Abstract
Since it is known that environmental contaminants have the potential to cause endocrine disorders in humans and animals, there is an urgent need for in vivo tests to assess possible effects of these endocrine disrupting chemicals (EDCs). Although there is no standardized guideline, the avian embryo has proven to be particularly promising as it responds sensitively to a number of EDCs preferentially impacting the reproductive axis. In the present study we examined the effects of in ovo exposure to fulvestrant and tamoxifen as antiestrogenic model compounds and co-exposure to both substances and the potent estrogen 17α-ethinylestradiol (EE2) regarding sex differentiation and embryonic development of the domestic fowl (Gallus gallus domesticus). The substances were injected into the yolk of fertilized eggs on embryonic day 1. On embryonic day 19 sex genotype and phenotype were determined, followed by gross morphological and histological examination of the gonads. Sole EE2-treatment (20 ng/g egg) particularly affected male gonads and resulted in an increased formation of female-like gonadal cortex tissue and a reduction of seminiferous tubules. In ovo exposure to tamoxifen (0.1/1/10 µg/g egg) strongly impaired the differentiation of female gonads, led to a significant size reduction of the left ovary and induced malformations of the ovarian cortex, while fulvestrant (0.1/1/10 µg/g egg) did not affect sexual differentiation. However, both antiestrogens were able to antagonize the feminizing effects of EE2in genetic males when administered simultaneously. Since both estrogens and antiestrogens induce concentration-dependent morphological alterations of the sex organs, the chick embryo can be regarded as a promising model for the identification of chemicals with estrogenic and antiestrogenic activity.
Keywords: Gonad, Chicken embryo, Estrogen, Fulvestrant, Sex differentiation, Antiestrogen, 17α-ethinylestradiol, Tamoxifen, Endocrine disruption
Introduction
In recent decades, reproductive disorders in animals and humans and the potential role of chemical substances that are suspected to cause these effects through their endocrine potential became of great interest for science and society. These so-called endocrine disrupting chemicals (EDCs) may alter sex-differentiation and reproduction by very different modes of action. If a chemical substance has the same effects as endogenous sex hormones at the estrogen or androgen receptor, this substance acts as an agonist and its effects are referred to as estrogenic or androgenic. On the contrary it is referred to as antiestrogenic or antiandrogenic when it inhibits the action of endogenous sex hormones as an antagonist at the corresponding steroid receptor. In view of the large number of constantly used chemicals, it is expected that potential EDCs end up in the environment and may affect humans and animals. These chemicals can originate from agriculture or industry, or may be used as pharmaceuticals. In the study of steroidal and non-steroidal substances, e.g., bisphenol A (BPA), 17α-ethinylestradiol (EE2), tributyltin (TBT) and many more, hormonal effects on different groups of organisms have already been identified (Peakall & Lincer, 1996; Berg et al., 1998; Berg et al., 1999; Watts, Pascoe & Carroll, 2001; Grote et al., 2004; Berg & Pettersson, 2006; Oehlmann et al., 2006; Pettersson et al., 2006; Ahn et al., 2007; Choi et al., 2007; Bodiguel et al., 2009; Scheider et al., 2018; Jessl, Scheider & Oehlmann, 2018). These studies underline the assumption that numerous chemicals have an endocrine potential and may pose a potential threat to the ecosystem and to animal and human health.
In order to assess possible effects and to weigh risks, the testing of chemicals for their endocrine potential is of great importance. So far only a small fraction of the circulating and constantly used chemicals have been tested for a potential effect on the hormonal system. According to REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) further chemicals are to be tested for their harmful potential in Europe. To implement this, a variety of animal experiments have to be executed. For the testing of androgenic and estrogenic EDCs two rodent-based tests, the Hershberger assay (OECD, 2009) and the uterotrophic assay (OECD, 2007), have been internationally standardized. Since mainly juvenile or adult animals with full pain perception are used, the search for a suitable animal replacement system is of great importance. In addition, the developing embryo, which is regarded as the most sensitive stage of life and thus a special subject of protection, is insufficiently considered for the testing of chemicals. Since its development is particularly vulnerable to environmental influences including chemicals, the testing of embryos can unfold possible effects of these substances that may not be detected in adult individuals (Duis et al., 2014).
Beside developmental stages of other animal taxa, avian embryos have been used for a long time to study sexual development and the potential impact of environmental pollutants, including EDCs (Fry & Toone, 1981; Berg et al., 1998; Eising et al., 2001; Berge et al., 2004; Biau et al., 2007). One advantage of working with fertile eggs is that the application of substances, often injected directly into the egg, allows the use of specific and standardized dosages (Berg et al., 1999). As the hen affects the development of its offspring by transferred genetic materials and hormones (Carere & Balthazart, 2007), substances incorporated by the mother may consequently also influence the development of the offspring even originally or as metabolites in the allantoic fluid (Kamata et al., 2006). However, in contrast to developing mammals or aquatic species, the chicken egg is a largely closed system lacking significant exchange with its external environment except for the interchange of gases. However, it should be noted that beyond gas exchange there is still a potential of interaction with the external environment since the embryo is sensitive to changes in temperature and humidity. In addition, the passage of metals (Ackerman et al., 2016) and highly lipophilic organic compounds (Bargar, Scott & Cobb, 2001; Zheng et al., 2014) from mother to offspring has been demonstrated. Thus, one injection of a test compound results in chronic chemical exposure, because no exchange or loss of the substance is possible except for metabolization, protein bonding or further modifications of the substance by the internal embryo environment. A single injection may therefore be sufficient to influence the developing embryo (Davies et al., 1997; Gooding et al., 2003; McAllister & Kime, 2003; Zhang et al., 2007; Scheider et al., 2018; Jessl, Scheider & Oehlmann, 2018).
It is already known that the exposure to xenobiotics during avian embryonic development may cause irreversible malformations of the sex organs and a disruption of gender-specific behavior in adult animals (Adkins-Regan, 1990; Ottinger & Abdelnabi, 1997). The embryo of the domestic fowl (Gallus gallus domesticus) is particularly suitable for our experiments as its developmental stages are fully described (Keibel & Abraham, 1900; Hamburger & Hamilton, 1992; Starck & Ricklefs, 1997). However, there is still no standardized procedure for experiments with chicken embryos available.
The present study is part of a project aiming to expedite a protocol to assess the potential effect of EDCs on early sexual differentiation in the chicken embryo. As part of this effort we analyzed the effects of different estrogenic and antiestrogenic compounds on embryonic development with special focus on potential gross morphological and histological changes of the gonads. EE2, a synthetic hormone primarily used for contraception was selected for the study of estrogenic substances and was used as a positive substance. It has already been widely used in the study of EDCs and has shown to affect sexual differentiation in bird embryos (Berg et al., 1998; Berg et al., 1999; Berg et al., 2001; Berg et al., 2004; Akazome & Mori, 1999; Watts, Pascoe & Carroll, 2001; Watts, Pascoe & Carroll, 2003; Berg & Pettersson, 2006; Pettersson et al., 2006; Biau et al., 2007; Brunstrom et al., 2009; Scheider et al., 2018; Jessl, Scheider & Oehlmann, 2018). For the testing of antiestrogenic substances tamoxifen and fulvestrant, two well-known drugs with desired hormonal action were selected. Both compounds are used for the first-line endocrine therapy of estrogen receptor-positive metastatic breast cancer (Henderson, 1991; Buzdar, 2001; Buzdar & Robertson, 2006). Furthermore, both have been used for the testing of potential effects on different non-target organisms including the bird embryo (Scheib & Baulieu, 1981; Cevasco et al., 2008; Sun, Zha & Wang, 2009; Hoffmann & Kloas, 2012; Yu et al., 2014).
Materials and Methods
Dosing
All experiments were carried out with respect for the principles of laboratory animal care, in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and the German Animal Welfare Act. Fertilized eggs of white Leghorn (G. domesticus) were obtained from a local breeder (LSL Rhein-Main Geflügelvermehrungsbetrieb, Dieburg, Germany). The total number of eggs per experiment and treatment-group are shown in Table 1 for fulvestrant and Table 2 for tamoxifen, including the treatment-groups with parallel sole and co-exposure to 17α-ethinylestradiol (EE2). The testing of tamoxifen was conducted in a series of four experiments while fulvestrant was tested in a single experiment. Tamoxifen was tested in four experiments to ensure a higher degree of replication for an up to then not tested class of EDCs with unknown effects. The eggs were incubated at 37.5 ± 0.5 °C and 60 ± 10% relative humidity and turned over eight times a day in a fully automated incubator (J. Hemel Brutgeräte, Verl, Germany).
Table 1. Mortality (%) and malformations (%) in chicken embryos after in ovo exposure to fulvestrant (Ful, 0.1, 1, 10 µg/g egg) and EE2 (20 ng/g egg) or co-exposure to all concentrations of fulvestrant and EE2.
| Test substance | Fulvestrant | |||
|---|---|---|---|---|
| ∑ experiments | 1 | |||
| ∑ eggs | 200 | |||
| Group | ∑ eggs | ∑ fertilized eggs | Mortality (%)a | Malformations (%)a |
| NC | 24 | 22 | 18.2 (4) | 0.00 (0) |
| SC | 22 | 20 | 15.0 (3) | 0.00 (0) |
| Ful 0.1 | 22 | 19 | 26.3 (5) | 10.5 (2) |
| Ful 1 | 22 | 22 | 22.7 (5) | 4.55 (1) |
| Ful 10 | 22 | 19 | 31.6 (6) | 5.26 (1) |
| EE2 | 22 | 22 | 45.5 (10) | 4.55 (1) |
| Ful 0.1 +EE2 | 22 | 20 | 35.0 (7) | 10.0 (2) |
| Ful 1 +EE2 | 22 | 22 | 18.2 (4) | 4.55 (1) |
| Ful 10 +EE2 | 22 | 18 | 33.3 (6) | 11.1 (2) |
Notes.
The number in parentheses represents the number of affected embryos.
TITLE
- NC
- untreated control
- SC
- solvent control
Table 2. Mortality (%) and malformations (%) in chicken embryos after in ovo exposure to tamoxifen (Tam, 0, 1, 1, 10 µg/g egg) and EE2 (20 ng/g egg) or co-exposure to all concentrations of tamoxifen (plus 0.001, 0.01 µg/g egg) and EE2.
| Test substance | Tamoxifen | |||
|---|---|---|---|---|
| ∑ experiments | 4 | |||
| ∑ eggs | 603 | |||
| Group | ∑ eggs | ∑ fertilized eggs | Mortality (%)a | Malformations (%)a |
| NC | 93 | 88 | 7.95 (7) | 0.00 (0) |
| SC | 83 | 75 | 28.0 (21) | 5.33 (4) |
| Tam 0.1 | 60 | 53 | 43.4 (23) | 5.66 (3) |
| Tam 1 | 60 | 51 | 39.2 (20) | 1.96 (1) |
| Tam 10 | 59 | 52 | 55.8 (29) | 1.92 (1) |
| EE2 | 47 | 45 | 24.4 (11) | 6.67 (3) |
| Tam 0.001 +EE2 | 30 | 28 | 7.14 (2) | 7.14 (2) |
| Tam 0.01 +EE2 | 31 | 30 | 20.0 (6) | 3.33 (1) |
| Tam 0.1 +EE2 | 46 | 44 | 34.1 (15) | 4.54 (2) |
| Tam 1 +EE2 | 47 | 45 | 40.0 (18) | 4.44 (2) |
| Tam 10 +EE2 | 47 | 43 | 30.2 (13) | 6.98 (3) |
Notes.
The number in parentheses represents the number of affected embryos.
TITLE
- NC
- untreated control
- SC
- solvent control
Fulvestrant (CAS: 129453-61-8; purity: ≥98%), tamoxifen (CAS: 10540-29-1; purity: ≥99%) and EE2 (CAS: 57-63-6; purity: ≥98%) were purchased from Sigma Aldrich Chemie GmbH (München, Germany). Fulvestrant (applied doses: 0.1, 1, 10 µg/g egg), tamoxifen (applied doses: 0.001, 0.01, 0.1, 1, 10 µg/g egg) and EE2 (applied dose: 20 ng/g egg) were dissolved alone or in combination in 60 µL of the solvent dimethyl sulfoxide (DMSO; CAS: 67-68-5; purity: 99.5%; Applichem, Darmstadt, Germany). Eggs were yolk-injected on day 1 of incubation and further processed until dissection as described elsewhere (Jessl, Scheider & Oehlmann, 2018).
Dissection, tissue preparation and evaluation
On day 19 of incubation embryos were dissected. Deformations of body and internal organs were recorded with special focus on ovaries and testes. Gonads were photographed (Diskus, Carl H. Hilgers, Königswinter, Germany) for further analysis of the gonad surface area, in which the entire visible surface of each single gonad was determined with an image editing program (Fiji is just ImageJ, Open Source). After dissection gonads were fixed in Bouin’s solution, which was removed by repeated rinsing with 80% ethanol after 24 h. Ethanol was removed by saccharose solution (10, 20 and 30% in phosphate buffered saline). Gonads were embedded in Tissue-Tek® (Sakura Finetek Europe B.V., Alphen aan den Rijn, Netherlands) and sectioned (6 µm) by a freeze microtome (Microm HM 500 O, Thermo Fisher Scientific Germany, Bonn, Germany) at −23 °C. Tissue sections were stained with hematoxylin and eosin.
Measurements and statistics
Histological examination was performed using a light microscope (Olympus BX50, Olympus, Tokyo, Japan) and a camera (JVC Digital Camera, KY-F75U, Yokohama, Japan). Gonadal cortex thickness of both sexes and male percentage of seminiferous tubules in left testes were measured (Fiji is just ImageJ, Open Source). Ten sections for each embryo were evaluated which were exclusively taken from the gonad’s middle sectional plane as described previously (Jessl, Scheider & Oehlmann, 2018).
For the test series with the combination of tamoxifen and EE2, the results of four test runs and for the combination of fulvestrant and EE2, the results of a single test run were merged and analyzed. For the endpoints, gonadal cortex thickness and percentage of seminiferous tubules as well as gonad surface area data were normalized to the solvent control. Data were analyzed using Fisher’s exact test, one-way ANOVA with Dunnett’s multiple comparison test or Kruskal-Wallis test with Dunn’s multiple comparison test with GraphPad Prism 5.01 (GraphPad Software Inc., San Diego, USA).
Determination of sexual genotype
DNA isolation was performed using a tissue sample from the heart taken during dissection. All embryos were typed for their sexual ZZ or ZW genotype, using the PCR-based method of Fridolfsson & Ellegren (1999) with the primers 2550F (5′-GTT ACT GAT TCG TCT ACG AGA-3′) and 2718R (5′-ATT GAA ATG ATC CAG TGC TTG-3′) and a modified protocol. Both primers mark two CHD1 introns, located on the Z (CHD1Z, 600 bp) and W chromosome (CHD1W, 450 bp). Thermal cycling was composed of DNA polymerase activation at 95 °C for 15 min followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 52 °C for 30 s, elongation at 72 °C for 40 s and a final extension step at 72 °C for 5 min followed by a melt curve (60–95 °C with a heating rate of 0.2 °C/s). Following qPCR-mediated amplification with EvaGreen® dye, all amplicons showed two different melting peaks based upon the different melting properties of double-stranded DNA, which resulted in characteristic bands for each sex (Chen et al., 2012). Both sexes had a single 600-bp CHD1-Z specific fragment with a melting temperature of ∼84 °C. Females had an additional 450-bp CHD1-W female-specific fragment with a melting temperature of ∼82 °C.
Results
Effects of in ovo exposure to fulvestrant and EE2
Embryonic mortality and malformations
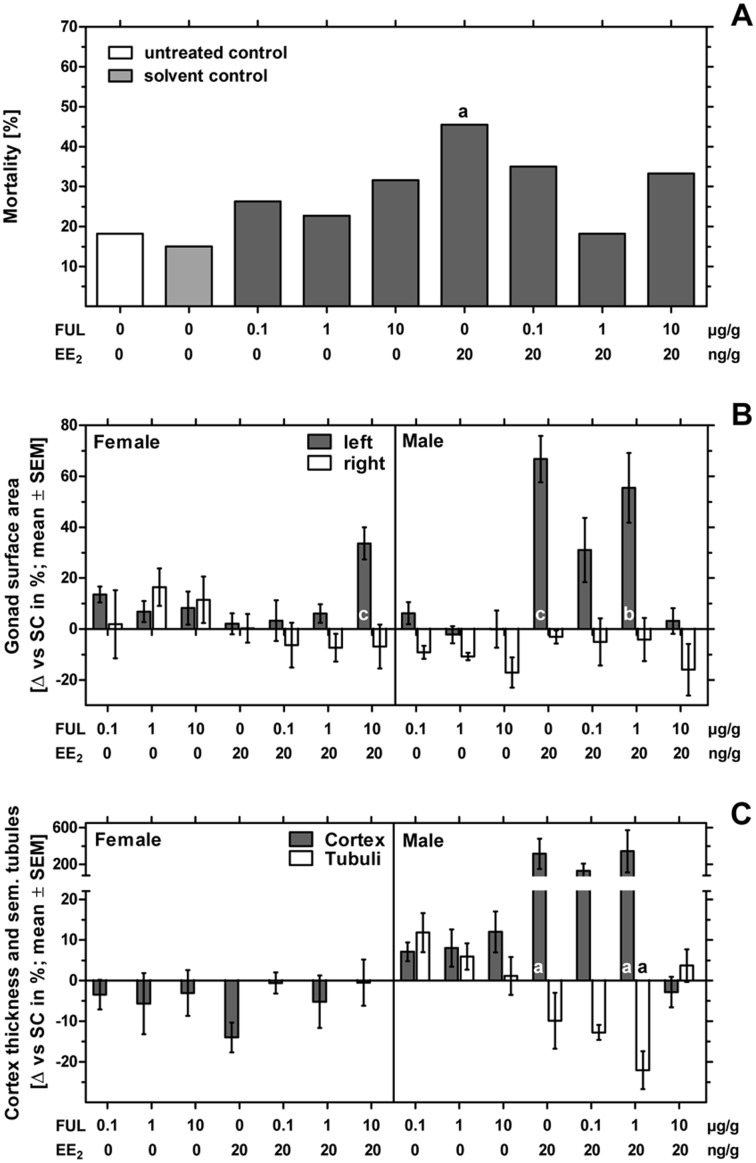
The number of fertilized eggs, embryonic mortality and malformations per treatment-group are presented in Table 1. The fertility rate of the individual groups was at least 82% with a mean total fertility rate of 92% for the whole experiment. The solvent control group showed the lowest mortality of 15%, followed by the untreated control group with 18% mortality. Sole treatment to fulvestrant as well as co-exposure to all concentrations of fulvestrant plus EE2 showed mortality rates up to 35%. Sole treatment to EE2 caused 46% mortality being significantly different from the control (p < 0.05) (Fig. 1A).
Figure 1. Effects of in ovo exposure to fulvestrant and 17α-ethinylestradiol on mortality, left and right gonad surface area, cortex thickness and percentage of seminiferous tubules of left gonad of embryos of the domestic fowl (Gallus g. domesticus).
Effects of in ovo exposure to fulvestrant (FUL, 0.1, 1, 10 µg/g egg) and 17α-ethinylestradiol (EE2; 20 ng/g egg) on mortality (A), left and right gonad surface area (B) and cortex thickness and percentage of seminiferous tubules of left gonad (C) of embryos of the domestic fowl (Gallus g. domesticus) on embryonic day 19. Statistical analysis by Fisher’s exact test (A) and one-way ANOVA with Dunnett’s multiple comparison test (B, C). Grey background distinguishes the co-exposure to fulvestrant and EE2. Lowercase indicate significant differences compared to the solvent control. Level of significance: a: p < 0.05; b: p < 0.01; c: p < 0.001.
While no malformations were detected in the control groups, all substance treated groups showed one to two malformed embryos. The EE2-treated group 1 of 22 embryos (4.55%) showed a single malformation which was found to be celosomia. Examining all groups receiving different concentrations of fulvestrant, four of 60 embryos (6.67%) showed malformations which were mainly found to be celosomia or malformations of the eyes or the beak. Examining all groups co-exposed to fulvestrant and EE2, five of 60 embryos (8.33%) showed malformations which were mainly found to affect the beak, the eyes, the limbs, the brain or celosomia. Compared to the control, none of the tested groups showed a substance-induced increase in the rate of malformation.
Morphological observation of the gonads—gonad surface area
In females sole exposure to EE2 or all concentrations of fulvestrant as well as co-exposure to EE2 and lower concentrations of fulvestrant (0.1 and 1 µg/g egg) had no statistically significant effect on left and right gonad surface area. Co-exposure to EE2 and 10 µg fulvestrant/g egg, however, resulted in a statistically significant increase of the left gonad surface area (p < 0.001) (Fig. 1B and Table 3).
Table 3. Gonad surface area, gonadal cortex thickness and percentage of seminiferous tubules of chicken embryos after in ovo exposure to fulvestrant (Ful, 0.1, 1, 10 µg/g egg) and EE2 (20 ng/g egg) or co-exposure to all concentrations of fulvestrant and EE2.
| Sex | Group | Gonad surface area | Cortex thickness (µm) | Seminiferous tubules (%) | |
|---|---|---|---|---|---|
| left (mm2) | right (mm2) | ||||
| Male | NC | 4.22 ± 0.95 | 3.92 ± 0.57 | 10.1 ± 0.40 | 28.3 ± 4.49 |
| SC | 3.99 ± 0.46 | 3.91 ± 0.30 | 10.7 ± 0.73 | 30.2 ± 1.83 | |
| Ful 0.1 | 4.24 ± 0.42 | 3.55 ± 0.24 | 11.5 ± 0.61 | 33.8 ± 3.56 | |
| Ful 1 | 3.90 ± 0.36 | 3.49 ± 0.13 | 11.6 ± 1.30 | 32.0 ± 2.60 | |
| Ful 10 | 3.99 ± 0.82 | 3.24 ± 0.66 | 12.0 ± 1.54 | 30.6 ± 3.98 | |
| EE2 | 6.66 ± 0.82c | 3.79 ± 0.23 | 44.6 ± 39.3a | 27.3 ± 4.65 | |
| Ful 0.1 +EE2 | 5.23 ± 1.23 | 3.71 ± 0.89 | 25.0 ± 18.3 | 26.4 ± 1.23 | |
| Ful 1 +EE2 | 6.21 ± 1.09b | 3.75 ± 0.67 | 47.6 ± 49.4a | 23.6 ± 2.82a | |
| Ful 10 +EE2 | 4.12 ± 0.53 | 3.29 ± 1.05 | 10.4 ± 0.91 | 31.4 ± 2.70 | |
| Female | NC | 10.3 ± 0.69c | 2.38 ± 0.34b | 143 ± 33.9 | – |
| SC | 8.23 ± 0.95 | 1.80 ± 0.34 | 154 ± 30.0 | – | |
| Ful 0.1 | 9.35 ± 0.72 | 1.84 ± 0.68 | 149 ± 15.0 | – | |
| Ful 1 | 8.80 ± 1.03 | 2.10 ± 0.40 | 145 ± 32.7 | – | |
| Ful 10 | 8.91 ± 1.20 | 2.01 ± 0.37 | 149 ± 19.5 | – | |
| EE2 | 8.40 ± 0.91 | 1.81 ± 0.27 | 133 ± 15.0 | – | |
| Ful 0.1 +EE2 | 8.50 ± 1.98 | 1.69 ± 0.45 | 153 ± 8.89 | – | |
| Ful 1 +EE2 | 8.74 ± 1.12 | 1.67 ± 0.37 | 146 ± 37.2 | – | |
| Ful 10 +EE2 | 11.0 ± 1.37c | 1.68 ± 0.41 | 153 ± 21.5 | – | |
Notes.
Statistical analysis by one-way ANOVA with Dunnett’s multiple comparison test. Lowercase indicate significant differences compared to the solvent control (SC). NC, untreated control. Level of significance: ap < 0.05; bp < 0.01; c0.001.
In males fulvestrant did not affect left and right gonad surface area at all applied sole concentrations. EE2 administered alone or in combination with lower concentrations of fulvestrant (1 µg/g egg) resulted in a significant increase in the left gonad surface area (p < 0.001 and p < 0.01, respectively). The affected left testes showed a female-like shape and a well visible female-typical thickened cortex region when viewed under a stereomicroscope (Fig. 2). However, a concentration of 10 µg fulvestrant/g egg completely antagonized the EE2-induced feminization of genetic males.
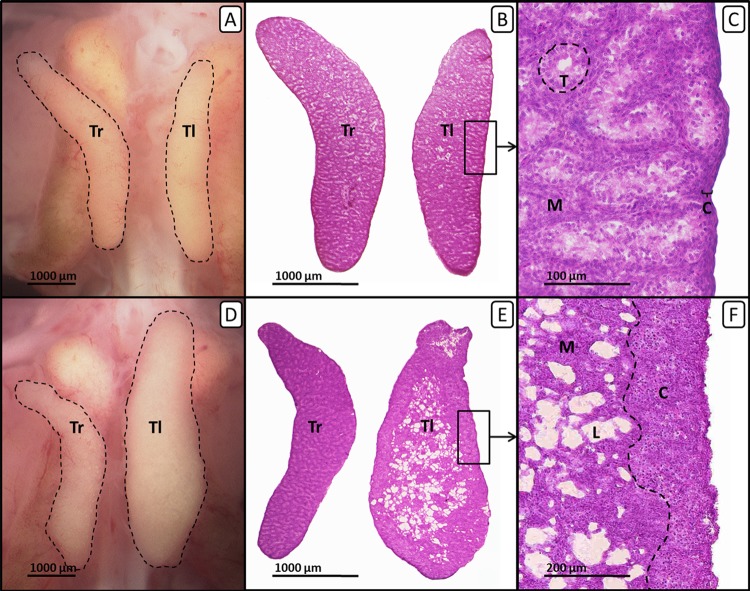
Figure 2. Right and left testis of genetic males of untreated control group and EE2-treated group on day 19 of embryonic development.
Right and left testis of genetic males of untreated control group (A, B, C) and EE2-treated group (20 ng/g egg; D, E, F). (A, D) Unfixed right (“Tr”) and left (“Tl”) testis (outlined in black) on day 19 of embryonic development. Untreated control males (A) show testes of nearly identical size. EE2-treated males (D) show an unaffected right testis and a significantly enlarged left testis. (B, E) Histological thin sections (6 µm) of right (“Tr”) and left (“Tl”) testis. Untreated control males (B) show testes of nearly identical size and structure. EE2-treated males (E) show an unaffected right testis and a significantly enlarged left testis with female-like shape and structure. (C, F) Histological thin sections (6 µm) of left testis in close-up. Untreated control males (C) show a thin gonadal cortex layer (“C”) of three to four cells and interstitial space and seminiferous tubules (“T”) in the medulla (“M”). Left testes of EE2-treated males (F) show female-typical structures as a well-differentiated gonadal cortex region (“C”) with oocyte-like cells and a loosely arranged medulla (“M”) crossed by lacunar channels (“L”).
Histological observation of the gonads—left testis and ovary
In females sole exposure to all concentrations of fulvestrant or co-exposure to fulvestrant and EE2 had no statistically significant effect on ovarian cortex thickness (Fig. 1C).
Administration of EE2 alone or in combination with 1 µg fulvestrant/g egg caused a significant increase in male gonadal cortex thickness by up to 344% (p < 0.05, respectively) while the percentage of seminiferous tubules decreased with increasing concentration of fulvestrant (co-exposure to EE2 and 1 µg fulvestrant/g egg: p < 0.05). The affected testicular tissue appeared significantly changed with female-typical structures such as lacunae and a differentiated female-like gonadal cortex region with oocyte-like cells. On the contrary, the number and degree of differentiation of seminiferous tubules were visibly lower. A concentration of 10 µg fulvestrant/g egg completely antagonized the feminization of genetic males caused by EE2 (Table 3).
Effects of in ovo exposure to tamoxifen and EE2
Embryonic mortality and malformations
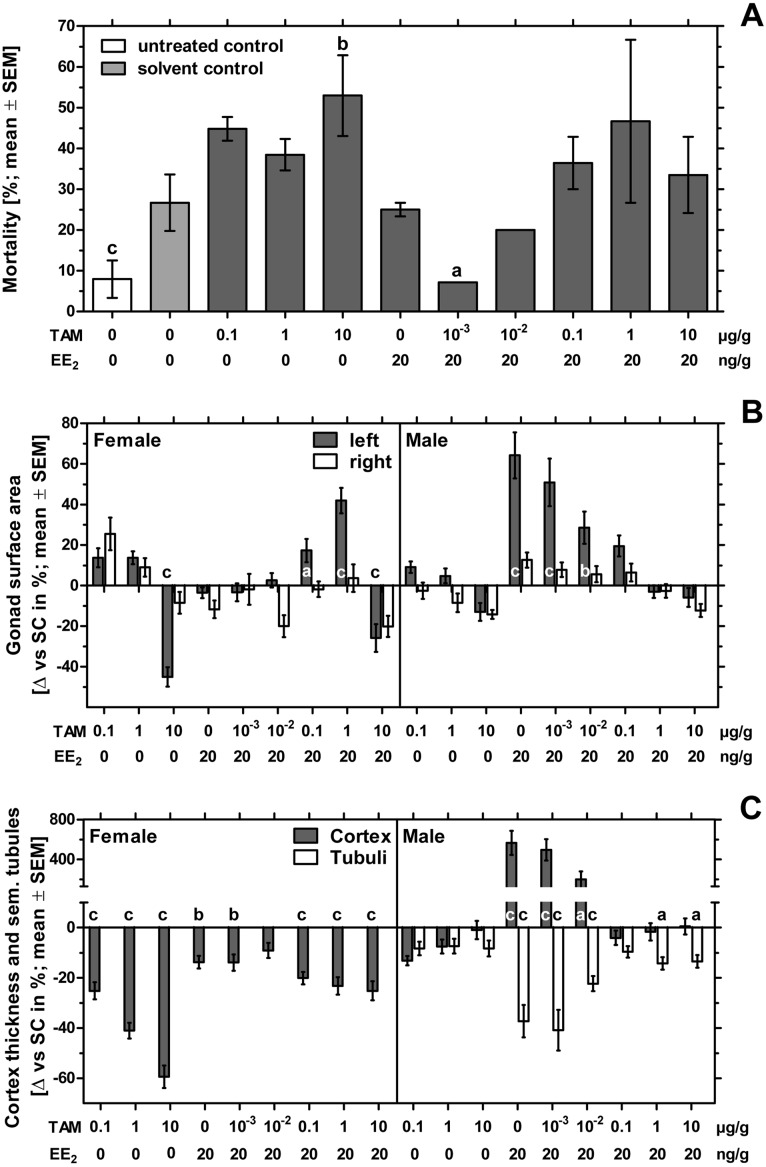
The number of fertilized eggs, embryonic mortality and malformations per treatment-group are presented in Table 2. The fertility rate of the individual groups was at least 88% with a total fertility rate of 92% for the whole experiment. The unmanipulated negative control showed the lowest mortality of nearly 8% and differed significantly from the solvent-treated control with 28% mortality (p < 0.001). Sole exposure to tamoxifen showed mortality rates up to 56%. Only the group treated with 10 µg tamoxifen/g egg showed a significant difference to the solvent-treated control (p < 0.01). Sole exposure to 20 ng EE2/g egg caused 24% mortality. Groups co-exposed to tamoxifen and EE2 showed mortality rates up to 40% with a significant deviation to the solvent-treated control for the group co-exposed to EE2 and 0.001 µg tamoxifen/g egg (p < 0.05) (Fig. 3A).
Figure 3. Effects of in ovo exposure to tamoxifen and 17α-ethinylestradiol on mortality, left and right gonad surface area, cortex thickness and percentage of seminiferous tubules of left gonad of embryos of the domestic fowl (Gallus g. domesticus).
Effects of in ovo exposure to tamoxifen (TAM, 0.001, 0.01, 0.1, 1, 10 µg/g egg) and 17α-ethinylestradiol (EE2, 20 ng/g egg) on mortality (A), left and right gonad surface area (B) and cortex thickness and percentage of seminiferous tubules of left gonad (C) of embryos of the domestic fowl (Gallus g. domesticus) on embryonic day 19. Statistical analysis by Fisher’s exact test (A), one-way ANOVA with Dunnett’s multiple comparisons test or Kruskal-Wallis test with Dunn’s multiple comparison test (B, C). Grey background distinguishes the co-exposure to tamoxifen and EE2. Lowercase indicates significant differences compared to the solvent control. Level of significance: a, p < 0.05; b, p < 0.01; c, p < 0.001.
While no malformations were detected in the untreated negative control group, four of 75 embryos (5.33%) of the solvent-treated control showed malformations which affected the eyes, the beak, the extremities or edema or celosomia. In the EE2-treated group three of 45 embryos (6.67%) showed malformations of the eyes, the beak or celosomia. Examining all groups receiving different concentrations of tamoxifen, five of 156 embryos (3.21%) showed malformations which were found to be celosomia, malformations of the vertebral column, the beak or the brain. There was also one egg containing two embryos conjoined at the head, showing various malformations. Examining all groups co-exposed to EE2 and different concentrations of tamoxifen, 10 of 190 embryos (5.26%) showed malformations which were found to be celosomia, malformations of the beak or the extremities or a missing mesonephros. Compared to the untreated negative control group, the solvent-treated group and the substance-treated groups receiving EE2 or a combination of EE2 and 10 µg tamoxifen/g egg showed a statistically significant effect (p < 0.05). On the contrary, there were no significant differences between the substance-treated groups and the solvent control.
Morphological observation of the gonads—gonad surface area
In females exposure to 10 µg tamoxifen/g egg resulted in a statistically significant decrease of the left gonad surface area (p < 0.001) (Fig. 3B). This effect could not be antagonized by the simultaneous administration of EE2. The combination of EE2 and 0.1 or 1 µg tamoxifen/g egg resulted in a statistically significant increase in the left gonad surface area (p < 0.05 and p < 0.001, respectively). Female right gonad surface areas were not significantly affected by sole exposure to EE2 or tamoxifen or the co-exposure to tamoxifen and EE2.
In comparison, male left and right gonad surface area were not significantly affected by sole exposure to all concentrations of tamoxifen. The EE2-induced statistically significant increase of the male left gonad surface area (p < 0.001) could be gradually reduced through simultaneous administration of increasing tamoxifen-concentrations. From a concentration of 0.1 µg tamoxifen/g egg EE2-related effects were largely antagonized and left gonad surface areas fluctuated around the control values (Table 4).
Table 4. Gonad surface area, gonadal cortex thickness and percentage of seminiferous tubules of chicken embryos after in ovo exposure to tamoxifen (Tam, 0.1, 1, 10 µg/g egg) and EE2 (20 ng/g egg) or co-exposure to all concentrations of tamoxifen (plus 0.001, 0.01 µg/g egg) and EE2.
| Sex | Group | Gonad surface area | Cortex thickness (µm) | Seminiferous tubules (%) | |
|---|---|---|---|---|---|
| left (mm2) | right (mm2) | ||||
| Male | NC | 3.96 ± 0.65 | 3.67 ± 0.58a | 8.67 ± 0.96b | 28.7 ± 3.11 |
| SC | 3.80 ± 0.61 | 3.35 ± 0.63 | 9.35 ± 1.08 | 29.8 ± 3.29 | |
| Tam 0.1 | 4.15 ± 0.41 | 3.26 ± 0.53 | 8.12 ± 0.61 | 27.3 ± 2.90 | |
| Tam 1 | 3.99 ± 0.54 | 3.06 ± 0.60 | 8.65 ± 1.00 | 27.6 ± 3.09 | |
| Tam 10 | 3.31 ± 0.50 | 2.87 ± 0.22 | 9.26 ± 0.84 | 27.3 ± 2.51 | |
| EE2 | 6.25 ± 1.67c | 3.77 ± 0.48 | 62.3 ± 45.6c | 18.7 ± 8.16c | |
| Tam 0.001 +EE2 | 5.74 ± 1.67c | 3.61 ± 0.45 | 55.7 ± 34.6c | 17.6 ± 8.38c | |
| Tam 0.01 +EE2 | 4.89 ± 1.04b | 3.54 ± 0.46 | 28.2 ± 22.1a | 23.1 ± 2.69c | |
| Tam 0.1 +EE2 | 4.55 ± 0.70 | 3.56 ± 0.53 | 8.97 ± 0.96 | 26.9 ± 2.42 | |
| Tam 1 +EE2 | 3.69 ± 0.42 | 3.27 ± 0.42 | 9.19 ± 1.19 | 25.5 ± 2.79a | |
| Tam 10 +EE2 | 3.58 ± 0.80 | 2.94 ± 0.49 | 9.40 ± 1.25 | 25.8 ± 3.11a | |
| Female | NC | 10.2 ± 1.48b | 2.20 ± 0.41 | 155 ± 11.4 | – |
| SC | 9.25 ± 1.32 | 1.99 ± 0.46 | 153 ± 15.7 | – | |
| Tam 0.1 | 10.5 ± 1.79 | 2.49 ± 0.64 | 115 ± 20.7c | – | |
| Tam 1 | 10.5 ± 1.31 | 2.16 ± 0.39 | 90.5 ± 20.3c | – | |
| Tam 10 | 5.08 ± 1.71c | 1.82 ± 0.41 | 70.4 ± 17.1c | – | |
| EE2 | 8.92 ± 0.95 | 1.75 ± 0.35 | 132 ± 13.7b | – | |
| Tam 0.001 +EE2 | 8.93 ± 1.42 | 1.95 ± 0.52 | 132 ± 16.4b | – | |
| Tam 0.01 +EE2 | 9.49 ± 1.14 | 1.59 ± 0.36 | 139 ± 16.1 | – | |
| Tam 0.1 +EE2 | 10.8 ± 2.13a | 1.95 ± 0.30 | 123 ± 14.5c | – | |
| Tam 1 +EE2 | 13.1 ± 2.09c | 2.06 ± 0.48 | 118 ± 17.8c | – | |
| Tam 10 +EE2 | 6.86 ± 2.00c | 1.59 ± 0.31 | 115 ± 18.3c | – | |
Notes.
Statistical analysis by one-way ANOVA with Dunnett’s multiple comparison test or Kruskal Wallis test with Dunn’s post test (female right gonad surface area). Lowercase indicate significant differences compared to the solvent control (SC). NC, untreated control. Level of significance: ap < 0.05; bp < 0.01; cp < 0.001.
Cortex thickness and percentage of seminiferous tubules
In ovo exposure to all concentrations of tamoxifen as well as co-exposure to tamoxifen and EE2 resulted in a statistically significant concentration-dependent decrease of the left ovarian cortex thickness in females (Fig. 3C). Remarkably, several females exposed to the highest concentration of tamoxifen (10 µg/g egg) also showed an altered distribution of the ovarian cortex region (Fig. 4), which could not be detected at lower concentrations. The affected left ovaries were no longer covered by a continuous ovarian cortex but exhibited larger regions of uncovered medulla, partly resembling a testis. In none of any other experiments, even with different endocrine active compounds, was such a phenomenon detected (Scheider et al., 2014; Scheider et al., 2018; Jessl, Scheider & Oehlmann, 2018).
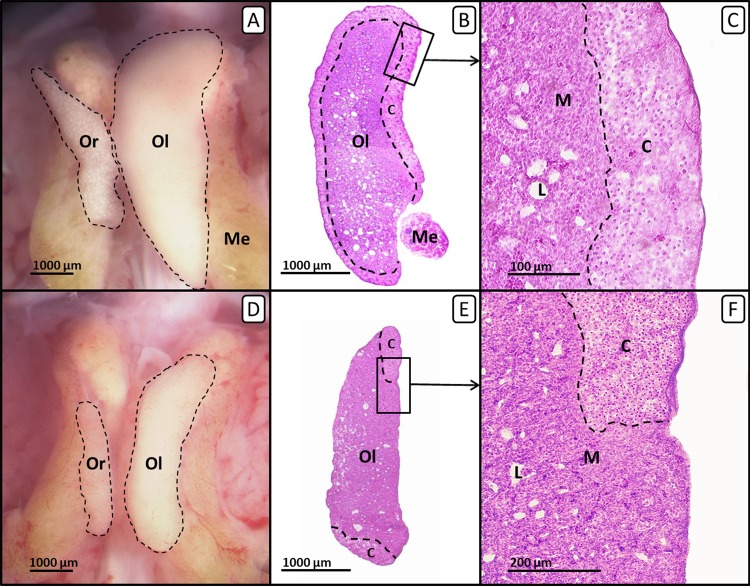
Figure 4. Right and left ovary of genetic females of untreated control group and tamoxifen-treated group on day 19 of embryonic development.
Right and left ovary of genetic females of untreated control group (A, B, C) and tamoxifen-treated group (10 µg/g egg; D, E, F). (A, D) Unfixed right (“Or”) and left (“Ol”) ovary (outlined in black) on day 19 of embryonic development. Untreated control females (A) show a regressed right ovary and a well-differentiated left ovary. Tamoxifen-treated females (D) show an unaffected right ovary and a significantly decreased left ovary. (B, E) Histological thin sections (6 µm) of right (“Or”) and left (“Ol”) ovary. Untreated control females (B) show a left ovary of female typical size and structure. The left ovarian cortex (“C”) is well-differentiated and covers almost the whole ovary (except the region close to the mesonephron (“Me”)). Tamoxifen-treated females (E) show a significantly decreased left ovary with an altered distribution of the cortex region (“C”). The left ovary is no longer covered by a continuous cortex but exposes large regions of uncovered medulla, partly resembling a male testis. (C, F) Histological thin sections (6 µm) of the left ovary in close-up. Untreated control females (C) show a well-differentiated continuous ovarian cortex region (“C”) and a loosely arranged medulla (“M”) crossed by lacunar channels (“L”). Tamoxifen-treated females (F) show a discontinuous irregular scattered ovarian cortex region (“C”) with larger regions of uncovered medulla (“M”) crossed by lacunar channels (“L”).
In males the testicular cortex thickness or the percentage of seminiferous tubules were not affected by sole tamoxifen-exposure. Administration of EE2 alone or in combination with lower concentrations of tamoxifen (0.001 and 0.01 µg/g egg) resulted in a statistically significant increase in testicular cortex thickness by up to 566%, which was completely suppressed at higher concentrations of tamoxifen (0.1, 1 and 10 µg/g egg). By administration of EE2 alone or in combination with lower concentrations of tamoxifen (0.001, 0.01 µg/g egg) the percentages of seminiferous tubules were significantly decreased (p < 0.001). However, higher concentrations of tamoxifen (0.1, 1 and 10 µg/g egg) could partly (percentage of seminiferous tubules) or completely (testicular cortex thickness) antagonize the EE2-induced feminization of genetic males (Table 4).
Discussion
Embryonic mortality and malformations
In the present study fertilization rates were comparable for all experiments with values around 90%. These values are consistent with the results of Romanoff & Romanoff (1972), which described a fertilization rate of almost 89% in an experiment examining 202 breeding hens. The detection of unfertilized eggs is of importance here since the calculation of mortality and the incidence of malformations is based on the number of fertile eggs. While the untreated control showed mortality rates below 20% in all experiments, in 80% (four of five) of the experiments the mortality in the solvent control was below 30%. Based on the data of various publications (Romanoff & Romanoff, 1972; Wyatt & Howarth, 1976; DeWitt, Meyer & Henshel, 2005a; DeWitt, Meyer & Henshel, 2005b) and our own investigations (Jessl, Scheider & Oehlmann, 2018), the natural mortality of unmanipulated chicken embryos is expected to be about 20%. Since it is known that carrier substances in general, or DMSO in the present case, can affect the survival of chicken embryos, for example through their intrinsic toxicity, volume, or density, an increase in mortality compared to the untreated control was expected (Caujolle et al., 1967; Carew & Foss, 1972; Landauer & Salam, 1972; Morgan, 1974; Wyatt & Howarth, 1976). Not only in the present study (110 untreated and 95 solvent-treated individuals in five experiments), but also in further investigations (Jessl, Scheider & Oehlmann, 2018; 256 untreated and 258 solvent-treated individuals in 15 experiments), all of the experiments showed mortality rates below 20% for the untreated control and a predominant proportion of the experiments (87%; 13 of 15 experiments) showed mortality rates below 30% for the solvent control. Due to the large sample size and the consistent results, we assume that the shown values can be reliably used for the comparison to substance-treated groups.
In general, the treatment of chicken embryos with any of the compounds fulvestrant or tamoxifen alone or in combination with EE2 led to an increase in mortality as expected. However, significant deviations in mortality from the solvent control were rare and found to be concentration-independent. Groups treated with a defined concentration of fulvestrant or tamoxifen responded similarly to groups that were co-exposed to the same substance plus EE2. The absence of a statistically significant difference between solely treated and co-exposed groups of the same substance and concentration suggests that the additional administration of EE2 does not necessarily result in an increase in mortality, as one might have expected. Despite the increase in mortality in some groups of the experiments, a sufficient number of vital embryos remained for subsequent histological studies.
Analyzing the frequency of malformations in the five experiments, no malformations were found in the untreated control group with 110 embryos while in the solvent-treated control group four of 95 embryos (4.21%) exhibited malformations. To some extent, this statistically significant increase in the rate of malformation from untreated to solvent-treated control group reflects a weak teratogenic potential of DMSO as noted by Wyatt & Howarth (1976). However, this value is still in the range of the reported spontaneous malformation rate in chicken embryos of 2–7% (Alsop, 1919; Byerly, 1930; Caujolle et al., 1967). In this context, the incidence of malformations in the solvent-treated control can be considered as inconspicuous.
Since there are no statistically significant differences in the frequency of malformations between the individual concentrations of the respective substances fulvestrant, tamoxifen and/or EE2 in all experiments, they are combined in groups representing the respective substance (EE2total, fulvestranttotal, fulvestrant + EE2total, tamoxifentotal and tamoxifen + EE2total). While almost all of the substance groupings show significantly higher malformation rates than the untreated control (EE2total, fulvestranttotal and tamoxifen + EE 2total: p < 0.05; fulvestrant + EE 2total: p < 0.01), none of them are significantly different from the solvent-treated control. In addition, there are no statistically significant differences between the respective substance groupings. However, some of the substance-treated groupings show a marginally (EE2total, tamoxifen + EE2total; p > 0.05) or significantly (fulvestranttotal: p < 0.05) increased incidence of celosomia, compared to the solvent-treated control. In addition, exencephalia are exclusively found in substance-treated groups, while the formation of edema is completely absent. Various malformations have already been described, among them, for example, terata of the eyes, the beak, the brain or the formation of celosomia (Byerly, 1930; Caujolle et al., 1967). These terata largely coincide with the malformations found in the solvent- and substance-treated groups. There is no statistical evidence that treatment with fulvestrant, tamoxifen and/or EE2 specifically favors particular terata or generally results in increased malformation rates.
Morphological observations of the gonads—gonad surface area
Since it is known that substances with endocrine potential are able to induce morphological changes in the sex organs of birds (Scheib, 1983; Berg et al., 1998; Berg et al., 1999; Berg et al., 2001; Berg, Halldin & Brunstrom, 2001; Matsushita et al., 2006; Razia et al., 2006), the chicken embryo appears as a suitable model for the study of early sexual development and the potential impact of EDCs impacting the reproductive axis. In birds, the genetic male is homozygous (ZZ), while the genetic female is heterozygous (ZW). During embryonic development the differentiation in one of the sexes depends on the level of circulating steroid hormones, mainly estrogen (Bruggeman, Van As & Decuypere, 2002). Without estrogen or external influences the undifferentiated gonads of genetic males develop into testes. In genetic females, the key enzyme P450 aromatase (P450arom) is synthesized and testosterone is metabolized to estrogen (Kagami & Hanada, 1997; Ayers, Sinclair & Smith, 2013; Scheider et al., 2014). Since P450arom is not synthesized in male gonads (Ayers, Sinclair & Smith, 2013; Scheider et al., 2014), constitutional estrogen concentration in testes is very low (Woods & Erton, 1978; Tanabe et al., 1979; Tanabe, Yano & Nakamura, 1983) and not sufficient to cause a feminization. Though, for a short time during embryonic development the estrogen receptor (ER) is detectable in male gonads, making males basically vulnerable to estrogen (Gasc, 1980; Smith, Andrews & Sinclair, 1997; Nakabayashi et al., 1998). The artificial presence of estrogen or estrogen-active EDCs at this critical time point therefore causes the differentiation of genetic males towards the phenotypically female sex as demonstrated in the present study and various other studies on domestic fowl (Gallus g. domesticus) and japanese quail (Coturnix japonica) (Sotonyi & Csaba, 1986; Etches & Kagami, 1997; Berg et al., 1998; Berg et al., 1999; Berg et al., 2001; Berg, Halldin & Brunstrom, 2001; Shibuya et al., 2004).
Furthermore, it is shown that the injection of interfering substances into fertilized eggs, such as aromatase inhibitors, can result in a stop of estrogen synthesis leading to a masculinization of ovaries (Elbrecht & Smith, 1992; Burke & Henry, 1999). Not only aromatase inhibitors, but also antiestrogens are capable of affecting female gonad differentiation in birds. Tamoxifen, a selective ER modulator, interacts with various proteins involved in the transcription of estrogen-regulated genes (MacGregor & Jordan, 1998). In the present study sole exposure to high concentrations of tamoxifen resulted in a significant decrease of the left ovarian surface area. This data coincide with the studies of Scheib (1983) on quail embryos, which show that treatment with tamoxifen reduces the size of the left ovary and disturbs the correct formation of the left ovarian cortex. Surprisingly, sole exposure to all concentrations of fulvestrant had no such effect on female ovarian surface area. Due to its considerably higher affinity to ER compared to tamoxifen (Wakeling & Bowler, 1987; Wakeling, Dukes & Bowler, 1991) and the fact that fulvestrant-treatment results in a complete inhibition of the estrogen signaling pathway (Osborne et al., 1995; Wakeling, 1995; Wardley, 2002) we expected fulvestrant as the more potent antiestrogen. The difference between fulvestrant and tamoxifen regarding their effect on embryonic ovarian development of Gallus domesticus could be due to their mode of action, since tamoxifen is a partial ER antagonist, depending on the target tissue, and thus has both antiestrogenic and estrogen-like activity while fulvestrant is a pure or competitive ER antagonist without intrinsic activity (Webb et al., 1995; MacGregor & Jordan, 1998; Bentrem et al., 2001; Shou et al., 2004). However, it is unknown whether these differences can impact the activity in ovo.
The present study also demonstrates a concentration-dependent neutralization of the feminizing effect of EE2 with increasing concentration of fulvestrant or tamoxifen. In genetic females co-exposure to EE2 and higher concentrations of fulvestrant or tamoxifen led to a statistically significant increase in female left gonad surface area. This data coincide with the study of Scheib (1983) which shows that the feminizing effect of diethylstilbestrol on male quail embryos could be largely compensated by simultaneous tamoxifen-treatment. While in the experiments of Scheib (1983) sole treatment with the estrogenic compound diethylstilbestrol reduces the volume of the left ovary but does not affect its differentiation, co-exposure to tamoxifen and the estrogen results in a reduction of the tamoxifen-related effects in females. It can be assumed that the competition between estrogen and antiestrogen at the binding site of the ER is influenced by their differing concentrations but also by their specific binding affinity to the receptor. Blair et al. (2000) reported relative binding affinities to the ER from rat uterine cytosol preparations of 190%, 1.6% and 37.5% for EE2, tamoxifen and fulvestrant, respectively. Because both antiestrogens were applied at doses up to factor 500 higher than the estrogen, it is likely that the estrogen is successively displaced from the ER, resulting in a neutralization of the estrogenic response.
Histological observations of the gonads—left testis and ovary
The results of the present study show that in ovo exposure to EE2 leads to a reduction of the ovarian cortex in females. In males EE2 causes a significantly thickened testicular cortex with oocyte-like cells and female-like structure.
Furthermore, sole treatment with fulvestrant or tamoxifen does not significantly affect differentiation of male testes, which coincides with the studies of Salzgeber, Reyssbrion & Baulieu (1981) and Scheib & Baulieu (1981). Their experiments on chicken and quail show that tamoxifen especially affects female but not male gonadogenesis. Thus, tamoxifen-treatment especially disturbed the formation of the left ovarian cortex (Scheib, 1983). In the present study, exposure to 10 µg tamoxifen/g egg results in an altered distribution of the left ovarian cortex which might be based on the specific mechanism of action of tamoxifen. Tamoxifen is a partial agonist of the ER whose effect always depends on the specific tissue along with the intrinsic activity of tamoxifen. As ER alpha is primarily expressed in the gonadal cortex (Nakabayashi et al., 1998), its blockage at high doses might have contributed to the suppression of the cortical hypertrophy in the ovarian differentiation and its unbalanced dispersal. This might lead to the speculation that in higher doses tamoxifen might improve sterility of the respecting females, as the oogonia develop in the border between cortex and medulla (Gonzalez-Moran, 2011).
Since the ER is largely irrelevant for the normal differentiation of the male sex, fulvestrant and tamoxifen remain without statistically significant effect for males. However, the feminization of genetic males of domestic fowl and quail caused by estrogenic substances can be effectively compensated by antiestrogens (Samsel, Zeis & Weniger, 1982; Scheib, 1983) which agrees with the data of the present study. It can be assumed that both estrogenic and antiestrogenic substances compete for the binding site of the ER. Accordingly, a high estrogen-concentration leads to its preferential binding to the ER and results in the feminization of male gonads. With increasing concentration of the antiestrogen the ER is successively blocked. This results in a compensation of the feminizing effects as the ER and subsequent signal cascades are not affected by the estrogen.
Overall Conclusions
The focus of the present work was on the study of the effects of the estrogenic compound EE2 as well as the antiestrogenic compounds fulvestrant and tamoxifen on embryonic sex development of chicken (Gallus g. domesticus). In ovo exposure to EE2 resulted in a distinct feminization of genetic males which formed female-like cortex tissue in their left gonads. In addition, EE2-treatment resulted in a reduction of the percentage of seminiferous tubules. The antiestrogen tamoxifen influenced female sex differentiation and led to a size reduction of the left ovary and malformations of the ovarian cortex. In contrast, fulvestrant did not affect sexual differentiation in chicken in the tested concentration range. However, both antiestrogens were able to antagonize the feminizing effects of a potent estrogen in genetic males when administered simultaneously with EE2. Since both estrogenic and antiestrogenic substances induce concentration-dependent morphological alterations of the sex organs, the chick embryo can be regarded as a promising model for the identification of chemicals with estrogenic and antiestrogenic activity. However, it should be considered that the chick embryo is not necessarily sensitive to all classes of EDCs impacting the reproductive axis. Therefore, pending studies of androgenic and antiandrogenic compounds will provide more information about the suitability of the chicken embryo test for these classes of EDCs.
Supplemental Information
Acknowledgments
We thank Andrea Misovic, Simone Ziebart, Alina Helmes and Katrin Collmar for technical assistance.
Funding Statement
This work was carried out in the framework of the project GenOvotox II, funded by the Federal Ministry of Education and Research (BMBF; project no 031A104B). There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Rebecca Lenz is an employee of Dr. Drexler and Dr. Fecher GmbH and Fabian G. Massing is an employee of ERM GmbH. Luzie Jessl is an employee of R-Biopharm AG. Jörg Oehlmann is an Academic Editor for PeerJ.
Author Contributions
Luzie Jessl conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Rebecca Lenz and Fabian G. Massing performed the experiments, analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Jessica Scheider and Jörg Oehlmann conceived and designed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All experiments were carried out with respect for the principles of laboratory animal care, in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and the German Animal Welfare Act. Fertilized eggs of white Leghorn (G. domesticus) were obtained from a local breeder (LSL Rhein-Main Geflügelvermehrungsbetrieb, Dieburg, Germany).
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.
References
- Ackerman et al. (2016).Ackerman JT, Eagles-Smith CA, Herzog MP, Hartman CA. Maternal transfer of contaminants in birds: mercury and selenium concentrations in parents and their eggs. Environmental Pollution. 2016;210(1):145–154. doi: 10.1016/j.envpol.2015.12.016. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan (1990).Adkins-Regan E. Hormonal basis of sexual differentiation in birds. Hormones, brain and behavior in vertebrates. Vol. 8. Karger; Basel: 1990. pp. 1–14. [Google Scholar]
- Ahn et al. (2007).Ahn RS, Han SJ, Kim SC, Kwon HB. Effects of butyltin compounds on follicular steroidogenesis in the bullfrog (Rana catesbeiana) Environmental Toxicology and Pharmacology. 2007;24(2):149–154. doi: 10.1016/j.etap.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Akazome & Mori (1999).Akazome Y, Mori T. Evidence of sex reversal in the gonads of chicken embryos after oestrogen treatment as detected by expression of lutropin receptor. Journal of Reproduction and Fertility. 1999;115(1):9–14. doi: 10.1530/jrf.0.1150009. [DOI] [PubMed] [Google Scholar]
- Alsop (1919).Alsop FM. The effect of abnormal temperatures upon the developing nervous system in the chick embryos. Anatomical Record. 1919;15(6):306–331. doi: 10.1002/ar.1090150604. [DOI] [Google Scholar]
- Ayers, Sinclair & Smith (2013).Ayers KL, Sinclair AH, Smith CA. The molecular genetics of ovarian differentiation in the avian model. Sexual Development. 2013;7(1–3):80–94. doi: 10.1159/000342358. [DOI] [PubMed] [Google Scholar]
- Bargar, Scott & Cobb (2001).Bargar TA, Scott GI, Cobb GP. Maternal transfer of contaminants: case study of the excretion of three polychlorinated biphenyl congeners and technical-grade endosulfan into eggs by white leghorn chickens (Gallus domesticus) Environmental Toxicology and Chemistry. 2001;20(1):61–67. doi: 10.1002/etc.5620200106. [DOI] [PubMed] [Google Scholar]
- Bentrem et al. (2001).Bentrem DJ, Dardes RC, Liu H, Maccgregor-Schafer J, Zapf JW, Jordan VC. Molecular mechanism of action at estrogen receptor alpha of a new clinically relevant antiestrogen (GW7604) related to tamoxifen. Endocrinology. 2001;142(2):838–846. doi: 10.1210/endo.142.2.7932. [DOI] [PubMed] [Google Scholar]
- Berg et al. (2004).Berg C, Blomqvist A, Holm L, Brandt I, Brunstrom B, Ridderstrale Y. Embryonic exposure to oestrogen causes eggshell thinning and altered shell gland carbonic anhydrase expression in the domestic hen. Reproduction. 2004;128(4):455–461. doi: 10.1530/rep.1.00211. [DOI] [PubMed] [Google Scholar]
- Berg, Halldin & Brunstrom (2001).Berg C, Halldin K, Brunstrom B. Effects of bisphenol A and tetrabromobisphenol A on sex organ development in quail and chicken embryos. Environmental Toxicology and Chemistry. 2001;20(12):2836–2840. doi: 10.1002/etc.5620201224. [DOI] [PubMed] [Google Scholar]
- Berg et al. (1998).Berg C, Halldin K, Brunstrom B, Brandt I. Methods for studying xenoestrogenic effects in birds. Toxicology Letters. 1998;103:671–676. doi: 10.1016/s0378-4274(98)00285-9. [DOI] [PubMed] [Google Scholar]
- Berg et al. (1999).Berg C, Halldin K, Fridolfsson AK, Brandt I, Brunstrom B. The avian egg as a test system for endocrine disrupters: effects of diethylstilbestrol and ethynylestradiol on sex organ development. Science of the Total Environment. 1999;233(1–3):57–66. doi: 10.1016/S0048-9697(99)00179-5. [DOI] [PubMed] [Google Scholar]
- Berg et al. (2001).Berg C, Holm L, Brandt I, Brunstrom B. Anatomical and histological changes in the oviducts of Japanese quail, Coturnix japonica, after embryonic exposure to ethynyloestradiol. Reproduction. 2001;121(1):155–165. doi: 10.1530/rep.0.1210155. [DOI] [PubMed] [Google Scholar]
- Berg & Pettersson (2006).Berg C, Pettersson I. Ethynyl estradiol causes female-biased sex ratio in the frog Xenopus tropicalis. Marine Environmental Research. 2006;62:S270–S270. doi: 10.1016/j.marenvres.2006.04.041. [DOI] [Google Scholar]
- Berge et al. (2004).Berge JA, Brevik EM, Bjorge A, Folsvik N, Gabrielsen GW, Wolkers H. Organotins in marine mammals and seabirds from Norwegian territory. Journal of Environmental Monitoring. 2004;6(2):108–112. doi: 10.1039/B311662J. [DOI] [PubMed] [Google Scholar]
- Biau et al. (2007).Biau S, Bayle S, Barbara PD, Roig B. The chick embryo: an animal model for detection of the effects of hormonal compounds. Analytical and Bioanalytical Chemistry. 2007;387(4):1397–1403. doi: 10.1007/s00216-006-0870-y. [DOI] [PubMed] [Google Scholar]
- Blair et al. (2000).Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong W, Shi L, Perkins R, Sheehan DM. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicological Sciences. 2000;54(1):138–153. doi: 10.1093/toxsci/54.1.138. [DOI] [PubMed] [Google Scholar]
- Bodiguel et al. (2009).Bodiguel X, Loizeau V, Le Guellec AM, Roupsard F, Philippon X, Mellon-Duval C. Influence of sex, maturity and reproduction on PCB and p,p’DDE concentrations and repartitions in the European hake (Merluccius merluccius, L.) from the Gulf of Lions (NW Mediterranean) Science of the Total Environment. 2009;408(2):304–311. doi: 10.1016/j.scitotenv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Bruggeman, Van As & Decuypere (2002).Bruggeman V, Van As P, Decuypere E. Developmental endocrinology of the reproductive axis in the chicken embryo. Comparative Biochemistry and Physiology a-Molecular and Integrative Physiology. 2002;131(4):839–846. doi: 10.1016/S1095-6433(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Brunstrom et al. (2009).Brunstrom B, Axelsson J, Mattsson A, Halldin K. Effects of estrogens on sex differentiation in Japanese quail and chicken. General and Comparative Endocrinology. 2009;163(1–2):97–103. doi: 10.1016/j.ygcen.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Burke & Henry (1999).Burke WH, Henry MH. Gonadal development and growth of chickens and turkeys hatched from eggs injected with an aromatase inhibitor. Poultry Science. 1999;78(7):1019–1033. doi: 10.1093/ps/78.7.1019. [DOI] [PubMed] [Google Scholar]
- Buzdar (2001).Buzdar AU. Endocrine therapy in the treatment of metastatic breast cancer. Seminars in Oncology. 2001;28(3):291–304. doi: 10.1016/S0093-7754(01)90122-8. [DOI] [PubMed] [Google Scholar]
- Buzdar & Robertson (2006).Buzdar AU, Robertson JFR. Fulvestrant: pharmacologic profile versus existing endocrine agents for the treatment of breast cancer. Annals of Pharmacotherapy. 2006;40(9):1572–1583. doi: 10.1345/aph.1G401. [DOI] [PubMed] [Google Scholar]
- Byerly (1930).Byerly TC. The effects of breed on the growth of the chick embryo. Journal of Morphology. 1930;50(2):341–359. doi: 10.1002/jmor.1050500203. [DOI] [Google Scholar]
- Carere & Balthazart (2007).Carere C, Balthazart J. Sexual versus individual differentiation: the controversial role of avian maternal hormones. Trends in Endocrinology and Metabolism. 2007;18(2):73–80. doi: 10.1016/j.tem.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Carew & Foss (1972).Carew LB, Foss DC. Tolerance of chicks for dimethyl sulfoxide. Poultry Science. 1972;51(1):206–211. doi: 10.3382/ps.0510206. [DOI] [PubMed] [Google Scholar]
- Caujolle et al. (1967).Caujolle FM, Caujolle DH, Cros SB, Calvet MMJ. Limits of toxic and teratogenic tolerance of dimethyl sulfoxide. Annals of the New York Academy of Sciences. 1967;141(A1):110–126. doi: 10.1111/j.1749-6632.1967.tb34871.x. [DOI] [PubMed] [Google Scholar]
- Cevasco et al. (2008).Cevasco A, Urbatzka R, Bottero S, Massari A, Pedemonte F, Kloas W, Mandich A. Endocrine disrupting chemicals (EDC) with (anti)estrogenic and (anti)androgenic modes of action affecting reproductive biology of Xenopus laevis: II. Effects on gonad histomorphology. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 2008;147(2):241–251. doi: 10.1016/j.cbpc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2012).Chen C-C, Liu Y-S, Cheng C-C, Wang C-L, Liao M-H, Tseng C-N, Chang H-W. High-throughput sex identification by melting curve analysis in blue-breasted quail and chicken. Theriogenology. 2012;77(9):1951–1958. doi: 10.1016/j.theriogenology.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Choi et al. (2007).Choi MJ, Kim SC, Kim AN, Kwon HB, Ahn RS. Effect of endocrine disruptors and ovulation in amphibians, integrative biosciences on the oocyte maturation Rana dybowskii. Integrative Biosciences. 2007;11(1):1–8. doi: 10.1080/17386357.2007.9647309. [DOI] [Google Scholar]
- Davies et al. (1997).Davies IM, Harding MJC, Bailey SK, Shanks AM, LÄnge R. Sublethal effects of tributyltin oxide on the dogwhelk Nucella lapillus. Marine Ecology Progress Series. 1997;158:191–204. doi: 10.3354/meps158191. [DOI] [Google Scholar]
- DeWitt, Meyer & Henshel (2005a).DeWitt JC, Meyer EB, Henshel DS. Environmental toxicity studies using chickens as surrogates for wildlife: effects of injection day. Archives of Environmental Contamination and Toxicology. 2005a;48(2):270–277. doi: 10.1007/s00244-004-2006-8. [DOI] [PubMed] [Google Scholar]
- DeWitt, Meyer & Henshel (2005b).DeWitt JC, Meyer EB, Henshel DS. Environmental toxicity studies using chickens as surrogates for wildlife: effects of vehicle volume. Archives of Environmental Contamination and Toxicology. 2005b;48(2):260–269. doi: 10.1007/s00244-004-1006-2. [DOI] [PubMed] [Google Scholar]
- Duis et al. (2014).Duis K, Scheider J, Warnecke D, Vander Veen A, Coors A, Knacker T, schäfers C. Substances of very high concern under REACh—an evaluation of uncertainties in the environmental risk assessment of endocrine active substances. Final report UBA-project FKZ 3710 63 416 2014
- Eising et al. (2001).Eising CM, Eikenaar C, Schwbl H, Groothuis TGG. Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proceedings of the Royal Society B-Biological Sciences. 2001;268(1469):839–846. doi: 10.1098/rspb.2001.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbrecht & Smith (1992).Elbrecht A, Smith RG. Aromatase enzyme-activity and sex determination in chickens. Science. 1992;255(5043):467–470. doi: 10.1126/science.1734525. [DOI] [PubMed] [Google Scholar]
- Etches & Kagami (1997).Etches R, Kagami H. Genotypic and phenotypic sex reversal. In: Harvey S, Etches RJ, editors. Perspectives in avian endocrinology. Journal of Endocrinology Ltd.; Bristol: 1997. pp. 57–67. [Google Scholar]
- Fridolfsson & Ellegren (1999).Fridolfsson AK, Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. Journal of Avian Biology. 1999;30(1):116–121. doi: 10.2307/3677252. [DOI] [Google Scholar]
- Fry & Toone (1981).Fry DM, Toone CK. DDT-induced feminization of gull embryos. Science. 1981;213(4510):922–924. doi: 10.1126/science.7256288. [DOI] [PubMed] [Google Scholar]
- Gasc (1980).Gasc JM. Estrogen target-cells in gonads of the chicken-embryo during sexual-differentiation. Journal of Embryology and Experimental Morphology. 1980;55(FEB):331–342. [PubMed] [Google Scholar]
- Gonzalez-Moran (2011).Gonzalez-Moran MG. Histological and stereological changes in growing and regressing chicken ovaries during development. Anatomical Record-Advances in Integrative Anatomy and Evolutionary Biology. 2011;294(5):893–904. doi: 10.1002/ar.21364. [DOI] [PubMed] [Google Scholar]
- Gooding et al. (2003).Gooding MP, Wilson VS, Folmar LC, Marcovich DT, LeBlanc GA. The biocide tributyltin reduces the accumulation of testosterone as fatty acid esters in the mud snail (Ilyanassa obsoleta) Environmental Health Perspectives. 2003;111(4):426–430. doi: 10.1289/ehp.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote et al. (2004).Grote K, Stahlschmidt B, Talsness CE, Gericke C, Appel KE, Chahoud I. Effects of organotin compounds on pubertal male rats. Toxicology. 2004;202(3):145–158. doi: 10.1016/j.tox.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Hamburger & Hamilton (1992).Hamburger V, Hamilton HL. A series of normal stages in the development of the chick-embryo (reprinted from Journal of Morphology, Vol. 88, 1951) Developmental Dynamics. 1992;195(4):231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Henderson (1991).Henderson IC, editor. Endocrine therapy of metastatic breast cancer. Harris, JR; Hellman, S; Henderson, IC; Philadelphia: 1991. [Google Scholar]
- Hoffmann & Kloas (2012).Hoffmann F, Kloas W. The antiestrogens tamoxifen and fulvestrant abolish estrogenic impacts of 17 alpha-ethinylestradiol on male calling behavior of Xenopus laevis. PLOS ONE. 2012;7(9):8. doi: 10.1371/journal.pone.0044715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessl, Scheider & Oehlmann (2018).Jessl L, Scheider J, Oehlmann J. The domestic fowl (Gallus gallus domesticus) embryo as an alternative for mammalian experiments—validation of a test method for the detection of endocrine disrupting chemicals. Chemosphere. 2018;196:502–513. doi: 10.1016/j.chemosphere.2017.12.131. [DOI] [PubMed] [Google Scholar]
- Kagami & Hanada (1997).Kagami H, Hanada H. Current knowledge of sexual differentiation in domestic fowl. Worlds Poultry Science Journal. 1997;53(2):111–123. doi: 10.1079/WPS19970012. [DOI] [Google Scholar]
- Kamata et al. (2006).Kamata R, Takahashi S, Shimizu A, Shiraishi F. Avian transgenerational reproductive toxicity test with in ovo exposure. Archives of Toxicology. 2006;80(12):846–856. doi: 10.1007/s00204-006-0118-9. [DOI] [PubMed] [Google Scholar]
- Keibel & Abraham (1900).Keibel F, Abraham K. Normentafel zur Entwicklungsgeschichte des Huhnes, Gallus domesticus. Fischer; Jena: 1900. [Google Scholar]
- Landauer & Salam (1972).Landauer W, Salam N. Aspects of dimethyl sulfoxide as solvent for teratogens. Developmental Biology. 1972;28(1):35–46. doi: 10.1016/0012-1606(72)90124-8. [DOI] [PubMed] [Google Scholar]
- MacGregor & Jordan (1998).MacGregor JI, Jordan VC. Basic guide to the mechanisms of antiestrogen action. Pharmacological Reviews. 1998;50(2):151–196. [PubMed] [Google Scholar]
- Matsushita et al. (2006).Matsushita S, Yamashita J, Iwasawa T, Tomita T, Ikeda M. Effects of in ovo exposure to imazalil and atrazine on sexual differentiation in chick gonads. Poultry Science. 2006;85(9):1641–1647. doi: 10.1093/ps/85.9.1641. [DOI] [PubMed] [Google Scholar]
- McAllister & Kime (2003).McAllister BG, Kime DE. Early life exposure to environmental levels of the aromatase inhibitor tributyltin causes masculinisation and irreversible sperm damage in zebrafish (Danio rerio) Aquatic Toxicology. 2003;65(3):309–316. doi: 10.1016/S0166-445X(03)00154-1. [DOI] [PubMed] [Google Scholar]
- Morgan (1974).Morgan W. Toxic effect of a radioprotectant (DMSO) on young chicken embryos. Poultry Science. 1974;53(5):1958–1958. [Google Scholar]
- Nakabayashi et al. (1998).Nakabayashi O, Kikuchi H, Kikuchi T, Mizuno S. Differential expression of genes for aromatase and estrogen receptor during the gonadal development in chicken embryos. Journal of Molecular Endocrinology. 1998;20(2):193–202. doi: 10.1677/jme.0.0200193. [DOI] [PubMed] [Google Scholar]
- OECD (2007).OECD . Test No. 440: uterotrophic bioassay in rodents: a short-term screening test for oestrogenic properties, OECD guidelines for the testing of chemicals, section 4. OECD Publishing; Paris: 2007. [DOI] [Google Scholar]
- OECD (2009).OECD . Test No. 441: hershberger bioassay in rats: a short-term screening assay for (Anti)Androgenic properties, OECD guidelines for the testing of chemicals, section 4. OECD Publishing; Paris: 2009. [DOI] [Google Scholar]
- Oehlmann et al. (2006).Oehlmann J, Schulte-Oehlmann U, Bachmann J, Oetken M, Lutz I, Kloas W, Ternes TA. Bisphenol A induces superfeminization in the ramshorn snail Marisa cornuarietis (Gastropoda : Prosobranchia) at environmentally relevant concentrations. Environmental Health Perspectives. 2006;114:127–133. doi: 10.1289/ehp.8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne et al. (1995).Osborne CK, Coronadoheinsohn EB, Hilsenbeck SG, McCue BL, Wakeling AE, McClelland RA, Manning DL, Nicholson RI. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast-cancer. Journal of the National Cancer Institute. 1995;87(10):746–750. doi: 10.1093/jnci/87.10.746. [DOI] [PubMed] [Google Scholar]
- Ottinger & Abdelnabi (1997).Ottinger MA, Abdelnabi MA. Neuroendocrine systems and avian sexual differentiation. American Zoologist. 1997;37(6):514–523. doi: 10.1093/icb/37.6.514. [DOI] [Google Scholar]
- Peakall & Lincer (1996).Peakall DB, Lincer JL. Do PCBs cause eggshell thinning? Environmental Pollution. 1996;91(1):127–129. doi: 10.1016/0269-7491(95)00012-G. [DOI] [PubMed] [Google Scholar]
- Pettersson et al. (2006).Pettersson I, Arukwe A, Lundstedt-Enkel K, Mortensen AS, Berg C. Persistent sex-reversal and oviducal agenesis in adult Xenopus (Silurana) tropicalis frogs following larval exposure to the environmental pollutant ethynylestradiol. Aquatic Toxicology. 2006;79(4):356–365. doi: 10.1016/j.aquatox.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Razia et al. (2006).Razia S, Maegawa Y, Tamotsu S, Oishi T. Histological changes in immune and endocrine organs of quail embryos: exposure to estrogen and nonylphenol. Ecotoxicology and Environmental Safety. 2006;65(3):364–371. doi: 10.1016/j.ecoenv.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Romanoff & Romanoff (1972).Romanoff AL, Romanoff AJ. Pathogenesis of the avian embryo—an analysis of causes of malformations and prenatal death. Wiley; New York: 1972. [Google Scholar]
- Salzgeber, Reyssbrion & Baulieu (1981).Salzgeber B, Reyssbrion M, Baulieu EE. Modification of the female gonads in the chick-embryo induced by tamoxifen. Comptes Rendus de l’Academie des Sciences Serie III-Sciences de la Vie-Life Sciences. 1981;293(2):133–138. [PubMed] [Google Scholar]
- Samsel, Zeis & Weniger (1982).Samsel J, Zeis A, Weniger JP. Feminization in the chick-embryo testis by diethylstilbestrol and antagonizing action of tamoxifen. Biochimie. 1982;64(5):369–376. doi: 10.1016/S0300-9084(82)80442-2. [DOI] [PubMed] [Google Scholar]
- Scheib (1983).Scheib D. Effects and role of estrogens in avian gonadal differentiation. Differentiation. 1983;23:87–92. doi: 10.1007/978-3-642-69150-8_15. [DOI] [PubMed] [Google Scholar]
- Scheib & Baulieu (1981).Scheib D, Baulieu EE. Inhibiting effects of tamoxifen on the female differentiation of the gonads of quail embryos. Comptes Rendus des Seances de l’Academie des Sciences Serie III Sciences de la Vie. 1981;294(7):513–518. [PubMed] [Google Scholar]
- Scheider et al. (2014).Scheider J, Afonso-Grunz F, Hoffmeier K, Horres R, Groher F, Rycak L, Oehlmann J, Winter P. Gene expression of chicken gonads is sex- and side-specific. Sexual Development. 2014;8(4):178–191. doi: 10.1159/000362259. [DOI] [PubMed] [Google Scholar]
- Scheider et al. (2018).Scheider J, Afonso-Grunz F, Jessl L, Hoffmeier K, Winter P, Oehlmann J. Morphological and transcriptomic effects of endocrine modulators on the gonadal differentiation of chicken embryos: the case of tributyltin (TBT) Toxicology Letters. 2018;284:143–151. doi: 10.1016/j.toxlet.2017.11.019. [DOI] [PubMed] [Google Scholar]
- Shibuya et al. (2004).Shibuya K, Mizutani M, Wada M, Sato K, Nunoya T. A new screening model using F1 (AWE ×WE) japanese quail embryo for evaluating sex reversal effects. Journal of Toxicologic Pathology. 2004;17(4):245–252. doi: 10.1293/tox.17.245. [DOI] [Google Scholar]
- Shou et al. (2004).Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. Journal of the National Cancer Institute. 2004;96(12):926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- Smith, Andrews & Sinclair (1997).Smith CA, Andrews JE, Sinclair AH. Gonadal sex differentiation in chicken embryos: expression of estrogen receptor and aromatase genes. (vol 60, pg 295, 1997) Journal of Steroid Biochemistry and Molecular Biology. 1997;62(4):361–361. doi: 10.1016/S0960-0760(97)80910-7. [DOI] [PubMed] [Google Scholar]
- Sotonyi & Csaba (1986).Sotonyi PT, Csaba G. Effect of prenatal and or neonatal diethylstilbestrol (DES) or allylestrenol (AE) treatment on the postnatal-development of the chicken ovary. Acta Biologica Hungarica. 1986;37(3–4):189–196. [PubMed] [Google Scholar]
- Starck & Ricklefs (1997).Starck M, Ricklefs R. Avian growth and development: evolution within the altricial-precocial spectrum. Vol. 1. Oxford University Press; Oxford: 1997. [Google Scholar]
- Sun, Zha & Wang (2009).Sun LW, Zha JM, Wang ZJ. Effects of binary mixtures of estrogen and antiestrogens on Japanese medaka (Oryzias latipes) Aquatic Toxicology. 2009;93(1):83–89. doi: 10.1016/j.aquatox.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Tanabe et al. (1979).Tanabe Y, Nakamura T, Fujioka K, Doi O. Production and secretion of sex steroid-hormones by the testes, the ovary, and the adrenal-glands of embryonic and young chickens (Gallus domesticus) General and Comparative Endocrinology. 1979;39(1):26–33. doi: 10.1016/0016-6480(79)90189-8. [DOI] [PubMed] [Google Scholar]
- Tanabe, Yano & Nakamura (1983).Tanabe Y, Yano T, Nakamura T. Steroid-hormone synthesis and secretion by testes, ovary, and adrenals of embryonic and post-embryonic ducks. General and Comparative Endocrinology. 1983;49(1):144–153. doi: 10.1016/0016-6480(83)90018-7. [DOI] [PubMed] [Google Scholar]
- Wakeling (1995).Wakeling AE. Use of pure antiestrogens to elucidate the mode of action of estrogens. Biochemical Pharmacology. 1995;49(11):1545–1549. doi: 10.1016/0006-2952(94)00528-T. [DOI] [PubMed] [Google Scholar]
- Wakeling & Bowler (1987).Wakeling AE, Bowler J. Steroidal pure antiestrogens. Journal of Endocrinology. 1987;112(3):R7–R10. doi: 10.1677/joe.0.112R007. [DOI] [PubMed] [Google Scholar]
- Wakeling, Dukes & Bowler (1991).Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Research. 1991;51(15):3867–3873. [PubMed] [Google Scholar]
- Wardley (2002).Wardley AM. Fulvestrant: a review of its development, pre-clinical and clinical data. International Journal of Clinical Practice. 2002;56(4):305–309. [PubMed] [Google Scholar]
- Watts, Pascoe & Carroll (2001).Watts MM, Pascoe D, Carroll K. Chronic exposure to 17 alpha-ethinylestradiol and bisphenol A-effects on development and reproduction in the freshwater invertebrate Chironomus riparius (Diptera : Chironomidae) Aquatic Toxicology. 2001;55(1-2):113–124. doi: 10.1016/S0166-445X(01)00148-5. [DOI] [PubMed] [Google Scholar]
- Watts, Pascoe & Carroll (2003).Watts MM, Pascoe D, Carroll K. Exposure to 17 alpha-ethinylestradiol and bisphenol A-effects on larval moulting and mouthpart structure of Chironomus riparius. Ecotoxicology and Environmental Safety. 2003;54(2):207–215. doi: 10.1016/S0147-6513(02)00029-5. [DOI] [PubMed] [Google Scholar]
- Webb et al. (1995).Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway—potential origin for the cell-specific estrogen-like effects of antiestrogens. Molecular Endocrinology. 1995;9(4):443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- Woods & Erton (1978).Woods JE, Erton LH. Synthesis of estrogens in the gonads of the chick-embryo. General and Comparative Endocrinology. 1978;36(3):360–370. doi: 10.1016/0016-6480(78)90117-X. [DOI] [PubMed] [Google Scholar]
- Wyatt & Howarth (1976).Wyatt RD, Howarth B. Effect of dimethyl-sulfoxide on embryonic survival and subsequent chick performance. Poultry Science. 1976;55(2):579–582. doi: 10.3382/ps.0550579. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2014).Yu MX, Wang JY, Liu W, Qin JW, Zhou Q, Wang YA, Huang HH, Chen WL, Ma C. Effects of tamoxifen on the sex determination gene and the activation of sex reversal in the developing gonad of mice. Toxicology. 2014;321:89–95. doi: 10.1016/j.tox.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2007).Zhang JL, Zuo ZH, Chen YX, Zhao Y, Hu S, Wang CG. Effect of tributyltin on the development of ovary in female cuvier (Sebastiscus marmoratus) Aquatic Toxicology. 2007;83(3):174–179. doi: 10.1016/j.aquatox.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Zheng et al. (2014).Zheng XB, Luo XJ, Zeng YH, Wu JP, Chen SJ, Mai BX. Halogenated flame retardants during egg formation and chicken embryo development: maternal transfer, possible biotransformation, and tissue distribution. Environmental Toxicology and Chemistry. 2014;33(8):1712–1719. doi: 10.1002/etc.2588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.