Abstract
Accurate and timely visualization of apoptotic status in response to radiation is necessary for deciding whether to continue radiation or change to another mode of treatment. This is especially critical in patients with colorectal cancer, which requires a delicate combination of surgery, radiation, and chemotherapy in order to achieve optimal outcome. In this study, we investigated the potential of phosphatidylserine-recognizing peptide 1 (PSP1) as an apoptosis-targeting probe, which identifies phosphatidylserine on cell surfaces. We first screened colon cancer cell lines for their sensitivity to radiation and selected two cell lines: HCT116 and HT29. Cell binding assay using fluorescence-activated cell sorting and optical imaging showed that HCT116 cells had better binding to PSP1 than HT29 cells. Thus, mouse xenograft model using HCT116 cells was generated and was topically irradiated with either single or fractionated dose of radiation followed by systemic administration of PSP1 for subsequent molecular optical imaging. We confirmed that the PSP1 probe was selectively bound to apoptosis-induced tumor in a radiation dose-dependent manner. We also observed that fractionated radiation regimen, which is recently being used in clinical situation, was more effective in inducing tumor apoptosis than corresponding single-dose radiation treatment. We then evaluated the correlation between tumor targeting of PSP1 and suppression effect of tumor development and found that tumor volume and fluorescence intensity were correlated before (correlation coefficient r2 = 0.534) and after (r2 = 0.848) radiation therapy. Our study shows that PSP1 peptide is an efficient index probe for deciding “go or no-go” for radiation therapy in colorectal cancer.
Introduction
Rectal cancer requires multimodal management in that a combination of surgery, chemotherapy, and radiotherapy is necessary to achieve optimal outcome. Surgery remains as the primary determinant of cure in patients with localized rectal cancer; however, in locally advanced rectal cancer, surgical resection after neoadjuvant concurrent radiotherapy (RT) or chemoradiotherapy (CRT) is the current standard treatment [1]. Radiotherapy along with surgical resection significantly improves local control for rectal cancer [2], [3]. Although short-course radiotherapy is a frequent option, long-course preoperative chemoradiotherapy in 5 weeks and concurrent chemotherapy for 3 days are widely implemented [3]. Despite low local relapse rates, systemic recurrence remains a significant problem, occurring in 30% to 40% of patients [4]. Therefore, it is crucial to determine the tumor response to RT in order to avoid unnecessary delay of surgical resection. Tumor response to treatment is typically evaluated by anatomical imaging methods such as computed tomography and magnetic resonance imaging. However, anatomical imaging methods are possibly inadequate for early detection of therapeutic response because the tumor architecture often remains unchanged for a prolonged time after radiation therapy, regardless of apoptotic changes occurring inside. Therefore, it is important to timely select patients who would benefit most from a particular therapy to minimize exposure to toxic and noneffective regimens. In this regard, it is necessary to utilize an imaging method that allows for making a “go or no-go” decision earlier than anatomical imaging methods do. Except for surgical resection, the most dependable cancer treatments to date include radiation and chemotherapy, which are subject to inflicting DNA damages. Such DNA-damaging regimens cause cell death through replication catastrophe [5] or apoptosis by inducing ionization that generates DNA-damaging reactive oxygen species [6]. Moreover, in cells undergoing chemo- and radiotherapy, phosphatidylserine (PS) is exposed during early primary stage of necrosis due to disruption of plasma membrane integrity [7].
Irradiation initiates DNA damage to induce apoptosis [8], and apoptotic cell death commonly occurs earlier than anatomical change or reduction in tumor size [9], [10]. As a result, direct visualization of apoptosis would provide essential information of tumor response to radiotherapy. Apoptotic cells are recognizable by surface biomarkers such as PS and histone H1, which are little or absent on the surface of healthy cells [11], [12]. Annexin V is a PS-binding protein with molecular weight of 36 kDa and is most extensively used to image apoptosis in diverse preclinical circumstances [13], [14]. Annexin V is a PS-binding protein and binds to externalized PS on both apoptotic and necrotic cells with high affinity as an early event in apoptosis [10].
But on the other hand, it also may cause immunogenicity when injected into the body as an optical imaging probe due to its large size [15]. Therefore, it is necessary to develop alternative optical imaging probes that can detect apoptotic cells. As an alternative to bulky proteins or antibodies, small peptides have advances as an ideal imaging probe: rapid clearance from blood circulation, efficient tissue penetration due to the small size, low immunogenicity, and low production cost [16]. Thapa and colleagues have identified a novel peptide, named PSP1 (PS-recognizing peptide: CLSYYPSYC), and showed specific targeting to PS on the apoptosis-induced tumor cells after chemotherapy [17]. In addition, they directly compared PSP1 with Annexin V with respect to PS binding kinetics, apoptotic cell-targeting ability, and the efficacy of homing to apoptotic tumor cells after chemotherapy in a mouse model and showed that PSP1 targeted apoptotic cells more efficiently than did Annexin V [17]. In this study, we examined whether PSP1 can be used for in vivo imaging acquiring the signal of radiation-induced apoptosis in colorectal cancer.
Methods
Cell Culture
HCT116 and HT29 human colon cancer cells were grown in RPMI 1640 containing 10% fetal bovine serum (GIBCO) 1% antibiotic-antimycotic solution (GIBCO) at 37°C in a humidified atmosphere of 5% CO2.
Quantification of Apoptosis In Vitro
HCT116 and HT29 (1 × 106 cells) cells were seeded in 6-well plates and incubated overnight to allow for attachment. Cell were irradiated at 0, 5, and 10 Gy, and at 48 hours after radiation, cells were collected, washed, and suspended in binding buffer. The cells were then stained with both Annexin V-FITC and propidium iodide (PI) (BD bioscience) for flow cytometry and with PSP1-FITC (BioActs Inc.) for fluorescence-activated cell sorting (FACS). PSP1 peptide at a final concentration of 20 μmol in binding buffer was incubated with normal or irradiated cells. Experiments were repeated three times.
Xenograft Model
All experiments were carried out under a protocol approved by the Institutional Animal Care and Use Committee, Asan Institute for Life Sciences. HCT116 (5 × 106 cells) cells suspended in RPMI medium containing 10% fetal bovine serum were subcutaneously injected on the flank of BALB/c nude mice (6 weeks old, male, Japan, SLC, Inc.). Tumors were allowed to be 60 to 80 mm3 of volume before the treatment was initiated. Mice were exposed following radiation regimens: single dose (2, 5, 10, and 15 Gy) or multifractionated dose (2 Gy × 5 for 5 days and 5 Gy × 2 for 2 days).
Treatment of Radiation
The cells were culture in six-well plates and exposed to irradiation once with 6-MV photons generated by a linear accelerator (CLINAC EX, Varian CP., Palo Alto, CA) at 0, 2, and 5 Gy/min. Mice bearing right flank tumors (approximately 10 mm in diameter) were exposed to irradiation once for single dose or several times for multifractionated dose, respectively, using 6-MV photons generated by a linear accelerator (CLINAC EX, Varian CP., Palo Alto, CA). Before the radiation treatment, mice were anesthetized by intraperitoneal injection with Zoletil/Rompun (80 mg/kg Zoletil, 20 mg/kg Rompun).
Probe Injection and Optical Imaging
Near-infrared fluorescence dye-conjugated PSP1 (PSP1 peptide+flamma-675 NHS ester) was purchased (BioAct, Korea). After anesthetizing each mouse with 2% to 2.5% isoflurane, PSP1-flamma-675 (PSP1, 100 μmol per 200 μl) or scramble-flamma-675 peptide (control peptide) was injected into the tail vein, and then in vivo molecular images were performed at 72 hours after radiation. In vivo and ex vivo images were obtained using eXplore Optix MX3 (ART Advanced Research Technologies Inc., Montreal, Canada) in which excitation and emission wavelength were 675 nm and 720 nm, respectively. The images were obtained by using eXplore Optix optiView Software. After in vivo imaging, the mice were sacrificed, tumors were excised, and ex vivo images were immediately obtained.
Correlation between Targeting to Tumor and Suppression Effect of Tumor Growth
Mice were divided into two treatment groups (n = 4 per group): 1) nonradiation control and 2) radiation (2 Gy × 5 for 5 days). Follow-up changes in tumor size were measured for 3 weeks. Tumor volumes were calculated as a × b2/2, where “a” is the largest and “b” is the smallest dimension. The optical imaging was performed on the 8th day after the end of 5 consecutive irradiations and again on the 23rd day, the last day of the follow-up study.
Histological Examination
For histological examination, tumor tissues were collected, fixed in 10% formalin, and paraffin embedded. Sections were prepared using conventional procedures and stained with hematoxylin-eosin. Histological analysis was evaluated by a qualified pathologist. Apoptosis in the tumor tissues was assessed by terminal deoxynucleotidyl transferase dUTP nick and labeling (TUNEL) assay (Roche, Indianapolis, IN). Tissue sections were stained with DAPI for nuclei visualization and mounting solution for observation under a fluorescent microscope. Fluorescence microscopic analyses for dissected tissues of irradiated or control tumors allowed to confirm PSP1-binding cells and TUNEL-positive cells. Histology evaluation was performed in a blind manner. TUNEL-stained slices were selected to determine the number of apoptotic cells in each slide. At least five fields of view (objective magnification at ×800) were selected randomly, photographed, and counted for TUNEL-positive cells under an inverted Zeiss LSM 710 confocal microscopy (Carl Zeiss, Thornwood, NY).
Statistical Analysis
All values are expressed as the mean ± SE. The statistical significance between the experimental and control groups was evaluated via ANOVA test. A P value of less than .05 was considered to be statistically significant and is indicated by an asterisk over the value.
Results
In Vitro Assessment of Radiation-Sensitive Colorectal Cancer Cell Lines
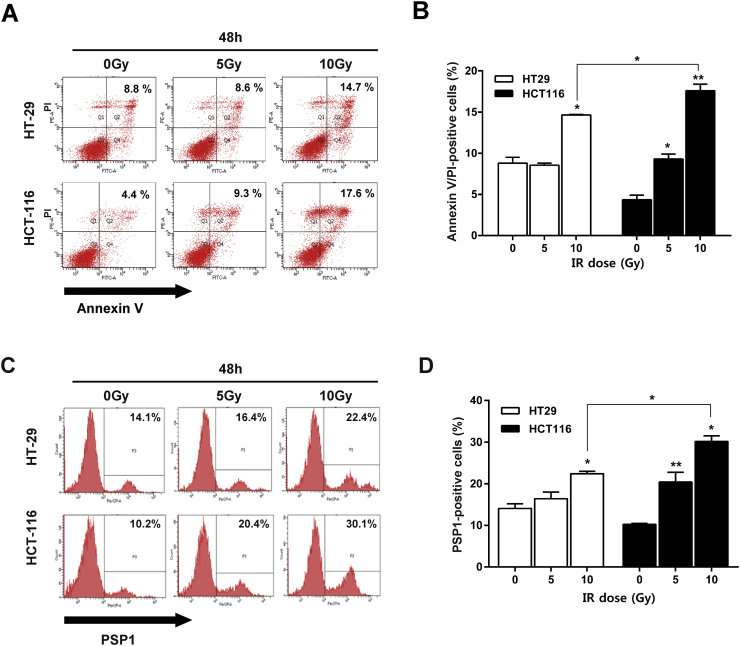
To examine the sensitivity to radiation treatment, two representative colorectal carcinoma cell lines (HT29 and HCT116) were treated with 0, 5, or 10 Gy of radiation. The percentages of cells detected with Annexin V/PI in response to each respective radiation dose at 48 hours were 8.8% ± 1.1%, 8.6% ± 0.4%, and 14.7% ± 0.1% in HT29 cells and 4.4% ± 0.6%, 9.3% ± 0.8%, and 17.6% ± 1.4% in HCT116 cells (Figure 1A). Thus, the cells were mostly in their late apoptotic [Annexin V (+)/PI (+)] stages, and radiation had significantly greater effect on apoptosis induction in HCT116 cells than in HT29 cells (Figure 1B). The percentages of cells treated with PSP1 in response to the individual irradiations at 48 hours were 14.1% ± 2.0%, 16.4% ± 2.8%, and 22.4% ± 1.0% in HT29 cells and 10.2% ± 0.5%, 20.4% ± 4.1%, and 30.1% ± 2.4% in HCT116 cells, respectively (Figure 2C). The result indicates that radiation had a significantly greater effect on apoptosis induction in HCT116 cells than in HT29 cells (Figure 2D). HT29 cells were resistant to irradiation at a dose of 10 Gy. Thus, PSP1 was supposed to be effective for the detection of apoptotic cells, and HCT116 cells were identified as radiosensitive and HT29 cells as radioresistant cell lines. Based on these sensitivity results, we chose HCT116 cell line for the following in vivo study.
Figure 1.
Radiation sensitivity in two colon cancer cell lines of HCT-116 and HT-29. (A) Cells were irradiated with 0, 5, and 10 Gy and then stained with Annexin V and PI for flow cytometry. Numbers represent the percent of cells in the fraction. (B) The Annexin V/PI-positive cells were calculated as the percentage. Columns, mean; error bars, SEM, from three independent experiments. *P < .05. (C) Cells were irradiated with 0, 5, and 10 Gy and then stained with PSP1 for FACS. Apoptosis was determined by FACS by using PSP1 in HT29 and HCT116 cells at 48 hours after radiation (0, 5, and 10 Gy). (D) The PSP1-positive cells were calculated as the percentage. Columns, mean; error bars, SEM, from three independent experiments. *P < .05.
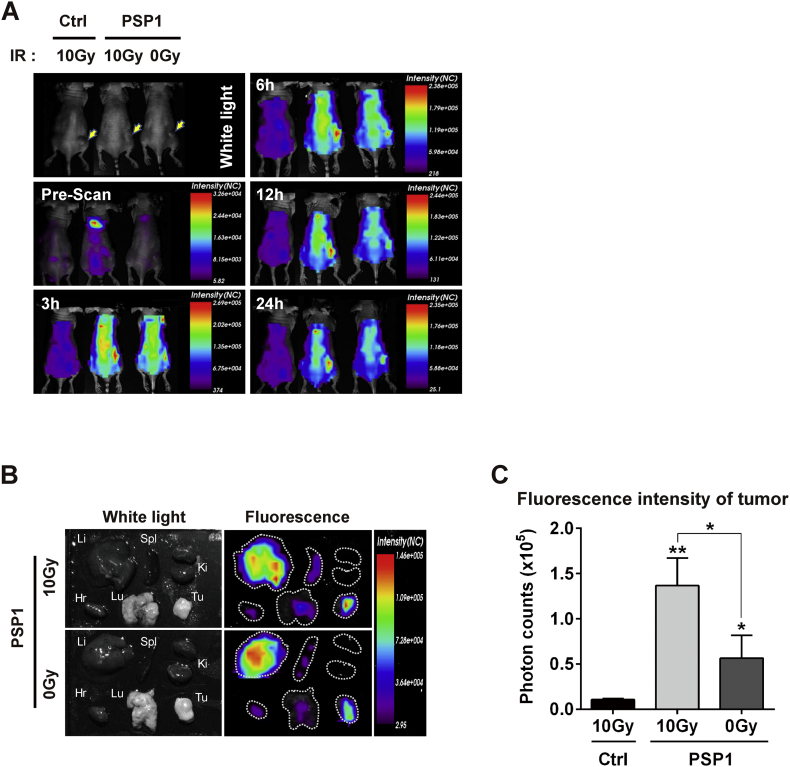
Figure 2.
In vivo time-dependent fluorescence images of HCT116 tumor-bearing mouse using PSP1. (A) PSP1-flamma675 or control peptide-flamma675 was injected into the tail vein of mice after receiving radiation therapy 10 Gy to tumor area. The yellow arrows indicate the tumors. (B) Ex vivo fluorescence images of major organs and tumors from PSP1-treated mouse after excision at 24 hours postinjection of PSP1. (C) Quantification of fluorescence intensities of excised tumor. Li = liver; Spl = spleen; Ki = kidney; Hr = Heart; Lu = Lung; Tu = Tumor. The results are expressed as the mean ± SD from three independent experiments. Asterisks represent statistical significance compared to control group. Asterisks on brackets represent significance in difference between the two groups. *P < .05 and **P < .01 by one-way ANOVA.
In Vivo Imaging of Apoptosis of Colorectal Cancer Using PSP1-Flamma-675
To examine the in vivo detection and imaging of apoptosis of colorectal cancer using PSP1, we used HCT116 cell tumor xenograft mouse model. Tumors on the flank of mouse were irradiated with 10 Gy, and the near-infrared fluorescence dye (flamma-675)-labeled peptide was introduced through the tail vein. We measured fluorescence intensity at the tumor by accumulation of the dye-labeled PSP1 after radiation treatment. After peptide injection, fluorescence images were obtained at indicated time intervals. Representative whole-body fluorescence images were shown in Figure 2A. The highest fluorescence signal in tumor was obtained at 1 hour postinjection (signal intensity: 2.66 × 105) and remained for more than 24 hours (Figure 2A). No fluorescence signal was detected at the tumors irradiated with 10 Gy in case of control probe-injected mice. In vivo images of PSP1-injected mice showed higher amount of fluorescence signals in the radiation-treated tumors than in nonirradiated tumors after 24 hours. Ex vivo fluorescence images of major organs and tumors from PSP1-treated mouse at 24 hours postinjection were obtained (Figure 2B), and the fluorescence intensity at tumor site was significantly higher in radiation-treated group compared with untreated control group (Figure 2C). The control peptide-injected mice showed negligible fluorescence signal at the tumor after radiation treatment, indicating specific targeting and determination of apoptotic cells via PSP1-based fluorescent probe.
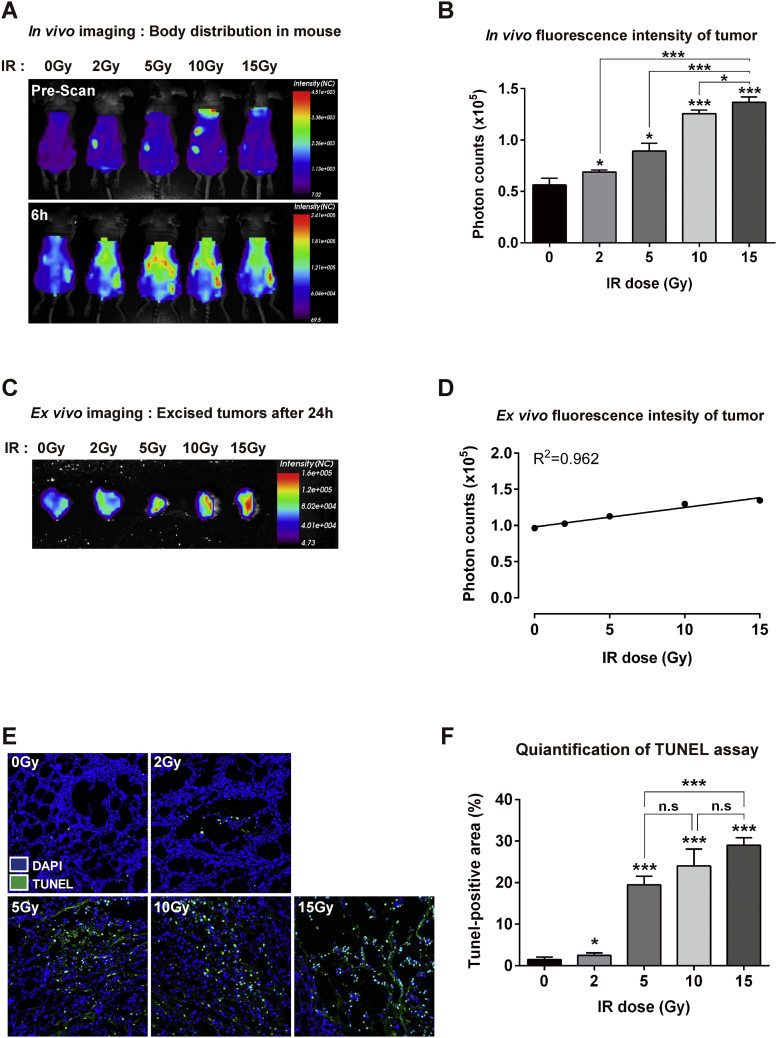
Binding of PSP1 to Tumor in Radiation Dose-Dependent Therapy and in Multifractionated Radiation Therapy
We next examined how PSP1 binding to tumor changed according to radiation dose. To compare fluorescence signal intensities in vivo and ex vivo, tumor in each mouse was treated with 2, 5, 10, and 15 Gy of radiation followed by injection of PSP1 through the tail vein and imaging at indicated times (Figure 3). After following up for 24 hours, tumors were excised, and ex vivo fluorescence imaging of tumors was performed (Figure 3C). The fluorescence intensities of images following PSP1 injection were strongly correlated with radiation dose (correlation coefficient r2 = 0.9624, Figure 3C). Level of apoptosis according to increment of radiation dose was further demonstrated by TUNEL staining of the tumor tissues (Figure 3, D and E). The number of TUNEL-positive cells drastically increased with the radiation doses used, particularly between 2 and 5 Gy (Figure 3E) (P < .001).
Figure 3.
Binding of PSP1 to tumor in radiation dose-dependent therapy. (A) PSP1-flamma675 was injected into the tail vein of mice after receiving radiotherapy 0, 2, 5, 10, and 15 Gy to tumor on right legs. In vivo optical imaging shows the distribution of PSP1 in mouse before and after administration of probe. (B) Quantification of fluorescence signal intensity in mice after administration of PSP1-flamma675 at 6 hours. The results are expressed as the mean ± SD from three independent experiments. Asterisks represent statistical significance compared to control group. Asterisks on brackets represent significance in difference between the two groups. *P < .05 and ***P < .001 by one-way ANOVA. (C) Ex vivo fluorescence images of tumors and (D) their quantification of fluorescence signal. (E) Histological analysis for apoptosis in tumor tissues and (F) quantification of apoptotic positive area. Asterisks represent statistical significance compared to control group. Asterisks on brackets represent significance in difference between the two groups. *P < .05 and ***P < .001 by one-way ANOVA. n.s. indicates not significant.
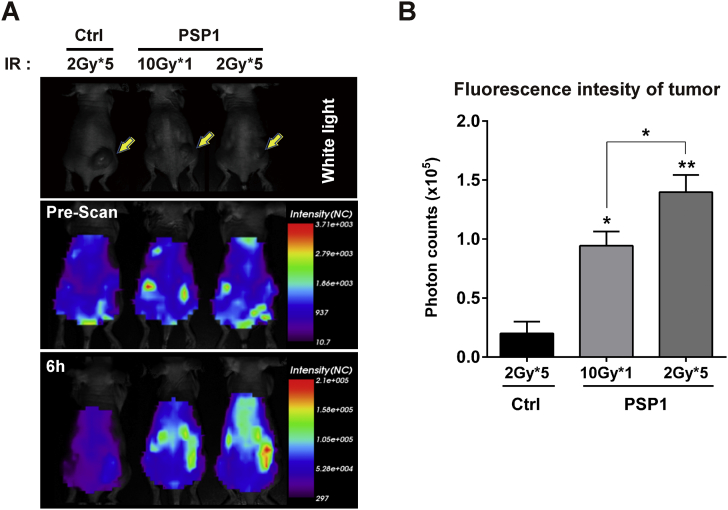
We also compared the efficacy of a single-dose radiation (10 Gy) with multifractionated-dose radiation (2 Gy × 5) in xenograft mice model of colorectal cancer using the peptide probes. Fluorescence intensities were significantly higher in multifractionated radiation group compared to corresponding single-dose radiation group (Figure 4). The fluorescence intensities at the tumors of control peptide-injected mice with 2 Gy × 5 were limited to 0.2, whereas the fluorescence intensities of PSP1 with 10 Gy or 2 Gy × 5 were 0.94 and 1.4 × 105, respectively (Figure 4B).
Figure 4.
Comparison of a single-dose and multifractionated doses of irradiation in mice administered with PSP1-flamma675. (A) Radiation therapies of 10 Gy, 5 Gy × 2 times, 5 Gy × 1 time, and 2 Gy × 5 times to right legs [IR (+)] are followed by administration of PSP1-flamma675 or control peptide-flamma675 to mice. The yellow arrows indicate the tumors. (B) Quantification of fluorescence signal intensity in mice after administration of PSP1-flamma675. Asterisks represent statistical significance compared to control group. Asterisks on brackets represent significance in difference between the two groups. *P < .05 and ***P < .001 by one-way ANOVA.
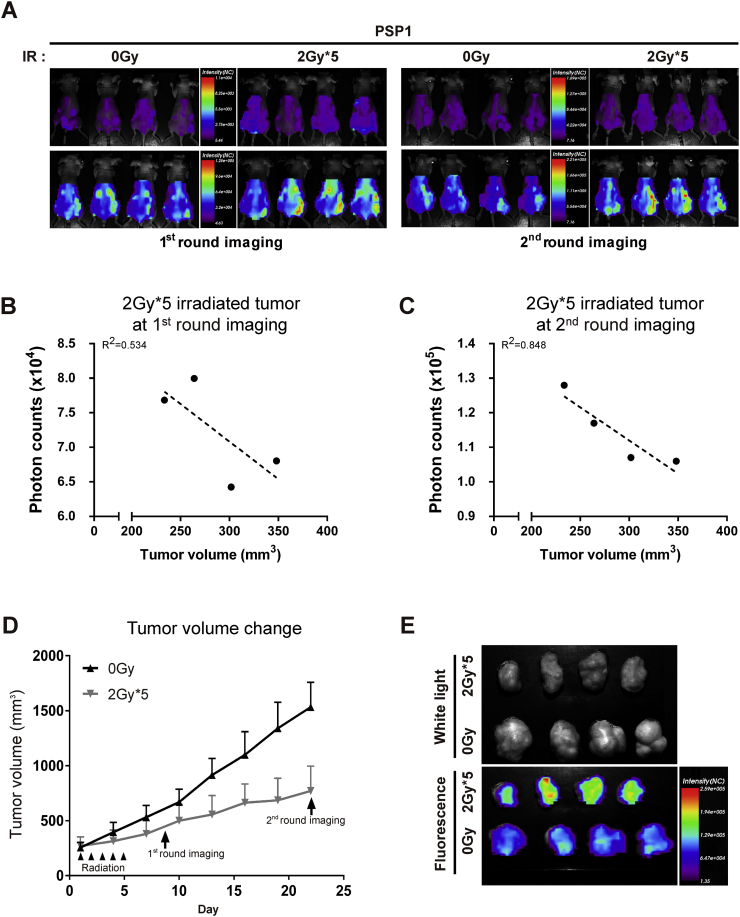
Correlation between Fluorescence Intensity and Tumor Volumes
We then examined the correlation between fluorescence intensity of in vivo apoptosis imaging after multifractionated radiation treatment (2 Gy × 5) and the changes in tumor volume. Mice treated with no (0 G) or multifractionated radiation (2 Gy × 5) were imaged on the 8th and the 23rd day, the latter of which was the last day of experiment (Figure 5A). Tumors treated with multifractionated radiation showed markedly higher fluorescence intensity than the control group of no radiation in both whole-body and excised tumor harvested at the 23rd day postradiation. The volume of the tumor in multifractionated group was also found to slowly enlarge compared to the control group (Figure 5D), which is in agreement with the fluorescence imaging (Figure 5A). The fluorescence intensities of tumor in mouse of 2Gy × 5 radiation after administration with PSP1 at 3 weeks after the initiation of radiotherapy were inversely correlated with tumor volumes (correlation coefficient r2 = 0.848), while showing weak correlation within 1 week postirradiation (r2 = 0.534). These results show that multifractionated radiation–treated tumor apoptosis can be thoroughly imaged using PSP1 probe; also, the increase in fluorescence intensity according suggests that cell apoptosis can be detected with fluorescence measurement (Figure 5D).
Figure 5.
Correlation between targeting to tumor and suppression effect of tumor growth. (A) Comparative imaging of a single-dose and multifractionated doses of irradiation in mice with before (Pre) and after 6 hours (6 h) of administration of PSP1-flamma675. (B-C) Correlation between fluorescence intensities and tumor volumes from the first and second round of imaging in mouse of 2 Gy × 5 radiation. The result was with tumor volumes (correlation coefficient r2 = 0.848) compared to those of tumor at 1 week (r2 = 0.534). (D) Changes in tumor volume in mouse with no radiation (0G: ◆) and 5 times 2 Gy radiation (2G*5: ■). The optical imaging was performed at 8 days (1 round imaging) and 23 days (2 round imaging) after the initiation of irradiation as shown in A. (E) Ex vivo fluorescence images of major organs and tumors from PSP1-treated mouse after excision at 24 hours postinjection of PSP1.
Discussion
Molecular imaging is a versatile tool for detection of tumors [11], [18], [19] and is also valuable for analyzing the expression and activity of specific molecules and biological processes such as apoptosis [20], [21], [22]. In the present study, we showed that the PSP1 peptide probe can efficiently identify radiation-induced apoptotic cells. The results are in line with a previous study which showed that PSP1 imaging is able to detect apoptotic cells generated in response to chemotherapy [17]. Our study shows that the number of apoptotic cells after radiotherapy increased with incremental radiation doses, signifying that high-dose radiation causes severe damage to cells. Radiation-induced apoptosis was accurately detected by molecular imaging of fluorescence-labeled PSP1 and TUNEL staining. The results suggest that fluorescent determination of apoptosis can be applied for evaluation and prediction of the cellular responses to radiation, as proposed in previous studies [3], [22], [23], [24].
Radiotherapy is a local treatment modality; thus, radiation-induced apoptosis of the tumors would be different depending on the type of treatment, time, and dosage. Functional molecular imaging methods such as positron emission tomography (PET) or single photon emission computed tomography are superior in visualizing the response of systemic chemotherapy because they have systems feasible for detecting both primary tumors and metastatic lesions, while, on the other hand, optical imaging may be more efficient for evaluating the treatment response for radiation because it can be used to detect local signals from the irradiated lesion. Fluorescence-based optical molecular imaging is also advantageous in that it is compatible with instruments for surveilling mucosal lesion in the gastrointestinal tract—the fluorescence imaging detector can be coupled to internal inspection devices such as endoscope. In this regard, the optical imaging has a competitive advantage over PET imaging system. Such optical imaging supported with endoscope enables acquisition of real-time in vivo image, which is not executable in PET system [23]. Thus, optical imaging accompanied with endoscopy is a powerful imaging modality for assessing tumor response after radiotherapy to intraluminal neoplasm including rectal cancer, cervical cancer, or head and neck cancer. Moreover, the response to radiotherapy could be immediately observed, and prompt decision to keep or change the therapeutic modality can be made by physicians. In that sense, we expect that optical imaging of apoptosis with fluorescence dye, conducted as a proof-of-concept in this study, holds great promise for potential applications to functional endoscopy.
Treatment with radiation is used in a variety of human cancers, and conventionally, radiation is applied in a single large dosage. However, in recent years, advanced radiotherapy with fractionation schedules has been developed as a new therapeutic strategy, equipped with devices capable of specific delivery of radiation to the targeting tumors while avoiding adjacent organs [24], [25]. Several studies have compared the efficacy and morbidity of short course of radiotherapy to those of conventional radiotherapy, which is administered at a total dose of 50.4 Gy over 28 times 1.8 Gy [26]. Here, we first assessed the efficacy of single fraction radiation and then compared it to that of a multifractionated regimen in a human colorectal cancer model using the fluorescence imaging method. As a result, we observed that fluorescence intensities were significantly higher in groups treated with multifractionation radiation compared to groups treated with a single dose of radiation. These results suggest that apoptosis imaging with PSP1 certainly acts as an effective tool for analyzing the degree of apoptosis induced by various fractionation regimens of radiotherapy in human colorectal cancer.
Although the extent of apoptosis against irradiation can be examined with apoptosis-targeted drug, the most cautious things are side effects from being exposed to radiation, whether it is acute pattern during treatment or chronic pattern that may occur months or even years after treatment [8]. Acute side effects include skin irradiation or damage to areas exposed to radiation beams, and possible chronic side effects of radiation include fibrosis; damage to the bowels resulting in diarrhea and bleeding; memory loss; infertility [25]; and, rarely, the induction of a second cancer [8]. In the present study, we compared the fluorescence intensities and the amount of apoptotic cells between single-dose irradiation group (10 Gy) and same multifractionated-dose irradiation group (2 × 5 Gy). Based on the effect of multifractionated dose irradiation, we confirmed the correlation between apoptosis-targeted imaging of PSP-1 by multifractionated dosing and tumor volume change. As a result, it was confirmed that the correlation between tumor fluorescence intensity and tumor volume size was higher in the secondary imaging than in the primary imaging. This is probably due to tumor cell apoptosis following the irradiation, which leads to increased tumor targeting, but it is time consuming to have an immediate effect on tumor growth and thus to a relatively higher correlation in secondary imaging over time.
However, the key advance of this study was to investigate the capability of PSP1 probe for predictive diagnosis after radiation treatment, and we plan to further analyze acute or chronic side effects in response to different fractionation radiation regimens separately.
In conclusion, our results suggest that PSP1 is a promising probe for in vivo imaging of tumor apoptosis after radiation therapy. This method may provide useful information on how radiation affects apoptosis of colorectal cancer cells, and could be developed into a molecular imaging probe for postradiotherapy monitoring. In addition, PSP1-based molecular imaging technique is considered to be used for early prediction of the therapeutic response to radiotherapy in a mouse model of colorectal cancer.
Conflicts of Interests
There are no potential conflicts of interest to disclose.
Acknowledgements
We thank Dr. Joon Seo Lim from the Scientific Publications Team at Asan Medical Center for his editorial assistance in preparing this manuscript, and the optical imaging core facility at the ConveRgence mEDIcine research cenTer (CREDIT), Asan Medical Center, for support and instrumentation.
This research was supported by the SK Chemical Research Fund of The Korean Society of Gastroenterology, the Student Research grant (14-15) of University of Ulsan College of Medicine, a grant (No. HI15C3078) of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, and a grant (No. 2015-585) from Asan Institute for Life Sciences, Asan Medical Center.
Contributor Information
Sang-Yeob Kim, Email: sykim3yk@amc.seoul.kr.
Seung-Jae Myung, Email: sjmyung@amc.seoul.kr.
References
- 1.Ooi K, Gibbs P, Faragher I. Surgical oncology issues in locally advanced rectal cancer. ANZ J Surg. 2011;81(11):790–796. doi: 10.1111/j.1445-2197.2011.05704.x. [DOI] [PubMed] [Google Scholar]
- 2.Haustermans K, Debucquoy A, Lambrecht M. The ESTRO Breur Lecture 2010: toward a tailored patient approach in rectal cancer. Radiother Oncol. 2011;100(1):15–21. doi: 10.1016/j.radonc.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Ngan SY. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30(31):3827–3833. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 4.Koukourakis GV. Role of radiation therapy in neoadjuvant era in patients with locally advanced rectal cancer. World J Gastrointest Oncol. 2012;4(12):230–237. doi: 10.4251/wjgo.v4.i12.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toledo LI. ATR prohibits replication catastrophe by preventing global exhaustion of RPA. Cell. 2013;155(5):1088–1103. doi: 10.1016/j.cell.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60(4):547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Krysko DV. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44(3):205–221. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 8.DeVita VT, Hellman S, Rosenberg S. 7th edn. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. Cancer, Principles & Practice of Oncology. [7th] [Google Scholar]
- 9.Stafford JH. Highly specific PET imaging of prostate tumors in mice with an iodine-124-labeled antibody fragment that targets phosphatidylserine. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0084864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belhocine T. Increased uptake of the apoptosis-imaging agent (99m)Tc recombinant human Annexin V in human tumors after one course of chemotherapy as a predictor of tumor response and patient prognosis. Clin Cancer Res. 2002;8(9):2766–2774. [PubMed] [Google Scholar]
- 11.Blankenberg FG, Strauss HW. Recent advances in the molecular imaging of programmed cell death: part I—pathophysiology and radiotracers. J Nucl Med. 2012;53(11):1659–1662. doi: 10.2967/jnumed.112.108944. [DOI] [PubMed] [Google Scholar]
- 12.Blankenberg FG, Norfray JF. Multimodality molecular imaging of apoptosis in oncology. AJR Am J Roentgenol. 2011;197(2):308–317. doi: 10.2214/AJR.11.6953. [DOI] [PubMed] [Google Scholar]
- 13.Blankenberg FG. In vivo detection of apoptosis. J Nucl Med. 2008;49(Suppl. 2):81S–95S. doi: 10.2967/jnumed.107.045898. [DOI] [PubMed] [Google Scholar]
- 14.In vivo detection of apoptosisJ Nucl Med. 2008;Suppl. 2:81S–95S. doi: 10.2967/jnumed.107.045898. [DOI] [PubMed] [Google Scholar]
- 15.Narula J. Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med. 2001;7(12):1347–1352. doi: 10.1038/nm1201-1347. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Xie J, Chen X. Peptides and peptide hormones for molecular imaging and disease diagnosis. Chem Rev. 2010;110(5):3087–3111. doi: 10.1021/cr900361p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thapa N. Discovery of a phosphatidylserine-recognizing peptide and its utility in molecular imaging of tumour apoptosis. J Cell Mol Med. 2008;12(5a):1649–1660. doi: 10.1111/j.1582-4934.2008.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weissleder R. Molecular imaging in cancer. Science. 2006;312(5777):1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 19.Schutters K, Reutelingsperger C. Phosphatidylserine targeting for diagnosis and treatment of human diseases. Apoptosis. 2010;15(9):1072–1082. doi: 10.1007/s10495-010-0503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K. In vivo imaging of tumor apoptosis using histone H1-targeting peptide. J Control Release. 2010;148(3):283–291. doi: 10.1016/j.jconrel.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Wang K. In situ dose amplification by apoptosis-targeted drug delivery. J Control Release. 2011;154(3):214–217. doi: 10.1016/j.jconrel.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 22.Kim S. Advantages of the phosphatidylserine-recognizing peptide PSP1 for molecular imaging of tumor apoptosis compared with annexin V. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmood U. Optical molecular imaging approaches in colorectal cancer. Gastroenterology. 2010;138(2):419–422. doi: 10.1053/j.gastro.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liauw SL, Connell PP, Weichselbaum RR. New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Sci Transl Med. 2013;5(173) doi: 10.1126/scitranslmed.3005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeVita, Hellman, Rosenberg's . 8th ed. 2008. Cancer: Principles and Practice of Oncology. [Google Scholar]
- 26.Glimelius B. Neo-adjuvant radiotherapy in rectal cancer. World J Gastroenterol. 2013;19(46):8489–8501. doi: 10.3748/wjg.v19.i46.8489. [DOI] [PMC free article] [PubMed] [Google Scholar]