Abstract
Allogenic stem cell transplantation (allo-SCT) has been considered the treatment of choice for high-risk patients with chronic lymphocytic leukemia (CLL) and the only approach offered with curative intent in this disease. The availability novel agents, including the B-cell receptor inhibitors ibrutinib, acalabrutinib, and idelalisib, as well as venetoclax, which targets the BCL2 pathway, and the success of these agents in treating high-risk disease patients have made it more difficult to assess who should be considered for allo-SCT and when in the treatment course. In this review, I will discuss the different treatment options available for the treatment of high-risk CLL and how allo-SCT fits into the treatment algorithm in the era of novel agents.
Introduction
In any disease, the choice of an allogeneic SCT (allo-SCT) must weigh both the risks of the morbidity of the transplant and its outcome compared with what can be achieved using other treatment approaches. Based upon these criteria, in 2007, a consensus paper identified groups of patients with chronic lymphocytic leukemia (CLL) who were considered at sufficiently high risk to undergo allo-SCT, namely, those patients whose CLL cells harbored del(17p) (deletion 17 p) or TP53 mutations or those who were refractory to (or relapsing within 2 years of receiving) purine analog combination treatment.1 These recommendations were widely accepted, and allo-SCT was considered the treatment of choice for patients with such high-risk disease and the only treatment that offers curative intent in CLL.1 However, the treatment algorithm for CLL has changed markedly over the past decade,2 firstly with chemoimmunotherapy replacing chemotherapy3,4 and more recently with the licensing for the treatment of CLL of the novel B-cell receptor inhibitors (BCRi’s) ibrutinib5,6 and idelalisib7 as well as the BCL2 inhibitor venetoclax.8-11 The availability of these novel agents and their high efficacy in those patients who previously were considered to be at high risk have changed the treatment landscape and altered the criteria for transplant in CLL from those defined in 2007.1,12 It is in this setting, where there is now widespread availability of novel agents, that we now have to make treatment decisions regarding who is a suitable candidate for allo-SCT and when in the course of disease is the optimal time to consider transplantation. CLL is not the only disease in which new drug development has had an impact on SCT. The chronic leukemias have already seen the biggest impact of novel agents on the use of transplantation, and imatinib has already largely replaced allo-SCT in the treatment of chronic myeloid leukemia.13 Here, I outline my approach to the clinical management of high-risk CLL patients on the basis of currently available treatment options.
Treatment of CLL and the role of transplant
CLL is an extremely heterogeneous disease, and patients do not merit treatment until their disease has progressed and become symptomatic.14 A number of prognostic factors have been identified that can help predict time from initial diagnosis to time of treatment and help identify patients more likely to require early treatment (Figure 1). Some of these factors can also be used to start to determine which younger CLL patients merit consideration for allo-SCT at some stage in their clinical course. None of these prognostic factors represent in themselves an indication to treat patients with CLL, and many clinicians perform analyses of these factors only at the time when patients have fulfilled the criteria for indication for treatment.14 Under these circumstances, these factors are being examined for their predictive value to determine response to treatment rather than as a prognostic factor. For younger, fit patients, the chemoimmunotherapy treatment of choice remains fludarabine, cyclophosphamide, and rituximab (FCR), based on the results of the German CLL Study Group CLL8 study, which demonstrated a survival advantage with FCR chemoimmunotherapy compared with fludarabine and cyclophosphamide chemotherapy alone.3 Many patients with CLL are too frail to be considered candidates for FCR, and other approved treatment approaches for these patients include bendamustine and rituximab,15 obinutuzumab and chlorambucil,4 or ibrutinib.6 A number of ongoing clinical trials are examining the role of chemoimmunotherapy vs novel agents alone or in combination. The results of these studies will help define the optimal front-line treatment of different patient groups in the future. As front-line treatments have improved, the number of patients with front-line refractory disease (previously a consideration for suitability for allo-SCT) has decreased.
Figure 1.
Selected prognostic markers in CLL. A number of factors have been shown to have prognostic significance in CLL, and a number of these are shown here.
Identification of high-risk patients with CLL
Conventional treatment approaches are not considered to be curative in CLL, although 2 recent studies have demonstrated that patients with mutated immunoglobulin heavy chain variable (IGHV) gene status can have very durable remissions following FCR chemotherapy and that a subset of these patients may indeed be cured of their disease.16,17 However, the majority of patients will at some stage relapse and subsequently require salvage treatment. We can assess risk on the basis of characteristics of the disease or on the duration of response or tolerability of treatment.
Short duration of response and the role of SCT in the era of chemoimmunotherapy
The International Workshop on CLL formulated practice recommendations and defined refractory disease as treatment failure (stable disease, nonresponse, progressive disease, or death from any cause) or disease progression within 6 months from the last antileukemic therapy.14 It is clear that the duration of first or subsequent response to therapy is an important prognostic indicator and patients who relapse early after last therapy are considered high risk.
Patient 1 is a 56-year-old man who presented with Rai stage IV disease and required immediate treatment. He had unmutated IGHV status and trisomy 12 with no evidence of del(17p) or TP53 mutation. He was commenced on treatment with FCR and completed 6 cycles. He had persistent thrombocytopenia and low-level but detectable minimal residual disease (MRD) at his outcome assessment. His disease was showing evidence of progression at his next clinic visit 5 months following completion of FCR. I met him with his family in clinic and discussed his poor prognosis. We performed tissue typing on him and his 3 brothers and identified an HLA-matched sibling. His disease progressed, and I repeated his cytogenetics and discovered that his CLL had acquired del(17p). At that time, he was treated with alemtuzumab and proceeded to a reduced intensity allo-SCT. He engrafted well, achieved full donor chimerism, and achieved complete remission (CR), with no evidence of GHVD.
In the era of chemoimmunotherapy, a 6-month duration of response after FCR is considered very poor, and 3 risk categories have been defined based upon genetic criteria of the disease, response, and duration of response to previous therapy (Table 1).18 The group of patients who require treatment who have the best outcome (“low risk”) are those with mutated IGHV, low β2-microglobulin, no del(11q), and no del(17p)/TP53 mutation. A second scenario of “high-risk” CLL patients are those that relapse relatively early with standard treatment, even if not in the “highest-risk” group. Analysis of patients within the German CLL Study Group CLL8 study identified factors that had independent prognostic impact on multivariable analyses for FCR-treated patients. For progression-free survival (PFS), these factors included elevated thymidine kinase (≥10 U/L), unmutated IGHV status, del(11q), del(17p), TP53 mutation, and SF3B1 mutation, whereas age ≥65 years, Eastern Cooperative Oncology Group performance status ≥1, β2-microglobulin ≥3.5 mg/L, thymidine kinase ≥10 U/L, unmutated IGHV status, del(17p), and TP53 mutation were predictive of overall survival (OS).19 The group of patients that had the worst outcome were those with del(17p), and based in this and similar analyses, patients with TP53 abnormalities (either del(17p) or TP53 mutations) are no longer considered candidates for chemoimmunotherapy and should be offered ibrutinib as the optimal front-line treatment. Lastly, the highest-risk category are those with TP53 loss/mutation, “purine analogue refractory,” very short (<24 months) response to prior chemoimmunotherapy, and no CR on prior exposure, and in these patients, treatment with FCR is unlikely to yield acceptable response rates or duration or survival. Patient 1 clearly fits into the highest-risk category. These patients are now prime candidates for drugs with proven activity in TP53 deleted/mutant cells and in refractory disease, which now include ibrutinib,20 idelalisib and rituximab,7 and venetoclax,8,9 none of which were available at the time that patient 1 was offered his allo-SCT.
Table 1.
Risk models and their potential impact on treatment of CLL
| Risk group | Definition | Treatment |
|---|---|---|
| Low | Mutated IGHV, no 11q or 17p deletion, no prior treatment | Conventional treatment based on fitness as per local guidelines |
| High | Unmutated IGHV, 11q deletion, high β2-microglobulin, no highest-risk features | Suitable for FCR alone or in clinical trial treatment with investigational agent in induction or maintenance.Ibrutinib treatment in first line for FCR-ineligible patients or those with unmutated IGHV. Consider allo-SCT later in disease after consideration of risk factors suggesting poor response to novel agents. |
| Highest | TP53 deletion/mutation and indication for treatment, chemoimmunotherapy refractory disease, early relapse (≤24 mo) after FCR (or FCR-like) treatment | Induction with novel agent; consider allogeneic SCT in suitable patients |
There are a number of prognostic models that have been developed, and these continue to be refined as new data on predictive factors for each of the approved agents in CLL are verified.21 The hope is that we can use these predictive models in a stratified medicine approach to find the optimal treatment for patients at each decision point, but it is still premature to use these models in clinical decisions outside the setting of clinical trials.
Patient 2 is a 46-year-old female with CLL with bulky lymphadenopathy. Her disease progressed to require treatment within 18 months of first diagnosis. Her CLL demonstrated del(11q). She was treated with FCR chemotherapy. She tolerated the treatment well and achieved CR but 11 months later had progressive disease. Her disease still had del(11q), and no del(17p) or TP53 mutation was identified. She had no HLA-matched siblings, but a suitable fully HLA-matched unrelated donor was identified. She has 3 young children and was not keen to undergo allo-SCT. She was treated on a clinical trial with venetoclax in combination with obinutuzumab. She achieved CR with eradication of MRD. She continues to be followed and does not want to undergo allo-SCT as she has young children and is very concerned about the treatment-related mortality associated with allo-SCT, and she continues to tolerate her treatment with venetoclax extremely well. The plan is to continue her venetoclax until disease progression and at that stage treat her with ibrutinib and plan for allo-SCT.
Patients 1 and 2 illustrate that in a few short years, we have moved from an era where allo-SCT was the only treatment option for very high-risk CLL patients to one where we have a number of highly effective treatment options.
Impact of novel agents
Ibrutinib, idelalisib, and venetoclax all demonstrate impressive activity in patients previously deemed to be high risk, including those with abnormalities in the TP53 pathway or with relapsed/refractory disease. Based upon the results of randomized clinical trials, ibrutinib received full US Food and Drug Administration approval for treatment of relapsed/refractory disease5 and all del(17p) CLL patients in July 2014 and for treatment of previously untreated CLL6 in March 2016. Ibrutinib is currently approved for use as single agent. The drug is very well tolerated in most patients, although some side effects have to be managed to optimize outcome.22 Although the drug is active in all patient groups, some studies have suggested that relapsed/refractory patients with TP53 mutation/deletion have a poorer outcome. However, in the largest series reported, the 24-month PFS for these patients was 63%, and 24-month OS was 75%,20 which are very different outcomes from the era of chemotherapy, when this setting was considered “very high risk,” as for patient 1. With 5-year follow-up, ibrutinib continues to yield a high overall response rate of 89%, with complete response rates increasing over time to 29% in treatment-naive (TN) patients and 10% in relapsed/refractory patients.23 The median PFS was not reached in TN patients and was 51 months in relapsed/refractory patients in the whole group and in those with del(11q) but 26 months in those with del(17p) and 43 months in those with unmutated IGHV. The 5-year PFS was 92% in TN and 44% in R/R patients. Del(17p) is often associated with a complex karyotype, and a study from the MD Anderson Cancer Center suggested that only complex karyotype was significantly associated with poorer event-free survival and OS on multivariate analysis, whereas the presence of del(17p) alone was not an independent factor for poorer outcome.24 In terms of assessing response to ibrutinib, baseline factors associated with failure to achieve CR on univariate analysis included bulky disease, higher clinical stage, higher number of previous therapies, and elevated β2-microglobulin concentration.25 The final multivariate model demonstrated that patients with no previous therapy and patients with largest lymph node <5 cm had an increased likelihood of CR.25
Idelalisib and rituximab were approved for the treatment of relapsed/refractory CLL on the basis of results of a randomized clinical trial and showed equivalent activity in patients with and without abnormalities of the TP53 pathway,7 although longer-term follow-up on this study is still awaited. The BCL2 inhibitor venetoclax demonstrated an overall response rate of 79% in heavily pretreated CLL patients, most of whom had high-risk disease.9 In a confirmatory study in 107 patients with relapsed or refractory CLL who had del(17p), the response rate as assessed by independent review was similarly high at 79.4%.8 Venetoclax received accelerated approval for relapsed/refractory del(17p) CLL in April 2016. The results of a phase 3 trial of venetoclax, delivered for a fixed duration of 2 years, in combination with rituximab compared with salvage treatment with bendamustine plus rituximab demonstrated clear superiority of the venetoclax-rituximab combination, and the venetoclax-rituximab combination was effective in patients with del(17p).10 We have therefore moved from an era where there were no effective treatments available for very high-risk CLL patients to one where we have 3 available agents that can be used in sequence26 and, in the setting of clinical trials, can be tested in combination.27
Role of allo-SCT in CLL
There have been no randomized clinical trials that compare the outcome of allo-SCT with conventional chemotherapy, immunochemotherapy, or novel non–chemotherapy-containing regimens. Similarly, although the vast majority of transplants are offered using reduced-intensity conditioning (RIC), no randomized clinical trial has compared myeloablative with RIC conditioning. Three nonrandomized trials have examined the potential impact of allo-SCT vs nontransplant (Table 2).28-30 All approaches suffer from the fact that the analyses were retrospective, do not always compare the same patient populations, and were all conducted prior to the approval of novel agents in CLL.
Table 2.
Studies comparing transplant vs no transplant in CLL
| Study | Transplant | No transplant | Model | Comments |
|---|---|---|---|---|
| Kharfan-Dabaja et al28 | RIC allogenic | Conventional chemotherapy | ||
| NRM | 22% | 8% | Markov decision model | Are patient populations sufficiently similar? |
| ORR | 81% | 57% | ||
| Relapse death | 70% | 86% | ||
| Life expectancy | 35 mo | 25 mo | ||
| Herth et al29 | Donor | No donor | ||
| 2-y survival | 78% | 55% | Donor vs no donor comparison | Small numbers in no-donor group |
| Median survival | Not reached | 30.6 mo | ||
| Poon et al30 | Transplant | No transplant | ||
| 2-y survival | 64% | 25% | Consulted for transplant | Del(17p) group only; 2 groups not comparable |
None of the studies listed are prospective randomized trials.
It is clear that the approval of novel agents has had an impact on the role of allo-SCT in CLL, and since the approval of ibrutinib and idelalisib, and now with the approval of venetoclax, the number of transplants being performed continues to decrease markedly in both Europe and the United States (Figure 2). This trend is likely to continue as other new agents are approved and as the existing agents are used earlier in the disease course and in combination.12,31
Figure 2.
Changing patterns over time of SCT in CLL. The number of autologous and allo-SCT reported to the EBMT and the number of allo-SCTs reported in the United States by year shows marked differences over time.
The first myeloablative treatment based transplantation strategies in CLL were performed >20 years ago. Although these studies showed that this approach demonstrated potent disease control, they were unsuitable for the majority of patients because of their substantial morbidity and mortality.32 It was then recognized that toxicities could be reduced by the use of nonmyeloablative RIC strategies without compromising engraftment and antitumor activity.33-35 This made allo-SCT accessible to a larger cohort of CLL patients, including more elderly and frail patients, and this resulted in a marked increase in the numbers of CLL allo-SCT that were performed in the first decade of this century (Figure 2). Several large prospective studies have been conducted, some of which have now reached a median follow-up of up to 6 years (Table 3). These long-term results indicate that RIC allo-SCT provides long-term disease control in ∼40% of patients and that allo-SCT is able to overcome the negative prognostic effect of TP53 abnormalities and fludarabine refractoriness,36-44 as well as of other poor prognostic features such as SF3B1 and NOTCH1 gene mutations.45 Long-term survival after allo-SCT in the largest series of patients who have undergone SCT for CLL has been reported from the European Society for Blood and Marrow Transplantation (EBMT).46 This study reports data from 2589 patients who underwent allo-SCT between 2000 and 2010 and used landmark analyses as well as relative survival analysis compared with an age- and sex-matched general population to assess outcome. Although allo-SCT certainly represents an advance over previous treatment and offers very long-term disease control for some patients, it is of interest to see that with longer-term follow-up, OS (62% at 2 years and 35% at 10 years) and event-free survival] (49% at 2 years and 28% at 10 years) continue to decrease over time (Table 4). The impact of baseline characteristics predicting for good outcome after allo-SCT in CLL has been reported in a series from EBMT.47 The presence or absence of del(17p) had no impact on outcome. Previous autologous SCT, no remission at the time of allo-SCT, and the use of a mismatched donor negatively impacted long-term PFS. The 10-year follow-up data of the German CLL Study Groups CLL3X study (NCT00281983) of allogeneic SCT in CLL have been reported and demonstrated a 10-year nonrelapse mortality of 20%, PFS of 34%, and OS of 51%.48 There was no prognostic impact of the presence of abnormalities of the TP53 pathway, but risk factors for relapse included active disease at time of allo-SCT and T-cell depletion using alemtuzumab, whereas absence of MRD at 12 months was highly prognostic for reduced relapse risk.
Table 3.
Summary of transplant characteristics and survival in selected prospective studies of RIC HSCT in CLL
| Fred Hutchinson Cancer Center38 | German CLL Study Group41,45 | MD Anderson Cancer Center40 | Dana Farber Cancer Institute39 | |
|---|---|---|---|---|
| Number of patients | 82 | 90 | 86 | 76 |
| Conditioning regimen | Flu/low-dose TBI | Flu/Cy ± ATG | Flu/Cy ± R | Flu/Bu |
| Donors, % (sibling/MUD) | 63/37 | 41/59 | 50/50 | 37/63 |
| Median follow-up, mo | 60 | 72 | 37 | 61 |
| Median PFS, % | 39 (5 y) | 38 (6 y) | 36 (6 y) | 43 (6 y) |
| Median OS, % | 50 (5 y) | 58 (6 y) | 51 (6 y) | 63 (6 y) |
| Early mortality, % (<100d) | <10 | <3 | <3 | <3 |
| NRM, % | 23 | 23 | 17 | 16 |
| Acute grade 3-4 GVHD, % | 20 | 14 | 7 | 17 |
| Severe chronic GVHD, % | 53 | 55 | 56 | 48 |
ATG, antithymocyte globulin; Bu, busulfan; Cy, cyclophosphamide; Flu, fludarabine; MUD, matched unrelated donor; R, rituximab; TBI, total body irradiation.
Table 4.
Outcome after allo-SCT for CLL in 2589 patients reported by EBMT
| Time after allo-SCT | ||||
|---|---|---|---|---|
| 1 y | 2 y | 5 y | 10 y | |
| OS, % (95% CI) | 71 (69-73) | 62 (60-64) | 45 (43-48) | 35 (32-38) |
| Nonrelapse mortality, % (95% CI) | 24 (23-26) | 30 (28-32) | 36 (34-38) | 40 (37-42) |
| Event-free survival, % (95% CI) | 62 (60-64) | 49 (47-52) | 35 (33-37) | 28 (25-31) |
| Incidence of relapse, % (95% CI) | 14 (13-25) | 21 (19-22) | 29 (27-30) | 32 (30-25) |
CI, confidence interval.
The decrease in allo-SCT has occurred despite the increased availability of suitable sources of stem cells though use of cord blood cells as well as haploidentical donors. The outcome of 68 patients (median age, 57 years) with poor-risk CLL/small lymphocytic lymphoma who underwent RIC unrelated cord blood transplantation from 2004 to 2012 have been reported.49 These patients had a number of poor prognostic features; 17 patients had del(17p)/TP53 mutation, 19 patients had fludarabine-refractory disease, 11 relapsed after autologous SCT, 8 had prolymphocytic leukemia, 4 had Richter syndrome, and 8 underwent transplantation with progressive or refractory disease. Most of the cord blood grafts were HLA mismatched, and 76% received a double unrelated cord blood transplantation. The median number of total nucleated cells collected was 4.7 × 107/kg. Day 100 graft-versus-host disease (GVHD) (grade II to IV) was 43%, and 3-year chronic GVHD was 32%. At 3 years, the cumulative incidence (CI) of relapse was 16%, nonrelapse mortality was 39%, PFS was 45%, and OS was 54%. The outcomes of 117 CLL patients who had received an allogeneic HCT with a haploidentical donor whose data were available in the EBMT registry have been reported.50 The CIs of nonrelapse mortality in the whole group at 2 and 5 years were 40% and 44%, and CIs of relapse at 2 and 5 years were 22% and 26%, respectively. PFS was 38% at 2 years and 31% at 5 years, whereas OS was 48% at 2 years and 38% at 5 years. The toxicity of haploidentical transplants has been greatly decreased by the use of posttransplant cyclophosphamide, and the results of haploidentical HCT in CLL in these patients appeared almost identical to those with HLA-matched donors.50
My approach to allo-SCT in CLL
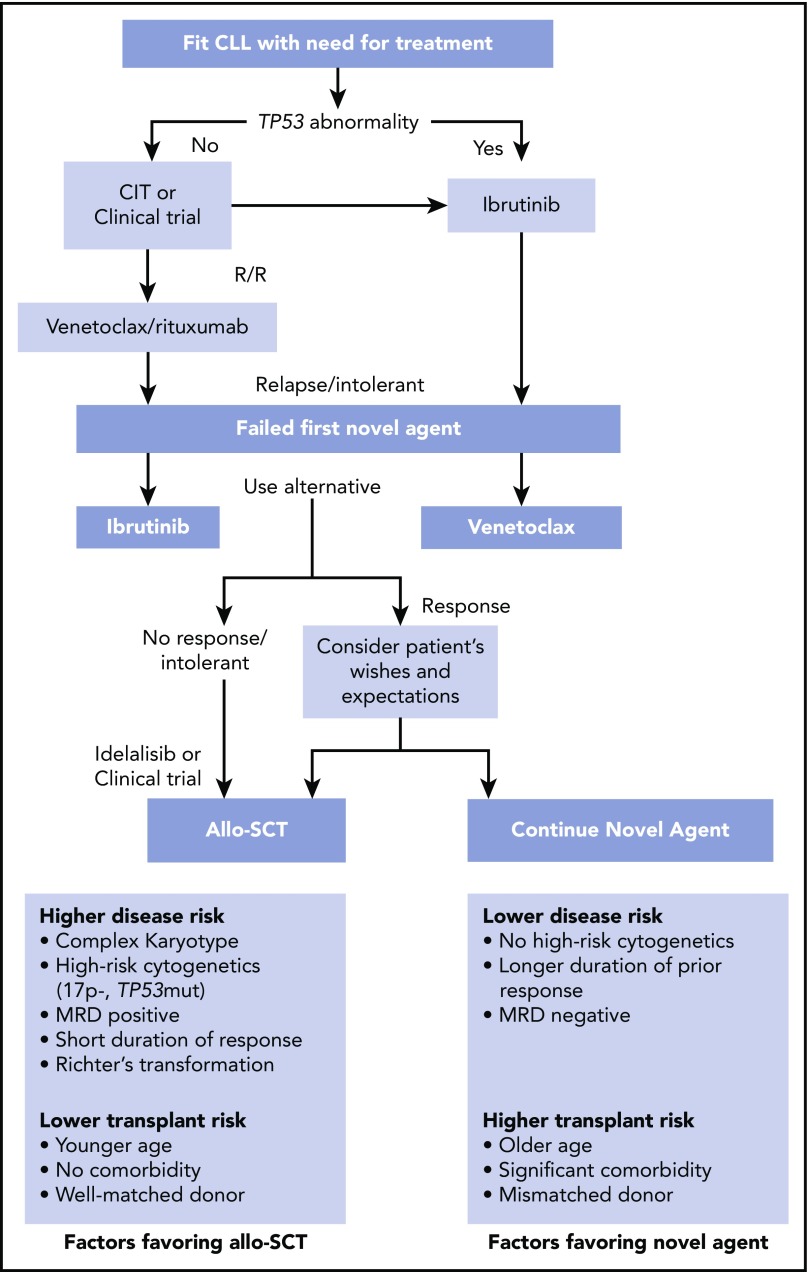
My treatment approach is outlined in Figure 3. The choice of first-line therapy is based upon age and comorbidity and the presence or absence of del(17p)/TP53 mutations, and some investigators consider the role of unmutated IGHV status when selecting between chemoimmunotherapy and front-line ibrutinib. Based upon the results that can be achieved using ibrutinib first line in patients and the availability of effective salvage therapies with venetoclax or idelalisib plus rituximab, I no longer offer allo-SCT as a treatment approach for CLL patients with del(17p) in first remission. In the relapsed setting, I explain to my patients that the role of allo-SCT has to be weighed against their previous treatments, their comorbidities, duration of response to previous treatment (and what that treatment was), and the current status of the del(17p)/TP53 mutation and potentially other mutations such as SF3B1 and NOTCH1, if this information is available. The outcomes with allo-SCT then have to be weighed against the side effect profile, response, and duration of response that can be obtained using novel agents compared with that achieved using allo-SCT. A final consideration is the cost of the continuous novel-agent administration compared with the cost of allo-SCT. Now that venetoclax plus rituximab is offered with a fixed duration of response of 2 years,10 this may impact the financial burden, but we do not have sufficient follow-up data for this treatment approach to be able to determine the relative risk of relapse at later time points.
Figure 3.
My approach to allo-SCT in CLL. Treatment algorithm for CLL patients who might be considered candidates for allo-SCT. Patients who require treatment who have no TP53 abnormality are candidates for chemoimmunotherapy (CIT) or a clinical trial, and those with TP53 abnormalities are candidates for ibrutinib front line. Patients who are relapsed or refractory (R/R) can be treated with ibrutinib or another BTKi or venetoclax plus rituximab. Patients who have relapsed after or are intolerant to ibrutinib are candidates for venetoclax, and those who have failed venetoclax plus rituximab are candidates for ibrutinib. Patients responding to second novel agent can either proceed to allo-SCT or continue the novel agent.
I recommend that patients who have relapsed, have poor prognostic features, and have a performance status where allo-SCT can be considered should be seen by an oncologist who has a specialist interest in transplant to consider this approach, assess suitability, and tissue type the patient and determine the best potential available donor. Because of the documented increased familial risk, sibling or family donors identified as suitable donors should undergo immunophenotypic analysis to rule out CLL or monoclonal B-cell lymphocytosis. Identifying suitable donors early means that if a patient has a poor response or is intolerant of or has a short duration of response to a novel agent, then an allo-SCT can proceed quickly, hopefully while the disease status remains under good control.
Excellent responses can be expected with novel agents in the setting of first relapse, and rarely do my patients opt for allo-SCT rather than continue with BCRi or venetoclax plus anti-CD20 monoclonal antibody. The duration of such response can be long, and there are available treatments and clinical trial options for these patients should they relapse or be intolerant of BCRi or venetoclax. A much more complex situation arises when patients are on second- or third-line novel agents and have an excellent response, including having eradication of MRD, as it is difficult to know when it is the right time to proceed with transplant, as there is a window of opportunity for optimal outcome with allo-SCT when it is offered with low disease burden.
Patient 3 is a 53-year-old man who presented with CLL with low blood count but significant lymphadenopathy. He was followed, and his lymphadenopathy progressed. He developed night sweats, and a positron emission scan was performed. This showed widespread adenopathy with low uptake, and although no areas of high standardized uptake values suggestive of Richter transformation (RT) were seen, lymph node biopsy was performed and showed changes consistent with CLL. Fluorescence in situ hybridization demonstrated del(17p). He was commenced on treatment with ibrutinib and had rapid resolution of his lymphadenopathy. He initially tolerated ibrutinib well, but 9 months later, he had evidence of progressive disease. His disease still demonstrated del(17p), and he was treated with venetoclax. He has a male sibling donor who is HLA matched, and the plan for him now is to undergo allo-SCT while his disease remains under good control.
With regards to the transplant procedure itself, I offer myeloablative allo-SCT very rarely and only in young patients with progressive/refractory disease. Most CLL patients are too frail to be considered for myeloablative allo-SCT, which is associated with increased morbidity and mortality. Like the vast majority of transplanters in this disease setting, I favor RIC regimens.51 The rational for allo-SCT in CLL is that there is clear evidence for a graft-versus-leukemia (GVL) effect in CLL.32 I therefore do not offer T-cell depletion as part of the conditioning regimen. There have been no direct comparisons of T-cell–depleted vs nondepleted transplants, but this remains an area of clinical investigation. Strong evidence for a GVL effect is the response seen with donor lymphocyte infusions (DLIs) as the only treatment offered after relapse following allo-SCT.32 In terms of posttransplant management, although there have also been no published studies that address the role, dose, or scheduling of DLI for mixed-chimerism, relapsed disease, either clinically or by MRD in CLL, I offer low-dose DLI when there is evidence of persistent mixed chimerism after stopping immunosuppressive therapy in patients with no evidence of GVHD. Similarly, I monitor my CLL transplant patients for detection of MRD by flow cytometry of peripheral blood and offer DLI for rising MRD disease or at time of detection of clinical relapse. There are emerging data on the use of ibrutinib in the setting of relapsed disease after allo-SCT, and this also appears useful in inducing a GVL effect while ameliorating GVHD.52,53 Venetoclax can also be offered at the time of relapsed disease.
Going back to patient 1, 3 years post allo-SCT, he demonstrated evidence of early disease relapse. He had no evidence of active GVHD. I treated him with ibrutinib, and 3 months later, he received DLI from his sibling donor, and I stopped his ibrutinib. He has achieved CR and is now MRD negative, although he did develop mild chronic GVHD.
RT remains a risk that can be associated with dismal outcome in CLL,54 even in the era of novel agents,55 and treatment of RT remains a challenge.56 If RT patients can be salvaged using conventional treatment approaches, then allo-SCT can be associated with good outcome.57 Practice guidelines recommend its use in this setting,51 and this is my own practice.
Alternative approaches
Although allo-SCT has proven successful, as discussed above, the results of allo-SCT appear less robust in CLL than in many other hematologic malignancies. However, there is clear evidence of a T-cell–mediated response that can be obtained with allo-SCT leading investigators to explore ways to exploit this activity. There is clear evidence of T-cell defects associated with CLL.58,59 A promising approach to overcome this and induce autologous T-cell responses against CLL is by the use of genetic engineering to introduce chimeric antigen receptors (CARs),60,61 and this remains the focus of ongoing clinical trials. Notably, T-cell function, even for CARs, can be enhanced by the use of ibrutinib.62 Whether CAR T cells can replace allo-SCT or be used an alternative in patients in whom no suitable donor can be found remains to be determined. Despite that fact that PDL1 is clearly implicated in T-cell defects in CLL,63 the results of checkpoint inhibitors, which have shown such efficacy in many solid tumors, have been rather disappointing in CLL and RT.64 However, this remains a focus of clinical investigation to determine whether checkpoint inhibitors will show benefit when used in combination.
Conclusions
The role of allo-SCT in CLL has been decreasing as novel agents have shown activity with less morbidity and mortality. It remains to be seen whether these agents can replace allo-SCT, as has been the case for imatinib in CML, or whether they are delaying the use of allo-SCT until later in the disease course. As the currently available novel agents become increasingly used in combination and earlier in the disease course, it is likely that unless new agents become available, allo-SCT will continue to have a role for patients who fail, are intolerant, or do not have access to these novel agents. The optimal timing of allo-SCT in the setting on these novel agents remains the focus of ongoing clinical investigation and should be the basis of randomized prospective clinical trials.
Acknowledgments
This study was funded by the National Institutes of Health, National Cancer Institute through the CLL Research Consortium (PO1-CA081534) (J.G.G.).
Authorship
Contribution: J.G.G. conceived and wrote the manuscript.
Conflict-of-interest disclosure: J.G.G. has received grant fudging from Janssen, Acerta, and Celgene and honoraria from Abbvie, Acerta, Celgene, Gilead, Janssen, Kite, Novartis, and Roche.
Correspondence: John G. Gribben, Barts Cancer Institute, Charterhouse Square, London EC1M6BQ, United Kingdom; e-mail j.gribben@qmul.ac.uk.
References
- 1.Dreger P, Corradini P, Kimby E, et al. ; Chronic Leukemia Working Party of the EBMT. Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia. 2007;21(1):12-17. [DOI] [PubMed] [Google Scholar]
- 2.Gribben JG. How I treat CLL up front. Blood. 2010;115(2):187-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallek M, Fischer K, Fingerle-Rowson G, et al. ; German Chronic Lymphocytic Leukaemia Study Group. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164-1174. [DOI] [PubMed] [Google Scholar]
- 4.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101-1110. [DOI] [PubMed] [Google Scholar]
- 5.Byrd JC, Brown JR, O’Brien S, et al. ; RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger JA, Tedeschi A, Barr PM, et al. ; RESONATE-2 Investigators. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373(25):2425-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768-778. [DOI] [PubMed] [Google Scholar]
- 9.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107-1120. [DOI] [PubMed] [Google Scholar]
- 11.Seymour JF, Ma S, Brander DM, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol. 2017;18(2):230-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dreger P, Schetelig J, Andersen N, et al. ; European Research Initiative on CLL (ERIC) and the European Society for Blood and Marrow Transplantation (EBMT). Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood. 2014;124(26):3841-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passweg JR, Baldomero H, Bader P, et al. Impact of drug development on the use of stem cell transplantation: a report by the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2017;52(2):191-196. [DOI] [PubMed] [Google Scholar]
- 14.Hallek M, Cheson BD, Catovsky D, et al. ; International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichhorst B, Fink AM, Bahlo J, et al. ; German CLL Study Group (GCLLSG). First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928-942. [DOI] [PubMed] [Google Scholar]
- 16.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208-215. [DOI] [PubMed] [Google Scholar]
- 17.Thompson PA, Tam CS, O’Brien SM, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2016;127(3):303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zenz T, Gribben JG, Hallek M, Döhner H, Keating MJ, Stilgenbauer S. Risk categories and refractory CLL in the era of chemoimmunotherapy. Blood. 2012;119(18):4101-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stilgenbauer S, Schnaiter A, Paschka P, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood. 2014;123(21):3247-3254. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409-1418. [DOI] [PubMed] [Google Scholar]
- 21.Mina A, Sandoval Sus J, Sleiman E, Pinilla-Ibarz J, Awan FT, Kharfan-Dabaja MA. Using prognostic models in CLL to personalize approach to clinical care: Are we there yet? Blood Rev. 2018;32(2):159-166. [DOI] [PubMed] [Google Scholar]
- 22.Gribben JG, Bosch F, Cymbalista F, et al. Optimising outcomes for patients with chronic lymphocytic leukaemia on ibrutinib therapy: European recommendations for clinical practice. Br J Haematol. 2018;180(5):666-679. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien S, Furman RR, Coutre S, et al. Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson PA, O’Brien SM, Wierda WG, et al. Complex karyotype is a stronger predictor than del(17p) for an inferior outcome in relapsed or refractory chronic lymphocytic leukemia patients treated with ibrutinib-based regimens. Cancer. 2015;121(20):3612-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien SM, Jaglowski S, Byrd JC, et al. Prognostic factors for complete response to ibrutinib in patients with chronic lymphocytic leukemia: a pooled analysis of 2 clinical trials. JAMA Oncol. 2018;4(5):712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones JA, Mato AR, Wierda WG, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19(1):65-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davids MS. How should we sequence and combine novel therapies in CLL? Hematology (Am Soc Hematol Educ Program). 2017;2017(1):346-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharfan-Dabaja MA, Pidala J, Kumar A, Terasawa T, Djulbegovic B. Comparing efficacy of reduced-toxicity allogeneic hematopoietic cell transplantation with conventional chemo-(immuno) therapy in patients with relapsed or refractory CLL: a Markov decision analysis. Bone Marrow Transplant. 2012;47(9):1164-1170. [DOI] [PubMed] [Google Scholar]
- 29.Herth I, Dietrich S, Benner A, et al. The impact of allogeneic stem cell transplantation on the natural course of poor-risk chronic lymphocytic leukemia as defined by the EBMT consensus criteria: a retrospective donor versus no donor comparison. Ann Oncol. 2014;25(1):200-206. [DOI] [PubMed] [Google Scholar]
- 30.Poon ML, Fox PS, Samuels BI, et al. Allogeneic stem cell transplant in patients with chronic lymphocytic leukemia with 17p deletion: consult-transplant versus consult- no-transplant analysis. Leuk Lymphoma. 2015;56(3):711-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montserrat E, Dreger P. Treatment of chronic lymphocytic leukemia with del(17p)/TP53 mutation: allogeneic hematopoietic stem cell transplantation or BCR-signaling inhibitors? Clin Lymphoma Myeloma Leuk. 2016;16 suppl:S74-S81. [DOI] [PubMed] [Google Scholar]
- 32.Gribben JG, Zahrieh D, Stephans K, et al. Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood. 2005;106(13):4389-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390-3400. [DOI] [PubMed] [Google Scholar]
- 34.Khouri IF, Keating M, Körbling M, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16(8):2817-2824. [DOI] [PubMed] [Google Scholar]
- 35.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91(3):756-763. [PubMed] [Google Scholar]
- 36.Moreno C, Villamor N, Colomer D, et al. Allogeneic stem-cell transplantation may overcome the adverse prognosis of unmutated VH gene in patients with chronic lymphocytic leukemia. J Clin Oncol. 2005;23(15):3433-3438. [DOI] [PubMed] [Google Scholar]
- 37.Gribben JG. Salvage therapy for CLL and the role of stem cell transplantation. Hematology (Am Soc Hematol Educ Program). 2005:292-298. [DOI] [PubMed] [Google Scholar]
- 38.Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111(1):446-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown JR, Kim HT, Armand P, et al. Long-term follow-up of reduced-intensity allogeneic stem cell transplantation for chronic lymphocytic leukemia: prognostic model to predict outcome. Leukemia. 2013;27(2):362-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khouri IF, Bassett R, Poindexter N, et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer. 2011;117(20):4679-4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dreger P, Döhner H, Ritgen M, et al. ; German CLL Study Group. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116(14):2438-2447. [DOI] [PubMed] [Google Scholar]
- 42.Schetelig J, de Wreede LC, Andersen NS, et al. ; CLL subcommittee, Chronic Malignancies Working Party. Centre characteristics and procedure-related factors have an impact on outcomes of allogeneic transplantation for patients with CLL: a retrospective analysis from the European Society for Blood and Marrow Transplantation (EBMT). Br J Haematol. 2017;178(4):521-533. [DOI] [PubMed] [Google Scholar]
- 43.Schetelig J, de Wreede LC, van Gelder M, et al. Risk factors for treatment failure after allogeneic transplantation of patients with CLL: a report from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2017;52(4):552-560. [DOI] [PubMed] [Google Scholar]
- 44.Schetelig J, van Biezen A, Brand R, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol. 2008;26(31):5094-5100. [DOI] [PubMed] [Google Scholar]
- 45.Dreger P, Schnaiter A, Zenz T, et al. TP53, SF3B1, and NOTCH1 mutations and outcome of allotransplantation for chronic lymphocytic leukemia: six-year follow-up of the GCLLSG CLL3X trial. Blood. 2013;121(16):3284-3288. [DOI] [PubMed] [Google Scholar]
- 46.van Gelder M, de Wreede LC, Bornhäuser M, et al. Long-term survival of patients with CLL after allogeneic transplantation: a report from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2017;52(3):372-380. [DOI] [PubMed] [Google Scholar]
- 47.van Gelder M, Ziagkos D, de Wreede L, et al. Baseline characteristics predicting very good outcome of allogeneic hematopoietic cell transplantation in young patients with high cytogenetic risk chronic lymphocytic leukemia: a retrospective analysis from the Chronic Malignancies Working Party of the EBMT. Clin Lymphoma Myeloma Leuk. 2017; 17(10):667-675. [DOI] [PubMed] [Google Scholar]
- 48.Krämer I, Stilgenbauer S, Dietrich S, et al. Allogeneic hematopoietic cell transplantation for high-risk CLL: 10-year follow-up of the GCLLSG CLL3X trial. Blood. 2017;130(12):1477-1480. [DOI] [PubMed] [Google Scholar]
- 49.Xavier E, Cornillon J, Ruggeri A, et al. Outcomes of cord blood transplantation using reduced-intensity conditioning for chronic lymphocytic leukemia: a study on behalf of Eurocord and Cord Blood Committee of Cellular Therapy and Immunobiology Working Party, Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation, and the Societé Française de Greffe de Moelle et Therapie Cellulaire. Biol Blood Marrow Transplant. 2015;21(8):1515-1523. [DOI] [PubMed] [Google Scholar]
- 50.van Gorkom G, van Gelder M, Eikema DJ, et al. Outcomes of haploidentical stem cell transplantation for chronic lymphocytic leukemia: a retrospective study on behalf of the chronic malignancies working party of the EBMT. Bone Marrow Transplant. 2018;53(3):255-263. [DOI] [PubMed] [Google Scholar]
- 51.Kharfan-Dabaja MA, Kumar A, Hamadani M, et al. Clinical practice recommendations for use of allogeneic hematopoietic cell transplantation in chronic lymphocytic leukemia on behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2016;22(12):2117-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127(8):3052-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryan CE, Sahaf B, Logan AC, et al. Ibrutinib efficacy and tolerability in patients with relapsed chronic lymphocytic leukemia following allogeneic HCT. Blood. 2016;128(25):2899-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chigrinova E, Rinaldi A, Kwee I, et al. Two main genetic pathways lead to the transformation of chronic lymphocytic leukemia to Richter syndrome. Blood. 2013;122(15):2673-2682. [DOI] [PubMed] [Google Scholar]
- 55.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1(1):80-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parikh SA, Kay NE, Shanafelt TD. How we treat Richter syndrome. Blood. 2014;123(11):1647-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kharfan-Dabaja MA, Kumar A, Stingo FE, et al. Allogeneic hematopoietic cell transplantation for richter syndrome: a single-center experience. Clin Lymphoma Myeloma Leuk. 2018;18(1):e35-e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Görgün G, Holderried TAW, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115(7):1797-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramsay AG, Johnson AJ, Lee AM, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118(7):2427-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turtle CJ, Hay KA, Hanafi LA, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fraietta JA, Beckwith KA, Patel PR, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127(9):1117-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120(7):1412-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding W, LaPlant BR, Call TG, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129(26):3419-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]