Abstract
TREK-2 (KCNK10/K2P10), a two-pore domain (K2P) potassium channel, is gated by polymodal stimuli such as stretch, fatty acids and pH, and by several drugs including the antidepressant fluoxetine (Prozac) and its metabolite norfluoxetine. However, the mechanisms that control channel gating are unclear. Here we present crystal structures of the human TREK-2 channel in two conformations and in complex with norfluoxetine, a state-dependent blocker. Norfluoxetine binds within intramembrane fenestrations found only in one of these two conformations, and channel activation by arachidonic acid and mechanical stretch involves conversion between these states via movement of the pore-lining helices. These results therefore not only provide a structural explanation for TREK channel mechanosensitivity, but also their regulation by other diverse stimuli and explain how Prozac inhibits TREK channels.
K2P channels contribute to the background leak potassium currents in nearly all cell types and exhibit one of the most versatile, polymodal patterns of regulation known for any class of ion channel. This functional diversity plays a key role in regulation of the resting membrane potential in many excitable and non-excitable tissues and K2P channels now represent important clinical targets for the treatment of cardiovascular disease and several neurological disorders including pain, migraine and depression (1).
The archetypal polymodal K2P channels TREK-1 and TREK-2 exhibit >55% sequence identity and are both regulated by physical factors such as mechanical stretch, voltage and temperature, and by natural ligands including polyunsaturated fatty acids such as arachidonic acid (AA), as well as both intra- and extracellular pH (1–4). Their activity can also be modulated by diverse pharmacological agents such as volatile anesthetics (5), neuroprotective drugs (6) and antidepressants such as fluoxetine (Prozac) (7, 8). Such diverse regulation allows these channels to couple cellular electrical activity to a remarkable variety of signaling pathways and consequently they represent important targets in pharmacology (9–11). In particular TREK channels are inhibited by the antidepressant fluoxetine (Prozac) and its active metabolite norfluoxetine (7, 8), at physiologically relevant concentrations (8, 12)). This selective serotonin reuptake inhibitor is used in the treatment of a range of depressive and anxiety disorders, and in addition to its principal effect of directly inhibiting serotonin transporters, fluoxetine also inhibits several GPCRs and ion channels (13–15). Intriguingly TREK-1 knockout mice have been reported to be resistant to depression, suggesting that TREK channel inhibition by fluoxetine could contribute to its antidepressant effects (7, 16). TREK channels in the cardiovascular system may also contribute to some of the adverse cardiac effects of the drug (17, 18).
The molecular and structural mechanisms that allow K2P channels to sense such diverse physiological and pharmacological stimuli are poorly understood. K2P channels share many basic structural features with classical tetrameric K+ channels, but they assemble as dimers, with a pseudo-tetrameric architecture around the central selectivity filter (19–21), and unlike many other K+ channels, they do not appear to gate via constriction of the cytoplasmic entrance to the pore. Instead, this part of the conduction pathway remains open even in the functionally closed channel, and gating occurs primarily within or near the selectivity filter (2, 22–25). However, the mechanisms that relay such intracellular and extracellular stimuli to the pore, and the way that drugs like fluoxetine modulate this process remain unclear.
Crystal structures of TREK-2 in two conformations
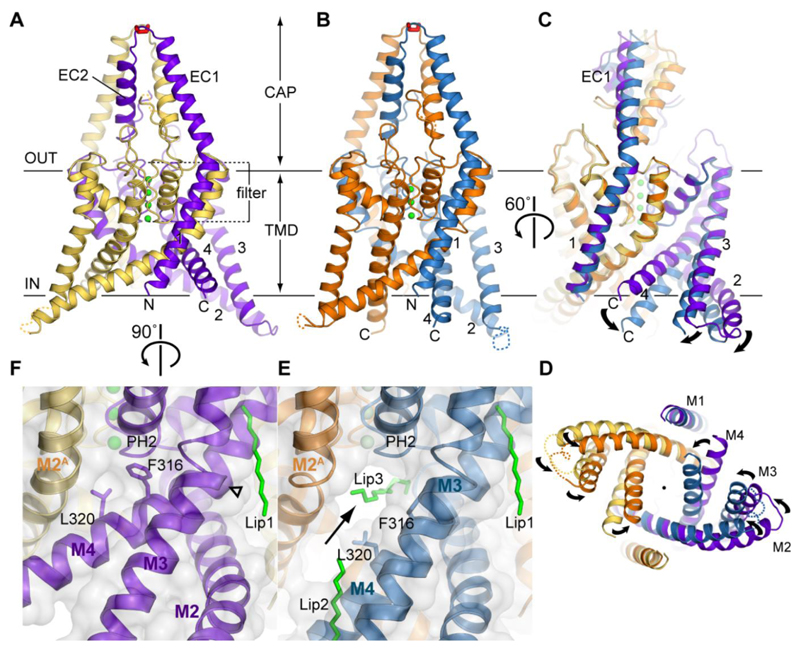
In order to understand the mechanisms of polymodal K2P channel gating and drug binding, we have solved the crystal structure of the human TREK-2 channel in two conformations at 3.4 and 3.9 Å resolution ((26), figs. S1 and S2, table S1). The truncated protein construct used for crystallization retains many of the functional properties exhibited by wild-type TREK-2, including activation by stretch and AA, and inhibition by norfluoxetine (fig. S3). The two TREK-2 structures show the classic K2P channel fold (19–21), with four transmembrane helices (M1-M4), an extracellular cap domain and a pseudo four-fold symmetry within the selectivity filter (Fig. 1A and 1B). Intriguingly, a higher resolution TRAAK/Fab-complex structure suggested a novel domain swap of the M1 helix at the apex of the cap domain where the two subunits are connected by a disulfide bond (19). Our TREK-2 structures also exhibit this domain swap (fig. S4), suggesting this feature may be common to most K2P channels.
Fig. 1. Crystal structures of two distinct conformations of TREK-2.
TREK-2 overall fold viewed parallel to the plane of the membrane, with views of (A) the up state, 3.4 Å structure; (B) the down state, 3.9 Å structure and (C) a superposition of the states and (D) a view of the superposition from the cytoplasmic side of the membrane. The up state chains A and B are colored yellow and purple, the down state chains A and B are colored orange and blue. Potassium ions are colored green and the disulfide bond in the cap is shown in red. (E) View of the lateral fenestration (indicated with an arrow) in the down state, showing the aliphatic chain which binds in the vestibule and the fenestration, and lipids (Lip1, Lip2, Lip3) bound to the structure (green sticks). (F) View of the same region as in E in the up state, showing the absence of the fenestration when the helices are in the up state, with Phe316 and Leu320 blocking the fenestration. Hinge point around G312 in M4 is indicated by an open triangle.
The differences between the two TREK-2 conformations are centered around the intramembrane and cytoplasmic sections of the M2, M3 and M4 helices (Figs. 1C and D, fig S5, Movie S1). In the 3.9 Å structure of TREK-2, these regions project further into the cytoplasm, an arrangement we refer to as the “down state”, whilst in the 3.4 Å structure, they move further up into the membrane (the “up state”). In the up state, M4 is kinked, with a hinge around Gly312, equivalent to the conserved ‘glycine hinge’ seen in many other K+ channels. In TREK-2 there are additional hinges in M2 at Gly201/Gly206 and Gly248 in M3, which allow these three helices to move into alternative positions in the up state (Fig. 1F). The down state of TREK-2 is similar to that previously observed in the TRAAK and TWIK-1 structures (20, 21). Although some movement of M4 was seen in one chain of the TRAAK/Fab structure (19), in the TREK-2 up state all three helices (M2, M3 and M4) move up (Movie S1 and fig. S5 and S6); this same movement is seen in both chains of the two dimers in the asymmetric unit. Molecular dynamics (MD) simulations of these structures in a phospholipid bilayer indicate that the overall conformations of both states are stable during a 100 ns simulation. Also, during simulation of the up state, the M2, M3 and M4 helices were all observed to move down and adopt a conformation similar to the down state, indicating that a transition can occur between states within a membrane (fig. S6).
A key feature of K2P channel structures are the intramembrane side fenestrations just below the selectivity filter (20, 21). These fenestrations are only present in the down state of TREK-2 (Fig. 1E). In the up state both fenestrations are closed, due to the elevation and rotation of M4 which places the side chains of Phe316 and Leu320 in the fenestration (Fig. 1F). In both the TREK-2 and TWIK-1 down state structures there is density for lipid (or PEG) like molecules extending across the top of the inner vestibule, below the pore filter (Fig. 1E and fig. S5A and B). Profiles of the inner pore show that the cytoplasmic entrance to the vestibule remains open in both conformations of TREK-2 (Fig. 1D and fig. S5D-F). Intriguingly, the down state only contains three K+ ions within the filter (S2-S4), whereas the up state contains four K+ ions, consistent with a fully conductive K+ channel filter (Figs. 1A and B) (27). This suggests that these two conformations may therefore represent different functional states even though both appear open at the cytoplasmic entrance to the inner pore.
TREK-2 in complex with Prozac derivatives
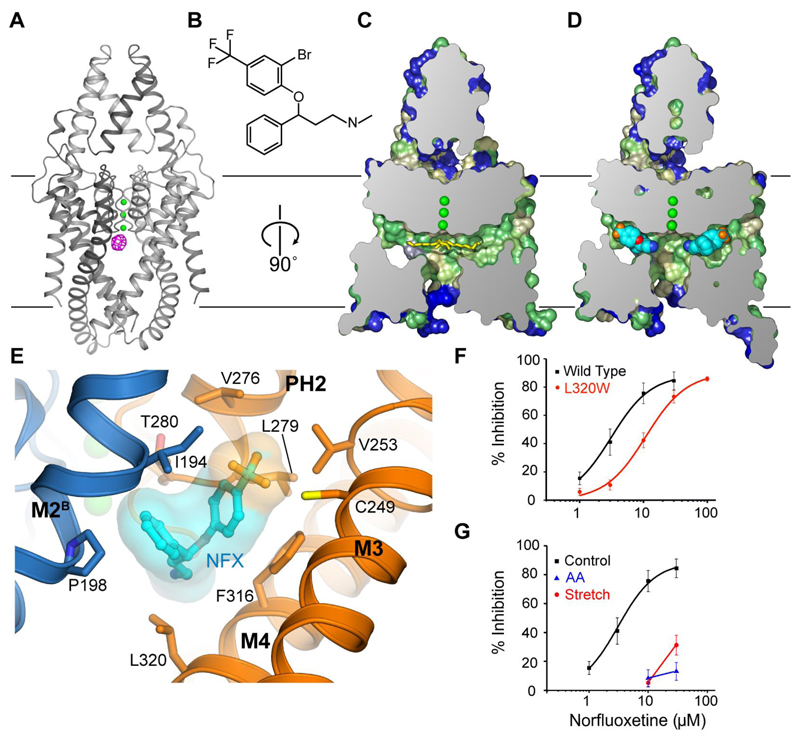
To probe the functional significance of these two states we examined TREK-2 interaction with derivatives of the inhibitor fluoxetine. Both fluoxetine and norfluoxetine exhibit state-dependent inhibition of TREK channel activity via a selective interaction with the closed state of the channel (8). We solved the structure of TREK-2 in complex with norfluoxetine and a brominated derivative of fluoxetine (3-(2-bromo-4-(trifluoromethyl)phenoxy)-N-methyl-3-phenylpropan-1-amine, Br-fluoxetine) at 3.7 and 3.64 Å resolution (Fig. 2 and fig. S7). The structures obtained were in the down state and there are clear peaks in anomalous difference maps for the bromine of the Br-fluoxetine in the fenestrations (Fig. 2A and fig. S7), which unequivocally identifies the binding site for Br-fluoxetine. Norfluoxetine also binds in the fenestration (Fig. 2C-E, Movie S1), but neither ligand extends into the vestibule and therefore they do not block the ion path directly (Fig. 2D and E). The fenestration provides a hydrophobic environment in which both Br-fluoxetine and norfluoxetine interact with residues Ile194 and Pro198 on M2 of chain B, Cys249 and Val253 on M3, Phe316 and Leu320 on M4 and Val276, Leu279 and Thr280 on PH2, close to the selectivity filter (Fig. 2E). There is some flexibility in the positioning of the fluoxetine derivative ligands (fig. S7), but this is not unexpected for such a relatively low-affinity ligand. Furthermore, a mutation within this binding site (L320W on M4) reduces the inhibition of TREK-2 by norfluoxetine (Fig. 2F) indicating the functional relevance of this site.
Fig. 2. Norfluoxetine and Br-Fluoxetine bind to TREK-2 in the fenestration adjacent to the pore filter entrance.
(A) The overall fold of TREK-2 down state is shown with the 5 Å anomalous difference map for the Br-fluoxetine/TREK-2 complex shown in pink (contoured at 4.5 sigma), indicating the location of the Br-fluoxetine in the fenestration. (B) The chemical structure of Br-fluoxetine is shown. Norfluoxetine, the active metabolite of fluoxetine, lacks the methyl group attached to the nitrogen atom. (C) Cross section of a surface view of TREK-2 in the down state, with the surface colored according to hydrophobicity (color ramped from green for most hydrophobic, through yellow to blue for least hydrophobic). (D) The complex of TREK-2 with norfluoxetine. Norfluoxetine is shown in cyan, blue, red and orange for carbon, nitrogen, oxygen and fluorine atoms. For clarity, only one enantiomer of norfluoxetine is shown in C/D. (E) The norfluoxetine binding site, with chain A shown in gold and chain B shown in blue, norfluoxetine is colored as in D. (F) Disruption of the binding site by the L320W mutation reduces norfluoxetine inhibition. (G) Stretch activation (-11 mmHg, red) at internal pH 7.3 dramatically reduces the efficacy of norfluoxetine inhibition, as does activation by 10 µM arachidonic acid (AA, blue).
Implications for polymodal K2P channel gating mechanisms
Closure of the fenestration in the up state removes the fluoxetine binding site and therefore suggests that fluoxetine cannot bind to this conformation and must therefore inhibits TREK-2 by interaction with a site that is only present in the down state. This is consistent not only with a state-dependent block of the closed channel by fluoxetine, but also predicts that the up state represents a more activated state of the channel, to which fluoxetine cannot bind. To test this idea we examined the effect of channel activation on norfluoxetine inhibition of TREK-2. We found that activation either by membrane stretch or by AA significantly reduced subsequent inhibition by norfluoxetine (Fig. 2G). The mechanisms underlying both stretch and AA activation are thought to be similar (28, 29), thus suggesting that the up state may represent both the stretch and AA activated conformation of the channel. Furthermore, we found that the presence of norfluoxetine dramatically reduces the rate of activation by membrane stretch (Fig. S8A). This finding is consistent with a binding site that would interfere with conversion between the two states. In addition to preventing this larger conformational change, fluoxetine binding close to the selectivity filter might also help stabilize the filter gate in a non-conductive state.
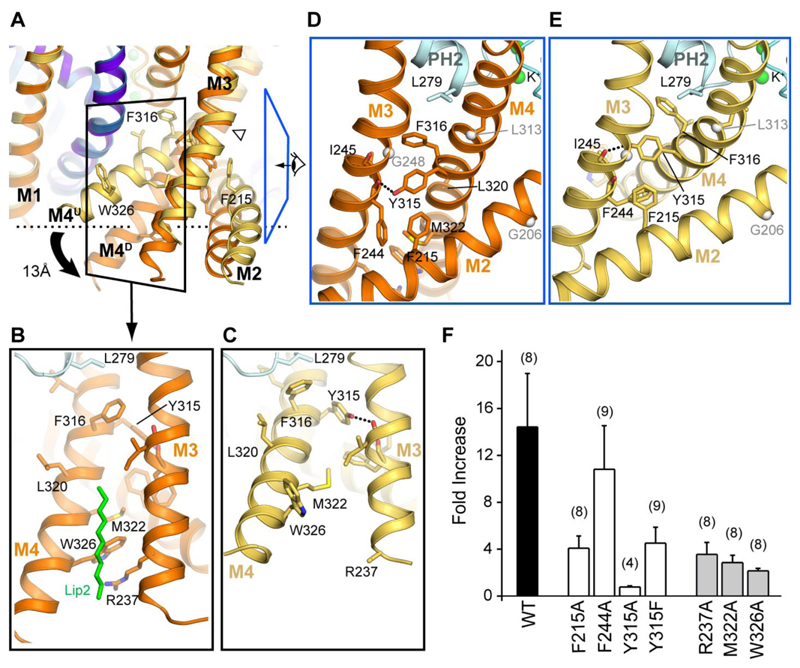
Movement between these two alternative conformations of TREK-2 provides a structural mechanism for the coupling of regulatory signals to the filter region where channel gating occurs. In particular, it would allow movement of M4 to be coupled directly to the cytoplasmic regulatory domain that is thought to move on and off the membrane surface in response to changes in intracellular pH and phosphorylation (16, 22, 28, 30). Comparison of the two conformations reveals a series of interactions between M2, M3 and M4 which would reorganize if the channel switched between up and down states; at the cytoplasmic end of M4, Trp326 is packed between the sidechains of Met322 on M4 and Arg237 on M3 in the down state (Fig. 3A and B). However, in the up state, M4 is rotated by 30° and kinked, allowing Trp326 to insert into the membrane, thus allowing the three helices to move further up into the membrane (Fig. 3A and C). Disruption of these interactions should therefore preferentially destabilize the down state, thus favoring the up state. We found that mutation of these three residues (W326A, M322A and R237A) all lead to a marked reduction in stretch activation, consistent with the idea that stretch activation involves movement between these two states (Fig. 3F).
Fig. 3. Concerted motion of transmembrane helices (M2, M3, M4).
(A) Superposition of the up and down states highlighting motion in M2-M4 transmembrane helices. (B) Interactions between helices M2/M3/M4 in the down state, showing the interactions between Trp326, Arg237 and Met322. View shown is indicated by black boxed region in A. The lipid chain that overlays this interface is shown in green. (C) View of corresponding region in the up state, showing disruption of distal M3/M4 interface due to shift in M4 orientation. (D-E) Interactions at the top of the M2/M3/M4 helices, proximal to the pore in the down (D) and up (E) states viewed from direction indicated by blue boxed region in A. TM helix hinge points are indicated by grey spheres. (F) Mutations at the interface between M2, M3 and M4 reduce the fold-activation by membrane stretch (-11 mmHg).
Immediately above Trp326 a set of hydrophobic residues (Phe215, Phe244, Ile245 and Tyr315) form another core of interactions between M2, M3 and M4 (Fig. 3D and E). The sidechains of these residues all rearrange as this helical bundle switches between conformations (Fig. 3A). Importantly, mutations within this region are also found to reduce stretch activation (Fig. 3F). In particular, Tyr315 on M4 hydrogen bonds with the backbone carbonyl of Phe244 on M3 in the up state, but then shifts to the backbone of Ile245 in the down state. We also find that both the Y315A and Y315F mutations markedly reduce stretch activation of TREK-2 supporting a functional role for this inter-helical hydrogen bond (Fig. 3D and E).
In addition to regulation by AA, TREK channels exhibit direct regulation by many other natural lipids including PIP2 (31). Interestingly, we observe a lipid-like density on the surface within a groove between M3 and M4 in both states, and between the lower section of M4 and M2/M3 in the down state. MD simulations confirm that these sites could accommodate lipids and thereby providing possible binding sites for such regulatory lipids. Alternatively, such lipid binding sites could contribute to the force from lipid principle of mechanosensitivity (Fig. 3B, fig. S9), (32–34).
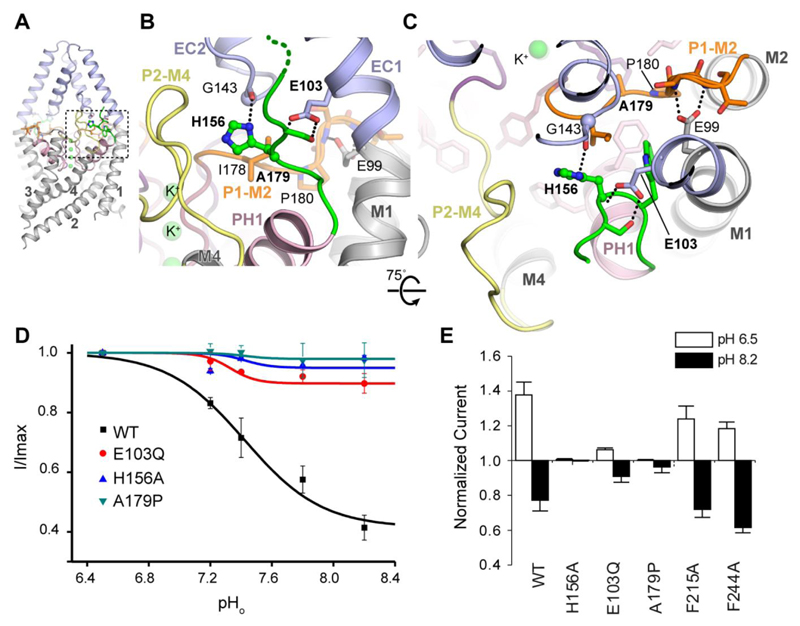
TREK channels are sensitive to changes in both external and intracellular pH. Extracellular pH (pHext) is thought to affect the filter gate via protonation of a conserved histidine (His156 in TREK-2). Previous studies have implicated a role for the P2-M4 linker of TREK-2 in this process, as well as many other extracellular residues in TREK-1 (35). These new structures reveal that His156 is located within a solvent accessible cavity adjacent to the extracellular P1-M2 and P2-M4 linkers (Fig. 4A-C). It is also at the center of an extensive hydrogen bond network which includes Glu103 and mutation of this residue (E103Q) reduces the pHext response (Fig. 4D). Another conserved glutamate within this network (Glu99) also contacts the backbone of the P1-M2 linker. A mutation within this linker that would alter its flexibility (A179P) also abolishes pHext-sensitivity (Fig. 4D), suggesting the structural dynamics of this network are involved in coupling external stimuli to the filter gate. Intracellular acidification (pHint) also activates TREK channels and protonation of a glutamate residue within the proximal C-terminus appears central to this process (Glu337 in TREK-2) (28, 36). However, this region is not resolved in any of these TREK-2 structures. Furthermore, we find that pHint-activation of TREK-2 does not reduce the efficacy of norfluoxetine inhibition (fig S8B), suggesting the structural mechanisms underlying pHint-activation may be different to stretch and AA induced activation. Nevertheless, this charged region is still well-positioned to influence movement of TREK-2 between the up and down states within the membrane and explains why it can influence so many regulatory pathways (16, 28, 36).
Fig. 4. The external pH sensor region centered on His156.
(A) The sensor histidine (His156, green) is located between the ECD (pale blue) and pore helix 1 (PH1) of the transmembrane domain adjacent to the pore region (B-C). The environment round His156 is shown with different structural elements colored. Dotted lines represent hydrogen bonds. The filter loops P1-M2 and P2-M4 are colored orange and yellow respectively. (D-E) Effects of mutating residues within either the His156 network (D) or the internal hydrophobic hinge between M2-M4 (E) on external pH-sensitivity.
Together, these structural and functional studies allow us to propose a gating mechanism (Fig. 5) in which movement of the pore-lining helices converts TREK-2 between different functional states. The down state representing a closed or ‘low-activity’ state of the channel that can be stabilized by the binding of norfluoxetine within the fenestration, whereas, activation by membrane stretch or AA stabilizes the channel in a more active up state, which is insensitive to inhibition by norfluoxetine (Fig. 5). Although it is possible that these two states represent the open and closed states of the channels, the situation is likely to be more subtle than this. The filter may be able to gate independently in both conformations, but does so with a higher probability in the up state, as shown in Fig. 5A. Regulation by pHext and pHint could modulate these different functional states conductivities through small movements at the pore filter, without a large scale change in conformation, whereas other modalities modulate channel activity by converting it between the up and down states. For example, our results provide an obvious mechanism for the coupling of mechanical forces within the membrane to TREK channel activity through the up/down movement of the transmembrane helices (Fig. 5B), and support recent evidence that mechanosensitive K2P channels sense force directly from interactions with lipid (32–34). Though, precisely how such conformational changes influence the selectivity filter gate (30), or the relative hydration status of the inner pore (37, 38), remains to be determined.
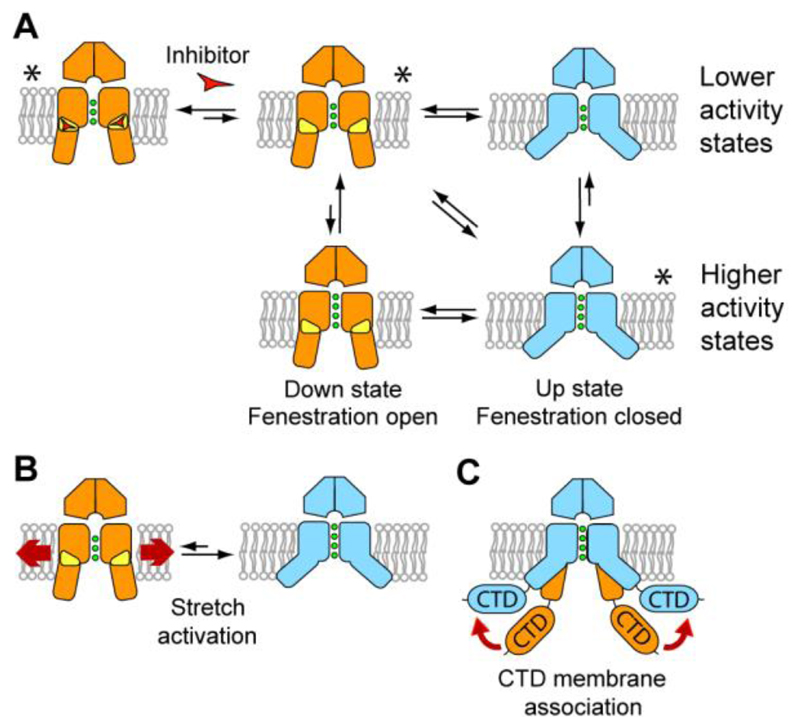
Fig. 5. Model of K2P channel gating and inhibition by norfluoxetine.
The down state, lower conductivity conformations are shown in orange, with the fenestrations indicated in yellow. The up state conformations are shown in blue. Inhibitors such as norfluoxetine are represented by a red triangle. (A) Overall scheme for K2P channel gating. Higher and lower conductivity states are shown for both conformations as the filter may gate independently of these larger changes, though with a different probability. Conformations for which we have crystal structures are indicated with an asterisk. (B) Schematic of activation of TREK channels by mechanical stretch. The direct interaction of TREK-2 with lipids in the membrane may allow lateral forces to facilitate conversion from the down to the up state which is fluoxetine insensitive. (C) Association of the C-terminal domain (CTD) with the membrane. The diverse cytoplasmic C-terminal extensions of K2P channels provide an additional site for modulation of channel activity. This regulation could operate in part though association of the CTD with the membrane after post-translational or pH changes that would favor the more active up state.
In summary, these results indicate how the movement of pore-lining helices observed in classical tetrameric K+ channels have been adapted as part of a unique gating mechanism for polymodal regulation of K2P channels, and also provides a structural insight into how a widely-deployed drug Prozac, can inhibit TREK ion channel activity.
Supplementary Material
One Sentence Summary.
Crystal structures of the TREK-2 K2P channel and a complex with norfluoxetine reveal mechanisms for K2P channel gating.
Acknowledgments
We thank members of the SGC Biotech team, including Claire Strain-Damerell for help with this project, including mutagenesis, small scale detergent selection techniques, preparation of baculovirus and insect cell cultures. We thank Brian Marsden and David Damerell for bioinformatics support, Rod Chalk and Georgina Berridge for help with mass spectrometry and Aled Edwards for suggesting the use of 8-bromo-fluoxetine for fluoxetine binding site identification. We are grateful to the staff at Diamond Light Source Ltd. and in particular the microfocus beamline I24 staff Danny Axford, Robin Owen and Gwyndaf Evans. The SGC is a registered charity (number 1097737) that receives funds from AbbVie, Bayer Pharma AG, Boehringer Ingelheim, the Canada Foundation for Innovation, Genome Canada, GlaxoSmithKline, Janssen, Lilly Canada, the Novartis Research Foundation, the Ontario Ministry of Economic Development and Innovation, Pfizer, Takeda, and the Wellcome Trust [092809/Z/10/Z]. MSPS is supported by the BBSRC and the Wellcome Trust. SJT is supported by a BBSRC Industrial Partnership Award, PA is Wellcome Trust OXION Training Fellow and MVC is supported by a Carlsberg Foundation Fellowship. AM is supported by an EPSRC Life Sciences Interface Doctoral Training Centre studentship (EP/I017909/1). Atomic coordinates and structure factors have been deposited with the Protein Data Bank under accession codes XXXX (down, fenestration-open form), 4BW5 (up, fenestration-closed form), XXXX (Br-fluoxetine complex) and XXXX (norfluoxetine complex).
Footnotes
The authors declare no competing financial interests.
References
- 1.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 2.Cohen A, Ben-Abu Y, Zilberberg N. Gating the pore of potassium leak channels. Eur Biophys J. 2009;39:61–73. doi: 10.1007/s00249-009-0457-6. [DOI] [PubMed] [Google Scholar]
- 3.Dedman A, et al. The mechano-gated K(2P) channel TREK-1. Eur Biophys J. 2009;38:293–303. doi: 10.1007/s00249-008-0318-8. [DOI] [PubMed] [Google Scholar]
- 4.Noel J, Sandoz G, Lesage F. Molecular regulations governing TREK and TRAAK channel functions. Channels (Austin) 2011;5:402–409. doi: 10.4161/chan.5.5.16469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel AJ, Honore E. 2P domain K+ channels: novel pharmacological targets for volatile general anesthetics. Adv Exp Med Biol. 2003;536:9–23. doi: 10.1007/978-1-4419-9280-2_2. [DOI] [PubMed] [Google Scholar]
- 6.Cadaveira-Mosquera A, Ribeiro SJ, Reboreda A, Perez M, Lamas JA. Activation of TREK currents by the neuroprotective agent riluzole in mouse sympathetic neurons. J Neurosci. 2011;31:1375–1385. doi: 10.1523/JNEUROSCI.2791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heurteaux C, et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- 8.Kennard LE. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br J Pharmacol. 2005;144:821–829. doi: 10.1038/sj.bjp.0706068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci. 2008;29:566–575. doi: 10.1016/j.tips.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Es-Salah-Lamoureux Z, Steele DF, Fedida D. Research into the therapeutic roles of two-pore-domain potassium channels. Trends Pharmacol Sci. 2010;31:587–595. doi: 10.1016/j.tips.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Mathie A, Veale EL. Therapeutic potential of neuronal two-pore domain potassium-channel modulators. Curr Opin Investig Drugs. 2007;8:555–562. [PubMed] [Google Scholar]
- 12.Henry ME, et al. A comparison of brain and serum pharmacokinetics of R-fluoxetine and racemic fluoxetine: A 19-F MRS study. Neuropsychopharmacology. 2005;30:1576–1583. doi: 10.1038/sj.npp.1300749. [DOI] [PubMed] [Google Scholar]
- 13.Thomas D, Gut B, Wendt-Nordahl G, Kiehn J. The antidepressant drug fluoxetine is an inhibitor of human ether-a-go-go-related gene (HERG) potassium channels. J Pharmacol Exp Ther. 2002;300:543–548. doi: 10.1124/jpet.300.2.543. [DOI] [PubMed] [Google Scholar]
- 14.Furutani K, Ohno Y, Inanobe A, Hibino H, Kurachi Y. Mutational and in silico analyses for antidepressant block of astroglial inward-rectifier Kir4.1 channel. Mol Pharmacol. 2009;75:1287–1295. doi: 10.1124/mol.108.052936. [DOI] [PubMed] [Google Scholar]
- 15.Jeong I, Choi JS, Hahn SJ. Effects of fluoxetine on cloned Kv4.3 potassium channels. Brain Res. 2013;1500:10–18. doi: 10.1016/j.brainres.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 16.Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- 17.Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. Eur Biophys J. 2009;38:305–318. doi: 10.1007/s00249-008-0326-8. [DOI] [PubMed] [Google Scholar]
- 18.Eckert M, Egenberger B, Doring F, Wischmeyer E. TREK-1 isoforms generated by alternative translation initiation display different susceptibility to the antidepressant fluoxetine. Neuropharmacology. 2011;61:918–923. doi: 10.1016/j.neuropharm.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Brohawn SG, Campbell EB, MacKinnon R. Domain-swapped chain connectivity and gated membrane access in a Fab-mediated crystal of the human TRAAK K+ channel. Proc Natl Acad Sci U S A. 2013;110:2129–2134. doi: 10.1073/pnas.1218950110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brohawn SG, del Marmol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335:436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller AN, Long SB. Crystal structure of the human two-pore domain potassium channel K2P1. Science. 2012;335:432–436. doi: 10.1126/science.1213274. [DOI] [PubMed] [Google Scholar]
- 22.Bagriantsev SN, Peyronnet R, Clark KA, Honore E, Minor DL., Jr Multiple modalities converge on a common gate to control K2P channel function. EMBO J. 2011;30:3594–3606. doi: 10.1038/emboj.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathie A, Al Moubarak E, Veale EL. Gating of two pore domain potassium channels. J Physiol. 2010;588:3149–3156. doi: 10.1113/jphysiol.2010.192344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piechotta PL, et al. The pore structure and gating mechanism of K2P channels. EMBO J. 2011;30:3607–3619. doi: 10.1038/emboj.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapedius M, et al. State-independent intracellular access of quaternary ammonium blockers to the pore of TREK-1. Channels (Austin) 2012;6:473–478. doi: 10.4161/chan.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Materials and methods are available as supplementary materials on Science Online.
- 27.Zhou M, Morais-Cabral JH, Mann S, MacKinnon R. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature. 2001;411:657–661. doi: 10.1038/35079500. [DOI] [PubMed] [Google Scholar]
- 28.Honore E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K(+) channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 30.Bagriantsev SN, Clark KA, Minor DL., Jr Metabolic and thermal stimuli control K(2P)2.1 (TREK-1) through modular sensory and gating domains. EMBO J. 2012;31:3297–3308. doi: 10.1038/emboj.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chemin J, et al. Up- and down-regulation of the mechano-gated K(2P) channel TREK-1 by PIP (2) and other membrane phospholipids. Pflugers Arch. 2007;455:97–103. doi: 10.1007/s00424-007-0250-2. [DOI] [PubMed] [Google Scholar]
- 32.Anishkin A, Loukin SH, Teng J, Kung C. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc Natl Acad Sci U S A. 2014;111:7898–7905. doi: 10.1073/pnas.1313364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brohawn SG, Su Z, MacKinnon R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc Natl Acad Sci U S A. 2014;111:3614–3619. doi: 10.1073/pnas.1320768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teng J, Loukin S, Anishkin A, Kung C. The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflugers Arch. 2014 doi: 10.1007/s00424-014-1530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandoz G, Douguet D, Chatelain F, Lazdunski M, Lesage F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc Natl Acad Sci U S A. 2009;106:14628–14633. doi: 10.1073/pnas.0906267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y, Gnatenco C, Bang H, Kim D. Localization of TREK-2 K+ channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pHi. Pflugers Arch. 2001;442:952–960. doi: 10.1007/s004240100626. [DOI] [PubMed] [Google Scholar]
- 37.Aryal P, Abd-Wahab F, Bucci G, Sansom MS, Tucker SJ. A hydrophobic barrier deep within the inner pore of the TWIK-1 K2P potassium channel. Nat Commun. 2014;5:4377. doi: 10.1038/ncomms5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aryal P, Sansom MS, Tucker SJ. Hydrophobic Gating in Ion Channels. J Mol Biol. 2014 doi: 10.1016/j.jmb.2014.07.030. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.