Abstract
The high prevalence and long latency period of prostate cancer (PCa) provide a unique opportunity to control disease progression with dietary and nutraceutical approaches. We developed ProFine, a standardized composition of luteolin, quercetin, and kaempferol, and investigated its potential as a nutraceutical for PCa in preclinical models. The three ingredients of ProFine demonstrated synergistic in vitro cytotoxicity and effectively induced apoptosis in PCa cells. ProFine markedly affected the transcriptome of PCa cells, suppressed the expression of androgen receptor, and inhibited androgen-regulated genes. Oral administration of ProFine did not exhibit obvious toxicities in mice, and the three ingredients retained their individual pharmacokinetic and bioavailability profiles. Importantly, ProFine significantly retarded the growth of PCa xenografts in athymic nude mice and extended the survival of animals. This study provides preclinical evidence supporting the promise of ProFine as a safe, efficacious, and affordable intervention to control PCa progression and improve clinical outcomes.
Introduction
Prostate cancer (PCa) is the most common nonskin cancer in American men, with a lifetime risk for diagnosis of approximately 15.9%. It is estimated that 164,690 new cases are diagnosed and 29,430 patients die in 2018 [1]. Most cases of PCa are low risk and have a good prognosis, even without any treatment. Nonetheless, about 30% of PCa patients harbor a higher-grade cancer and eventually progress to metastatic and castration-resistant status, which has no cure [2]. Currently available therapies can only extend the median survival by approximately 3 months. These expensive treatments (usually ranging from $21,500 to $93,000 for a typical course of treatment) pose a huge burden on patients, their families, and the healthcare system.
The high prevalence and long latency period of PCa provide a unique opportunity to control disease progression, reduce mortality, and improve the quality of life of patients using dietary or nutraceutical approaches [3]. Numerous epidemiologic studies have indicated an important role of diet in PCa progression and therapeutic response, and dietary management of PCa is being actively pursued due to low dose-limiting toxicities and negligible side effects [4]. Promising efficacy has been reported in several trials. For example, in a double-blind, placebo-controlled randomized study, an oral capsule (Pome-T) containing a blend of pomegranate, green tea, broccoli, and turmeric demonstrated a significant short-term and favorable effect on the median prostate-specific antigen (PSA) levels in PCa patients [5]. Despite these encouraging clinical results, however, most studies using dietary supplements still suffer from low patient number, short treatment duration, and absence of proper placebo control. Importantly, the lack of standardized formulations and nonspecific effects of dietary supplements make it difficult to validate and compare their clinical efficacy among various trials. Therefore, a nutraceutical with defined composition and potent anticancer activity is highly desired to provide a safe, efficacious, and affordable therapy for early-stage and low-risk PCa.
Luteolin, quercetin, and kaempferol are among the most common flavonoids found in plants, including some vegetables and fruits that have been thought to have anticancer benefits, such as onions, olives, grapes, tea, pomegranate, broccoli, and cauliflower [6]. Epidemiological evidence has associated the dietary consumption of these flavonoids with reduced risk of developing various diseases, including cancer [7]. Molecular studies demonstrated that luteolin, quercetin, and kaempferol have diverse pharmacological activities, including antioxidant, anti-inflammatory, and anticancer effects [8]. Despite a large body of epidemiological and preclinical evidence suggesting potential preventative and therapeutic benefits of flavonoids in human cancers, very few clinical trials have been or are being conducted using pure flavonoid compounds or defined compositions. To provide a standardized formulation of luteolin, quercetin, and kaempferol for clinical evaluation of their therapeutic efficacy in PCa patients, we developed ProFine, a unique combination of the three flavonoids at a specific ratio. In this study, we determined the in vitro and in vivo activities of ProFine in preclinical models of PCa and investigated the mechanism of action of ProFine against PCa progression.
Materials and Methods
Composition of ProFine
For in vitro studies, ProFine was prepared as a stock solution of 100 mg/ml, containing 24.68 mg/ml luteolin, 26.06 mg/ml quercetin, and 49.35 mg/ml kaempferol in 100% dimethyl sulfoxide (DMSO). The composition of ProFine formulation for oral gavage administration includes inactive ingredients hydroxypropyl methylcellulose (50%, w/v), corn oil (35%, v/v), Tween 80 (5%, v/v), and ethanol (10%, v/v). Ultrasonication was used to form a yellow-colored, well-dispersed colloid.
Microarray and Gene Set Enrichment Analysis (GSEA)
Total RNAs from triplicate preparations of ProFine- and DMSO-treated C4-2 cells as well as reference total RNA samples were amplified and hybridized to Agilent 44 K whole human genome expression oligonucleotide microarray slides. Spots of poor quality or average intensity levels <300 were removed from further analysis. Analysis of Microarrays (SAM) program was used to analyze expression differences between groups using unpaired, two-sample t tests and controlled for multiple testing by estimation of q-values using the false discovery rate method. The genes were ranked according to their t test scores and used to conduct GSEA to estimate pathway enrichment. We utilized the Hallmark pathways from within the MSigDBv6.0.
In Vivo Efficacy of Oral ProFine in PCa Xenograft Models
All animal procedures were approved by Augusta University Institutional Animal Care and Use Committee. For the subcutaneous (s.c.) model, male athymic nude mice (5 weeks; Harlan Laboratories) were randomly divided into three groups (n = 5 in control group, n = 5 in 100 mg/kg ProFine group, n = 7 in 200 mg/kg ProFine group). A total of 2 × 106 C4-2-luc cells were mixed with Matrigel and inoculated subcutaneously into two flanks of each mouse. Twenty-two days following tumor inoculation, mice were treated with ProFine (100 mg/kg or 200 mg/kg) or vehicle control, three times per week, via oral gavage. Tumors were measured three times per week using a caliper, and tumor volume was calculated using the formula: (width)2 × length/2. Bioluminescence imaging of s.c. C4-2-Luc tumors was also performed.
For the intratibial model, male athymic nude mice (5 weeks) were randomized and evenly divided into two groups (n = 8 in control group, n = 9 in ProFine group). For each mouse, a total of 2.0 × 106 C4-2 cells were inoculated into bilateral tibia. Tumor-bearing mice were treated with ProFine (100 mg/kg) or vehicle control, three times per week, via oral gavage. Mice were weighed twice per week, and tumor growth in bilateral tibia was followed by serum PSA once a week. At the end point, X-ray radiography was performed using MX-20 System (Faxitron, Tucson, AZ).
Statistics
Two-way analysis of variance was performed to test the overall difference across the control and treatment groups during the entire study period. The effects on animal survival were determined by log-rank survival test. Errors are SE values of averaged results, and values of P < .05 were taken as a significant difference between means. All in vitro data represent three or more experiments. GraphPad Prism 7.0 program (GraphPad Software Inc., La Jolla, CA) was used to perform the statistical analyses.
Results
In Vitro Cytotoxicity of ProFine in PCa Cells
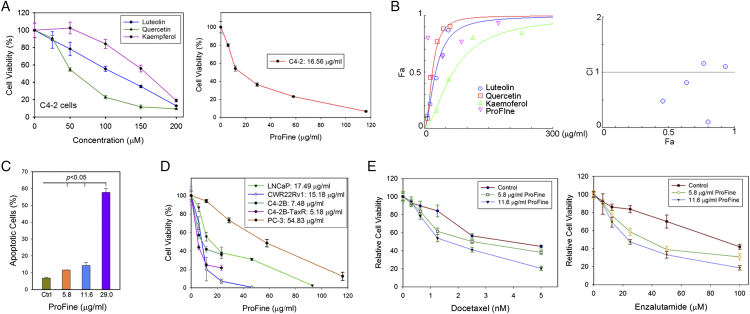
ProFine is a defined composition consisting of luteolin, quercetin, and kaempferol at the molar ratio of 1:1:2. In vitro cytotoxicity assays found that, as single compounds, luteolin, quercetin, and kaempferol have weak to modest activities in PCa cells. For example, the half minimal inhibitory concentration (IC50) of luteolin, quercetin, and kaempferol in androgen receptor (AR)-positive C4-2 cells is 114.02, 55.25, and 157.81 μM, respectively. In comparison, ProFine exhibited enhanced cytotoxicity compared to any of the three individual components, with the IC50 of 16.56 μg/ml in C4-2 cells (equivalent to 14.28, 14.28, and 28.60 μM of luteolin, quercetin, and kaempferol, respectively) (Figure 1A). Indeed, isobologram analysis showed that the combination index (CI) achieved as low as 0.11 when the three ingredients were used at low concentrations, indicating a strong synergy among them (Figure 1B; Table S1). Fluorescence-activated cell sorting analysis demonstrated that ProFine dose-dependently increased the surface expression of Annexin V, suggesting that ProFine effectively induced apoptosis in PCa cells (Figure 1C). Significantly, ProFine exhibited potent cytotoxicity in a panel of AR-positive PCa cell lines (IC50 ranging between 5.18 and 17.49 μg/ml), including androgen-dependent (LNCaP), androgen-independent (C4-2B, CWR22Rv1), and docetaxel-resistant (C4-2B-TaxR) cells. In comparison, ProFine was less cytotoxic in AR-negative PCa cells, such as PC-3 cells (Figure 1D). The presence of low concentrations of ProFine also significantly enhanced the in vitro cytotoxicity of docetaxel and enzalutamide in C4-2 cells (Figure 1E).
Figure 1.
In vitro cytotoxicity of ProFine in PCa cells. (A) Left: MTS cell proliferation assay of the in vitro cytotoxicity of luteolin, quercetin, and kaempferol in C4-2 cells (72 hours); right: MTS assay of the in vitro cytotoxicity of ProFine in C4-2 cells (72 hours). (B) CompuSyn analysis of the synergistic effect among the ingredients of ProFine. Left: Dose-effect curve of ProFine and the three individual ingredients in C4-2 cells; right: combination index plot of ProFine in C4-2 cells. Fa: fraction affected; CI: combination index. (C) Fluorescence-activated cell sorting analysis of Annexin V expression in C4-2 cells treated with varying concentrations of ProFine (48 hours). (D) MTS assay of the in vitro cytotoxicity of ProFine in PCa cell lines (72 hours); (E) left: MTS assay of the in vitro cytotoxicity of docetaxel in the presence of varying concentrations of ProFine in C4-2 cells (72 hours); right: MTS assay of the in vitro cytotoxicity of enzalutamide in the presence of varying concentrations of ProFine in C4-2 cells (72 hours).
ProFine Affects Multiple Genes
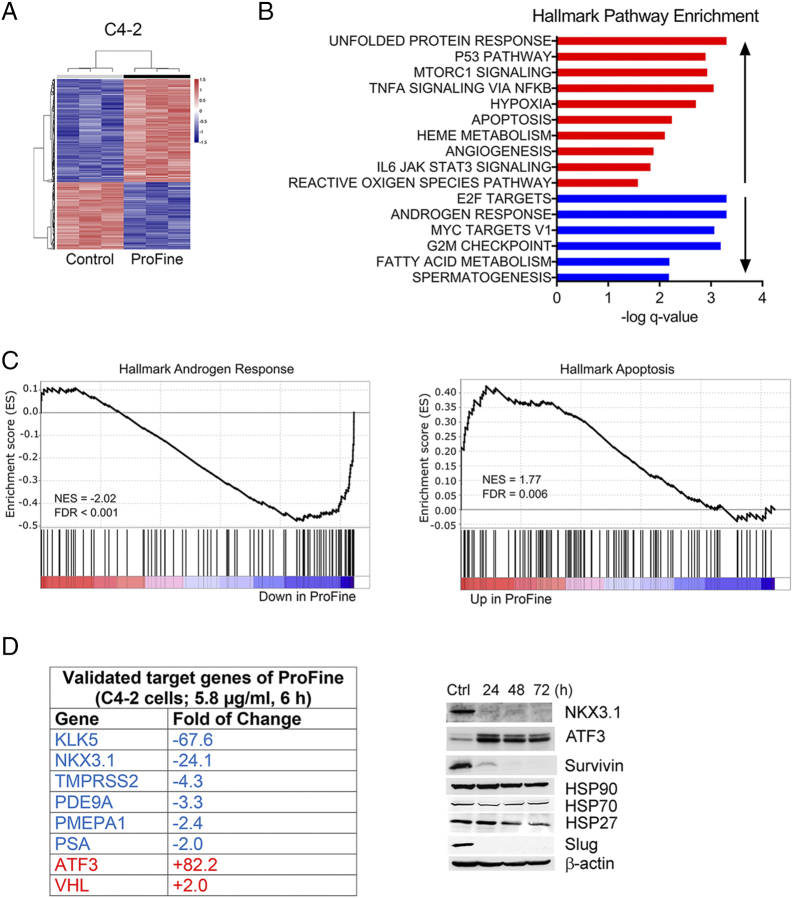
To identify potential molecular targets of ProFine, C4-2 cells were treated with ProFine at a low concentration of 5.8 μg/ml (equivalent to 5.0 μM luteolin, 5.0 μM quercetin, and 10.0 μM kaempferol) for 6 hours, and total RNAs were collected for microarray analysis. The data showed that 656 genes were significantly upregulated and 428 genes were significantly downregulated (q-value < 0.0001) (Figure 2A). GSEA found that ProFine affected multiple pathways (Figure 2B). Among them, androgen-regulated genes were negatively enriched, whereas apoptosis-related genes were positively enriched, with a false discovery rate less than 0.01 (Figure 2C). Quantitative real-time PCR analyses were further performed to validate the selected genes (Figure 2D, left). Expression of several androgen-responsive genes, including kallikrein related peptidase 5 (KLK5) [9], [10], NK3 homeobox 1 (NKX3.1) [11], TMPRSS2 [12], kallikrein related peptidase 3 (KLK3, also known as PSA) [13], prostate transmembrane protein, and androgen induced 1 (PMEPA1) [14], was significantly suppressed upon ProFine treatment. ProFine also markedly increased the mRNA expression of activating transcription factor 3 (ATF3) and Von Hippel–Lindau (VHL), two repressors of AR transcriptional activity in PCa cells [15], [16]. Western blot analysis confirmed the significant changes in the protein expression of NKX3.1 and ATF3. Expression of survivin, a crucial survival factor that is implicated in PCa progression and therapeutic resistance [17], was considerably suppressed. ProFine treatment also led to a significant reduction in the protein expression of Slug, a unique androgen-regulated zinc-finger transcription factor involved in AR transactivation and PCa metastasis [18] (Figure 2D, right).
Figure 2.
ProFine affects multiple genes in PCa cells. (A) Heat map of C4-2 transcriptome following the treatment with ProFine (5.8 μg/ml, 6 hours) or control (DMSO). Total RNAs were extracted from triplicate preparations. (B) Hallmark pathway gene set enrichment analysis of C4-2 cells treated with ProFine (5.8 μg/ml, 6 hours). (C) Pathway enrichment plots of androgen-responsive and apoptosis-related genes, respectively, in C4-2 cells treated with ProFine (5.8 μg/ml, 6 hours) or DMSO. (D) Selected target genes of ProFine in C4-2 cells, as validated by quantitative real-time PCR (left) and Western blot analyses (right, ProFine: 11.6 μg/ml; Ctrl: DMSO).
ProFine Targets AR Signaling
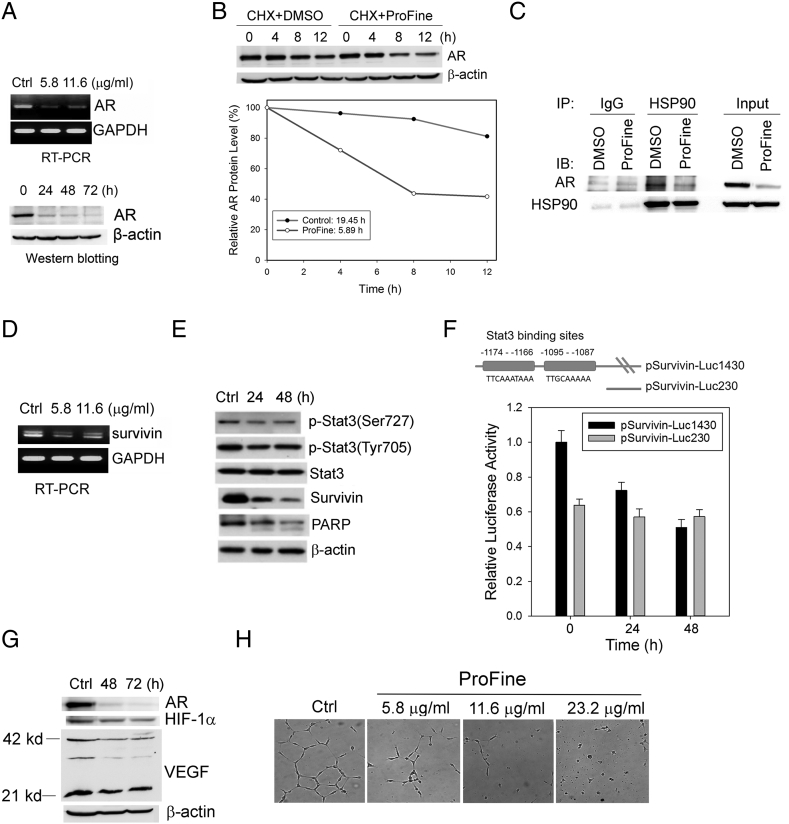
The effects of ProFine on androgen-responsive genes suggested that ProFine may interfere with AR signaling in PCa cells. Indeed, ProFine effectively inhibited AR expression at both the mRNA and protein levels (Figure 3A). Since there was a rapid reduction in AR protein level following ProFine treatment, we postulated that posttranslational regulation may be a major mechanism by which ProFine inhibits AR expression. To that end, we determined the half-life of AR protein in PCa cells treated with ProFine or vehicle control. In the presence of cycloheximide (CHX), an inhibitor of de novo protein synthesis, ProFine treatment significantly reduced the half-life of AR protein from 19.45 to 5.89 hours (Figure 3B). These results indicated that ProFine may promote AR degradation in a proteasome-dependent manner.
Figure 3.
ProFine inhibits AR and survivin signaling in PCa cells. (A) RT-PCR (upper, 24 hours treatment) and Western blot analyses (bottom, 11.6 μg/ml) of AR expression in C4-2 cells treated with ProFine. (B) Upper: Western blot analysis of AR protein expression in C4-2 cells pretreated with CHX (50 μg/ml, 1 hour) and further treated with ProFine (11.6 μg/ml) or DMSO for the indicated times. Lower: Plot of AR protein degradation in C4-2 cells treated with ProFine or DMSO. (C) Western blot analysis of AR protein expression in HSP90 immunoprecipitates from C4-2 cells treated with ProFine (11.6 μg/ml, 24 hours) or DMSO. (D) RT-PCR analysis of survivin expression in C4-2 cells treated with ProFine (24 hours); (E) Western blot analysis of p-Stat3, Stat3, survivin, and PARP in C4-2 cells treated with ProFine (11.6 μg/ml) at indicated times. (F) Upper: Schematic diagram of the Stat3 cis-elements in human survivin promoter; lower: luciferase activity of human survivin promoters in C4-2 cells treated with ProFine (11.6 μg/ml). (G) Western blot analysis of protein expression of AR, HIF-1α, and VEGF in C4-2 cells treated with ProFine (11.6 μg/ml). (H) In vitro tube formation of HUVECs treated with varying concentrations of ProFine (72 hours).
Several heat-shock proteins (HSPs), including HSP90, function as chaperones of AR protein and protect it from degradation [19]. Western blot analyses showed that ProFine only marginally reduced the protein expression of HSP90 in C4-2 cells (Figure 2D). We further performed co-immunoprecipitation experiments and found that the presence of AR protein in the HSP90 precipitates was significantly lower than that in the IgG precipitates (Figure 3C), indicating that ProFine may promote the disassociation of AR and HSP90 and subsequently subject AR protein to the degradation machinery.
ProFine Inhibits Survivin Transcription
ProFine treatment significantly reduced the expression of survivin at both mRNA and protein levels (Figure 3, D and E). At the posttranslational level, the stability of survivin protein is controlled by the ubiquitin-proteasome pathway [20]. We first examined the effect of ProFine on survivin protein expression in the presence of CHX. Unlike its effect on AR protein stability, however, ProFine treatment did not induce protein degradation of survivin (Figure S1), suggesting that the suppression of survivin expression may occur mainly at the RNA level. An examination of potential upstream regulators of survivin transcription showed that ProFine inhibited the phosphorylation of signal transducer and activator of transcription 3 (Stat3) at Serine 727 (Figure 3E). Indeed, it has been demonstrated that two cis-binding elements (located between −1174 and −1166 and between −1095 and −1087) in the human survivin promoter are critical to Stat3-dependent survivin transcription [21]. A promoter assay using a human survivin reporter (pSurvivin-Luc1430) showed that ProFine effectively inhibited survivin transcription but had a negligible effect on the reporter activity of pSurvivin-Luc230, a deletion mutant of pSurvivin-Luc1430 that does not contain two putative Stat3-binding elements (Figure 3F). These results indicate that ProFine may inhibit survivin transcription via Stat3-dependent signaling.
ProFine Inhibits Tube Formation of Human Umbilical Vein Endothelial Cells (HUVECs)
PCa cells are usually proangiogenic and express high levels of vascular endothelial cell growth factor (VEGF) [22]. Previous studies have found that luteolin, quercetin, and kaempferol can have an inhibitory effect on VEGF expression via hypoxia inducible factor 1α (HIF-1α) [23], [24]. In C4-2 cells, however, Western blotting showed that ProFine treatment only marginally inhibited the expression of HIF-1α and VEGF (Figure 3G). On the other hand, ProFine effectively inhibited the in vitro formation of capillary-like tubes on a basement membrane matrix by HUVECs (Figure 3H). These results suggested that ProFine does not affect VEGF expression in PCa cells but may act as an inhibitor of angiogenesis by directly targeting endothelial cells.
Formulation and Acute Toxicity of Oral ProFine
In a “proof-of-concept” study, intraperitoneal injection of ProFine (50 mg/kg, 3 times per week) significantly inhibited the s.c. growth of C4-2-Luc xenografts, as demonstrated by reduced tumor burden and serum PSA level in the ProFine treatment group (Figure S2, A-C). ProFine treatment also significantly extended the survival of animals (Figure S2D), without obvious toxicities (Figure S3A).
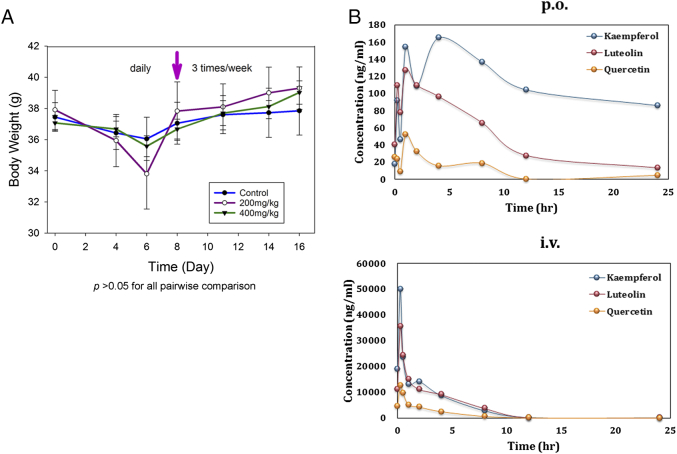
We further developed a corn oil–based formulation of ProFine that can be administered via oral gavage in animals and serve as a prototype for nutraceutical manufacturing. The repeated-dose toxicity of daily oral ProFine (at 200 and 400 mg/kg) was tested in healthy, male CD-1 mice. Body weights in the ProFine and vehicle control groups were found to be all reduced during the first week, probably from the stress of daily oral administration on the animals. However, when the schedule of administration changed to three times per week, all mice gained weight (Figure 4A). Complete blood count and chemistry panel on liver and kidney functions did not show obvious abnormalities, and major organs appeared normal (data not shown). These results indicated that oral ProFine is a safe regimen in rodent models when administered at high doses up to 400 mg/kg.
Figure 4.
In vivo toxicity and pharmacokinetics of ProFine in rodent models. (A) Average body weights of CD-1 mice treated with ProFine or vehicle control (n = 5 per group) via oral gavage, daily for the first week, then three times per week for the second week (arrow indicates the time of schedule change). (B) Plasma levels of luteolin, quercetin, and kaempferol at the indicated times in rats administered with ProFine via oral gavage (p.o.) or tail vein injection (i.v.). n = 3 per group.
Pharmacokinetics and Bioavailability of Oral ProFine Formulation
Flavonoids are well known for their lack of drug-like physicochemical properties such as poor bioavailability, which partially contributes to the limited success in chemoprevention studies [25]. We determined the pharmacokinetic parameters and bioavailability of the three individual constituents of ProFine in Sprague-Dawley rats. Figure 4B showed the mean concentration-time profiles of the three flavonoids in rat plasma after oral gavage or intravenous (i.v.) administration. The pharmacokinetic results (Table S2) showed rapid absorption for luteolin and quercetin, and a slower rate of absorption for kaempferol. Considering the administration concentrations, luteolin and kaempferol were absorbed more efficiently than quercetin in rat plasma. Compared with the i.v. route, the oral administration of ProFine resulted in a bioavailability of 1.30%, 0.91%, and 2.89% for luteolin, quercetin, and kaempferol, respectively. These results are in agreement with the reported bioavailability data of the three flavonoids after oral administration in rodent models [26], [27], [28], suggesting that co-administration of the three ingredients did not significantly alter their in vivo absorption and stability in the circulation.
In Vivo Effect of Oral ProFine on the s.c. Growth of C4-2-Luc Tumors and Animal Survival
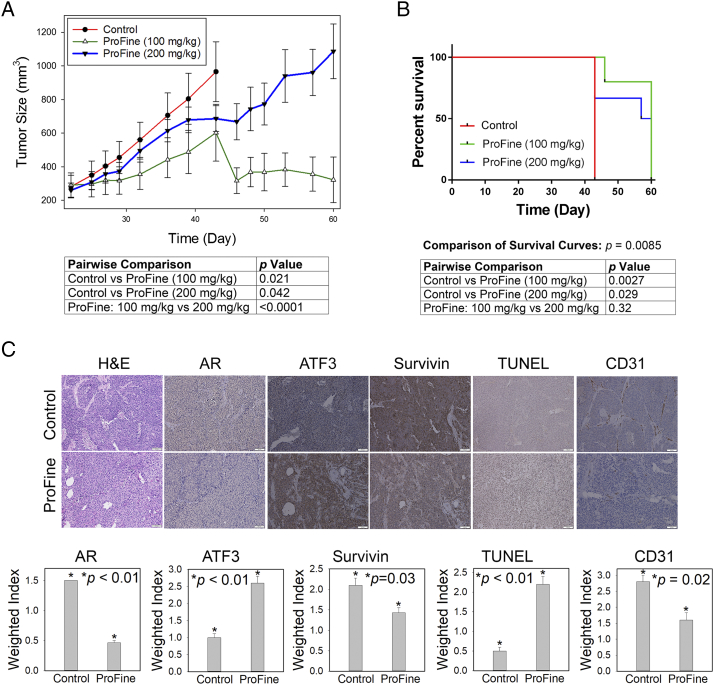
Two doses of ProFine (100 and 200 mg/kg) or vehicle control were administered via the oral route to male athymic nude mice carrying s.c. xenografts of C4-2-Luc cells (Figure S4). Tumor sizes were measured as the primary indicator of tumor growth and response to treatments (Figure 5A). On day 43 following tumor inoculation, all mice in the control group had to be euthanized because their tumors exceeded the allowable sizes. ProFine treatment was continued until day 60. Statistical analyses showed that there was a significant interaction between time and treatments, and the tumor sizes in the ProFine groups were significantly reduced with treatment time. Compared with the control, treatment with ProFine at both doses significantly inhibited the growth of C4-2-Luc tumors. Two-way analysis of variance indicated that both doses resulted in a significant regression of tumors until day 43. Interestingly, the 100-mg/kg dose more effectively reduced the C4-2-Luc tumor burden than the 200-mg/kg dose (Figure 5A). The median survival time of animals in each group was determined to be 43 days (control), 60 days (100 mg/kg ProFine), and 58.5 days (200 mg/kg ProFine), respectively. Compared with the vehicle control, both doses of ProFine significantly extended the survival of tumor-bearing mice, as determined by log-rank test. There was no significant difference in the survival of animals treated with two doses of ProFine (Figure 5B). Comparison of the body weights in the three treatment groups showed slightly reduced body weights of tumor-bearing mice with ProFine treatment at both doses (Figure S3B), although no obvious adverse effects on animal behaviors were observed. Ex vivo examination of the major organs did not find significant abnormalities.
Figure 5.
In vivo effect of oral ProFine on the subcutaneous growth of C4-2-Luc tumors in athymic nude mice. (A) Tumor size and pairwise comparison of C4-2-Luc xenografts treated with ProFine (100 mg/kg or 200 mg/kg) or vehicle control at the indicated times. (B) Log-rank survival curve and pairwise comparison of C4-2-Luc tumor-bearing animals treated with ProFine or vehicle control. (C) Upper: H&E, IHC staining of putative ProFine targets, and TUNEL expression in C4-2-Luc xenograft tissues. Scale bar: 100 μm. Lower: quantitation of IHC and TUNEL expression. Weighted index was calculated as the average (intensity × percentage of positive cells) from three random tissue areas. P values were calculated using Student's t test.
The in vivo effects of ProFine on the tissue expression of several putative targets were analyzed with immunohistochemistry (IHC) in C4-2-Luc tumor specimens harvested at the specified end points. Compared with the control group, the 100 mg/kg ProFine–treated group showed reduced expression of AR and survivin but increased expression of ATF3. Consistently, ProFine treatment showed increased staining of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), indicating activation of apoptosis. Significantly, the expression of CD31, an indicator of angiogenesis, and the presence of tumor-associated microvessels were reduced upon ProFine treatment (Figure 5C). These data indicate that oral administration of ProFine was effective in affecting the in vivo expression of selected target genes, inducing apoptosis and inhibiting tumor growth and angiogenesis.
In Vivo Effect of Oral ProFine on the Skeletal Growth of C4-2-Luc Tumors
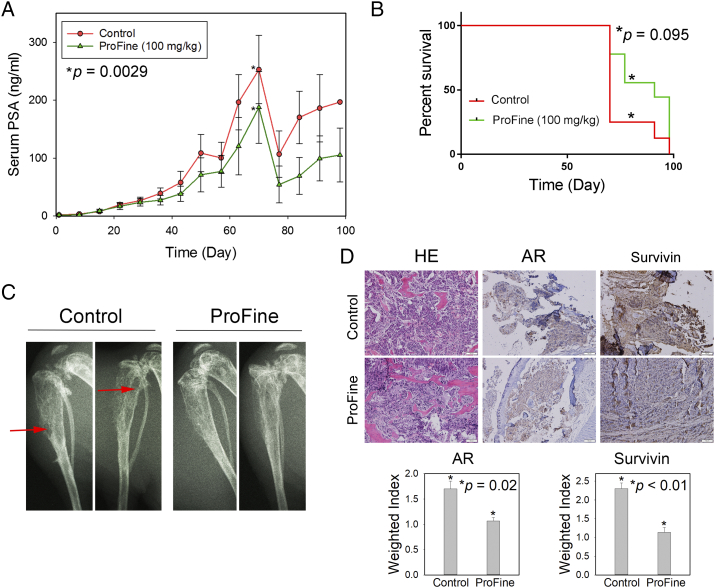
Previous studies have shown that flavonoids have physiological effects on bone mineral density and bone metabolism, stimulating osteoblast activities and inhibiting bone loss [29], [30], [31]. To test whether ProFine has therapeutic benefits in treating bone metastasis, a major cause of PCa morbidity and mortality, we evaluated the in vivo efficacy of oral ProFine in the intratibial model of C4-2-Luc cells. Statistical analysis showed that the average PSA expression in the ProFine group was significantly reduced with treatment time. There was a significant difference in PSA values between the two groups of mice, with the average PSA level at the end point being 196.84 ± 0.0 ng/ml (control) and 105.21 ± 46.67 ng/ml (ProFine), respectively (Figure 6A). ProFine treatment was also associated with higher body weights at the end point (Figure S3C). The median survival time of animals in each group was determined as 70 days (control) and 91 days (100 mg/kg ProFine). However, in contrast to the observed effects in the subcutaneous model, there was no significant difference in the survival rates between the control and ProFine groups (Figure 6B). ProFine treatment resulted in reduced osteolytic lesions and improved bone architecture in mice bearing C4-2-Luc tumors (Figure 6C). IHC studies demonstrated that ProFine reduced the expression of two major targets, i.e., AR and survivin, in C4-2-Luc bone tumors (Figure 6D). Taken together, these results indicated that, as a monotherapy, ProFine retards the progression of bone metastatic PCa and improves pathological characteristics (such as bone architecture) but does not demonstrate survival benefits in tumor-bearing mice.
Figure 6.
In vivo effect of oral ProFine on the intratibial growth of C4-2-Luc tumors in athymic nude mice. (A) Serum PSA levels in C4-2-Luc tumor-bearing mice treated with ProFine (100 mg/kg) or vehicle control. (B) Log-rank survival curve of C4-2-Luc tumor-bearing animals treated with ProFine or vehicle control. (C) X-ray radiography of C4-2-Luc tumor-bearing tibias of mice treated with ProFine or vehicle control, collected at end points. Red arrows: osteolytic lesions. (D) Upper: H&E and IHC staining of AR and survivin expression in C4-2-Luc bone tumors. Scale bar: 100 μm. Lower: Quantitation of IHC expression of AR and survivin in bone tumors. P values were calculated using Student's t test.
Discussion
Epidemiological studies have suggested an association between the dietary consumption of luteolin, quercetin, and kaempferol and reduced risk of human cancers [32]. A recent meta-analysis of multiple case-control and cohort studies revealed that for the three flavonoids combined, there is a significant reduction of overall risk of lung, colorectal, gastric, breast, ovarian, endometrial, renal, and pancreatic cancers [33]. On the other hand, these flavonoids may affect cancer risk differentially, and epidemiological evidence of their effects in certain cancer types (such as PCa) remains inconsistent and inconclusive [33], [34]. In this study, we sought to develop a defined nutraceutical composition of the three flavonoids for further evaluation of their clinical benefits in PCa management. Our results demonstrate that ProFine exhibits potent cytotoxicity in PCa cells and targets multiple genes implicated in PCa progression, including AR and survivin. In rodent models, ProFine is biologically available and does not exhibit significant toxicity and side effects. Significantly, oral administration of ProFine effectively retards the in vivo growth of PCa and extends the survival of tumor-bearing animals.
The in vitro and in vivo effects of luteolin, quercetin, and kaempferol as single agents have been, respectively, evaluated in various experimental models of human cancers [25]. However, their anticancer activities appear to be suboptimal in most studies [35], [36], [37], [38]. These observations point to a need to improve the anticancer efficacy of flavonoids for further translational application [25], [39]. We have utilized a strategy of “combinational treatment” and formulated ProFine as a specific mixture of luteolin, quercetin, and kaempferol, with the expectation to achieve a complementary and synergistic interference with multiple oncogenic signals implicated in PCa pathogenesis. Indeed, the combination synergistically enhanced the in vitro cytotoxicities of the three individual components, as demonstrated by the isobologram analysis (Figure 1, A and B, Table S1). Consistently, the intraperitoneal injection of ProFine at 50 mg/kg significantly suppressed the s.c. growth of C4-2-luc tumors (Figure S2). The equivalent amounts of luteolin, quercetin, and kaempferol at this dosage are 12.34, 13.03, and 24.68 mg/kg, respectively, which are markedly lower than those used as single agents in other studies (50-150 mg/kg) [35], [36], [37], [38]. Impressively, oral administration of ProFine at a low dose of 100 mg/kg effectively inhibited tumor growth and extended the survival of tumor-carrying mice (Figure 5). These data demonstrated that the combinational approach could improve the anticancer efficacy of these natural compounds without increasing dosage.
As a group of closely related polyphenolic metabolites, luteolin, quercetin, and kaempferol exert their anticancer activities by inducing cell cycle arrest, activating apoptosis, and inhibiting migratory and invasive capabilities [8]. Our microarray studies showed that at a low concentration of 5.8 μg/ml, ProFine significantly inhibited AR-dependent genes, which may contribute to the potent activity in AR-positive PCa cells (Figure 1D). Given the central role of AR signaling in PCa progression, we further performed detailed studies to understand the mechanism by which ProFine suppresses AR expression and activity. The results showed that, at the protein levels, ProFine may promote AR protein degradation by interfering in the physical interaction between AR and HSP90 (Figure 3, B and C). This is particularly interesting since it has been demonstrated that luteolin can directly bind HSP90 and promote the protein degradation of HSP90-interacting partners, such as p-Stat3(Ser727) [40]. Therefore, it is plausible that the luteolin component of ProFine may play a major role in interfering the HSP90-AR interaction and facilitating AR protein degradation. At the transcriptional level, ProFine effectively inhibited AR mRNA in PCa cells (Figure 3A). Although the exact mechanism remains to be delineated, a possible clue is that ProFine can suppress the expression of HSP27 (Figure 2D), a factor implicated in the transcriptional regulation of AR [41]. Interestingly, it has been shown that quercetin is capable of decreasing HSP27 in breast cancer cells [42]. ProFine also significantly increased the expression of the AR suppressor ATF3 and reduced the expression of the AR coactivators p-Stat3 and Slug [15], [18], [43]. Taken together, these results indicated that ProFine has a profound impact on AR signaling, a prominent target for PCa therapy. These molecular effects, in addition to the inhibition of Stat3-survivin signaling and angiogenesis, may collectively contribute to the potent anticancer activities of ProFine in cellular and animal models (Figure S5).
Naturally occurring flavonoids are extensively metabolized on ingestion and affected by phase 2 metabolism, therefore usually having low bioavailability [44]. Interestingly, the circulating concentrations of many flavonoids have been reported in the range of 1-5 μM, whereas most in vitro studies used concentrations of 50-100 μM in cell culture [45], [46]. In our studies, the in vitro concentrations of the three flavonoids are 5-10 μM (luteolin), 5-10 μM (quercetin), and 10-20 μM (kaempferol), respectively. Following oral gavage administration of ProFine at the dose of 100 mg/kg, the maximum plasma concentrations of these flavonoids in rats are 0.44 μM (127.33 ng/ml, luteolin), 0.17 μM (51.94 ng/ml, quercetin), and 0.57 μM (164.85 ng/ml, kaempferol) (Table S1), which are consistent with the reported bioavailability [26], [27], [28]. These observations indicate that the in vitro effects of pure compounds or active ingredients may not directly reflect the physiology levels and in vivo effects of the components, and caution must be taken when trying to make a correlation between the in vitro cytotoxicity and in vivo efficacy of ProFine or its flavonoid ingredients in animals or humans.
Flavonoids are generally thought to be safe in animals and humans, as supported by a large body of preclinical and clinical observations. The human daily consumption of flavonoids ranges from 23 to 1000 mg, depending on the sources of foods [25]. Specifically, the acute oral toxicity (LD50) of luteolin in rats has been calculated as >5000 mg/kg. Quercetin is on the FDA Generally Recognized as Safe list, and human daily intakes of up to 1000 mg for several months have produced no adverse effects. Oral administration of kaempferol at a daily dose of 200 mg/kg did not result in any toxicity in rats. These results are consistent with our study in which oral ProFine at doses up to 400 mg/kg did not have obvious adverse effects in rodents (Figure 4A). The therapeutic dosage of 100 mg/kg used in our study is equivalent to 8.3 mg/kg (or 308 mg/m2) in adult humans, which falls well within the safe dosage range of dietary flavonoids and can be readily translated into clinical testing in humans.
Optimal dose selection has been a significant challenge in oncology trials. Traditionally, the maximum tolerated dose is used to determine the dosage for clinical phases. However, accumulating evidence indicates that the maximum tolerated dose approach is likely associated with unnecessarily high doses, thereby increasing toxicity and compromising efficacy [47]. This complex, nonlinear dose-response relationship of investigational drugs has been observed in multiple human and animal studies. Of particular interest, the high-dose intakes of dietary supplements have shown little or even harmful effects in clinical trials, such as the Selenium and Vitamin E Cancer Prevention Trial [48]. These disappointing data have raised serious concerns over the assumption that high-dose use of antioxidants, such as vitamin E, β-carotene, and other natural compounds, can prevent cancer initiation and progression by scavenging free radicals. In fact, recent studies have challenged the notion of “more is better” and suggested that low-dose use of antioxidants may be more beneficial in chemoprevention, presumably by maintaining certain levels of reactive oxidant species and triggering cellular defense mechanisms [49], [50]. Intriguingly, our results showed that low concentrations of ProFine exhibited significant effects on the expression of multiple targets at the molecular level, and demonstrated stronger synergy in cytotoxicity assays when the three components were all used at low doses (Figure 1B, Table S1). Further, low-dose (100 mg/kg) administration of ProFine is more effective than high-dose (200 mg/kg) treatment in suppressing tumor growth in animal studies (Figure 5A). These observations are in line with the concept of “less is better” or hormesis in cancer therapy, which proposes that low-dose treatment can elicit better efficacy than high dosage [51]. Given the fact that the three ingredients of ProFine are well-known antioxidants [7], it is plausible that the greater anticancer activity of low-dose ProFine compared to the high dose may have a similar mechanistic basis to that of other natural compounds, such as resveratrol in colorectal cancer [49]. With the identification of putative targets of ProFine (e.g., AR, ATF3, survivin), it is possible to more precisely evaluate the dose-dependent effect on tumor progression and provide a basis for defining safe and biologically efficacious doses for clinical trials [47].
Several large-scale and long-term follow-up studies found that in patients with low-risk PCa, there are no significant differences in mortality between men who underwent radical prostatectomy and those treated with observation or active monitoring only [52], [53]. These studies support the expanding use of active surveillance as a major approach for the management of low-risk PCa. On the other hand, patients receiving observation or active surveillance have a higher frequency of treatment for disease progression, and among them, those with intermediate-risk PCa are at higher risk for progression to metastatic status and mortality [2]. Clearly, there is a need to develop new interventions to retard disease progression, prevent the occurrence of metastasis, and improve the quality of life in patients with low- to intermediate-risk PCa. Interestingly, in addition to its anticancer activity against the in vivo growth of PCa, ProFine significantly inhibited the in vitro migration and invasion of PCa cells in “wound-healing” and Transwell studies (Figure S6), indicating that ProFine may suppress or retard the metastatic spread of PCa cells, the major cause of PCa mortality. Currently, we are planning a phase I/II trial to evaluate the safety and efficacy of ProFine in the clinical setting. These future studies, in addition to the evidence presented here, will support the promise of ProFine as a safe, efficacious, and affordable nutraceutical with standardized composition to control PCa progression, reduce metastasis, and improve clinical outcomes.
Footnotes
This work was supported by National Cancer Institute grants 1R41CA186498-01A1, 1R41CA206725-01A1, and 1R41CA217491-01A1; Georgia Cancer Center Startup Fund (D.W.), and University of Georgia-Augusta University Cancer Research Initiative Award (D.W. and M.G.B.).
Conflicts of Interest: D. W. has ownership interests (including patent and trademark) in MetCure Therapeutics LLC. No potential conflicts of interest were disclosed by the other authors.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2018.06.003.
Appendix A. Supplementary data
Supplementary Materials
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Van der Kwast TH, Roobol MJ. Defining the threshold for significant versus insignificant prostate cancer. Nat Rev Urol. 2013;10:473–482. doi: 10.1038/nrurol.2013.112. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute online publication; Bethesda (MD): 2002. Prostate Cancer, Nutrition, and Dietary Supplements (PDQ(R)): Health Professional Version. ( https://www.cancer.gov/about-cancer/treatment/cam/hp/prostate-supplements-pdq) [Google Scholar]
- 4.Kallifatidis G, Hoy JJ, Lokeshwar BL. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin Cancer Biol. 2016;40-41:160–169. doi: 10.1016/j.semcancer.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas R, Williams M, Sharma H, Chaudry A, Bellamy P. A double-blind, placebo-controlled randomised trial evaluating the effect of a polyphenol-rich whole food supplement on PSA progression in men with prostate cancer-the UK NCRN Pomi-T study. Prostate Cancer Prostatic Dis. 2014;17:180–186. doi: 10.1038/pcan.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozlowska A, Szostak-Wegierek D. Flavonoids—food sources and health benefits. Rocz Panstw Zakl Hig. 2014;65:79–85. [PubMed] [Google Scholar]
- 7.Hollman PC, Katan MB. Health effects and bioavailability of dietary flavonols. Free Radic Res. 1999;31 Suppl:S75–S80. doi: 10.1080/10715769900301351. [DOI] [PubMed] [Google Scholar]
- 8.Weng CJ, Yen GC. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012;31:323–351. doi: 10.1007/s10555-012-9347-y. [DOI] [PubMed] [Google Scholar]
- 9.Korbakis D, Gregorakis AK, Scorilas A. Quantitative analysis of human kallikrein 5 (KLK5) expression in prostate needle biopsies: an independent cancer biomarker. Clin Chem. 2009;55:904–913. doi: 10.1373/clinchem.2008.103788. [DOI] [PubMed] [Google Scholar]
- 10.Mavridis K, Talieri M, Scorilas A. KLK5 gene expression is severely upregulated in androgen-independent prostate cancer cells after treatment with the chemotherapeutic agents docetaxel and mitoxantrone. Biol Chem. 2010;391:467–474. doi: 10.1515/BC.2010.026. [DOI] [PubMed] [Google Scholar]
- 11.Bieberich CJ, Fujita K, He WW, Jay G. Prostate-specific and androgen-dependent expression of a novel homeobox gene. J Biol Chem. 1996;271:31779–31782. doi: 10.1074/jbc.271.50.31779. [DOI] [PubMed] [Google Scholar]
- 12.Lin B, Ferguson C, White JT, Wang S, Vessella R, True LD, Hood L, Nelson PS. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999;59:4180–4184. [PubMed] [Google Scholar]
- 13.Young CY, Montgomery BT, Andrews PE, Qui SD, Bilhartz DL, Tindall DJ. Hormonal regulation of prostate-specific antigen messenger RNA in human prostatic adenocarcinoma cell line LNCaP. Cancer Res. 1991;51:3748–3752. [PubMed] [Google Scholar]
- 14.Xu LL, Shanmugam N, Segawa T, Sesterhenn IA, McLeod DG, Moul JW, Srivastava S. A novel androgen-regulated gene, PMEPA1, located on chromosome 20q13 exhibits high level expression in prostate. Genomics. 2000;66:257–263. doi: 10.1006/geno.2000.6214. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Yan C. Emerging roles of ATF3 in the suppression of prostate cancer. Mol Cell Oncol. 2016;3 doi: 10.1080/23723556.2015.1010948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Zhang W, Ji W, Liu X, Ouyang G, Xiao W. The von hippel-lindau protein suppresses androgen receptor activity. Mol Endocrinol. 2014;28:239–248. doi: 10.1210/me.2013-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo SI, Gera L, Zhau HE, Qian WP, Iqbal S, Johnson NA, Zhang S, Zayzafoon M, Stewart J, Wang R. BKM1740, an acyl-tyrosine bisphosphonate amide derivative, inhibits the bone metastatic growth of human prostate cancer cells by inducing apoptosis. Clin Cancer Res. 2008;14:6198–6206. doi: 10.1158/1078-0432.CCR-08-1023. [DOI] [PubMed] [Google Scholar]
- 18.Wu K, Gore C, Yang L, Fazli L, Gleave M, Pong RC, Xiao G, Zhang L, Yun EJ, Tseng SF. Slug, a unique androgen-regulated transcription factor, coordinates androgen receptor to facilitate castration resistance in prostate cancer. Mol Endocrinol. 2012;26:1496–1507. doi: 10.1210/me.2011-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Li J, Farah E, Sarkar S, Ahmad N, Gupta S, Larner J, Liu X. Cotargeting HSP90 and its client proteins for treatment of prostate cancer. Mol Cancer Ther. 2016;15:2107–2118. doi: 10.1158/1535-7163.MCT-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Tenev T, Martins LM, Downward J, Lemoine NR. The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J Cell Sci. 2000;113(Pt 23):4363–4371. doi: 10.1242/jcs.113.23.4363. [DOI] [PubMed] [Google Scholar]
- 21.Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 22.de Brot S, Ntekim A, Cardenas R, James V, Allegrucci C, Heery DM, Bates DO, Odum N, Persson JL, Mongan NP. Regulation of vascular endothelial growth factor in prostate cancer. Endocr Relat Cancer. 2015;22:R107–R123. doi: 10.1530/ERC-15-0123. [DOI] [PubMed] [Google Scholar]
- 23.Luo H, Jiang BH, King SM, Chen YC. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr Cancer. 2008;60:800–809. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]
- 24.Lee DH, Lee YJ. Quercetin suppresses hypoxia-induced accumulation of hypoxia-inducible factor-1alpha (HIF-1alpha) through inhibiting protein synthesis. J Cell Biochem. 2008;105:546–553. doi: 10.1002/jcb.21851. [DOI] [PubMed] [Google Scholar]
- 25.Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. 2014;8:122–146. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Liu L, Sun Z, Liu Y, Xu J, Liu S, Huang B, Ma L, Yu Z, Bi K. Pharmacokinetics of luteolin and tetra-acetyl-luteolin assayed by HPLC in rats after oral administration. Biomed Chromatogr. 2010;24:826–832. doi: 10.1002/bmc.1370. [DOI] [PubMed] [Google Scholar]
- 27.Dong X, Lan W, Yin X, Yang C, Wang W, Ni J. Simultaneous determination and pharmacokinetic study of quercetin, luteolin, and apigenin in rat plasma after oral administration of Matricaria chamomilla L. extract by HPLC-UV. Evid Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/8370584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barve A, Chen C, Hebbar V, Desiderio J, Saw CL, Kong AN. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm Drug Dispos. 2009;30:356–365. doi: 10.1002/bdd.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim TH, Jung JW, Ha BG, Hong JM, Park EK, Kim HJ, Kim SY. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J Nutr Biochem. 2011;22:8–15. doi: 10.1016/j.jnutbio.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Liang W, Luo Z, Ge S, Li M, Du J, Yang M, Yan M, Ye Z, Luo Z. Oral administration of quercetin inhibits bone loss in rat model of diabetic osteopenia. Eur J Pharmacol. 2011;670:317–324. doi: 10.1016/j.ejphar.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Takai H, Utsunomiya T, Li X, Li Z, Wang Z, Wang S, Sasaki Y, Yamamoto H, Ogata Y. Kaempferol stimulates bone sialoprotein gene transcription and new bone formation. J Cell Biochem. 2010;110:1342–1355. doi: 10.1002/jcb.22649. [DOI] [PubMed] [Google Scholar]
- 32.Neuhouser ML. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer. 2004;50:1–7. doi: 10.1207/s15327914nc5001_1. [DOI] [PubMed] [Google Scholar]
- 33.Tena JD, Burgos-Morón E, Calderón-Montaño JM, Sanz I, Sainz J, Lopez-Lazaro M. Consumption of the dietary flavonoids quercetin, luteolin and kaempferol and overall risk of cancer — a review and meta-analysis of the epidemiological data. WebmedCentral CANCER. 2013;4 [Google Scholar]
- 34.Guo K, Liang Z, Liu L, Li F, Wang H. Flavonoids intake and risk of prostate cancer: a meta-analysis of observational studies. Andrologia. 2016;48:1175–1182. doi: 10.1111/and.12556. [DOI] [PubMed] [Google Scholar]
- 35.Chiu FL, Lin JK. Downregulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate. 2008;68:61–71. doi: 10.1002/pros.20690. [DOI] [PubMed] [Google Scholar]
- 36.Hashemzaei M, Delarami Far A, Yari A, Heravi RE, Tabrizian K, Taghdisi SM, Sadegh SE, Tsarouhas K, Kouretas D, Tzanakakis G. Anticancer and apoptosisinducing effects of quercetin in vitro and in vivo. Oncol Rep. 2017;38:819–828. doi: 10.3892/or.2017.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dang Q, Song W, Xu D, Ma Y, Li F, Zeng J, Zhu G, Wang X, Chang LS, He D. Kaempferol suppresses bladder cancer tumor growth by inhibiting cell proliferation and inducing apoptosis. Mol Carcinog. 2015;54:831–840. doi: 10.1002/mc.22154. [DOI] [PubMed] [Google Scholar]
- 38.Luo H, Rankin GO, Liu L, Daddysman MK, Jiang BH, Chen YC. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr Cancer. 2009;61:554–563. doi: 10.1080/01635580802666281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Ahmad A, Kong D, Bao B, Sarkar FH. Recent progress on nutraceutical research in prostate cancer. Cancer Metastasis Rev. 2014;33:629–640. doi: 10.1007/s10555-013-9478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu J, Chen D, Zhao B, Zhao Z, Zhou J, Xu Y, Xin Y, Liu C, Luo L, Yin Z. Luteolin induces carcinoma cell apoptosis through binding Hsp90 to suppress constitutive activation of STAT3. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stope MB, Schubert T, Staar D, Ronnau C, Streitborger A, Kroeger N, Kubisch C, Zimmermann U, Walther R, Burchardt M. Effect of the heat shock protein HSP27 on androgen receptor expression and function in prostate cancer cells. World J Urol. 2012;30:327–331. doi: 10.1007/s00345-012-0843-z. [DOI] [PubMed] [Google Scholar]
- 42.Hansen RK, Oesterreich S, Lemieux P, Sarge KD, Fuqua SA. Quercetin inhibits heat shock protein induction but not heat shock factor DNA-binding in human breast carcinoma cells. Biochem Biophys Res Commun. 1997;239:851–856. doi: 10.1006/bbrc.1997.7572. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto T, Sato N, Sekine Y, Yumioka T, Imoto S, Junicho A, Fuse H, Matsuda T. Molecular interactions between STAT3 and protein inhibitor of activated STAT3, and androgen receptor. Biochem Biophys Res Commun. 2003;306:610–615. doi: 10.1016/s0006-291x(03)01026-x. [DOI] [PubMed] [Google Scholar]
- 44.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 45.Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balentine DA, Dwyer JT, Erdman JW, Jr., Ferruzzi MG, Gaine PC, Harnly JM, Kwik-Uribe CL. Recommendations on reporting requirements for flavonoids in research. Am J Clin Nutr. 2015;101:1113–1125. doi: 10.3945/ajcn.113.071274. [DOI] [PubMed] [Google Scholar]
- 47.Sachs JR, Mayawala K, Gadamsetty S, Kang SP, de Alwis DP. Optimal dosing for targeted therapies in oncology: drug development cases leading by example. Clin Cancer Res. 2016;22:1318–1324. doi: 10.1158/1078-0432.CCR-15-1295. [DOI] [PubMed] [Google Scholar]
- 48.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai H, Scott E, Kholghi A, Andreadi C, Rufini A, Karmokar A, Britton RG, Horner-Glister E, Greaves P, Jawad D. Cancer chemoprevention: evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murakami A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch Biochem Biophys. 2014;557:3–10. doi: 10.1016/j.abb.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 51.Gaya A, Akle CA, Mudan S, Grange J. The concept of hormesis in cancer therapy — is less more? Cureus. 2015;7 doi: 10.7759/cureus.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilt TJ, Jones KM, Barry MJ, Andriole GL, Culkin D, Wheeler T, Aronson WJ, Brawer MK. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132–142. doi: 10.1056/NEJMoa1615869. [DOI] [PubMed] [Google Scholar]
- 53.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, Davis M, Peters TJ, Turner EL, Martin RM. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials