Abstract
The aim of this study was to assess the potential of 99mTc-Hynic-TOC imaging in the primary diagnosis and follow-up of midgut neuroendocrine tumors (NETs). In comparison to 111In-octreotide, 99mTc-Hynic-TOC has a higher imaging quality and leads to a lower radiation absorption in patients. 99mTc-Hynic-TOC was used for assessing primary diagnosis (n = 14) and during follow-up (n = 17) in patients with NETs. The scintigraphic findings were compared with computed tomography scans and follow-up. In 31 patients, 34 somatostatin receptor scans using 99mTc-Hynic-TOC were performed. The primary diagnoses were midgut NET. The scintigraphy was true positive in 17 patients, true negative in 9, false negative in 4, and false positive in 1. From these data, a sensitivity of 81%, specificity of 90%, positive predictive value of 94%, and negative predictive value of 69% were calculated. In summary, 99mTc-TOC represents a useful radiotracer in imaging SSTR-expressing tumor lesions with slightly higher sensitivity, higher imaging quality, and lower radiation exposure for patients compared to 111In-octreotide. A 1-day double-acquisition protocol should be used to reduce false-positive findings of the gut.
Keywords: 99mTc-Hynic-TOC imaging, neuroendocrine tumor, octreotide, somatostatin receptor
INTRODUCTION
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are a rare disease. As with all NETs, they derive from ectodermal cells of the diffuse endocrine system. The majority of these tumors are hormonally inactive and they are usually diagnosed at an advanced stage as comparatively large tumor masses with distant metastases, as their clinical impact is often less impressive as with other tumor entities of the pancreas.[1] The cumulative 5-year survival rate is over 50% for the nonmetastasized stages and drops to roughly one-fourth in the presence of liver metastases. The incidence in Western countries is estimated around 1/100,000 with the majority of cases diagnosed between 50 and 80 years of age.[2]
Overexpression of cell surface somatostatin receptor (SSRs) in well-differentiated NETs can be exploited for imaging and therapy with radiolabeled somatostatin analogs. A Phase III trial investigated the therapeutic effect of 177Lu-DOTATATE administration in 229 patients with well-differentiated, metastatic midgut NETs. The patients were randomized in a therapeutic group (n = 116) which had a dose of 7.4 GBq every 8 weeks (four intravenous (IV) infusion) plus long-acting octreotide (LAR) and in a control group which had only LAR intramuscular application. The response rate was 18% in the 177Lu-DOTATATE group versus 3% in the control group (P < 0.001). In the planned interim analysis, 14 deaths occurred in the 177Lu-DOTATATE group and 26 in the control group (P = 0.004).[3] In the IEO Milan trial (1997–2013), 793 patients were included in the study. Different application protocols were used: 278 patients received 177Lu-octreotate (34.4%), 358 patients 90Y-octreotide (44.4%), and 157 patients a combination of 177Lu-octreotate plus 90Y-octreotide (19.5%), respectively. Nephrotoxicity is an important side effect of peptide receptor radionuclide therapy (PRRT). The data analysis showed acute nephrotoxicity in 279 patients (34.6%), and in 197 patients (24.3%), the impairment of renal function was persistent.90 Y treatment was associated with significantly higher rates of nephrotoxicity (43.9%) and persistent renal impairment (33.6%) compared to 177Lu treatment (25.5% and 13.4%; P < 0.001).[4]
Essential for PRRT is a pretherapeutic imaging of the somatostatin receptor (SSTR, especially the SSTR2. Since 1992, 111In-[DTPA-D-Phe1]-octreotide was used for SSTR scintigraphy, and the most data are available for this radiopharmaceutical.[5,6] Unfortunately, the physical characteristics of 111In are not optimal for gamma camera imaging. Optimal methods for diagnosis of GEP-NETs are 68Ga-DOTATATE positron emission tomography/computed tomography (CT)[7,8] or 64Cu-DOTATATE.[9] Unfortunately, a 68Ge/68Ga generator for the labeling of 68Ga-DOTATATE or DOTATOC is very expensive and so the availability of a 99mTc-labeled SSTR analogs allows a wide use of SSTR scintigraphy with good imaging quality.[8,10]
In this study, the diagnostic accuracy of 99mTc-Hynic-TOC (Tectrotyd©) was investigated.
PATIENTS AND METHODS
Patients
Octreotide scintigraphy was used during normal clinical routine. All patients gave their informed consent for somatostatin receptor scintigraphy. In 31 patients, 34 somatostatin receptor scans using 99mTc-Hynic-TOC were performed. The primary diagnosis was GEP-NET in all patients. 99mTc-Hynic-octreotide scintigraphy was performed in 13 cases for primary staging and in 18 cases during a follow-up imaging.
Radiopharmaceuticals and imaging
99mTc-Hynic-TOC was used as a commercial kit (Rotop, Rossendorf, Germany). For labeling the sodium pertechnetate-99mTc solution for injection, it should be obtained up to hours before the start of labeling. The radioactivity ≤2.2 GBq was injected at a maximum volume of 1 ml into the vial with Hynic-[D-Phe1, Tyr3-Octreotide] (Tectrotyd©) (99mTc-TOC). The solution should be placed in a water bath or within a heated block with a temperature of 80°C for 20 min, maintaining the vial in an upright position. Each patient received the radiopharmaceutical at an average activity of 728 ± 25 MBq IV.
Whole-body imaging was performed using a double-headed camera (Discovery NM630, GE Healthcare, Solingen, Germany). For the whole-body studies and single-photon emission computed tomography (SPECT), the camera was equipped with a low-energy high-resolution parallel-hole collimator, window setting 140 keV, width 10%. Whole-body scintigrams were obtained at 1 and 4 h after administration of the radiopharmaceutical with SPECT following the 4 h whole-body scan in each patient.
Imaging and data analysis
The observer of the scans was blinded and had no information about the primary diagnosis of results of other diagnostic modalities. All the patients were diagnosed by the same reader. Any focal tracer uptake exceeding physiological uptake was regarded as a pathologic finding. Linear, nonfocal slight increase uptake of the intestine was also stated as a physiological uptake. Of 23 patients, 22 underwent a previous CT and another one patient had a magnetic resonance imaging (MRI) of the abdomen within 1 month before 99mTc-TOC scan. In addition, in 3 patients ultrasounds of the abdomen and in 4 MRI of the brain were performed. The clinical follow-up was at least 2 years after first 99mTc-TOC scan.
Whole-body scans and SPECT images were classified as true positive (correlation between other diagnostic modalities with sign of tumor, clinical findings, and follow-up which evidence the positive finding in 99mTc-TOC), true negative (no pathologic finding in 99mTc-TOC and negative finding in diagnostic modalities and no sign of tumor in the follow-up), false positive (sign of tumor in 99mTc-TOC scan, but negative findings in other diagnostic modalities; no sign of tumor in follow-up), and false negative according to histopathology (primary diagnosis) or other diagnostic modalities (CT, MRI) in follow-up. From this data analysis, the sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of 99mTc-TOC scan were calculated.[11]
RESULTS
The primary tumor was detected correctly with 99mTc-TOC in 6 cases (small bowel in 2 cases, pancreas in 3 cases, and 1 within the rectum). In 4 symptomatic patients with suspicion of a NET, the findings were correctly negative, and in the 2-year follow-up, the signs for tumor presence could be evaluated. In 11 cases, the metastases were correctly visualized (liver tumor in 7, soft tissue of abdomen in 3 and one in lung). Four tumor sites of primary or recurrent tumors were detected by SPECT but were missed in the whole-body scan.
In summary, the scans were true positive in 17 patients (6 in primary disease and 11 in recurrent disease), true negative in 9 (4 in primary disease and 5 in recurrent disease), false negative in 4 (3 in primary disease and 1 in recurrent disease), and 1 false positive (in recurrent disease). From these data, a sensitivity of 73%, specificity of 88%, PPV of 92%, and NPV of 54% were calculated. In subgroup analysis of patients with primary staging, the sensitivity was lower with 67% compared to a sensitivity of 92% for using of 99mTc-TOC in the follow-up.
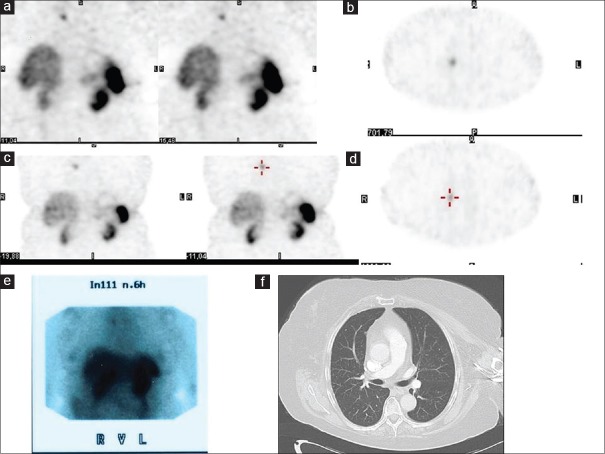
The patient with a false-positive finding had a GEP-NET within the pancreas. After surgery in 2005, the chromogranin A value was persistently elevated. With respect to the elevated chromogranin A levels, 99mTc-TOC scans were performed in 2015 and 2016. Both imagings showed the same lung lesion in the right upper lung lobe. The previous scan using 111In-octreotide in 2006 had shown the same lesion. However, over the whole period, the CT was negative in this area and the chromogranin A levels showed no further rise [Figure 1].
Figure 1.
patient with false-positive finding in 99mTc-TOC and also in 111In-octreoscan; (a and b) 99mTc-TOC scan in 2015 with positive lesion in the right upper lung; (c and d) 99mTc-TOC scan in 2016 with the same lesion; (e) 111In-octreoscan in 2006 with same lesion as in the first 99mTc-TOC; and (f) computed tomography chest in 2016 with no pathologic finding as correlate to the positive lesion in 99mTc-TOC
DISCUSSION
Somatostatin (SSTR) scintigraphy using 111In-octreotide is an established diagnostic modality in the imaging of different SSTR-expressing tumors.[10,12,13,14] However, the physical characteristics of 111In are not optimal and 111In-octreotide is relative expensive. Several researchers have tried to develop 99mTc-labeled somatostatin analogs to improve availability and imaging quality of SSTR scintigraphy as well as to reduce the radiation burden of patients.[10,11] 99mTc-[HYNIC, Tyr(3)]octreotide (99mTc-TOC) and 99mTc-[HYNIC, Tyr(3), Thr(8)]octreotide (99mTc-TATE) were two interesting candidates designated to replace the indium-labeled octreotide in SSTR imaging. Cwikla et al.[15] published data on the uptake of both radiopharmaceuticals in 12 patients with proven NETs. A slightly higher number of SSTR-expressing lesions was documented, especially in lymph nodes using 99mTc-TATE. However, only 99mTc-TOC is commercially available and most data are published about this 99mTc-labeled SSTR analog. In 2000, Bangard et al.[16] compared 111In-octreotide and 99mTc-TOC and found a superior capability of 99mTc-TOC as well to visualize extrahepatic lesions. The authors stated that 99mTc-TOC has a favorable clinical characteristic in the detection of SSTR-positive tumors due to specific and high receptor affinity, good biodistribution, faster renal excretion, lower radiation exposure, high imaging quality, and on-demand availability.
In the present study, a 1-day acquisition protocol was used with a double acquisition at 1 and 4 h after administration of 99mTc-TOC. This differs from protocols of other groups where 1-day single-acquisition protocols resorted to imaging at 2[17] or 4 h after injection.[11,18] However, the double acquisition can reduce the risk of false findings in the gut by avoiding the misinterpretation of physiological uptake.
In different papers,[15,16,17,18,19] the accuracy of 99mTc-TOC in GEP-NETs was documented, for example, Chrapko et al.[20] showed the usefulness of 99mTc-TOC in 117 NET patients, especially in primary diagnosis and restaging after primary tumor surgery. Another group[17] investigated the usefulness of 99mTc-TOC scans in solitary pulmonary nodules (SPNs) (n = 84). Positive scintigraphic results were found in 37 of 40 (93%) patients with malignant SPNs including 34 of 35 (97%) patients with primary lung carcinoma. Two remaining false-negative cases turned out to be metastatic lesions of malignant melanoma and leiomyosarcoma. Among 45 benign tumors, negative results were obtained in 31 cases (69%) and positive results in 14. False-positive findings were in 6 cases by inflammatory lesion, 3 by tuberculosis, 3 by hamartomas, and 2 by unknown etiology. The authors calculated an accuracy of the method of 80%. Another study[10] included 88 patients with GETNEPs. They reported true-positive findings in 56 patients, true negative in 17, false negative in 14, and false positive in 1. The false-positive finding was caused by a colonic adenoma. The authors calculated a 99mTc-TOC scan sensitivity of 80%, specificity of 95%, and accuracy of 83%. These data are comparable with the results of our study with a sensitivity of 73%, specificity of 88%, PPV of 92%, and NPV of 54%. In our study, we also found one false-positive patient. However, the exact cause of the false-positive signal in the patient's lung could not be solved over a 10-year period where the finding was constant and thus was left without resection and treatment. Physiologic uptake in the uncinate process of the pancreas can also mimic a (false) positive finding in SSTR scans. Yamaga et al.[21] presented a focal uptake within the uncinate process of the pancreas, mimicking malignancy in 20% of the investigated patients. However, this was a single report, and we did not observe any patients with false-positive uptake of 99mTc-TOC in this area.
Artiko et al.[19] published preliminary results from a multicenter trial that included 495 patients with different NETs. There were 334 true positive, 73 true negative, 6 false positive, and 82 false-negative results. The authors calculated a sensitivity of 80%, specificity of 92%, PPV of 98%, NPV of 47, and accuracy of 82%. Unfortunately, the paper included different NETs such as medullary thyroid carcinomas (48), lung (50), mediastinal (12), ovarian (6), kidney (4), hypophysis (10), brain (5), breast (7), paraganglioma (12), parathyroid (1), pheochromocytoma (7), NET of unknown origin (95), pancreatic (97), gastric (32), colorectal (21), small bowel (71), carcinoid of appendix (10), and liver (7).
Patients with SSTR-positive lesions are potential candidates for therapy with radiolabeled PRRT. One monocentric retrospective study from Bad Berka[22] examined the effectiveness of PRRT labeled with Lutetium-177 and Yttrium-90. In a total of 450 patients, a high effectiveness of PRRT for patients with low-to-intermediate neuroendocrine neoplasms with minor adverse effects was shown, with a complete remission in 5.6% of patients, a partial response in 22.4%, and stable disease in 47.3%. One of the patients in our study also had two cycles of Lutetium-177-DOTATOC with a partial response [Figures 2 and 3]. A follow-up using 99mTc-TOC was not performed.
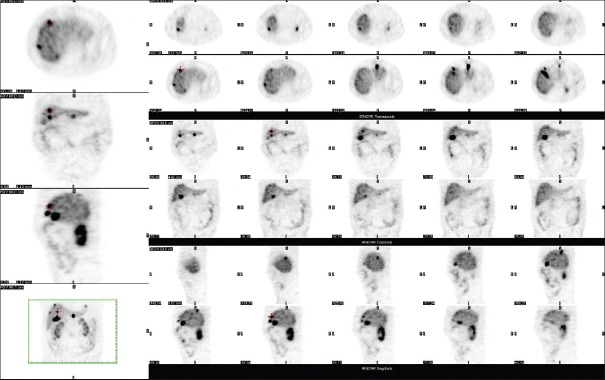
Figure 2.
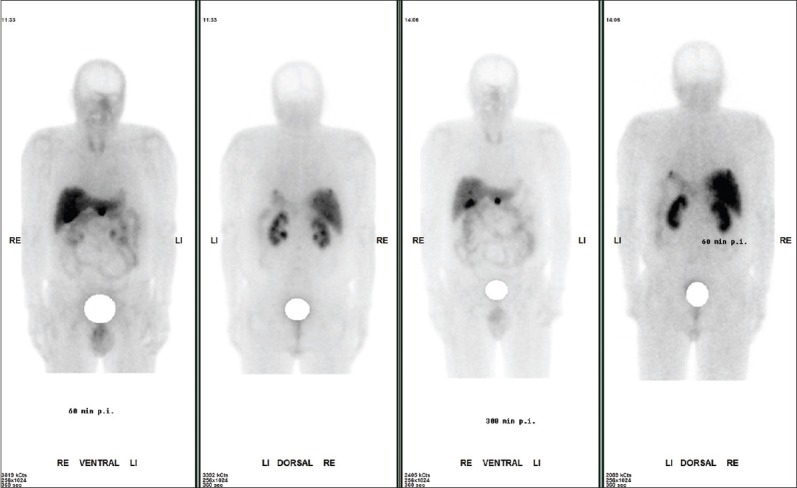
Male patient; 64 years; neuroendocrine tumor of pancreas, after surgery of pancreas splenectomy two years ago; actual multiple liver metastases and in the area of pancreas. Whole body imaging one and four hours after 736 MBq of 99mTc –TOC is shown. The tumor lesions showed a high target to background ratio indicating a high SSTR receptor expression. In the follow-up this patient had two cycles of Lutetium DOTATOC therapy with partial response and without severe side-effects
Figure 3.
The single-photon emission computed tomography imaging using 736 MBq of 99mTc-TOC in the same patients as in Figure 2
Unfortunately, only limited data on kinetics and dosimetry are available. Grimes et al.[23] observed the uptake in critically normal organs for radiation exposure (kidney, liver, and spleen) in 28 patients. The maximum uptake in the kidney occurred after 5–10 min, followed by the washout phase. In the liver, maximum uptake occurred in <5 min, followed by a rapid washout. Uptake in the spleen was slower. From these data, an effective half-life of 5.3 h and biological half-life of 47.9 h in tumor lesions were calculated with similar effective half-life/biological half-life of 5.4 h/51.5 h for kidney, 5.4 h/51.0 h for liver, 5.3 h/57.9 h for spleen, and 4.6 h/19.7 h for thyroid, respectively. The relative absorbed doses of 0.021 ± 0.007 mGy/MBq for kidney (15 mGy for 740MBq of 99mTc-TOC), 0.012 ± 0.005 mGy/MBq for liver (9 mGy for 740MBq), and 0.014 ± 0.004 mGy/MBq for the urinary bladder wall (10 mGy for 740MBq) are tolerable for diagnostic modalities.
CONCLUSION
99mTc-TOC represents a useful imaging radiotracer in SSTR-expressing tumor lesions with slightly higher sensitivity, higher imaging quality, and lower radiation exposure for patients compared to 111In-octreotide. A 1-day double-acquisition protocol should be used to reduce false-positive findings in the gut. In the present study, we did not record any patients with a false-positive finding in the gut. The 4 h imaging in 99mTc-TOC is sufficient to exclude physiological uptake in this area.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wu Y, Tedesco L, Lucia K, Schlitter AM, Garcia JM, Esposito I, et al. RSUME is implicated in tumorigenesis and metastasis of pancreatic neuroendocrine tumors. Oncotarget. 2016;7:57878–7893. doi: 10.18632/oncotarget.11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiruvella A, Kooby DA. Surgical management of pancreatic neuroendocrine tumors. Surg Oncol Clin N Am. 2016;25:401–21. doi: 10.1016/j.soc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177 Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19. doi: 10.1007/s00259-014-2893-5. [DOI] [PubMed] [Google Scholar]
- 5.Reubi JC, Waser B, van Hagen M, Lamberts SW, Krenning EP, Gebbers JO, et al. In vitro and in vivo detection of somatostatin receptors in human malignant lymphomas. Int J Cancer. 1992;50:895–900. doi: 10.1002/ijc.2910500613. [DOI] [PubMed] [Google Scholar]
- 6.Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:R53–73. doi: 10.1677/ERC-09-0078. [DOI] [PubMed] [Google Scholar]
- 7.Poeppel TD, Binse I, Petersenn S, Lahner H, Schott M, Antoch G, et al. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J Nucl Med. 2011;52:1864–70. doi: 10.2967/jnumed.111.091165. [DOI] [PubMed] [Google Scholar]
- 8.Etchebehere EC, de Oliveira Santos A, Gumz B, Vicente A, Hoff PG, Corradi G, et al. 68Ga-DOTATATE PET/CT, 99mTc-HYNIC-octreotide SPECT/CT, and whole-body MR imaging in detection of neuroendocrine tumors: A prospective trial. J Nucl Med. 2014;55:1598–604. doi: 10.2967/jnumed.114.144543. [DOI] [PubMed] [Google Scholar]
- 9.Pfeifer A, Knigge U, Binderup T, Mortensen J, Oturai P, Loft A, et al. 64Cu-DOTATATE PET for neuroendocrine tumors: A prospective head-to-head comparison with 111In-DTPA-octreotide in 112 patients. J Nucl Med. 2015;56:847–54. doi: 10.2967/jnumed.115.156539. [DOI] [PubMed] [Google Scholar]
- 10.Baum RP, Hofmann M. Nuclear Medicine diagnostik of neuroendocrine tumors. Onkologe. 2004;6:598–610. [Google Scholar]
- 11.Gabriel M, Muehllechner P, Decristoforo C, von Guggenberg E, Kendler D, Prommegger R, et al. 99mTc-EDDA/HYNIC-Tyr(3)-octreotide for staging and follow-up of patients with neuroendocrine gastro-entero-pancreatic tumors. Q J Nucl Med Mol Imaging. 2005;49:237–44. [PubMed] [Google Scholar]
- 12.Pauwels S, Leners N, Fiasse R, Jamar F. Localization of gastroenteropancreatic neuroendocrine tumors with 111indium-pentetreotide scintigraphy. Semin Oncol. 1994;21:15–20. [PubMed] [Google Scholar]
- 13.Jamar F, Fiasse R, Leners N, Pauwels S. Somatostatin receptor imaging with indium-111-pentetreotide in gastroenteropancreatic neuroendocrine tumors: Safety, efficacy and impact on patient management. J Nucl Med. 1995;36:542–9. [PubMed] [Google Scholar]
- 14.Valkema R, Steens J, Cleton FJ, Pauwels EK. The diagnostic utility of somatostatin receptor scintigraphy in oncology. J Cancer Res Clin Oncol. 1996;122:513–32. doi: 10.1007/BF01213548. [DOI] [PubMed] [Google Scholar]
- 15.Cwikla JB, Mikolajczak R, Pawlak D, Buscombe JR, Nasierowska-Guttmejer A, Bator A, et al. Initial direct comparison of 99mTc-TOC and 99mTc-TATE in identifying sites of disease in patients with proven GEP NETs. J Nucl Med. 2008;49:1060–5. doi: 10.2967/jnumed.107.046961. [DOI] [PubMed] [Google Scholar]
- 16.Bangard M, Béhé M, Guhlke S, Otte R, Bender H, Maecke HR, et al. Detection of somatostatin receptor-positive tumours using the new 99mTc-tricine-HYNIC-D-phe1-tyr3-octreotide:First results in patients and comparison with 111In-DTPA-D-phe1-octreotide. Eur J Nucl Med. 2000;27:628–37. doi: 10.1007/s002590050556. [DOI] [PubMed] [Google Scholar]
- 17.Płachcińska A, Mikołajczak R, Kozak J, Rzeszutek K, Kuśmierek J. Differential diagnosis of solitary pulmonary nodules based on 99mTc-EDDA/HYNIC-TOC scintigraphy: The effect of tumour size on the optimal method of image assessment. Eur J Nucl Med Mol Imaging. 2006;33:1041–7. doi: 10.1007/s00259-006-0117-3. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez Londoño GA, García Vicente AM, Soriano Castrejon AM, Gómez López OV, Palomar Muñoz A, Vega Caicedo CH, et al. Role of 99mTc-HYNIC-Tyr3-octreotide scintigraphy in neuroendocrine tumors based on localization of the primary tumor. Minerva Endocrinol. 2016;41:10–8. [PubMed] [Google Scholar]
- 19.Artiko V, Afgan A, Petrović J, Radović B, Petrović N, Vlajković M, et al. Evaluation of neuroendocrine tumors with 99mTc-EDDA/HYNIC TOC. Nucl Med Rev Cent East Eur. 2016;19:99–103. doi: 10.5603/NMR.2016.0020. [DOI] [PubMed] [Google Scholar]
- 20.Chrapko BE, Nocuń A, Gołebiewska R, Stefaniak B, Korobowicz E, Czekajska-Chehab E, et al. 99mTc-EDDA/HYNIC-TOC somatostatin receptor scintigraphy in daily clinical practice. Med Sci Monit. 2010;16:MT35–44. [PubMed] [Google Scholar]
- 21.Yamaga LY, Neto GC, da Cunha ML, Osawa A, Oliveira JC, Fonseca RQ, et al. 99mTc-HYNIC-TOC increased uptake can mimic malignancy in the pancreas uncinate process at somatostatin receptor SPECT/CT. Radiol Med. 2016;121:225–8. doi: 10.1007/s11547-015-0593-2. [DOI] [PubMed] [Google Scholar]
- 22.Hörsch D, Ezziddin S, Haug A, Gratz KF, Dunkelmann S, Miederer M, et al. Effectiveness and side-effects of peptide receptor radionuclide therapy for neuroendocrine neoplasms in germany: A multi-institutional registry study with prospective follow-up. Eur J Cancer. 2016;58:41–51. doi: 10.1016/j.ejca.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Grimes J, Celler A, Birkenfeld B, Shcherbinin S, Listewnik MH, Piwowarska-Bilska H, et al. Patient-specific radiation dosimetry of 99mTc-HYNIC-Tyr3-octreotide in neuroendocrine tumors. J Nucl Med. 2011;52:1474–81. doi: 10.2967/jnumed.111.088203. [DOI] [PubMed] [Google Scholar]