Abstract
Error-monitoring abnormalities in stimulant-dependent individuals (SDIs) may be due to reduced awareness of committed errors, or to reduced sensitivity upon such awareness. The distinction between these alternatives remains largely undifferentiated, but may have substantial clinical relevance. We sought to better characterize the nature, and clinical relevance, of SDIs' error-monitoring processes by comparing carefully isolated neural responses during the presentation of negative feedback to a) stimulant dependence status and b) lifetime stimulant use. Forty-eight SDIs and twenty-three non-SDIs performed an fMRI-based time-estimation task specifically designed to isolate neural responses associated with the presentation (versus expectation) of contingent negative feedback. SDIs showed reduced dACC response compared to non-SDIs following the presentation of negative feedback, but only when error expectancies were controlled. Moreover, lifetime stimulant use correlated negatively with magnitude of expectancy-controlled dACC attenuation. While this finding was minimized after controlling for age, these results suggest that SDIs may be characterized by a core reduction in neural activity following error feedback, in the context of intact feedback expectancies. Correlations with lifetime stimulant use suggest that this neural attenuation may hold clinical significance.
Keywords: Stimulant dependence, Cocaine, Negative feedback, Anterior cingulate, Relapse, Biomarker
Highlights
-
•
Examined whether SDIs error-related deficits are due to reduced error-awareness, or reduced sensitivity upon such awareness.
-
•
A time-estimation task was used that isolated SDI's and non-SDI's neural responses to the presentation and expectation of negative feedback.
-
•
SDIs showed reduced dACC response following the presentation of negative feedback, but only when error-expectancies were controlled.
-
•
dACC signal change to the presentation of negative feedback distinguished SDI/non-SDI, and predicted stimulant use history.
1. Introduction
Stimulant dependent individuals (SDIs) are characterized by a persistent use of drugs despite repeated negative consequences, and this has been interpreted in terms of a potentially deficient action-monitoring system (Franken et al., 2007; Hester et al., 2013, Hester et al., 2007). A growing body of work has provided both electrophysiological and hemodynamic evidence indicating that substance abuse is characterized by abnormal ACC/mFC responses to rewards and errors (Hester et al., 2013; Kaufman et al., 2003; Steele et al., 2014; but see Alexander et al., 2015; Baker et al., 2016; Castelluccio et al., 2013), as well as to feedback indicating rewards and errors (Fedota et al., 2015; Parvaz et al., 2015; Patel et al., 2013). Moreover, a handful of recent studies have demonstrated significant relationships between magnitude of dACC/mFC response to error-related information and clinically-relevant downstream metrics of abuse (Luo et al., 2013; Marhe et al., 2013; Moeller et al., 2014; Steele et al., 2014). Nonetheless, the precise nature of these error-monitoring abnormalities is still unclear. For instance, it remains to be determined whether SDIs are characterized by a reduced recognition of committed errors, or by a reduced sensitivity following such recognition. Work in normative populations has consistently validated this distinction (e.g. (Delgado et al., 2000), and has highlighted its importance for characterizing the nature of error-monitoring failures when they occur. Applied to SDIs, evidence of reduced error awareness may imply a central impairment in the ability to recognize relevant behavioral outcomes (Hester et al., 2007). Alternately, evidence of an attenuated neural response subsequent to recognition of the error may imply a more basic insensitivity to the presentation of error-related information. These subtle differences in the nature of SDI's error-monitoring abnormalities may underlie important details regarding the pathogenesis of the disorder, and may help inform the development of targeted treatment protocols.
Of significant inconvenience, precise isolation of neural responses underlying the presentation versus recognition of error-related feedback is not so easily accomplished, as the magnitude of neural response to error is generally reciprocal to that error's prior expectancy (Holroyd and Coles, 2002; Holroyd et al., 2004); thus, the mere expectation of error-related feedback may interfere with the accurate characterization of presentation-phase sensitivity. Several approaches devised to reduce the impact of this reciprocity have attempted to remove the contingency between response and outcome (e.g. guessing tasks, random-outcome gambling tasks; (Delgado et al., 2000); however, in so doing, these tasks have difficulty evaluating critical relationships between outcome processing and subsequent behavioral change (Shane and Weywadt, 2014). Other approaches have parsed the ‘expectation’ versus ‘presentation’ phases of outcome processing into distinct temporal units (e.g. monetary incentive delay tasks; MID; (Knutson et al., 2001a, Knutson et al., 2001b, Knutson et al., 2005)); while this retains important response-outcome contingencies, it is unclear whether the temporal isolation of expectation/presentation phases truly controls for the reciprocal nature of error-related activity during each phase (Shane and Weywadt, 2014).
Perhaps due to these shortcomings, we are aware of only three studies that have attempted isolation of presentation-phase activity in SDIs, and these have yielded inconsistent results. Patel et al. (2013) failed to find cocaine-related dACC reductions during either phase of a MID task. Similarly, Rose et al. (2017) failed to find any differences in dACC in cocaine users compared to controls, but did report reduced reward outcome-related responses in the right habenula in cocaine users. In contrast, a third study reported that cocaine-dependent individuals showed electrophysiological evidence of reduced sensitivity to unexpected, but not expected, error feedback on a gambling-type task (Parvaz et al., 2015). The latter result suggests that SDIs' error-monitoring abnormalities may be caused by fundamental insensitivity to the presentation of error-related feedback. However, additional research would aid firm conclusions, and help determine the extent to which reduction in error-sensitivity are associated with downstream metrics of abuse.
To these ends, the present study utilized an fMRI-based time-estimation task specifically designed to evaluate neural responses following the presentation of outcome feedback in a way that could control for prior expectancies (Shane and Weywadt, 2014). Of primary interest was the extent to which SDIs and non-SDIs would show differential neural responses following presentation of error feedback when variance associated with prior expectations was, and was not, experimentally controlled. Consistent with the extant literature (Hester et al., 2013; Kaufman et al., 2003; Parvaz et al., 2015), we hypothesized that SDIs would demonstrate reduced error-related responses compared to non-SDIs following presentation of contingent negative feedback. Of import, and consistent with Parvaz et al. (2015), we predicted that this reduced error-related response would occur only when error-expectancies were controlled, and would disappear when error-expectancies were allowed to vary. This result would suggest that SDIs' error-monitoring abnormalities are the result of reduced sensitivity to unexpected negative feedback, rather than by reduced tendency to form such expectations.
An additional goal of the present study was to serve as a preliminary test of the clinical utility of any witnessed error-related abnormalities in the SDI group. Only a handful of such studies have been conducted to date, and none have attempted to parse expectation/presentation phases of outcome processing (Parvaz et al., 2015; Patel et al., 2013). With this in mind, we evaluated the extent to which the magnitude of error-related responses would relate to lifetime stimulant use (Parvaz et al., 2015). Following our broader hypothesis, we predicted that these clinically-relevant relationships would also present only when error-expectancies were controlled.
2. Methods and materials
2.1. Participants and diagnostic categories
Eighty-four participants were recruited through advertisements placed in probation/parole offices throughout the Albuquerque metropolitan area. Advertisements specifically targeted individuals with a criminal history who were currently on probation or parole with or without a history of cocaine use. Participants were phone-screened regarding key inclusion/exclusion components of the study, and were further screened in person to determine eligibility. After obtaining consent, SDI status was determined via the Structured Clinical Interview for DSM-IV Disorders (SCID; (First et al., 2002)). SDIs met dependence criteria for cocaine or stimulant dependence; non-SDIs met criteria for neither. To best compare SDI and non-SDI groups, and isolate differences specifically associated to stimulant dependence, both SDI and non-SDI groups were allowed to meet for other co-morbid abuse/dependence diagnoses. Thus, any differences seen between groups can be more confidently attributed to the stimulant dependence diagnosis, rather than to some other comorbid condition. Exclusionary criteria included: history of head injury that caused >30 min lost consciousness, past or current history of brain lesion or CNS disease (e.g. stroke, MS, epilepsy or repeated seizure history), Axis I DSM-IV lifetime history of psychotic disorder in self, report of psychotic disorder in first degree relative, history of alcohol-induced seizures, significant major medical disease, or current mood disorder (past six months), hypertension or diabetes, current pregnancy, mental retardation, left handedness, suicidal ideation, and positive urine drug screen on day of scan.
Comprehensive substance abuse histories were obtained via a modified version of the Addiction Severity Index – Expanded (ASI-X; (McLellan et al., 1980)). All but four participants had been abstinent for at least the previous 30 days; all reported results remain significant if these four participants are removed from analyses. In addition, scores on the Beck Depression Inventory-II (BDI-II; (Beck et al., 1996)), the Spielberger State-Trait Anxiety Scale (STAI; (Spielberger et al., 1983)), and the two-subtest Weschler Abbreviated Intelligence Scale for Adults (WASI; (Wechsler, 1999)) were collected and included as covariates in relevant analyses. Groups were carefully monitored for differences in gender, age, IQ and comorbid diagnoses, with targeted recruitment of individuals with specific characteristics sometimes employed to minimize significant differences across important demographic and clinical variables (see Table 1 for full demographic/clinical details).
Table 1.
Demographics and DSM-IV diagnoses across stimulant dependent individuals (SDI) and non-stimulant dependent individuals (non-SDI).
| SDI | Control | Test statistic | p | |
|---|---|---|---|---|
| n | 48 | 23 | ||
| Females | 10 (21%) | 1 (4%) | 2.09 | 0.15 |
| Age | 35.5 (8.3) | 28.9 (8.7) | 3.14 | 0.003 |
| Hispanic/Latinoa | 26 (54%) | 11 (48%) | 0.06 | 0.81 |
| IQ | 102.8 (12.5) | 103.7 (11.8) | 0.30 | 0.77 |
| Mood disorders | ||||
| Bipolar (any) | 0% | 0% | ||
| Major depression | 13 (27%) | 3 (13%) | 1.04 | 0.31 |
| Substance induced mood disorder | 5 (10%) | 0 (0%) | 1.23 | 0.27 |
| Schizophrenia/psychotic | ||||
| Schizophrenia/psychotic | 0% | 0% | ||
| Substance-related | ||||
| Alcohol dependence | 29 (60%) | 7 (30%) | 4.57 | 0.03 |
| Sedative/hypnotic/anxiolytic | 1 (2%) | 0 (0%) | 0 | 1 |
| Cannabis dependence | 23 (48%) | 9 (39%) | 0.19 | 0.66 |
| Stimulant dependence | 23 (48%) | 0 (0%) | 14.18 | <0.001 |
| Opioid dependence | 21 (44%) | 5 (22%) | 2.37 | 0.12 |
| Cocaine dependence | 40 (83%) | 0 (0%) | 40.57 | <0.001 |
| Hallucinogen/PCP | 6 (12%) | 0 (0%) | 1.73 | 0.19 |
| Poly drug | 3 (6%) | 0 (0%) | 0.35 | 1 |
| Anxiety | ||||
| Panic disorder | 7 (15%) | 0 (0%) | 2.26 | 0.13 |
| Agoraphobia | 1 (2%) | 0 (0%) | 0 | 1 |
| Social phobia | 4 (8%) | 0 (0%) | 0.77 | 0.38 |
| Specific phobia | 4 (8%) | 0 (0%) | 0.77 | 0.38 |
| OCD | 1 (2%) | 0 (0%) | 0 | 1 |
| PTSD | 14 (29%) | 2 (9%) | 2.65 | 0.10 |
| Generalized anxiety | 5 (10%) | 0 (0%) | 1.23 | 0.27 |
| Beck Depression Inventory-IIb | 15.39 (12.40) | 7.00 (7.27) | 2.94 | 0.006 |
| Cigarette smoker | 19 (40%) | 8 (35%) | 0.15 | 0.70 |
Statistical tests include t-test for age, and z-test of proportions for all other variables.
One non-SDI and two SDIs failed to report ethnicity.
Two non-SDIs and seven SDIs did not have BDI-II data available for analysis.
2.2. Study design
2.2.1. Time estimation task
The time estimation task was modified from Shane and Weywadt (2014) (see also van der Veen et al., 2011), and designed to evaluate neural responses to the presentation of contingent performance feedback with and without variance associated with performance expectancies controlled. Each trial began with an asterisk, presented centrally on a video screen (visual angle ~3°).
Participants were instructed to wait for the asterisk to disappear, and to press a button with their index finger when they felt 1 s had transpired from the asterisk's offset. Next, participants rated their level of confidence in their estimate. Finally, following a jittered interval, participants received one of several feedback symbols regarding the accuracy of their estimate. Responses were deemed successful if they fell within a desired window surrounding 1000 ms. This window was initially set at +/−250 ms; thus time estimates between 750 ms and 1250 ms were initially deemed successful, and any estimate outside this window was deemed unsuccessful. The size of the window was adaptive, on a trial-by-trial basis, depending on participants' performance throughout the course of the task. Every time a successful estimate was made, the window decreased by 30 ms; every time an unsuccessful estimate was made, the window increased by 30 ms. Previous work with this type of task has provided substantial evidence of the validity of this adaptive algorithm for balancing trial presentation in both healthy and clinical populations (Becker et al., 2014; Shane and Weywadt, 2014). The task was self-paced. Each participant completed 56 trials in each of two runs, for a total of 112 trials.
On half of the trials, feedback was Informative: participants received a ‘+’ sign to indicate an accurate estimate, or a ‘−’ sign to indicate an inaccurate estimate. On the other half of the trials, feedback was Uninformative: participants received a ‘?’ that provided no accuracy information. The ‘?’ feedback was presented randomly on 50% of accurate trials, and 50% of inaccurate trials; thus, on each trial, participants had an equal expectation of either Informative or Uninformative feedback. The uninformative feedback trials served as a critical design feature: unlike the IncorrectINFORMED > CorrectINFORMED contrast (within which performance and performance feedback are necessarily confounded), the IncorrectINFORMED > IncorrectUNINFORMED contrast differs only in the type of feedback provided. Thus observed differences in neural response in the IncorrectINFORMED > IncorrectUNINFORMED contrast can be specifically attributed to the presentation of the informed feedback.
2.2.2. MRI acquisition
All participants completed a urine screen prior to scanning. MRI data was collected on a 3 T Siemens Trio (Erlangen, Germany) whole body scanner with gradient-echo pulse sequence (TR = 2000 ms, TE = 29, flip angle = 75, 33 axial slices, 64 × 64 matrix, 3.75 × 3.75 mm2, 3.5 mm thickness, 1 mm gap) acquired with a 12-channel head coil.
2.3. Data analytic strategies
2.3.1. Behavior analyses
Behavioral measures were collected on each trial, and included estimation accuracy, estimation accuracy on trial n + 1 (to evaluate post-feedback behavioral adjustments), and confidence ratings (on a 1–4 Likert scale). Time estimations were extracted for each condition of interest. Time estimations >10 s were removed, followed by all estimations >3 standard deviations from individual subject means, to prevent undue influence from outliers. This process resulted in 104 total trials being removed from the analysis (1.4% of all data).
2.3.2. Neuroimaging analyses
All image analyses were undertaken with FMRIB's Software Library (FSL) version 4.1.0 (Smith et al., 2004), using standard preprocessing parameters. FSL's Motion Correction using FMRIBs Linear Image Registration Tool (MCFLIRT; (Jenkinson et al., 2002)) was used to realign functional images within a given run to the middle volume within the run. Images were deskulled using FSL's Brain Extraction Tool (BET; (Smith, 2002)), spatially smoothed with a 5 mm full-width half-max Gaussian kernel, temporally filtered using a high-pass filter of 50 s, prewhitened using FMRIBs Improved Linear Model (FILM) and grand mean intensity normalized; all of these steps were performed using FMRIB's Expert Analysis Tool (FEAT; Woolrich et al., 2004).
All analyses were run as part of a 3-stage process. In the first stage, customized regressors were created for each participant for four events: cue, estimate (correct, incorrect), confidence ratings, and feedback (informed-correct, informed-incorrect, uninformed-correct, uninformed-incorrect). In addition, six first-order motion parameters were included in the statistical model. Statistical analyses were performed using the general linear model as implemented in FEAT. Time series analyses were conducted using FILM (Woolrich et al., 2004) with local autocorrelation estimation. This first level analysis generated parameter estimates for each condition of interest. Contrast maps were registered to the participant's high-resolution anatomical image and the MNI 152 brain template using FLIRT (Jenkinson et al., 2002). Next, analyses from each run within a participant were combined using a fixed effects model in FEAT. This second level analysis generated mean effects and within subject variance estimates that were used in third level analyses. Within the task, we were interested in 4 conditions of interest (i.e. Informed Correct, Informed Incorrect, Uninformed Correct, Uninformed Incorrect), which were analyzed using a 2 (Performance: Correct, Incorrect) × 2 (Feedback Type: Informed, Uninformed) × 2 (Group: SDI, non-SDI) mixed-model ANOVA. In addition, two primary contrasts were computed at the individual subject level to support hypothesis driven analyses of group differences in feedback processing: IncorrectINFORMED > CorrectINFORMED (within which performance expectancies necessarily varied), and IncorrectINFORMED > IncorrectUNINFORMED (within which performance expectancies were effectively controlled); each of these contrasts were compared across groups using an independent samples t-test.
Whole brain group analyses were conducted using permutation testing with the randomise program within FSL. Threshold free cluster enhancement (TFCE) was used to correct for multiple comparisons at p < 0.05.
2.3.3. Years of use
Lifetime stimulant use assessment was consistent with general PhenX guidelines (Conway et al., 2014), and total years of use equaled the summed total years of use for all stimulants queried via the ASI-X. Correlation analyses investigated the relationship between lifetime years of use and percent signal change within each of two 10 mm spherical ROIs drawn around the peak dACC voxels within the two clusters that emerged in the comparison of SDI and non-SDI for the IncorrectINFORMED > IncorrectUNINFORMED contrast.
3. Results
3.1. Sample characterization
Following exclusions for motion, performance and psychotropic drug use (see Supplement for full details regarding exclusion criteria), 71 participants (48 SDIs and 23 non-SDIs) were included in the final dataset. Table 1 provides full demographics.
3.2. Behavioral results
3.2.1. Accuracy
Necessarily, a main effect of Accuracy indicated that participants' time estimates were more accurate on correct than incorrect trials, F(1, 69) = 414.38, p < 0.001. Importantly, however, the main effect of Information did not reach significance, indicating that performance was similar across Informed and Uninformed trials. Similarly, no group effects, nor interactions, reached significance (see Table 2 and Fig. S1a in Supplement).
Table 2.
Means and standard deviations for performance measures in the time estimation task.
| Time estimation | SDI |
Non-SDI |
|||
|---|---|---|---|---|---|
| Informative | Uninformative | Informative | Uninformative | ||
| Deviation from 1000 ms | Correct | 143 (65) | 146 (78) | 158 (76) | 154 (83) |
| Incorrect | 432 (137) | 425 (136) | 425 (168) | 432 (202) | |
| Post-feedback change | Correct | 167 (64) | 182 (83) | 157 (77) | 160 (60) |
| Incorrect | 225 (81) | 210 (71) | 231 (116) | 209 (143) | |
Deviation from 1000 ms and post-feedback change values are presented in milliseconds. Deviation scores represent the difference between the 1000 ms target estimation and the mean absolute value of deviations from 1 s. Post-feedback change scores represent the change in magnitude of deviation for trial n to n + 1. To compute change, the difference in the absolute value of the deviation from 1 s on trial (n) to the absolute value of the deviation from 1 s on trial (n + 1) was computed. Main effects for performance (i.e. Correct vs. Incorrect) were significant, but main effects for Feedback Type (Informative vs. Uninformative) and group (SDI vs. non-SDI) were not significant.
3.2.2. Confidence
The time estimation task was specifically designed to maximize neural response to informative feedback by minimizing the development of outcome awareness. Consistent with this goal, while participants showed fairly high confidence in their estimation attempts (mean confidence rating = 1.78 (0.87)), the correlation between confidence and accuracy, while significant, was only low-to-moderate, r(68) = 0.14, p < 0.01. Importantly, this was true for both SDI (r = 0.17, p < 0.01) and non-SDI (r = 0.10, p < 0.01) groups (between group t-test: t(67) = 1.61, p = ns); moreover, no significant group differences in confidence were found in any condition (all p's > 0.20).
3.2.3. Post-feedback behavioral adjustments
Trial-to-trial adjustments in estimation attempts were evaluated by calculating the deviation from 1000 ms on trial n + 1. A 2 (Accuracy) × 2 (Information) × 2 (Group) mixed-model ANOVA indicated that participants' estimation adjustments were greater following incorrect compared to correct trials, F(1, 69) = 62.94, p < 0.001. While main effects of Information and Group did not reach significance, a significant Accuracy × Information interaction, F(1, 69) = 3.86, p = 0.05, indicated that the difference between performance adjustments following correct/incorrect feedback was greater for Informed compared to Uninformed feedback (see Table 2 and Fig. S1b in Supplement).
3.3. Neuroimaging results
3.3.1. Main effects and interactions
The 2 (Accuracy) × 2 (Information) × 2 (Group) mixed-model ANOVA revealed a main effect of Accuracy, within several clusters including dorsal and pregenual ACC (d/pg ACC), bilateral nucleus accumbens, and bilateral occipital cortex. A main effect of Information was also identified within several regions including d/pg ACC, bilateral anterior insula, and nucleus accumbens. Finally, a main effect of group was identified within right cerebellum and left lingual gyrus. An Accuracy × Feedback interaction revealed significant clusters within bilateral occipital pole, ventral striatum, and left inferior parietal lobe. The Information × Group interaction, Group × Accuracy interaction and three-way Accuracy × Information × Group interaction revealed no significant clusters. See Table S1 in Supplement for all regions reaching significance within these higher-order analyses.
3.3.2. Contrasts of interest
IncorrectINFORMED > IncorrectUNINFORMED and IncorrectINFORMED > CorrectINFORMED served as primary contrasts of interest. Here we report effects within these contrasts across all participants. Below we present the group-relevant distinctions. Other contrast-level effects can be found in the Supplement.
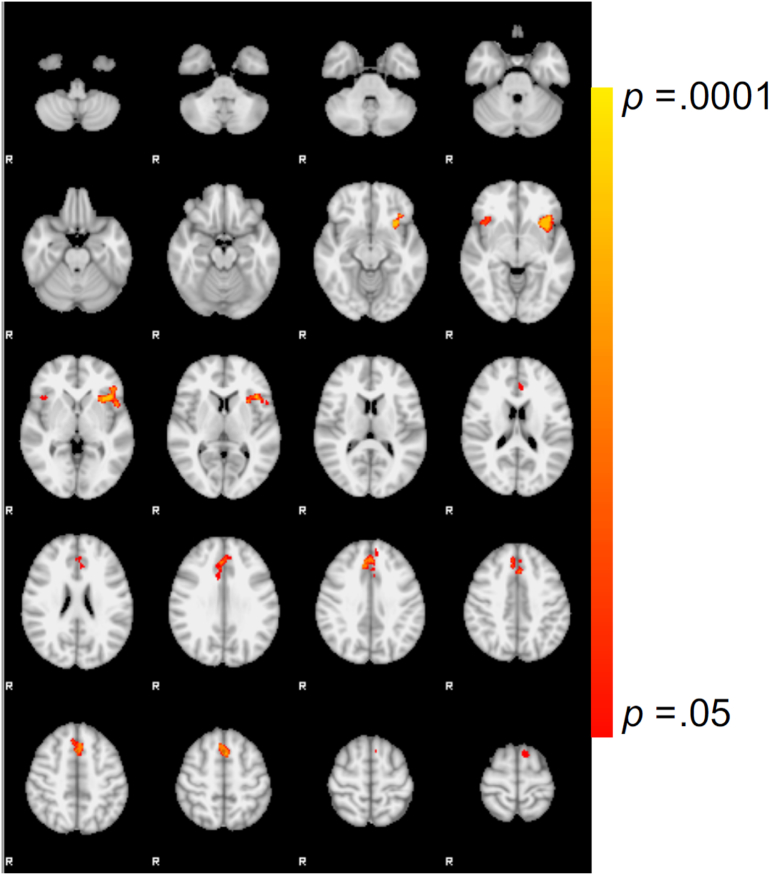
IncorrectINFORMED > IncorrectUNINFORMED. By controlling for expectancy effects related to differential performance, this contrast provides a well-controlled evaluation of the brain's response to the specific presentation of negative feedback. Results revealed greater response following the presentation of IncorrectINFORMED feedback within several regions, including d/pg ACC and bilateral insula (see Fig. 1 and Table 3). No regions showed greater activity following the presentation of IncorrectUNINFORMED feedback within this contrast.
Fig. 1.
IncorrectINFORMED > IncorrectUNINFORMED feedback. Main effect of IncorrectINFORMED > IncorrectUNINFORMED (TFCE corrected at p < 0.05).
Table 3.
Brain regions showing significant differences in the IncorrectINFORMED > IncorrectUNINFORMED and IncorrectINFORMED > CorrectINFORMED contrasts. Analyses were corrected for multiple comparisons using TFCE with 5000 permutations at p < 0.05.
| Contrast | Region | Voxels | x | y | z | p |
|---|---|---|---|---|---|---|
| IncorrectINFORMED > IncorrectUNINFORMED | preSMA/SFG | 851 | −2 | 14 | 52 | 0.018 |
| L OFC/Insula | 727 | −38 | 22 | −8 | 0.01 | |
| R OFC/insula | 124 | 44 | 20 | −6 | 0.033 | |
| IncorrectINFORMED > CorrectINFORMED | R OFC/insula/IFG | 4528 | −32 | 18 | −14 | <0.001 |
| L OFC/insula/IFG | 3112 | 34 | 20 | −16 | <0.001 | |
| dACC/pgACC | 2771 | −4 | 36 | 24 | 0.001 | |
| L IPL/angular gyrus | 1181 | −52 | −56 | 40 | 0.001 | |
| R IPL/angular gyrus | 663 | 54 | −46 | 46 | 0.007 | |
| L occipital pole | 383 | −24 | −98 | −8 | 0.002 | |
| R occipital pole | 135 | 28 | −92 | −4 | 0.011 | |
| R middle frontal/IFG | 7 | 62 | 22 | 26 | 0.049 | |
| Non-SDI > SDI (IncorrectINFORMED > IncorrectUNINFORMED) | pgACC | 71 | 4 | 40 | 0 | 0.032 |
| Frontal pole | 54 | 42 | 56 | 4 | 0.037 | |
| dACC | 46 | 6 | 36 | 20 | 0.043 |
IncorrectINFORMED > CorrectINFORMED. No regions showed greater response following IncorrectINFORMED feedback in this contrast. However, several regions, including bilateral insula, bilateral ventral striatum and pgACC, showed greater response to CorrectINFORMED feedback (see Table 3).
3.3.3. Group Effects (SDI vs. non-SDI)
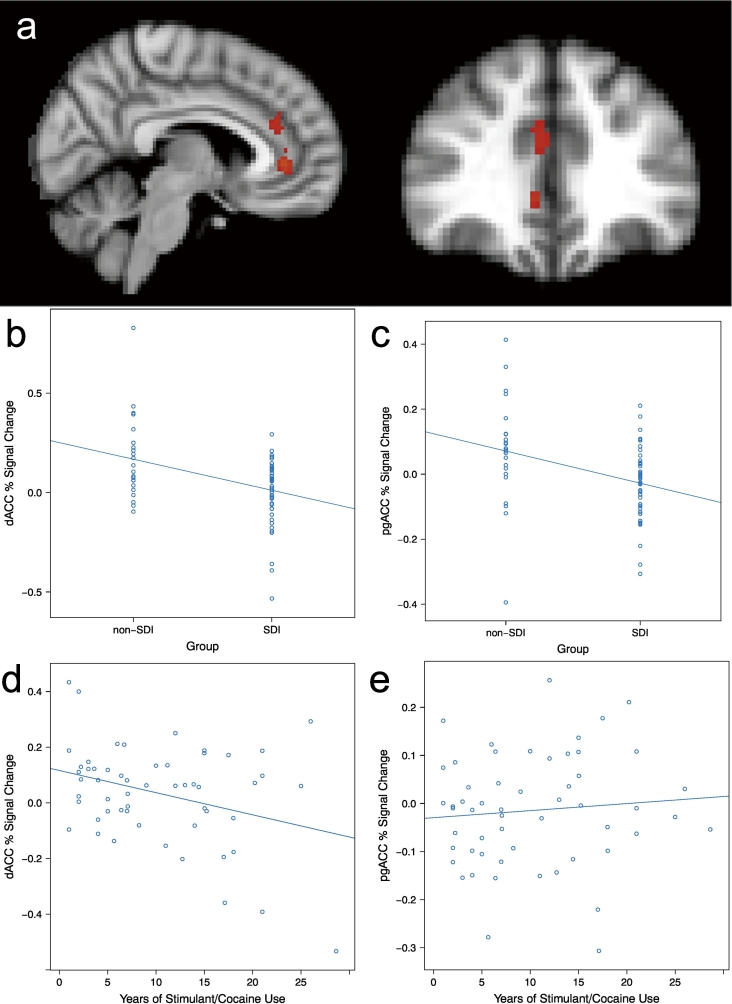
A priori hypotheses centered around differences in post-error neural responses between the SDI and non-SDI groups. To this end, we undertook targeted two-sample t-tests to evaluate group differences in the IncorrectINFORMED > IncorrectUNINFORMED and CorrectINFORMED contrasts. These analyses indicated that SDIs showed reduced ACC response compared to non-SDIs in the IncorrectINFORMED > IncorrectUNINFORMED contrast in clusters within both dorsal and pregenual portions of the ACC (see Fig. 2a, b; TFCE corrected p < 0.05). However, no such group differences were revealed in the IncorrectINFORMED > CorrectINFORMED contrast. Thus, only when performance expectancies were controlled did SDIs show attenuated ACC response compared to non-SDIs following presentation of negative feedback. Importantly, repeating this group level analysis with relevant covariates included (i.e. drug use, BDI, STAI, and age) did not alter the direction of these effects (see Supplement for details).
Fig. 2.
Association between error-related BOLD response, stimulant dependence and use history. a) d/pg ACC region within which SDIs (n = 48) showed reduced response compared to non-SDIs (n = 23 in the IncorrectINFORMED > IncorrectUNINFORMED contrast (TFCE corrected at p < 0.05). b and c) Percent signal change in b) dACC and c) pgACC by group. d and e) Correlation between years of stimulant use and BOLD response during the IncorrectINFORMED > IncorrectUNINFORMED contrast in the d) dACC and e) pgACC.
Group differences were also evaluated in the CorrectINFORMED > IncorrectINFORMED and the CorrectINFORMED > CorrectUNINFORMED contrasts, to evaluate potential differences associated with reward processing. However, only one region within left lateral occipital cortex reached significance in the CorrectINFORMED > IncorrectINFORMED contrast, and no regions reached significance in the CorrectINFORMED > CorrectUNINFORMED contrast. Thus, while broad dACC activation was seen to both positive and error feedback, only error-related responses manifested as group differences.
3.3.4. Relationship with years of use
Lifetime years of stimulant use was correlated with percent signal change in the dACC ROI derived from the group difference analysis. This analysis revealed a negative association between lifetime use and dACC response in the IncorrectINFORMED > IncorrectUNINFORMED contrast, r(53) = −0.33, p < 0.02, (see Fig. 2c). This correlation remained after controlling for IQ and lifetime years of other drug use, and dips slightly to p = 0.13 when further controlling for age., Parallel analyses with the pgACC showed no associations with years of use.
4. Discussion
The current study compared neural responses following the presentation of exogenous feedback in individuals with and without a stimulant dependence disorder. Results indicated that SDIs exhibited reduced dACC and pgACC response following the presentation of negative feedback; moreover, the magnitude of SDIs' dACC reductions was correlated with the length of stimulant use history. While the latter effect was minimized after taking age into account, these findings converge with a growing body of work indicating that SDIs are characterized by identifiable error-related processing abnormalities (Franken et al., 2007; Hester et al., 2013, Hester et al., 2007; Kaufman et al., 2003; Patel et al., 2013), and emphasize the role that these abnormalities may play in the development and maintenance of stimulant use behaviors. Consistent with the proposed role of dACC in signaling to lateral prefrontal regions the need for phasic increases in cognitive control (Botvinick et al., 2001; Holroyd and Coles, 2002), a reduced dACC response to error feedback in SDIs may signify insufficient engagement of control mechanisms in the face of relevant error-related information.
The present study's primary goal was to better characterize the extent to which SDIs' error-processing abnormalities were the result of reduced awareness, or reduced sensitivity, to error-related information. While prior studies have sought similar goals, an inability to control for outcome expectancies has hindered a full understanding of the pathophysiology of SDIs' error processing abnormalities. Importantly, the present results demonstrated that SDIs' attenuated d/pgACC response following negative feedback occurred only when variance associated with prior behavior (and thus behavioral expectancies) was fully controlled. This effect is subtle, but may provide clarification to a nascent, and currently inconsistent, literature (Parvaz et al., 2015; Patel et al., 2013; Rose et al., 2017). By controlling for expectancy effects, the task affords a pure measure of sensitivity to the mere presentation of negative feedback. In this context, SDIs showed a significantly attenuated dACC response. The fact that this effect disappeared when expectancies were allowed to vary further suggests that this reduced sensitivity may exist within the context of otherwise intact expectancy formation (see also (Parvaz et al., 2015)).
Of equal import, associations with SDIs' lifetime stimulant use were also identified only when performance metrics were held constant. While this effect was somewhat mitigated after controlling for age, it nonetheless suggests that SDIs' attenuated responses following presentation of negative feedback may in fact play an important role in the pathophysiology of the disease state. A handful of previous studies have reported similar associations between error-related responses and clinically-relevant abuse metrics (Luo et al., 2013; Marhe et al., 2013; Moeller et al., 2014; Steele et al., 2014); however, the driving force behind these associations has not yet been characterized. The present results suggest that a core insensitivity to the presentation of negative feedback may be an important factor in this process. One possibility is that a reduced sensitivity to negative feedback may interfere with the ability to incorporate the information held within that feedback into existing behavioral repertoires (see Shane and Peterson, 2004).
It is relevant to note that both groups showed increased dACC response following presentation of positive feedback, but that these activations did not manifest as group differences. Thus, group differences in d/pgACC response were specific to the presentation of error-related feedback. While coinciding with a growing body of work that has positioned ACC as a core structure within the mesocorticolimbic reward system, it does conflict with some previous work that has reported reward-related associations with drug use behaviors (e.g. (Baker et al., 2016)). Much of this previous work has focused on evaluating cortical and sub-cortical responses to drug- and non-drug rewards (Goldstein et al., 2009; Volkow et al., 2011), and to engagement of cognitive control mechanisms towards inhibition of a desired reward (Garavan and Hester, 2007; Motzkin et al., 2014; Volkow et al., 2010). It may be that engagement of dACC in these inhibitory contexts is also abnormal in substance abusers; however, the mere presentation of positive feedback in the present study did not elicit similar group differences.
Some limitations of this study should be noted. First, despite our best attempts, the frequency with which SDI and non-SDI participants expressed comorbid dependencies differed somewhat across the two groups. While such differential comorbidities could have contributed to differences between SDI and non-SDI groups (Hester et al., 2007; Luo et al., 2013), it is important to note that all reported results remained significant when lifetime years of other drugs was included as a covariate (see Supplementary results). Second, because both SDI and non-SDI groups were recruited through probation/parole, they all had significant criminal histories. To some extent, this may be expected, given the high rate of comorbidity between substance use disorders and criminal activity (Fazel et al., 2006). Nonetheless, it is important to note that the extent to which our results may generalize to SDIs without significant criminal histories, or less antisocial personalities (see Cope et al., 2012), remains an open question. Third, we did not observe a similar relationship between dACC response and magnitude of post-feedback behavioral change. We note that while correlations between error-related responses and post-error changes are commonly reported within healthy populations (Danielmeier and Ullsperger, 2011), they are less reliably elicited within clinical populations including SDIs (Hester et al., 2007; Moeller et al., 2014). One possibility is that the effects of reduced error processing sum cumulatively, such that each indication of reduced processing may impose only minimal influence on subsequent behavior. Finally, as with most studies of this nature, it is difficult to rule out the possibility that participants' dACC attenuations predated their stimulant use. Nonetheless, the fact that lifetime use correlated with magnitude of these attenuations suggests that longer-term, or more severe, use may further intensify alterations to this feedback processing system.
In summary, the current study demonstrated that dACC response following presentation of negative feedback, when unconfounded by error expectancies, was 1) attenuated in SDIs and 2) negatively associated with lifetime years of use. Negative feedback-related ACC response may provide a useful biomarker of stimulant dependence, and a potential target for treatments.
Financial disclosures
Drs. Claus and Shane report no financial conflicts of interest.
Acknowledgements
This work was supported by a grant from the National Institute on Drug Abuse (R01DA026932) to Dr. Shane. The National Institute on Drug Abuse did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. We would like to acknowledge Mr. Ben Wasserott and Program Evaluation Services at the Center on Alcoholism, Substance Abuse, and Addictions at the University of New Mexico for recruitment of participants and data collection.
Footnotes
This work was presented previously at the annual meeting of the Society of Biological Psychiatry, May 14-16, 2015, Toronto, ON.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.05.007.
Appendix A. Supplementary data
Supplementary material
References
- Alexander W.H., Fukunaga R., Finn P., Brown J.W. Reward salience and risk aversion underlie differential ACC activity in substance dependence. NeuroImage: Clin. 2015;8:59–71. doi: 10.1016/j.nicl.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T.E., Stockwell T., Barnes G., Haesevoets R., Holroyd C.B. Reward sensitivity of ACC as an intermediate phenotype between DRD4-521T and substance misuse. J. Cogn. Neurosci. 2016;28:460–471. doi: 10.1162/jocn_a_00905. [DOI] [PubMed] [Google Scholar]
- Beck A., Steer R., Brown G. 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- Becker M.P.I., Nitsch A.M., Miltner W.H.R., Straube T. A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. J. Neurosci. 2014;34:3005–3012. doi: 10.1523/JNEUROSCI.3684-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M., Braver T., Barch D., Carter C., Cohen J. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Castelluccio B.C., Meda S.A., Muska C.E., Stevens M.C., Pearlson G.D. Error processing in current and former cocaine users. Brain Imaging Behav. 2013;8:87–96. doi: 10.1007/s11682-013-9247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway K.P., Vullo G.C., Kennedy A.P., Finger M.S. Data compatibility in the addiction sciences: an examination of measure commonality. Drug Alcohol Depend. 2014;141:153–158. doi: 10.1016/j.drugalcdep.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope L.M., Shane M.S., Segall J.M., Nyalakanti P.K., Stevens M.C., Pearlson G.D., Calhoun V.D., Kiehl K.A. Examining the effect of psychopathic traits on gray matter volume in a community substance abuse sample. Psychiatry Res. 2012;204:91–100. doi: 10.1016/j.pscychresns.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C., Ullsperger M. Post-error adjustments. Front. Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R., Nystrom L.E., Fissell C., Noll D.C., Fiez J.A. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- van der Veen F.M., Röder C.H., Mies G.W., van der Lugt A., Smits M. Remedial action and feedback processing in a time-estimation task: evidence for a role of the rostral cingulate zone in behavioral adjustments without learning. NeuroImage. 2011;54:447–454. doi: 10.1016/j.neuroimage.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Fazel S., Bains P., Doll H. Substance abuse and dependence in prisoners: a systematic review. Addiction. 2006;101:181–191. doi: 10.1111/j.1360-0443.2006.01316.x. [DOI] [PubMed] [Google Scholar]
- Fedota J.R., Sutherland M.T., Salmeron B.J., Ross T.J., Hong L.E., Stein E.A. Reward anticipation is differentially modulated by varenicline and nicotine in smokers. Neuropsychopharmacology. 2015;40:2038–2046. doi: 10.1038/npp.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Spitzer R., Gibbon M., Williams J. 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/PSY SCREEN) [Google Scholar]
- Franken I.H.A., Van Strien J.W., Franzek E.J., van de Wetering B.J. Error-processing deficits in patients with cocaine dependence. Biol. Psychol. 2007;75:45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Garavan H., Hester R. The role of cognitive control in cocaine dependence. Neuropsychol. Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Goldstein R., Alia-Klein N., Tomasi D., Carrillo J., Maloney T., Woicik P., Wang R., Telang F., Volkow N. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9453–9458. doi: 10.1073/pnas.0900491106. (doi:0900491106 [pii] 281073/pnas.0900491106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R., Simões-Franklin C., Garavan H. Post-error behavior in active cocaine users: poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology. 2007;32:1974–1984. doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- Hester R., Bell R.P., Foxe J.J., Garavan H. The influence of monetary punishment on cognitive control in abstinent cocaine-users. Drug Alcohol Depend. 2013:1–8. doi: 10.1016/j.drugalcdep.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd C., Coles M. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Nieuwenhuis S., Yeung N., Nystrom L., Mars R.B., Coles M.G.H., Cohen J.D. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat. Neurosci. 2004;7:497–498. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. (doi:S1053811902911328 [pii]) [DOI] [PubMed] [Google Scholar]
- Kaufman J.N., Ross T.J., Stein E.A., Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J. Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Adams C., Fong G., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21:15. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Fong G., Adams C., Varner J., Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B., Taylor J., Kaufman M., Peterson R., Glover G. Distributed neural representation of expected value. J. Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Zhang S., Hu S., Bednarski S.R., Erdman E., Farr O.M., Hong K.I., Sinha R., Mazure C.M., Li C.S.R. Error processing and gender-shared and -specific neural predictors of relapse in cocaine dependence. Brain. 2013;136:1231–1244. doi: 10.1093/brain/awt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R., van de Wetering B.J.M., Franken I.H.A. Error-related brain activity predicts cocaine use after treatment at 3-month follow-up. BPS. 2013;73:782–788. doi: 10.1016/j.biopsych.2012.12.016. [DOI] [PubMed] [Google Scholar]
- McLellan A.T., Luborsky L., Woody G.E., O'Brien C.P. An improved diagnostic evaluation instrument for substance abuse patients. The addiction severity index. J. Nerv. Ment. Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Moeller S.J., Konova A.B., Parvaz M.A., Tomasi D., Lane R.D., Fort C., Goldstein R.Z. Functional, structural, and emotional correlates of impaired insight in cocaine addiction. JAMA Psychiat. 2014;71:61. doi: 10.1001/jamapsychiatry.2013.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin J.C., Baskin-Sommers A., Newman J.P., Kiehl K.A., Koenigs M. Neural correlates of substance abuse: reduced functional connectivity between areas underlying reward and cognitive control. Hum. Brain Mapp. 2014;35:4282–4292. doi: 10.1002/hbm.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz M.A., Konova A.B., Proudfit G.H., Dunning J.P., Malaker P., Moeller S.J., Maloney T., Alia-Klein N., Goldstein R.Z. Impaired neural response to negative prediction errors in cocaine addiction. J. Neurosci. 2015;35:1872–1879. doi: 10.1523/JNEUROSCI.2777-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K.T., Stevens M.C., Meda S.A., Muska C., Thomas A.D., Potenza M.N., Pearlson G.D. Robust changes in reward circuitry during reward loss in current and former cocaine users during performance of a monetary incentive delay task. Biol. Psychiatry. 2013;74:529–537. doi: 10.1016/j.biopsych.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose E.J., Salmeron B.J., Ross T.J., Waltz J., Schweitzer J.B., Stein E.A. Dissociable effects of cocaine dependence on reward processes: the role of acute cocaine and craving. Neuropsychopharmacology. 2017;42:736–747. doi: 10.1038/npp.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane M.S., Peterson J.B. Defensive copers show a deficit in passive avoidance learning on Newman's go/no-go task: implications for self-deception and socialization. J. Pers. 2004;72:939–966. doi: 10.1111/j.0022-3506.2004.00286.x. [DOI] [PubMed] [Google Scholar]
- Shane M.S., Weywadt C.R. Voluntary modulation of anterior cingulate response to negative feedback. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Jenkinson M., Woolrich M., Beckmann C., Behrens T., Johansen-Berg H., Bannister P., De Luca M., Drobnjak I., Flitney D., Niazy R., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J., Matthews P. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. (doi:S1053-8119(04)00393-3 [pii] 10.1016/j.neuroimage.2004.07.051) [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the State-trait Anxiety Inventory. [Google Scholar]
- Steele V.R., Fink B.C., Maurer J.M., Arbabshirani M.R., Wilber C.H., Jaffe A.J., Sidz A., Pearlson G.D., Calhoun V.D., Clark V.P., Kiehl K.A. Brain potentials measured during a Go/NoGo task predict completion of substance abuse treatment. BPS. 2014;76:75–83. doi: 10.1016/j.biopsych.2013.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Fowler J.S., Wang G.-J., Telang F., Logan J., Jayne M., Ma Y., Pradhan K., Wong C., Swanson J.M. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Fowler J.S., Tomasi D., Telang F. Addiction: beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation: Harcourt Brace & Company; New York, NY: 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Woolrich M., Behrens T., Beckmann C., Jenkinson M., Smith S. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material