Abstract
BACKGROUND:
The extent to which omega-3 fatty acid status is related to risk for death from any cause and for incident cardiovascular disease (CVD) remains controversial.
OBJECTIVE:
To examine these associations in the Framingham Heart Study.
DESIGN:
Prospective and observational.
SETTING:
Framingham Heart Study Offspring cohort.
MEASUREMENTS:
The exposure marker was red blood cell levels of eicosapentaenoic and docosahexaenoic acids (the Omega-3 Index) measured at baseline. Outcomes included mortality (total, CVD, cancer, and other) and total CVD events in participants free of CVD at baseline. Follow-up was for a median of 7.3 years. Cox proportional hazards models were adjusted for 18 variables (demographic, clinical status, therapeutic, and CVD risk factors).
RESULTS:
Among the 2500 participants (mean age 66 years, 54% women), there were 350 deaths (58 from CVD, 146 from cancer, 128 from other known causes, and 18 from unknown causes). There were 245 CVD events. In multivariable-adjusted analyses, a higher Omega-3 Index was associated with significantly lower risks (P-values for trends across quintiles) for total mortality (P = .02), for non-CVD and non-cancer mortality (P = .009), and for total CVD events (P = .008). Those in the highest (>6.8%) compared to those in the lowest Omega-3 Index quintiles (<4.2%) had a 34% lower risk for death from any cause and 39% lower risk for incident CVD. These associations were generally stronger for docosahexaenoic acid than for eicosapentaenoic acid. When total cholesterol was compared with the Omega-3 Index in the same models, the latter was significantly related with these outcomes, but the former was not.
LIMITATIONS:
Relatively short follow-up time and one-time exposure assessment.
CONCLUSIONS:
A higher Omega-3 Index was associated with reduced risk of both CVD and allcause mortality.
Keywords: Epidemiology, Prospective cohort study, Eicosapentaenoic acid, Docosahexaenoic acid, Omega-3 fatty acids
Introduction
Several recent studies have linked higher blood levels and/or dietary intakes of the long-chain n-3 polyunsaturated fatty acids (PUFAs) with greater longevity. Plasma phospholipid n-3 PUFA levels were inversely associated with total mortality rates in the Cardiovascular Health Study,1 and similar associations were seen for this endpoint with the red blood cell (RBC) content of eicosapentaenoic acid (EPA) plus docosahexaenoic acid (DHA) in the Heart and Soul Study.2 This latter metric, called for simplicity the Omega-3 Index, has been proposed as a risk factor for death from cardiovascular disease (CVD).3,4 Consistent with these observations, there is an inverse relationship between the Omega-3 Index and the rate of telomere attrition, a marker of cellular aging.5 Although early randomized controlled trials with n-3 PUFAs found reduced overall mortality,6,7 other more recent studies8–11 have not confirmed such a protective effect. Some of the potential reasons why recent studies may have yielded null results for intervention with n-3 PUFAs (background use of statins,12 short follow-up periods, low n-3 PUFA doses, improvements in acute care, etc.) have been reviewed.13,14 In the present investigation, we examined the relations between RBC n-3 PUFA levels in participants in the Framingham Heart Study’s (FHS’s) Offspring cohort and total mortality as the primary endpoint, with secondary endpoints of death from CVD and other causes, and of incident coronary heart disease (CHD), CVD, and ischemic stroke.
Methods
The FHS is a longitudinal community-based cohort study that was initiated in 1948. Adult children of the original cohort were recruited in 1971 into the Framingham Offspring Cohort. The selection criteria for this cohort (and for the more racially diverse Framingham Omni Cohort) have previously been described.15,16 We evaluated Framingham Offspring/Omni participants (n = 3021) who attended their 8th/3rd examination cycles (2005–2008). Participants were excluded in hierarchical order if they were missing RBC FA measurements or relevant clinical covariates (n = 122), leaving 2899. We also excluded 399 with a history of CVD (ie, nonfatal CHD or stroke), leaving 2500 for the present investigation. The study protocol was approved by the Institutional Review Board of the Boston University Medical Center. Informed consent was provided by all participants.
Covariates and mortality outcomes
We considered 18 primary baseline demographic and CV risk covariates: sex, age, body mass index, marital status, education level, employment status, health insurance status, regular aspirin user, prevalent hypertensive status, use of cholesterol-lowering drugs, prevalent diabetes, history of CVD, alcohol consumption, smoking status, physical activity (in metabolic equivalent units), the total cholesterol to high-density lipoprotein cholesterol ratio, systolic blood pressure, and C-reactive protein. Four mortality endpoints were examined: total, CVD [fatal myocardial infarction (MI), CHD death, sudden cardiac death, fatal ischemic stroke, or other CVD death), cancer, and other (ie, non-CVD, non-cancer; Note that in the Framingham Heart Study, “other” causes of death were not specifically identified]. Incident CVD-related endpoints were also examined: total CVD (total stroke, total CHD, or CVD mortality), total stroke (any fatal or nonfatal ischemic stroke), and total CHD (fatal or nonfatal MI, CHD death or sudden cardiac death).
RBC FA analysis
Blood was drawn after a 10- to 12-hour fast into an EDTA tube, and RBCs were separated from plasma by centrifugation. The RBC fraction was frozen at −80°C immediately after collection. RBC FA composition was determined as described previously.17 Briefly, RBCs were incubated at 100°C using boron trifluoride methanol and hexane to generate FA methyl esters that were then analyzed by gas chromatography with flame ionization detection. N-3 FAs analyzed included α-linolenic acid (ALA; 18:3n3), EPA (20:5n3), docosapentaenoic acid (DPA; 22:5n3), DHA (22:6n3), and the Omega-3 Index (EPA + DHA).3 The coefficients of variation for these were 10.7%, 7.8%, 3.8%, 3.3%, and 2.4%, respectively.
Statistical analysis
Sample characteristics were summarized using standard statistical metrics (eg, means, standard deviations (SDs), and correlations). Hazard ratios were estimated using the survival package in R.18 Primary analyses related incident clinical outcomes by date of event (or censoring) to quintiles of the Omega-3 Index, with follow-up analyses adjusting for demographic and medical history covariates. Secondary analyses explored relationships of outcomes by quintiles of individual n-3 PUFA levels, of a modified Omega-3 Index that included DPA, and by risk category of the Omega-3 Index (high risk, ≤4%; intermediate risk, >4% to <8%; and low risk, ≥8%).19 In other analyses, we included the n-6 FAs as covariates and also compared the n-6:n-3 ratio with the Omega-3 Index. Finally, baseline total cholesterol levels were compared with the Omega-3 Index in the same models to get a sense of how this emerging risk factor compared with an established one. All analyses used 2-sided tests at the P < .05 statistical significance level.
Results
Cohort description
This analysis was performed on 2500 Framingham Offspring study participants who were free of CVD at baseline and for whom FAs, clinical outcomes, and demographic covariates were available. Follow-up ended on December 31, 2016 (maximum, 11.2 years; median, 7.3 years). Table 1 provides the baseline characteristics of the cohort by mortality status. The average age was 66 years, and there were more women (57%) than men (43%). There were 350 deaths during the follow-up period: 58 were attributable to CVD (17%), 146 to cancer (42%), 128 to other known causes (37%), and 18 from unknown causes (5%). Overall, there were 245 incident cases of CVD, 119 of CHD, and 105 ischemic strokes. (Case and control numbers and length of follow-up for each outcome are in Table S1).
Table 1. Demographic overview (n = 2500).
| Died (n = 350) | Still living (n = 2150) | Total | |
|---|---|---|---|
|
|
|||
| Variable | % (n) or mean (SD) | ||
| Sex | |||
| Male | 52.9% (185) | 41.5% (893) | 43.1% (1078) |
| Female | 47.1% (165) | 58.5% (1257) | 56.9% (1422) |
| Age | 72.9 (8.6) | 64.4 (8.2) | 65.56 (8.76) |
| BMI | 27.9 (5.9) | 28.3 (5.4) | 28.2 (5.4) |
| Marital status | |||
| Single/never married | 4.3% (15) | 6.7% (144) | 6.4% (159) |
| Married | 63.7% (223) | 70.7% (1519) | 69.7% (1742) |
| Separated/divorced | 12.0% (42) | 12.7% (273) | 12.6% (315) |
| Widowed | 18.3% (64) | 9.4% (203) | 10.7% (267) |
| Education | |||
| Some high school or less | 6.0% (21) | 2.4% (51) | 2.9% (72) |
| High school graduate | 33.7% (118) | 24.8% (533) | 26.0% (651) |
| Some college or vocational | 22.9% (78) | 21.9% (471) | 22.0% (549) |
| College graduate | 36.6% (128) | 50.5% (1086) | 48.6% (1214) |
| Employment | |||
| Employed | 31.1% (109) | 56.3% (1210) | 52.8% (1319) |
| Disabled/unemployed | 3.1% (11) | 2.5% (53) | 2.6% (64) |
| Retired | 64.3% (225) | 40.8% (877) | 44.1% (1102) |
| Health insurance status | |||
| No insurance | 2.3% (8) | 1.9% (40) | 1.9% (48) |
| Insurance, but no prescription | 11.4% (40) | 8.4% (181) | 8.8% (221) |
| Full insurance | 84.0% (294) | 88.7% (1906) | 88.0% (2200) |
| Regular aspirin use | 49.7% (174) | 38.6% (830) | 40.2% (1004) |
| Prevalent hypertension | 57.1% (200) | 42.2% (908) | 44.3% (1108) |
| Cholesterol medication | 41.4% (145) | 36.9% (794) | 37.6% (939) |
| Prevalent diabetes | 22.3% (78) | 11.6% (249) | 13.1% (327) |
| Alcohol consumption | |||
| None | 34.0% (119) | 23.2% (499) | 24.7% (618) |
| <1 drink per day | 36.0% (126) | 50.7% (1089) | 48.6% (1215) |
| 1–2 drinks per day | 22.6% (79) | 20.2% (434) | 20.5% (513) |
| >2 drinks per day | 6.9% (24) | 5.7% (123) | 5.9% (147) |
| Smoking | |||
| Not current smoker | 89.1% (312) | 90.5% (1946) | 90.3% (2258) |
| Current | 10.6% (37) | 9.3% (200) | 9.5% (237) |
| METS | 4.7 (15.4) | 3.3 (8.6) | 3.5 (9.9) |
| Total to HDL cholesterol ratio | 3.5 (1.1) | 3.5 (1.0) | 3.5 (1.1) |
| Systolic BP | 133.6 (19.8) | 128.2 (16.9) | 129.0 (17.4) |
| C-reactive protein | 4.9 (12.7) | 3.0 (6.1) | 3.3 (7.4) |
BMI, body mass index; METS, metabolic equivalents; HDL, high-density lipoprotein; BP, blood pressure.
The Omega-3 Index and total and cause-specific mortality
We estimated the associations between adjusted risk for fatal outcomes across quintiles of the Omega-3 Index (Fig. 1, with details in Table S2). In the multivariable-adjusted model, death from any cause was significantly and inversely associated with the Omega-3 Index (P for trend = .02). When comparing individuals in the lowest quintile of the Omega-3 Index (<4.2%) to those in the highest (>6.8%), risk was 34% lower. Median values for the extreme quintiles were 3.7% and 7.8%. The modified Omega-3 Index (which included DPA) performed no differently from the original metric (Table S3), and an examination of the relations between risk and the three Omega-3 Index risk categories found a significant trend across the categories (P = .04), but only the intermediate category differed significantly from the high-risk category (possibly because there were 10-fold more subjects in the intermediate than the highest categories; Table S4). When we controlled for RBC n-6 PUFA levels in the original analysis, or substituted the n-6:n-3 ratio for the Omega-3 Index, there was no material improvement in mortality hazard ratios (Table S5). In neither case did the n-6 PUFAs alter the n-3 PUFA associations.
Figure 1.
Relations between quintiles of the Omega-3 Index and hazard ratios (+95% confidence interval) for death from CVD (n = 58), cancer (n = 146), other causes (n = 128), and all causes (n = 350, including 18 of unknown causes). Data are from 2500 participants free of baseline CVD followed for a median of 7.3 years. Adjusted for all variables in Table 1. P-values for trend are shown above the columns. CVD, cardiovascular disease; CHD, coronary heart disease.
Regarding cause-specific mortality, the Omega-3 Index was only significantly associated with risk for death from “other” (non-CVD, non-cancer) causes (P for trend = .009), with the participants in the first (reference) quintile being at significantly higher risk than those in quintiles 2–5. There was a trend for reduced risk for CVD across Omega-3 Index levels (eg, there was a 61% lower risk between extreme quintiles), but with wide confidence intervals, the differences were nonsignificant. The results for total and cause-specific mortality were similar in analyses including all 2899 participants (ie, including those with a history of CVD; Table S6).
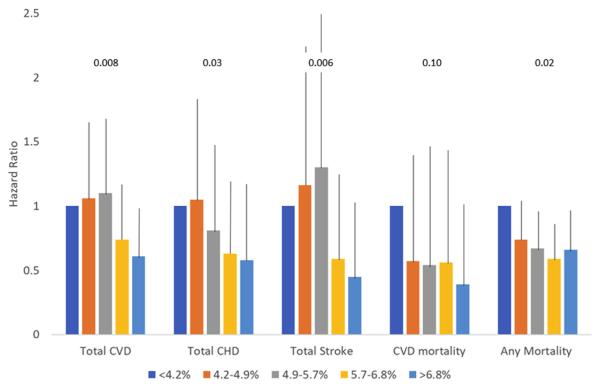
The Omega-3 Index and CVD outcomes
The Omega-3 Index was significantly and inversely associated with total CVD, total CHD, and total stroke after adjusting for covariates in a linear trend analysis across quintiles (Table 2; P values for trends = .008, .03, and .006, respectively). Unadjusted hazard ratios are shown in Table S7. The hazard ratio for total CVD was significantly reduced in quintile 5 vs 1, but for total CHD or total stroke, no individual quintile differed significantly from the reference. Similar findings were observed in the full cohort (n = 2899) for total CHD and total stroke, but the relationship with total CVD was no longer statistically significant (Table S8). Using the modified Omega-3 Index (with DPA) did not change the pattern of results (Table S3), and when Omega-3 risk categories were tested, the general pattern of relationships remained the same, but they were mostly nonsignificant (Table S4). Finally, neither including n-6 PUFAs as covariates nor replacing the Omega-3 Index with the n-6:n-3 ratio altered the original results (Table S5).
Table 2. Adjusted risk of events by Omega-3 Index (n = 2500)§.
| Hazard ratios (95% CIs) |
|||
|---|---|---|---|
| Total CVD | Total CHD | Total stroke | |
| Omega-3 Index | |||
| <4.2% (n = 508) | 1.0 | 1.0 | 1.0 |
| 4.2%–4.9% (n = 500) | 1.06 (0.69, 1.63) | 1.05 (0.60, 1.83) | 1.16 (0.60, 2.24) |
| 4.9%–5.7% (n = 501) | 1.10 (0.72, 1.68) | 0.81 (0.44, 1.48) | 1.30 (0.68, 2.49) |
| 5.7%–6.8% (n = 502) | 0.74 (0.47, 1.17) | 0.63 (0.34, 1.20) | 0.59 (0.28, 1.25) |
| >6.8% (n = 489) | 0.61 (0.37, 0.99) * | 0.58 (0.29, 1.18) | 0.45 (0.20, 1.03) |
| P-value for linear trend | .008 † | .03 * | .006 † |
CVD, cardiovascular disease; CHD, coronary heart disease; CI, confidence interval.
P < .05
P < .01.
All significant hazard ratios/P-values are shown in bold italics.
Adjusted for all variables in Table 1 except baseline CVD.
Individual RBC n-3 PUFAs and outcomes
The adjusted risk for experiencing the endpoints of interest by each of the four n-3 PUFAs separately is shown in Table 3 (unadjusted hazard ratios in Table S9). The plant-derived n-3 PUFA, ALA, was not significantly associated with reduced risk for any of the outcomes tested (Table 3). In general, as the levels of the EPA and (especially) DHA increased, risk for disease outcomes and total (and other) mortality decreased (Table 3). The only outcome that DPA was (marginally, P = .059) associated with was death from other causes (Table 3).
Table 3. Adjusted risk of events and mortality by individual omega-3 fatty acids (n = 2500)§.
| Hazard ratios (95% CIs) |
|||||||
|---|---|---|---|---|---|---|---|
| Total Events |
Mortality |
||||||
| CVD | CHD | Stroke | CVD | Cancer | Other | Total | |
| α-linolenic acid | |||||||
| <4.2% (n = 483) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 4.2%–4.9% (n = 496) | 1.45 (0.94, 2.22) | 1.44 (0.78, 2.65) | 1.59 (0.77, 3.25) | 1.76 (0.62, 4.93) | 0.71 (0.41, 1.21) | 0.51 (0.26, 1.02) | 0.84 (0.58, 1.21) |
| 4.9%–5.7% (n = 506) | 0.98 (0.62, 1.56) | 1.00 (0.52, 1.92) | 0.93 (0.43, 2.02) | 1.79 (0.59, 5.44) | 1.05 (0.65, 1.71) | 0.92 (0.49, 1.70) | 1.04 (0.72, 1.50) |
| 5.7%–6.8% (n = 505) | 1.00 (0.63, 1.61) | 0.78 (0.40, 1.53) | 1.27 (0.62, 2.64) | 3.06 (1.09, 8.60) * | 0.54 (0.28, 1.03) | 0.84 (0.46, 1.54) | 0.96 (0.67, 1.39) |
| >6.8% (n = 510) | 1.16 (0.75, 1.80) | 1.03 (0.55, 1.94) | 1.42 (0.69, 2.92) | 1.78 (0.62, 5.06) | 0.91 (0.54, 1.53) | 0.85 (0.48, 1.52) | 1.04 (0.73, 1.47) |
| P-value for linear trend . | 84 | .43 | .59 | .16 | .48 | .94 | .64 |
| Eicosapentaenoic acid | |||||||
| <0.44% (n = 494) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 0.44%–0.55% (n = 495) | 1.34 (0.90, 1.99) | 1.13 (0.66, 1.93) | 1.61 (0.83, 3.15) | 0.72 (0.30, 1.73) | 0.50 (0.27, 0.93) * | 0.98 (0.54, 1.77) | 0.78 (0.55, 1.10) |
| 0.55%–0.68% (n = 510) | 1.20 (0.77, 1.87) | 0.74 (0.30, 1.38) | 1.78 (0.88, 3.60) | 0.64 (0.26, 1.62) | 0.69 (0.40, 1.19) | 0.81 (0.47, 1.41) | 0.66 (0.47, 0.95) * |
| 0.68%–0.92% (n = 508) | 0.77 (0.49, 1.20) | 0.45 (0.24, 0.86) * | 1.13 (0.54, 2.35) | 0.81 (0.32, 2.05) | 0.58 (0.35, 0.97) * | 0.41 (0.22, 0.79) † | 0.58 (0.41, 0.84) † |
| >0.92% (n = 493) | 0.87 (0.55, 1.39) | 0.69 (0.37, 1.29) | 0.95 (0.43, 2.11) | 0.58 (0.21, 1.61) | 0.83 (0.49, 1.41) | 0.71 (0.39, 1.30) | 0.74 (0.52, 1.06) |
| P-value for linear trend | .11 | .02 * | .57 | .36 | .63 | .03 * | .03 |
| Docosapentaenoic acid | |||||||
| <0.44% (n = 493) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 0.44%–0.55% (n = 504) | 1.17 (0.78, 1.77) | 0.86 (0.49, 1.51) | 2.09 (1.05, 4.16)* | 0.64 (0.24, 1.70) | 1.02 (0.60, 1.73) | 1.34 (0.76, 2.34) | 0.93 (0.66, 1.29) |
| 0.55%–0.68% (n = 520) | 0.83 (0.55, 1.27) | 0.64 (0.36, 1.15) | 1.24 (0.60, 2.54) | 0.92 (0.37, 2.27) | 0.52 (0.27, 0.99)* | 0.89 (0.48, 1.67) | 0.71 (0.50, 1.01) |
| 0.68%–0.92% (n = 497) | 0.87 (0.56, 1.36) | 0.83 (0.47, 1.48) | 1.22 (0.57, 2.63) | 0.40 (0.14, 1.14) | 0.86 (0.49, 1.51) | 0.91 (0.45, 1.85) | 0.74 (0.51, 1.07) |
| >0.92% (n = 486) | 0.92 (0.58, 1.46) | 0.60 (0.31, 1.15) | 1.17 (0.52, 2.66) | 0.90 (0.34, 2.37) | 1.15 (0.68, 1.94) | 0.60 (0.30, 1.17) | 0.81 (0.56, 1.17) |
| P-value for linear trend | .31 | .13 | .65 | .58 | .87 | .059 | .10 |
| Docosahexaenoic acid | |||||||
| <3.69% (n = 513) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 3.69%–4.36% (n = 498) | 0.98 (0.64, 1.50) | 0.90 (0.51, 1.57) | 1.14 (0.60, 2.17) | 0.67 (0.26, 1.71) | 0.70 (0.41, 1.19) | 0.43 (0.24, .0.78) † | 0.78 (0.55, 1.11) |
| 4.36%–5.01% (n = 498) | 0.99 (0.65, 1.50) | 0.68 (0.37, 1.25) | 1.27 (0.68, 2.38) | 0.61 (0.22, 1.72) | 0.62 (0.35, 1.11) | 0.38 (0.20, 0.71) † | 0.61 (0.42, 0.88) † |
| 5.01%–5.96% (n = 504) | 0.71 (0.44, 1.12) | 0.61 (0.32, 1.14) | 0.57 (0.27, 1.20) | 0.76 (0.29, 1.97) | 0.77 (0.45, 1.31) | 0.47 (0.26, 0.87) * | 0.72 (0.50, 1.05) |
| >5.96% (n = 487) | 0.57 (0.35, 0.92) * | 0.54 (0.27, 1.07) | 0.41 (0.18, 0.93) * | 0.43 (0.17, 1.10) | 0.92 (0.54, 1.56) | 0.47 (0.27, 0.81) † | 0.68 (0.47, 0.99) * |
| P-value for linear trend | .004 † | .03 | .002 † | .14 | .98 | .054 | .06 |
CVD, cardiovascular disease; CHD, coronary heart disease; CI, confidence interval.
P < .05
P < .01.
All significant hazard ratios/P-values are shown in bold italics.
Adjusted for all variables in Table 1 except history of CVD.
Omega-3 Index vs total cholesterol
In the head-to-head comparison of the Omega-3 Index and total cholesterol for total and CVD mortality and for the 3 CVD outcomes, the former metric was significantly associated with risk for 4 of the 5 outcomes, whereas the total cholesterol level, in the same models, was not associated with risk for any of these outcomes (Table 4).
Table 4. Omega-3 Index and total cholesterol: Associations with risk for disease outcomes (n = 2500).
| Hazard ratios (95% CIs) |
|||||
|---|---|---|---|---|---|
| Biomarker | Total CVD | Total CHD | Total Stroke | CVD mortality | Any Mortality |
| Omega-3 Index§ | |||||
| <4.2% (n = 506) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 4.2%–4.9% (n = 500) | 1.08 (0.70, 1.65) | 1.06 (0.61, 1.85) | 1.20 (0.63, 2.27) | 0.65 (0.27, 1.54) | 0.74 (0.53, 1.03) |
| 4.9%–5.7% (n = 500) | 1.11 (0.73, 1.68) | 0.81 (0.44, 1.47) | 1.32 (0.69, 2.50) | 0.53 (0.19, 1.49) | 0.67 (0.47, 0.97) |
| 5.7%–6.8% (n = 502) | 0.74 (0.47, 1.17) | 0.63 (0.34, 1.19) | 0.61 (0.29, 1.27) | 0.58 (0.22, 1.55) | 0.58 (0.41, 0.84) † |
| >6.8% (n = 489) | 0.63 (0.39, 1.01) | 0.59 (0.30, 1.17) | 0.47 (0.21, 1.06) | 0.44 (0.16, 1.91) | 0.65 (0.45, 0.94) |
| P-value from linear trend testǁ | .009 † | .03 * | .006 † | .19 | .01 * |
| Total cholesterol§ | |||||
| <154 (n = 406) | 1.0 | 1.00 | 1.0 | 1.0 | 1.0 |
| 154–175 (n = 491) | 1.03 (0.69, 1.56) | 1.02 (0.55, 1.89) | 0.88 (0.47, 1.66) | 1.22 (0.53, 2.77) | 0.73 (0.50, 1.05) |
| 176–194 (n = 520) | 0.95 (0.62, 1.45) | 1.29 (0.71, 2.37) | 0.63 (0.31, 1.27) | 0.67 (0.26, 1.77) | 0.72 (0.49, 1.06) |
| 195–218 (n = 551) | 0.89 (0.56, 1.39) | 1.01 (0.53, 1.92) | 0.69 (0.32, 1.40) | 1.07 (0.30, 3.79) | 0.91 (0.64, 1.31) |
| >218 (n = 530) | 1.09 (0.66, 1.80) | 1.59 (0.81, 3.11) | 0.89 (0.41, 1.93) | 0.31 (0.72, 1.34) | 0.96 (0.66, 1.40) |
| P-value from linear trend testǁ | .99 | .26 | .50 | .27 | .11 |
CVD, cardiovascular disease; CHD, coronary heart disease; CI, confidence interval.
P < .05
P < .01
All significant hazard ratios/P-values are shown in bold italics.
Hazard ratios presented here were adjusted for all variables in Table 1 with the addition of grouped total cholesterol (and removing total cholesterol to high-density lipoprotein cholesterol ratio) and the grouped Omega-3 Index.
Linear trend test models were fit for both the Omega-3 Index and TC simultaneously, after adjusting for variables as described in footnote “§”.
Discussion
In this study of participants in the Framingham Offspring study, the baseline Omega-3 Index at about 66 years of age was inversely associated with total mortality over the next 7 years—with values of the Omega-3 Index significantly lower in those who died during follow-up than those who did not. The same was generally true for the 2 components of the Index, EPA and DHA, but not for RBC DPA n-3 or ALA. Findings for the Omega-3 Index persisted in a model adjusted for 18 other relevant variables including plasma lipids and C-reactive protein.
We recently reported similar findings to these in the Women’s Health Initiative Memory Study where there was a significant inverse trend across quartiles of the Omega-3 Index for total mortality, with the highest quartile being at 22% lower risk for death compared with the lowest.20 That study followed a nationwide sample of 6500, approximately 70-year-old women for 15 years. Kleber et al. also found a 9% decrease in risk for all-cause mortality per 1 SD increase in the Omega-3 Index after 10 years of follow-up in 3259 German CHD patients.21 In the present study, we followed up 2500 men and women for half as long and found an interquintile risk reduction of 34%. We also found reduced risk for total CVD events, CHD events, and stroke. The approximately 50% lower risk for CVD mortality was, however, not statistically significant, most likely due to having only 58 events in the analysis (see below). Nevertheless, these findings are generally consistent with those of several meta-analyses that all linked higher n-3 PUFA biomarker levels with reduced risk for “coronary events”22 or fatal CHD.19,23 This study was somewhat unique, however, in exploring relations between RBC n-3 PUFA levels instead of whole plasma or plasma phospholipid n-3 PUFAs. But since these markers of n-3 PUFA status are strongly intercorrelated,24 this coherence is expected.
The causes of death most strongly associated with the Omega-3 Index were non-CVD and non-cancer, ie, “other” causes. By design, the FHS focused primarily on CVD outcomes, so details of the other causes of death are unavailable. An 11% reduction (per 1 SD increase in the Omega-3 Index) in risk for death from other causes was also seen in the women’s study mentioned previously.20 It is unclear what mechanism might explain this observation; but since n-3 PUFAs are known to have a wide variety of effects on cell membrane biology,25,26 a systemic impact of higher levels of these FAs on overall cellular health is possible. As noted earlier, several recent randomized trials of fish oil with total mortality as an endpoint have been null, but they typically showed trends toward a benefit.27,28 More consistent with our observations was a 2017 meta-analysis,29 which found a significant 19% reduction in risk for coronary death associated with fish oil treatment. Two nonrandomized retrospective studies using governmental databases to track patient outcomes (one from England30 and another from Italy31) reported lower risk for all-cause mortality in those CHD patients who had been prescribed 1 g of fish oil at discharge. Finally, higher plasma long-chain n-3 FA levels were strongly associated with reduced risk for total mortality and CHD outcomes in renal transplant patients in Norway.32
A somewhat surprising observation in this study was the low rate of CVD in this population, with only 17% of deaths being attributable to CVD (as opposed to 42% from cancer). Part of this is clearly the exclusion from the analysis set of participants with CVD at baseline, but even including these individuals, the CVD death rate was only 22%. This relatively low CVD death rate should perhaps not be surprising given the reduction in heart disease mortality in the United States over the last several decades. For example, the age-standardized death rate from heart disease per 100,000 people dropped from 520 in 1969 to 167 in 2014, a 68% decline.33
With regard to the individual RBC n-3 PUFAs studied here, in the multivariable-adjusted model, ALA was not significantly related to any measured outcome in the quintile analysis. The null mortality findings for ALA agree with some previous studies,34,35 but higher circulating ALA levels (primarily plasma and plasma phospholipids) have been favorably associated with CVD death,23 and ALA intake was associated with reduced total mortality in a large randomized clinical trial (RCT) from Spain.36 So, evidence for ALA remains mixed. Turning to DPA, in the final model, it was not related with any examined outcome (although trends were favorable). This contrasts with a previous meta-analysis in which this FA (again, more often measured in whole plasma or plasma phospholipids) was inversely associated with risk for CVD death.23 In light of this and other prior reports (eg, in heart failure37), the utility of RBC DPA as a risk marker remains unclear. Nevertheless, because of the growing evidence for a beneficial role of DPA,38 it is reasonable to ask why this FA is not a component of the Omega-3 Index. Including it would be reasonable if adding it to EPA and DHA improved the utility of the metric in risk assessment. In the present study, we compared the original and the modified Omega-3 Index (Table S3) and found essentially the same associations with outcomes for both. This would be expected since the correlation between these 2 predictor variables is 0.98. Hence, our findings provide no compelling reason to alter the original definition/components of the Omega-3 Index.
We found that the risk of stroke was inversely associated with the Omega-3 Index, but not in a linear manner, ie, hazard ratios were reduced only in the upper 2 quintiles; however, the trend across quintiles was significant. Risk reduction was largely associated with DHA, where risk was 59% lower in the fifth (vs the first) quintile (P < .05). An association between serum long-chain n-3 PUFA levels and stroke risk has been reported previously in cross-sectional studies.39,40 The impact of marine n-3 PUFA levels on ischemic stroke may extend beyond the risk of incident event. Lower plasma proportions of EPA and DHA have been shown to be independently associated with increased ischemic stroke severity and, for DHA, poor functional outcomes at 90 days after stroke.41 In addition, a higher ratio of EPA or DHA to arachidonic acid in serum was associated with lower likelihood of early neurologic deterioration after ischemic stroke.42 A 2012 meta-analysis of 32 observational cohorts and 13 n-3 RCTs reported inverse associations with stroke and fish intake, but no significant relations with either circulating biomarkers or with supplementation,43 although a 2017 study of plasma phospholipid omega-3 FA and incident stroke from 3 cohorts found inverse relations with DHA as we did here.44 A randomized, open-label study in patients surviving myocardial infarction45 showed a reduction in risk of nonfatal stroke with n-3 FA supplementation, and an open-label study in Japan demonstrated no effect on primary prevention of stroke but a 20% relative reduction in recurrent total stroke with EPA supplementation.46 However, some studies have found no relations between n-3 PUFA levels and stroke,47,48 so the n-3 PUFA and stroke literature is mixed, and more studies are needed.
A variety of exploratory analyses were conducted here. Given the controversies regarding the CVD effects of the n-6 PUFAs and the frequent use of the n-6:n-3 ratio, in one analysis we controlled for RBC n-6 PUFA levels and in another we directly compared the ratio with the Omega-3 Index. In neither case did the n-6 PUFAs add or detract from the omega-3 effect. Hence, this study found no support for using a combined metric with n-6 and n-3 PUFAs. Finally, we compared associations with disease outcomes for the Omega-3 Index with that of total cholesterol, one of the most well-known CVD risk factors. This analysis was undertaken to give some perspective of the relative sensitivity of these 2 biomarkers. In head-to-head comparisons with the same multivariable models, a higher Omega-3 Index was significantly associated with reduced risk for 4 of the 5 outcomes studies, but cholesterol was unrelated to risk for any outcome. This may be because about 38% of participants were on a cholesterol-lowering medication, and so cholesterol levels at baseline were therapeutically reduced; because 399 subjects with a diagnosis of CVD at baseline were excluded; or because those who were particularly susceptible to cholesterol-induced CHD may have already died. There is indeed some controversy regarding the predictive power of serum cholesterol in older individuals.49 Nevertheless, in the unique context of this study in which we controlled for 17 other variables, cholesterol levels were unassociated with these outcomes. Whatever the reasons, this observation supports the need for further research to more clearly define the relative roles of traditional and emerging biomarkers in risk stratification.
The originally proposed (in 2004) clinical cut-points for the Omega-3 Index were <4% (high risk) and >8% (low risk).3 Although these have recently been confirmed in a meta-analysis including 10 cohorts and over 27,000 subjects,19 it was of interest to explore their utility in the present study as well. We found a significant trend across risk categories for total mortality and significant reductions in risk for the intermediate group compared with the high group, but not for the >8% category. This may reflect a nonlinear relationship between the Omega-3 Index and risk as has been suggested by others,25 or the fact that only 10% of the sample was in the >08% category. Nevertheless, the hazard ratio of 0.69 for any death in the >8% category is at least suggestive of lower risk.
As alluded to earlier, mechanisms that may explain the associations between higher RBC n-3 PUFA and improved longevity and reduced CVD risk are not clearly understood, but there are beneficial effects of these FAs on a variety of CV risk factors. These include reductions in serum triglyceride levels,50 blood pressure,51 platelet aggregation,52 heart rate,53 susceptibility to ventricular fibrillation (in some settings),54 inflammatory markers,55,56 and plaque vulnerability57,58 along with improvements in endothelial function.59 The observation that blood EPA + DHA levels are independently associated with slower rates of telomere shortening,5 a purported marker of “cellular aging,” is yet another favorable biomarker relationship, but it is not a molecular mechanism.
From an observational study, we cannot conclude that raising the Omega-3 Index will have heart benefits and/or prolong life. Nevertheless, it is instructive to consider how much more EPA + DHA an individual might need to consume to move the median Omega-3 Index in quintile 1 (3.7%) to that in quintile 5 (7.8%). Based on a recent dose-response study,60 one can estimate that it would take about 1300 more mg of EPA + DHA per day to do this. This amount of EPA + DHA could be obtained from a single serving (100 g) of farmed salmon61 daily. Alternatively, 4 standard fish oil pills per day would suffice. Several early omega-3 RCTs used 800–1000 mg/d and reported reduced total mortality,6,7,62 whereas other more recent studies at the same dose have not.9,11,63 Several possible reasons for this discrepancy have been proposed.64,65
Strengths and limitations
The strengths of our investigation include the relatively large sample size from a well-characterized community, the unambiguous nature of the primary endpoint, and the use of an objective and standardized biomarker of PUFA exposure that has low biological variability.66 Weaknesses include the assessment of PUFA exposure at only one point in time (which cannot capture changes in PUFA status that may have occurred during follow-up) and the largely Caucasian cohort (which precludes firm conclusions regarding other races). Also, the relatively low event rates, especially for CVD death, limited our ability to detect associations with this endpoint. Although clearly affected by omega-3 PUFA intake, other factors can influence the Omega-3 Index such as smoking and body weight,67 both of which were controlled for here. What we did not (and could not) control for is heritability, which we showed previously to explain about 24% of the variability in this biomarker.67 In the same vein, we did not include in our models the intake of key nutrients (kilocalories, saturated FAs, salt, fiber, etc.), which might contribute to the relationships reported here. However, by controlling for some of the physiological/metabolic consequences of consuming diets of poor quality (body weight, blood pressure, hyperlipidemia, and CRP levels), as well as for socioeconomic variables (which themselves track with poor diets), it is unlikely that including individual nutrients would have altered the outcome of the study. As always, the presence of residual or unmeasured confounding also precludes inferences about causality. For example, despite controlling for multiple lifestyle/health factors, higher omega-3 PUFA levels may simply be a marker for a healthier overall diet and behaviors, which themselves, independent of the omega-3 levels, may be cardioprotective.
In conclusion, we observed that higher RBC levels of n-3 PUFAs were associated with greater longevity and reduced risk for several CVD-related endpoints in this community-based sample. These findings, in the context of the totality of available evidence on this topic, provide further support for a role for the use of the Omega-3 Index in risk stratification algorithms.
Acknowledgments
Funding Sources: This study was supported by NIH contracts N01-HC25195 and HHSN268201500001l (Dr. Vasan) and R01 HL089590 (Dr. Harris). Statistical analysis was supported by OmegaQuant Analytics, LLC, in part through a grant from the Global Organization for EPA and DHA. The NHLBI played no role in the design, conduct, and reporting of these data.
APPENDIX
Table S1. Distribution of outcomes (n = 2500).
| Number of people with events (cases) |
Number of people without events (controls) |
Median follow-up days* |
Maximum follow-up days* |
|
|---|---|---|---|---|
| Total CVD | 245 | 2255 | 2351 | 3833 |
| Total CHD | 119 | 2381 | 2342 | 3815 |
| Ischemic stroke | 105 | 2395 | 2342 | 3833 |
| CVD mortality | 58 | 2442 | 2673 | 3815 |
| Death from any cause† | 350 | 2150 | 2686 | 3815 |
CVD, cardiovascular disease; CHD, coronary heart disease; CI, confidence interval.
Days to event or days to censoring.
The causes of the 350 observed deaths, besides CVD, were 146 cancer (42%), 128 other (37%), and 18 unknown (5%).
Table S2. Risk of fatal events by Omega-3 Index (n = 2500).
| Hazard ratios (95% CIs) |
||||
|---|---|---|---|---|
| CVD mortality | Cancer mortality | Other mortality | Any mortality | |
| Omega-3 Index–unadjusted | ||||
| <4.2% (n = 508) | 1.0 | 1.0 | 1.0 | 1.0 |
| 4.2%–4.9% (n = 500) | 0.97 (0.41, 2.30) | 0.94 (0.58, 1.55) | 0.59 (0.34, 1.02) | 0.81 (0.59, 1.12) |
| 4.9%–5.7% (n = 501) | 0.96 (0.43, 2.16) | 0.88 (0.50, 1.57) | 0.63 (0.36, 1.09) | 0.78 (0.56, 1.10) |
| 5.7%–6.8% (n = 502) | 1.10 (0.46, 2.62) | 0.72 (0.42, 1.23) | 0.67 (0.40, 1.11) | 0.73 (0.53, 1.02) |
| >6.8% (n = 489) | 0.88 (0.39, 2.01) | 1.19 (0.72, 1.97) | 0.53 (0.31, 0.91) * | 0.81 (0.58, 1.12) |
| P-value for linear trend | .86 | .85 | .07 | .16 |
| Omega-3 Index–adjusted§ | ||||
| <4.2% (n = 508) | 1.0 | 1.0 | 1.0 | 1.0 |
| 4.2%–4.9% (n = 500) | 0.57 (0.23, 1.40) | 0.66 (0.38, 1.14) | 0.54 (0.31, 0.94) * | 0.74 (0.53, 1.04) |
| 4.9%–5.7% (n = 501) | 0.54 (0.20, 1.47) | 0.73 (0.42, 1.28) | 0.38 (0.20, 0.73) † | 0.67 (0.46, 0.96) * |
| 5.7%–6.8% (n = 502) | 0.56 (0.22, 1.43) | 0.60 (0.35, 1.03) | 0.44 (0.25, 0.79) † | 0.59 (0.41, 0.86) † |
| >6.8% (n = 489) | 0.39 (0.15, 1.02) | 0.96 (0.56, 1.64) | 0.44 (0.24, 0.79) † | 0.66 (0.45, 0.96) * |
| P-value for linear trend | .10 | .88 | .009 † | .02 * |
CVD, cardiovascular disease; CI, confidence interval.
P < .05
P < .01
P < .001.
All significant hazard ratios/P-values are shown in bold italics.
Adjusted for all variables in Table 1 except baseline CVD.
Table S3. Risk of events by Omega-3 Index modified to include docosapentaenoic acid (n = 2500).
| Hazard ratios (95% CIs |
||||
|---|---|---|---|---|
| Total CVD | Total CHD | Total stroke | Any mortality | |
| Omega-3 Index–adjusted | ||||
| <4.2% (n = 508) | 1.0 | 1.0 | 1.0 | 1.0 |
| 4.2%–4.9% (n = 500) | 1.06 (0.69, 1.63) | 1.05 (0.60, 1.83) | 1.16 (0.60, 2.24) | 0.74 (0.53, 1.04) |
| 4.9%–5.7% (n = 501) | 1.10 (0.72, 1.68) | 0.81 (0.44, 1.48) | 1.30 (0.68, 2.49) | 0.67 (0.46, 0.96) * |
| 5.7%–6.8% (n = 502) | 0.74 (0.47, 1.17) | 0.63 (0.34, 1.20) | 0.59 (0.28, 1.25) | 0.59 (0.41, 0.86) † |
| >6.8% (n = 489) | 0.61 (0.37, 0.99) * | 0.58 (0.29, 1.18) | 0.45 (0.20, 1.03) | 0.66 (0.45, 0.96) * |
| P-value for linear trend | .008 † | .03 * | .006 † | .02 * |
| Modified Omega-3 Index (+DPA)–adjusted | ||||
| <6.7% (n = 510) | 1.0 | 1.0 | 1.0 | 1.0 |
| 6.7%–7.5% (n = 497) | 1.01 (0.66, 1.54) | 0.93 (0.54, 1.61) | 1.04 (0.52, 2.07) | 0.75 (0.53, 1.06) |
| 7.5%–8.4% (n = 502) | 0.83 (0.54, 1.27) | 0.58 (0.32, 1.05) | 1.09 (0.57, 2.08) | 0.48 (0.40, 0.84) † |
| 8.4%–9.7% (n = 507) | 0.69 (0.44, 1.08) | 0.54 (0.29, 1.01) | 0.65 (0.31, 1.35) | 0.72 (0.50, 1.04) |
| >9.7% (n = 484) | 0.64 (0.40, 1.03) | 0.54 (0.27, 1.05) | 0.51 (0.23, 1.14) | 0.61 (0.41, 0.90) * |
| P-value for linear trend | .012 * | .013 * | .024 * | .02 * |
CVD, cardiovascular disease; CHD, coronary heart disease; CI, confidence interval.
P < .05
P < .01
P < .001.
All significant hazard ratios/P-values are shown in bold italics.
Adjusted for all variables in Table 1.
Table S4. Risk of events by Omega-3 Index risk categories (n = 2500).
| Hazard ratios (95% CIs) |
||||
|---|---|---|---|---|
| Total CVD | Total CHD | Total stroke | Any mortality | |
| Omega-3 Index–unadjusted | ||||
| <4% (n = 416) | 1.0 | 1.0 | 1.0 | 1.0 |
| 4%–8% (n = 1876) | 1.03 (0.72, 1.48) | 0.94 (0.57, 1.54) | 1.12 (0.64, 1.94) | 0.73 (0.56, 0.96) * |
| >8% (n = 208) | 0.77 (0.43, 1.38) | 0.73 (0.34, 1.58) | 0.88 (0.33, 2.32) | 0.85 (0.68, 1.07) |
| P-value for linear trend | .45 | .48 | .93 | .15 |
| Omega-3 Index–adjusted§ | ||||
| <4% (n = 416) | 1.0 | 1.0 | 1.0 | 1.0 |
| 4%–8% (n = 1876) | 0.88 (0.59, 1.32) | 0.85 (0.49, 1.47) | 0.91 (0.48, 1.71) | 0.61 (0.45, 0.83) † |
| >8% (n = 208) | 0.68 (0.36, 1.27) | 0.83 (0.35, 1.94) | 0.50 (0.17, 1.52) | 0.69 (0.43, 1.13) |
| P-value for linear trend | .59 | .23 | .21 | .04 * |
CVD, cardiovascular disease; CHD, coronary heart disease; CI, confidence interval.
P < .05
P < .01
P < .001.
All significant hazard ratios/P-values are shown in bold italics.
Adjusted for all variables in Table 1.
Table S5. The Omega-3 Index vs the n-6:n-3 ratio and vs the Omega-3 Index adjusting for all n-6 fatty acids (n = 2500).
| Hazard ratios (95% CIs) |
|||||||
|---|---|---|---|---|---|---|---|
| Total CVD | Total CHD | Total stroke | CVD mortality | Cancer mortality | Other mortality | Any Mortality | |
| Omega-3 Index § | |||||||
| <4.2% | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 4.2%–4.9% | 1.06 (0.69, 1.63) | 1.05 (0.60, 1.83) | 1.16 (0.60, 2.24) | 0.57 (0.23, 1.40) | 0.66 (0.38, 1.14) | 0.54 (0.31, 0.94) * | 0.74 (0.53, 1.04) |
| 4.9%–5.7% | 1.10 (0.72, 1.68) | 0.81 (0.44, 1.48) | 1.30 (0.68, 2.49) | 0.54 (0.20, 1.47) | 0.73 (0.42, 1.28) | 0.38 (0.20, 0.73) † | 0.67 (0.46, 0.96) * |
| 5.7%–6.8% | 0.74 (0.47, 1.17) | 0.63 (0.34, 1.20) | 0.59 (0.28, 1.25) | 0.56 (0.22, 1.43) | 0.60 (0.35, 1.03) | 0.44 (0.25, 0.79) † | 0.59 (0.41, 0.86) † |
| >6.8% | 0.61 (0.37, 0.99) * | 0.58 (0.29, 1.18) | 0.45 (0.20, 1.03) | 0.39 (0.15, 1.02) | 0.96 (0.56, 1.64) | 0.44 (0.24, 0.79) † | 0.66 (0.45, 0.96) * |
| P-value for trend | .008 † | .03 * | .006 † | .10 | .88 | .009 † | .02 * |
| n6:n3 ratio§ | |||||||
| <3.31 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 3.32–3.99 | 1.11 (0.70, 1.78) | 0.93 (0.33, 1.96) | 1.34 (0.56, 3.19) | 1.17 (0.45, 3.02) | 0.65 (0.37, 1.15) | 1.20 (0.66, 2.20) | 0.98 (0.67, 1.41) |
| 3.99–4.56 | 1.69 (1.10, 2.62) * | 1.26 (0.65, 2.46) | 3.08 (1.45, 6.54) † | 1.41 (0.58, 3.46) | 0.83 (0.48, 1.42) | 0.83 (0.43, 1.59) | 1.00 (0.69, 1.45) |
| 4.56–5.20 | 1.64 (1.06, 2.53) * | 1.62 (0.86, 3.06) | 2.52 (1.15, 5.54) * | 1.12 (0.45, 2.75) | 0.75 (0.42, 1.35) | 1.12 (0.62, 2.00) | 1.11 (0.78, 1.59) |
| >5.20 | 1.61 (0.99, 2.62) | 1.88 (0.95, 3.74) | 2.02 (0.88, 4.65) | 2.46 (0.93, 6.51) | 1.12 (0.65, 1.93) | 2.43 (1.36, 4.35) † | 1.58 (1.08, 2.31) * |
| P-value for trend | .008 † | .014 * | .012 * | .14 | .68 | .023 † | .019* |
| Omega-3 Index + all n-6 fatty acids§ | |||||||
| <4.2% | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 4.2%–4.9% | 1.03 (0.67, 1.57) | 1.04 (0.59, 1.83) | 1.15 (0.59, 2.24) | 0.52 (0.20, 1.38) | 0.66 (0.38, 1.14) | 0.43 (0.23, 0.78) | 0.67 (0.47, 0.95)* |
| 4.9%–5.7% | 1.04 (0.67, 1.62) | 0.85 (0.45, 1.58) | 1.22 (0.60, 2.47) | 0.46 (0.16, 1.35) | 0.72 (0.39, 1.32) | 0.32 (0.17, 0.62) ‡ | 0.63 (0.43, 0.92) * |
| 5.7%–6.8% | 0.65 (0.40, 1.08) | 0.65 (0.32, 1.34) | 0.50 (0.22, 1.15) | 0.52 (0.18, 1.46) | 0.55 (0.28, 1.10) | 0.33 (0.16, 0.68) † | 0.52 (0.34, 0.80) † |
| >6.8% | 0.48 (0.26, 0.91) * | 0.62 (0.26, 1.44) | 0.33 (0.10, 1.04) | 0.37 (0.10, 1.31) | 0.92 (0.41, 2.10) | 0.32 (0.13, 0.78) * | 0.61 (0.36, 1.04) |
| P-value from trend | .014 | .14 | .02 * | .16 | .58 | .006 † | .025 * |
CVD, cardiovascular disease; CHD, coronary heart disease; CI, confidence interval.
P < .05
P < .01
P < .001.
All significant hazard ratios/P-values are shown in bold italics.
Adjusted for all variables in Table 1 except baseline CVD.
Table S6. Risk of fatal events by Omega-3 Index (n = 2899).
| Hazard ratios (95% CIs) |
||||
|---|---|---|---|---|
| Cancer mortality | Other mortality | CVD mortality | Any mortality | |
| Omega-3 Index–unadjusted | ||||
| <4.2% (n = 580) | 1.0 | 1.0 | 1.0 | 1.0 |
| 4.2%–4.9% (n = 579) | 0.88 (0.58, 1.33) | 0.66 (0.41, 1.07) | 0.84 (0.44, 1.61) | 0.83 (0.63, 1.09) |
| 4.9%–5.7% (n = 580) | 0.86 (0.53, 1.38) | 0.62 (0.39, 1.01) | 0.93 (0.50, 1.73) | 0.78 (0.58, 1.04) |
| 5.7%–6.8% (n = 580) | 0.61 (0.37, 1.00) | 0.65 (0.41, 1.03) | 1.06 (0.56, 2.00) | 0.72 (0.54, 0.96) * |
| >6.8% (n = 480) | 0.97 (0.62, 1.51) | 0.58 (0.37, 0.92) * | 0.92 (0.51, 1.68) | 0.79 (0.60, 1.04) |
| P-value for linear trend | .51 | .046 * | .96 | .05 |
| Omega-3 Index–adjusted | ||||
| <4.2% (n = 580) | 1.0 | 1.0 | 1.0 | 1.0 |
| 4.2%–4.9% (n = 579) | 0.70 (0.43, 1.12) | 0.65 (0.41, 1.03) | 0.57 (0.23, 1.42) | 0.79 (0.59, 1.06) |
| 4.9%–5.7% (n = 580) | 0.75 (0.46, 1.21) | 0.38 (0.22, 0.67) ‡ | 0.90 (0.45, 1.76) | 0.70 (0.52, 0.95) * |
| 5.7%–6.8% (n = 580) | 0.55 (0.33, 0.91) * | 0.52 (0.31, 0.87) * | 0.88 (0.42, 1.85) | 0.66 (0.49, 0.90) † |
| >6.8% (n = 480) | 0.87 (0.55, 1.39) | 0.49 (0.29, 0.84) † | 0.74 (0.36, 1.50) | 0.71 (0.52, 0.97) * |
| P-value for linear trend | .45 | .01 * | .87 | .02 * |
CVD, cardiovascular disease; CHD, coronary heart disease; CI, confidence interval.
P < .05
P < .01
P < .001.
All significant hazard ratios/P-values are shown in bold italics.
Adjusted for all variables in Table 1 except baseline CVD.
Table S7. Unadjusted risk of events by Omega-3 Index (n = 2500).
| Hazard ratios (95% CIs) |
|||
|---|---|---|---|
| Total CVD | Total CHD | Total stroke | |
| Omega-3 Index–unadjusted | |||
| <4.2% (n = 508) | 1.0 | 1.0 | 1.0 |
| 4.2%–4.9% (n = 500) | 1.08 (0.72, 1.63) | 1.07 (0.63, 1.82) | 1.19 (0.65, 2.20) |
| 4.9%–5.7% (n = 501) | 1.33 (0.90, 1.96) | 0.88 (0.50, 1.53) | 1.64 (0.92, 2.93) |
| 5.7%–6.8% (n = 502) | 0.88 (0.57, 1.34) | 0.73 (0.41, 1.33) | 0.77 (0.39, 1.53) |
| >6.8% (n = 489) | 0.70 (0.45, 1.10) | 0.59 (0.32, 1.07) | 0.68 (0.32, 1.45) |
| P-value for linear trend | .07 | .03 * | .16 |
CVD, cardiovascular disease; CHD, coronary heart disease; CI, confidence interval.
P < .05
P < .01
P < .001.
All significant hazard ratios/P-values are shown in bold italics.
Table S8. Risk of CVD-related events by Omega-3 Index (n = 2899).
| Hazard ratios (95% CIs) |
|||
|---|---|---|---|
| Total CVD | Total CHD | Total stroke | |
| Omega-3 Index–unadjusted | |||
| <4.2% (n = 580) | 1.0 | 1.0 | 1.0 |
| 4.2%–4.9% (n = 579) | 1.07 (0.76, 1.52) | 0.81 (0.51, 1.28) | 1.53 (0.88, 2.66) |
| 4.9%–5.7% (n = 580) | 1.24 (0.89, 1.73) | 0.74 (0.47, 1.18) | 1.77 (1.03, 3.02) * |
| 5.7%–6.8% (n = 580) | 0.99 (0.70, 1.41) | 0.80 (0.51, 1.27) | 1.20 (0.67, 2.14) |
| >6.8% (n = 580) | 0.88 (0.62, 1.26) | 0.65 (0.41, 1.04) | 1.19 (0.65, 2.20) |
| P-value for linear trend | .40 | .10 | .92 |
| Omega-3 Index–adjusted§ | |||
| <4.2% (n = 580) | 1.0 | 1.0 | 1.0ǁ |
| 4.2%–4.9% (n = 579) | 1.11 (0.74, 1.67) | 1.08 (0.67, 1.74) | 1.36 (0.77, 2.45) |
| 4.9%–5.7% (n = 580) | 1.31 (0.90, 1.93) | 0.85 (0.50, 1.44) | 1.20 (0.63, 2.28) |
| 5.7%–6.8% (n = 580) | 1.00 (0.68, 1.46) | 0.73 (0.46, 1.17) | 0.76 (0.41, 1.40) |
| >6.8% (n = 580) | 0.82 (0.54, 1.25) | 0.56 (0.32, 0.99) * | 0.51 (0.26, 1.02) |
| P-value for linear trend | .25 | .01 * | .003 |
CVD, cardiovascular disease; CHD, coronary heart disease; CI, confidence interval.
P < .05
P < .01
P < .001.
All significant hazard ratios/P-values are shown in bold it.
Adjusted for all variables in Table 1.
Table S9. Unadjusted risk of events and mortality by individual omega-3 fatty acids (n = 2500).
| Hazard ratios (95% CIs) |
|||||||
|---|---|---|---|---|---|---|---|
| Total Events |
Mortality |
||||||
| CVD | CHD | Stroke | CVD | Cancer | Other | Total | |
| α-linolenic acid | |||||||
| <4.2% (n = 483) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 4.2–4.9% (n = 496) | 1.36 (0.91, 2.03) | 1.33 (0.77, 2.32) | 1.69 (0.88, 3.26) | 1.81 (0.72, 4.56) | 0.76 (0.46, 1.28) | 0.52 (0.28, 0.97) * | 0.77 (0.54, 1.09) |
| 4.9–5.7% (n = 506) | 0.92 (0.60, 1.43) | 0.88 (0.48, 1.61) | 0.99 (0.48, 2.04) | 1.59 (0.49, 4.26) | 0.99 (0.60, 1.62) | 0.81 (0.48, 1.36) | 0.95 (0.68, 1.32) |
| 5.7–6.8% (n = 505) | 0.89 (0.57, 1.38) | 0.73 (0.40, 1.36) | 1.24 (0.62, 2.47) | 1.99 (0.75, 5.27) | 0.58 (0.32, 1.04) | 0.78 (0.46, 1.34) | 0.84 (0.60, 1.19) |
| >6.8% (n = 510) | 1.26 (0.84, 1.90) | 1.10 (0.61, 1.97) | 1.68 (0.88, 3.21) | 1.32 (0.52, 3.35) | 0.95 (0.58, 1.57) | 0.97 (0.57, 1.65) | 1.04 (0.75, 1.45) |
| P-value for linear trend | .26 | .51 | .34 | .53 | .52 | .81 | .73 |
| Eicosapentaenoic acid | |||||||
| <0.44% (n = 494) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 0.44–0.55% (n = 495) | 1.32 (0.90, 1.94) | 1.04 (0.62, 1.74) | 1.67 (0.91, 3.07) | 1.09 (0.52, 2.29) | 0.64 (0.37, 1.10) | 1.12 (0.66, 1.91) | 0.89 (0.63, 1.22) |
| 0.55–0.68% (n = 510) | 1.07 (0.69, 1.64) | 0.62 (0.34, 1.12) | 1.71 (0.86, 3.38) | 0.70 (0.27, 1.81) | 0.74 (0.44, 1.27) | 0.91 (0.53, 1.56) | 0.74 (0.52, 1.05) |
| 0.68–0.92% (n = 508) | 0.85 (0.57, 1.28) | 0.53 (0.29, 0.95) * | 1.22 (0.64, 2.33) | 1.02 (0.47, 2.23) | 0.65 (0.40, 1.06) | 0.54 (0.30, 0.97) * | 0.64 (0.46, 0.89) † |
| >0.92% (n = 493) | 0.78 (0.50, 1.21) | 0.57 (0.32, 1.00) * | 1.08 (0.52, 2.23) | 0.72 (0.30, 1.74) | 0.93 (0.56, 1.53) | 0.62 (0.34, 1.12) | 0.77 (0.55, 1.08) |
| P-value for linear trend | .048 * | .005 † | .84 | .45 | .68 | .009 † | .02 |
| Docosapentaenoic acid | |||||||
| <0.44% (n = 493) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 0.44–0.55% (n = 504) | 1.24 (0.84, 1.84) | 0.88 (0.51, 1.51) | 2.10 (1.12, 3.96) * | 0.90 (0.43, 1.88) | 1.06 (0.62, 1.81) | 1.36 (0.81, 2.28) | 1.06 (0.77, 1.46) |
| 0.55–0.68% (n = 520) | 0.95 (0.63, 1.44) | 0.73 (0.41, 1.28) | 1.37 (0.71, 2.63) | 0.71 (0.33, 1.55) | 0.65 (0.37, 1.15) | 0.82 (0.46, 1.46) | 0.72 (0.51, 1.03) |
| 0.68–0.92% (n = 497) | 0.99 (0.66, 1.49) | 0.81 (0.46, 1.44) | 1.48 (0.79, 2.78) | 0.59 (0.24, 1.44) | 0.99 (0.59, 1.66) | 0.73 (0.39, 1.35) | 0.83 (0.59, 1.18) |
| >0.92% (n = 486) | 0.90 (0.58, 1.40) | 0.60 (0.33, 1.09) | 1.21 (0.56, 2.60) | 0.78 (0.32, 1.89) | 1.33 (0.80, 2.20) | 0.62 (0.34, 1.12) | 0.87 (0.62, 1.23) |
| P-value for linear trend | .36 | .10 | .98 | .35 | .43 | .01 * | .15 |
| Docosahexaenoic acid | |||||||
| <3.69% (n = 513) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 3.69–4.36% (n = 498) | 1.07 (0.72, 1.60) | 1.0 (0.59, 1.70) | 1.26 (0.68, 2.32) | 1.09 (0.45, 2.60) | 0.98 (0.60, 1.61) | 0.50 (0.28, 0.88) * | 0.84 (0.61, 1.16) |
| 4.36–5.01% (n = 498) | 1.23 (0.83, 1.82) | 0.81 (0.46, 1.43) | 1.53 (0.86, 2.75) | 1.05 (0.46, 2.38) | 0.73 (0.41, 1.32) | 0.53 (0.31, 0.93) * | 0.69 (0.48, 0.97) * |
| 5.01–5.96% (n = 504) | 0.86 (0.56, 1.31) | 0.71 (0.40, 1.28) | 0.78 (0.39, 1.55) | 1.28 (0.53, 3.11) | 0.88 (0.52, 1.48) | 0.68 (0.41, 1.13) | 0.84 (0.60, 1.16) |
| >5.96% (n = 487) | 0.72 (0.46, 1.12) | 0.59 (0.33, 1.07) | 0.67 (0.31, 1.42) | 1.03 (0.46, 2.34) | 1.16 (0.70, 1.92) | 0.70 (0.36, 0.99) * | 0.86 (0.62, 1.19) |
| P-value for linear trend | .07 | .03 | .13 | .93 | .77 | .25 | .41 |
CVD, cardiovascular disease; CHD, coronary heart disease; CI, confidence interval.
All significant hazard ratios/P-values are shown in bold italics.
P < .05
P < .01
P < .001.
Footnotes
Registration: None.
Authors’ contributions: WSH wrote the original grant that supported the laboratory analysis of the blood samples and wrote the first draft of the manuscript. NLT performed the statistical analysis and drafted the Methods and Results sections. MRE provided expertise in the interpretation of the stroke outcomes. RSV contributed substantially to the preparation of the initial grant proposal, and like all authors, provided substantive input in drafting this manuscript.
Financial disclosure
Dr. Harris is the owner of OmegaQuant Analytics, LLC. The other authors have nothing to disclose.
Contributor Information
William S. Harris, Department of Internal Medicine, Sanford School of Medicine, University of South Dakota; OmegaQuant Analytics, LLC, Sioux Falls, SD, USA.
Nathan L. Tintle, Department of Mathematics & Statistics, Dordt College, Sioux Center, IA, USA.
Mark R. Etherton, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Ramachandran S. Vasan, National Heart Lung and Blood Institute’s, Boston University’s Framingham Heart Study, Framingham, MA, USA; Departments of Cardiology and Preventive Medicine, Department of Medicine, Boston University School of Medicine, Boston, MA, USA; Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA.
References
- 1. Mozaffarian D Lemaitre RN King IB et al. Plasma phospholipid long-chain omega-3 fatty acids and total and cause-specific mortality in older adults: a cohort study Ann Intern Med 2013. 158 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pottala JV Garg S Cohen BE Whooley MA Harris WS Blood eicosapentaenoic and docosahexaenoic acids predict all-cause mortality in patients with stable coronary heart disease: The Heart and Soul Study Circ Cardiovasc Qual Outcomes 2010. 3 406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harris WS von Schacky C The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 2004. 39 212–220 [DOI] [PubMed] [Google Scholar]
- 4. Harris WS The Omega-3 Index: from biomarker to risk marker to risk factor Curr Atheroscler Rep 2009. 11 411–417 [DOI] [PubMed] [Google Scholar]
- 5. Farzaneh-Far R Lin J Epel ES Harris WS Blackburn EH Whooley MA Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease JAMA 2010. 303 250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marchioli R Barzi F Bomba E et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione Circulation 2002. 105 1897–1903 [DOI] [PubMed] [Google Scholar]
- 7. Investigators G-H Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial Lancet 2008. 372 1223–1230 [DOI] [PubMed] [Google Scholar]
- 8. Rauch B Schiele R Schneider S et al. Highly purified omega-3 fatty acids for secondary prevention of sudden cardiac death after myocardial infarction-aims and methods of the OMEGA-study Cardiovasc Drugs Ther 2010. 20 365–375 [DOI] [PubMed] [Google Scholar]
- 9. Roncaglioni MC Tombesi M Avanzini F et al. n-3 fatty acids in patients with multiple cardiovascular risk factors N Engl J Med 2013. 368 1800–1808 [DOI] [PubMed] [Google Scholar]
- 10.Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bosch J Gerstein HC Dagenais GR et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia N Engl J Med 2012. 367 309–318 [DOI] [PubMed] [Google Scholar]
- 12. Eussen SR Geleijnse JM Giltay EJ Rompelberg CJ Klungel OH Kromhout D Effects of n-3 fatty acids on major cardiovascular events in statin users and non-users with a history of myocardial infarction Eur Heart J 2012. 33 1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris WS Are n-3 fatty acids still cardioprotective? Curr Opin Clin Nutr Metab Care 2013. 16 141–149 [DOI] [PubMed] [Google Scholar]
- 14. Deckelbaum RJ Calder PC Different outcomes for omega-3 heart trials: why? Curr Opin Clin Nutr Metab Care 2012. 15 97–98 [DOI] [PubMed] [Google Scholar]
- 15. Kannel WB Feinleib M McNamara PM Garrison RJ Castelli WP An investigation of coronary heart disease in families The Framingham offspring study Am J Epidemiol 1979. 110 281–290 [DOI] [PubMed] [Google Scholar]
- 16. Splansky GL Corey D Yang Q et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination Am J Epidemiol 2007. 165 1328–1335 [DOI] [PubMed] [Google Scholar]
- 17. Harris WS Pottala JV Vasan RS Larson MG Robins SJ Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans J Nutr 2012. 142 1297–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Version 3. www.r-project.org R-Project.
- 19. Harris WS Del Gobbo L Tintle NL The Omega-3 Index and relative risk for coronary heart disease mortality: estimation from 10 cohort studies Atherosclerosis 2017. 262 51–54 [DOI] [PubMed] [Google Scholar]
- 20. Harris WS Luo J Pottala JV et al. Red blood cell polyunsaturated fatty acids and mortality in the Women’s Health Initiative Memory Study J Clin Lipidol 2017. 11 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleber ME Delgado GE Lorkowski S Marz W von Schacky C Omega-3 fatty acids and mortality in patients referred for coronary angiography. The Ludwigshafen Risk and Cardiovascular Health Study Atherosclerosis 2016. 252 175–181 [DOI] [PubMed] [Google Scholar]
- 22. Chowdhury R Warnakula S Kunutsor S et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis Ann Intern Med 2014. 160 398–406 [DOI] [PubMed] [Google Scholar]
- 23. Del Gobbo LC Imamura F Aslibekyan S et al. Omega-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies JAMA Intern Med 2016. 176 1155–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu XF Sandhu SK Harris WS Chan HM Conversion ratios of n-3 fatty acids between plasma and erythrocytes: a systematic review and meta-regression Br J Nutr 2017. 117 1162–1173 [DOI] [PubMed] [Google Scholar]
- 25. Mozaffarian D Wu JH Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events J Am Coll Cardiol 2011. 58 2047–2067 [DOI] [PubMed] [Google Scholar]
- 26. Endo J Arita M Cardioprotective mechanism of omega-3 polyunsaturated fatty acids J Cardiol 2016. 67 22–27 [DOI] [PubMed] [Google Scholar]
- 27. Rizos EC Ntzani EE Bika E Kostapanos MS Elisaf MS Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis JAMA 2012. 308 1024–1033 [DOI] [PubMed] [Google Scholar]
- 28. Casula M Soranna D Catapano AL Corrao G Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: A meta-analysis of randomized, double blind, placebo controlled trials Atheroscler Suppl 2013. 14 243–251 [DOI] [PubMed] [Google Scholar]
- 29. Alexander DD Miller PE Van Elswyk ME Kuratko CN Bylsma LC A meta-analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long-chain omega-3 fatty acids and coronary heart disease risk Mayo Clin Proc 2017. 92 15–29 [DOI] [PubMed] [Google Scholar]
- 30. Poole CD Halcox JP Jenkins-Jones S et al. Omega-3 Fatty acids and mortality outcome in patients with and without type 2 diabetes after myocardial infarction: a retrospective, matched-cohort study Clin Therap 2013. 35 40–51 [DOI] [PubMed] [Google Scholar]
- 31. Greene SJ Temporelli PL Campia U et al. Effects of polyunsaturated fatty acid treatment on postdischarge outcomes after acute myocardial infarction Am J Cardiol 2016. 117 340–346 [DOI] [PubMed] [Google Scholar]
- 32. Eide IA Jenssen T Hartmann A et al. The association between marine n-3 polyunsaturated fatty acid levels and survival after renal transplantation Clin J Am Soc Nephrol 2015. 10 1246–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weir HK, Anderson RN, Coleman King SM. et al. Heart Disease and Cancer Deaths - Trends and Projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157. doi: 10.5888/pcd13.160211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marklund M Leander K Vikstrom M et al. Polyunsaturated fat intake estimated by circulating biomarkers and risk of cardiovascular disease and all-cause mortality in a population-based cohort of 60-year-old men and women Circulation 2015. 132 586–594 [DOI] [PubMed] [Google Scholar]
- 35. Warensjo E Sundstrom J Vessby B Cederholm T Riserus U Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study Am J Clin Nutr 2008. 88 203–209 [DOI] [PubMed] [Google Scholar]
- 36.Sala-Vila A, Guasch-Ferre M, Hu FB. et al. Dietary alpha-linolenic acid, marine omega-3 fatty acids, and mortality in a population with high fish consumption: Findings from the PREvencion con DIeta MEDiterranea (PREDIMED) Study. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mozaffarian D Lemaitre RN King IB et al. Circulating long-chain omega-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study Ann Intern Med 2011. 155 160–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaur G Guo XF Sinclair AJ Short update on docosapentaenoic acid: a bioactive long-chain n-3 fatty acid Curr Opin Clin Nutr Metab Care 2016. 19 88–91 [DOI] [PubMed] [Google Scholar]
- 39. Park Y Park S Yi H et al. Low level of n-3 polyunsaturated fatty acids in erythrocytes is a risk factor for both acute ischemic and hemorrhagic stroke in Koreans Nutr Res 2009. 29 825–830 [DOI] [PubMed] [Google Scholar]
- 40. Ikeya Y Fukuyama N Mori H Low plasma eicosapentaenoic acid concentration as a possible risk factor for intracerebral hemorrhage Nutr Res 2015. 35 214–220 [DOI] [PubMed] [Google Scholar]
- 41. Song TJ Chang Y Shin MJ Heo JH Kim YJ Low levels of plasma omega 3-polyunsaturated fatty acids are associated with cerebral small vessel diseases in acute ischemic stroke patients Nutr Res 2015. 35 368–374 [DOI] [PubMed] [Google Scholar]
- 42. Suda S Katsumata T Okubo S et al. Low serum n-3 polyunsaturated fatty acid/n-6 polyunsaturated fatty acid ratio predicts neurological deterioration in Japanese patients with acute ischemic stroke Cerebrovasc Dis 2013. 36 388–393 [DOI] [PubMed] [Google Scholar]
- 43.Chowdhury R, Stevens S, Gorman D. et al. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. BMJ. 2012;345:e6698. doi: 10.1136/bmj.e6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saber H Yakoob MY Shi P et al. Omega-3 fatty acids and incident ischemic stroke and its atherothrombotic and cardioembolic subtypes in 3 US cohorts Stroke 2017. 48 2678–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Investigators G-P Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E in 11,324 patients with myocardial infarction: Results of the GISSI-Prevenzione trial Lancet 1999. 354 447–455 [PubMed] [Google Scholar]
- 46. Tanaka K Ishikawa Y Yokoyama M et al. Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial Stroke 2008. 39 2052–2058 [DOI] [PubMed] [Google Scholar]
- 47.de Goede J, Verschuren WM, Boer JM, Verberne LD, Kromhout D, Geleijnse JM. N-6 and N-3 fatty acid cholesteryl esters in relation to fatal CHD in a Dutch adult population: a nested case-control study and meta-analysis. PLoS One. 2013;8:e59408. doi: 10.1371/journal.pone.0059408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wennberg M Bergdahl IA Stegmayr B et al. Fish intake, mercury, long-chain n-3 polyunsaturated fatty acids and risk of stroke in northern Sweden Br J Nutr 2007. 98 1038–1045 [DOI] [PubMed] [Google Scholar]
- 49. Felix-Redondo FJ Grau M Fernandez-Berges D Cholesterol and cardiovascular disease in the elderly. Facts and gaps Aging Dis 2013. 4 154–169 [PMC free article] [PubMed] [Google Scholar]
- 50. Balk EM Lichtenstein AH Chung M Kupelnick B Chew P Lau J Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review Atherosclerosis 2006. 189 19–30 [DOI] [PubMed] [Google Scholar]
- 51. Geleijnse JM Giltay EJ Grobbee DE Donders AR Kok FJ Blood pressure response to fish oil supplementation: meta-regression analysis of randomized trials J Hypertens 2002. 20 1493–1499 [DOI] [PubMed] [Google Scholar]
- 52. Violi F Pignatelli P Basili S Nutrition, supplements, and vitamins in platelet function and bleeding Circulation 2010. 121 1033–1044 [DOI] [PubMed] [Google Scholar]
- 53. Mozaffarian D Geelen A Brouwer IA Geleijnse JM Zock PL Katan MB Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials Circulation 2005. 112 1945–1952 [DOI] [PubMed] [Google Scholar]
- 54. Rauch B Senges J The effects of supplementation with omega-3 polyunsaturated Fatty acids on cardiac rhythm: anti-arrhythmic, pro-arrhythmic, both or neither? It depends Front Physiol 2012. 3 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fontes JD Rahman F Lacey S et al. Red blood cell fatty acids and biomarkers of inflammation: A cross-sectional study in a community-based cohort Atherosclerosis 2015. 240 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li K, Huang T, Zheng J, Wu K, Li D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor alpha: a meta-analysis. PLoS One. 2014;9:e88103. doi: 10.1371/journal.pone.0088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cawood AL Ding R Napper FL et al. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability Atherosclerosis 2010. 212 252–259 [DOI] [PubMed] [Google Scholar]
- 58. Thies F Garry JM Yaqoob P et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial Lancet 2003. 361 477–485 [DOI] [PubMed] [Google Scholar]
- 59. Pase MP Grima NA Sarris J Do long-chain n-3 fatty acids reduce arterial stiffness? A meta-analysis of randomised controlled trials Br J Nutr 2011. 106 974–980 [DOI] [PubMed] [Google Scholar]
- 60.Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of Erythrocyte Omega-3 Fatty Acid Content in Response to Fish Oil Supplementation: A Dose-Response Randomized Controlled Trial. J Am Heart Assoc. 2013;2:e000513. doi: 10.1161/JAHA.113.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sprague M, Dick JR, Tocher DR. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006-2015. Sci Rep. 2016;6:21892. doi: 10.1038/srep21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Burr ML Fehily AM Gilbert JF et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet 1989. 2 757–761 [DOI] [PubMed] [Google Scholar]
- 63. Rauch B Schiele R Schneider S et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction Circulation 2010. 122 2152–2159 [DOI] [PubMed] [Google Scholar]
- 64. von Schacky C Omega-3 fatty acids in cardiovascular disease–an uphill battle Prostaglandins Leukot Essent Fatty Acids 2015. 92 41–47 [DOI] [PubMed] [Google Scholar]
- 65.Meyer BJ, Groot RHM. Effects of Omega-3 long chain polyunsaturated fatty acid supplementation on cardiovascular mortality: The importance of the dose of DHA. Nutrients. 2017;9:1305. doi: 10.3390/nu9121305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Harris WS Thomas RM Biological variability of blood omega-3 biomarkers Clin Biochem 2010. 43 338–340 [DOI] [PubMed] [Google Scholar]
- 67. Harris WS Pottala JV Lacey SM Vasan RS Larson MG Robins SJ Clinical correlates and heritability of erythrocyte eicosapentaenoic and docosahexaenoic acid content in the Framingham Heart Study Atherosclerosis 2012. 225 425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]