Visual Abstract
Key Words: heart failure, relaxin, vascular function
Abbreviations and Acronyms: AngII, angiotensin II; HF, heart failure; L-NAME, L-NG-nitroarginine methyl ester; LV, left ventricular; SBP, systolic blood pressure
Highlights
-
•
Temporary administration of recombinant relaxin-2 (serelaxin) in patients hospitalized with HF was associated with improved mortality 6 months after discharge.
-
•
The specific effects of serelaxin on vascular and myocardial structure and function in HF have not been studied.
-
•
In mice subjected to continuous 28-day heart failure stimulus of AngII and L-NAME, serelaxin was administered for 3 days (days 7 to 9), and both the acute effects during serelaxin infusion and the delayed effects after termination of serelaxin on cardiovascular structure and function were studied.
-
•
Temporary serelaxin improved vascular fibrosis and myocardial capillary density and reduced resistance vessel constriction to potassium chloride during administration. These effects unexpectedly persisted 19 days after discontinuation of serelaxin, despite continued exposure to AngII/L-NAME. Serelaxin did not alter cardiac hypertrophy, geometry, or dysfunction at either time point.
-
•
These findings support that serelaxin predominantly affects vascular structure and function in the setting of HF.
Summary
In patients hospitalized with acute heart failure, temporary serelaxin infusion reduced 6-month mortality through unknown mechanisms. This study therefore explored the cardiovascular effects of temporary serelaxin administration in mice subjected to the angiotensin II (AngII)/L-NG-nitroarginine methyl ester (L-NAME) heart failure model, both during serelaxin infusion and 19 days post–serelaxin infusion. Serelaxin administration did not alter AngII/L-NAME-induced cardiac hypertrophy, geometry, or dysfunction. However, serelaxin-treated mice had reduced perivascular left ventricular fibrosis and preserved left ventricular capillary density at both time points. Furthermore, resistance vessels from serelaxin-treated mice displayed decreased potassium chloride–induced constriction and reduced aortic fibrosis. These findings suggest that serelaxin improves outcomes in patients through vascular-protective effects.
Although mortality in heart failure (HF) remains high, neurohormonal antagonists have significantly improved prognosis and survival of patients with chronic compensated HF with reduced ejection fraction. In patients specifically hospitalized with acute HF, 6-month mortality approaches 20% 1, 2, 3, with 30-day readmission as high as 25% 3, 4. The mechanisms promoting mortality after acute HF remain poorly understood, and consequently specific therapies to improve outcomes have not been successful.
The hormone relaxin has been identified recently as a potential therapy for acute HF. Relaxin also regulates multiple cardiovascular processes through interaction with the relaxin receptor, which is widely expressed in cardiovascular tissues (5). In vascular tissue, relaxin induces potent dilation of systemic and coronary vessels, at least in part by a nitric oxide–dependent mechanism, leading to increased arterial compliance, cardiac output, and renal blood flow 6, 7, 8, 9. In patients with HF, acute treatment with recombinant human relaxin-2 (serelaxin) decreases systemic blood pressure and pulmonary capillary wedge pressure (10). Circulating relaxin concentrations are elevated in humans with HF (8), further suggesting that relaxin normally opposes the maladaptive vasoconstricting effects of the renin angiotensin aldosterone system. In the recent serelaxin trial for treatment of acute HF, RELAX-AHF (Efficacy and Safety of Relaxin for the Treatment of Acute Heart Failure), patients hospitalized with acute HF were randomized to a 48-h infusion of serelaxin, which improved 1 of the 2 coprimary endpoints assessing dyspnea (3). Moreover, serelaxin treatment was associated with reduced mortality at 6 months after infusion, suggesting a prolonged benefit derived from short-term serelaxin treatment.
The delayed reduction in mortality after serelaxin infusion in patients with acute HF suggests that serelaxin confers benefits beyond acute vasodilation. Accordingly, in addition to acute vasorelaxing effects, serelaxin has been shown to attenuate vascular and cardiac fibrosis in several disease models and to exert direct effects on cultured cardiac myocytes and fibroblasts (11). Additionally, in humans with HF, short-term serelaxin treatment improved markers of renal function as well producing favorable effects on renal hemodynamic status 12, 13. Supporting this, in the RELAX-AHF study, serum markers of cardiac injury, renal dysfunction, and liver damage were improved after serelaxin administration 13, 14. Whether temporary administration of serelaxin can produce prolonged benefits in HF, as was observed in humans, has not been tested experimentally, and the specific vascular and cardiac effects of serelaxin in the setting of acute and chronic HF remain unknown.
To address these questions, we examined the effects of serelaxin in the angiotensin II (AngII)/L-NG-nitroarginine methyl ester (L-NAME) experimental model of HF, at time points designed to recapitulate the on-treatment and 6-month post-treatment time points reported in RELAX-AHF, but on a time scale suitable for mice 3, 15. We chose the well-established 28-day AngII/L-NAME model using published concentrations of AngII/L-NAME that are well validated to produce a rise in systolic blood pressure (SBP). These doses induce myocardial damage within 4 days of administration 16, 17, with myocardial fibrosis, cardiac myocyte hypertrophy, and reduction of LV systolic function present by 7 days and further progressive dysfunction after 28 days of AngII/L-NAME. In the present study, we investigated: 1) the vascular and cardiac effects during serelaxin infusion initiated on day 7 during the acute phase of AngII/L-NAME HF; and 2) the effects on these parameters at day 28 of AngII/L-NAME, 18 days after cessation of serelaxin.
Methods
A detailed description of methods is found in the Supplemental Appendix. Primer sequences can be found in Supplemental Table 1.
Animal studies and experimental design
All mice were handled in accordance with National Institutes of Health standards, and all procedures were approved by the Tufts Medical Center Institutional Animal Care and Use Committee. The experimental timelines and designs are described in Figure 1A. We used 2 timelines: an “Acute:on-treatment” study and a “Chronic:post- treatment” study. Male, C57Bl6 mice 10 to 12 weeks of age were used in all studies. At day 0, wild-type mice received AngII via osmotic pump and L-NAME in drinking water continuously for 9 or 28 days. At day 7, mice were randomized to serelaxin (500 μg/kg/day) or vehicle (saline) administered by a second, 3-day osmotic pump. Mice were sacrificed on day 9 (2 days into serelaxin or vehicle infusion, “Acute:on-treatment” group) or day 28 (19 days after the 3-day serelaxin or vehicle infusion, “Chronic:post-treatment” group). In both the Acute:on-treatment and the Chronic:post-treatment studies, separate groups of mice were sacrificed and used for cardiac (n = 20) or vascular (n = 22) studies.
Figure 1.
Serelaxin Temporarily Decreases Blood Pressure in the Angiotensin II/L-NG-Nitroarginine Methyl Ester Experimental Model
(A) In the Chronic:post-treatment study, telemeters were implanted on day 7. Blood pressure (BP) was recorded at baseline and throughout the duration of the experiment. In each experiment, angiotensin II (AngII) (800 ng/kg/min)/L-NG-nitroarginine methyl ester (L-NAME) (30 mg/kg/day in drinking water) treatment began at day 0. On day 7, mice were randomized to serelaxin or vehicle infusion by 3-day osmotic pump. Mice were sacrificed, and cardiac and vascular parameters were measured on day 9 in the Acute:on-treatment groups and on day 28 in the Chronic:post-treatment groups. (B) Telemetric average 24-h systolic blood pressure on day 0 and daily from day 7 to day 28. (C) Systolic blood pressure changes from baseline to day 28 during AngII/L-NAME. *p = 0.028. (B, C) Two-factor repeated-measures analysis of variance with Tukey post hoc test.
Statistical analysis
For repeated measures within treatment groups (Figure 1B), statistical differences were assessed using 2-factor repeated-measures analysis of variance to account for treatment effects at experimental time points and to measure main effects of drug treatment. For all other experiments, data were analyzed using 2-factor analysis of variance with factors of time (day 9, day 28) and treatment (vehicle, serelaxin). We included an interaction term for treatment within time. Multiple comparisons were performed using the Tukey post hoc test. Main effects of time point and drug treatment are also reported when significant; p values < 0.05 were considered to indicate statistical significance. Although normality assessment by Shapiro-Wilk testing confirmed parametric distribution of the data in most cases, because of the relatively small sample sizes, we performed analysis using 2-factor analysis of variance even in experimental groups that did not meet strict normality assumptions.
Data were analyzed using SigmaPlot version 12.0 (Systat Software, San Jose, California).
Results
Effects of AngII/L-NAME administration on blood pressure and mortality
Sixty-one mice were treated with AngII/L-NAME to produce hypertension-induced HF. As expected in a model of acute HF, there were 17 deaths (26%), which occurred in the first 7 days, prior to randomization, with only 2 deaths occurring after randomization to serelaxin or vehicle (1 vehicle-treated, 1 serelaxin-treated), resulting in 42 experimental animals. After 28 days, AngII/L-NAME treatment increased SBP to the same extent in mice randomized to either serelaxin or vehicle (Figure 1B).
Serelaxin temporarily reduces blood pressure in the AngII/L-NAME HF model
Serelaxin infusion induced a modest and transient decrease in SBP (Figure 1B). The serelaxin-treated group tended to have lower SBP until 6 days after cessation of serelaxin infusion (day 16), but this was statistically significant only at day 13. After cessation of serelaxin infusion, blood pressure rose in the serelaxin-treated animals such that overall, long-term AngII/L-NAME administration resulted in similar SBP in both groups (Figure 1C).
No effect of serelaxin treatment on acute and chronic left ventricular structure or function in the AngII/L-NAME model
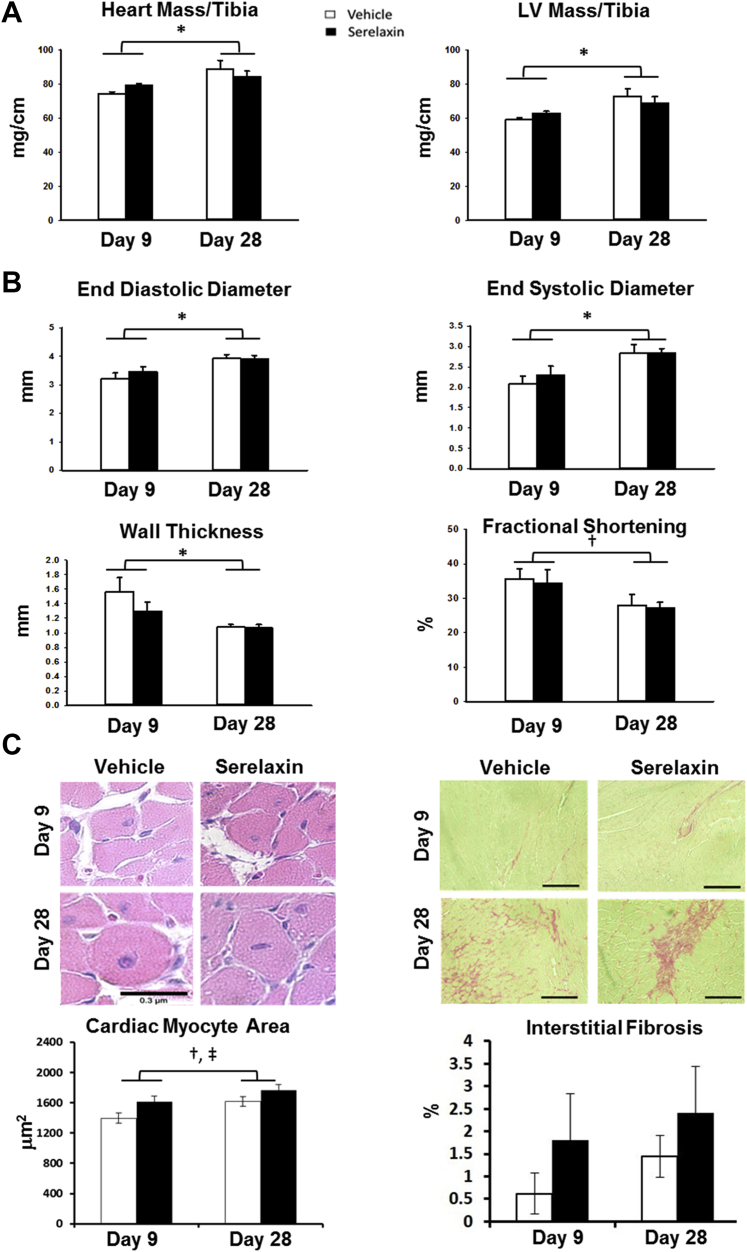
Complete organ mass and histological data are reported in Supplemental Table 2. Echocardiographic data from the Acute:on-treatment and Chronic:post-treatment studies are reported in Supplemental Table 3. As expected, both heart weight and left ventricular (LV) mass, each normalized to tibia length, increased significantly from day 9 to day 28. However, these parameters did not differ between vehicle- and serelaxin-treated groups at either time point (Figure 2A). Similarly, echocardiographic measures of end-diastolic diameter and end-systolic diameter increased globally from day 9 to 28, with a decrease in LV wall thickness and ejection fraction, indicating that AngII/L-NAME induced progressive LV remodeling, dilation, and systolic dysfunction comparable with previous studies at the same time point (18). Compared with historical untreated control mice of the same age (15), LV mass was increased, and fractional shortening was impaired, indicating that AngII/L-NAME induces pathological remodeling by day 9. No differences were observed in these ultrasound parameters between vehicle- and serelaxin-treated groups at either time point (Figure 2B). Cardiomyocyte cross sectional area increased from day 9 to day 28 (Figure 2C) and did not differ significantly between serelaxin- or vehicle-treated groups at either time point.
Figure 2.
Serelaxin Does Not Reduce Left Ventricular Hypertrophy or Remodeling in the Angiotensin II/L-NG-Nitroarginine Methyl Ester Model
Cardiac ultrasound was performed followed by organ harvest and histology in the Acute:on-treatment (day 9) and Chronic:post-treatment (day 28) groups in angiotensin II (AngII)/L-NG-nitroarginine methyl ester (L-NAME)–treated mice. (A) Heart and left ventricular (LV) mass normalized to tibia length. (B) Cardiac dimensions and function as measured by echocardiography. (C) Representative and summary data of cardiac myocyte cross-sectional area from hematoxylin and eosin–stained LV sections (scale bar represents 0.3 μm) and of interstitial fibrosis from picrosirius red–stained LV sections (scale bar represents 1 μm). n = 6 (day 9) or 5 (day 28) per treatment. Connected single bars over days 9 and 28 indicate analysis of main effects of time factor for vehicle and serelaxin in combination. *p < 0.01 and †p = 0.024, day 9 versus day 28; ‡p = 0.027 for main effects of treatment factor serelaxin versus vehicle. Two-factor analysis of variance with Tukey post hoc test.
Brief serelaxin infusion reduces perivascular but not interstitial cardiac fibrosis and enhances cardiac angiogenesis in the AngII/L-NAME model
Cardiac fibrosis was already evident at day 9 of AngII/L-NAME treatment, though the extent of interstitial fibrosis, measured by picrosirius red staining, did not differ between vehicle- and serelaxin-treated groups at day 9 or day 28 (Figure 2C). This is consistent with LV gene expression of markers of fibrosis (type-1 collagen, matrix metalloproteinase-2, and transforming growth factor-β), which did not differ between serelaxin- and vehicle-treated groups (Supplemental Figure 1). Perivascular fibrosis, however, increased substantially from day 9 to day 28, but this was significantly attenuated in the serelaxin-treated group compared with vehicle (Figure 3A). As a result, at day 28, perivascular fibrosis was significantly reduced in the serelaxin-treated compared with the vehicle-treated group.
Figure 3.
Serelaxin Reduces Coronary Perivascular Fibrosis and Increases Left Ventricular Capillary Density in the Angiotensin II/L-NG-Nitroarginine Methyl Ester Model
Left ventricular (LV) histology in the Acute:on-treatment group (day 9, n = 6 per group) and Chronic:post-treatment group (day 28, n = 5 per group) in angiotensin II (AngII)/L-NG-nitroarginine methyl ester (L-NAME)–treated mice. (A) Representative and summary data of perivascular fibrosis from picrosirius red–stained LV sections. Scale bars represent 1 μm. (B) Representative and summary data of capillary density from CD31 staining. Black arrows denote examples of CD31-positive capillaries. Scale bars represent 0.3 μm. Connected single bars over days 9 and 28 indicate analysis of main effects of time factor for vehicle and serelaxin in combination. *p < 0.01 treatment within day; †p < 0.001 for main effects of time factor, day 9 versus day 28; ‡p = 0.009 for main effects of treatment factor serelaxin versus vehicle. Two-factor analysis of variance with Tukey post hoc test.
Serelaxin-treated mice also displayed a significant increase in cardiac capillary density, as assessed by the density of CD31-positive capillaries per area of myocardium, at both day 9 and day 28 compared with vehicle-treated mice (Figure 3B).
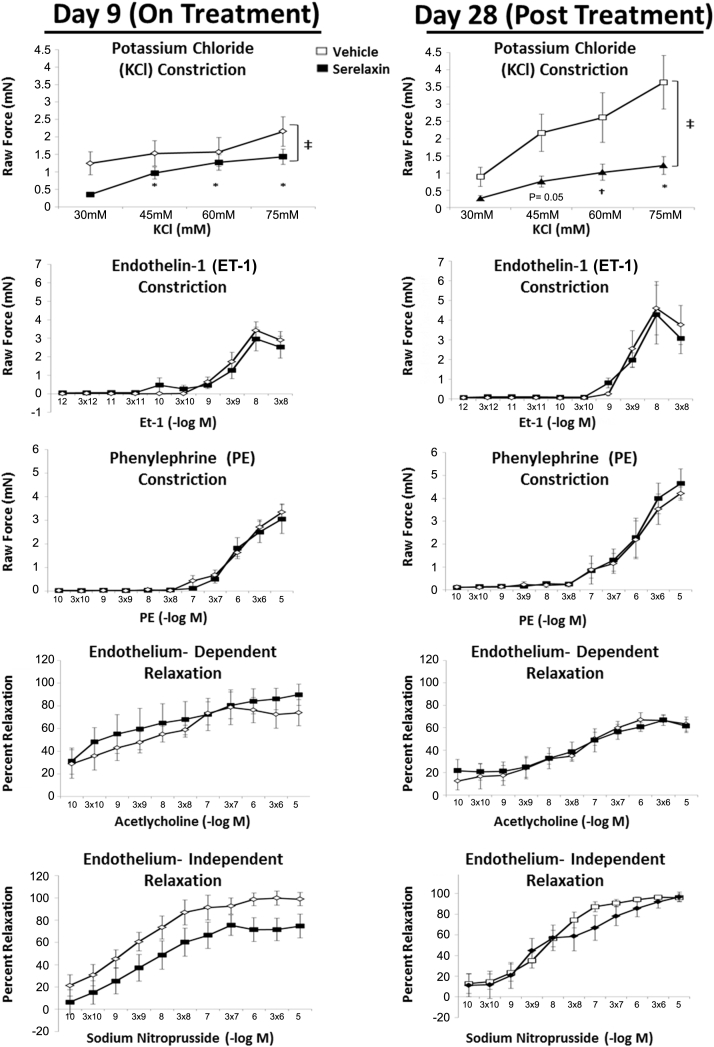
Acute and sustained effects of serelaxin on resistance artery function in the AngII/L-NAME model
We next examined the effects of serelaxin on systemic vascular structure and function. The acute and sustained effects of serelaxin on mesenteric resistance arteriole contraction and relaxation were examined by wire myography (Figure 4). Compared with vehicle-treated mice at day 9, mesenteric resistance arteries from mice exposed to serelaxin displayed reduced constriction to the depolarization-dependent vasoconstrictor potassium chloride. At 18 days after cessation of serelaxin treatment (day 28), this reduction in potassium chloride constriction persisted and was even more pronounced (Figure 4). Exposure to serelaxin during AngII/L-NAME treatment did not affect constriction to phenylephrine or endothelin-1, nor did it affect endothelium-independent relaxation to sodium nitroprusside or endothelium-dependent relaxation to acetylcholine.
Figure 4.
Serelaxin Treatment Decreases the Mesenteric Arteriolar Contractile Response to Potassium Chloride in the Angiotensin II/L-NG-Nitroarginine Methyl Ester Heart Failure Model
Mesenteric resistance arterioles were isolated on day 9 or day 28 from mice treated with AngII/L-NAME, and with either serelaxiin or vehicle. Vasoconstrictor and vasorelaxer responses were measured by wire myography. *p < 0.01 and †p < 0.05 for treatment effect within potassium chloride dose; ‡p < 0.05 for overall main effects of serelaxin versus vehicle (p = 0.004, day 9; p = 0.034, day 28). Data were compared using 2-factor repeated-measures analysis of variance with Tukey post hoc test.
Serelaxin attenuates the development and progression of vascular fibrosis in the AngII/L-NAME model
Vascular hypertrophy and fibrosis were quantified in sections of abdominal aorta from vehicle- and serelaxin-treated animals on day 9 and day 28 of AngII/L-NAME infusion (Figure 5A). Aortic medial thickness measured from elastin-stained sections did not differ between serelaxin- and vehicle-treated mice at either time. From day 9 to 28, the area of fibrosis in the vessel media increased 8-fold in vehicle treated vessels. Overall, aortic medial fibrosis area was significantly decreased in serelaxin-treated compared with vehicle-treated mice. Although serelaxin was infused only temporarily, the progression of fibrosis was attenuated such that at day 28, there was a profound and significant decrease in vascular fibrosis in mice treated with serelaxin (p < 0.001) (Figure 5B).
Figure 5.
Serelaxin Infusion Attenuates Vascular Fibrosis and Fibrotic Gene Expression in the Angiotensin II/L-NG-Nitroarginine Methyl Ester Heart Failure Model
The abdominal aorta was isolated on day 9 (n = 6) or on day 28 (n = 5) of angiotensin II (AngII)/L-NG-nitroarginine methyl ester (L-NAME) infusion. Representative and summary data of aortic medial area from elastin-stained aortas (A) and aortic medial fibrosis from trichrome-stained aortas (B). Black arrows define the tunica media (medial area and degree of fibrosis was measured between arrows). Scale bars represent 1 μm. *p < 0.001; †p < 0.001 for main effects of treatment factor serelaxin versus vehicle; ‡p < 0.001 for main effects of time factor, day 9 versus day 28. (C) Messenger ribonucleic acid was isolated from aortas of mice treated with serelaxin or vehicle, and expression of fibrosis genes was measured by quantitative reverse transcription polymerase chain reaction. *p = 0.018 serelaxin versus vehicle day 9; †p < 0.001, serelaxin day 28 versus vehicle day 28; ‡p = 0.022 for main effects of time factor, day 9 versus day 28; §p < 0.001 for main effects of treatment factor serelaxin versus vehicle; ‖p = 0.049 and ¶p = 0.003, serelaxin day 9 versus serelaxin day 28. Connected single bars over days 9 and 28 indicate analysis of main effects between time points for vehicle and serelaxin in combination. Groups were compared using 2-factor analysis of variance with Tukey post hoc test. Col1 = type 1 collagen; CTGF = connective tissue growth factor; MMP2 = matrix metalloproteinase 2; TGF = transforming growth factor.
Effects of serelaxin administration on vascular inflammatory and fibrosis gene expression in the AngII/L-NAME model
To begin to examine mechanisms of decreased vascular fibrosis in the serelaxin-treated group, we examined gene expression in the thoracic aortas of serelaxin- and vehicle-treated mice by quantitative reverse transcription polymerase chain reaction. Expression of inflammatory genes did not differ significantly between serelaxin- and vehicle-treated groups at day 9 or day 28 (Supplemental Figure 2). Vascular expression of the fibrosis regulators connective tissue growth factor and transforming growth factor β also did not change with time or treatment in this model (Figure 5C). Interestingly, however, in the serelaxin-treated group, vascular expression of type-1 collagen declined significantly from day 9 to day 28, resulting in a highly significant decrease in collagen expression in vessels treated with serelaxin compared with vehicle, at day 28 (Figure 5C). Furthermore, expression of matrix metalloproteinase-2, an enzyme that degrades collagen and hence opposes fibrosis, was increased 9-fold at day 9 in serelaxin-treated aortas compared with vehicle-treated aortas and then diminished compared with the vehicle-treated aortas by day 28 (Figure 5C).
Discussion
In the present study, we examined the cardiac and vascular effects of serelaxin in the AngII/L-NAME experimental model of HF, both during serelaxin infusion (Acute:on-treatment) and 18 days after cessation of serelaxin (Chronic:post-treatment). Our combined data reveal predominantly vascular, as opposed to direct cardiac, protective actions of serelaxin in the AngII/L-NAME HF model. Furthermore, in all cases we observed that these vascular effects were sustained or even enhanced well after completion of serelaxin infusion. Taken together these data support that serelaxin induces important biologic effects on vascular structure and function in the setting of HF that persist long after treatment and thus may contribute to the observed long-term benefits of brief serelaxin therapy during HF exacerbation in humans.
Lack of serelaxin effect on AngII/L-NAME -induced LV hypertrophy, dysfunction, and interstitial fibrosis
Prior work has revealed that the relaxin receptor is expressed in the cardiac myocyte, and its expression increases in the failing left ventricle (19). However, in that study, short-term augmentation of relaxin did not alter the general pathological cardiac effects of increased AngII and decreased nitric oxide. Other studies in rodents have demonstrated reduction of interstitial fibrosis with serelaxin, but these studies involved chronic, rather than temporary, serelaxin infusion 20, 21. Interestingly, 1 such study demonstrated reduced fibrosis in a myocardial infarction model, though similar to our findings, detected no significant alteration of LV function, hypertrophy, or structural remodeling with relaxin treatment (22). We therefore interpret our findings to support that temporary serelaxin infusion does not exert direct effects on myocardial hypertrophy and remodeling in the AngII/L-NAME model. An alternative interpretation is that serelaxin does exert direct effects on cardiomyocyte function and remodeling in vivo, but that these effects were overwhelmed by the high dose and prolonged duration of AngII/L-NAME used in our study. As described earlier, we chose these doses and time points because they have been well validated previously to produce chronic HF and progressive cardiac and vascular remodeling. To our knowledge no other short-term pharmacological therapy has demonstrated long-term cardiac or vascular benefits in the AngII/L-NAME model. Thus, the impressive vascular benefits described here suggest that short-term serelaxin therapy can prevent the progression of vascular fibrosis and dysfunction despite persistent AngII/L-NAME treatment. However, future studies examining effects of serelaxin on cardiac and vascular dysfunction in additional models of acute and chronic HF will be informative.
Serelaxin enhances capillary density and attenuates the progression of perivascular fibrosis in response to AngII/L-NAME
Unlike interstitial fibrosis, which predominantly occurs as replacement for apoptotic myocytes (23) or as a reactive process of interstitial fibroblasts to neurohormonal stimulation (24), cardiac perivascular fibrosis likely results predominantly from coronary vascular interaction with and recruitment of circulating leukocytes 24, 25. Perivascular fibrosis also predisposes the left ventricle to ischemic events both in experimental models (24) and in humans with nonischemic cardiomyopathy 26, 27.
The preservation of capillary density observed in serelaxin-treated left ventricles further supports a predominantly vascular effect of serelaxin, in this case on angiogenesis, in the modulation of the HF phenotype. In other HF experimental models, failure to increase capillary density in the left ventricle to the same degree as myocardial mass promotes the transition from compensated to decompensated HF (28). Preservation of LV capillary density in the face of cardiac hypertrophy may therefore represent a protective mechanism of serelaxin in HF.
Serelaxin improves vascular structure and function in the AngII/L-NAME model
Brief serelaxin treatment induced significant and persistent improvements in vascular fibrosis. Gene expression data suggest that the mechanism may involve simultaneous down-regulation of collagen expression and up-regulation of collagen degrading enzymes. Systemic vascular fibrosis increases in response to renin-angiotensin-aldosterone system activation and likely plays an important role in the pathophysiology of HF (18). We therefore interpret our findings to suggest that relaxin normally opposes neurohormone-induced arterial fibrosis and thus may represent an important intrinsic protective mechanism in HF.
Unexpectedly, we observed that ex vivo resistance vessels from serelaxin-treated mice displayed reduced vascular constriction to potassium chloride in both the intra- and post-infusion settings. These findings appear specific to the depolarizing vasoconstrictor potassium chloride, as constriction to neurohormonal agonists phenylephrine and endothelin-1 and relaxation to vasodilators did not differ between vessels isolated from serelaxin- and vehicle-treated mice. These results indicate that serelaxin attenuates vascular constriction by altering smooth muscle cell polarization, likely through direct effects on arteriolar ion channel function, as opposed to a more general structural effect on extracellular matrix content. The fact that these vasomotor effects persist after cessation of serelaxin further supports direct long-term effects of serelaxin on resistance vessel function. We hypothesize that relaxin normally promotes vascular smooth muscle cell polarization through either regulation of membrane channels or through effects on resistance endothelium to modulate endothelial-derived hyperpolarization. Further studies beyond the scope of this study are needed to clarify the mechanism, but this hypothesis is supported by previous observations in rats that long-term relaxin administration increases transcription of cardiovascular ion channels such as the calcium-sensitive potassium channel and the cardiac sodium channel Nav1.5 (11). We interpret our findings to support an important protective mechanism through which acute serelaxin administration might oppose the increased vascular resistance and afterload that occur in the setting of HF.
More broadly, the observed effects of serelaxin in the AngII/L-NAME model also provide mechanistic support that serelaxin specifically opposes the pro-fibrotic and pro-constrictive effects of angiotensin and of impaired nitric oxide availability. Prior work has demonstrated that relaxin-induced vasorelaxation occurs through promotion of endothelial nitric oxide synthase transcription and inhibition of endothelin-induced vasoconstriction (29). Dysregulation of angiotensin and nitric oxide occurs in humans with HF and is generally accepted to contribute to acute HF exacerbation and to drive pathological cardiovascular remodeling as HF progresses. Taken together, this supports that relaxin opposes the deleterious effects of AngII and reduced nitric oxide on the vasculature during acute HF. Interestingly, the fact that serelaxin improved vascular structure and function in the setting of decreased nitric oxide availability also suggests that some of the vascular effects of serelaxin in HF may occur independently of its regulation of nitric oxide synthesis.
Clinical implications
The recent RELAX-AHF trial observed that patients treated with 48-h serelaxin infusion during an acute HF hospitalization experienced lower all-cause and cardiovascular mortality at 6 months after treatment (3). Based on the promising results of RELAX-AHF, the RELAX-AHF-2 trial enrolled over 6,600 patients admitted for AHF and evaluated the effects of serelaxin compared to placebo on the independently powered primary endpoints of worsening heart failure through 5 days and 180-day cardiovascular mortality (30). Serelaxin did not improve either the primary endpoint of cardiovascular mortality at 180 days or worsening heart failure through day 5 compared to placebo. Ongoing analyses of data from RELAX-AHF-2 and prior studies of serelaxin may provide additional insights into subgroup analyses of these results. The pre-clinical studies shown herein demonstrate that serelaxin can produce protective biological effects in an HF model that persist after a short-term treatment and thus support plausible mechanisms through which serelaxin could improve long-term outcomes in subgroups of patients with acute HF. The observation that serelaxin improved vascular, as opposed to cardiac, structure and function in mice is interesting because these findings correlate with those in the RELAX-AHF trial, but not in the RELAX-AHF-2 trial. The results of the present study may help to inform the ongoing analysis of the RELAX-AHF-2 trial.
Study limitations
First, although we directly compared serelaxin with vehicle treatment at each experimental time point, we did not include corresponding groups treated with no AngII/L-NAME, raising the question of the degree of HF induced at the time serelaxin was initiated. The early mortality in AngII/L-NAME-treated mice in our study supports the severity of the phenotype after 7 days of this treatment. Furthermore, the normalized lung masses of surviving mice at day 9 were increased compared with normal values in mice, and comparable with other published HF models (15), indicating the induction of pulmonary edema, a hallmark of HF. Our demonstration of progressive LV hypertrophy, interstitial fibrosis, increased LV chamber diameters, as well as reduced LV systolic function between day 9 and day 28, further indicates that the AngII/L-NAME treatment induced progressive remodeling and HF.
In addition, although we aimed to recapitulate the conditions from the human trial, it should be acknowledged that direct comparison between a 28-day time point in mice versus a 6-month endpoint in humans is inherently hard to interpret. However, on the basis of the much shorter life span of the mouse compared with humans, 28 days of AngII/L-NAME treatment produces chronic changes that would require several months in humans (15). It should be noted that we analyzed the Acute:on-treatment study during the third day of serelaxin infusion, before the full 72 h of treatment. Although we designed this to ensure that the animals were studied while the drug was still infusing, it remains possible that a complete 72 h of serelaxin could have produced more detectable effects on cardiovascular structure and function. It is also important to note this difference in duration of serelaxin administration when comparing the findings from the acute and chronic studies. Regardless, our results provide potential mechanistic insight into the effects of relaxin observed in humans with HF, though it remains possible that the mechanisms of clinical benefit of serelaxin differ between human patients and rodents and may differ with different causes of HF. Further studies are needed to explore these possibilities.
Conclusions
We have identified selective beneficial effects of serelaxin on vascular fibrosis, angiogenesis, and vascular contractile function in an experimental HF model and have demonstrated that these benefits persist after discontinuation of serelaxin. These findings support novel vascular mechanisms through which serelaxin opposes the deleterious effects of neurohormonal dysregulation in HF. Finally, these findings suggest that the clinical benefits observed in the RELAX-AHF trial may arise from prolonged protective effects of serelaxin the vasculature.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Our experimental study addresses the clinical findings of the RELAX-AHF study, in which temporary infusion of serelaxin (recombinant human relaxin-2) improved symptoms in patients with acute HF but also was associated with improved mortality 6 months after infusion. We demonstrated, in a preclinical experimental HF model, that serelaxin improved vascular, rather than cardiac, structure and function. Furthermore, the vascular benefits of serelaxin during acute HF were sustained after discontinuation of treatment. These experimental findings provide a potential mechanistic explanation for the clinical observations in RELAX-AHF and suggest that relaxin improves outcomes after acute HF predominantly through improving vascular structure and function.
TRANSLATIONAL OUTLOOK: Although initial clinical trials of serelaxin in acute HF observed a 6-month mortality reduction associated with serelaxin infusion, phase 3 clinical trials of serelaxin reportedly did not reduce similar clinical endpoints in acute HF patients. These potentially discrepant findings highlight the need to better understand biological mechanisms regulated by serelaxin in acute HF. By using an experimental approach, our study identified that serelaxin predominantly improved vascular structure and function in a model of HF, whereas it did not directly affect or improve cardiac parameters. These findings support further investigation of the relative efficacy of serelaxin in improving vascular versus cardiac pathophysiology in acute HF, and may provide mechanistic insight into the clinical trial outcomes described above.
Footnotes
This study was funded by Novartis Pharmaceuticals through an investigator initiated study award (RLX030AUSNC14T). The authors have reported that they have no relationships relevant to the contents of this paper to disclose. Drs. Jaffe and Blanton contributed equally to this work.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Chen J., Normand S.T., Wang Y., Krumholz H.M. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries 1998–2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2139. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.Teerlink J.R., Cotter G., Davison B.A. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 4.Elixhauser A, Steiner C. Readmissions to U.S. hospitals by diagnosis 2010: Statistical Brief #153. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb153.pdf. Accessed May 19, 2017.
- 5.Sarwar M., Du X.J., Dschietzig T.B., Summers R.J. The actions of relaxin on the human cardiovascular system. Br J Pharmacol. 2017;174:933–949. doi: 10.1111/bph.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bani-Sacchi T., Bigazzi M., Bani D., Mannaioni P.F., Masini E. Relaxin-induced increased coronary flow through stimulation of nitric oxide production. Br J Pharmacol. 1995;116:1589–1594. doi: 10.1111/j.1476-5381.1995.tb16377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debrah D.O., Conrad K.P., Jeyabalan A., Danielson L.A., Shroff S.G. Relaxin increases cardiac output and reduces systemic arterial load in hypertensive rats. Hypertension. 2005;46:745–750. doi: 10.1161/01.HYP.0000184230.52059.33. [DOI] [PubMed] [Google Scholar]
- 8.Dschietzig T., Teichman S., Unemori E. Intravenous recombinant human relaxin in compensated heart failure: a safety, tolerability, and pharmacodynamic trial. J Card Fail. 2009;15:182–190. doi: 10.1016/j.cardfail.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Novak J., Danielson L.A., Kerchner L.J. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J Clin Invest. 2001;107:1469–1475. doi: 10.1172/JCI11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponikowski P., Mitrovic V., Ruda M. A randomized, double-blind, placebo- controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur Heart J. 2014;35:431–441. doi: 10.1093/eurheartj/eht459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry B.L., Gabris B., Li Q. Relaxin suppresses atrial fibrillation in aged rats by reversing fibrosis and upregulating Na+ channels. Heart Rhythm. 2016;13:983–991. doi: 10.1016/j.hrthm.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voors A.A., Dahlke M., Meyer S. Renal hemodynamic effects of serelaxin in patients with chronic heart failure. Circ Heart Fail. 2014;7:994. doi: 10.1161/CIRCHEARTFAILURE.114.001536. [DOI] [PubMed] [Google Scholar]
- 13.Teerlink J.R., Metra M., Felker G.M. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo- controlled, parallel-group, dose-finding phase IIb study. Lancet. 1925;373:1429–1439. doi: 10.1016/S0140-6736(09)60622-X. [DOI] [PubMed] [Google Scholar]
- 14.Metra M., Cotter G., Davison B.A. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the relaxin in acute heart failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Blanton R.M., Takimoto E., Lane A.M. Protein kinase G iα inhibits pressure overload induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J Am Heart Assoc. 2012;1:e003731. doi: 10.1161/JAHA.112.003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda J., Ichiki T., Matsuura H. Deletion of Phd2 in myeloid lineage attenuates hypertensive cardiovascular remodeling. J Am Heart Assoc. 2013;2:e000178. doi: 10.1161/JAHA.113.000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pojoga L.H., Romero J.R., Yao T.M. Caveolin-1 ablation reduces the adverse cardiovascular effects of N-w-nitro-L-arginine methyl ester and angiotensin II. Endocrinology. 2010;151:1236–1246. doi: 10.1210/en.2009-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton D.J., Zhang A., Li S. Combination of angiotensin II and l-NG- nitroarginine methyl ester exacerbates mitochondrial dysfunction and oxidative stress to cause heart failure. Am J Physiol Heart Circ Physiol. 2016;310:H667–H680. doi: 10.1152/ajpheart.00746.2015. [DOI] [PubMed] [Google Scholar]
- 19.Dschietzig T., Richter C., Bartsch C. The pregnancy hormone relaxin is a player in human heart failure. FASEB J. 2001;15:2187–2195. doi: 10.1096/fj.01-0070com. [DOI] [PubMed] [Google Scholar]
- 20.Wang D., Zhu H., Yang Q., Sun Y. Effects of relaxin on cardiac fibrosis, apoptosis, and tachyarrhythmia in rats with myocardial infarction. Biomed Pharmacother. 2016;84:348–355. doi: 10.1016/j.biopha.2016.09.054. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J., Qi Y.F., Geng B. Effect of relaxin on myocardial ischemia injury induced by isoproterenol. Peptides. 2005;26:1632–1639. doi: 10.1016/j.peptides.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Samuel C.S., Cendrawan S., Gao X.M. Relaxin remodels fibrotic healing following myocardial infarction. Lab Invest. 2011;91:675–690. doi: 10.1038/labinvest.2010.198. [DOI] [PubMed] [Google Scholar]
- 23.Samuel C.S., Royce S.G., Hewitson T.D., Denton K.M., Cooney T.E., Bennett R.G. Anti-fibrotic actions of relaxin. Br J Pharmacol. 2017;174:962–976. doi: 10.1111/bph.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan R., Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor B1 in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicoletti A., Michel J.B. Cardiac fibrosis and inflammation. Cardiovasc Res. 1999;41:532–543. doi: 10.1016/s0008-6363(98)00305-8. [DOI] [PubMed] [Google Scholar]
- 26.Bart B.A., Shaw L.K., McCants J. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J Am Coll Cardiol. 1997;30:1002–1008. doi: 10.1016/s0735-1097(97)00235-0. [DOI] [PubMed] [Google Scholar]
- 27.Dai Z., Aoki T., Fukumoto Y., Shimokawa H. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. Journal of Cardiology. 2012;60:416–421. doi: 10.1016/j.jjcc.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Perrino C., Prasad S.V.N., Mao L. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116:1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson S.S., Ayaz S.I., Levy P.D. Relaxin: a novel agent for the treatment of acute heart failure. Pharmacotherapy. 2015;35:315–327. doi: 10.1002/phar.1548. [DOI] [PubMed] [Google Scholar]
- 30.Teerlink J.R., Voors A.A., Ponikowski P. Serelaxin in addition to standard therapy in acute heart failure: rationale and design of the RELAX-AHF-2 study. Eur J Heart Fail. 2017;19:800–809. doi: 10.1002/ejhf.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.