Among 316 participants with T1DM who follow a VLCD, the mean HbA1c was 5.67%, with low rates of hypoglycemia and other acute complications.
Abstract
OBJECTIVES:
To evaluate glycemic control among children and adults with type 1 diabetes mellitus (T1DM) who consume a very low–carbohydrate diet (VLCD).
METHODS:
We conducted an online survey of an international social media group for people with T1DM who follow a VLCD. Respondents included adults and parents of children with T1DM. We assessed current hemoglobin A1c (HbA1c) (primary measure), change in HbA1c after the self-reported beginning of the VLCD, total daily insulin dose, and adverse events. We obtained confirmatory data from diabetes care providers and medical records.
RESULTS:
Of 316 respondents, 131 (42%) were parents of children with T1DM, and 57% were of female sex. Suggestive evidence of T1DM (based on a 3-tier scoring system in which researchers took into consideration age and weight at diagnosis, pancreatic autoimmunity, insulin requirement, and clinical presentation) was obtained for 273 (86%) respondents. The mean age at diagnosis was 16 ± 14 years, the duration of diabetes was 11 ± 13 years, and the time following a VLCD was 2.2 ± 3.9 years. Participants had a mean daily carbohydrate intake of 36 ± 15 g. Reported mean HbA1c was 5.67% ± 0.66%. Only 7 (2%) respondents reported diabetes-related hospitalizations in the past year, including 4 (1%) for ketoacidosis and 2 (1%) for hypoglycemia.
CONCLUSIONS:
Exceptional glycemic control of T1DM with low rates of adverse events was reported by a community of children and adults who consume a VLCD. The generalizability of these findings requires further studies, including high-quality randomized controlled trials.
What’s Known on This Subject:
Despite pharmacological and technological advances, optimal glycemic control of type 1 diabetes remains elusive, putting millions of people worldwide at increased risk of micro- and macrovascular complications. One conceptually promising but poorly studied approach is dietary carbohydrate restriction.
What This Study Adds:
Exceptional glycemic control of type 1 diabetes without high rates of acute complications may be achievable among children and adults with a very low–carbohydrate diet. However, the generalizability of these findings and long-term safety of carbohydrate restriction remain unknown.
Before the discovery of insulin, the lives of children with type 1 diabetes mellitus (T1DM) were extended, sometimes for years, by severe carbohydrate restriction.1 After the advent of insulin treatment, the recommended carbohydrate intake was increased without clinical trial proof of superiority. By the 1980s, a low-fat diet containing up to 60% of energy from carbohydrates became the standard of care.2 More recently, the American Diabetes Association (ADA) has emphasized the individualization of diet rather than focusing on macronutrients.3
Despite major medical and technological advances, the management of T1DM remains suboptimal. With an average overall hemoglobin A1c (HbA1c) of 8.2%, only 20% of children and 30% of adults achieve the glycemic targets of HbA1c <7% for adults and <7.5% for children as set forth by the ADA to reduce long-term complications.4 The greatest challenge in this regard involves difficulty controlling postprandial glycemia, which is a major determinant of HbA1c.5 Even with modern insulin analogs and technical advances, a mismatch between carbohydrate absorption and insulin action typically exists after meals. Beyond a point, measures to lower postprandial hyperglycemia inevitably increase risk for hypoglycemia, with potentially life-threatening consequences.6–9
The source and amount of carbohydrates consumed affect postprandial hyperglycemia and glycemic variability more than any other dietary factor,3,10–12 providing a conceptual basis for interest in carbohydrate-modified diets for T1DM. Regarding carbohydrate source, a diet with a low versus high glycemic index can be used to reduce HbA1c moderately (by ∼0.5%).13 Case series and pilot studies reveal more substantial improvements in HbA1c and other benefits (less hypoglycemia and reduced glycemic variability) with a very low–carbohydrate diet (VLCD).14–21 Although varying to some degree among studies, a VLCD is typically defined as ≤20 to 50 g per day of carbohydrates or ≤5% to 10% carbohydrates as a proportion of calories.22–24 In T1DM, small sample sizes and methodological issues limit the significance of VLCD benefits, and little is known about prevalence, practice, and sustainability. In the absence of larger studies in pragmatic settings, a VLCD is generally discouraged out of concern for potential diabetic ketoacidosis (DKA), hypoglycemia, dyslipidemia, nutrient deficiency, growth failure in children, and sustainability.25,26 Our aim in this study was to characterize glycemic control and acute adverse events among children and adults who have adopted this approach for the long-term self-management of T1DM.
Methods
Design
Using an online survey, we collected primary data from respondents and confirmatory medical information from a secondary survey of health care providers or a review of medical records. Our goals were to (1) establish that adult respondents and children for whom an adult respondent completed the survey (both henceforth referred to as participants) were formally and accurately diagnosed with T1DM, (2) characterize glycemic control (ie, HbA1c, total daily insulin dose, average blood glucose concentrations and SD as measured by a continuous glucose monitor [CGM] or glucose meter), (3) determine adverse event rates (eg, DKA, hypoglycemia, diabetes-related hospitalizations and emergency department visits), (4) assess anthropometrics (eg, weight, height, and BMI) and metabolic health parameters (eg, serum lipids), (5) compare longitudinal changes in glycemic control (pre- to post-VLCD), and (6) characterize participants’ satisfaction with their diabetes management and relationship with the health care system. The study was approved by the Boston Children’s Hospital Institutional Review Board and is registered at www.clinicaltrials.gov (identifier NCT02839174). Electronic consent was obtained from the respondents.
Participants and Enrollment
A volunteer sample was recruited from TypeOneGrit, an online Facebook community for people with T1DM who follow a VLCD and diabetes management method as recommended in the book Dr Bernstein’s Diabetes Solution.20,27 This method comprises a VLCD with weight-based carbohydrate prescription of up to 30 g per day derived from fibrous vegetables and nuts with a low glycemic index. High-protein foods with associated fat are substituted for carbohydrates and adjusted on the basis of outcomes, including glycemic control and weight. Participants adhere to a structured meal plan and adjust bolus insulin empirically according to postprandial glycemia. Basal insulin is adjusted according to fasting glycemia. The group was established in April 2014, with ∼1900 members at the time of the survey.
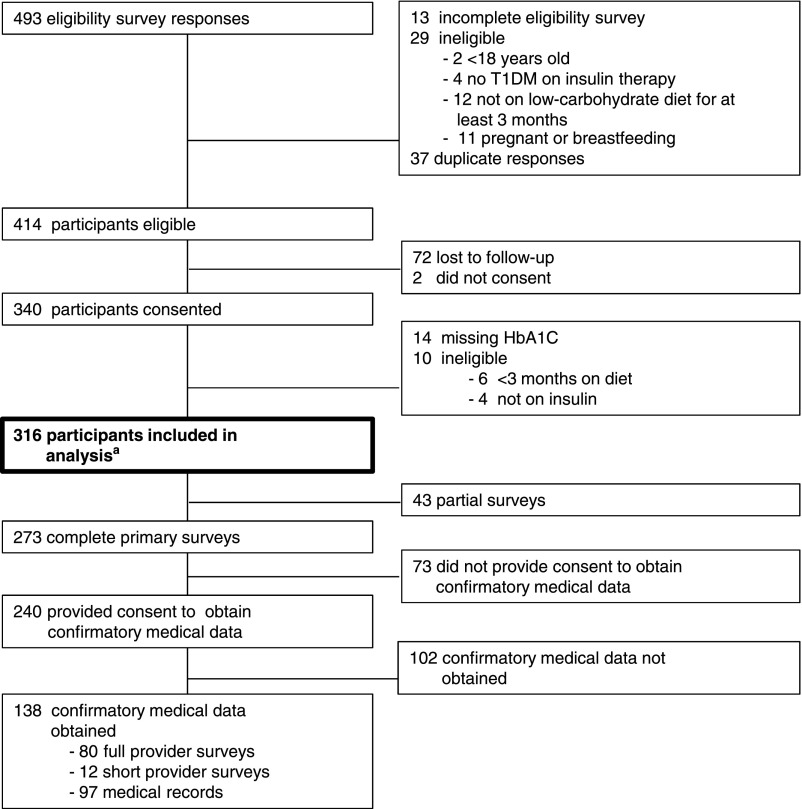
We used an eligibility survey of community members between September 2016 and November 2016. Members aged ≥18 years were eligible if they or a child in their care satisfied these 3 self-reported criteria: having T1DM, receiving insulin therapy, and consuming a carbohydrate-restricted diet for at least 3 months. Women who were pregnant or breastfeeding were excluded. Of 493 eligibility survey responses, 414 (84%) individuals were eligible to participate and 316 (76%) provided sufficient information to be included in the study (Fig 1). With respondent permission, we contacted 182 providers; 97 (53%) completed the full or short provider survey. Of 238 participants who agreed to provide medical records, 101 records were received. Primary data acquisition continued until January 2017. Confirmatory medical information was collected until March 2017.
FIGURE 1.
Enrollment. a The primary end point, current HbA1c, was available in the appropriate time frame (at least 3 months after starting the VLCD) for 300 participants. Data from all 316 eligible participants are included in the other analyses.
Data Collection and Categorization
When possible, survey questions were modified from the T1D Exchange Clinic Registry (https://t1dexchange.org/pages/resources/our-data/studies-with-data)28 and covered several domains: (1) diabetes diagnosis and treatment, (2) diet, (3) insulin regimen, (4) other diabetes-related care, (5) glycemic control, (6) diabetes complications, (7) general health and health care, (8) interactions between the patient and the diabetes care provider, and (9) sociodemographic data. Survey instruments are avalable at https://osf.io/d6wrj/. Respondents were asked to provide consent so that their or their children’s primary diabetes care providers could be contacted or to provide confirmatory medical records themselves. Data were collected and managed by using research electronic data capture (version 7.3.5; Vanderbilt University, Nashville, TN) tools hosted at Boston Children’s Hospital.29
Ascertainment of T1DM Diagnosis
We created a 3-tiered scoring system to ascertain T1DM diagnosis with varying levels of confidence using participant-reported and confirmatory medical information. Participants were classified as having diagnostic evidence of T1DM if they had a diabetes diagnosis at age <20 years, a nonobese body weight (BMI <30 in adults or BMI standard deviation score [SDS] <1.645 [∼95th percentile] in children), and positive diabetes antibody test results. A classification of strong evidence was assigned to participants with a diabetes diagnosis at age <10 years; a diagnosis between ages ≥10 and <20 years, immediate insulin requirement, and a nonobese body weight; or a diagnosis between ages ≥20 and <40 years, immediate insulin requirement, positive diabetes antibody test results, and a nonobese body weight. A classification of suggestive evidence was assigned to participants with a diagnosis at ages ≥20 and <40 years, immediate insulin requirement, a nonobese body weight, and 1 additional factor (including low C-peptide level at diagnosis, physician-specified diagnosis of T1DM, or other evidence [abrupt onset with consistent symptoms, history of DKA, or negative genetic test results for maturity onset diabetes of the young (MODY)]); or diagnosis at age >40 years, immediate insulin requirement, a nonobese body weight, and positive diabetes antibody test results or 1 of the aforementioned additional factors.
Statistical Analysis
Analyses were performed by using SAS version 9.3 (SAS Institute, Inc, Cary, NC). Statistical significance was defined as P < .01, which is a conservative threshold chosen to take into account multiple secondary outcomes. Exact P values are reported. To assess agreement among data sources, we performed Lin’s concordance correlation between patient-provider pairs of each clinical measurement. The following statistical comparisons were made according to an a priori analysis plan. Comparisons between participants were made by independent sample t test or χ2 test for participants with versus without confirmatory medical information and adults versus children. A within-subject, paired, 2-tailed t test was used for pre- and postdiet data and height SDS comparison. Multiple comparisons for diagnostic evidence groups were made by analysis of variance. We performed a linear regression of current, self-reported HbA1c on age, years with diabetes, years on VLCD, carbohydrate intake goal, educational status, and income class.
Post hoc analyses were performed with Pearson’s correlation between pediatric height SDS and carbohydrate intake and by Wilcoxon signed-rank test and McNemar’s test to compare participant- and provider-satisfaction data.
Results
Participants
Descriptive characteristics are listed in Table 1. Most participants were from the United States, Canada, Europe, or Australia; 57% were of female sex, 42% were children, 88% were white and non-Hispanic, and 84% of all respondents (adults or parents of children with T1DM) completed college or the equivalent. The mean age at diabetes diagnosis was 16 ± 14 years, the duration of diabetes was 11 ± 13 years, and the time following a VLCD was 2.2 ± 3.9 years.
TABLE 1.
Participant-Reported Descriptive Characteristics
| Characteristics | No. Responses | Finding, No. (%) or Mean ± SD |
|---|---|---|
| Anthropometrics | ||
| Female sex | 281 | 161 (57) |
| Age, y | 316 | 27 ± 19 |
| Pediatric | 316 | 131 (42) |
| Height SDS | 272 | 0.37 ± 1.1 |
| BMI (adult) | 168 | 24 ± 3 |
| BMI SDS (pediatric) | 106 | 0.44 (0.96) |
| Diabetes-related data | ||
| Age at diagnosis, y | 316 | 16 ± 14 |
| Years with T1DM | 316 | 11 ± 13 |
| Diagnostic category | ||
| Diagnostic evidence of T1DM | 316 | 85 (27) |
| Strong evidence of T1DM | 316 | 153 (48) |
| Suggestive evidence of T1DM | 316 | 35 (11) |
| T1DM unascertained | 316 | 43 (14) |
| HbA1c at diagnosis | 173 | 11.20% ± 2.72% |
| Diet | ||
| Years on VLCDa | 313 | 2.2 ± 2.9 |
| Use of a specific carbohydrate intake goal | 313 | 223 (71) |
| Carbohydrate intake goal, g | 223 | 36 ± 15 |
| Goal achieved, d per wk | 216 | 6.4 ± 1.0 |
| Sociodemographic | ||
| Country | 284 | |
| United States, Canada | 193 (68) | |
| Europe, United Kingdom | 40 (14) | |
| Australia | 36 (13) | |
| Otherb | 15 (5) | |
| Race and/or ethnicity | 284 | |
| White, non-Hispanic | 250 (88) | |
| Hispanic or Latino | 8 (3) | |
| Black | 0 (0) | |
| Asian | 2 (1) | |
| Other | 24 (8.5) | |
| Education | 283 | |
| Primary or less | 1 (0.4) | |
| Secondary | 13 (5) | |
| Upper, postsecondary | 31 (11) | |
| Tertiary | 238 (84) | |
| Income | 284 | |
| Lower | 22 (8) | |
| Middle | 200 (70) | |
| Upper | 62 (22) | |
| Diabetes care provider subspecialty | 288 | |
| Endocrinology | 234 (81) | |
| Pediatrics, family medicine | 38 (13) | |
| Other | 16 (6) |
The median was 1.7 y; the range was 0.2–31.7 y.
New Zealand, South Africa, United Arab Emirates, Saudi Arabia, Singapore, Israel, India, and Aruba.
Validation of Participant-Reported Data
Confirmatory medical information (from providers and/or medical records) was available for 148 (47%) participants (Fig 1, Supplemental Table 4). Participant- and provider-reported data revealed good agreement for relevant clinical variables. Participants with and without confirmatory medical information did not differ (Supplemental Table 5). Therefore, only participant-reported information is reported below.
Ascertainment of T1DM
At least suggestive evidence for T1DM was reported by participants or providers in 273 (86%) patients, at least strong evidence was reported in 238 (75%) patients, and diagnostic evidence was reported in 85 (27%) patients. Evidence was unavailable for 36 (10%) patients, and 7 (2%) did not meet criteria only because of obesity (Table 1). Apart from expected differences related to the scoring system (eg, age, age at diagnosis, and obesity), participants with and without supportive evidence of T1DM did not differ (Supplemental Table 6). Therefore, data for all participants regardless of evidence category are presented together.
Clinical Outcomes
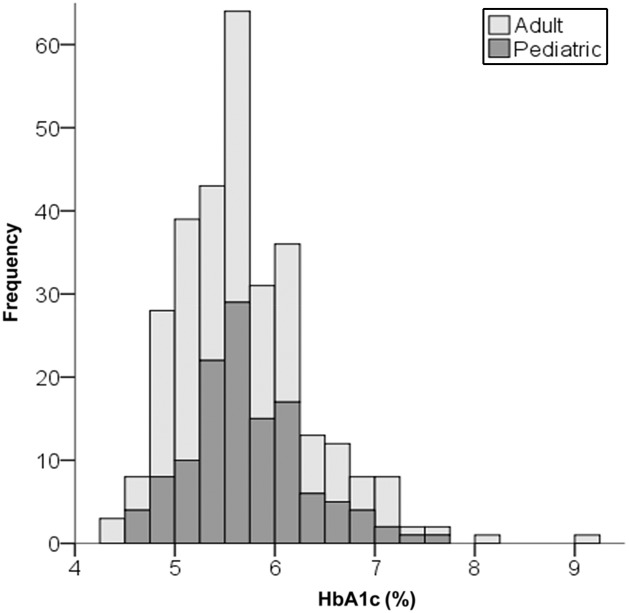
Participants reported a mean daily carbohydrate intake of 36 ± 15 g (n = 223). The mean participant-reported current HbA1c was 5.67% ± 0.66% among the 300 participants who provided this information in the acceptable time frame (Table 2, Fig 2), and 97% of participants achieved the ADA glycemic targets. The participant-reported change in HbA1c from pre- to post-VLCD was −1.45% ± 1.04% (n = 127; P < .001). Of the 137 respondents who reported CGM mean blood glucose values and the 115 who reported CGM blood glucose SD, average blood glucose was 104 ± 16 mg/dL and SD was 28 ± 12 mg/dL. In the regression analysis, a priori covariates explained little of the variation in HbA1c (r2 = 0.06). Carbohydrate intake goal was the only significant predictor (F = 10.4; P = .001), with an increase in HbA1c of 0.1% per 10 g of carbohydrate consumed (Supplemental Table 9). The mean daily insulin dose was 0.40 ± 0.19 U/kg per day.
TABLE 2.
Participant-Reported Clinical Variables
| Clinical Variables | No. Responses | Finding, Mean ± SD or No. (%) |
|---|---|---|
| Glycemic control | ||
| HbA1c | 300 | 5.67% ± 0.66% |
| CGM average, mg/dLa | 137 | 104 ± 16 |
| CGM SD, mg/dLa | 115 | 28 ± 12 |
| Blood glucose meter average, mg/dLb | 77 | 106 ± 21 |
| Blood glucose meter SD, mg/dLb | 36 | 36 ± 35 |
| Insulin daily dose, U/kg per d | 282 | 0.40 ± 0.19 |
| Insulin % basal | 198 | 64% ± 21% |
| Adverse events | ||
| Diabetes-related hospitalizations, persons per yc | 300 | 7 (2) |
| DKA | 4 (1) | |
| Hypoglycemia | 2 (1) | |
| Other | 4 (1) | |
| Diabetes-related emergency encounters, persons per y | 301 | 10 (3) |
| DKA | 3 (1) | |
| Hypoglycemia | 2 (1) | |
| Other | 7 (2) | |
| Hypoglycemia with seizure and/or coma, persons per y | 298 | 7 (2) |
| Hypoglycemia requiring help from others, adults per y | 174 | 20 (12) |
| Hypoglycemia requiring glucagon, persons per yd | 299 | 11 (4) |
| Symptomatic hypoglycemic episodes, persons per mo | 297 | |
| 0 | 92 (31) | |
| 1–5 | 112 (38) | |
| 5–10 | 40 (13) | |
| 10–20 | 30 (10) | |
| ≥21 | 23 (8) | |
| Monthly symptomatic hypoglycemic episodes | 101 | 6.1 (8.5) |
| Lipids, fasting | ||
| Total cholesterol, mg/dL | 79 | 234 ± 89 |
| Total cholesterol ≥200 mg/dL | 47 (60) | |
| LDLc, mg/dL | 81 | 147 ± 83 |
| >130 mg/dL | 39 (48) | |
| HDLc, mg/dL | 80 | 74 ± 21 |
| <35 mg/dL | 0 (0) | |
| TG, mg/dL | 81 | 74 ± 37 |
| >150 mg/dL | 5 (6) | |
| Dyslipidemia (TG >130 mg/dL, LDLc >130, or HDLc <35 mg/dL) | 82 | 51 (62) |
Obtained over 20 ± 18 d.
Obtained over 20 ± 18 d; 8 ± 3 measurements per day.
Data overlap because some participants reported events in different categories.
Only assessed in adults because help from others is always required in children.
FIGURE 2.
HbA1c distribution. The adult (light gray) and pediatric (dark gray) age groups are shown (n = 300).
Participant-reported rates of adverse events were low, and many declined after the initiation of a VLCD (Supplemental Table 7). Of 300 participants, 7 (2%) reported hospitalizations in the past 12 months (14 separate occurrences; 0.05 hospitalizations per person per year), 4 (1%) had 4 (0.01 per person per year) hospitalizations for DKA, and 6 (2%) had 9 hospitalizations (0.03 per person per year) for other reasons. Symptomatic hypoglycemia within the past month was reported by 205 (69%) participants, with the majority (112; 55%) having few (1–5) episodes per month. Likewise, rates of severe hypoglycemia were low, with 7 (2%) reporting hypoglycemia with seizure or coma and 11 (4%) requiring glucagon in the past year. Conventional chronic disease risk factors revealed a mixed profile, with low triglycerides (TG) and elevated high-density lipoprotein cholesterol (HDLc) but high total and low-density lipoprotein cholesterol (LDLc) (Table 2).
Pediatric Age Group and Growth
Children, compared with adults, had similar participant-reported HbA1c and other clinical parameters (Supplemental Table 8). Participant- and provider-reported height SDSs were 0.26 ± 1.21 (n = 107; 82% of children) and 0.25 ± 1.00 (n = 49; 37%), respectively. There was no correlation of height SDS with carbohydrate intake goal (r = 0.15; P = .20) or diet duration (r = 0.14; P = .16). Provider-reported, current height SDS compared with height SDS at diagnosis was 0.20 ± 1.02 vs 0.41 ± 1.27 (P = .05) among the small subset of children for whom data were available (n = 34; 26%). Of the interval of 2.3 ± 2.0 years since diagnosis, these children had followed a VLCD for 1.2 ± 0.8 years.
Health and Health Care Satisfaction
Participants reported high levels of overall health and satisfaction with diabetes management but not with their professional diabetes care (Table 3), and 27% did not discuss their adherence to a VLCD with their diabetes care providers. Of those who did discuss their diet, only 49% agreed or strongly agreed that their diabetes care providers were supportive. Narrative explanations by participants for not discussing their diet included disagreement on treatment goals and approach, perceived provider disinterest or unfamiliarity with a VLCD, a desire to avoid conflicts with the provider, and (for parents) fear of being accused of child abuse. Participating providers corroborated the overall health ratings and reported even greater satisfaction with diabetes control compared with participants (Z −4.09; P < .001). The therapeutic relationship was perceived as very good or excellent by 82% of providers. Interestingly, providers perceived themselves as more supportive of the VLCD compared with participant perception (Z −2.69; P .007).
TABLE 3.
Participant- and Provider-Reported Health, Satisfaction With Diabetes Control and Care
| Clinical Variables | All Participants | Participant With Provider Response | Provider | Z; Pa |
|---|---|---|---|---|
| Health, overall rating | ||||
| No. responses | 288 | 79 | 65 | −1.87; .06 |
| Excellent, n (%) | 119 (41) | 40 (51) | 50 (77) | |
| Very good, n (%) | 130 (45) | 34 (43) | 10 (15) | |
| Good, n (%) | 32 (11) | 5 (6) | 4 (6) | |
| Fair, n (%) | 5 (2) | 0 (0) | 0 (0) | |
| Poor, n (%) | 2 (1) | 0 (0) | 1 (1.5) | |
| Diabetes control satisfaction | ||||
| No. responses | 288 | 79 | 66 | −4.09; <.001 |
| Very satisfied, n (%) | 104 (36) | 25 (32) | 51 (77) | |
| Satisfied, n (%) | 147 (51) | 39 (49) | 11 (17) | |
| Neutral, n (%) | 22 (8) | 12 (15) | 3 (4.5) | |
| Dissatisfied, n (%) | 14 (5) | 3 (4) | 1 (1.5) | |
| Very dissatisfied, n (%) | 1 (0) | 0 (0) | 0 (0) | |
| Satisfaction with professional diabetes care | ||||
| No. responses | 287 | 78 | 64 | — |
| Very satisfied, n (%) | 53 (19) | 16 (21) | — | — |
| Satisfied, n (%) | 89 (31) | 36 (46) | — | — |
| Neutral, n (%) | 67 (23) | 12 (15) | — | — |
| Dissatisfied, n (%) | 50 (17) | 8 (10) | — | — |
| Very dissatisfied, n (%) | 28 (10) | 6 (8) | — | — |
| Therapeutic relationship | ||||
| Excellent, n (%) | — | — | 35 (55) | — |
| Very good, n (%) | — | — | 17 (27) | — |
| Good, n (%) | — | — | 10 (16) | — |
| Fair, n (%) | — | — | 2 (3) | — |
| Poor, n (%) | — | — | 0 (0) | — |
| Diet and/or lifestyle not discussed with diabetes care provider | ||||
| No. responses | 287 | 79 | 65 | .12 |
| n (%) | 77 (27) | 15 (19) | 4 (6) | |
| Diabetes care provider supportive of diet | ||||
| No. responses | 210 | 64 | 61 | −2.69; .007 |
| Strongly agree, n (%) | 34 (16) | 12 (19) | 22 (36) | |
| Agree, n (%) | 70 (33) | 22 (34) | 21 (34) | |
| Neutral, n (%) | 64 (30) | 19 (30) | 12 (20) | |
| Disagree, n (%) | 27 (13) | 8 (13) | 5 (8) | |
| Strongly disagree, n (%) | 14 (7) | 3 (5) | 1 (2) | |
—, not applicable.
Wilcoxon signed-rank test for ordinal variables and McNemar’s test for binomial variables.
Discussion
In this survey of children and adults who follow a VLCD for the long-term treatment of T1DM, we observed measures of glycemic control in the near-normal range, low rates of hypoglycemia and other adverse events, and generally high levels of satisfaction with health and diabetes control. These findings are without precedent among people with T1DM, revealing a novel approach to the prevention of long-term diabetes complications.
Researchers in the Diabetes Control and Complications Trial achieved an average HbA1c of 7.2% in the intensively treated group but with increased rates of hypoglycemia.6,7,30 In a recent survey of 3 international pediatric registries, an HbA1c <7% was not associated with higher rates of hypoglycemia.31 Nevertheless, targeting a near-normal HbA1c is generally not recommended because of concern for hypoglycemia. The participants in our survey had an average HbA1c in the normal range and low rates of hypoglycemia compared with those in other surveys.32,33 Likewise, hospitalizations for DKA or all diabetes-related causes compared favorably with prevailing rates.32–34
The effect of a VLCD on cardiovascular disease risk has been subject to debate. Consistent with the known effects of low carbohydrate and (presumed) associated higher saturated fat intakes, participants had low TG and high HDLc and LDLc levels. The remarkably low ratio of TG to HDLc of 1:1, together with the low total daily insulin requirement, indicate high insulin sensitivity and good cardiometabolic health.35 In contrast, total LDLc is considered a conventional cardiovascular risk factor. However, total LDLc elevation on a VLCD (associated with low TG) may reflect large, buoyant lipoprotein particles, which is a relatively low-risk subtype.36 Furthermore, in the Diabetes Control and Complications Trial cohort of 1441 adolescents and young adults, HbA1c had the largest effects on cardiovascular risk, followed by TG and LDLc.37 Postprandial hyperglycemia has been proposed as an independent cardiovascular disease risk factor38 for which a VLCD would plausibly provide benefit. Another major cardiovascular risk factor, BMI, was significantly below population averages for study participants, possibly reflecting another benefit of a VLCD.39
Children generally did as well as adults, which is a promising finding in view of the adverse effects of diabetes-related hyper- and hypoglycemia on brain development40,41 and growth.42–46 The commonly reported growth deceleration in T1DM is generally ascribed to poor glycemic control.42–46 Concerns have also been raised that a VLCD or chronic ketosis may adversely affect growth and pubertal development.25 Although pubertal development was not assessed in this survey, we obtained children’s height data from parents and medical providers. Participant-reported, current mean height was modestly above average for age and sex (SDS 0.26). Provider-reported data were used to corroborate this finding and also revealed a marginal decrease in height SDS since diabetes diagnosis. This possible growth deceleration may have preceded or occurred during the diet and is comparable in magnitude to the previously described decreases in height SDS in T1DM. Taken together, these data do not reveal an adverse effect of a VLCD on growth, but additional research into this possibility is warranted.
Although participants reported high levels of satisfaction with health and diabetes control, relationships with diabetes care providers were often fraught. A minority of participants did not disclose their adherence to a VLCD to their providers, citing concerns for being criticized, pressured to change behavior, or accused of child abuse. This distrust may increase the risk for a catastrophic adverse event if patients feel unable to seek medical support at times of need (eg, impending DKA) and instead make diabetes management decisions beyond their competencies. Notably, most providers described the therapeutic relationship as very good or excellent and perceived themselves as more supportive of a VLCD than how they were described by the participants. This discrepancy warrants follow-up in qualitative research.
Strengths of this study include verification of self-reported information by independent sources (diabetes care providers and medical records), a rigorous approach to establish T1DM diagnosis, and the pragmatic setting. Our study has 3 main limitations. First, we cannot prove that all participants had T1DM. However, we found no important discrepancies between those who did and did not have diagnostic evidence (eg, childhood onset, diabetes antibodies, and nonobese body weight). Second, the generalizability of the findings is unknown. We cannot determine how many of the members of the online group are active, have T1DM (versus being health care providers, family members, or others with general interest), and would be eligible to participate in the study. In addition, children and adults adhering to a VLCD and remaining in this online community may represent a special subpopulation with high levels of motivation and other health-related behaviors (eg, physical activity), presenting another source of selection bias. Therefore, the study sample may not be representative of all people with T1DM in the social media group and may differ from the general T1DM population in ways that could influence the safety, effectiveness, and practicality of a VLCD. Third, we did not obtain detailed information on participants’ diet and other components of this diabetes management approach, nor did we assess factors contributing to glycemic control before the self-reported start of the VLCD.
Conclusions
We suggest that a VLCD may allow for exceptional control of T1DM without increased risk of adverse events. This possibility is mechanistically plausible because of the dominant effects of dietary carbohydrates on postprandial glycemia and the lower insulin doses required with a VLCD. The results, if confirmed in clinical trials, indicate that the chronic complications of T1DM might be prevented by diet. In light of study limitations, these findings by themselves should not be interpreted as sufficient to justify a change in diabetes management. Additional research is needed to determine the degree of carbohydrate restriction (and other dietary aspects) necessary to achieve these benefits, optimal insulin regimen to accompany a VLCD (specifically, with regard to avoiding severe hypoglycemia), safety and efficacy (in randomized controlled trials). If this work is a success, trials to evaluate effectiveness in preventing long-term diabetes complications should be conducted.
Acknowledgments
We thank the TypeOneGrit community for participating in this study, Paul Lakin for assistance with statistical analyses, Mallory Mandel for assistance with programming the surveys, Victoria Ravenelle for help with study management, and Tessa Graham for help with data management.
Glossary
- ADA
American Diabetes Association
- CGM
continuous glucose monitor
- DKA
diabetic ketoacidosis
- HbA1c
hemoglobin A1c
- HDLc
high-density lipoprotein cholesterol
- LDLc
low-density lipoprotein cholesterol
- MODY
maturity onset diabetes of the young
- SDS
standard deviation score
- TG
triglycerides
- T1DM
type 1 diabetes mellitus
- VLCD
very low–carbohydrate diet
Footnotes
Dr Lennerz conceptualized and designed the study, created the data collection instruments, collected the data, reviewed the medical records, conducted the statistical analyses, drafted the initial manuscript, and maintained full control over the database and all statistical analysis; Drs Barton and Diulus conceptualized the study, participated in the design of the data collection instruments, and revised the manuscript; Drs Bernstein, Dikeman, Hallberg, Rhodes, and Ebbeling participated in the design of the data collection instruments and revised the manuscript; Drs Westman and Yancy participated in the scientific design and revised the manuscript; Dr Ludwig conceptualized and designed the study, drafted the initial manuscript, and maintained full control over the database and all statistical analysis; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: Dr Yancy received research grants from the National Institutes of Health and the US Department of Veterans Affairs for projects involving veterans’ health related to a low-carbohydrate diet. Drs Ebbeling and Ludwig received research grants (to Boston Children’s Hospital) from the National Institutes of Health, the Nutrition Science Initiative, the Laura and John Arnold Foundation, and other philanthropic organizations unaffiliated with the food industry; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grants K24DK082730 (Dr Ludwig) and K12DK094721 (Dr Lennerz) from the National Institute of Diabetes and Digestive and Kidney Diseases. No other honorarium, grant, or other form of payment was given to anyone to produce the article. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Bernstein reported receiving royalties for books on the management of diabetes (which were used by members of the online social media group surveyed in this study). Dr Hallberg reported stock options and research support from Virta Health, a company that provides health care services for type 2 diabetes, and consulting fees from Atkins. Dr Rhodes is the site principal investigator in clinical trials for pediatric type 2 diabetes that are sponsored by Merck and AstraZeneca. Dr. Westman has ownership interest in companies using low-carbohydrate principles, and he receives royalties for books related to low-carbohydrate diets. Dr Ludwig reported receiving royalties from books on nutrition and obesity; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Newburgh LH, Marsh PL. The use of a high fat diet in the treatment of diabetes mellitus. Arch Intern Med (Chic). 1921;27(6):699–705 [Google Scholar]
- 2.American Diabetes Association Nutritional recommendations and principles for individuals with diabetes mellitus: 1986. American Diabetes Association. Diabetes Care. 1987;10(1):126–132 [DOI] [PubMed] [Google Scholar]
- 3.Armstrong C. ADA updates standards of medical care for patients with diabetes mellitus. Am Fam Physician. 2017;95(1):40–43 [PubMed] [Google Scholar]
- 4.Miller KM, Foster NC, Beck RW, et al. ; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38(6):971–978 [DOI] [PubMed] [Google Scholar]
- 5.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25(2):275–278 [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes. 1997;46(2):271–286 [PubMed] [Google Scholar]
- 7.The DCCT Research Group Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group. Am J Med. 1991;90(4):450–459 [PubMed] [Google Scholar]
- 8.Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2014;(2):CD009122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes. 2014;63(7):2188–2195 [DOI] [PubMed] [Google Scholar]
- 10.Sheard NF, Clark NG, Brand-Miller JC, et al. . Dietary carbohydrate (amount and type) in the prevention and management of diabetes: a statement by the American diabetes association. Diabetes Care. 2004;27(9):2266–2271 [DOI] [PubMed] [Google Scholar]
- 11.Bell KJ, King BR, Shafat A, Smart CE. The relationship between carbohydrate and the mealtime insulin dose in type 1 diabetes. J Diabetes Complications. 2015;29(8):1323–1329 [DOI] [PubMed] [Google Scholar]
- 12.Emami A, Willinska ME, Thabit H, et al. . Behavioral patterns and associations with glucose control during 12-week randomized free-living clinical trial of day and night hybrid closed-loop insulin delivery in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(7):433–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26(8):2261–2267 [DOI] [PubMed] [Google Scholar]
- 14.Dressler A, Reithofer E, Trimmel-Schwahofer P, et al. . Type 1 diabetes and epilepsy: efficacy and safety of the ketogenic diet. Epilepsia. 2010;51(6):1086–1089 [DOI] [PubMed] [Google Scholar]
- 15.Nielsen JV, Gando C, Joensson E, Paulsson C. Low carbohydrate diet in type 1 diabetes, long-term improvement and adherence: a clinical audit. Diabetol Metab Syndr. 2012;4(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen JV, Jönsson E, Ivarsson A. A low carbohydrate diet in type 1 diabetes: clinical experience–a brief report. Ups J Med Sci. 2005;110(3):267–273 [DOI] [PubMed] [Google Scholar]
- 17.Ranjan A, Schmidt S, Damm-Frydenberg C, Holst JJ, Madsbad S, Nørgaard K. Short-term effects of a low carbohydrate diet on glycaemic variables and cardiovascular risk markers in patients with type 1 diabetes: a randomized open-label crossover trial. Diabetes Obes Metab. 2017;19(10):1479–1484 [DOI] [PubMed] [Google Scholar]
- 18.Tóth C, Clemens Z. Type 1 diabetes mellitus successfully managed with the paleolithic ketogenic diet. Int J Case Rep Images. 2014;5(10):699–703 [Google Scholar]
- 19.Feinman RD, Pogozelski WK, Astrup A, et al. . Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2015;31(1):1–13 [DOI] [PubMed] [Google Scholar]
- 20.O’Neill DF, Westman EC, Bernstein RK. The effects of a low-carbohydrate regimen on glycemic control and serum lipids in diabetes mellitus. Metab Syndr Relat Disord. 2003;1(4):291–298 [DOI] [PubMed] [Google Scholar]
- 21.Westman EC, Feinman RD, Mavropoulos JC, et al. . Low-carbohydrate nutrition and metabolism. Am J Clin Nutr. 2007;86(2):276–284 [DOI] [PubMed] [Google Scholar]
- 22.Astrup A, Meinert Larsen T, Harper A. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet. 2004;364(9437):897–899 [DOI] [PubMed] [Google Scholar]
- 23.Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westman EC, Mavropoulos J, Yancy WS, Volek JS. A review of low-carbohydrate ketogenic diets. Curr Atheroscler Rep. 2003;5(6):476–483 [DOI] [PubMed] [Google Scholar]
- 25.de Bock M, Lobley K, Anderson D, et al. . Endocrine and metabolic consequences due to restrictive carbohydrate diets in children with type 1 diabetes: an illustrative case series. Pediatr Diabetes. 2018;19(1):129–137 [DOI] [PubMed] [Google Scholar]
- 26.Kanikarla-Marie P, Jain SK. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic Biol Med. 2016;95:268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernstein RK. Dr. Bernstein’s Diabetes Solution: The Complete Guide to Achieving Normal Blood Sugars. 4th ed. Boston, MA: Little, Brown & Company; 2011 [Google Scholar]
- 28.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA; T1D Exchange Clinic Network . The T1D Exchange clinic registry. J Clin Endocrinol Metab. 2012;97(12):4383–4389 [DOI] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986 [DOI] [PubMed] [Google Scholar]
- 31.Haynes A, Hermann JM, Miller KM, et al. ; T1D Exchange, WACDD and DPV Registries . Severe hypoglycemia rates are not associated with HbA1c: a cross-sectional analysis of 3 contemporary pediatric diabetes registry databases. Pediatr Diabetes. 2017;18(7):643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinstock RS, Xing D, Maahs DM, et al. ; T1D Exchange Clinic Network . Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D exchange clinic registry. J Clin Endocrinol Metab. 2013;98(8):3411–3419 [DOI] [PubMed] [Google Scholar]
- 33.Cengiz E, Xing D, Wong JC, et al. ; T1D Exchange Clinic Network . Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D exchange clinic registry. Pediatr Diabetes. 2013;14(6):447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angus VC, Waugh N. Hospital admission patterns subsequent to diagnosis of type 1 diabetes in children: a systematic review. BMC Health Serv Res. 2007;7:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quispe R, Manalac RJ, Faridi KF, et al. . Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: the Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis. 2015;242(1):243–250 [DOI] [PubMed] [Google Scholar]
- 36.Ahmadi SA, Boroumand MA, Gohari-Moghaddam K, Tajik P, Dibaj SM. The impact of low serum triglyceride on LDL-cholesterol estimation. Arch Iran Med. 2008;11(3):318–321 [PubMed] [Google Scholar]
- 37.Diabetes Control and Complications Trial, Epidemiology of Diabetes Interventions and Complications Research Group Risk factors for cardiovascular disease in type 1 diabetes. Diabetes. 2016;65(5):1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ceriello A, Hanefeld M, Leiter L, et al. . Postprandial glucose regulation and diabetic complications. Arch Intern Med. 2004;164(19):2090–2095 [DOI] [PubMed] [Google Scholar]
- 39.Mansoor N, Vinknes KJ, Veierød MB, Retterstøl K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr. 2016;115(3):466–479 [DOI] [PubMed] [Google Scholar]
- 40.Malone JI. Diabetic central neuropathy: CNS damage related to hyperglycemia. Diabetes. 2016;65(2):355–357 [DOI] [PubMed] [Google Scholar]
- 41.Mazaika PK, Weinzimer SA, Mauras N, et al. ; Diabetes Research in Children Network . Variations in brain volume and growth in young children with type 1 diabetes. Diabetes. 2016;65(2):476–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parthasarathy L, Khadilkar V, Chiplonkar S, Khadilkar A. Longitudinal growth in children and adolescents with type 1 diabetes. Indian Pediatr. 2016;53(11):990–992 [DOI] [PubMed] [Google Scholar]
- 43.Khadilkar VV, Parthasarathy LS, Mallade BB, Khadilkar AV, Chiplonkar SA, Borade AB. Growth status of children and adolescents with type 1 diabetes mellitus. Indian J Endocrinol Metab. 2013;17(6):1057–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hannon TS, Rogol AD. Diabetes mellitus and growth in children and adolescents. J Pediatr. 2012;160(6):893–894 [DOI] [PubMed] [Google Scholar]
- 45.Bonfig W, Kapellen T, Dost A, et al. ; Diabetes Patienten Verlaufsdokumentationssystem Initiative of the German Working Group for Pediatric Diabetology; German Bundesministerium für Bildung und Forschung Competence Net for Diabetes Mellitus . Growth in children and adolescents with type 1 diabetes. J Pediatr. 2012;160(6):900–903.e2 [DOI] [PubMed] [Google Scholar]
- 46.Frohlich-Reiterer EE, Kaspers S, Hofer S, et al. ; Diabetes Patienten Verlaufsdokumentationssystem-Wiss Study Group . Anthropometry, metabolic control, and follow-up in children and adolescents with type 1 diabetes mellitus and biopsy-proven celiac disease. J Pediatr. 2011;158(4):589–593.e2 [DOI] [PubMed] [Google Scholar]