Graphical abstract
Keywords: Mitral valve regurgitation, MitraClip, Intracardiac echocardiography, PMVR, 3D ICE
Highlights
-
•
First in human experience: percutaneous mitral valve repair (PMVR) procedure guided by three-dimensional volume intracardiac echocardiography (ICE).
-
•
Volume ICE adds valuable information to two-dimensional ICE
-
•
Further improvements such as wider opening angle of the volume ICE probe are needed for routine use in PMVR.
Introduction
Percutaneous mitral valve repair (PMVR) using the MitraClip system is a novel method to treat mitral regurgitation (MR) in patients not eligible for conventional mitral valve surgery. The procedure is mainly guided by transesophageal echocardiography (TEE). The advent of three-dimensional (3D) TEE with the possibility of simultaneous visualization of two orthogonal planes (X-plane) has facilitated the positioning of the clip. However, TEE is not possible in all patients.
We have demonstrated before that under certain circumstances, intracardiac echocardiography (ICE) can serve as an imaging modality for PMVR.1, 2 A significant step to improve imaging by ICE during PMVR would be to have ICE technology with real-time 3D imaging functionality and the possibility to generate X-plane views. In this case, we used a volume ICE probe (Acuson AcuNav V, Siemens Healthcare, Mountain View, CA). Real-time 3D ICE was only recently introduced to visualize heart structures in three dimensions3 but has not been used so far to guide a PMVR procedure.
Case Presentation
A 51-year-old patient presented with progressive dyspnea caused by MR grade IV. Transthoracic echocardiography revealed primary MR; left ventricular function was normal. Thirty-three years earlier, the patient had undergone radiation therapy for Hodgkin's disease with resulting lung fibrosis and esophageal stenosis, which required repeated endoscopic balloon dilations. The patient had coronary artery disease with involvement of the left main coronary artery, for which she had undergone multiple coronary interventions. Furthermore, she had nonsevere aortic regurgitation. Due to these comorbidities, a decision for PMVR using the MitraClip system was made by our interdisciplinary heart team. As TEE was not possible due to the aforementioned esophageal stenosis, we decided to carry out the procedure using ICE.
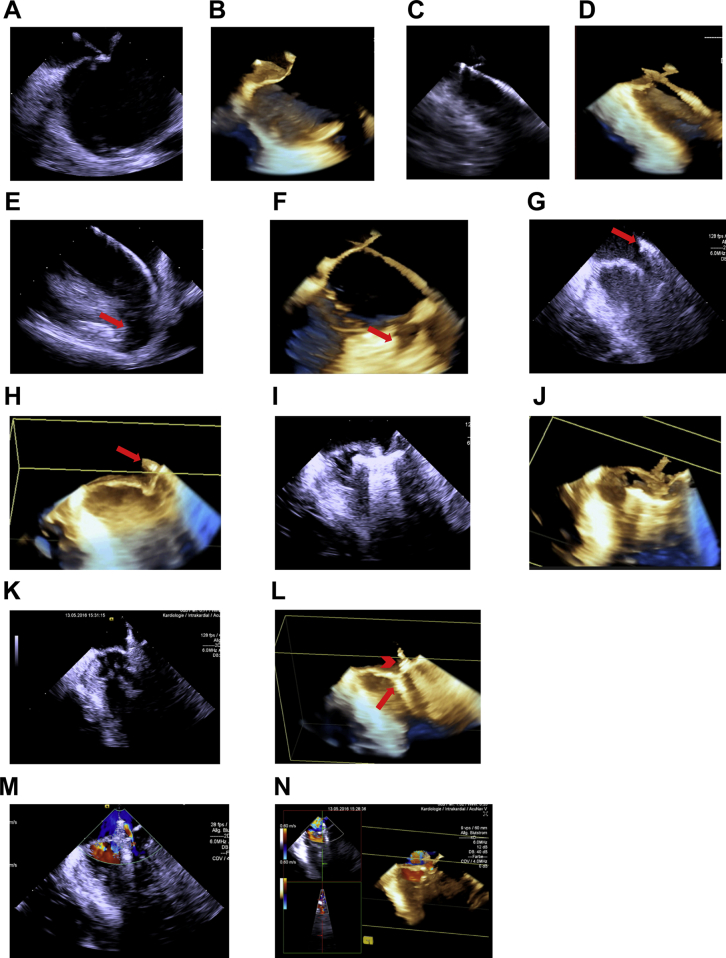
The procedure was carried out under deep sedation using propofol and midazolam. For imaging, we used the AcuNav Volume ICE catheter, which is a 10F intracardiac ultrasound catheter that—when connected to the Acuson SC2000 ultrasound platform (Siemens Healthcare)—uses InFocus technology to generate real-time 3D images with a field of view of 90° by 24° at high frame rate. Figure 1 depicts imaging sequences generated during this PMVR procedure.
Figure 1.
Images of ICE during PMVR; see also Video 1. (A-F) Right atrial ICE. (A) Two-dimensional ICE and (B) 3D ICE confirming position of the Brockenbrough needle at the interatrial septum before transseptal puncture. (C, D) Two-dimensional and 3D images showing the guidewire crossing the interatrial septum. (E, F) Two-dimensional and 3D images confirming position of the guidewire in the left upper pulmonary vein. The red arrow indicates the LA appendage (LAA). (G-N) LA ICE. (G, H) Two-dimensional and 3D images showing the position of the closed clip (red arrow) over the mitral valve plane. (I, J) Two-dimensional and 3D images showing that both mitral leaflets are visible on the extended clip arms, after the clip has been advanced into the left ventricle. (K) Two-dimensional imaging of the fully closed clip still attached to the catheter and (L) 3D imaging of the fully closed clip (red arrow) after deployment from the catheter (red arrowhead). (M) Color flow mapping showing residual MR lateral to the clip (Nyquist setting 0.6 m/sec). (N) Real-time 3D color flow image of residual MR.
First, the volume ICE probe was advanced into the right atrium and subsequently into the left atrium (LA) as described elsewhere.1, 2 Three-dimensional imaging guidance was used for transseptal puncture (Figure 1A-D) and positioning of a superstiff wire in the left upper pulmonary vein (Figure 1E-F). Then, the MitraClip delivery system was advanced into the LA, the ICE catheter was switched to the LA, and the clip was positioned over the mitral valve plane (Figure 1G, H). After that, the ICE catheter was switched to the LA. The clip was advanced into the left ventricle, opened to 160°, and positioned under the mitral valve plane (Figure 1I, J). Clip alignment was adjusted, the clip was closed, and a sufficient grasp was confirmed with the volume ICE catheter (Figure 1K, L). Color flow mapping confirmed substantial reduction of MR with only slight residual regurgitation lateral to the clip (Figure 1M, N).
Discussion
Currently, the Acuson AcuNav V catheter (Siemens Healthcare) is the only available ICE probe with real-time 3D imaging functionality. While first experience has been gathered with volume ICE in electrophysiology4 and transcatheter aortic valve replacement,5 this is to the best of our knowledge the first published PMVR procedure guided by volume ICE. Imaging by ICE provides very good spatial resolution. Sedation is not needed, thereby minimizing the risk of hypotensive periods or drops in oxygen saturation. The added value of 3D ICE over two-dimensional (2D) ICE includes the good visualization of the ridge between the LA appendage and the left pulmonary veins, which is very easy, uniformly successful, and excellent in resolution.4 Furthermore, it enables real-time 3D documentation of target structures, facilitates interpretation of 3D structures, and may reduce procedure time.4 As experience in other interventions suggests that tracking small structures such as thin wires, catheters, and devices with real-time 3D ICE is generally easier compared with 2D ICE,6 this technique may facilitate precise positioning of the MitraClip in relation to the underlying pathology.
Yet there are also disadvantages such as the additional puncture of the iliac vein and a second transseptal access, which, however, can usually be achieved with a blunt approach. Future studies will have to define the optimal position for the LA ICE probe in the interatrial septum in relation to the MitraClip guide catheter. In our case, we used the volume ICE probe in the right atrial position for guiding the transseptal puncture and for positioning the clip over the mitral valve plane. We then introduced the probe into the LA for visualization of MR and for guiding the grasping maneuver as well as for the final evaluation of residual MR. Visualization of mitral valve leaflets during the grasping maneuver was very good in the 2D as well as in the 3D mode.
Conclusions
All steps of the PMVR procedure could be visualized by ICE. The real-time 3D images provided valuable information on mitral valve morphology and helped to validate a sufficient grasp. Because the field of view of the volume ICE is still limited (90° × 24°), the whole mitral annulus cannot be captured in one view. A wider angle might be of particular importance in MR of primary origin with calcifications or a prolapse to address the underlying pathology. More experience and further technical developments are urgently needed to render ICE a complementary or even alternative imaging modality to TEE during the PMVR procedure.
Footnotes
This work was supported by grants from the German Heart Foundation.
Conflicts of Interest: Dr. Schreieck has received speaker fees from Medtronic and St. Jude Medical. Dr. Langer was reimbursed by Abbott Vascular for training courses in the percutaneous mitral valve repair procedure. The remaining authors had no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.case.2017.01.006.
Supplementary Data
Video reconstruction of volume ICE catheter sequences acquired during the PMVR procedure.
References
- 1.Henning A., Mueller, Mueller K., Zuern C., Walker T., Gawaz M. Percutaneous edge-to-edge mitral valve repair escorted by left atrial intracardiac echocardiography (ICE) Circulation. 2014;130:e173–e174. doi: 10.1161/CIRCULATIONAHA.114.012504. [DOI] [PubMed] [Google Scholar]
- 2.Patzelt J., Seizer P., Zhang Y.Y., Walker T., Schreieck J., Gawaz M. Percutaneous mitral valve edge-to-edge repair with simultaneous biatrial intracardiac echocardiography: first-in-human experience. Circulation. 2016;133:1517–1519. doi: 10.1161/CIRCULATIONAHA.115.020923. [DOI] [PubMed] [Google Scholar]
- 3.Fontes-Carvalho R., Sampaio F., Ribeiro J., Gama Ribeiro V. Three-dimensional intracardiac echocardiography: a new promising imaging modality to potentially guide cardiovascular interventions. Eur Heart J Cardiovasc Imaging. 2013;14:1028. doi: 10.1093/ehjci/jet047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brysiewicz N., Mitiku T., Haleem K., Bhatt P., Al-Shaaraoui M., Clancy J.F. 3D real-time intracardiac echocardiographic visualization of atrial structures relevant to atrial fibrillation ablation. JACC Cardiovasc Imaging. 2014;7:97–100. doi: 10.1016/j.jcmg.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Dhoble A., Nakamura M., Makar M., Castellanos J., Jilaihawi H., Cheng W. 3D intracardiac echocardiography during TAVR without endotracheal intubation. JACC Cardiovasc Imaging. 2016;9:1014–1015. doi: 10.1016/j.jcmg.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Bartel T., Muller S., Biviano A., Hahn R.T. Why is intracardiac echocardiography helpful? Benefits, costs, and how to learn. Eur Heart J. 2014;35:69–76. doi: 10.1093/eurheartj/eht411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video reconstruction of volume ICE catheter sequences acquired during the PMVR procedure.