary stent implantation in hypercholesterolemic minipigs. Circulation. 1992;86:531–537. doi: 10.1161/01.cir.86.2.531. [DOI] [PubMed] [Google Scholar]
- 98.Clopath P. The effect of acetylsalicylic acid (ASA) on the development of atherosclerotic lesions in miniature swine. Br J Exp Pathol. 1980;61:440–443. [PMC free article] [PubMed] [Google Scholar]
- 99.Legendre C.M., Licht C., Muus P. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 100.Kelly R.J., Höchsmann B., Szer J. Eculizumab in Pregnant Patients with Paroxysmal Nocturnal Hemoglobinuria. N Engl J Med. 2015;373:1032–1039. doi: 10.1056/NEJMoa1502950. [DOI] [PubMed] [Google Scholar]
- 101.Yehoshua Z., de Amorim Garcia Filho C.A. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology. 2014;121:693–701. doi: 10.1016/j.ophtha.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shaddinger B.C., Xu Y., Roger J.H. Platelet aggregation unchanged by lipoprotein-associated phospholipase A2 inhibition: results from an in vitro study and two randomized phase I trials. PLoS One. 2014;9:e83094. doi: 10.1371/journal.pone.0083094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brucato A., Imazio M., Gattorno M. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA. 2016;316:1906–1912. doi: 10.1001/jama.2016.15826. [DOI] [PubMed] [Google Scholar]
- 104.Scott I.C., Ibrahim F., Simpson G. A randomised trial evaluating anakinra in early active rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:88–93. [PubMed] [Google Scholar]
- 105.Tzanetakou V., Kanni T., Giatrakou S. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52–59. doi: 10.1001/jamadermatol.2015.3903. [DOI] [PubMed] [Google Scholar]
- 106.Ataga K.I., Kutlar A., Kanter J. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376:429–439. doi: 10.1056/NEJMoa1611770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schreiber S., Feagan B., D'Haens G. Oral p38 mitogen-activated protein kinase inhibition with BIRB 796 for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:325–334. doi: 10.1016/j.cgh.2005.11.013. [DOI] [PubMed] [Google Scholar]