Abstract
Objectives
To identify the types of cancer patients admitted to inpatient medical rehabilitation and to describe their rehabilitation outcomes. Design: Retrospective cohort study.
Setting
U.S. inpatient rehabilitation facilities (IRFs).
Participants
Adult patients (N=27,952) with a malignant cancer diagnosis admitted to an IRF with a cancer-related impairment between October 2010 and September 2012 were identified from the Uniform Data System for Medical Rehabilitation database.
Interventions
Not applicable.
Main Outcome Measures
Demographic, medical, and rehabilitation characteristics for patients with various cancer tumor types were summarized using data collected from the Inpatient Rehabilitation Facility–Patient Assessment Instrument. Rehabilitation outcomes included the percentage of patients discharged to the community and acute care settings, and functional change from admission to discharge. Functional status was measured using the FIM instrument.
Results
Cancer patients constituted about 2.4% of the total IRF patient population. Cancer types included brain and nervous system (52.9%), digestive (12.0%), bone and joint (8.7%), blood and lymphatic (7.6%), respiratory (7.1%), and other (11.7%). Overall, 72% were discharged to a community setting, and 16.5% were discharged back to acute care. Patients with blood and lymphatic cancers had the highest frequency of discharge back to acute care (28%). On average, all cancer patient groups made significant functional gains during their IRF stay (mean FIM total change ± SD, 23.5±16.2).
Conclusions
In a database representing approximately 70% of all U.S. patients in IRFs, we found that patients with a variety of cancer types are admitted to inpatient rehabilitation. Most cancer patients admitted to IRFs were discharged to a community setting and, on average, improved their function. Future research is warranted to understand the referral patterns of admission to postacute care rehabilitation and to identify factors that are associated with rehabilitation benefit in order to inform the establishment of appropriate care protocols.
Keywords: Hospitalization, Neoplasms, Rehabilitation, Survivors
Cancer is a chronic disease and a major source of morbidity and mortality in the United States. Increasing cancer incidence, coupled with an aging population, has vastly increased cancer prevalence. Currently, there are approximately 14.5 million cancer survivors living in the United States.1 It is estimated that there will be 18 million by 2022.2 In addition, the 5-year survival rates for all cancer types has increased from 49% during the years of 1975 to 1977, to 69% during 2005 to 2011.1 Although cancer patients are surviving longer, many have physical and cognitive impairments resulting from the etiology of cancer and from cancer treatments such as surgery, chemotherapy, radio-therapy, and hormonal therapy.3 Common types of impairments in cancer patients include pain, fatigue, neurologic dysfunction, bone metastasis, soft tissue disruption, sexual dysfunction, and cognitive deficits.4 These conditions can appear suddenly, but can also be a gradual accumulation of multiple disease and treatment effects.3
The American College of Surgeons Commission on Cancer requires accredited facilities to ensure that cancer patients have access to rehabilitation services and that a survivorship care plan is developed for comprehensive cancer care.5 However, standard guidelines for follow-up care among cancer survivors do not currently exist,6 and there is increased recognition that an increase in the coordination of care among cancer survivors is needed.7 Rehabilitation services are widely underused among cancer patients,8 and many patients with functional disability needs do not receive postacute rehabilitative care.8–10
Patients with diagnosed cancer are more likely to report poorer health outcomes, have a higher burden of illness, and a lower physical and mental quality of life than patients without cancer.11,12 Among cancer survivors, poor physical quality of life is reported more frequently than poor mental quality of life,12 and patient distress is strongly related to impaired physical functioning.11 Rehabilitation programs may serve to improve functional capacity lost as a result of cancer etiology and treatment. Early intervention through participation in comprehensive rehabilitation therapies across the care continuum may be effective in restoring functional deficits and preventing long-term disability related to cancer.3
Inpatient rehabilitation facilities (IRFs) provide interdisciplinary postacute care inpatient rehabilitation services consisting of medical management, physical therapy, occupational therapy, and speech-language therapy. The goal of IRF care is to restore or improve function and to help patients achieve a level of independence that allows them to return to a community setting. Admission to an IRF requires the patient to be medically stable and able to tolerate a minimum of 3 hours of therapy per day for at least 5 days per week.13 For cancer patients, IRF care focuses on addressing acute physical impairments related to treatment.3
There is little known about the characteristics of cancer patients receiving inpatient rehabilitation services. A better understanding of the types of cancer patients that present to inpatient rehabilitation and their outcomes could inform targeted approaches to address their functional needs and enhance management of complex disease sequelae. In this study, we describe the demographic, medical, and rehabilitation characteristics of cancer patients who were admitted to U.S. IRFs over a 3-year period using a large national database. We hypothesized that there would be differences in demographic characteristics as well as rehabilitation outcomes between patients with different tumor types.
Methods
Study design and population
We conducted a retrospective cohort study using 3 years of de-identified data from the Uniform Data System for Medical Rehabilitation (UDSMR) database. The UDSMR maintains the world’s largest nongovernmental database for rehabilitation outcomes14 and includes data from more than 70% of the IRFs in the United States. The dataset includes demographic, medical, and rehabilitation data collected from the Inpatient Rehabilitation Facility–Patient Assessment Instrument (IRF-PAI). These data are collected from each patient at admission and discharge from the participating IRF, and data collection procedures are standardized for all facilities.15
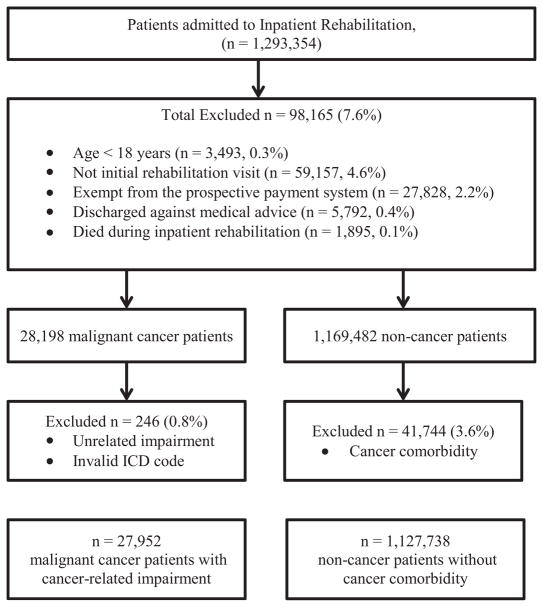
The inclusion and exclusion criteria for the study population are depicted in figure 1. In these analyses, patients were included if they were at least 18 years of age, admitted to the IRF for an initial rehabilitation visit, and discharged between October 2010 and September 2012. Some IRFs in the UDSMR database were exempt from the prospective payment system and were not subject to the requirements regarding data collection and reporting time frames associated with the IRF-PAI (n=16 institutions). Patients seen in those facilities were excluded from the analysis (n=27,828). In addition, patients were excluded if they were discharged against medical advice (n=5792) or if they died while in the IRF (n=1895).
Fig 1.
Study population inclusion and exclusion criteria of the UDSMR dataset, October 2010 through September 2012. Abbreviation: ICD, International Classification of Diseases.
Patients with malignant cancers were identified and classified according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes 140–209.36 and 209.72–209.79, abstracted from the etiologic diagnosis code field on the IRF-PAI. Patients with a benign or in situ tumor were excluded from the sample. Cancer patients were excluded if they were admitted for a rehabilitation impairment that was not associated with their cancer diagnosis or treatment. These conditions included traumatic brain injury, multiple sclerosis, Parkinson disease, Guillain-Barré syndrome, cerebral palsy, traumatic spinal cord injury, arthritis, hip and knee replacements, burn injury, congenital deformities, major multiple trauma, and developmental disability. In addition to examination of the entire group of cancer patients, we examined characteristics by cancer subgroups. Cancer sites were determined by ICD-9-CM code and included brain and nervous system, digestive, bone and joint, blood and lymphatic, respiratory, and other cancers. This study was approved by the Institutional Review Board of the University at Buffalo.
Study variables
Demographic, medical, and rehabilitation characteristics were examined. Demographic variables included age (years), race (white, black, other), marital status (married, not married), employment status before admission to the hospital (employed, not employed, retired), prehospital living setting (community, long-term care, acute care, rehabilitation, other), and living situation before hospitalization (living alone, living with others). Medical and rehabilitation variables considered included rehabilitation impairment type (brain dysfunction, debility, spinal cord dysfunction, medically complex condition, and other impairments), impairment onset days to rehabilitation (defined as the time between the onset of the impairment needing rehabilitation and admission to the rehabilitation facility), length of stay in inpatient rehabilitation, mobility status at admission (walking, wheelchair, or both), and Centers for Medicare and Medicaid Services comorbidity tier (tier A, no cost; tier B, high cost; tier C, moderate cost; tier D, low cost). Up to 10 comorbid condition codes can be indicated on the IRF-PAI using ICD-9-CM codes. Comorbidities are defined as conditions reported at admission to the IRF that affect the principal diagnosis or rehabilitation impairment. Patients in comorbidity tier A do not have comorbidities that significantly affect resource utilization, or have comorbid conditions of the least severity. Tier B patients have the most severe comorbidities, tier C have moderately severe comorbidities, and tier D have mild comorbidities.
Functional independence was measured using the FIM instrument (“FIM”). This instrument measures the severity of disability of patients by rating their ability to perform basic life activities. It includes 18 items in 2 major domains (motor, cognitive) and 6 subdomains (self-care, sphincter control, transfers, locomotion, communication, social cognition). Motor items include eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bladder control, bowel control, bed/chair/wheelchair transfers, toilet transfers, tub/shower transfers, walking/wheelchair locomotion, and stair climbing. Cognitive items include comprehension, expression, social interaction, problem solving, and memory. Each item is rated on a scale from 1 (complete dependence) to 7 (complete independence). The scores for the 18 items are summed, resulting in a composite score ranging from 18 to 126, with higher scores reflecting greater functional independence.
Functional variables derived from the FIM instrument include FIM admission total, FIM discharge total, FIM change, and length of stay efficiency. These rehabilitation outcome measures have been used in studies16–21 of other inpatient rehabilitation patient populations. Admission FIM total and discharge FIM total are the total summed ratings derived at admission and discharge, respectively. FIM change is the difference between the FIM discharge rating and the FIM admission rating and is an indicator of functional improvement over the course of inpatient rehabilitation. Length of stay efficiency is calculated as FIM change divided by the rehabilitation length of stay, and is interpreted as FIM points gained per day, broadly reflecting a measure of the efficiency of inpatient rehabilitation.
Statistical analysis
Demographic, medical, and rehabilitation variables were summarized using means and SDs for continuous variables and frequency counts and percentages for categorical variables. To test the differences in admission FIM total, discharge FIM total, rehabilitation length of stay, and length of stay efficiency between cancer subgroups, analysis of variance was used. Analysis of variance was also used to test the difference in FIM subdomain scores between cancer patient groups. Chi-square tests were used to test the differences in discharge to the community setting and discharge to the acute care setting between cancer subgroups. A P value of <.05 was considered statistically significant. Data were analyzed using SPSS version 22.a
Results
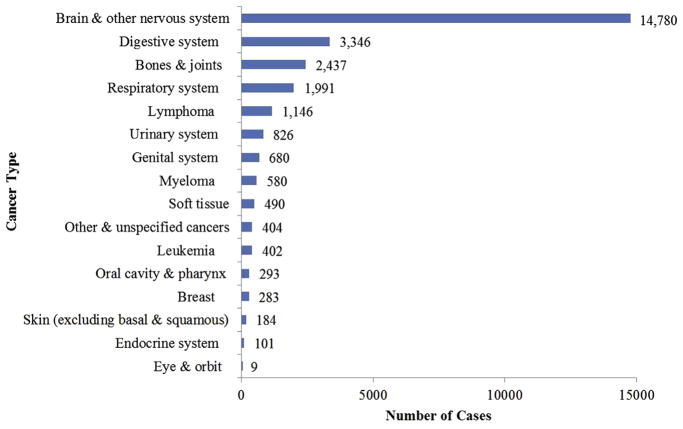
A total of 27,952 patients with malignant cancer were identified from among the 1,155,690 patients who met inclusion/exclusion criteria. Prevalence of cancer categories among patients are shown in figure 2. Cancer patients were grouped into the following categories: brain and nervous system cancers (52.9%), digestive system cancers (12.0%), bone and joint cancers (8.7%), blood and lymphatic cancers (7.6%), respiratory system cancers (7.1%), and other cancers (11.7%). Demographic and medical characteristics of the study sample are shown in table 1. The mean age ± SD of the overall cancer population was 65.1±14.1 years, 52% were men, 79% were white, 58% were married, 66% were retired before hospitalization, 59% had Medicare as the primary insurance payer, and 78% were living with others before hospitalization. Patients with digestive or respiratory cancers and those in the group of other cancers tended to be older, most likely as a result of an older average age at diagnosis for those cancer types. Cancer patients were admitted most frequently for impairments related to brain dysfunction (48%), debility (24%), and spinal cord dysfunction (12%). Most cancer patients (81%) were walking at admission to the IRF. There was a median of 9 days (interquartile range, 9d) between the onset of the rehabilitation impairment and admission to the IRF.
Fig 2.
Distribution of cancer patients in U.S. IRFs, October 2010 through September 2012 (N=27,952).
Table 1.
Demographic and medical characteristics of cancer patients in IRFs (UDSMR), October 2010– September 2012 (N=27,952)
| Characteristic | All Patients (N=27,952) | Brain and Nervous System (n=14,780) | Digestive (n=3346) | Bone and Joint (n=2437) | Blood and Lymphatic (n=2128) | Respiratory (n=1991) | Other* (n=3270) |
|---|---|---|---|---|---|---|---|
| Age (y) | 65.1±14.1 | 61.4±13.7 | 74.3±10.6 | 62.1±14.7 | 66.1±13.6 | 71.8±7.1 | 69.6±13.7 |
| 18–44 | 8.3 | 11.2 | 1.0 | 11.7 | 6.7 | 1.1 | 5.4 |
| 45–64 | 35.8 | 44.1 | 16.6 | 41.0 | 34.4 | 19.5 | 24.8 |
| 65–74 | 28.5 | 27.8 | 28.3 | 27.2 | 30.2 | 35.6 | 27.6 |
| 75–89 | 27.4 | 16.9 | 54.1 | 20.1 | 28.7 | 43.8 | 42.2 |
| Sex | |||||||
| Male | 52.2 | 52.0 | 51.8 | 56.1 | 56.9 | 54.0 | 46.7 |
| Female | 47.8 | 48.0 | 48.2 | 43.9 | 43.1 | 46.0 | 53.3 |
| Missing (n) | 5 | 4 | 0 | 0 | 0 | 0 | 1 |
| Ethnicity | |||||||
| White | 79.4 | 78.8 | 82.2 | 74.1 | 77.9 | 85.0 | 80.7 |
| Black | 9.4 | 8.8 | 9.1 | 13.0 | 10.3 | 7.8 | 10.2 |
| Hispanic | 5.2 | 5.8 | 4.1 | 6.2 | 5.4 | 3.1 | 3.9 |
| Other/unknown | 6.0 | 6.6 | 4.6 | 6.7 | 6.5 | 4.1 | 5.2 |
| Marital status | |||||||
| Married | 58.2 | 62.1 | 50.2 | 58.9 | 60.8 | 52.0 | 50.2 |
| Not married | 41.8 | 37.9 | 49.8 | 41.1 | 39.2 | 48.0 | 49.8 |
| Missing (n) | 441 | 236 | 35 | 43 | 46 | 24 | 57 |
| Employment status | |||||||
| Employed | 20.4 | 25.8 | 9.0 | 25.0 | 20.6 | 6.9 | 12.6 |
| Not employed (%) | 14.1 | 17.4 | 5.9 | 17.4 | 13.0 | 7.6 | 10.0 |
| Retired | 65.5 | 56.8 | 85.2 | 57.7 | 66.5 | 85.6 | 77.4 |
| Missing (n) | 465 | 274 | 51 | 37 | 31 | 25 | 47 |
| Primary payer | |||||||
| Medicare | 59.0 | 48.4 | 83.3 | 50.1 | 61.2 | 81.9 | 73.1 |
| Medicaid | 6.2 | 7.4 | 2.6 | 8.7 | 5.5 | 3.7 | 4.9 |
| Commercial | 24.2 | 30.5 | 10.3 | 29.1 | 22.0 | 10.7 | 15.6 |
| Other | 10.6 | 13.6 | 3.9 | 12.1 | 11.4 | 3.8 | 6.4 |
| Prehospital living situation | |||||||
| Living with others | 77.7 | 82.2 | 67.9 | 78.9 | 78.4 | 70.4 | 70.6 |
| Living alone | 22.3 | 17.8 | 32.1 | 21.1 | 21.6 | 29.6 | 29.4 |
| Missing (n) | 382 | 156 | 91 | 17 | 30 | 29 | 59 |
| Onset days to rehabilitation† | 14.6±24.2 | 12.9±25.1 | 17.9±20.1 | 13.4±21.0 | 22.3±29.7 | 11.8±9.7 | 14.5±22.0 |
| Median (IQR) | 9.0 (9.0) | 8.0 (7.0) | 12.0 (12.0) | 8.0 (8.0) | 13.0 (20.0) | 9.0 (10.0) | 9.0 (11.0) |
| Rehabilitation impairment | |||||||
| Brain dysfunction | 47.9 | 86.0 | 0.2 | 2.2 | 17.7 | 2.5 | 5.9 |
| Debility | 24.3 | 0.7 | 76.4 | 8.3 | 39.7 | 60.5 | 57.7 |
| Spinal cord dysfunction | 12.1 | 11.0 | 0.6 | 51.2 | 16.8 | 0.9 | 3.3 |
| Medically complex conditions | 7.1 | 1.0 | 15. | 5.2 | 17.9 | 17.3 | 14.2 |
| Other | 8.5 | 1.3 | 6.9 | 33.1 | 8.0 | 18.8 | 18.9 |
| Comorbidity tier‡ | |||||||
| Tier A, no cost | 50.8 | 47.9 | 50.8 | 69.3 | 45.4 | 53.9 | 51.9 |
| Tier D, low cost | 33.3 | 37.0 | 31.6 | 21.5b | 37.7 | 26.4 | 28.0 |
| Tier C, medium cost | 12.9 | 14.1 | 14.1 | 6.8 | 13.4 | 10.5 | 12.1 |
| Tier B, high cost | 3.0 | 0.9 | 3.5 | 2.5 | 3.4 | 9.2 | 8.0 |
| Mobility status at admission | |||||||
| Walk | 81.1 | 79.2 | 92.3 | 67.8 | 76.0 | 91.7 | 85.1 |
| Wheelchair | 15.2 | 16.9 | 5.3 | 27.8 | 19.5 | 5.4 | 11.4 |
| Walk and wheelchair | 3.7 | 3.9 | 2.4 | 4.4 | 4.6 | 2.9 | 3.5 |
NOTE. Values are mean ± SD, percentages, or as otherwise indicated. Cancer patients were identified by ICD codes 140–209.36 and 209.72–209.79. Abbreviation: IQR, interquartile range.
Other cancers include patients with urinary, genital, soft tissue, oral cavity and pharynx, breast, skin, endocrine, and eye and orbit cancers.
Number of days from onset of rehabilitation impairment until date of admission to IRF.
Comorbid conditions grouped into those that affect resource demands and increase the cost of the rehabilitation stay.
Rehabilitation characteristics are shown in table 2. On average, cancer patients had a mean ± SD admission FIM total score of 65.1±14.1, were discharged with a mean ± SD FIM total score of 84.7±22.1, and gained approximately a mean ± SD of 23.5±16.2 FIM points over the course of inpatient rehabilitation. Cancer patients had a mean ± SD length of stay of 12.6±7.4 days in the inpatient rehabilitation unit and gained a mean of 2.2 FIM points per day. Analysis of variance test results indicated that there were significant differences between cancer subgroups with regard to admission FIM total (F5,27946=151.9, P<.001; r=.16), discharge FIM total (F5,27946=187.8, P<.001; r=.18), FIM total change (F5,27946=78.2, P<.001; r=.12), rehabilitation length of stay (F5,27946=96.2, P<.001; r=.13), and length of stay efficiency (F5,27946=117.2, P<.001; r=.14). Patients with brain and nervous system cancers were most functionally dependent on admission (mean admission FIM total, 58.9), whereas patients with respiratory cancers had higher functional scores (mean admission FIM total, 66.4). Patients with digestive cancers made the largest functional gains during their rehabilitation stay (mean FIM total change, 26.9). On average, all cancer patient groups made meaningful functional change over the course of inpatient rehabilitation. Approximately 72% of cancer patients admitted to inpatient rehabilitation were discharged to a community setting, and 16.5% were discharged to an acute care setting. Blood and lymphatic cancer patients experienced the lowest discharge to community (62%) and the highest average discharge to acute care settings (28.4%). Age, admission FIM total, discharge FIM total, FIM gain, and discharge to the community setting are summarized for each tumor site in table 3. FIM score changes for cancer patients ranged from 17.5 to 32.5 points gained, and rates of discharge to the community ranged from 44.4% to 100%.
Table 2.
Rehabilitation characteristics of cancer patients in IRFs (UDSMR), October 2010–September 2012 (N=27,952)
| Characteristic | All Patients*,† (N=27,952) | Brain and Nervous System (n=14,780) | Digestive (n=3346) | Bones and Joints (n=2437) | Blood and Lymph (n=2128) | Respiratory (n=1991) | Other‡ (n=3270) |
|---|---|---|---|---|---|---|---|
| Admission FIM total | 65.1±14.1 | 58.9±17.1 | 62.8±15.2 | 64.7±14.4 | 63.1±15.5 | 66.4±14.9 | 63.4±15.5 |
| Motor | 38.1±12.4 | 37.9±13.2 | 37.9±11.3 | 36.9±11.3 | 37.9±11.6 | 41.5±11.3 | 38.8±11.5 |
| Cognitive | 23.1±7.4 | 21.0±7.4 | 24.9±6.3 | 27.8±6.0 | 25.2±6.9 | 24.9±6.1 | 25.1±6.7 |
| Discharge FIM total | 84.7±22.1 | 81.2±23.1 | 89.7±19.4 | 86.9±20.5 | 84.6±21.2 | 91.5±20.0 | 89.6±19.9 |
| Motor | 57.8±17.9 | 56.3±18.7 | 60.8±16.1 | 56.4±17.6 | 56.3±17.2 | 62.7±16.5 | 60.6±16.4 |
| Cognitive | 26.9±6.9 | 24.9±7.2 | 28.9±5.5 | 30.5±5.2 | 28.3±6.4 | 28.8±5.5 | 29.0±5.8 |
| FIM total change | 23.5±16.2 | 22.3±16.3 | 26.9±16.0 | 22.2±15.8 | 21.5±15.5 | 25.1±15.9 | 26.2±15.8 |
| Motor | 19.7±13.6 | 18.4±13.5 | 22.9±13.5 | 19.4±13.5 | 18.4±13.1 | 21.2±13.4 | 22.3±13.3 |
| Cognitive | 3.8±4.9 | 3.9±5.0 | 4.0±5.0 | 2.8±4.9 | 3.1±4.8 | 3.9±4.9 | 3.9±4.8 |
| Rehabilitation length of stay (d) | 12.6±7.4 | 13.2±7.8 | 11.3±5.5 | 13.7±8.3 | 12.7±8.0 | 10.4±5.0 | 11.8±6.2 |
| Length of stay efficiency§ | 2.2±1.9 | 2.1±1.9 | 2.6±2.0 | 2.0±2.0 | 2.0±1.7 | 2.7±2.1 | 2.6±2.0 |
| Discharge to community (%)|| | 72.0 | 72.5 | 73.7 | 70.8 | 62.0 | 75.2 | 75.4 |
| Discharge to acute care (%) | 16.5 | 14.9 | 16.8 | 18.1 | 28.4 | 17.0 | 14.7 |
NOTE. Values are mean ± SD. Cancer patients were identified by ICD codes 140–209.36 and 209.72–209.79.
One-way analysis of variance was used to test the difference in means for continuous variables between cancer subgroups. All P values were P<.001.
Chi-square test of independence was used to test the difference in discharge settings between cancer subgroups. All P values were P<.001.
Other cancers include urinary, genital, soft tissue, oral cavity and pharynx, breast, skin, endocrine, and eye and orbit cancers.
Length of stay efficiency is the discharge FIM change divided by the rehabilitation length of stay (ie, the number of FIM points gained per day).
Community venues include discharges to home, transitional living, board and care, and assisted living.
Table 3.
Age, functional status, and discharge setting for cancer patients in IRFs (UDSMR), October 2010–September 2012 (N=27,952)
| Cancer Type | n | % | Age (y) | Admission FIM Total | Discharge FIM Total | FIM Gain | Discharge to Community | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | % | |||||||
| All sites | 27,952 | 100.0 | 65.1±14.1 | 61.2±16.4 | 84.7±22.1 | 23.5±16.2 | 20,195 | 72.2 |
| Oral cavity and pharynx | 293 | 1.0 | 68.6±11.9 | 59.8±16.1 | 90.2±19.6 | 30.4±15.5 | 223 | 76.1 |
| Tongue | 74 | 0.3 | 66.5±12.2 | 61.9±17.6 | 92.7±21.3 | 30.7±16.0 | 55 | 74.3 |
| Mouth | 76 | 0.3 | 67.9±11.4 | 58.7±14.1 | 91.2±19.4 | 32.5±14.3 | 58 | 76.3 |
| Pharynx | 81 | 0.3 | 68.0±12.5 | 60.2±16.9 | 88.1±19.6 | 27.9±15.5 | 62 | 76.5 |
| Other oral cavity | 62 | 0.2 | 72.8±10.6 | 57.9±15.5 | 88.5±17.5 | 30.7±16.3 | 48 | 77.4 |
| Digestive system | 3,346 | 12.0 | 74.3±10.6 | 62.8±15.2 | 89.7±19.4 | 26.9±16.0 | 2,465 | 73.7 |
| Esophagus | 261 | 0.9 | 69.4±9.8 | 61.9±15.0 | 88.1±20.4 | 26.1±16.0 | 177 | 67.8 |
| Stomach | 206 | 0.7 | 73.4±10.9 | 64.2±15.9 | 90.0±20.7 | 25.8±17.1 | 148 | 71.8 |
| Small intestine | 74 | 0.3 | 73.3±10.2 | 63.9±15.0 | 89.8±23.4 | 25.8±18.5 | 52 | 70.3 |
| Colon | 1,449 | 5.2 | 77.6±9.6 | 62.5±15.5 | 90.4±19.1 | 27.9±15.3 | 1,125 | 77.6 |
| Rectum | 283 | 1.0 | 71.9±11.5 | 60.8±13.3 | 89.8±18.5 | 29.1±16.4 | 198 | 70.0 |
| Anus, anal canal, and anorectum | 230 | 0.8 | 73.3±11.4 | 61.6±15.1 | 89.6±20.6 | 28.0±17.0 | 171 | 74.3 |
| Liver and intrahepatic bile duct | 263 | 0.9 | 68.6±11.1 | 64.3±14.7 | 87.6±20.2 | 23.4±16.6 | 179 | 68.1 |
| Gallbladder and other biliary | 72 | 0.3 | 74.2±9.8 | 63.9±17.1 | 86.6±18.8 | 22.8±16.2 | 46 | 63.9 |
| Pancreas | 350 | 1.3 | 73.6±8.6 | 65.1±14.9 | 89.8±18.0 | 24.7±16.3 | 250 | 71.4 |
| Other digestive organs | 158 | 0.6 | 70.9±10.8 | 61.1±14.8 | 89.8±19.5 | 28.8±15.3 | 119 | 75.3 |
| Respiratory system | 1,991 | 7.1 | 71.8±9.7 | 66.4±14.9 | 91.5±20.0 | 25.1±15.9 | 1,497 | 75.2 |
| Larynx | 148 | 0.5 | 69.3±9.5 | 61.6±16.3 | 90.2±20.0 | 28.6±15.5 | 101 | 68.2 |
| Lung and bronchi | 1,756 | 6.3 | 72.3±9.4 | 67.0±14.6 | 91.8±19.9 | 24.8±15.9 | 1,335 | 76.0 |
| Other respiratory organs | 87 | 0.3 | 66.4±13.3 | 62.9±16.7 | 88.0±21.9 | 25.1±16.4 | 61 | 70.1 |
| Bones and joints | 2,437 | 8.7 | 62.1±14.7 | 64.7±14.4 | 86.7±20.5 | 22.2±15.8 | 712 | 70.8 |
| Soft tissue | 490 | 1.8 | 61.0±16.6 | 68.8±14.7 | 93.7±17.8 | 25.0±14.2 | 384 | 78.4 |
| Skin | 184 | 0.7 | 73.3±13.1 | 62.7±15.5 | 89.7±20.3 | 27.0±14.4 | 138 | 75.0 |
| Melanoma | 76 | 0.3 | 71.3±14.0 | 65.4±15.1 | 93.0±18.5 | 27.7±13.7 | 56 | 73.7 |
| Other nonepithelial skin | 108 | 0.4 | 74.7±12.3 | 60.9±15.6 | 87.4±21.3 | 26.5±14.9 | 82 | 75.9 |
| Breast | 283 | 1.0 | 69.3±13.0 | 63.6±15.5 | 90.0±20.0 | 26.4±16.5 | 216 | 76.3 |
| Genital system | 680 | 2.4 | 72.5±11.1 | 62.6±14.2 | 88.8±19.4 | 26.2±16.1 | 493 | 72.5 |
| Uterine cervix | 37 | 0.1 | 63.5±14.1 | 64.9±11.4 | 89.0±17.6 | 24.1±16.0 | 26 | 70.3 |
| Uterine corpus | 172 | 0.6 | 71.5±10.7 | 63.2±13.7 | 89.2±19.1 | 26.0±16.0 | 124 | 72.1 |
| Ovary | 234 | 0.8 | 72.9±10.1 | 65.2±13.3 | 92.4±18.3 | 27.2±15.7 | 178 | 76.1 |
| Vulva | 23 | 0.1 | 76.9±9.9 | 62.7±12.7 | 94.2±15.3 | 31.4±13.8 | 16 | 69.6 |
| Vagina and other genital, female | 18 | 0.1 | 68.3±11.1 | 61.6±18.6 | 79.1±24.6 | 17.5±14.4 | 8 | 44.4 |
| Prostate | 172 | 0.6 | 75.0±10.6 | 58.7±15.0 | 84.9±20.4 | 26.3±17.3 | 128 | 74.4 |
| Testis | 1 | 0.0 | 29.0* | 74.0* | 104.0* | 30.0* | 1 | 100.0 |
| Penis and other genital, male | 23 | 0.1 | 71.7±11.8 | 57.7±16.2 | 81.1±20.0 | 23.4±15.7 | 12 | 52.2 |
| Urinary system | 826 | 3.0 | 74.1±10.2 | 62.8±15.1 | 89.2±20.0 | 26.4±16.6 | 632 | 76.5 |
| Urinary bladder | 366 | 1.3 | 75.6±9.7 | 59.2±14.6 | 86.1±20.6 | 26.9±17.5 | 269 | 73.5 |
| Kidney and renal pelvis | 387 | 1.4 | 72.1±10.6 | 65.8±15.2 | 91.7±19.7 | 25.9±16.1 | 307 | 79.3 |
| Ureter and other urinary organs | 73 | 0.3 | 76.8±9.1 | 64.4±13.0 | 91.3±16.8 | 26.9±15.0 | 55 | 76.7 |
| Eye and orbit | 9 | 0.0 | 72.8±16.1 | 70.6±11.1 | 97.2±14.0 | 26.7±10.7 | 9 | 100.0 |
| Brain and other nervous system | 14,780 | 52.9 | 61.4±13.7 | 58.9±17.1 | 81.2±23.1 | 22.3±16.3 | 10,721 | 72.5 |
| Endocrine system | 101 | 0.4 | 60.3±17.8 | 57.8±18.1 | 82.1±22.2 | 24.3±14.7 | 70 | 69.3 |
| Thyroid | 24 | 0.1 | 69.8±12.3 | 67.1±13.7 | 91.3±21.3 | 24.2±13.4 | 16 | 66.7 |
| Other endocrine | 77 | 0.3 | 57.4±18.3 | 54.9±18.4 | 79.3±21.9 | 24.3±15.2 | 54 | 70.1 |
| Lymphoma | 1,146 | 4.1 | 66.8±13.2 | 61.4±16.0 | 81.8±21.8 | 20.4±15.5 | 650 | 56.7 |
| Hodgkin lymphoma | 43 | 0.2 | 57.9±21.0 | 64.8±15.9 | 84.0±21.7 | 19.2±17.7 | 29 | 67.4 |
| Non-Hodgkin lymphoma | 1,103 | 3.9 | 67.1±12.7 | 61.3±16.0 | 81.7±21.8 | 20.4±15.5 | 621 | 56.3 |
| Myeloma | 580 | 2.1 | 66.5±11.6 | 64.7±14.1 | 86.4±19.9 | 21.6±14.6 | 397 | 68.4 |
| Leukemia | 402 | 1.4 | 63.4±16.8 | 65.6±15.4 | 89.9±20.0 | 24.3±16.1 | 273 | 67.9 |
| Acute lymphocytic leukemia | 69 | 0.2 | 51.1±17.1 | 68.7±14.0 | 90.7±18.6 | 22.1±15.2 | 45 | 65.2 |
| Chronic lymphocytic leukemia | 84 | 0.3 | 77.7±8.2 | 62.7±16.0 | 88.7±19.7 | 26.0±15.2 | 57 | 67.9 |
| Acute myeloid leukemia | 170 | 0.6 | 60.4±15.3 | 66.6±15.1 | 91.6±19.2 | 25.0±15.9 | 120 | 70.6 |
| Chronic myeloid leukemia | 33 | 0.1 | 65.6±13.6 | 65.6±14.9 | 88.4±22.9 | 22.8±19.8 | 23 | 69.7 |
| Other leukemia | 46 | 0.2 | 65.7±16.7 | 62.2±16.9 | 85.4±23.3 | 23.2±17.0 | 28 | 60.9 |
| Other and unspecified cancers | 404 | 1.4 | 67.3±14.1 | 63.7±16.2 | 87.4±21.1 | 23.7±15.5 | 302 | 74.8 |
NOTE. Values are mean ± SD or as otherwise indicated.
No SD with value because only 1 case.
The FIM ratings for functional subdomains of the FIM instrument by cancer type are displayed in table 4. Analysis of variance test results were significant for all FIM subdomains, with statistically significant differences between cancer subgroups between self-care (F5,27946=95.1, P<.001; r=.13), sphincter control (F5,27946=18.9, P<.001; r=.06), mobility (F5,27946=70.0, P<.001; r=.11), locomotion (F5,27946=60.2, P<.001; r=.10), communication (F5,27946=33.9, P<.001; r=.08), and the social interaction domains (F5,27946=26.3, P<.001; r=.07). Large qualitative differences in the functional subdomains of the FIM instrument between cancer groups were not present. However, patients with digestive cancers had the greatest FIM change in all subdomains.
Table 4.
Functional subdomains of cancer patients in IRFs (UDSMR), October 2010–September 2012 (N=27,952)
| Characteristic | Mean ± SD of Patients, by Group | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| All Patients (N=27,952) | Brain and Nervous System (n=14,780) | Digestive (n=3346) | Bones and Joints (n=2437) | Blood and Lymph (n=2128) | Respiratory (n=1991) | Other* (n=3270) | |
| Self-care (range, 6–42) | |||||||
| Admission FIM total | 19.8±6.3 | 19.4±6.6 | 20.0±6.0 | 20.3±5.7 | 20.2±6.0 | 21.2±6.1 | 20.1±6.1 |
| Discharge FIM total | 28.7±8.4 | 27.7±8.3 | 30.4±7.6 | 28.9±8.1 | 28.5±8.0 | 30.9±7.9 | 30.3±7.7 |
| FIM gain | 8.9±6.8 | 8.3±6.8 | 10.4±6.9 | 8.6±6.7 | 8.4±6.5 | 9.8±7.0 | 10.2±6.9 |
| Sphincter control (range, 2–14) | |||||||
| Admission FIM total | 7.2±3.7 | 7.3±3.7 | 6.6±3.7 | 6.6±3.7 | 7.0±3.6 | 8.2±3.4 | 7.1±3.7 |
| Discharge FIM total | 9.4±3.8 | 9.4±3.8 | 9.3±3.7 | 8.8±3.9 | 9.0±3.8 | 10.3±3.3 | 9.4±3.8 |
| FIM gain | 2.2±3.4 | 2.1±3.4 | 2.6±3.5 | 2.2±3.4 | 2.1±3.5 | 2.1±3.2 | 2.2±3.4 |
| Transfers (range, 3–21) | |||||||
| Admission FIM total | 7.7±3.3 | 7.6±3.4 | 7.9±3.1 | 6.8±3.2 | 7.5±3.3 | 8.7±3.0 | 7.9±3.1 |
| Discharge FIM total | 12.6±4.6 | 12.2±4.7 | 13.5±4.1 | 11.9±4.9 | 12.1±4.6 | 14.0±4.2 | 13.4±4.2 |
| FIM gain | 4.9±3.8 | 4.5±3.8 | 5.6±3.8 | 5.1±4.1 | 4.7±3.8 | 5.3±3.8 | 5.5±3.8 |
| Locomotion (range, 2–14) | |||||||
| Admission FIM total | 3.4±1.9 | 3.5±2.0 | 3.3±1.7 | 3.1±1.6 | 3.2±1.7 | 3.4±1.8 | 3.4±1.8 |
| Discharge FIM total | 7.1±3.4 | 7.0±3.5 | 7.6±3.4 | 6.7±3.2 | 6.5±3.2 | 7.5±3.5 | 7.4±3.7 |
| FIM gain | 3.7±3.0 | 3.5±3.0 | 4.2±3.0 | 3.6±3.0 | 3.3±2.9 | 4.1±3.2 | 4.1±3.1 |
| Communication (range, 2–14) | |||||||
| Admission FIM total | 9.7±3.0 | 8.9±3.1 | 10.3±2.6 | 11.5±2.4 | 10.6±2.8 | 10.4±2.5 | 10.4±2.8 |
| Discharge FIM total | 11.2±2.8 | 10.5±3.0 | 11.9±2.1 | 12.5±2.0 | 11.7±2.5 | 11.9±2.2 | 11.9±2.7 |
| FIM gain | 1.5±2.2 | 1.5±2.2 | 1.5±2.1 | 1.0±2.1 | 1.2±2.0 | 1.5±2.1 | 1.5±2.1 |
| Social cognition (range, 3–21) | |||||||
| Admission FIM total | 13.4±4.6 | 12.1±4.6 | 14.5±4.0 | 16.2±3.8 | 14.6±4.3 | 14.6±3.8 | 14.7±4.2 |
| Discharge FIM total | 15.7±4.4 | 14.4±4.5 | 17.0±3.6 | 18.0±3.4 | 16.5±4.2 | 17.0±3.6 | 17.1±3.8 |
| FIM gain | 2.3±3.2 | 3.4±3.2 | 2.5±3.2 | 1.7±3.2 | 2.0±3.1 | 2.4±3.2 | 2.4±3.1 |
NOTE. Cancer patients were identified by ICD codes 140–209.36 and 209.72–209.79.
Includes urinary, genital, soft tissue, oral cavity and pharynx, breast, skin, endocrine, and eye and orbit cancers.
Discussion
We examined demographic, medical, and rehabilitation characteristics of cancer patients in a study sample that included most of the patients admitted to IRFs in the United States. We found that patients with cancer in our study made clinically significant functional improvement from admission to discharge, with a mean gain in FIM total score of 23.5 points. Beninato et al22 found that a difference of 22 between the FIM at admission and discharge among stroke patients was the smallest increment with clinical significance. Wallace et al23 also reported on the minimal clinically important difference among stroke patients and found that a motor FIM change score of 11 points was clinically important; patients with cancer in our study made changes in FIM of this magnitude on average. Previous research has indicated that a 1-point increase in the total FIM instrument rating is associated with a reduction of 3 to 6 minutes of care-giver help per day, which on average would equate to approximately 1 to 2 hours less of caregiver help per day in our sample.24–27 Although previous work on the clinical meaningfulness of the FIM instrument is based on patients with various diseases, these guidelines provide a reference point for the clinical utility of inpatient rehabilitation in cancer patients. Our results provide a useful framework for clinicians and hospital administrators for setting appropriate goals and expectations for functional outcomes among cancer patients admitted to inpatient rehabilitation programs.
Our findings are consistent with previous smaller studies performed among cancer patients in IRFs, which have shown positive functional improvement. Marciniak et al28 performed a retrospective chart review of 159 patients with a variety of cancer diagnoses and found significant functional gains in mean motor FIM total from admission to discharge (42.6 vs 56.0, P<.001). All cancer groups examined in the study (brain, breast, spinal cord, other) experienced similar functional improvements and efficiency in functional gain. In a study29 of 200 cancer patients, significant functional improvements from admission to discharge were reported, with equivalent motor and cognitive gains across all 9 cancer subgroups. In studies30–34 of patients with brain tumors, patients were shown to make significant functional improvement from admission to discharge. Significant functional gains have also been reported for patients with malignant spinal cord compression35 and cancer-related asthenia.36 Given the predisposition for marked functional debility among patients with cancer, the observed functional improvement reported after inpatient rehabilitation has important implications for therapeutic efficacy during cancer treatment as well as for enhanced quality of life thereafter. Furthermore, a few small studies37–40 suggest that functional improvement is associated with prolonged survival for cancer patients.
Because of the recent Hospital Readmissions Reduction Program provision of the Affordable Care Act,41 the prevention of hospital readmissions and the identification of factors predictive of rehospitalization are increasingly important to medical institutions. Reported readmission rates among cancer patients in inpatient rehabilitation in the United States have ranged from 17% to 35%.8,42–44 We found that among all cancer patients in our cohort, 16% were discharged to acute care. In addition, we also found that patients with blood and lymphatic cancers were most likely to be discharged back to the acute care setting (28%); other studies8,44,45 have also reported leukemia and lymphoma patients as having a higher likelihood of discharge from inpatient rehabilitation to acute care settings (33% and 38%, respectively). However, we do not have data as to how many of these cancer patients would have been readmitted to acute care if they had not received the inpatient rehabilitation, nor do we know how many of these readmissions were planned for additional cancer treatment. Future study in this population should focus on understanding the predictors of unplanned discharges to acute care from inpatient rehabilitation.
The characteristics of cancer patients seen in IRFs do not reflect the general population of cancer patients, particularly in regards to tumor type. Patients are admitted to IRFs for functional impairments that require intensive inpatient rehabilitation with medical management. Patients must meet certain eligibility criteria that include medical stability, the need for intensive rehabilitation therapies, and the ability to tolerate rehabilitation therapy at the required duration. Cancer patients without the need for more than one rehabilitation therapy, or those who have unrecognized impairments in acute care would be less likely to be referred to an IRF. The high percentage of patients with brain and nervous system cancer in this sample reflects the higher burden of physical and cognitive disabilities that accompany these diagnoses, and the higher likelihood that these patients were referred to IRFs. In addition, restrictions related to Medicare reimbursement may limit admission of cancer patients to IRFs who do not have rehabilitation impairments that qualify for the IRF compliance threshold.8
Study limitations
Potential limitations of these data are important to consider when interpreting these findings. This study was a retrospective study limited to the variables collected on the IRF-PAI, and to the quality of methods used to obtain this information across multiple institutions. The IRF-PAI did not include information regarding the details of the cancer diagnosis. Specifically, primary and metastatic cancer cases were not differentiated. While there may be differences in outcomes between primary and metastatic cancers, there is evidence that cancer patients are able to make functional improvements even with metastatic lesions.28,33,46 In addition, we were unable to examine the influence of tumor characteristics such as stage and grade, as well as type of cancer treatment received, on rehabilitation outcomes. However, there is evidence that receiving cancer treatments during rehabilitation does not impede functional progress.28,46 We were unable to determine if the type of rehabilitation therapy or the frequency or duration of rehabilitation therapy was related to cancer type, as this data was not available.
Another limitation in the interpretation of the results of this study is that most of the study population is white (79%). This lack of diversity in our sample brings forth several potential issues for consideration including referral bias, inequity of health insurance, and cultural factors that may favor other forms of rehabilitation care outside the inpatient setting. Research in other patient populations has suggested that minority patients are less likely to use postacute care47 and are less likely to be discharged to inpatient rehabilitation.48 In addition, rehabilitation outcomes have been reported to differ between racial and ethnic groups in inpatient rehabilitation populations.49,50 Further research needs to be performed to explore variables that influence racial and ethnic disparities in cancer rehabilitation research in order to improve health care access, quality, and outcomes.
An important strength of this study is its size and generaliz-ability to cancer patients admitted to IRFs. The UDSMR dataset is unique in that it includes data collected from more than 70% of the IRFs in the United States. Cancer patients represent a little more than 2% of the IRF population; there were close to 28,000 cancer patients in our analytic sample over a 3-year period. In addition, detailed standardized data on functional status are collected at admission and discharge from the IRF, and extensive training and credentialing protocols are followed to ensure reproducibility of the measurement across institutions. The validity and reliability of the FIM instrument have been demonstrated in previous studies. In a meta-analysis of 11 studies published between 1993 and 1995 with a total sample of 1568 patients with various diagnoses, Ottenbacher et al51 reported a median z-transformed interrater reliability coefficient of .95 for the FIM total rating. In addition, the FIM total rating has been shown to be negatively correlated with the amount of time required from a caregiver to assist the patient in performing activities of daily living in the home setting.24,25,27,52 These data provide information with significant clinical implications regarding expectations for functional improvement in cancer patients. Although previous studies on this topic exist, they have been at single institution settings with smaller sample sizes. The larger volume of data in this examination helps to corroborate findings of previous studies and also provides useful benchmarking information for rehabilitation outcomes in a variety of cancer types.
Conclusions
The prevalence of cancer survivors in the United States is expected to continue to increase with the aging population. At present, cancer patients constitute more than 2% of patients seen in IRFs, a proportion that could grow as the population ages, as treatment of cancer allows for an increased number of survivors. We found that most of the cancer patients with malignant tumors admitted to an inpatient rehabilitation program were discharged to a community setting and, on average, were able to make functional improvements during their IRF stay. Future research is warranted to understand the referral patterns of admission to postacute care rehabilitation and to identify factors that are associated with rehabilitation benefit. Increased awareness is needed among cancer care professionals of the potential for improving function in cancer patients.
List of abbreviations
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- IRF
inpatient rehabilitation facility
- IRF-PAI
Inpatient Rehabilitation Facility–Patient Assessment Instrument
- UDSMR
Uniform Data System for Medical Rehabilitation
Footnotes
SPSS version 22; IBM Corp.
References
- 1.American Cancer Society. [Accessed November 30, 2016];Cancer facts and figures. 2016 Available at: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- 2.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA Cancer J Clin. 2013;63:295–317. doi: 10.3322/caac.21186. [DOI] [PubMed] [Google Scholar]
- 4.Naughton MJ, Weaver KE. Physical and mental health among cancer survivors: considerations for long-term care and quality of life. N C Med J. 2014;75:283–6. doi: 10.18043/ncm.75.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silver JK, Raj VS, Fu JB, Wisotzky EM, Smith SR, Kirch RA. Cancer rehabilitation and palliative care: critical components in the delivery of high-quality oncology services. Support Care Cancer. 2015;23:3633–43. doi: 10.1007/s00520-015-2916-1. [DOI] [PubMed] [Google Scholar]
- 6.Aziz NM. Cancer survivorship research: state of knowledge, challenges and opportunities. Acta Oncol. 2007;46:417–32. doi: 10.1080/02841860701367878. [DOI] [PubMed] [Google Scholar]
- 7.McCabe MS, Bhatia S, Oeffinger KC, et al. American Society of Clinical Oncology statement: achieving high-quality cancer survivorship care. J Clin Oncol. 2013;31:631–40. doi: 10.1200/JCO.2012.46.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang ME, Sliwa JA. Inpatient rehabilitation of patients with cancer: efficacy and treatment considerations. PM R. 2011;3:746–57. doi: 10.1016/j.pmrj.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 Suppl 1):S27–37. doi: 10.1097/PHM.0b013e31820be3be. [DOI] [PubMed] [Google Scholar]
- 10.Movsas SB, Chang VT, Tunkel RS, Shah VV, Ryan LS, Millis SR. Rehabilitation needs of an inpatient medical oncology unit. Arch Phys Med Rehabil. 2003;84:1642–6. doi: 10.1053/s0003-9993(03)00345-9. [DOI] [PubMed] [Google Scholar]
- 11.Banks E, Byles JE, Gibson RE, et al. Is psychological distress in people living with cancer related to the fact of diagnosis, current treatment or level of disability? Findings from a large Australian study. Med J Aust. 2010;193(5 Suppl):S62–7. doi: 10.5694/j.1326-5377.2010.tb03931.x. [DOI] [PubMed] [Google Scholar]
- 12.Weaver KE, Forsythe LP, Reeve BB, et al. Mental and physical health-related quality of life among U.S. cancer survivors: population estimates from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21:2108–17. doi: 10.1158/1055-9965.EPI-12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Medicare and Medicaid Services; Department of Health and Human Services, editor. Medicare program; inpatient rehabilitation facility prospective payment system for federal fiscal year 2016. 80. 2015. 42 CFR Part 412. [Google Scholar]
- 14.Uniform Data System for Medical Rehabilitation. The Center for Functional Assessment Research (CFAR); 2013. [Accessed July 15, 2013]. Available at: http://www.udsmr.org/WebModules/UDSMR/Com_CFAR.aspx. [Google Scholar]
- 15.Centers for Medicare and Medicaid Services. The IRF-PAI training manual. Washington (DC): Department of Health and Human Services; 2004. [Google Scholar]
- 16.Granger CV, Karmarkar AM, Graham JE, et al. The Uniform Data System for Medical Rehabilitation: report of patients with traumatic spinal cord injury discharged from rehabilitation programs in 2002–2010. Am J Phys Med Rehabil. 2012;91:289–99. doi: 10.1097/PHM.0b013e31824ad2fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granger CV, Markello SJ, Graham JE, Deutsch A, Ottenbacher KJ. The Uniform Data System for Medical Rehabilitation: report of patients with stroke discharged from comprehensive medical programs in 2000–2007. Am J Phys Med Rehabil. 2009;88:961–72. doi: 10.1097/PHM.0b013e3181c1ec38. [DOI] [PubMed] [Google Scholar]
- 18.Granger CV, Markello SJ, Graham JE, Deutsch A, Reistetter TA, Ottenbacher KJ. The Uniform Data System for Medical Rehabilitation: report of patients with traumatic brain injury discharged from rehabilitation programs in 2000–2007. Am J Phys Med Rehabil. 2010;89:265–78. doi: 10.1097/PHM.0b013e3181d3eb20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granger CV, Markello SJ, Graham JE, Deutsch A, Reistetter TA, Ottenbacher KJ. The Uniform Data System for Medical Rehabilitation: report of patients with lower limb joint replacement discharged from rehabilitation programs in 2000–2007. Am J Phys Med Rehabil. 2010;89:781–94. doi: 10.1097/PHM.0b013e3181f1c83a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granger CV, Reistetter TA, Graham JE, et al. The Uniform Data System for Medical Rehabilitation: report of patients with hip fracture discharged from comprehensive medical programs in 2000–2007. Am J Phys Med Rehabil. 2011;90:177–89. doi: 10.1097/PHM.0b013e31820b18d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottenbacher KJ, Smith PM, Illig SB, Linn RT, Ostir GV, Granger CV. Trends in length of stay, living setting, functional outcome, and mortality following medical rehabilitation. JAMA. 2004;292:1687–95. doi: 10.1001/jama.292.14.1687. [DOI] [PubMed] [Google Scholar]
- 22.Beninato M, Gill-Body KM, Salles S, Stark PC, Black-Schaffer RM, Stein J. Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Arch Phys Med Rehabil. 2006;87:32–9. doi: 10.1016/j.apmr.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 23.Wallace D, Duncan PW, Lai SM. Comparison of the responsiveness of the Barthel Index and the motor component of the Functional Independence Measure in stroke: the impact of using different methods for measuring responsiveness. J Clin Epidemiol. 2002;55:922–8. doi: 10.1016/s0895-4356(02)00410-9. [DOI] [PubMed] [Google Scholar]
- 24.Granger CV, Cotter AC, Hamilton BB, Fiedler RC. Functional assessment scales: a study of persons after stroke. Arch Phys Med Rehabil. 1993;74:133–8. [PubMed] [Google Scholar]
- 25.Granger CV, Cotter AC, Hamilton BB, Fiedler RC, Hens MM. Functional assessment scales: a study of persons with multiple sclerosis. Arch Phys Med Rehabil. 1990;71:870–5. [PubMed] [Google Scholar]
- 26.Granger CV, Hamilton BB, Linacre JM, Heinemann AW, Wright BD. Performance profiles of the Functional Independence Measure. Am J Phys Med Rehabil. 1993;72:84–9. doi: 10.1097/00002060-199304000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton BB, Deutsch A, Russell C, Fiedler RC, Granger CV. Relation of disability costs to function: spinal cord injury. Arch Phys Med Rehabil. 1999;80:385–91. doi: 10.1016/s0003-9993(99)90274-5. [DOI] [PubMed] [Google Scholar]
- 28.Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996;77:54–7. doi: 10.1016/s0003-9993(96)90220-8. [DOI] [PubMed] [Google Scholar]
- 29.Cole RP, Scialla SJ, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81:623–7. doi: 10.1016/s0003-9993(00)90046-7. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85:568–73. doi: 10.1097/01.phm.0000223218.38152.53. [DOI] [PubMed] [Google Scholar]
- 31.Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79:1386–90. doi: 10.1016/s0003-9993(98)90232-5. [DOI] [PubMed] [Google Scholar]
- 32.Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79:327–35. doi: 10.1097/00002060-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82:457–63. doi: 10.1053/apmr.2001.21862. [DOI] [PubMed] [Google Scholar]
- 34.O’Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79:1530–4. doi: 10.1016/s0003-9993(98)90414-2. [DOI] [PubMed] [Google Scholar]
- 35.Fortin CD, Voth J, Jaglal SB, Craven BC. Inpatient rehabilitation outcomes in patients with malignant spinal cord compression compared to other non-traumatic spinal cord injury: a population based study. J Spinal Cord Med. 2015;38:754–64. doi: 10.1179/2045772314Y.0000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y, Shin KY, Hainley S, Bruera E, Palmer JL. Inpatient rehabilitation improved functional status in asthenic patients with solid and hematologic malignancies. Am J Phys Med Rehabil. 2011;90:265–71. doi: 10.1097/PHM.0b013e3182063ba6. [DOI] [PubMed] [Google Scholar]
- 37.Saotome T, Klein L, Faux S. Cancer rehabilitation: a barometer for survival? Support Care Cancer. 2015;23:3033–41. doi: 10.1007/s00520-015-2673-1. [DOI] [PubMed] [Google Scholar]
- 38.Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008;255:820–7. doi: 10.1007/s00415-008-0695-z. [DOI] [PubMed] [Google Scholar]
- 39.Wedding U, Rohrig B, Klippstein A, Pientka L, Hoffken K. Age, severe comorbidity and functional impairment independently contribute to poor survival in cancer patients. J Cancer Res Clin Oncol. 2007;133:945–50. doi: 10.1007/s00432-007-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quist M, Rorth M, Langer S, et al. Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: a pilot study. Lung Cancer. 2012;75:203–8. doi: 10.1016/j.lungcan.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 41.The Patient Protection and Affordable Care Act, Pub.L. No. 111–148 § 3502, 124 Stat. 119, 124 (2010).
- 42.Alam E, Wilson RD, Vargo MM. Inpatient cancer rehabilitation: a retrospective comparison of transfer back to acute care between patients with neoplasm and other rehabilitation patients. Arch Phys Med Rehabil. 2008;89:1284–9. doi: 10.1016/j.apmr.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC) PM R. 2014;6:808–13. doi: 10.1016/j.pmrj.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Fu JB, Lee J, Smith DW, Shin K, Guo Y, Bruera E. Frequency and reasons for return to the primary acute care service among patients with lymphoma undergoing inpatient rehabilitation. PM R. 2014;6:629–34. doi: 10.1016/j.pmrj.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu JB, Lee J, Smith DW, Bruera E. Frequency and reasons for return to acute care in patients with leukemia undergoing inpatient rehabilitation: a preliminary report. Am J Phys Med Rehabil. 2013;92:215–22. doi: 10.1097/PHM.0b013e3182744151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tay SS, Ng YS, Lim PA. Functional outcomes of cancer patients in an inpatient rehabilitation setting. Ann Acad Med Singapore. 2009;38:197–201. [PubMed] [Google Scholar]
- 47.Englum BR, Villegas C, Bolorunduro O, et al. Racial, ethnic, and insurance status disparities in use of posthospitalization care after trauma. J Am Coll Surg. 2011;213:699–708. doi: 10.1016/j.jamcollsurg.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meagher AD, Beadles CA, Doorey J, Charles AG. Racial and ethnic disparities in discharge to rehabilitation following traumatic brain injury. J Neurosurg. 2015;122:595–601. doi: 10.3171/2014.10.JNS14187. [DOI] [PubMed] [Google Scholar]
- 49.Ottenbacher KJ, Campbell J, Kuo YF, Deutsch A, Ostir GV, Granger CV. Racial and ethnic differences in postacute rehabilitation outcomes after stroke in the United States. Stroke. 2008;39:1514–9. doi: 10.1161/STROKEAHA.107.501254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shafi S, Marquez de la Plata C, Diaz-Arrastia R, et al. Racial disparities in long-term functional outcome after traumatic brain injury. J Trauma. 2007;63:1263–8. doi: 10.1097/TA.0b013e31815b8f00. discussion 1668–1670. [DOI] [PubMed] [Google Scholar]
- 51.Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the Functional Independence Measure: a quantitative review. Arch Phys Med Rehabil. 1996;77:1226–32. doi: 10.1016/s0003-9993(96)90184-7. [DOI] [PubMed] [Google Scholar]
- 52.Corrigan JD, Smith-Knapp K, Granger CV. Validity of the Functional Independence Measure for persons with traumatic brain injury. Arch Phys Med Rehabil. 1997;78:828–34. doi: 10.1016/s0003-9993(97)90195-7. [DOI] [PubMed] [Google Scholar]