Abstract
Background
Most children who are exposed to threat-related adversity (e.g., violence, abuse, neglect) are resilient - that is, they show stable trajectories of healthy psychological development. Despite this, most research on neurodevelopmental changes following adversity has focused on the neural correlates of negative outcomes, such as psychopathology. The neural correlates of trait resilience in pediatric populations are unknown, and it is unclear whether they are distinct from those related to adversity exposure and the absence of negative outcomes (e.g., depressive symptomology).
Methods
This functional magnetic resonance imaging (fMRI) study reports on a diverse sample of 55 children and adolescents (ages 6–17 years) recruited from a range of stressful environments (e.g., lower income, threat-related adversity exposure). Participants completed a multi-echo multi-band resting-state fMRI scan and self-report measures of trait resilience and emotion-related symptomology (e.g., depressive symptoms). Resting-state data were submitted to an independent component analysis (ICA) to identify core neurocognitive networks (salience and emotion network [SEN], default mode network [DMN], central executive network [CEN]). We tested for links among trait resilience and dynamic (i.e., time-varying) as well as conventional static (i.e., averaged across the entire session) resting-state functional connectivity (rsFC) of core neurocognitive networks.
Results
Youth with higher trait resilience spent a lower fraction of time in a particular dynamic rsFC state, characterized by heightened rsFC between the anterior DMN and right CEN. Within this state, trait resilience was associated with lower rsFC of the SEN with the right CEN and anterior DMN. There were no associations among trait resilience and conventional static rsFC. Importantly, although more resilient youth reported lower depressive symptoms, the effects of resilience on rsFC were independent of depressive symptoms and adversity exposure.
Conclusions
The present study is the first to report on the neural correlates of trait resilience in youth, and offers initial insight into potential adaptive patterns of brain organization in the context of environmental stressors. Understanding the neural dynamics underlying positive adaptation to early adversity will aid in the development of interventions that focus on strengthening resilience rather than mitigating already-present psychological problems.
Keywords: Youth, Resting-state fMRI, Dynamic connectivity, Default mode network, Salience network, Central executive network
Highlights
-
•
This is the first study to examine neural correlates of trait resilience in youth.
-
•
More resilient children spent a lower fraction of time in a certain rsFC state.
-
•
More resilient children showed a state-specific reduction in rsFC of the SEN with the CEN and DMN.
-
•
Effects of resilience on rsFC were independent of depression and early adversity.
1. Introduction
Prospective studies show that most individuals who are exposed to threat-related adversity (e.g., violence, abuse) are resilient - that is, they show stable trajectories of healthy psychological and interpersonal functioning over time (Bonanno, 2005). Children appear to be particularly resilient, with research suggesting that only a subset of children exposed to adversity will subsequently develop negative outcomes, such as psychopathology (Collishaw et al., 2007; Green et al., 2010; Kessler et al., 2010; Masten, 2001; McLaughlin et al., 2012; Trentacosta et al., 2016). Despite this, most research on neurodevelopmental changes following adversity has focused on the neural correlates of negative outcomes, such as psychopathology. Research into the neurobiological mechanisms underlying resilience may provide new insights into positive neural adaptation following adversity, and may open new avenues for interventions - informed by developmental neuroscience – to promote positive outcomes for children exposed to adversity.
Interest in the underlying neurobiology of resilience has grown over the past decade (see review by Russo et al., 2012). Studies in humans have associated various peripheral neuroendocrine markers, including cortisol, testosterone, and dehydroepiandrosterone (DHEA), with a more resilient phenotype. Research in laboratory animals suggests that these neuroendocrine findings are accompanied by various neural and molecular adaptations. Interestingly, these studies indicate that resilient individuals not only show an absence of key molecular abnormalities that occur in susceptible individuals, but also show a separate set of active molecular adaptations that promote coping and adaptive behavioral functioning. These active neuroadaptations are consistent with the definition of resilience in the psychological literature - a multidimensional trait that reflects an individual's ability to cope with stressful or adverse experiences (Connor and Davidson, 2003; Luthar et al., 2000). Empirical data suggest that trait resilience, as measured by the widely used Connor-Davidson Resilience Scale (CD-RISC; Connor and Davidson, 2003), is comprised of several adaptive psychological processes and characteristics (e.g., persistence, hardiness; Campbell-Sills and Stein, 2007).
Despite the conceptualization of resilience as a collection of positive trait attributes and neuroadaptations, most research into the underlying neural bases has operationally defined resilience as the non-emergence of psychopathology or symptomology following adversity (e.g., Peres et al., 2011; van der Werff et al., 2017; van der Werff et al., 2013a; Vythilingam et al., 2009). These neuroimaging studies have documented reduced threat-related amygdala and insula reactivity, altered reward-related responding in corticostriatal reward circuitry, and widespread changes in regional brain volumes in individuals who did not develop psychopathology. To our knowledge, only five neuroimaging studies have assessed the neural correlates of trait resilience (Daniels et al., 2011; New et al., 2009; Reynaud et al., 2013; van Rooij et al., 2016; Waugh et al., 2008). Of note, these studies are all in adults. These studies similarly implicate several brain areas in trait resilience, including the insula, cingulate cortex, medial temporal lobe, and prefrontal cortex. Given that these regions span core neurocognitive networks for emotion regulation, attention, and executive control, it has been hypothesized that resilience is related to dynamic interactions of distributed brain areas operating in large-scale neurocognitive networks (Gupta et al., 2016). For example, trait resilience may relate to more flexible and efficient responding across brain networks that regulate coping behavior, affective processing, and attentional control (Southwick and Charney, 2012; van der Werff et al., 2013b).
One way to measure flexibility in interactions between brain networks is to assess dynamic, or time-varying, patterns of resting-state functional connectivity (rsFC). For example, in an independent pediatric sample, we have recently demonstrated several distinct patterns of rsFC between core neurocognitive networks that re-occur over time and across participants (Marusak et al., 2016). The present study aimed to examine, for the first time, the functional neural correlates of trait resilience in a pediatric sample. We tested for links among trait resilience and dynamic as well as conventional static (i.e., averaged across the entire session) rsFC of core neurocognitive networks in children and adolescents. Based on previous findings in adults, we focused on three neurocognitive core networks: (1) the default mode network (DMN), involved in self-reference, introspection, and social cognition (see review by Raichle, 2015); (2) the central executive network (CEN), involved in working memory, inhibitory control, and higher-order reasoning (Zanto and Gazzaley, 2013), and (3) the salience and emotion network (SEN), involved in attention, homeostatic and emotion-related processing, and regulating interactions within and between the DMN and CEN (Seeley et al., 2007; Sridharan et al., 2008). For example, an adult neuroimaging study found a positive association between a more resilient personality profile (i.e., low neuroticism and above average openness, extraversion, agreeableness, and conscientiousness) and rsFC between the SEN and DMN (Kilpatrick et al., 2015). Based on these findings and the view that higher trait resilience is related to lower susceptibility to rumination and/or mind-wandering (Min et al., 2013), and increased SEN-DMN rsFC has been observed in depressed individuals (Manoliu et al., 2013), we predicted more resilient children to show lower SEN-DMN rsFC, which may reflect better attentional control over mind-wandering. In addition, consistent with the view that individuals with higher trait resilience have greater inhibitory control over emotion processing regions (Gupta et al., 2016), we predicted that more resilient children would show increased rsFC between the SEN and CEN across the entire scan (i.e., static rsFC). Further, based on findings that individuals with emotion dysregulation (e.g., depression) demonstrate higher within-network rsFC of the DMN (Kaiser et al., 2015; Patriat et al., 2016), we predict lower within-DMN rsFC in youth with higher trait resilience. By measuring dynamic rsFC, these neural patterns may be more prominent in certain brain configurations – or ‘connectivity states’, measured during the scan. Of note, although the basic structure and function of the DMN, CEN, and SEN are established early in life (see review by Menon, 2013), the interactions within and between these networks continue to develop across the first two decades of life (Marusak et al., 2016; Uddin et al., 2011). Thus, it is also possible that resilience relates to different patterns of rsFC in adults than in a pediatric sample.
In addition, given (1) that studies consistently report negative associations between trait resilience and psychopathology (e.g., depressive symptoms; Laird et al., 2018; Lee et al., 2017; Sharpley et al., 2016), and (2) that depressive symptoms are related to altered rsFC within and between the DMN, CEN, and SEN (e.g., Kaiser et al., 2015; Menon, 2011; Sheline et al., 2010), we tested associations between depressive symptoms and static and dynamic rsFC, to assess whether functional neural correlates differ from those associated with resilience. These results would support the notion that neural mechanisms of positive adaptations (e.g., trait resilience) may be distinct from those associated with pathology (e.g., depression). Similarly, although the effects of early adversity on resilience are mixed (see for e.g., Scali et al., 2012; Sexton et al., 2015), previous studies indicate that early adversity alters rsFC within and between the CEN, DMN, and SEN (e.g., Klapwijk et al., 2013; Marusak et al., 2015; Patriat et al., 2016). Thus, we additionally tested for potential effects of early adversity on rsFC, and tested whether the observed effects differ from those associated with resilience.
2. Material and methods
2.1. Participants
This functional magnetic resonance imaging (fMRI) study reports on 55 children and adolescents (ages 6–17 years, M = 10.59, SD = 3.23, 51% female). Although participants were not recruited for race or economic standing, the study was conducted in an urban setting (Detroit, Michigan, USA). As a result, the study sample was economically and racially diverse (see Table 1), with a large percentage of youth at risk for emotional psychopathology via socioeconomic disadvantage (i.e., lower income). In addition, several participants were recruited for increased risk of emotional psychopathology via exposure to early threat-related adversity (violence, abuse exposure, intensive medical treatments; see Table 1). This served to improve our ability to draw initial links among resilience, brain connectivity, and indices of psychological health. In addition, evaluation of resilience in an at-risk sample is consistent with the notion of ‘stress inoculation’ – in that moderate levels of adversity may be needed to promote adaptive coping responses (see Russo et al., 2012). Children with and without histories of adversity exposure did not differ in trait resilience, motion (mean framewise displacement [FD]), depression, or age (p's > 0.07).
Table 1.
Participant demographics.
| n (%) | m (SD) | Range | |
|---|---|---|---|
| Gender | |||
| Female | 28 (50.9) | ||
| Male | 27 (49.1) | ||
| Age (years) | 10.59 (3.23) | 6.33–17.83 | |
| Pubertal stage | |||
| Tanner stage | 2.46 (1.31) | 1–5 | |
| Early/mid pubertal | 32 (58.18) | 1–2 | |
| Mid/late pubertal | 20 (36.36) | 3–5 | |
| Not reported | 3 (5.45) | ||
| Race/ethnicity | |||
| African American | 23 (41.82) | ||
| Caucasian | 23 (41.82) | ||
| Latino/Latina | 2 (3.64) | ||
| Other | 3 (5.45) | ||
| Not reported | 4 (7.27) | ||
| IQ | 102.02 (15.59) | 58–131 | |
| Household annual income | |||
| <10,000 | 4 (7.27) | ||
| $10–20,000 | 4 (7.27) | ||
| $20–30,000 | 10 (18.18) | ||
| $30–40,000 | 4 (7.27) | ||
| $40–50,000 | 2 (3.64) | ||
| $50–60,000 | 5 (9.09) | ||
| $60–80,000 | 7 (12.73) | ||
| $80–100,000 | 2 (3.64) | ||
| $100–120,000 | 1 (1.82) | ||
| $120,000+ | 11 (20) | ||
| Not reported | 5 (9.09) | ||
| Community distress score | 60.48 (36.70) | 1.0–99.4 | |
| Low distress (0−20) | 12 (21.81) | ||
| 20–40 | 5 (9.09) | ||
| 40–60 | 10 (18.18) | ||
| 60–80 | 2 (3.64) | ||
| Distressed (80–100) | 26 (47.27) | ||
| Highly distressed (>90) | 23 (41.82) | ||
| Exposure to threat-related adversity | 21 (38.18) | ||
| Threat-related adversity type endorsed | |||
| Exposure to domestic violence | 3 (14.29) | ||
| Exposure to other violence | 5 (23.81) | ||
| Physical abuse | 7 (33.33) | ||
| Sexual abuse | 2 (9.52) | ||
| Emotional abuse | 4 (19.05) | ||
| Childhood cancer | 9 (42.86) | ||
| More than one type | 6 (28.57) | ||
| Trait resilience | |||
| CD-RISC-10 total score | 29.75 (7.37) | 10–40 | |
| Not reported | 4 (7.27) | ||
| Depressive symptoms | |||
| CDI-S total score | 2.40 (2.39) | 0–10 | |
| Above threshold | 23 (41.82) | ||
| Movement during scan (mm) | |||
| Mean FD | 0.25 (0.25) | 0.07–1.68 | |
Participants were recruited locally through advertisements, mental health service providers, or a pediatric oncology clinic (Children's Hospital of Michigan). Study exclusionary criteria included: English as a second language, significant learning disorder, history of traumatic brain injury, neurological or movement disorders, or presence of MRI contraindications. Participants recruited from the oncology clinic had finished active treatment for at least two months prior to their participation in the study.
The present study was approved by the Institutional Review Board of Wayne State University. Parental informed written consent and child/ adolescent assent were obtained prior to study participation.
2.2. Self-report measures
Due to the relatively wide age range, all participants were assisted in completing self-report measures by clinically trained research staff (postdoctoral fellow or advanced clinical psychology doctoral student). Participants completed a 10-item version of the CD-RISC (Campbell-Sills and Stein, 2007), which evaluates trait resilience over the past month. The CD-RISC has been previously used in 6–16 year old children and adolescents (Fu et al., 2014; Vetter et al., 2010), and assesses the ability to adapt or cope with stress and adversity. All youth endorsed understanding the questions and, to rule out potential effects of age, we computed internal consistency across the sample and within the younger age group specifically (median split). Cronbach's alpha was 0.86 across the sample, and 0.80 within the younger age group (6–9.75 years old), suggesting good internal consistency. These consistency values are similar to those reported among young adults (alpha value of 0.85; Campbell-Sills and Stein, 2007). Possible CD-RISC-10 scores range from 0 to 40, with higher scores corresponding to higher levels of trait resilience. Prior factor analysis of CD-RISC-10 scores in adults suggests two separate dimensions (i.e., persistence and hardiness; Campbell-Sills and Stein, 2007). In studies with pediatric samples, however, only one factor emerges, suggesting that these separate dimensions observed in adults may be less distinct in children (Duong and Hurst, 2016; Notario-Pacheco et al., 2011). In line with these results, factor analysis of CD-RISC-10 scores here, using Varimax rotation, supported a one-factor solution (eigenvalue = 4.51). This single factor explained 45% of the variance, and all items loaded highly onto this factor (all r's > 0.5).
Using the self-reported Tanner stages questionnaire (Marshall and Tanner, 1968), average Tanner stage was 2.46 (‘early-mid’ pubertal; SD = 1.31). The sample was average in IQ (M = 102.02, SD = 15.59), estimated using the KBIT-2 (Kaufman and Kaufman, 2004). Participants also completed a standardized self-report measure of depressive symptoms over the past two weeks (Children's Depression Inventory - short form, CDI-S; Kovacs, 1992).
2.3. MRI data acquisition, preprocessing, and Denoising
MRI data were acquired on a 3 T Siemens MAGNETOM Verio scanner (MR Research Facility, Wayne State University), using a 32-channel head coil. Participants completed a 10 min 17 s resting-state scan, during which they were instructed to lay awake with their eyes closed. Foam padding was used to reduce motion during the scan. Whole-brain fMRI data were collected using a multi-echo/multi-band (ME/MB) echo-planar imaging sequence, customized for the scanner and for pediatric neuroimaging: 51 slices, 186 mm field of view (FOV), 64 × 65 matrix size yielding 2.90 mm isotropic resolution, in-plane GRAPPA acceleration factor 2, flip angle (FA) = 83 degrees, repetition time (TR) = 1.50 s, and echo time triplet (TEs) = 15, 31, 46 ms. A whole-brain anatomical image was collected during the scan session, using a magnetization prepared rapid acquisition GRE (MP-RAGE) sequence: 128 slices, 256 mm FOV, 384 × 384 matrix size yielding 0.70 × 0.70 × 1.3 mm resolution, in-plane GRAPPA acceleration factor 2, FA = 9 degrees, TR = 1.68 s, TE = 3.51 ms.
ME/MB fMRI data were preprocessed and denoised using custom ME-ICA software (v3, beta 1; https://bitbucket.org/prantikk/me-ica). ME-ICA applies independent components analysis (ICA) to decompose ME fMRI datasets into independent components, that are then categorized as BOLD or non-BOLD (noise) based on their TE-dependence (Kundu et al., 2012). These components are then optimally combined into a denoised fMRI timeseries that reflects the T2* weighted averaging of timeseries from each echo. Of note, previous studies demonstrate that ME-ICA is an effective means for reducing non-BOLD artifact, enhancing specificity, and improving fMRI effect sizes and thus statistical power (Kundu et al., 2015; Lombardo et al., 2016).
The preprocessing pipeline in ME-ICA includes: (1) skull-stripping and warping of the anatomical image to the Montreal Neurological Institute (MNI) template, (2) co-registration of the first echo timeseries for motion correction and for anatomical-functional co-registration, (3) de-obliquing of the functional data, and (4) 12-parameter affine anatomical-functional co-registration. In addition, the first 15 s of data were removed to allow for signal equilibration. No temporal filtering or smoothing was applied to the data. See Kundu et al. (2012) for further information.
We took several steps to reduce the potential influence of motion-related artifact in the data. First, participants viewed a movie about MRI to prepare them for their visit (available at www.tnp2lab.org/think-study-video), underwent pre-training in a mock MRI scanner, and a research assistant remained in the room with the child during the entire length of the scan. Second, we used multi-echo fMRI acquisition and ME-ICA denoising strategies (Kundu et al., 2012). ME-ICA allows for a more principled removal of non-BOLD signals from fMRI data by acquiring both fMRI signal time series and their NMR signal decay (Kundu et al., 2012). This circumvents the need for the application of traditional denoising strategies that apply arbitrary spatial or temporal smoothing (e.g., bandpass filtering) and/or result in the removal of large amounts of data (e.g., scrubbing). Of note, even prior to ME-ICA denoising, head motion was relatively low across the sample (see Table 1). For participants in the higher range of motion, ME-ICA sufficiently flattens out the DVARS trace (i.e., rate of change of BOLD signal across the entire brain at each time point; Power et al., 2012), see Marusak et al. (2017) for demonstration. Additionally, mean FD was not related to our main variable of interest, trait resilience (r = 0.16, p = 0.27), nor depressive symptoms (r = −0.04, p = 0.76). Next, we used group ICA (described below) to identify network components of interest (i.e., DMN, CEN, and SEN), thus effectively discounting non-BOLD signal components. Additional steps were taken to address potential motion-related artifact in the derived component timeseries, following group ICA (component “postprocessing”; Calhoun et al., 2001). In particular, high-motion frames were replaced with the best estimate using a third-order spline fit to the clean portions of the timeseries, following prior work (Allen et al., 2014). Outliers were detected based on the median absolute deviation, as implemented in 3dDespike (http://afni.nimh.nih.gov/afni). Of note, we chose to replace, rather than remove, high-motion frames, as removing frames would compromise the subsequent sliding window dynamic rsFC approach. This approach also ensures that participants contribute an equal number of time points to the analysis.
2.4. Group ICA and component identification
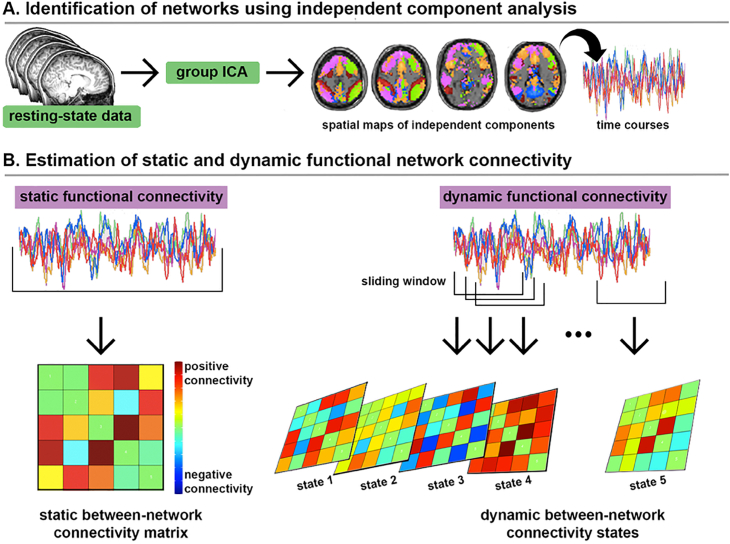
The dimensionality of each participant's dataset was estimated using ME-ICA, as the number of BOLD components derived from that individual's timeseries data. This information was used to determine the number of components to be derived at the group level. For all participants, each individuals' optimally combined and denoised timeseries data were submitted to a group-level spatial ICA to identify DMN, SEN, and CEN. ICA was implemented in the GIFT toolbox (v.4.a; http://mialab.mrn.org/software/gift/), and 12 components were derived using the Infomax algorithm, repeated 20 times in ICASSO to estimate stable components. Twelve components were chosen based on the dimensionality of individual subjects' datasets, and also to limit the number of comparisons by deriving 1–2 components per network of interest. Group ICA results in a set of group aggregate spatial maps that are then back reconstructed into single subject space. Each back-reconstructed component consists of a spatial Z-map reflecting the network's coherent activity across space and an associated time course reflecting network activity over time. See Fig. 1a for overview of the ICA approach.
Fig. 1.
Schematic representation of group-level independent component analysis (ICA) and static and dynamic resting-state functional connectivity (rsFC) estimation. a) Preprocessed and de-noised resting-state datasets (N = 55) were submitted to a group-level ICA to identify 12 spatially-independent and temporally synchronous components (networks). Five of these components were identified as neurocognitive networks of interest (i.e., DMN, SEN, CEN; see Fig. 2). Components were then back reconstructed into individual participant space to produce single-participant network time courses and spatial maps; b) Conventional static rsFC was computed as Fisher's Z-transformed Pearson correlations between network components of interest, averaged across the entire resting-state scan. Dynamic rsFC was computed using a sliding windows analysis and k-means clustering of Fisher's Z-transformed Pearson correlations. Here, five dynamic rsFC states were identified that re-occurred across the scan and across participants.
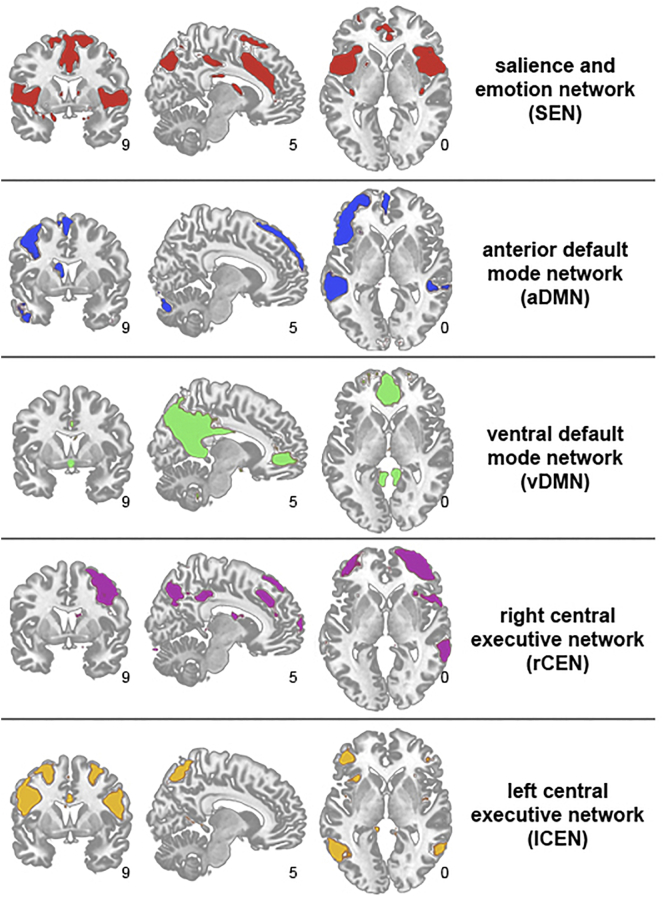
Following group ICA, five components of interest were identified using a combination of visual inspection and spatial template-matching (i.e., correlation between the template image and component map of interest): ventral DMN (vDMN), anterior DMN (aDMN), left CEN (lCEN), right CEN (rCEN), and SEN (see Fig. 2). Templates and further details regarding component identification is described in detail in our prior work (Marusak et al., 2016, 2017). Observed group aggregate spatial maps of the 5 components of interest (Fig. 2) align with neurocognitive networks reported in prior work (Damoiseaux et al., 2012).
Fig. 2.
Spatial maps of five core neurocognitive networks of interest identified using group-level independent components analysis. Coordinates are provided in MNI convention.
2.5. Dynamic and static rsFC estimation
Individual participant component network maps were used to estimate dynamic and conventional static rsFC between network components (see Fig. 1b), following our prior work (Marusak et al., 2016, 2017). Static rsFC was measured using estimates of covariance between network components, averaged across the entire resting-state scan. Dynamic rsFC was estimated using a sliding windows approach and k-means clustering (see Fig. 2b and Marusak et al., 2016 for description of parameters).
2.6. Statistical analysis
Using Pearson bivariate correlation analyses in IBM SPSS v.23, we tested for associations between resilience scores and measures of dynamic and conventional static rsFC. Given the relatively wide age range (6–17 years) and a negative association between trait resilience and depressive symptoms in this sample, (r = −0.470, p < 0.001), age and depressive symptoms were controlled for in all analyses to better isolate the effects of resilience. For static rsFC, we evaluated strength of rsFC between network components, averaged across the experiment. For dynamic rsFC, we investigated (1) the number of state transitions, as well as (2) mean dwell time (i.e., how long a participant is in each state), and (3) fraction of (total) time spent in each state. To limit the number of comparisons, we evaluated effects of resilience on (4) strength of between-network rsFC only for states showing an association between resilience and dwell time or fraction of time spent in that particular state. In addition, to test whether effects of depressive symptoms were overlapping with those of resilience, we also tested for main effects of depressive symptoms on static and dynamic rsFC. All results were considered significant at p < 0.05, two-tailed.
3. Results
3.1. Trait resilience and depressive symptoms
Trait resilience scores in the sample ranged from 10 to 40 (see Table 1) and were comparable to previous findings in adults (Campbell-Sills et al., 2009). Trait resilience was negatively associated with depressive symptomology (r[51] = −0.47, p < 0.001), but not related to trauma exposure, or other demographic variables (i.e., age, pubertal maturation, IQ, gender, race, income). Depressive symptom scores ranged from 0 to 10 (see Table 1), and 42% of participants exceeded the threshold suggested for detecting pathological depression (CDI-S ≥ 3; Allgaier et al., 2012). Thus, although formal diagnostic testing was not performed here, this standardized measure suggests a significant number of youth at risk for emotional psychopathology.
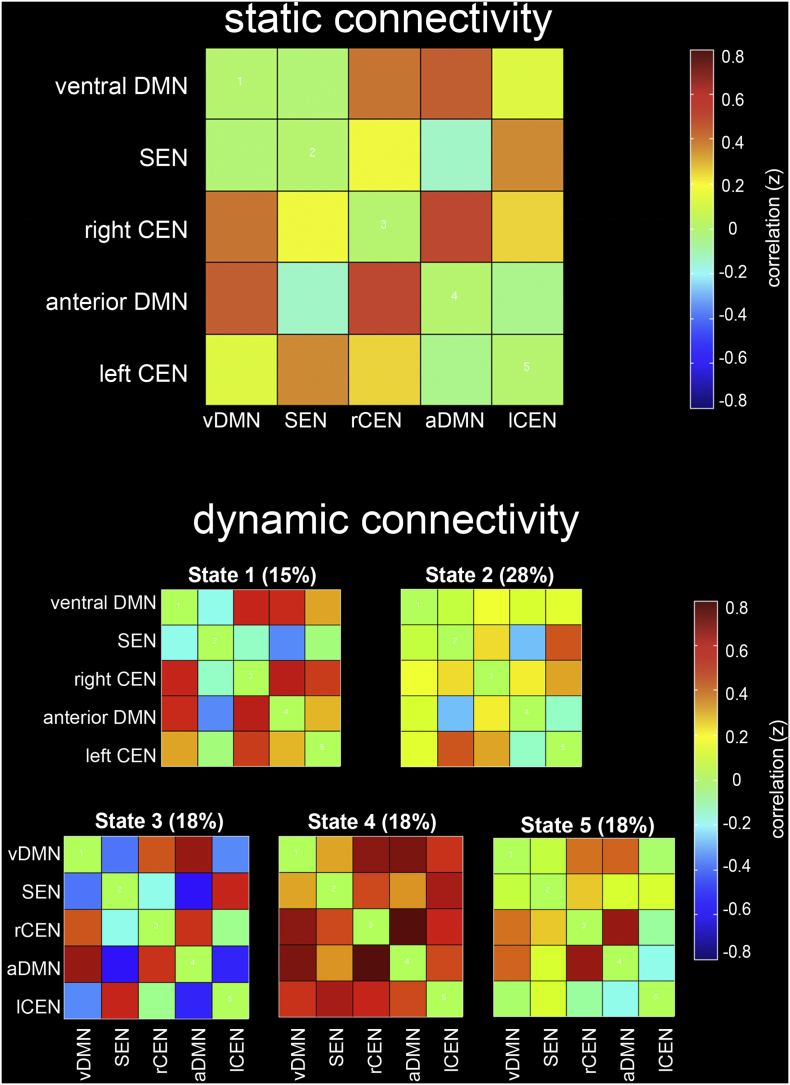
3.2. Static rsFC across the sample
Static rsFC across the sample was characterized by positive rsFC within the DMN (i.e., between the vDMN and aDMN components), between SEN and lCEN, and between rCEN and both DMN components (see Fig. 3, top). Negative rsFC was observed between the aDMN and both the SEN and lCEN. These patterns resemble those observed in prior rsFC studies in adults and in children (Manoliu et al., 2013; Marusak et al., 2017).
Fig. 3.
Static (top) and dynamic (bottom) resting-state functional connectivity (rsFC) across the entire youth sample (N = 55). Static rsFC is computed as the correlation between core neurocognitive network components, averaged across the entire resting-state scan. Dynamic rsFC is computed using a sliding-windows analysis and k-means clustering. Percentage of occurrence is listed for each dynamic state, over the course of the scan and averaged across participants. Abbreviations: ventral default mode network, vDMN; anterior default mode network, aDMN; salience and emotion network, SEN; right central executive network, rCEN; left central executive network, lCEN.
3.3. Dynamic rsFC across the sample
The dynamic rsFC analysis identified 5 connectivity states that re-occurred throughout the scan and across participants (see Fig. 3, bottom). The observed dynamic states were replicated across time and over participants, and importantly, diverged in part from the pattern of static rsFC (compare with Fig. 3, top). Connectivity State 1 was characterized by positive rsFC of the rCEN with the vDMN, aDMN, and lCEN, and positive rsFC between the two DMN components. State 2 was characterized by overall weak rsFC among all network components, apart from positive rsFC between the SEN and lCEN. State 3 was characterized by positive rsFC of the aDMN with the vDMN and rCEN, positive rsFC between SEN and lCEN, and negative rsFC between the SEN and lCEN. State 4 was characterized by positive rsFC between all networks; a state that has been observed in prior dynamic rsFC studies in adults (Allen et al., 2014) and in an independent youth sample (Marusak et al., 2016). In State 5, rsFC among network components was generally diffuse apart from positive rsFC between the aDMN and rCEN.
3.4. Trait resilience and static rsFC
There was no significant association between trait resilience scores and static rsFC between networks, p's > 0.05. Of note, there were no direct effects of age on static rsFC.
3.5. Trait resilience and dynamic rsFC
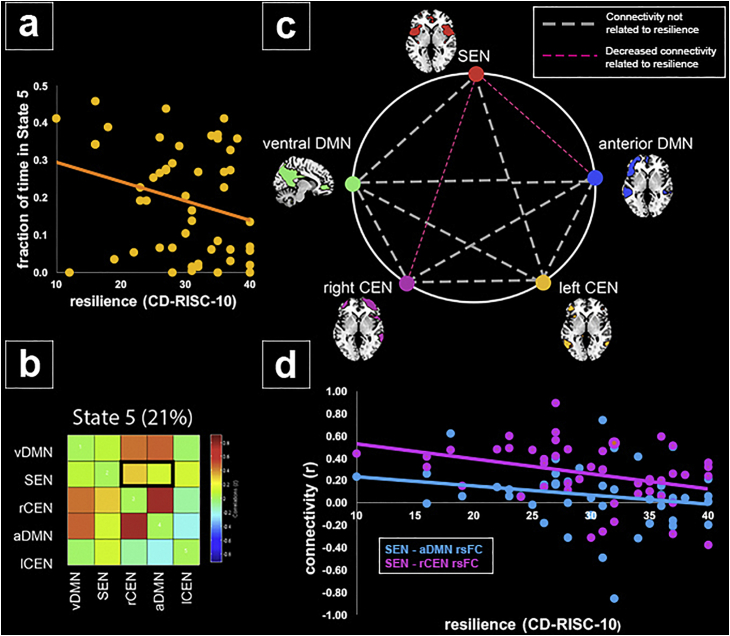
Dynamic rsFC analyses revealed that children with higher trait resilience score overall spent a significantly lower fraction of time in State 5 (r(47) = −0.35, p = 0.01; see Fig. 4a–b). There was no significant association between trait resilience score and overall number of dynamic state transitions or with dwell time in any State. Given the observed association between trait resilience and fraction of time spent in State 5, we tested for associations between resilience and strength of between-network rsFC within this State. Within State 5, higher trait resilience was associated with lower SEN rsFC with both the rCEN (r(43) = −0.49, p = 0.001) and aDMN (r(43) = −0.32, p = 0.03; see Fig. 4c–d). Follow-up analyses additionally controlling for income, age, pubertal stage, and early adversity found that the effects of resilience on fraction of time in State 5 and on SEN-right CEN connectivity within State 5 remained significant (p = .04 and p = .001, respectively). However, the effect of resilience on SEN-anterior DMN connectivity in State 5 became p = .054.
Fig. 4.
Effects of resilience on state-specific SEN resting-state functional connectivity (rsFC) and fraction of time spent in State 5. a) Trait resilience was negatively associated with fraction of time spent in State 5; b) Within State 5, resilience was associated with a state-specific reduction in SEN rsFC with aDMN and rCEN; c) Visualization of state-specific reduction in SEN rsFC with aDMN and rCEN; d) Visualization of SEN-aDMN and SEN-rCEN rsFC values plotted by participants' trait resilience scores. Abbreviations: ventral default mode network, vDMN; anterior default mode network, aDMN; salience and emotion network, SEN; right central executive network, rCEN; left central executive network, lCEN.
3.6. Depressive symptoms and static rsFC
There was a significant association between static rsFC and depressive symptoms such that higher depressive symptoms were associated with lower static rsFC between the aDMN and vDMN components (r = −0.30, p = 0.03). However, this effect appeared to be driven by an outlier (CDI Z score > 3) and the effect became nonsignificant after removing that individual.
3.7. Depressive symptoms and dynamic rsFC
There was no direct effect of depression on dwell time or fraction of time spent in any dynamic state for the full sample, or after the potential outlier was removed. Taken together, these results suggest that effects of resilience on rsFC are also not associated with depressive symptoms. Moreover, there were no effects of depressive symptoms on dynamic or static rsFC.
3.8. Adversity exposure and static rsFC
There were no significant differences in static rsFC between groups (adversity-exposed vs. control, p's > 0.3).
3.9. Adversity exposure and dynamic rsFC
Although there was no group difference (adversity, control) in trait resilience, there were group differences in rsFC during State 5. Interestingly, effects of adversity on rsFC within State 5 were distinct from those associated with resilience. In particular, youth exposed to adversity demonstrated increased rsFC between the SEN and lCEN (t(49) = 2.92, p = 0.005), and between the vDMN and rCEN (t(49) = 2.31, p = 0.025) in State 5, relative to their unexposed peers. There were no group differences in dwell time or fraction of time spent in any dynamic state (p's > 0.1).
4. Discussion
To date, most research on neurodevelopmental consequences of childhood adversity has focused on neural consequences of negative outcomes (e.g., psychopathology) rather than resilience. This study is the first to examine the neural correlates of trait resilience in youth, and we applied complementary measures of static and dynamic rsFC in a sample of at-risk children and adolescents (i.e., sociodemographic risk, frequent exposure to adversity). In line with previous reports, higher trait resilience was related to lower levels of depressive symptomology in our sample (see meta-analysis by Hu et al., 2015). In addition, we found that trait resilience was related to functional neural dynamics and interactions between neurocognitive networks over time. In particular, dynamic rsFC analyses revealed that children with higher trait resilience spent less time in a dynamic state characterized by positive rsFC between the aDMN and rCEN, a pattern that may reflect increased higher-order control over spontaneous processing of internal stimuli (e.g., passive autobiographical memory recall, prospection; Spreng and Andrews-Hanna, 2015). Within this dynamic state, more resilient children exhibit lower rsFC of the SEN, involved in emotional and attentional control, with other core neurocognitive networks. Of note, the effects of resilience on rsFC were significant when controlling for depressive symptoms, and there were no significant direct effects of depression on dynamic rsFC, suggesting unique patterns. Similarly, although adversity exposure was not related to static rsFC, there were effects of adversity on dynamic rsFC; however, these effects were independent of those associated with resilience. Research into the brain systems underlying resilience in children and adolescents may lead to better means of promoting positive adaptation following exposure to stress or adversity.
4.1. Trait resilience is related to fraction of time spent in a dynamic state
We found that children and adolescents with higher trait resilience spent a lower fraction of time in dynamic State 5, characterized by heightened aDMN connectivity with the vDMN and rCEN. Prior findings suggest these functional connectivity patterns, namely, hyperconnectivity of the DMN (see review by Hamilton et al., 2015) and heightened rsFC between regions of the DMN and CEN (Jacobs et al., 2014), may underlie rumination and sustained attention to negative cognitions that hallmark depression. Thus, our results suggest that more resilient youth may be less likely to visit a neurocognitive configuration that might reflect an immersion into negative internalized experience. Interestingly, however, these effects were observed when controlling for levels of depression, and direct effects of depression were not detected for fraction of time spent in State 5. This suggests that the observed effects are driven by positive attributes (i.e., resilience) rather than by the presence or absence of depressive symptomology.
4.2. Trait resilience is related to a state-specific reduction in rsFC of the SEN
We found that more trait resilient children and adolescents demonstrate lower rsFC of the SEN with the rCEN and aDMN within State 5. Interestingly, these associations were not significant for static rsFC, averaged across the scan, suggesting a state-specific effect of resilience. Prior research examining structural neural markers of trait resilience in adults have documented volumetric differences in core regions of the CEN (e.g., parietal lobe) and the SEN (e.g., anterior cingulate cortex, amygdala; Gupta et al., 2016), implicated in executive control and emotional arousal, respectively. In addition, a functional neuroimaging study of trait resilience in adults demonstrated that higher trait resilience was associated with less prolonged response of the insula, a core SEN region, to neutral images presented after a “threat” cue (Waugh et al., 2008). The authors interpreted this finding as indicating that more resilient individuals are better able to control the SEN to adaptively use emotional resources. Similarly, one study in trauma-exposed adults found that high trait resilience was related to increased activity in SEN and CEN areas involved in emotion regulation (i.e., thalamus, middle frontal gyrus) while reading trauma vs. neutral scripts (Daniels et al., 2011). Given the role of the SEN and the CEN in emotional arousal in executive control, respectively, the observed negative association between resilience and SEN-rCEN rsFC may reflect a better ability to engage executive control processes without emotional inputs when evaluating potential threats, or better higher-order control over emotional responding.
During State 5, more resilient children and adolescents also demonstrated lower SEN-aDMN rsFC. Heightened SEN-DMN rsFC is observed during acute detection of threats (Gold et al., 2015) and exaggerations in this circuitry are implicated in vulnerability to PTSD and other emotion-related disorders among adults (see review by MacNamara et al., 2016; Sripada et al., 2012). Therefore, elevated SEN-DMN rsFC may contribute to aberrant detection of threat or abnormal saliency processing, a hallmark of several emotion-related disorders. In support of the notion that reduced SEN-DMN rsFC may be protective, a recent study demonstrated that, among trauma-exposed adults, those with higher trait resilience demonstrated reduced rsFC between key nodes of the DMN and SEN (Brunetti et al., 2017). Another recent study in adolescents with histories of depression found a positive association between self-reported rumination and rsFC between core SEN (i.e., amygdala) and DMN (i.e., PCC) regions (Peters et al., 2016). Therefore, lower state-specific SEN-aDMN rsFC observed here among more resilient children may reflect lower susceptibility to (or, protective against) patterns of both maladaptive/altered saliency processing and negative self-referential cognitions.
4.3. Trait resilience is not related to within-network rsFC of the DMN
Studies have repeatedly reported changes in intrinsic functional connectivity of the DMN in emotion dysregulation (e.g., depression; Kaiser et al., 2015). We did not find effects of trait resilience on within-DMN rsFC. This is consistent with the idea that the effects of trait resilience may be independent of those associated with emotion dysregulation.
4.4. Effects of depressive symptoms differ from effects of resilience on rsFC
Importantly, the neural correlates of resilience were not related to depressive symptoms. These results support the view that neural correlates of positive adaptations (e.g., trait resilience) may be distinct from those associated with pathology (e.g., depression).
4.5. Null effects of early adversity on resilience in this sample
Here, we did not find direct effects of early adversity or pubertal stage on resilience. The findings in the literature on the effect of early adversity and puberty on resilience (as measured by the CD-RISC) are mixed (see for e.g., Scali et al., 2012; Sexton et al., 2015). Effects of adversity on resilience may depend on severity, developmental stage, or type of adversity (see Russo et al., 2012). Nonetheless, given evidence that early adversity and pubertal stage have been shown to affect rsFC within and between the DMN, SEN, and CEN (e.g., Klapwijk et al., 2013; Marusak et al., 2015; Patriat et al., 2016), we performed additional analyses controlling for these variables. These additional analyses suggested that the observed effects of resilience on fraction of time in State 5 and on SEN-right CEN connectivity within State 5 remained significant. However, the effect of resilience on SEN-anterior DMN connectivity in State 5 became p = .054. In addition, we examined potential direct effects of adversity on static and dynamic rsFC. Although resilience did not differ between groups (adversity, control), there were group differences in dynamic rsFC within State 5. However, the effects of adversity on dynamic rsFC differed from those associated with resilience (i.e., affected SEN-lCEN connectivity and vDMN-rCEN). These findings reinforce the notion that neural correlates of resilience and adversity are separate.
4.6. Limitations
Our findings must be interpreted considering several limitations. First, we examined the relationship between trait resilience and brain function during a resting-state condition, rather than during an experimental task (Waugh et al., 2008). However, patterns of brain connectivity during the resting-state have been shown to correspond with task-related brain activation across a wide range of psychological and cognitive tasks (Smith et al., 2009; Tavor et al., 2016). Given that resilience is considered to be a multi-dimensional trait, it is unclear whether the neural correlates of resilience will be best captured within the context of a single experimental paradigm. Second, our indices of resilience and depressive symptoms were trait measures, and it may be that state-like measures are associated with different effects on rsFC. However, rsFC is considered to be a trait-like measure of functional brain organization (e.g., Angelides et al., 2017). Third, although we took several measures to account for developmental effects on results, including assistance with self-report measures, evaluation of internal consistency in reporting among younger youth, controlling for age and additionally for pubertal stage, the age range was relatively wide (ages 6–17 years). Future studies with larger sample sizes are needed to evaluate potential age-related changes in neural correlates of resilience. Fourth, the study sample included a large portion of youth at risk for emotional psychopathology by way of sociodemographic risk factors (e.g., lower income) and/or exposure to early adversity, which may limit the generalizability of findings to lower risk populations. Yet, at-risk populations are largely understudied in the context of neuroscientific research, and research on the neural correlates of resilience in at-risk youth may pave the way for future interventions aimed at improving outcomes for populations with the highest need.
4.7. Conclusions
While both task-related and resting-state functional neuroimaging have been used to investigate the neural correlates of trait resilience in adults, parallel studies do not yet exist in children or adolescents (see review by van der Werff et al., 2013b). This is despite evidence that the brain systems involved in trait resilience in adults (i.e., CEN, SEN, DMN) undergo substantial development across the first two decades of life (Marusak et al., 2016; Uddin et al., 2011). The present study is the first report on the neural correlates of trait resilience in a pediatric sample, and offers initial insight into potential adaptive patterns of brain organization in the context of environmental stressors. Future studies will be crucial to elucidate the relationship between trait resilience and both static and dynamic rsFC of key neurocognitive networks in other at-risk pediatric groups as well as clinical populations. Understanding the neural dynamics underlying positive adaptation to trauma and stress in early life will aid in the development of interventions that focus on strengthening resilience, in addition to mitigating already-present psychological and emotional problems.
Acknowledgments
Acknowledgments
The authors would like to thank Pavan Jella, Limi Sharif, Sajah Fakhoury, Xhenis Brahimi, Klaramara Gellci, and Farah Sheikh of Wayne State University (WSU) for assistance in participant recruitment and data collection, and for Dr. Moriah Thomason for sharing some of the included data. Thank you also to the children and families who generously shared their time to participate in this study.
Funding
Research reported in this publication was supported, in part, by the WSU Departments of Pharmacy Practice and Pediatrics, the WSU Merrill Palmer Skillman Institute, and American Cancer Society14-238-04-IRG and Karmanos Cancer Institute Institutional Research Grant14-238-04-IRG CAR). Dr. Marusak is supported by American Cancer Society award 129368-PF-16-057-01-PCSM. Dr. Rabinak is supported by National Institute of Mental Health grants K01MH101123 and R61MH111935. Funding sources had no involvement in study design; in collection, analysis and interpretation or data; in the writing of the report; and in the decision to submit the article for publication.
References
- Allen E.A., Damaraju E., Plis S.M., Erhardt E.B., Eichele T., Calhoun V.D. Tracking whole-brain connectivity dynamics in the resting state. Cereb. Cortex. 2014;24:663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgaier A.K., Fruhe B., Pietsch K., Saravo B., Baethmann M., Schulte-Korne G. Is the Children's depression inventory short version a valid screening tool in pediatric care? A comparison to its full-length version. J. Psychosom. Res. 2012;73:369–374. doi: 10.1016/j.jpsychores.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Angelides N.H., Gupta J., Vickery T.J. Associating resting-state connectivity with trait impulsivity. Soc. Cogn. Affect. Neurosci. 2017:nsx031. doi: 10.1093/scan/nsx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno G.A. Resilience in the face of potential trauma. Curr. Dir. Psychol. Sci. 2005;14:135–138. [Google Scholar]
- Brunetti M., Marzetti L., Sepede G., Zappasodi F., Pizzella V., Sarchione F., Vellante F., Martinotti G., Di Giannantonio M. Resilience and cross-network connectivity: a neural model for post-trauma survival. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;77:110–119. doi: 10.1016/j.pnpbp.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L., Stein M.B. Psychometric analysis and refinement of the connor–Davidson resilience scale (CD-RISC): validation of a 10-item measure of resilience. J. Trauma. Stress. 2007;20:1019–1028. doi: 10.1002/jts.20271. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L., Forde D.R., Stein M.B. Demographic and childhood environmental predictors of resilience in a community sample. J. Psychiatr. Res. 2009;43:1007–1012. doi: 10.1016/j.jpsychires.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Collishaw S., Pickles A., Messer J., Rutter M., Shearer C., Maughan B. Resilience to adult psychopathology following childhood maltreatment: evidence from a community sample. Child Abuse Negl. 2007;31:211–229. doi: 10.1016/j.chiabu.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Connor K.M., Davidson J.R. Development of a new resilience scale: the Connor-Davidson resilience scale (CD-RISC) Depression Anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Prater K.E., Miller B.L., Greicius M.D. Functional connectivity tracks clinical deterioration in Alzheimer's disease. Neurobiol. Aging. 2012;33(828):e819–e828. doi: 10.1016/j.neurobiolaging.2011.06.024. (e830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels J.K., Hegadoren K.M., Coupland N.J., Rowe B.H., Densmore M., Neufeld R.W., Lanius R.A. Neural correlates and predictive power of trait resilience in an acutely traumatized sample: a pilot investigation. J. Clin. Psychiatry. 2011;73:327–332. doi: 10.4088/JCP.10m06293. [DOI] [PubMed] [Google Scholar]
- Duong C., Hurst C.P. Reliability and validity of the Khmer version of the 10-item Connor-Davidson resilience scale (Kh-CD-RISC10) in Cambodian adolescents. BMC Res. Notes. 2016;9:297. doi: 10.1186/s13104-016-2099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C., Leoutsakos J.-M., Underwood C. An examination of resilience cross-culturally in child and adolescent survivors of the 2008 China earthquake using the Connor–Davidson Resilience Scale (CD-RISC) J. Affect. Disord. 2014;155:149–153. doi: 10.1016/j.jad.2013.10.041. [DOI] [PubMed] [Google Scholar]
- Gold A.L., Morey R.A., McCarthy G. Amygdala–prefrontal cortex functional connectivity during threat-induced anxiety and goal distraction. Biol. Psychiatry. 2015;77:394–403. doi: 10.1016/j.biopsych.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J.G., McLaughlin K.A., Berglund P.A., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch. Gen. Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Love A., Kilpatrick L.A., Labus J.S., Bhatt R., Chang L., Tillisch K., Naliboff B., Mayer E.A. Morphological brain measures of cortico-limbic inhibition related to resilience. J. Neurosci. Res. 2016;95:1760–1775. doi: 10.1002/jnr.24007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol. Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T., Zhang D., Wang J. A meta-analysis of the trait resilience and mental health. Personal. Individ. Differ. 2015;76:18–27. [Google Scholar]
- Jacobs R.H., Jenkins L.M., Gabriel L.B., Barba A., Ryan K.A., Weisenbach S.L., Verges A., Baker A.M., Peters A.T., Crane N.A. Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A.S., Kaufman N.L. Pearson; 2004. Kaufman Brief Intelligence Test: KBIT 2; Manual. [Google Scholar]
- Kessler R.C., McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M., Aguilar-Gaxiola S., Alhamzawi A.O., Alonso J., Angermeyer M. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry. 2010;197:378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick L.A., Istrin J.J., Gupta A., Naliboff B.D., Tillisch K., Labus J.S., Mayer E.A. Sex commonalities and differences in the relationship between resilient personality and the intrinsic connectivity of the salience and default mode networks. Biol. Psychol. 2015;112:107–115. doi: 10.1016/j.biopsycho.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapwijk E.T., Goddings A.-L., Heyes S.B., Bird G., Viner R.M., Blakemore S.-J. Increased functional connectivity with puberty in the mentalising network involved in social emotion processing. Horm. Behav. 2013;64:314–322. doi: 10.1016/j.yhbeh.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Incorporated; 1992. Children's Depression Inventory. Multi-Health Systems. [Google Scholar]
- Kundu P., Inati S.J., Evans J.W., Luh W.M., Bandettini P.A. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. NeuroImage. 2012;60:1759–1770. doi: 10.1016/j.neuroimage.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P., Benson B.E., Baldwin K.L., Rosen D., Luh W.M., Bandettini P.A., Pine D.S., Ernst M. Robust resting state fMRI processing for studies on typical brain development based on multi-echo EPI acquisition. Brain Imaging Behav. 2015;9:56–73. doi: 10.1007/s11682-014-9346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird K.T., Lavretsky H., Paholpak P., Vlasova R.M., Roman M., Cyr N.S., Siddarth P. Clinical correlates of resilience factors in geriatric depression. Int. Psychogeriatr. 2018:1–10. doi: 10.1017/S1041610217002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Blackmon B.J., Cochran D.M., Kar B., Rehner T.A., Gunnell M.S. Community resilience, psychological resilience, and depressive symptoms: an examination of the Mississippi Gulf Coast 10 years after hurricane Katrina and 5 years after the Deepwater Horizon Oil Spill. Disaster Med. Public Health Preparedness. 2017:1–8. doi: 10.1017/dmp.2017.61. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Auyeung B., Holt R.J., Waldman J., Ruigrok A.N., Mooney N., Bullmore E.T., Baron-Cohen S., Kundu P. Improving effect size estimation and statistical power with multi-echo fMRI and its impact on understanding the neural systems supporting mentalizing. NeuroImage. 2016;142:55–66. doi: 10.1016/j.neuroimage.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar S.S., Cicchetti D., Becker B. The construct of resilience: a critical evaluation and guidelines for future work. Child Dev. 2000;71:543–562. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A., Digangi J., Phan K.L. Aberrant spontaneous and task-dependent functional connections in the anxious brain. Biol. Psychiatry. 2016;1:278–287. doi: 10.1016/j.bpsc.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A., Meng C., Brandl F., Doll A., Tahmasian M., Scherr M., Schwerthöffer D., Zimmer C., Förstl H., Bäuml J. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Growth and physiological development during adolescence. Annu. Rev. Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- Marusak H.A., Etkin A., Thomason M.E. Disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth. NeuroImage Clin. 2015;8:516–525. doi: 10.1016/j.nicl.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak H.A., Calhoun V.D., Brown S., Crespo L.M., Sala-Hamrick K., Gotlib I.H., Thomason M.E. Dynamic functional connectivity of neurocognitive networks in children. Hum. Brain Mapp. 2016;39:97–108. doi: 10.1002/hbm.23346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak H.A., Thomason M.E., Elrahal F., Peters C.A., Kundu P., Lombardo M.V., Calhoun V.D., Goldberg E.K., Cohen C., Taub J.W. Mindfulness and dynamic functional neural connectivity in children and adolescents. bioRxiv. 2017:106021. doi: 10.1016/j.bbr.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten A.S. Ordinary magic: resilience processes in development. Am. Psychol. 2001;56:227. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch. Gen. Psychiatry. 2012;69:1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V. Developmental pathways to functional brain networks: emerging principles. Trends Cogn. Sci. 2013;17:627–640. doi: 10.1016/j.tics.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Min J.-A., Yu J.J., Lee C.-U., Chae J.-H. Cognitive emotion regulation strategies contributing to resilience in patients with depression and/or anxiety disorders. Compr. Psychiatry. 2013;54:1190–1197. doi: 10.1016/j.comppsych.2013.05.008. [DOI] [PubMed] [Google Scholar]
- New A.S., Fan J., Murrough J.W., Liu X., Liebman R.E., Guise K.G., Tang C.Y., Charney D.S. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol. Psychiatry. 2009;66:656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Notario-Pacheco B., Solera-Martínez M., Serrano-Parra M.D., Bartolomé-Gutiérrez R., García-Campayo J., Martínez-Vizcaíno V. Reliability and validity of the Spanish version of the 10-item Connor-Davidson resilience scale (10-item CD-RISC) in young adults. Health Qual. Life Outcomes. 2011;9:63. doi: 10.1186/1477-7525-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriat R., Birn R.M., Keding T.J., Herringa R.J. Default-mode network abnormalities in pediatric posttraumatic stress disorder. J. Am. Acad. Child Adol. Psychiatry. 2016;55:319–327. doi: 10.1016/j.jaac.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres J.F., Foerster B., Santana L.G., Fereira M.D., Nasello A.G., Savoia M., Moreira-Almeida A., Lederman H. Police officers under attack: resilience implications of an fMRI study. J. Psychiatr. Res. 2011;45:727–734. doi: 10.1016/j.jpsychires.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Peters A.T., Burkhouse K., Feldhaus C.C., Langenecker S.A., Jacobs R.H. Aberrant resting-state functional connectivity in limbic and cognitive control networks relates to depressive rumination and mindfulness: a pilot study among adolescents with a history of depression. J. Affect. Disord. 2016;200:178–181. doi: 10.1016/j.jad.2016.03.059. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. The brain's default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Reynaud E., Guedj E., Souville M., Trousselard M., Zendjidjian X., El Khoury-Malhame M., Fakra E., Nazarian B., Blin O., Canini F. Relationship between emotional experience and resilience: an fMRI study in fire-fighters. Neuropsychologia. 2013;51:845–849. doi: 10.1016/j.neuropsychologia.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Russo S.J., Murrough J.W., Han M.-H., Charney D.S., Nestler E.J. Neurobiology of resilience. Nat. Neurosci. 2012;15:1475. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scali J., Gandubert C., Ritchie K., Soulier M., Ancelin M.-L., Chaudieu I. Measuring resilience in adult women using the 10-items Connor-Davidson resilience scale (CD-RISC). Role of trauma exposure and anxiety disorders. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M.B., Hamilton L., McGinnis E.W., Rosenblum K.L., Muzik M. The roles of resilience and childhood trauma history: main and moderating effects on postpartum maternal mental health and functioning. J. Affect. Disord. 2015;174:562–568. doi: 10.1016/j.jad.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpley C.F., Bitsika V., Jesulola E., Fitzpatrick K., Agnew L.L. The association between aspects of psychological resilience and subtypes of depression: implications for focussed clinical treatment models. Int. J. Psychiatry Clin. Pract. 2016;20:151–156. doi: 10.1080/13651501.2016.1199810. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z., Mintun M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., MacKay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick S.M., Charney D.S. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338:79–82. doi: 10.1126/science.1222942. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Andrews-Hanna J.R. Brain Mapping: An Encyclopedic Reference. Vol. 1316. 2015. The default network and social cognition; pp. 165–169. [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Welsh R.C., Garfinkel S.N., Wang X., Sripada C.S., Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom. Med. 2012;74:904. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor I., Jones O.P., Mars R., Smith S., Behrens T., Jbabdi S. Task-free MRI predicts individual differences in brain activity during task performance. Science. 2016;352:216–220. doi: 10.1126/science.aad8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentacosta C.J., Harper F.W., Albrecht T.L., Taub J.W., Phipps S., Penner L.A. Pediatric Cancer Patients' treatment-related distress and longer-term anxiety: an individual differences perspective. J. Dev. Behav. Pediatr. 2016;37:753–761. doi: 10.1097/DBP.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K.S., Ryali S., Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J. Neurosci. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff S.J., Pannekoek J.N., Veer I.M., van Tol M.-J., Aleman A., Veltman D.J., Zitman F.G., Rombouts S.A., Elzinga B.M., van der Wee N.J. Resilience to childhood maltreatment is associated with increased resting-state functional connectivity of the salience network with the lingual gyrus. Child Abuse Negl. 2013;37:1021–1029. doi: 10.1016/j.chiabu.2013.07.008. [DOI] [PubMed] [Google Scholar]
- van der Werff S.J., van den Berg S.M., Pannekoek J.N., Elzinga B.M., Van Der Wee N.J. Neuroimaging resilience to stress: a review. Front. Behav. Neurosci. 2013;7 doi: 10.3389/fnbeh.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff S.J., Elzinga B.M., Smit A.S., van der Wee N.J. Structural brain correlates of resilience to traumatic stress in Dutch police officers. Psychoneuroendocrinology. 2017;85:172–178. doi: 10.1016/j.psyneuen.2017.08.019. [DOI] [PubMed] [Google Scholar]
- van Rooij S.J., Stevens J.S., Ely T.D., Fani N., Smith A.K., Kerley K.A., Lori A., Ressler K.J., Jovanovic T. Childhood trauma and COMT genotype interact to increase hippocampal activation in resilient individuals. Front. Psychiatry. 2016;7 doi: 10.3389/fpsyt.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter S., Dulaev I., Mueller M., Henley R.R., Gallo W.T., Kanukova Z. Impact of resilience enhancing programs on youth surviving the Beslan school siege. Child Adolesc. Psychiatry Mental Health. 2010;4:11. doi: 10.1186/1753-2000-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M., Nelson E.E., Scaramozza M., Waldeck T., Hazlett G., Southwick S.M., Pine D.S., Drevets W., Charney D.S., Ernst M. Reward circuitry in resilience to severe trauma: an fMRI investigation of resilient special forces soldiers. Psychiatry Res. Neuroimaging. 2009;172:75–77. doi: 10.1016/j.pscychresns.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh C.E., Wager T.D., Fredrickson B.L., Noll D.C., Taylor S.F. The neural correlates of trait resilience when anticipating and recovering from threat. Soc. Cogn. Affect. Neurosci. 2008;3:322–332. doi: 10.1093/scan/nsn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto T.P., Gazzaley A. Fronto-parietal network: flexible hub of cognitive control. Trends Cogn. Sci. 2013;17:602–603. doi: 10.1016/j.tics.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]