Corresponding Author

Key Words: heart failure, interleukin-1β, myocardial infarction
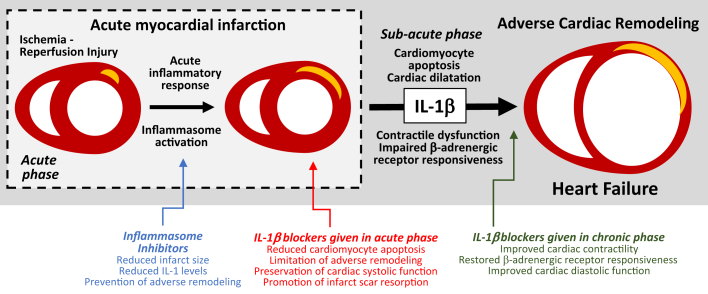
Acute myocardial infarction (AMI) and heart failure (HF) are characterized by an intense inflammatory response that contributes to progression of the injury and dysfunction (1). Tissue injury stimulates the formation of the inflammasome and the production of interleukin (IL)-1β 2, 3, the prototypical cytokine involved in virtually every local and systemic inflammatory response, also known as the “fever molecule” (4) (Figure 1).
Figure 1.
Role of IL-1β in Adverse Cardiac Remodeling and Heart Failure
Acute myocardial infarction (AMI) results from an abrupt oxygen supply/demand imbalance deriving from coronary atherothrombosis. Myocardial ischemia and/or ischemia-reperfusion injury determine loss of functional myocardium and induce an intense acute inflammatory response, characterized by the activation of the inflammasome and maturation of interleukin (IL)-1β and other pro-inflammatory cytokines. IL-1β induces further loss of viable myocardium, promoting cardiac dilation and dysfunction in the sub-acute phase of AMI, and suppressing cardiac contractility and β-adrenergic receptor responsiveness. Inhibitors of the inflammasome or of IL-1β given during AMI have the potential to prevent adverse cardiac remodeling and/or heart failure.
In this issue of JACC: Basic to Translational Science, Harouki et al. (5) show that neutralization of IL-1β using the monoclonal antibody gevokizumab initiated shortly after reperfusion (1 h) or late (7 days) significantly improved cardiac remodeling and systolic and diastolic function in nondiabetic as well as diabetic rats with AMI due to ischemia-reperfusion injury. Furthermore, when gevokizumab was administered 83 days after reperfusion, and when cardiac dilation and dysfunction had already been established, IL-1β blockade resulted in an improvement in systolic function. These beneficial effects of IL-1β blockade in preventing adverse cardiac remodeling after AMI had been, in part, already described (3). A murine analog of gevokizumab was tested in mice with large nonreperfused AMI showing similar results (6). Another IL-1β-blocking antibody, a murine analog of canakinumab, was also shown to improve cardiac function in mice early and late after large nonreperfused AMI 7, 8. The current study by Harouki et al. (5) confirms prior findings, deriving for the most part from a single laboratory and significantly expanded prior findings by exploring the AMI model with reperfusion and including experiments in rats with diabetes. The addition of reperfusion adds a clear clinical translational value as most of the patients with AMI nowadays receive reperfusion. It is important to note that the treatment occurred 1 h after reperfusion and not before reperfusion. This makes it a clinically relevant model, avoiding the dilemma of whether treatment after reperfusion, as occurs in practice, would jeopardize benefit (9). They also added 2 treatment groups for rats with established cardiac dysfunction at 7 and 83 days after reperfusion, confirming that the beneficial effects of IL-1β blockade on cardiac contractility are, at least in part, time-independent. They show also a smaller infarct scar size measured on day 90, with gevokizumab given early, which, combined with prior data of no-infarct sparing effect of another IL-1β antibody (10), suggests an effect on infarct resorption. Studying rats with diabetes is also very clever because patients with AMI are often diabetic. Gevokizumab also improved acetylcholine-induced coronary relaxation and significantly reduced oxidative stress, providing a potential molecular mechanism. The reported effects of the IL-1β pathway in AMI are described in Figure 1.
The data presented by Harouki et al. (5) come at a very appropriate time in the clinical development of IL-1β blockers in heart disease (3). Anakinra, a recombinant IL-1 receptor antagonist, has been tested in a pilot feasibility study of 10 patients with ST-segment elevation AMI (11) and in an additional follow-up proof-of-concept study of 30 patients (12), showing a favorable safety and tolerability profile, a significant blunting of the inflammatory response, and a promising signal of heart failure incidence (13). Small proof-of-concept studies in patients with systolic heart failure treated with anakinra also showed a promising improvement in exercise capacity and quality of life 14, 15. On June 22, 2017, Novartis Pharma announced in a press release that the large phase III clinical trial of canakinumab, an IL-1β antibody, in 10,061 patients with prior AMI (CANTOS [Canakinumab Anti-inflammatory Thrombosis Outcomes Study]), met its primary endpoint, meaning that the treatment significantly reduced the composite endpoint of cardiovascular death, nonfatal AMI, and nonfatal stroke (16). The bench-to-bedside translation for IL-1β blockade is therefore becoming a reality. Based on preclinical data in AMI and HF, including the data presented herein (5) and the promising data in the CANTOS trial (16), it is foreseeable that IL-1β blockers will be further explored as a treatment strategy also for patients with AMI and/or symptomatic HF in the near future.
Footnotes
Drs. Abbate and Van Tassell are supported in part by National Institutes of Health/National Heart Lung and Blood Institute grant R34 HL121402. Dr. Toldo has received research grants from Olatec. Drs. Abbate and Van Tassell have received research grants from Novartis Pharma and Swedish Orphan Biovitrum.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Seropian I.M., Toldo S., Van Tassell B.W., Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63:1593–1603. doi: 10.1016/j.jacc.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Toldo S., Mezzaroma E., Mauro A.G., Salloum F., Van Tassell B.W., Abbate A. The inflammasome in myocardial injury and cardiac remodeling. Antioxid Redox Signal. 2015;22:1146–1161. doi: 10.1089/ars.2014.5989. [DOI] [PubMed] [Google Scholar]
- 3.Van Tassell B.W., Toldo S., Mezzaroma E., Abbate A. Targeting interleukin-1 in heart disease. Circulation. 2013;128:1910–1923. doi: 10.1161/CIRCULATIONAHA.113.003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harouki N., Nicol L., Remy-Jouet I. The IL-1β antibody gevokizumab limits cardiac remodeling and coronary dysfunction in rats with heart failure. J Am Coll Cardiol Basic Trans Science. 2017;2:418–430. doi: 10.1016/j.jacbts.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbate A., Van Tassell B.W., Seropian I.M. Interleukin-1beta modulation using a genetically engineered antibody prevents adverse cardiac remodeling following acute myocardial infarction in the mouse. Eur J Heart Fail. 2010;12:319–322. doi: 10.1093/eurjhf/hfq017. [DOI] [PubMed] [Google Scholar]
- 7.Toldo S., Mezzaroma E., Van Tassell B.W. Interleukin-1β blockade improves cardiac remodelling after myocardial infarction without interrupting the inflammasome in the mouse. Exp Physiol. 2013;98:734–745. doi: 10.1113/expphysiol.2012.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toldo S., Mezzaroma E., Bressi E. Interleukin-1β blockade improves left ventricular systolic/diastolic function and restores contractility reserve in severe ischemic cardiomyopathy in the mouse. J Cardiovasc Pharmacol. 2014;64:1–6. doi: 10.1097/FJC.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 9.Trankle C., Thurber C.J., Toldo S., Abbate A. Mitochondrial membrane permeability inhibitors in acute myocardial infarction: still awaiting translation. J Am Coll Cardiol Basic Trans Science. 2016;1:524–535. doi: 10.1016/j.jacbts.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sager H.B., Heidt T., Hulsmans M. Targeting interleukin-1β reduces leukocyte production after acute myocardial infarction. Circulation. 2015;132:1880–1890. doi: 10.1161/CIRCULATIONAHA.115.016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbate A., Kontos M.C., Grizzard J.D., for the VCU-ART Investigators Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study) Am J Cardiol. 2010;105:1371–1377. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 12.Abbate A., Van Tassell B.W., Biondi-Zoccai G. Effects of Interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction (from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2 pilot study) Am J Cardiol. 2013;111:1394–1400. doi: 10.1016/j.amjcard.2013.01.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbate A., Kontos M.C., Abouzaki N.A. Comparative safety of Interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies) Am J Cardiol. 2015;115:288–292. doi: 10.1016/j.amjcard.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Van Tassell B.W., Arena R.A., Toldo S. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One. 2012;7:e33438. doi: 10.1371/journal.pone.0033438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Tassell B., Canada J., Carbone S. Interleukin-1 blockade in recently decompensated systolic heart failure: the recently decompensated heart failure anakinra response trial (REDHART) Eur J Heart Fail. 2017;19(Suppl):368. doi: 10.1161/CIRCHEARTFAILURE.117.004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novartis. Novartis phase III study shows ACZ885 (canakinumab) reduces cardiovascular risk in people who survived a heart attack. Available at: https://www.novartis.com/news/media-releases/novartis-phase-iii-study-shows-acz885-canakinumab-reduces-cardiovascular-risk. Accessed July 13, 2017.