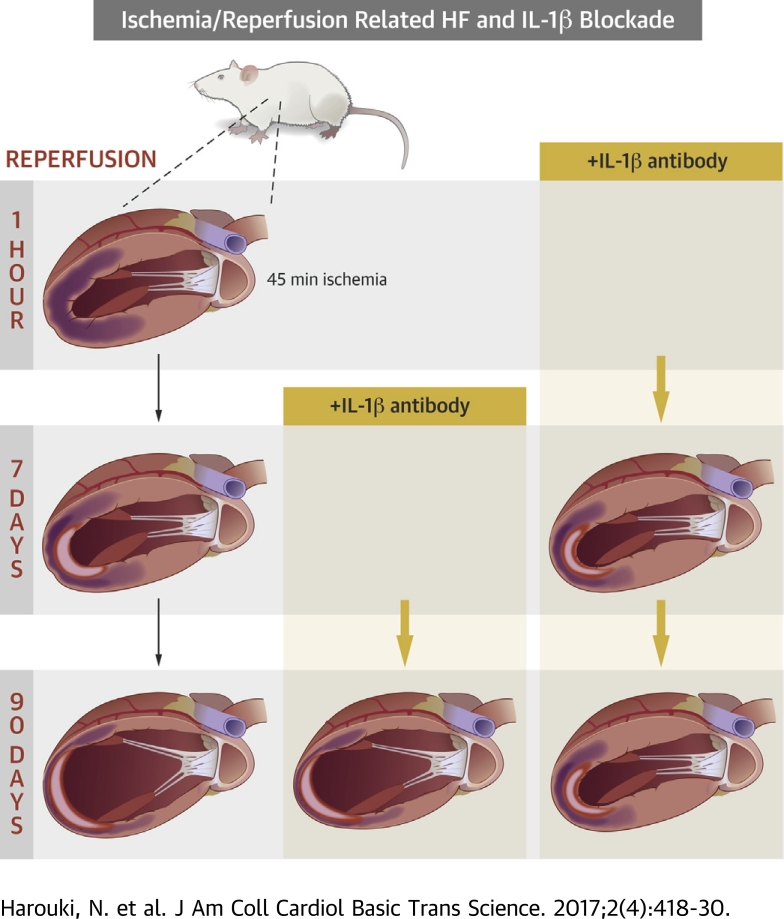
Visual Abstract
Key Words: cardiovascular function, heart failure, IL-1β, ischemia/reperfusion
Abbreviations and Acronyms: GK, Goto-Kakisaki; I/R, ischemia/reperfusion; IL, interleukin; LV, left ventricle/ventricular; LVEDP, left ventricular end-diastolic pressure; LVEDPV, left ventricular end-diastolic pressure–volume relationship; LVESP, left ventricular end-systolic pressure; LVESPVR, left ventricular end-systolic pressure–volume relationship; ROS, reactive oxygen species; SOD, superoxide dismutase
Highlights
-
•
Immediate IL-1β antibody gevokizumab administration reduces ischemia/reperfusion related infarct size.
-
•
Immediate and late IL-1β antibody gevokizumab administration improves heart failure related left ventricular remodeling.
-
•
IL-1β antibody gevokizumab improves heart failure related coronary dysfunction.
Summary
This study reports preclinical data showing that the interleukin (IL)-1β modulation is a new promising target in the pathophysiological context of heart failure. Indeed, in nondiabetic Wistar and diabetic Goto-Kakizaki rats with chronic heart failure induced by myocardial infarction, administration of the IL-1β antibody gevokizumab improves ‘surrogate’ markers of survival (i.e., left ventricular remodeling, hemodynamics, and function as well as coronary function). However, whether IL-1β modulation per se or in combination with standard treatments of heart failure improves long-term outcome in human heart failure remains to be determined.
Enhanced interleukin-1β (IL-1β) levels are involved in the immediate and long-term consequences of myocardial ischemia/reperfusion (I/R), as suggested by acute as well as chronic increases in IL-1β plasma levels observed in patients and rodents following myocardial infarction 1, 2. Indeed, after the abrupt but transient increase of cardiac the IL-1β gene expression within the first hour to 1 day after ischemia 3, 4, a second and sustained IL-1β upregulation is observed 5, 6. This specific time course of IL-1β expression may be important in terms of left ventricular (LV) remodeling and/or LV dysfunction as IL-1β signaling is essential both during 5, 7 and beyond the infarct healing period (8). Indeed, IL-1β plays an orchestrating role in the inflammatory response to myocardial injury, including enhanced synthesis of other proinflammatory mediators, activation of profibrotic pathways (5), and promotion of cytokine-induced cardiomyocyte apoptosis (9) but also exerts direct negative inotropic effects on myocyte contractility 9, 10, 11.
Although the deleterious role of IL-1β in the acute consequences of cardiac I/R injury has been well established 7, 12, the neutralization and modulation of IL-1β after myocardial infarction in mice has been suggested as a therapeutic target in the prevention of long-term consequences of I/R (i.e., development of heart failure [HF]) 7, 13, 14. Thus, the aim of this study was to determine the short- and long-term effects of treatment with the IL-1β modulating antibody gevokizumab 15, 16, initiated either 1 h following reperfusion after myocardial ischemia and continued for 7 (early short-term) or 90 (early long-term) days or initiated 7 days following reperfusion after myocardial ischemia and continued for 83 (delayed long-term) days on I/R-induced LV remodeling or dysfunction and coronary dysfunction. Moreover, as several pathologies, notably diabetes, are known to aggravate myocardial I/R injury, we also evaluated Gevokizumab in the setting of type 2 diabetes, using Goto-Kakisaki (GK) rats.
Our results showed that the IL-1β antibody gevokizumab induces an immediate but sustained improvement of I/R-induced cardiac and coronary dysfunctions in “healthy” as well as diabetic rat models. However, whether IL-1β modulation may be a therapeutic option for the acute and long-term consequences (i.e., survival of cardiac I/R in humans remains to be determined).
Materials and Methods
The investigation conforms to the Guide for the Care and Use of Laboratory Animals (U.S. National Institutes of Health publication 85-23, revised 1996) and was approved by the local ethical committee (CENOMEXA number 54; ref. 0871.01).
Animals and experimental design
This study was performed in either 12-week-old male Wistar (Janvier Labs, Saint Berthevin, France) or GK (Metabrain, Chilly-Mazarin, France) rats, anesthetized with intraperitoneal administration of ketamine/xylazine (150 and 5 mg ∙ kg−1, respectively), subjected to either sham surgery or transient ischemia followed by reperfusion, the latter being verified visually before closing the chest, as previously described (17), provoked by temporary left coronary artery occlusion (Wistar rats: 45 min; GK rats: 20 min), followed by reperfusion for 2, 7, or 90 days.
Independent protocols were performed to study the short- and long-term effects of gevokizumab. A first protocol assessed long-term gevokizumab treatment initiated (10 mg∙kg−1, intraperitoneally) either 1 h (early long-term) or 7 days (delayed long-term) following reperfusion and continued for 90 or 83 days, respectively, at a dose of 10 mg∙kg−1 subcutaneously once per week. This dosage regimen resulted in an effective “neutralization” of IL1-β between 2 subsequent subcutaneous administrations. A second and a third protocol assessed early short-term gevokizumab treatments initiated 1 h following reperfusion at an intraperitoneal dose of 10 mg∙kg−1 and continued for 2 or 7 days. A fourth protocol assessed the effects of delayed short-term (7-day) gevokizumab treatment initiated in a context of established HF, that is, 83 days following reperfusion at an intraperitoneal dose of 10 mg∙kg−1. All “untreated” control rats received immunoglobulin G (IgG).
Measured parameters
Statistical analysis
All results are mean ± SEM. Left ventricular diastolic/systolic diameters and hemodynamic parameters were assessed as primary endpoints, whereas all other parameters, that is, molecular mechanisms, were assessed as secondary endpoints. Because no data were available for the possible effect of gevokizumab on remodeling and hemodynamics, we made a simulation, using our historical data 18, 19, 20 for each parameter obtained in untreated animals to demonstrate statistical significance (p < 0.05) with a minimal power of 80%. The minimal expected effect size or difference between untreated and treated was fixed at 10% and 30%, with the coefficient of variation to 25% and 15% for echocardiographic infarct size studies and hemodynamic studies, respectively. For all significant differences concerning primary endpoints, a posteriori powers higher than 80% were also checked.
In order to evaluate the effect of I/R, all parameters obtained in Wistar rats subjected to I/R and sham surgery were compared using Student unpaired 2-tailed t test. Before applying parametric tests, such as Student unpaired 2-tailed t test, the Gaussian distribution of data was assessed by Shapiro-Wilk normality test and Kolmogorov-Smirnov test and graphically by QQplot and normal probability plot.
In order to evaluate the effects of long-term early and delay-initiated gevokizumab, all parameters obtained in placebo, early and delayed long-term gevokizumab-treated rats were compared using one-way ANOVA followed, in case of significance, by Tukey test for multiple comparisons.
In order to evaluate the effects of short-term (2- or 7-day) gevokizumab treatment, all parameters obtained in IgG placebo, and 2- or 7-day gevokizumab-treated rats were compared by unpaired, 2-tailed Student t test.
Identical statistical analyses were used for the GK protocols. All statistical analysis was conducted using SPSS version 20.0 software (IBM, Armonk, New York) and R Statistic version 3.1.4 (R Foundation for Statistical Computing, Vienna, Austria). Differences were considered significant at a p level of <0.05.
Results
Effects of early and delayed long-term gevokizumab
LV remodeling and function
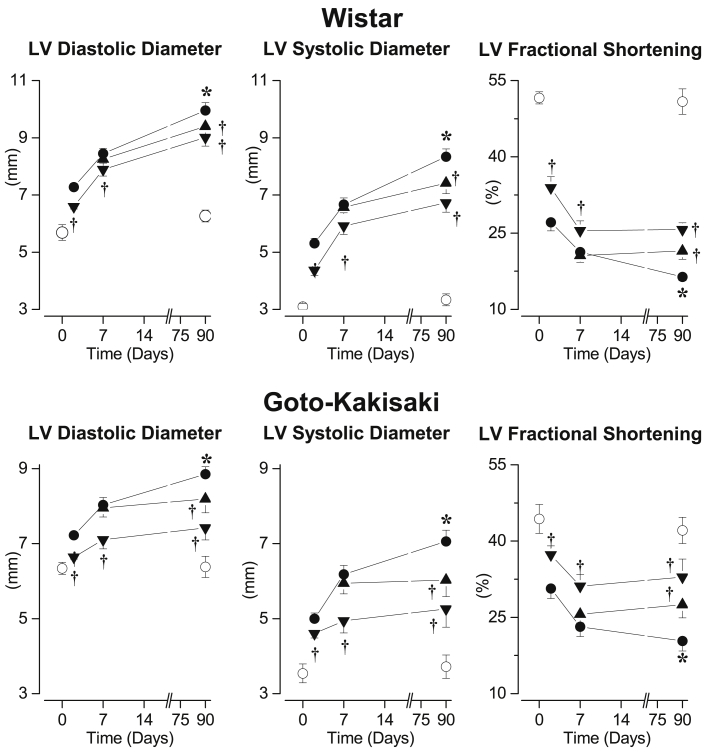
In untreated Wistar rats, 2 days after I/R, LV systolic and diastolic diameters were increased resulting in a reduced LV fractional shortening compared with that in sham-operated rats. Further increases in LV systolic and diastolic diameters were observed 7 and 90 days after I/R, resulting in a progressive impairment of LV fractional shortening (Figure 1, upper panel). These effects were associated with an increase in pulmonary weight (sham: 0.89 ± 0.01 g vs. I/R: 1.22 ± 0.03 g; p < 0.05) 90 days after I/R. Early gevokizumab, initiated 1 h after I/R, induced rapid and persistent reduction of LV diastolic and systolic diameters after 2, 7, and 90 days, resulting in an improved LV fractional shortening. Similarly, compared to untreated Wistar I/R rats, delayed gevokizumab initiated 7 days after I/R reduced the progressive LV dilation and the impairment of LV fractional shortening (Figure 1, upper panel). Pulmonary weight was not significantly modified by gevokizumab (early: 1.17 ± 0.05 and delayed: 1.15 ± 0.04, nonsignificant vs. I/R, respectively).
Figure 1.
Time Course of Left Ventricular Dilatation
(Upper panel) Left ventricular (LV) diastolic, LV systolic diameter, and LV fractional shortening determined, before and 90 days after sham surgery, in untreated Wistar (open circles; n = 10) and 2, 7, and 90 days after 45-min transient ischemia in placebo-treated I/R Wistar (filled circles; n = 15), early long-term (down-triangles; n = 14) or delayed long-term gevokizumab-treated I/R Wistar (up-triangles; n = 15). (Lower panel) LV diastolic, LV systolic diameter, and LV fractional shortening determined, before and 90 days after sham surgery in untreated Goto-Kakisaki (open circles; n = 12) and 2, 7, and 90 days after 20-min transient ischemia in placebo-treated I/R Goto-Kakisaki (filled circles; n = 16), early long-term (down-triangles; n = 15) or delayed long-term gevokizumab-treated I/R Goto-Kakisaki (up-triangles; n = 15). *p < 0.05 versus untreated sham. †p < 0.05 versus untreated I/R.
In GK rats, I/R induced progressive LV dilation and impairment of LV fractional shortening, as it did in Wistar rats. Early and delayed long-term gevokizumab limited, as in Wistar rats, LV dilation and impairment of LV fractional shortening (Figure 1, lower panel).
LV hemodynamics
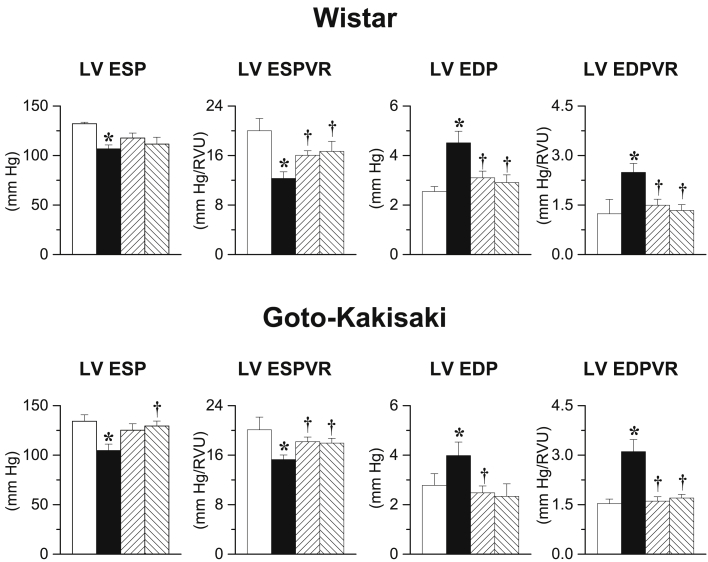
After 90 days, I/R Wistar rats displayed decreases in LV end-systolic pressure (LVESP) as well as in LV end-systolic pressure–volume relationship (LVESPVR) and increases in LV end-diastolic pressure (LVEDP) as well as in LV EDP volume relationship (LVEDPVR) compared with sham-operated animals (Figure 2, upper panel). Early and delayed gevokizumab increased LVESPVR and decreased LVEDP as well as LVEDPVR.
Figure 2.
Left Ventricular Hemodynamics
(Upper panel) Left ventricular end-systolic pressure (LVESP), left ventricular end-systolic pressure-volume relation (LVESPVR), left ventricular end-diastolic pressure (LVEDP), and left ventricular end-diastolic pressure-volume relation (LVEDPVR) determined 90 days after sham surgery in untreated Wistar (white bars; n = 14) and 90 days after 45-min transient ischemia in placebo-treated I/R Wistar (black bars; n = 16), early long-term (up-hatched bars; n = 14) or delayed long-term gevokizumab-treated I/R Wistar (down-hatched bars; n = 15). (Lower panel) LVESP, LVESPVR, LVEDP, and LVEDPVR determined 90 days after sham-surgery in untreated Goto-Kakisaki (white bars; n = 10) and 90 days after 20-min transient ischemia in placebo-treated I/R Goto-Kakisaki (black bars; n = 15), early long-term (up-hatched bars; n = 15) or delayed long-term gevokizumab-treated I/R Goto-Kakisaki (down-hatched bars; n = 15). *p < 0.05 versus untreated sham. †p < 0.05 versus untreated I/R.
In GK rats, after 90 days, I/R decreased LVESP and LVESPVR but increased LVEDP and LVEDPVR (Figure 2, lower panel). Early and delayed long-term gevokizumab increased LVESP as well as LVESPVR and decreased LVEDP as well as LVEDPVR.
LV hypertrophy, tissue perfusion, and infarct size
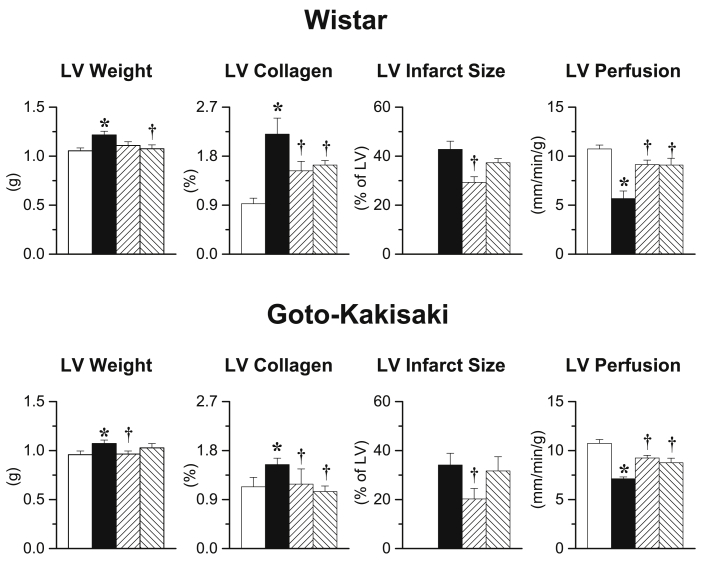
After 90 days, untreated I/R Wistar rats showed major infarct size associated with increased LV weight and collagen density, whereas pulmonary weight was increased (sham: 0.90 ± 0.03 g vs. I/R: 0.97 ± 0.01 g; p < 0.05) but reduced LV tissue perfusion. Early and delayed gevokizumab initiation decreased LV weight and collagen density by increased tissue perfusion 90 days post I/R, while only early gevokizumab reduced infarct size (Figure 3). Pulmonary weight was not significantly modified by gevokizumab (early: 0.97 ± 0.09; delayed: 1.00 ± 0.09, NS vs. I/R, respectively).
Figure 3.
Left Ventricular Remodeling
(Upper panel) Left ventricular (LV) weight, collagen density, infarct size, and tissue perfusion in the ‘viable’ part of the LV determined 90 days after sham surgery in untreated Wistar (white bars; n = 14) and 90 days after 45-min transient ischemia in placebo-treated I/R Wistar (black bars; n = 16), early long-term (up-hatched bars; n = 14) or delayed long-term gevokizumab-treated I/R Wistar (down-hatched bars; n = 15). (Lower panel) LV weight, collagen density, infarct size, and tissue perfusion in the ‘viable’ part of the LV determined 90 days after sham surgery in untreated Goto-Kakisaki (white bars; n = 10) and 90 days after 20-min transient ischemia in placebo-treated I/R Goto-Kakisaki (black bars; n = 15), early long-term (up-hatched bars; n = 15) or delayed long-term gevokizumab-treated I/R Goto-Kakisaki (down-hatched bars; n = 15). *p < 0.05 versus untreated sham. †p < 0.05 versus untreated I/R.
Ischemia/reperfusion induced large infarcts in GK rats, as it did in Wistar rats, which were associated with increased LV weight, collagen density, and reduced tissue perfusion. Again, early and delayed long-term gevokizumab decreased LV weight and collagen density and increased tissue perfusion, only early gevokizumab reduced infarct size (Figure 3).
Coronary artery relaxation
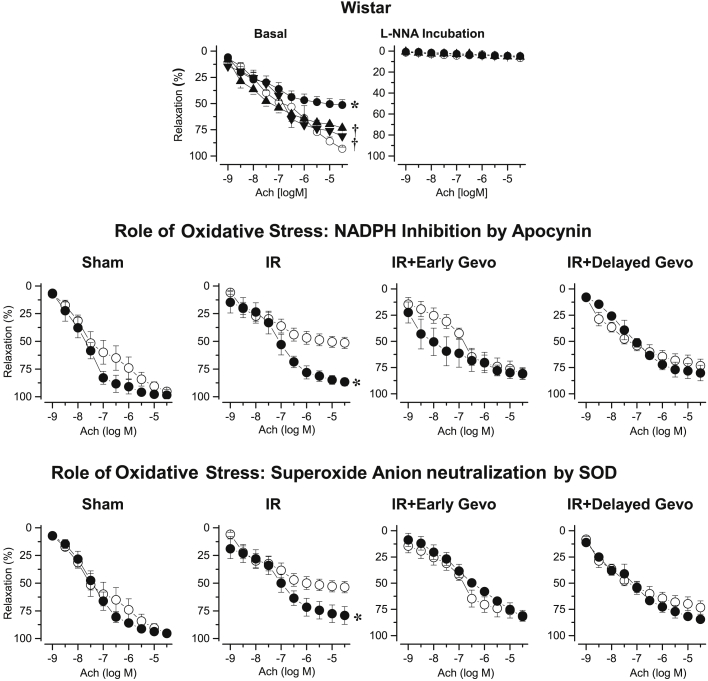
After 90 days, acetylcholine-induced relaxation of coronary arteries was impaired after I/R. Early and delayed long-term gevokizumab prevented this I/R-induced impaired coronary relaxation. Furthermore, L-NG-nitro-arginine abolished the responses to acetylcholine in all groups, whereas apocynin or superoxide dismutase (SOD) restored acetylcholine-induced relaxation only in coronary arteries from untreated I/R rats (Figure 4).
Figure 4.
Coronary Relaxation in Wistar Rats
(Upper panel) Coronary relaxation induced by acetylcholine 90 days after sham surgery in untreated Wistar (open circles; n = 5) or 90 days after 45-min transient ischemia in placebo-treated I/R Wistar (filled circles; n = 6), early long-term (down-triangles; n = 15) or delayed long-term gevokizumab-treated I/R Wistar (up-triangles; n = 5). *p < 0.05 versus untreated sham. †p < 0.05 versus untreated I/R. (Middle and lower panel) Coronary relaxation induced by acetylcholine 90 days after sham surgery in untreated Wistar or 90 days after 45-min transient ischemia in untreated I/R Wistar, early long-term or delayed long-term gevokizumab-treated I/R Wistar before (open circles) and after incubation with apocyin or SOD (filled circles). *p < 0.05 versus before incubation.
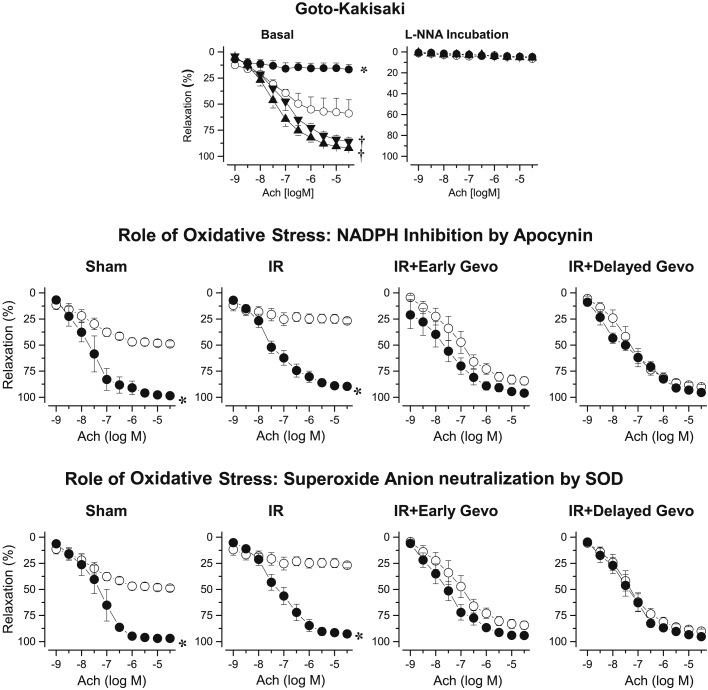
In sham-operated GK rats, the acetylcholine-induced coronary relaxation was impaired compared with that in sham-operated Wistar rats, and this response was further impaired 90 days after I/R (Figure 5). Early and delayed long-term gevokizumab supra-normalized, acetylcholine induced coronary vasorelaxation, whereas the effects of L-NNA, apocynin, and SOD were similar to those observed in coronary arteries of Wistar rats.
Figure 5.
Coronary Relaxation in Gotokakisaki Rats
(Upper panel) Coronary relaxation induced by acetylcholine 90 days after sham surgery in untreated Goto-Kakisaki (open circles; n = 5) or 90 days after 20-min transient ischemia in placebo-treated I/R Goto-Kakisaki (filled circles; n = 8), early long-term (down-triangles; n = 6) or delayed long-term gevokizumab-treated I/R Goto-Kakisaki (up-triangles; n = 6). *p < 0.05 versus untreated sham. †p < 0.05 versus placebo-treated I/R. (Middle and lower panels) Coronary relaxation induced by acetylcholine 90 days after sham-surgery in IgG-treated Goto-Kakisaki or 90 days after 20-min transient ischemia in placebo-treated I/R Goto-Kakisaki, early long-term or delayed long-term gevokizumab-treated I/R Goto-Kakisaki before (open circles) and after incubation with apocyin or SOD (filled circles). *p < 0.05 versus before incubation.
LV oxidative stress
Ninety days post I/R, the levels of reactive oxygen species (ROS) increased in Wistar rats (sham: 15.0 ± 0.9 AU/mg prot; untreated I/R: 18.2 ± 1.0 AU/mg prot; p < 0.05). Early and delayed long-term gevokizumab reduced cardiac ROS levels (early: 12.8 ± 0.6 AU/mg prot; delayed: 13.4 ± 0.8 AU/mg prot; both p < 0.05 vs. untreated I/R).
Early short-term effects of gevokizumab
LV remodeling and hemodynamics
Reductions in LV systolic and diastolic diameters and resulting increase in LV fractional shortening after 2 and 7 days of gevokizumab treatment (Figure 1) were associated with increased LVESP and LVESPVR and decreased LVEDP as well as LVEDPVR (Table 1). Simultaneously, 2-day gevokizumab initiated 1 h after I/R in Wistar rats reduced the surface of the necrotic area, whereas the area at risk was not modified.
Table 1.
Infarct Size and Hemodynamics in 2- or 7-Day Gevokizumab Treatments in Wistar Rats
| Time | Sham Treatment | Ischemia/Reperfusion |
||
|---|---|---|---|---|
| Untreated | Gevokizumab Treated | |||
| LV weight, g | D0–2 | – | 0.79 ± 0.07 | 0.66 ± 0.04 |
| D0–7 | 0.65 ± 0.02 | 0.64 ± 0.02 | 0.66 ± 0.03 | |
| Area at risk, % of LV | D0–2 | – | 45.2 ± 3.4 | 45.6 ± 1.9 |
| Necrotic area, % of LV | D0–2 | – | 25.9 ± 2.7 | 8.5 ± 0.3† |
| Infarct size, % of LV | D0–7 | – | 42.9 ± 2.6 | 34.0 ± 2.9† |
| LV ESP, mm Hg | D0–2 | – | 84.6 ± 5.5 | 100.4 ± 6.8† |
| D0–7 | 130.1 ± 1.6 | 101.9 ± 7.0∗ | 119.6 ± 5.1† | |
| LV ESVRP, mm Hg/RVU | D0–2 | – | 14.4 ± 0.8 | 16.8 ± 0.5† |
| D0–7 | 24.1 ± 0.64 | 12.3 ± 1.2∗ | 15.5 ± 0.9† | |
| LV EDP, mm Hg | D0–2 | – | 2.68 ± 0.53 | 1.96 ± 0.19† |
| D0–7 | 1.87 ± 0.59 | 4.09 ± 0.57∗ | 3.19 ± 0.59 | |
| LV EDPVR, mm Hg/RVU | D0–2 | – | 1.81 ± 0.15 | 1.43 ± 0.09† |
| D0–7 | 0.89 ± 0.11 | 2.10 ± 0.22∗ | 1.66 ± 0.16† | |
Values are mean ± SD.
D0–2 = 2-day treatment initiated 1 h after I/R; D0–7 = 7-day treatment initiated 1 h after I/R; LV EDP = left ventricular end-diastolic pressure; LV EDPVR = left ventricular end-diastolic pressure-volume relationship; LV ESP = left ventricular end-systolic pressure; LV ESPVR = left ventricular end-systolic pressure-volume relationship; LV = left ventricular; RVU = relative volume units.
p < 0.05 versus sham.
p < 0.05 versus untreated I/R. n = 4 to 17 per group.
Similarly, in GK rats, reductions in LV systolic and diastolic diameters and resulting increase in LV fractional shortening after 2 days of gevokizumab (Figure 1) were associated with increased LVESPVR and decreased LVEDP, as well as LVEDPVR. Similar to Wistar rats, the 2-day gevokizumab treatment in GK rats reduced the necrotic area, whereas the area at risk was not modified (Table 2).
Table 2.
Effect of 2-Day Gevokizumab Treatment in Goto-Kakisaki Rats
| Ischemia/Reperfusion |
||
|---|---|---|
| Untreated | Gevokizumab Treated | |
| LV weight, g | 0.90 ± 0.02 | 0.86 ± 0.01 |
| Area at risk, % of LV | 43.4 ± 3.0 | 45.2 ± 4.9 |
| Infarct size, % of LV | 16.6 ± 1.8 | 7.6 ± 1.9∗ |
| LV ESP, mm Hg | 93.9 ± 6.8 | 90.0 ± 5.2 |
| LV ESPVR, mm Hg/RVU | 12.5 ± 1.0 | 16.4 ± 1.0∗ |
| LV EDP, mm Hg | 2.06 ± 0.31 | 1.27 ± 0.15∗ |
| LV EDPVR, mm Hg/RVU | 2.15 ± 0.21 | 1.44 ± 0.17∗ |
Values are mean ± SD.
Abbreviations as in Table 1.
p <0.05 versus untreated I/R. n = 10 to 15 per group.
LV oxidative stress
Two days post I/R, cardiac ROS levels were not modified either in untreated rats (sham: 2.47 ± 0.38 AU/mg prot; untreated: I/R: 2.10 ± 0.15 AU/mg prot) or in gevokizumab-treated rats (2.36 ± 0.11 AU/mg prot). However, the increased LV ROS levels observed 7 days after I/R (sham: 1.53 ± 0.21 AU/mg prot; I/R: 2.23 ± 0.19 AU/mg prot; p < 0.05) were reduced by 7-day gevokizumab treatment (1.84 ± 0.16 AU/mg prot; p < 0.05 vs. I/R).
Furthermore, cardiac glutathione peroxide, catalase, and CuZn SOD enzymatic activities were not modified 2 days post I/R, but mitochondrial SOD activity was reduced in untreated I/R rats compared with that in sham-operated animals. Two-day gevokizumab reduced catalase activity and tended to normalize mitochondrial SOD (Table 3).
Table 3.
Cellular Effects of 2-Day Gevokizumab Treatment in Wistar Rats With I/R
| Sham Treated | Ischemia/Reperfusion |
||
|---|---|---|---|
| Untreated | Gevokizumab Treated | ||
| Glutathione peroxidase activity, μmol/min/mg prot | 219 ± 9 | 175 ± 21 | 168 ± 15 |
| Catalase activity, μmol/min/mg prot | 13.4 ± 1.1 | 16.39 ± 1.8 | 10.8 ± 1.1† |
| Mitochondrial SOD activity, U SOD Mn/mg prot | 7.2 ± 0.9 | 2.8 ± 0.7∗ | 4.9 ± 0.8 |
| Cytosolic SOD activity, U SOD CuZn/mg prot | 4.5 ± 1.1 | 3.7 ± 0.7 | 4.4 ± 1.2 |
| Total lymphocytes, nb/field | 11. 7 ± 2.1 | 44. ± 6.9∗ | 38.9 ± 8.1 |
| Lymphocytes in infarct area, nb/field | 2.9 ± 0.5 | 26.9 ± 4.4∗ | 22.1 ± 5.1 |
| Total leukocytes, nb/field | 56.3 ± 15.5 | 241.2 ± 23.2∗ | 115.3 ± 41.2† |
| Leucocytes in infarct area, nb/field | 13.5 ± 3.6 | 89.4 ± 12.0∗ | 31.8 ± 11.9† |
| Cleaved caspase-1 in infarct area, nb/field | 0.00 ± 0.00 | 0.75 ± 0.25∗ | 0.30 ± 0.10† |
Values are mean ± SD.
nb = 9 per group; SOD = superoxide dismutase.
p < 0.05 versus sham.
p < 0.05 versus untreated I/R. n = 6 TO 10 per group.
Cardiac inflammation
Two days post I/R, lymphocytes and leukocytes accumulated in the infarcted area, while activated caspase-1 activity increased and gevokizumab reduced lymphocyte and leukocyte accumulation as well as caspase-1 activity (Table 2).
Short-term effects of gevokizumab in well-established heart failure
Short-term gevokizumab initiated 83 days post I/R reduced LV systolic diameter but did not modify LV diastolic diameter, resulting in improved LV fractional shortening. This was associated with a marked increase in LVESP and LVESPVR, although the latter did not reach statistical significance. Furthermore, LVEDP and LVEDPVR were decreased. Moreover, neither LV weight nor collagen accumulation were modified by short-term gevokizumab initiated in a context of established HF, but myocardial tissue perfusion of the “viable” part of the LV was increased (Table 4). At the vascular level, short-term gevokizumab also restored the HF-induced impairment of coronary relaxation.
Table 4.
Hemodynamics of 7-Day Gevokizumab in Wistar Rats With CHF
| Chronic Heart Failure |
||
|---|---|---|
| Untreated | Gevokizumab Treated | |
| LV diastolic diameter, mm | 9.74 ± 0.24 | 9.61 ± 0.46 |
| LV systolic diameter, mm | 8.20 ± 0.19 | 7.18 ± 0.36∗ |
| LV fractional shortening, % | 15.8 ± 0.7 | 25.3 ± 1.1∗ |
| LV ESP, mm Hg | 108.0 ± 9.7 | 126.8 ± 2.9∗ |
| LV ESPVR, mm Hg/RVU | 12.2 ± 1.0 | 14.3 ± 0.5 |
| LV EDP, mm Hg | 4.40 ± 0.58 | 2.43 ± 0.16∗ |
| LV EDPVR, mm Hg/RVU | 2.45 ± .21 | 1.96 ± 0.10∗ |
| LV Weight, g | 1.20 ± 0.04 | 1.19 ± 0.08 |
| LV collagen density, % | 3.15 ± 0.44 | 3.01 ± 0.52 |
| LV infarct size, % | 39.2 ± 8.8 | 40.1 ± 6.1 |
| Myocardial perfusion, ml/min/100 g | 7.92 ± 0.16 | 8.57 ± 0.18∗ |
| Coronary relaxation, % | ||
| Basal | 37.1 ± 5.9 | 78.0 ± 9.0∗ |
| Apocin incubation | 80.4 ± 13.4 | 87.2 ± 7.1 |
| SOD incubation | 81.0 ± 6.9 | 82.8 ± 8.7 |
Discussion
Our results, obtained in a rat model of HF induced by cardiac I/R, showed that neutralization of IL-1β by the IL-1β antibody gevokizumab is associated not only with immediate but also sustained long-term improvement of cardiac and vascular functions involving multiple direct and indirect mechanisms, such as reduction of IL-1β’s direct negative inotropic effects, LV oxidative stress, and LV tissue inflammation and/or reduction of infarct size.
Although extensive research has clearly established that the inflammatory response is one of the major determinants involved in I/R-induced adverse cardiac remodeling or dysfunction, most clinical trials evaluating anti-inflammatory or immune modulatory drugs in HF, such as the anti-tumor necrosis factor (TNF)-α antibody etanercept (21) and colchicine (22) have been disappointing. However, IL-1β neutralization could be a new treatment of HF because in our study chronic neutralization of IL-1β by the IL-1β anti-body gevokizumab limits the progression of HF, shown by the limitation of LV dilation and LV dysfunction and improved LV hemodynamics. Our results, obtained in rats, which are similar to those obtained in mice (8), clearly show that IL-1β neutralization is beneficial beyond the “acute” phase of myocardial infarction, where IL-1β neutralization by the antibody anakinra/IL-1 trap has already shown improvement in LV function and remodeling in acute experimental 7, 23, 24 and human myocardial infarction 25, 26, 27.
Concerning the pathways involved in the improvement of cardiac function, our results clearly show that multiple direct and indirect mechanisms act in concert. First, reduction of IL-1β’s direct negative inotropic properties 10, 28 will lead to an immediate and sustained improvement of LV function, as illustrated by the increase of LV end-systolic pressure–volume relationship after 2, 7, and 90 days of gevokizumab treatment. Indeed, such direct mechanism of IL-β antagonism has already been suggested in mice 8, 13, and gevokizumab may thus oppose the impaired Ca2+ handling in HF (29) by limiting IL-1β-induced Ca2+ leakage from sarcoplasmic reticulum (11). Second, reduction of IL-1β-induced production of ROS (30), resulting in a reduced LV oxidative stress, as illustrated by the reduction of LV ROS observed after 7 or 90 days of gevokizumab treatment, should be beneficial. Indeed, it may reduce oxidative stress-induced production of local vasoconstrictors, such as endothelin (31), which is implicated in the deterioration of LV function and/or remodeling 32, 33. Moreover, although this has not been assessed in our study, the reduction of ROS by gevokizumab per se will diminish oxidative stress-induced modifications of sarcoplasmic reticulum Ca2+–ATPase protein structure and its activity, resulting in abnormal Ca2+ handling and impaired excitation-contraction coupling 34, 35. Third, the reduction of myocardial oxidative stress by gevokizumab may induce indirect long-term effects interfering with LV diastolic function. Indeed, oxidative stress induces cardiac remodeling, such as accumulation of interstitial collagen 36, 37. Hence, reduction of oxidative stress by IL-1β neutralization may partly explain the reduction of LV collagen density observed after long-term gevokizumab administration. This reduced fibrosis will contribute to the improvement of LV diastolic function, as LV collagen accumulation provokes LV diastolic dysfunction. However, the exact role of the modifications in myocardial collagen remains to be elucidated because the 7-day gevokizumab initiated in established HF, that is, after 83 days after I/R, still improves LV diastolic function without any modification of LV collagen accumulation, probably involving, as discussed above, immediate effects on inotropism/calcium handling. Fourth, the reduction in LV ROS levels should, theoretically, improve LV nitric oxide (NO) bioavailability. This could contribute to the improvement of LV diastolic dysfunction observed after both short- and long-term gevokizumab administration, because reduced myocardial NO bioavailability increases myocyte resting tension and impairs LV function 38, 39, 40, 41, while an increase in NO bioavailability and production, such as that induced through administration of the eNOS cofactor tetrahydrobiopterin (42) or NO donors (43) or eNOS enhancers (44) reduces LV diastolic dysfunction.
In addition to the beneficial effects of gevokizumab on LV function and structure, our results show for the first time that neutralization of IL-1β by gevokizumab also prevents HF-related coronary dysfunction, illustrated by the complete normalization of coronary endothelium-dependent relaxation. This normalization of coronary relaxation by gevokizumab may be the direct consequence of the prevention of I/R-induced vascular (endothelial) oxidative stress and the subsequent increase in vascular NO bioavailability, as illustrated by the fact that the normalized coronary relaxation in gevokizumab-treated animals is abolished by the NO synthase inhibitor L-NNA.
The restoration of coronary relaxation observed in gevokizumab-treated animals could indirectly contribute to the improvement of LV function. Indeed, the restoration of coronary relaxation by gevokizumab probably contributes to the improved myocardial tissue perfusion, as observed after gevokizumab, which will prevent LV tissue hypoxia. Moreover, as hypoxia induces production of ROS through enhanced pro-oxidant systems such as NADPH oxidase 45, 46, 47, imbalanced eNOS-to-iNOS ratio (48), and/or hypoxia-induced production of local cytokines, including IL-1β, the increased LV tissue perfusion observed with gevokizumab might rupture the vicious circles of IL-1β-induced ROS production (30) and/or IL-1β-induced IL-1β release, as well as ROS-induced IL-1β release and/or ROS-induced ROS production but also IL-1β induced activation of the renin-angiotensin system (49).
Finally, the reduction of infarct size observed after early but not delayed gevokizumab contributes to the improvement of LV function. This might be related to the reduced activation of the IL-1β pathway as suggested by the reduction of (activated) caspase-1 activity in our experiments, as already reported with the human recombinant IL-1β receptor antagonist anakinra (23). However, it cannot be excluded that the caspase-1 pathway is reduced because infarct size is reduced, as IL-1β blockade in a severe ischemic myocardial infarction does not reduce inflammatory activation (5). Furthermore, other pathways, such as enhanced NO bioavailability due to reduced oxidative stress may be involved in the reduction of infarct size (50). Although not determined, reduced IL-1β-induced production of ROS during the infarct healing period, that is, the first 7 days after I/R, will reduce LV oxidative stress and increase NO bioavailability, which are both clearly observed after 7 and 90 days in our experiments. However, whether a reduction of infarct size occurs in humans is currently unknown. Although a reduction of infarct size per se will have a beneficial effect, gevokizumab also exerts beneficial effects in terms of cardiovascular function even when started after completion of the infarct healing. It is thus tempting to state that treatment should be initiated as early as possible and continued beyond the infarct healing period but might also be initiated after infarct healing. This is clinically important because it suggests that a number of patients may benefit from gevokizumab or other IL-1β antagonism rather than being reserved for patients with acute myocardial infarction treated with early primary percutaneous coronary intervention 12, 51. However, it must be stressed that, in this experimental study, the duration of the ischemic period is longer in nondiabetic animals in order to reproduce similar I/R injury since diabetes renders prone to I/R injury. However, in the clinical setting diabetic patients will have similar time of ischemia to nondiabetic patients, which could limit the efficacy of gevokizumab in humans. These critical points needs to be evaluated in humans.
It must be stressed that several pathologies, such as diabetes, render the heart more prone to I/R injury, which may be related in part to the modest but significant increase in IL-1β concentrations observed in humans with diabetes (52). Thus, we also evaluated the efficiency of gevokizumab in I/R injury in a model of diabetes (i.e., GK rats). In this pathological context, gevokizumab’s short- and long-term cardiac effects, that is, improved LV hemodynamics and function, reduced LV remodeling, and so forth, are similar to those observed in Wistar rats. However, at the vascular level, gevokizumab restores the coronary endothelial-dependent relaxation beyond the relaxation observed in the untreated GK group and reaches even the value of the relaxation of coronary artery from Wistar rats. This suggests that, in diabetes, increased IL-1β levels per se reduce endothelium-dependent vasorelaxation.
Surprisingly, in GK rats, gevokizumab improves cardiovascular function and structure independently from possible metabolic modifications, as all metabolic parameters, especially glucose and insulin tolerance are not statistically significantly modified by gevokizumab. Whether this is related to the dosage regimen used in our study remains to be investigated, as short-term anakinra therapy improves insulin sensitivity in patients with type 1 diabetes (53).
Study limitations
Although this study did not evaluate long-term survival, our results suggest that gevokizumab may improve long-term survival after I/R. Indeed, as the magnitude of infarct size is correlated with the relative risk of death (54), the reduction of infarct size observed when gevokizumab treatment is initiated “immediately” after I/R should be associated with an increase in long-term survival. Furthermore, although intermediate “surrogate” markers of survival (i.e., LV dilation) are improved by long-term gevokizumab, other major determinants of survival in this model of HF (i.e., reductions in arterial blood pressure and heart rate) 32, 55, are not observed in gevokizumab-treated animals. Thus, the effect of IL-1β modulation per se or in combination with standard treatments of HF on long-term survival after I/R remains to be determined.
Conclusions
The IL-1β antibody gevokizumab limits the progression of HF due to cardiac I/R. The improvement of cardiac and coronary functions involves immediate effects (i.e., reduction of IL-1β-related negative inotropic effects, improved myocardial perfusion, reduced oxidative stress, reduced infarct size, and so forth, and long-term effects such as modifications in the myocardial structure, all independent from the pathological metabolic status). Furthermore, the fact that, in I/R GK diabetic rats, gevokizumab’s improvement of coronary endothelium-dependent relaxation reaches relaxation of coronary arteries from nondiabetic Wistar suggests a possible role for not only secondary but also primary protection in diabetes.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Heart failure is a clinical syndrome characterized by dyspnea, fatigue, and poor exercise capacity and has become a major health problem because, despite medical treatment, its incidence continues to increase. We report pre-clinical data showing that IL-1β modulation is a new, promising target in the pathophysiological context of HF. Indeed, the IL-1β antibody gevokizumab improves intermediate “surrogate” markers of survival, that is, left ventricular remodeling and function and coronary function.
TRANSLATIONAL OUTLOOK: However, whether IL-1β modulation per se or in combination with standard treatments for HF improves long-term outcome in human HF remains to be determined.
Footnotes
This work funded in part by Servier France and by European Union and Région Normandie. The European Union is associated with Normandy through the European Regional Development Fund (ERDF). Drs. Bolduc, Bouly, and Roussel are employees of Servier. Ms. Golding was a recipient of an Erasmus grant. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Antonio Abbate, MD, PhD, served as Guest Editor for this paper.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Van Tassell B.W., Arena R.A., Toldo S. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One. 2012;7:e33438. doi: 10.1371/journal.pone.0033438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guillen I., Blanes M., Gomez-Lechon M.J., Castell J.V. Cytokine signaling during myocardial infarction: sequential appearance of IL-1beta and IL-6. Am J Physiol. 1995;269:R229–R235. doi: 10.1152/ajpregu.1995.269.2.R229. [DOI] [PubMed] [Google Scholar]
- 3.Herskowitz A., Choi S., Ansari A.A., Wesselingh S. Cytokine mRNA expression in postischemic/reperfused myocardium. Am J Pathol. 1995;146:419–428. [PMC free article] [PubMed] [Google Scholar]
- 4.Nian M., Lee P., Khaper N., Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 5.Hwang M.W., Matsumori A., Furukawa Y. Neutralization of interleukin-1beta in the acute phase of myocardial infarction promotes the progression of left ventricular remodeling. J Am Coll Cardiol. 2001;38:1546–1553. doi: 10.1016/s0735-1097(01)01591-1. [DOI] [PubMed] [Google Scholar]
- 6.Orn S., Ueland T., Manhenke C. Increased interleukin-1beta levels are associated with left ventricular hypertrophy and remodelling following acute ST segment elevation myocardial infarction treated by primary percutaneous coronary intervention. J Intern Med. 2012;272:267–276. doi: 10.1111/j.1365-2796.2012.02517.x. [DOI] [PubMed] [Google Scholar]
- 7.Van Tassell B.W., Varma A., Salloum F.N. Interleukin-1 trap attenuates cardiac remodeling after experimental acute myocardial infarction in mice. J Cardiovasc Pharmacol. 2010;55:117–122. doi: 10.1097/FJC.0b013e3181c87e53. [DOI] [PubMed] [Google Scholar]
- 8.Toldo S., Mezzaroma E., Bressi E. Interleukin-1beta blockade improves left ventricular systolic/diastolic function and restores contractility reserve in severe ischemic cardiomyopathy in the mouse. J Cardiovasc Pharmacol. 2014;64:1–6. doi: 10.1097/FJC.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 9.Bujak M., Frangogiannis N.G. The role of IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp (Warsz) 2009;57:165–176. doi: 10.1007/s00005-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulick T., Chung M.K., Pieper S.J., Lange L.G., Schreiner G.F. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci U S A. 1989;86:6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan D.J., Yang Z., Hopkins P.M., Steele D.S., Harrison S.M. TNF-alpha and IL-1beta increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium. 2010;47:378–386. doi: 10.1016/j.ceca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westman P.C., Lipinski M.J., Luger D. Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol. 2016;67:2050–2060. doi: 10.1016/j.jacc.2016.01.073. [DOI] [PubMed] [Google Scholar]
- 13.Van Tassell B.W., Toldo S., Mezzaroma E., Abbate A. Targeting interleukin-1 in heart disease. Circulation. 2013;128:1910–1923. doi: 10.1161/CIRCULATIONAHA.113.003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbate A., Van Tassell B.W., Seropian I.M. Interleukin-1beta modulation using a genetically engineered antibody prevents adverse cardiac remodelling following acute myocardial infarction in the mouse. Eur J Heart Fail. 2010;12:319–322. doi: 10.1093/eurjhf/hfq017. [DOI] [PubMed] [Google Scholar]
- 15.Owyang A.M., Issafras H., Corbin J. XOMA 052, a potent, high-affinity monoclonal antibody for the treatment of IL-1beta-mediated diseases. MAbs. 2011;3:49–60. doi: 10.4161/mabs.3.1.13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roell M.K., Issafras H., Bauer R.J. Kinetic approach to pathway attenuation using XOMA 052, a regulatory therapeutic antibody that modulates interleukin-1beta activity. J Biol Chem. 2010;285:20607–20614. doi: 10.1074/jbc.M110.115790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richard V., Kaeffer N., Tron C., Thuillez C. Ischemic preconditioning protects against coronary endothelial dysfunction induced by ischemia and reperfusion. Circulation. 1994;89:1254–1261. doi: 10.1161/01.cir.89.3.1254. [DOI] [PubMed] [Google Scholar]
- 18.Henri O., Pouehe C., Houssari M. Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction. Circulation. 2016;133:1484–1497. doi: 10.1161/CIRCULATIONAHA.115.020143. discussion 1497. [DOI] [PubMed] [Google Scholar]
- 19.Mulder P., Barbier S., Chagraoui A. Long-term heart rate reduction induced by the selective I(f) current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109:1674–1679. doi: 10.1161/01.CIR.0000118464.48959.1C. [DOI] [PubMed] [Google Scholar]
- 20.Mulder P., Mellin V., Favre J. Aldosterone synthase inhibition improves cardiovascular function and structure in rats with heart failure: a comparison with spironolactone. Eur Heart J. 2008;29:2171–2179. doi: 10.1093/eurheartj/ehn277. [DOI] [PubMed] [Google Scholar]
- 21.Mann D.L., McMurray J.J., Packer M. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 22.Deftereos S., Giannopoulos G., Panagopoulou V. Anti-inflammatory treatment with colchicine in stable chronic heart failure: a prospective, randomized study. J Am Coll Cardiol HF. 2014;2:131–137. doi: 10.1016/j.jchf.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Abbate A., Salloum F.N., Vecile E. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 24.Salloum F.N., Chau V., Varma A. Anakinra in experimental acute myocardial infarction: does dosage or duration of treatment matter? Cardiovasc Drugs Ther. 2009;23:129–135. doi: 10.1007/s10557-008-6154-3. [DOI] [PubMed] [Google Scholar]
- 25.Abbate A., Kontos M.C., Abouzaki N.A. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies) Am J Cardiol. 2015;115:288–292. doi: 10.1016/j.amjcard.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Abbate A., Kontos M.C., Grizzard J.D. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study) Am J Cardiol. 2010;105:1371–1377. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 27.Abbate A., Van Tassell B.W., Biondi-Zoccai G. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study] Am J Cardiol. 2013;111:1394–1400. doi: 10.1016/j.amjcard.2013.01.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Tassell B.W., Seropian I.M., Toldo S., Mezzaroma E., Abbate A. Interleukin-1beta induces a reversible cardiomyopathy in the mouse. Inflam Res. 2013;62:637–640. doi: 10.1007/s00011-013-0625-0. [DOI] [PubMed] [Google Scholar]
- 29.Kho C., Lee A., Hajjar R.J. Altered sarcoplasmic reticulum calcium cycling: targets for heart failure therapy. Nat Rev Cardiol. 2012;9:717–733. doi: 10.1038/nrcardio.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radeke H.H., Meier B., Topley N., Floge J., Habermehl G.G., Resch K. Interleukin 1-alpha and tumor necrosis factor-alpha induce oxygen radical production in mesangial cells. Kidney Int. 1990;37:767–775. doi: 10.1038/ki.1990.44. [DOI] [PubMed] [Google Scholar]
- 31.Yoshizumi M., Kurihara H., Morita T. Interleukin 1 increases the production of endothelin-1 by cultured endothelial cells. Biochem Biophys Res Commun. 1990;166:324–329. doi: 10.1016/0006-291x(90)91948-r. [DOI] [PubMed] [Google Scholar]
- 32.Mulder P., Richard V., Derumeaux G. Role of endogenous endothelin in chronic heart failure: effect of long- term treatment with an endothelin antagonist on survival, hemodynamics, and cardiac remodeling. Circulation. 1997;96:1976–1982. doi: 10.1161/01.cir.96.6.1976. [DOI] [PubMed] [Google Scholar]
- 33.Sakai S., Miyauchi T., Kobayashi M., Yamaguchi I., Goto K., Sugishita Y. Inhibition of myocardial endothelin pathway improves long-term survival in heart failure. Nature. 1996;384:353–355. doi: 10.1038/384353a0. [DOI] [PubMed] [Google Scholar]
- 34.Jain M., Brenner D.A., Cui L. Glucose-6-phosphate dehydrogenase modulates cytosolic redox status and contractile phenotype in adult cardiomyocytes. Circ Res. 2003;93:e9–e16. doi: 10.1161/01.RES.0000083489.83704.76. [DOI] [PubMed] [Google Scholar]
- 35.Kuster G.M., Lancel S., Zhang J. Redox-mediated reciprocal regulation of SERCA and Na+-Ca2+ exchanger contributes to sarcoplasmic reticulum Ca2+ depletion in cardiac myocytes. Free Radic Biol Med. 2010;48:1182–1187. doi: 10.1016/j.freeradbiomed.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siwik D.A., Pagano P.J., Colucci W.S. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 37.Zhao W., Zhao T., Chen Y., Ahokas R.A., Sun Y. Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor-beta1 in hypertensive rats. Mol Cell Biochem. 2008;317:43–50. doi: 10.1007/s11010-008-9803-8. [DOI] [PubMed] [Google Scholar]
- 38.Gillebert T.C., De Hert S.G., Andries L.J., Jageneau A.H., Brutsaert D.L. Intracavitary ultrasound impairs left ventricular performance: presumed role of endocardial endothelium. Am J Physiol. 1992;263:H857–H865. doi: 10.1152/ajpheart.1992.263.3.H857. [DOI] [PubMed] [Google Scholar]
- 39.van Heerebeek L., Hamdani N., Handoko M.L. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 40.van Heerebeek L., Somsen A., Paulus W.J. The failing diabetic heart: focus on diastolic left ventricular dysfunction. Curr Diab Rep. 2009;9:79–86. doi: 10.1007/s11892-009-0014-9. [DOI] [PubMed] [Google Scholar]
- 41.El-Omar M.M., Lord R., Draper N.J., Shah A.M. Role of nitric oxide in posthypoxic contractile dysfunction of diabetic cardiomyopathy. Eur J Heart Fail. 2003;5:229–239. doi: 10.1016/s1388-9842(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 42.Silberman G.A., Fan T.H., Liu H. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–528. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matter C.M., Mandinov L., Kaufmann P.A., Vassalli G., Jiang Z., Hess O.M. Effect of NO donors on LV diastolic function in patients with severe pressure-overload hypertrophy. Circulation. 1999;99:2396–2401. doi: 10.1161/01.cir.99.18.2396. [DOI] [PubMed] [Google Scholar]
- 44.Fraccarollo D., Widder J.D., Galuppo P. Improvement in left ventricular remodeling by the endothelial nitric oxide synthase enhancer AVE9488 after experimental myocardial infarction. Circulation. 2008;118:818–827. doi: 10.1161/CIRCULATIONAHA.107.717702. [DOI] [PubMed] [Google Scholar]
- 45.Serpillon S., Floyd B.C., Gupte R.S. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol Heart Circ Physiol. 2009;297:H153–H162. doi: 10.1152/ajpheart.01142.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen P., Osmers A. Oxidative stress in young Zucker rats with impaired glucose tolerance is diminished by acarbose. Horm Metab Res. 2006;38:575–586. doi: 10.1055/s-2006-950397. [DOI] [PubMed] [Google Scholar]
- 47.Sauve M., Ban K., Momen M.A. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes. 2010;59:1063–1073. doi: 10.2337/db09-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toblli J.E., Cao G., Giani J.F., Angerosa M., Dominici F.P., Gonzalez-Cadavid N.F. Antifibrotic effects of pioglitazone at low doses on the diabetic rat kidney are associated with the improvement of markers of cell turnover, tubular and endothelial integrity, and angiogenesis. Kidney Blood Press Res. 2012;34:20–33. doi: 10.1159/000320380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sekiguchi K., Li X., Coker M. Cross-regulation between the renin-angiotensin system and inflammatory mediators in cardiac hypertrophy and failure. Cardiovasc Res. 2004;63:433–442. doi: 10.1016/j.cardiores.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Otani H. The role of nitric oxide in myocardial repair and remodeling. Antioxid Redox Signal. 2009;11:1913–1928. doi: 10.1089/ars.2009.2453. [DOI] [PubMed] [Google Scholar]
- 51.Luger D., Lipinski M.J., Westman P.C. Intravenously delivered mesenchymal stem cells: systemic anti-inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ Res. 2017;120:1598–1613. doi: 10.1161/CIRCRESAHA.117.310599. [DOI] [PubMed] [Google Scholar]
- 52.Ozer G., Teker Z., Cetiner S. Serum IL-1, IL-2, TNFalpha and INFgamma levels of patients with type 1 diabetes mellitus and their siblings. J Pediatr Endocrinol Metab. 2003;16:203–210. doi: 10.1515/jpem.2003.16.2.203. [DOI] [PubMed] [Google Scholar]
- 53.van Asseldonk E.J., van Poppel P.C., Ballak D.B., Stienstra R., Netea M.G., Tack C.J. One week treatment with the IL-1 receptor antagonist anakinra leads to a sustained improvement in insulin sensitivity in insulin resistant patients with type 1 diabetes mellitus. Clin Immunol. 2015;160:155–162. doi: 10.1016/j.clim.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 54.Pfeffer M.A., Pfeffer J.M., Steinberg C., Finn P. Survival after an experimental myocardial infarction: beneficial effects of long-term therapy with captopril. Circulation. 1985;72:406–412. doi: 10.1161/01.cir.72.2.406. [DOI] [PubMed] [Google Scholar]
- 55.Wollert K.C., Studer R., von Bulow B., Drexler H. Survival after myocardial infarction in the rat. Role of tissue angiotensin-converting enzyme inhibition. Circulation. 1994;90:2457–2467. doi: 10.1161/01.cir.90.5.2457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.