Abstract
Gut microbiota exert a pivotal influence on various functions including gastrointestinal (GI) motility, metabolism, nutrition, immunity, and the neuroendocrine system in the host. These effects are mediated by not only short-chain fatty acids produced by microbiota but also gut hormones and inflammatory signaling by enteroendocrine and immune cells under the influence of the microbiota. GI motility is orchestrated by the enteric nervous system and hormonal networks, and disturbance of GI motility plays an important role in the pathophysiology of functional gastrointestinal disorders (FGIDs). In this context, microbiota-associated mediators are considered to act on specific receptors, thus affecting the enteric nervous system and, subsequently, GI motility. Thus, the pathophysiology of FGIDs is based on alterations of the gut microbiota/gut hormone axis, which have crucial effects on GI motility.
Keywords: Enteric nervous system, Functional gastrointestinal disorders, Gastrointestinal hormones, Irritable bowel syndrome, Microbiome
Introduction
Although the pathogenesis of functional gastrointestinal disorders (FGIDs) is multifactorial, gastrointestinal (GI) dysmotility, and visceral hypersensitivity play a central role.1 Recently, much attention has been focused on gut microbiota, as it has become clear that alterations in the gut microenvironment are significantly involved in the pathophysiology of various diseases such as GI disease, metabolic syndrome, autoimmune disease, and nervous/endocrine system disorders.2 Gut microbiota have a complex influence on metabolism, nutrition and immune function in the host, and therefore disruption or alteration of the microbiota plays a pivotal role in GI inflammatory and/or FGIDs. Gut hormones also have central mediating roles in GI motility, appetite and body energy metabolism via the brain-gut axis, and thus also have pivotal involvement in FGIDs whose characteristic symptoms are closely associated with food intake. The present review provides a broad outline of interactions between the gut microbiota and gut hormone axis in relation to GI motility in the pathophysiology of FGIDs.
Role of Gut Hormones in the Pathophysiology of Functional Gastrointestinal Disorders
The motility of the GI tract is mediated by both neural and hormonal networks. Gut hormones are released from enteroendocrine cells scattered along the GI tract (comprising fewer than 1% of all GI epithelial cells),3 which play prominent roles in the hormonal networks during the interdigestive and postprandial periods. At present, more than 30 gut hormones have been isolated. Interestingly, the gut hormones that function in GI motility also affect appetite and body energy metabolism, suggesting that feeding behavior and GI motility are cooperatively regulated by gut hormone secretion (Table 1).
Table 1.
Profile of Gut Hormones
| Gut hormones | Site of secretion | Endocrine cells | Localization of receptors | Roles in gastrointestinal motility |
|---|---|---|---|---|
| Motilin | Duodenum, jejunum | M-cells | Vagal nerve | Promotes phase III MMC activity |
| CNS | Accelerates gastric contraction | |||
| Ghrelin | Stomach, duodenum, jejunum | X/A-cells | Vagal nerve | Suppresses motilin release |
| CNS | Suppresses phase III MMC activity | |||
| CCK | Duodenum, jejunum | I-cells | Gastrointestine | Triggers gallbladder emptying |
| Gallbladder | Slows gastric emptying | |||
| Vagal nerve | Accelerates small intestinal transit | |||
| Enteric neurons | ||||
| CNS | ||||
| GIP | Duodenum, jejunum | K-cells | Enteric neurons | Reduces phase III MMC activity |
| CNS | Slows small intestinal transit | |||
| GLP-1 | Ileum, colon | L-cells | Enteric neurons | Slows gastric emptying |
| Immune cells | Slows small intestinal transit | |||
| CNS | Inhibits colonic transit | |||
| PYY | Ileum, colon | L-cells | Enteric neurons | Slows gastric emptying |
| CNS | Slows small intestinal transit | |||
| Inhibits colonic transit | ||||
| Serotonin (5-HT) | Whole GI tract | EC cell | Enteric neurons | Accelerates gastric emptying |
| Muscle cells | Accelerates gastric accommodation | |||
| Immune cells | Initiates peristaltic reflex and propulsive motility | |||
| Vagal nerve | Induces slow excitatory postsynaptic potentials | |||
| CNS | Triggers colonic migrating motor complexes |
CCK, cholecystokinin; GIP, glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide; GLP-1, glucagon-like peptide 1; PYY, peptide YY; 5-HT, 5-hydroxytryptamine; GI, gastrointestinal; EC, enterochromaffin; CNS, central nervous system; MMC, migrating motor complex.
Gut Hormones Affecting Interdigestive Motility
Motilin
Motilin, which is produced mainly by M cells in the duodenum during the interdigestive period, promotes phase III activity of the migrating motor complex and gastric contraction. Erythromycin, a motilin receptor agonist, increases gastric emptying and not only alleviates gastroparesis but also mediates blood glucose control in diabetic patients.4 However, clinical use of erythromycin for dysmotility is thought to be difficult, as its continuous use causes dysbiosis of the gut flora.
Ghrelin
In 1999, ghrelin was isolated from the stomach as the endogenous ligand of growth hormone secretagogue receptor 1a.5 Ghrelin is produced and secreted by X/A-like cells in the stomach (especially the fundus)5 and stimulates appetite and food intake.6 In addition, accumulating evidence has clarified that ghrelin stimulates both gastric motility and gastric acid secretion.7,8 Ghrelin stimulates GI motility by acting on not only neuropeptide Y, the preganglionic dorsal vagal complex and vagal afferent neurons, but also intrinsic cholinergic neurons in the GI tract,9–12 and these effects are remarkably abolished by bilateral vagotomy.7
Gut Hormones Affecting Postprandial Motility
Cholecystokinin
Cholecystokinin (CCK) was discovered in jejunal extracts as a gallbladder contraction factor.13 CCK is abundantly synthesized in small-intestinal I-cells and cerebral neurons. In addition, CCK is expressed in various endocrine glands (pituitary, thyroid, pancreatic islets, adrenal, and testis), peripheral nerves and kidney.14 Indeed, CCK plays roles in not only digestive function (pancreatic enzyme secretion and gut motility) but also neurotransmission in the cerebral and peripheral neuron systems.14 In the context of gut motility, it has been reported that exogenous CCK suppresses antral and duodenal motility,15 whereas CCK receptor antagonist accelerates gastric emptying.16 CCK is able to excite mucosal vagal afferent fibers in the stomach and regulate postprandial gastric emptying and satiation largely via the vagal pathway.17–19 On the other hand, small-intestinal transit time is shortened by stimulation with exogenous CCK, and prolonged by CCK receptor antagonist.20 In the colon, CCK administration does not affect human rectal motor function.21
Glucose-dependent insulinotropic polypeptide
Glucose-dependent insulinotropic polypeptide (GIP; also known as gastric inhibitory polypeptide) is produced mainly by K cells in the duodenum22 and stimulates insulin secretion as an in-cretin hormone in a glucose-dependent manner. In addition, GIP is likely to inhibit gastric acid secretion and gastric emptying in animals, whereas these inhibitory effects remain unclear in humans.22 On the other hand, triglyceride disposal and adipose uptake of fatty acids may be important functions of GIP in humans.23
Glucagon-like peptide 1
Glucagon-like peptide 1 (GLP-1) is secreted predominantly from L cells in the ileum and colon and released as an incretin hormone in response to enteral nutrient exposure.24 GLP-1 as well as GIP stimulates insulin secretion and is rapidly degraded by dipeptidylpeptidase-4 (DPP4).25 In this context, not only GLP-1 agonist but also the DPP4 inhibitor was developed as a therapeutic medicine for diabetes. Of note, GLP-1 also plays a role in postprandial GI motility. Studies using endogenous/exogenous GLP-1 and/or DPP4 inhibitors have revealed that GLP-1 slows gastric emptying and intestinal motility.26 Moreover, recent evidence has suggested that GLP-1 inhibits postprandial GI motility through the GLP-1 receptor at myenteric neurons, involving nitrergic and cAMP-dependent mechanisms.27,28
Peptide YY
Peptide YY (PYY) is secreted mainly from L cells in the ileum and colon29 and degraded by DPP4,30 similarly to GLP-1. Furthermore, the function of PYY resembles that of GLP-1; thus, PYY is likely to suppress appetite, slow gastric emptying and inhibit small-intestinal motility.31 Endogenous PYY acts via neuronal Y2 receptors to inhibit colonic transit.32
Serotonin
Serotonin, also termed 5-hydroxytryptamine (5-HT), functions as both a neurotransmitter in the CNS and a local hormone in the GI tract. More than 90% of the body’s 5-HT is synthesized in the gut (approximately 90% of 5-HT originates from enterochromaffin (EC) cells and 10% from enteric neuron cells).33 Tryptophan hydroxylase-1 is the rate-limiting enzyme for biosynthesis of 5-HT, and serotonin reuptake transporter (SERT) terminates the actions of 5-HT by removing it from the interstitial space.34 5-HT has motor function through interaction with neurons within the myenteric and submucosal plexuses, intrinsic and extrinsic sensory neurons, and EC cells. Among the 7 subtypes of serotonin receptors, 5-HT3 and 5-HT4 receptors have been most studied in the context of GI motility.35 5-HT released by mucosal mechanical and chemical stimuli is capable of inducing the mucosal peristaltic reflex, and hence propulsive peristalsis, and also affects the colonic migrating motor complexes.35
Dysregulation of Gut Hormone in Functional Gastrointestinal Disorders
FGIDs are defined by symptom-based diagnostic criteria that combine chronic or recurrent symptoms attributable to the GI tract in the absence of other pathologically based disorders. A number of factors are involved in the pathophysiology of FGIDs, including visceral sensitivity, GI motility, GI mucosal immunity, gut microbiota, and psychosocial stress in brain-gut interaction.1 Gut hormones have been proposed as key mediators of these factors in the brain-gut axis and are indeed involved in the development and/or exacerbation of FGID symptoms (Tables 2 and 3), as described below.
Table 2.
Dysregulation of Gut Hormones in Functional Dyspepsia
| Gut hormone | Published year | Clinical evidences for gut hormone in FD | Reference No. |
|---|---|---|---|
| Motilin | 2000 | ABT-229 (motilin agonist) does not relieve the symptoms in FD patients. | 38 |
| 2005 | Exogenous motilin stimulation inhibits proximal gastric accommodation in FD patients. | 36 | |
| 2008 | Camicinal accelerates gastric emptying (35–60%) in patients with gastroparesis. | 40 | |
| 2016 | Motilin receptor agonist (camicinal; GSK962040) accelerates gastric emptying and increases glucose absorption in feed-intolerant critically ill patients. | 39 | |
| Ghrelin | 2004 | The plasm ghrelin concentration may be decreased in accordance with the progression of gastric atrophy due to H. pylori infection. | 45 |
| 2005 | Plasma acylated ghrelin levels are correlated with symptom score in FD patients. | 43 | |
| 2006 | Ghrelin, a novel appetite-promoting gastrointestinal peptide that also promotes gastric motility or basal acid secretion may be a therapeutic target for FD treatment. | 44 | |
| 2007 | Fasting desacyl and total ghrelin levels are significantly lower in FD patients than in controls, but active ghrelin levels are similar between 2 groups in both fasting and postprandial periods. | 41 | |
| 2008 | Ghrelin treatment tends to increase daily food intake in FD patients. | 46 | |
| 2009 | Acylated ghrelin levels are significantly lower in NERD and PDS patients than in healthy volunteers. | 173 | |
| Abnormally low preprandial ghrelin levels and absence of significant postprandial decrease of ghrelin levels are present in a subset of dysmotility-like FD patients. | 42 | ||
| 2013 | The preproghrelin 3056TT genotype is significantly associated with the acylated ghrelin levels and the feeling of hunger in H. pylori-negative FD patients. | 174 | |
| 2015 | The serum ghrelin level 30 minutes after breakfast is significantly higher in dyspepsia patients than in controls. | 175 | |
| FD-PDS is associated with lower fasting and maximum acyl ghrelin concentrations and dampened acyl ghrelin flux. | 176 | ||
| CCK | 1994 | FD patients with CCK-8 stimulation show stronger symptoms of dyspepsia compared with healthy control. | 48 |
| 2008 | Fasting and postprandial plasma CCK is greater in FD patients. | 47 | |
| 2014 | Following lipid infusion, the mean mucosal CCK concentration is lower in FD patients compared with healthy volunteers. | 177 | |
| GIP and GLP-1 | 2014 | Increased sensitivity to enteral dextrose and lipid infusions is associated with greater plasma GIP and GLP-1 concentrations in FD. | 51 |
| 2016 | GLP-1 concentration is similar in FD patients and controls, but postprandial GLP-1 secretion may correlate with nausea in FD patients. | 52 | |
| PYY | 2008 | Fasting and postprandial PYY are lower in FD patients than in healthy subjects. | 47 |
| 2014 | PYY concentrations in response to dextrose and lipid infusions are higher in FD patients with impaired glucose tolerance. | 51 | |
| Serotonin (5-HT) | 2011 | Serotonin receptor 3A polymorphism HTR3A c.-42T is associated with severe dyspepsia. | 54 |
| Serotonin transporter gene polymorphism may be associated with functional dyspepsia in a Japanese population. | 55 | ||
| 2013 | Patients with FD have lower basal and postprandial plasma levels of serotonin. | 56 |
FD, functional dyspepsia; H. pylori, Helicobacter pylori; NERD, non-erosive reflux disease; PDS, postprandial distress syndrome; CCK, cholecystokinin; GIP, glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide; GLP-1, glucagon-like peptide 1; PYY, peptide YY; 5-HT, 5-hydroxytryptamine.
Table 3.
Dysregulation of Gut Hormones in Irritable Bowel Syndrome
| Gut hormone | Published year | Clinical evidences for gut hormone in IBS | Reference No. |
|---|---|---|---|
| Motilin | 1985 | Circulating motilin is positively correlated with symptoms in functional bowel disorders. | 59 |
| 1996 | The IBS patients have reduced motilin secretion after both water intake and the fat meal. | 60 | |
| 2005 | Higher motilin levels are observed in IBS in both interdigestive and postprandial periods. | 61 | |
| Ghrelin | 2009 | The number of ghrelin-positive cells is increased in IBS-D patients. | 66 |
| The low densities of ghrelin cell is found in IBS-C patients. | 66 | ||
| CCK | 2006 | IBS patients have increased fasting and postprandial plasma levels of CCK | 178 |
| 2010 | Post-infectious IBS patients have increased numbers of CCK cells in the duodenum. | 70 | |
| 2015 | The densities of duodenal CCK cells are significantly lower in patients with IBS-D. | 69 | |
| GIP | 2015 | The GIP cell density is significantly reduced in IBS-C. | 69 |
| GLP-1 | 2009 | GLP-1 analog (ROSE-010) relieves acute pain attacks in IBS patients. | 76 |
| 2012 | GLP-1 analog (ROSE-010) delays gastric emptying of solids in IBS-C patients. | 75 | |
| 2014 | Exogenous glucagon-like peptide 1 reduces contractions in human colon circular muscle. | 72 | |
| 2017 | Decreased serum GLP-1 correlates with abdominal pain in patients with IBS-C | 71 | |
| PYY | 2010 | The increased PYY is observed in IBS-C patients whose colonic transit is delayed. | 80 |
| 2014 | The expression of PYY is increased in the ileum in patients with IBS-C. | 79 | |
| The densities of PYY cells is significantly lower in IBS patients than controls. | 81 | ||
| PYY expression is higher in the colon in post-infectious IBS. | 82 | ||
| 2017 | PYY cell density is increased in IBS-C relative to controls. | 94 | |
| Serotonin (5-HT) | 2003 | Plasma serotonin levels is increased in IBS-D. | 91 |
| 2006 | Postprandial plasma serotonin level is decreased in IBS-C. | 90 | |
| 2007 | IBS patients have elevated concentrations of platelet depleted plasma 5-HT under fasting and fed conditions compared with controls. | 87 | |
| 2009 | Fasting and postprandial plasma 5-HT concentrations are significantly higher in IBS patients. | 86 | |
| 2010 | In the IBS samples, higher 5-HT content and lower SERT mRNA are detected as compared with controls. | 179 | |
| Post-infectious IBS patients have significantly lower plasma 5-HIAA. | 70 | ||
| 2011 | Compared with healthy controls, patients with IBS show a significant increase in 5-HT-positive cell counts and 5-HT release. | 92 | |
| 2012 | The frequency of SLC6A4-polymorphism and higher levels of 5-HT are significantly associated with IBS | 180 | |
| Serotonin and PYY cell densities are reduced in the colon of IBS patients. | 181 | ||
| 2014 | The intensity of serotonin transporter immunoreactivity is increased in the ileum of patients with IBS. | 182 | |
| The density of the serotonin-immunoreactive cells is significantly decreased in the IBS-M patients and increased in the IBS-C patients relative to the controls. | 93 | ||
| 2016 | The 5-HIAA concentrations and 5-HT acetic acid/5-HT ratio are significantly lower in IBS compared to HC. | 88 | |
| 2017 | The densities of serotonin cells are reduced in IBS patients. | 94 | |
| IBS patients show increased 5-HT compared to healthy volunteers. | 85 |
IBS, irritable bowel syndrome; IBS-D, irritable bowel syndrome with diarrhea; IBS-C, irritable bowel syndrome with constipation; CCK, cholecystokinin; GIP, glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide; GLP-1, glucagon-like peptide 1; PYY, peptide YY; 5-HT, 5-hydroxytryptamine; SERT, serotonin reuptake transporter; 5-HIAA, 5-hydroxyindole acetic acid.
Functional dyspepsia
Functional dyspepsia (FD) is a heterogeneous disorder associated with abnormalities of gut motor function, including initially accelerated or delayed gastric emptying, impaired proximal gastric relaxation, increased perception of gastric distension, and disordered antro-duodenal motility.36
Motilin and ghrelin, which may accelerate gastric emptying, are potential pathogenetic factors targets for clinical management of FD. For instance, although fasting motilin levels in FD patients do not differ from those in healthy subjects, exogenous motilin stimulation produces greater inhibition of proximal gastric accommodation in FD patients.36 In connection with gastric emptying, mitemcinal (a motilin receptor agonist) and ABT-229 (a motilin agonist) have been administered to patients with gastroparesis or FD, but it is still unclear whether they relieve symptoms such as abdominal satiety and pain in patients with FD.37,38 Another motilin receptor agonist (camicinal; GSK962040) has been developed and used in a phase II trial for critically ill patients with food intolerance.39 Camicinal has been shown to accelerate gastric emptying by 35–60% in patients with gastroparesis,40 and therefore further clinical studies of camicinal may be justified in patients with FD.
As for ghrelin, a few studies have reported that the level of ghrelin in plasma is decreased in FD patients,41,42 whereas others have indicated that it is elevated in such patients and related to the severity of their symptoms,43,44 and thus opinion is still divided. The plasma ghrelin concentration may decrease in accordance with the progression of gastric atrophy due to Helicobacter pylori infection.45 In addition to gastric atrophy, obesity and stress also affect the plasma ghrelin level, thus complicating our understanding of how ghrelin is involved in the pathophysiology of FD. Ghrelin may be a potentially promising therapeutic agent for FD, and Akamizu et al46 have reported that ghrelin administration improves appetite in affected patients. However, as their study was preliminary and did not include a placebo group, further large scale clinical studies including FD symptoms and GI motility assessments will be needed.
In patients with FD, both fasting and postprandial plasma CCK concentrations are higher. Interestingly, intake of a high-fat diet increases the CCK level significantly and is related to the severity of nausea, suggesting that fat diet-associated CCK is involved in the development of FD symptoms.47 Furthermore, Chua et al48 have reported that FD patients stimulated with CCK-8 showed more severe symptoms of dyspepsia than healthy controls, suggesting that FD patients are hypersensitive to CCK stimulation. Also, as CCK promotes serotonin secretion in the hypothalamus,49 FD patients likely have central nervous system hypersensitivity to serotonin.50 Thus, postprandial CCK may affect serotonin signaling in the central nervous system in FD patients and participate in the development of their symptoms.
The hormone incretin plays a role in not only postprandial glucose metabolism but also GI motility, strongly suggesting significant involvement of incretin in the food-intake-associated pathophysiology of FD. Although the fasting plasma GIP and GLP-1 concentrations do not differ between FD patients and healthy controls, FD patients show hypersensitive responses to lipid infusion into the duodenum.51 Moreover, FD patients with severe symptoms show higher GIP and GLP-1 levels in response to lipid stimulation, supporting the contention that incretin mediates increased intestinal sensitivity to nutrients in FD. Witte et al52 have also reported that although the GLP-1 concentration is altered in FD, postprandial GLP-1 secretion correlates with nausea in affected patients. GIP and GLP-1 may be important targets for the treatment of not only diabetes/metabolic syndrome but also FD, and therefore further clinical studies should be encouraged.
PYY as well as GLP-1 is known to act as an “ileal brake” by suppressing GI motility, implying its pathophysiologic involvement in FD. In this connection, it is tempting to speculate that plasma PYY might be increased in FD patients. However, Pilichiewicz et al47 have reported that both the fasting and postprandial PYY levels are lower in FD patients than in healthy subjects, and Bharucha et al51 have found no difference between the two. Thus, although plasma PYY is not increased in patients with FD, this issue requires further investigation.
Data on 5-HT abnormalities are relatively fewer for FD than for IBS patients. It has been reported that 5-HT4 receptor agonists may improve symptoms of dyspepsia, particularly in patients with delayed gastric emptying.53 Previous studies have shown that 5-HT receptor 3A polymorphism54 and SERT gene polymorphism are associated with dyspeptic symptoms.55 Cheung et al56 recently showed that low levels of baseline and postprandial 5-HT are associated with early satiation, lower calorie intake, and more severe postprandial dyspeptic symptoms in FD patients.
Irritable bowel syndrome
Irritable bowel syndrome (IBS) is characterized by symptoms such as abdominal pain or discomfort, bloating, and stool irregularities, without any structural or organic lesions.57 Many factors are involved in the pathogenesis of IBS, and indeed gut hormones are key players, as described below.
The levels of motilin reported in IBS patients have been conflicting, various studies indicating that they are higher,58 similar to,59 or reduced60 in comparison with healthy controls. Although dysmotility and visceral hypersensitivity are thought to play a crucial role in the pathophysiology of IBS, a high level of motilin may not reflect alteration of GI motility in IBS patients.61 In addition, exogenous motilin does not affect rectal sensation, at least in healthy volunteers.62 These findings suggest that alterations of the motilin level may be a consequence rather than a cause of IBS. The effect of erythromycin (a motilin receptor agonist) on colonic motility is also controversial; some studies have demonstrated that erythromycin accelerates the intestinal and/or colonic transit time,63,64 whereas others have not confirmed this.64,65
It is interesting that the ghrelin cell density in the gastric oxyntic mucosa is altered in patients with IBS. On the other hand, since IBS and FD frequently overlap, it is not unlikely that endocrine cells may be altered in the upper GI in IBS. Although evidence is still insufficient, it has been reported that ghrelin cell density is decreased in patients with IBS with constipation (IBS-C).66 This finding may not be surprising because the delay in intestinal transit time can be explained by inhibition of motility-promoting ghrelin. In contrast, ghrelin-positive cells are increased in patients with IBS with diarrhea (IBS-D),66 supporting the fact that such patients show rapid gastric emptying.67 However, gastric emptying in IBS is still controversial.68
El-Salhy M et al69 have shown that the densities of CCK are reduced in the duodenum of IBS-D patients, whereas they are not altered in IBS-C patients. In IBS-D patients, reduction of CCK may be involved in the rapid gastric emptying.67 A subgroup of IBS patients are known to have a history of infectious colitis, and show an increased number of CCK-positive cells.70 Furthermore, the plasma level of CCK is likely increased in the fasting and postprandial periods in IBS patients,70 similarly to the situation in FD patients.47 In contrast to the stomach, CCK stimulates intestinal motility, and therefore high CCK levels may be associated with the diarrhea seen in post-infectious IBS.
The density of duodenal endocrine cells differs between healthy controls and IBS patients. Indeed, GIP cell density is significantly decreased in both IBS-C and IBS-D,69 but other information on GIP in IBS is sparse. Clinical trials of a GLP-1 analog have been performed in IBS patients. Li et al71 have shown that the serum GLP-1 level was significantly decreased in IBS-C patients and negatively correlated with the severity of abdominal pain/discomfort. However, this seems paradoxical because GLP-1 inhibits intestinal motility and reduction of GLP-1 would lead to acceleration of GI motility. In this connection, opinion regarding the action of GLP-1 on colonic motility is still divided, both inhibitory and stimulatory effects having been reported.72–74 In a placebo-controlled trial, Camilleri et al75 showed that a GLP-1 receptor agonist, ROSE-010, improved colonic transit time in IBS-C patients, and Hellström et al76 found that ROSE-010 administration relieved acute pain in patients with IBS. Additionally, the GLP-1 receptor agonist also decreases visceral sensitivity and accelerates colonic transit via the central corticotropin-releasing factor and peripheral vagal pathways, although these findings were obtained in animal experiments.77,78
The expression of PYY is increased in the ileum of patients with IBS-C.79 As PYY inhibits colonic transit, it seems logical that IBS-C patients with delayed colonic transit show increased expression of PYY.80 However, the number of PYY-positive endocrine cells is smaller in the colon and rectum in both IBS-D and IBS-C patients,79,81 and PYY expression is high in the colon of patients with post-infectious IBS.82 These discrepancies may reflect the complicated pathophysiology of IBS. In fact, it is known that IBS patients switch from one subtype to another at least once yearly,83,84 probably as a result of the complex behavior of their enteroendocrine cells.
Data on 5-HT in IBS patients have been conflicting (Table 2). Some studies have reported that in both fasting and postprandial states, the plasma 5-HT level is increased in IBS patients relative to healthy volunteers,85–87 whereas others have found no differences in the plasma 5-HT level between the 2 groups.88 When IBS patients are subdivided, the plasma serotonin level is decreased in those with IBS-C89,90 and increased in those with IBS-D.90,91 A few histological studies have demonstrated that the number of 5-HT-positive cells is increased in IBS (especially post-infectious IBS),89,92 although conflicting data have also been reported.93,94 Genetic analyses of SERT polymorphism have also yielded mixed results.95–97 However, such discrepancies are not surprising because 5-HT is not the only factor operating in the development of IBS. On the other hand, the receptors for 5-HT are widely expressed in various types of cells including neurons and smooth muscle cells,35 and indeed 5-HT signaling is involved in GI motility and the development of IBS-related symptoms. In this context, it is noteworthy that release of 5-HT from the colonic mucosa correlated with the severity of abdominal pain in IBS patients.92
Role of Gut Microbiota in the Pathophysiology of Functional Gastrointestinal Disorders
The human microbiota comprises approximately 1200 different bacterial species whose number (1013–1014) in the gut is 10 times greater than the total number of cells in the human body.2 Gut microbiota exert a marked influence on host physiology including metabolism, nutrition, and immune function, and therefore, its disruption or alteration has been linked with GI inflammatory and functional disease.98 It has become increasingly evident that host-gut microbial interactions have an extremely important role in the pathogenesis of FGIDs (Table 4), especially IBS.99 Recent advances in sequencing technology have revealed that the profile of gut microbiota differs between patients with IBS and healthy subjects, and this has promoted researchers to investigate the role of the gut microbiota in IBS.
Table 4.
Data by Microbiota Analyses in Functional Gastrointestinal Disorders Patients
| Published year | Main findings of microbiota analyses in FGID patients | Reference No. |
|---|---|---|
| Functional dyspepsia | ||
| 2016 | The overall structure of the bacterial community and the abundance of genus Prevotella in the gastric fluid of the FD patients were significantly different from those in the HC. | 123 |
| 2017 | Alteration in the gastric fluid microbiota characterized by Bacteroidetes > Proteobacteria abundance and the absence of Acidobacteria was found in the gastric fluid of patients with FD. | 124 |
| Irritable bowel syndrome | ||
| 1994 | IBS is frequently occurred in patients after Salmonella-induced enteritis. | 125 |
| 2005 | Lower amounts of Lactobacillus spp. were present in the samples of IBS-D patients whereas IBS-C patients carried increased amounts of Veillonella spp. | 183 |
| 2007 | Significant differences between IBS and control were found in several bacterial genera which belong to Coprococcus, Collinsella, and Coprobacillus. | 184 |
| 2009 | The microbial communities of IBS-D patients were enriched in Proteobacteria and Firmicutes, but reduced in the number of Actinobacteria and Bacteroidetes compared to HC. | 185 |
| 2010 | IBS patients showed significantly higher counts of Veillonella and Lactobacillus than controls. | 186 |
| A significant difference between IBS and HC was indicated with significantly more variation in the gut microbiota of healthy volunteers than that of IBS patients. | 187 | |
| Quantitative PCR analysis demonstrated a significant 3.6 fold increase in concentrations of fecal Lactobacillus species between IBS-D patients and HC. | 188 | |
| 2011 | Pseudomonas aeruginosa was significantly more abundant in faeces of IBS patients than in faeces of healthy subjects. | 189 |
| The microbiota of IBS had a 2-fold increased ratio of the Firmicutes to Bacteroidetes compared with controls. | 130 | |
| Microbiomes associated with pediatric IBS were characterized by a significantly greater percentage of the class γ-proteobacteria; one prominent component of this group was Haemophilus parainfluenzae. | 190 | |
| The biodiversity of microbes within fecal samples from IBS-D patients is lower (1.2-fold) than that from HC. | 191 | |
| 2012 | IBS microbiota showed an increase in relative abundance of lactobacilli, B. cereus and B. clausii, bifidobacteria, Clostridium cluster IX and E. rectale, and a decrease in abundance of Bacteroides/Prevotella group and Veillonella genus. | 192 |
| IBS-D patients had significantly higher levels of Enterobacteriaceae and lower levels of Fecalibacterium genera compared to HC. | 193 | |
| An increased Firmicutes:Bacteroidetes ratio best characterises those IBS subjects who differ from normal populations. | 194 | |
| Bifidobacteria were lower in the IBS-D group than in the IBS-C group and controls. The maximum number of stools per day negatively correlated with the number of mucosa-associated Bifidobacteria and Lactobacilli in IBS. | 195 | |
| 2013 | The sensitivity to colonic distension of IBS patients can be transferred to rats by the fecal microbiota. | 131 |
| 2014 | Several members of Bacteroidetes phylum were increased 12-fold in PI-IBS patients, while HC had 35-fold more uncultured Clostridia. | 196 |
| 2015 | Differences in the mucosal-associated microbiota between healthy individuals and IBS patients are minimal (one bacterial group) compared to differences in the faecal microbiota of both groups (53 bacterial groups). | 197 |
| Validation confirms dysbiosis was detected in 73% of IBS patients, 70% of treatment-naïve IBD patients and 80% of IBD patients in remission, vs. 16% of healthy individuals. | 198 | |
| 16S rDNA sequencing confirmed microbial overgrowth and its diversity-reduction in the small bowel of IBS patients. | 199 | |
| Number of Bacteroides thetaiotamicron and Pseudomonas aeruginosa were higher among IBS patients. Number of Bacteroides thetaiotamicron and segmented filamentous bacteria (SFB) was higher among IBS-D than IBS-C. Abdominal distension was associated with higher number of Bacteroides thetaiotamicron, Clostridium coccoides, P. aeruginosa, SFB, and Gram-negative bacteria (GNB); bloating was associated with Clostridium coccoides and GNB. | 200 | |
| Fecal microbiota composition of PI-IBS patients differed significantly from both general IBS patients and HC. Both mucosal and fecal microbial diversity were reduced in PI-IBS compared to HC. | 201 | |
| 2016 | Higher abundance of colonic Veillonellaceae and small intestinal Prevotellaceae, and lower amount of oral cavity normal flora in proximal small intestine were found in IBS patients. | 202 |
| Bacteroidetes was predominant in fecal samples from HC and IBS-D and IBS-M subjects, whereas Firmicutes was predominant in samples from IBS-C subjects. Species richness, but not community diversity, differentiated all IBS patients from HC. | 203 | |
| In IBS-C patients, Bacteroides, Roseburia-Eubacterium rectale and Bifidobacterium were decreased, and Enterobacteriaceae, Desulfovibrio sp., and mainly Akkermansia muciniphila were increased compared to healthy individuals. | 204 | |
| 2017 | Mice receiving the IBS-D fecal microbiota, exhibited faster gastrointestinal transit, intestinal barrier dysfunction, innate immune activation, and anxiety-like behavior. | 205 |
| Down-regulation of bacterial colonization including Lactobacillus, Bifidobacterium, and F. prausnitzii was observed in IBS patients, particularly in IBS-D. | 206 | |
| IBS symptom severity was associated negatively with microbial richness, exhaled CH4, presence of methanogens, and enterotypes enriched with Clostridiales or Prevotella species. | 207 | |
| Compared with healthy controls, the standardized mean differences of Bifidobacteria, Lactobacillus, Escherichia Coli, and Enterobacter were significant in Chinese IBS patients. | 208 | |
| Using linear discriminant analysis effect size method, gut dysbiosis was observed in subjects with IBS (Plesiomonas and Trabulsiella, effect size 3.0). | 209 | |
| 2018 | The fecal microbiota dysbiosis was more prevalent in IBS than in healthy volunteers. | 210 |
| FMT significantly relives the symptom in IBS patients. | 211 | |
| In IBS, Dialister spp. and Faecalibacterium prausnitzii were the most representative species. | 212 | |
FGID, functional gastrointestinal disorders; HC, healthy controls; FD, functional dyspepsia; IBS, irritable bowel syndrome; IBS-D, IBS with diarrhea; IBS-C, IBS with constipation; PCR, polymerase chain reaction; IBS-M, mixed IBS; PI-IBS, post-infectious IBS; FMT, fecal microbiota transplantation.
Gut Microbiota Production of Serotonin and SCFA
Evolutionarily-oriented studies have shown that many enzymes involved in human hormone metabolism have evolved through gene transfer from bacteria.100 Interestingly, serotonin and other hormones such as epinephrine, norepinephrine and dopamine are produced and/or secreted from specific bacterial strains.101 Furthermore, it has been revealed that microorganisms harbor hormone receptors,102 suggesting that gut hormones act as possible mediators of communication between the host and gut microbiota.
Depending on the substrates (amino acids, lipids or carbohydrates) present in the intestinal lumen, gut microbiota can generate specific metabolites. Short-chain fatty acids (SCFAs) are produced by microbiota in the ileum and colon from carbohydrates with low digestibility (resistant starch and soluble oligo- and polysaccharides). The main components of SCFAs in the human colon are acetate (2-carbon), propionate (3-carbon), and butyrate (4-carbon), at a ratio of about 3:1:1.103,104 Butyrate is considered a major energy source for the colonic epithelium. Propionate, entering the portal circle, is primarily utilized for gluconeogenesis in the liver. Consequently, SCFAs in plasma are largely acetate.105,106 Approximately 95% of the produced SCFAs are rapidly absorbed by colonocytes in the large intestine while the remaining 5% are secreted in the stools.107
Short-chain Fatty Acids as a Mediator between Gut Microbiota and Endocrine Cells
SCFAs serve as not only an important energy source but also chemical messengers or signaling molecules for various types of cells. GPR41 and GPR43, which are classified as G protein-coupled receptors and known as free fatty acid receptor (FFAR)3 and FFAR2, respectively, have recently been identified as receptors for SCFAs. Since these receptors are expressed in a variety of cell types, including colonic endocrine L cells, mucosal mast cells, adipose tissue, neutrophils, and monocytes, activation of these receptors may elicit distinct functions in various fields. Both GPR41 and GPR43 exhibit coupling to the Gi/o family and inhibit cAMP production, whereas GPR43, but not GPR41, also couples efficiently through the Gq protein family.108
In the ileum and colon, GPR41 and GPR43 are expressed in enteroendocrine L-cells that secrete GLP-1 and PYY.109 Therefore, it had been expected that SCFA would promote GLP-1 and PYY secretion by activating these GPRs, and indeed several in vivo studies have demonstrated that intraluminal injection of SCFAs induced the release of PYY and GLP-1 into the circulating blood.110 Furthermore, in vitro studies have shown that activation of GPR43 by SCFAs promotes GLP-1 secretion111 and PYY expression112 in enteroendocrine cells. On the other hand, individual GPR41- or GPR43-deficient mice show low GLP-1 release in response to SCFA stimulation, indicating that both GPR41 and GPR43 are involved in SCFA-evoked GLP-1 release.111 These findings strongly suggest that SCFA signaling to GPR41 and GPR43 on L cells is crucial for controlling the levels of GLP-1 and PYY, thus playing a pivotal role in the “ileal brake.” However, in GF mice, Wichmann et al113 have demonstrated that total SCFA in the cecal content is decreased whereas the plasma GLP-1 level is increased. They showed that colonization of GF mice with microbiota or treatment with SCFAs reduced the expression of GLP-1 in the colon.113 Although these findings are difficult to reconcile, the explanation proposed is that GF mice are deprived of SCFAs produced by gut microbiota and serve as an important energy source for colonocytes. Subsequently, in response to this insufficiency of available energy in the colon, the GLP-1 level is increased to slow intestinal transit. In this context, GPRs may act as sensors of the amount of SCFA rather than being receivers of SCFA signaling.
SCFAs may be involved in the production of not only GLP-1 and PYY, but also other gut hormones such as 5-HT, GIP, ghrelin, and CKK. Histological studies have clarified that GPR43 is co-expressed with 5-HT,114 and that SCFAs stimulate the release of 5-HT by EC cells in the colonic mucosa.115 On the other hand, GPR40 and/or GPR120 are co-localized in cells expressing GIP, GLP-1, PYY, CCK, ghrelin, or 5HT,116–118 although their ligands are predominantly medium- to long-chain fatty acids.119 Taken together, the available data suggest that GPRs are key tools involved in the interaction between endocrine cells and fatty acids produced from food and gut microbiota.
Alterations of Gut Microbiota in the Pathophysiology of Functional Gastrointestinal Disorders
The Rome Foundation has recently provided an excellent overview of the importance of microbiota in health and disease, especially functional bowel diseases.99 It is widely accepted that GI infections caused by bacteria, viruses, or parasites are strong risk factors for the development of FGIDs.120 With regard to the association between bacterial infection and FD, almost all current knowledge is based on H. pylori infection. H. pylori causes chronic atrophic gastritis, gastroduodenal ulcers and gastric cancers, suggesting a close involvement in the development of organic disease. In this context, whether or not patients with H. pylori infection should be excluded from the category of FD has been discussed. However, possible pathogenetic concepts to explain the link between H. pylori infection and dyspeptic symptoms have been positively incorporated into the recent Rome IV classification121 and management guidelines for H. pylori infection.122 In response to the recent focus on gut microbiota and FGIDs, several studies have begun to investigate the gastric microbiota in FD patients. For instance, as dysbiosis has been observed in the gastric fluid of patients with FD,123 it has been suggested that normalization of the gastric microbiota by probiotics might be an effective treatment.124 However, studies of the gastric microbiota in FGIDs are still at an early stage.
The link between GI infection and FGIDs has been studied mainly in IBS. In 1994, a group in the United Kingdom reported that IBS frequently occurs in patients after Salmonella-induced enteritis.125 Thereafter, similar observations were reported worldwide, and a recent meta-analysis has clearly shown that IBS occurs frequently after bacterial enteritis caused by pathogenic strains of Escherichia coli, Salmonella, and Campylobacter jejuni.126 Moreover, it is interesting that FD also occurs frequently in patients after bacterial enteritis, being compatible with the fact that IBS and FD often overlap in a single patient.126 After remission of bacterial enteritis, no endoscopic and/or microscopic abnormalities are detectable in the GI tract of such FGID patients. Why, then, do patients with post-infectious IBS show visceral hypersensitivity and GI dysmotility? Accumulating evidence has led to a hypothesis that pathogens disrupt the mucosal barrier and that subsequently mucosal immune cells are persistently activated as a result of increased exposure to luminal antigens.127 This low-grade mucosal inflammation is able to affect the immune system, endocrine cell behavior, and subsequently visceral sensitivity and GI motility. This modification of the gut environment may be central to the development of post-infectious IBS.
As only about 10% of IBS patients have a history of infectious colitis,128 what is the role of gut microbiota in IBS patients without such a history? Recent advances in sequencing technology have made it easier to obtain information on gut microbiota at the genus level. Comprehensive analyses of fecal samples have demonstrated that the gut microbiota profile in IBS patients is largely different from that in healthy subjects, and has reduced diversity.99,129 Specifically, several studies have demonstrated increased Firmicutes to Bacteroidetes ratios at the phylum level in IBS patients.99,129,130 At the genus level, Lactobacillus, Bifidobacterium and uncultured Clostridium are decreased in patients with IBS, whereas Ruminococcus species are enriched.130 Although it seems impossible to determine which bacterial strain is responsible for the development of IBS, dysbiosis of the microbiota is definitely a contributing factor. This is supported by experimental evidence that germ-free (GF) animals given microbiota transplants from IBS patients show visceral hypersensitivity and GI dysmotility.131,132 On the other hand, although stress events in early life are closely associated with IBS development, the gut microbiota profile may be possibly modified by such events.133,134 Moreover, antibiotic-induced dysbiosis (especially in childhood) has also been shown to be involved in the development of IBS.133,135 These findings suggest that events in early life may be crucial in determining the IBS-related profile of gut microbiota. This invites speculation that manipulation of the gut microbiota may be useful for the treatment of IBS. Interestingly, the antibiotic rifaximin has been demonstrated to relieve the symptoms of IBS,136 although the mechanism responsible remains unclear. A meta-analysis has also indicated that probiotics may reduce IBS symptoms, although there was significant heterogeneity among the studies investigated,129,137,138 and the mechanism through which such probiotics act remains to be elucidated.
Possible Axis of Gut Microbiota and Enteroendocrine System Interaction in Functional Gastrointestinal Disorders Dysmotility
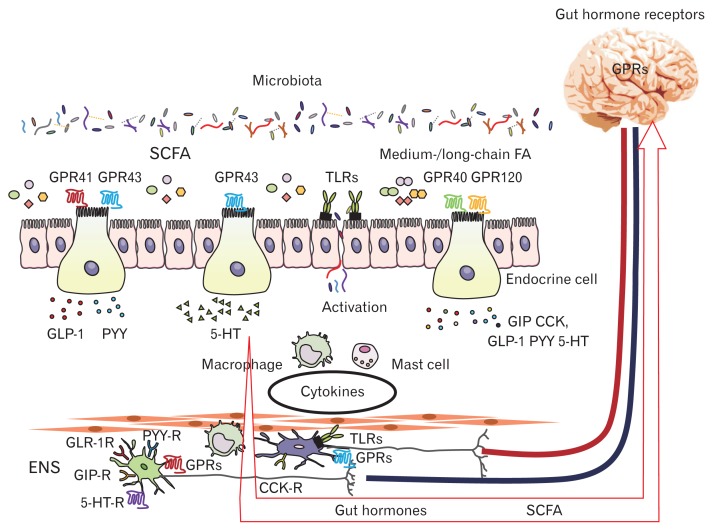
FGIDs are diagnosed on the basis of characteristic symptoms that are closely associated with food intake. The pathogenesis of FGIDs is multifactorial, and includes the neuroendocrine system, gut microbiota, neuroimmune reactions, interactions, psychological factors, and dietary factors.129 The complexity of FGID pathophysiology may be due to the fact that through their interaction, these factors may become a cause and/or a consequence of the pathophysiology. The physiological alterations in FGIDs are also complicated, but there is little doubt that GI dysmotility and visceral hypersensitivity are critical. Here, we would like to focus on the interrelationships existing among gut microbiota, gut hormones and GI dysmotility in FGIDs (Figure).
Figure.
Interaction among gut microbiota, enteroendocrine cells, immune cells, and enteric nervous system (ENS). SCFA, short-chain fatty acid; FA, fatty acid; GPR, G protein-coupled receptor; TLR, toll-like receptor; GLP-1, glucagon-like peptide 1; PYY, peptide YY; 5-HT, 5-hydroxytryptamine; CCK, cholecystokinin; GIP, glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide; -R, receptor.
Enteric Nervous System Receptors
The enteric nervous system (ENS) in the GI tract comprises a network of 200–600 million neurons and is involved in multiple aspects of host physiology including motility, metabolism, and behavior. This neural network is arranged in distinct units between the longitudinal and circular muscle layers of the intestine or in the submucosa as ganglionated plexi.139 Gut microbiota may interact with the ENS directly or via afferent nerves (vagal sensory neurons, spinal sensory neurons and intrinsic primary afferent neurons) and the CNS indirectly through neurotransmitters. The link between microbiota and the ENS has been demonstrated in GF mice, which show a reduced number of enteric neurons and associated deficits of gut motility,140 whereas reconstitution of GF mice with conventional microbiota normalizes the density of the ENS network and, subsequently, gut physiology.141
How, then, does the gut microbiota affect the ENS? Importantly, the ENS expresses pattern recognition receptors known as Toll-like receptors (TLRs) by which the microbiota communicate with the host.142 Anitha et al140 have demonstrated that TLR4-deficient mice show a significant decrease of both the ENS network and GI transit time, and similar findings have been observed in TLR2-deficient mice.143 Another important ENS receptor type are the GPRs such as GPR41 and GPR43, which respond to SCFA signaling. Since the gut microbiota produce SCFAs, they can use them as chemical messengers or signaling molecules to communicate with the host ENS. At present, although little is known about a molecular alteration of the ENS by SCFA stimulation, treatment with butyrate enhances histone H3 acetylation in enteric neurons and increases the contraction of cholinergic-mediated colonic circular muscle.144
Gut Microbiota, Immune System Activation, and Gastrointestinal Motility
Not only the direct but also the indirect action of gut micro-biota on the ENS is important, and this interaction is complicated. Microbiota-derived SCFAs play roles in the maintenance of intestinal barrier function,145,146 and indeed, butyrate, a microbiota-derived SCFA, prevents bacterial translocation by increasing the expression of tight junction proteins such as claudin, occludin, and the zonula occludens.147 In this context, it is interesting to note that in IBS patients butyrate-producing bacteria may be reduced148 and intestinal permeability is increased.149,150 Thus, leakage of pathogens into the lamina propria of the intestinal mucosa is a significant trigger for activation of the mucosal immune system. In IBS patients, increased amounts of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) and decreased amounts of the anti-inflammatory IL-10 have been reported.151 Th1 cytokines such as TNFα, IL-1β, and IL-6 may act on the ENS and smooth muscle, resulting in suppression of GI motility.152,153 On the other hand, Th2 cytokines including IL-10 may inhibit the expression of the above cytokines, thus accelerating of GI motility.152,154 In IBS, it is still unclear whether these cytokines simply show this pattern of interaction. However, imbalance of not only gut microbiota but also the cytokine profile must be associated with intestinal low-grade inflammation, which is a significant pathophysiologic alteration in IBS.
Among the various immune cells, mast cells have been highlighted in the pathophysiology of IBS as their numbers are increased in colonic tissues of affected patients, and this increase is correlated with the severity of the clinical symptoms.155,156 Mast cells are able to release histamine, serotonin, tryptase, and prostaglandins, and these mediators may act on their specific receptors on myenteric neural cells, leading to altered motor function.155 Recent evidence has emphasized the pivotal roles of macrophages in GI motility through their action on myenteric neural cells,152,153,157,158 and indeed increased infiltration of macrophages into colonic tissues is observed in IBS patients,156,159 implying that macrophages, like mast cells, may affect the ENS and smooth muscle through various mediators.152,153,157,158 Recently, macrophages have been classified into the M1 and M2 types that produce mainly Th1 and Th2 cytokines, respectively.160,161 Thus, M1 macrophages release proinflammatory cytokines such as TNFα, IL-1β, and IL-6, whereas M2 macrophages may suppress M1 macrophages by releasing anti-inflammatory cytokines. Interestingly, M2 macrophages are known to infiltrate into the muscle layer of the intestine and may accelerate GI motility through stimulation with Th2 cytokines.155 Furthermore, it has been reported that serotonin plays a role in polarization of macrophages toward an M2 phenotype.160,162 Supporting these findings, we have clarified that 5-HT expression is increased in the colon of GF mice after fecal transplantation, and that moreover M2 macrophages in the colonic muscular layer are increased in those mice.163 Furthermore, it is noteworthy that the numbers of muscularis M2 macrophages and 5-HT-positive endocrine cells are significantly correlated throughout the GI tract, and that their increase is associated with acceleration of GI motility. Thus, the gut microbiota plays a role in the association between accelerated GI motility and induction of the 5 HT/muscularis mannose receptor positive macrophage axis in the GI tract.163 Specifically, Muller et al164 have demonstrated that M2 macrophages migrating adjacent to the ENS may be involved in the control of GI motility through cross-talk with enteric neurons via bone morphogenetic protein 2 signaling.
Gut Microbiota and Gastrointestinal Motility-associated Hormones
The gut microbiota is able to affect GI motility via gut hormones. As shown in Tables 2 and 3, many investigators have intensively studied the gut microbiota and 5-HT in patients with IBS. However, to clarify the role of gut microbiota in the gut hormone/GI motility axis in IBS, experimental animal studies are needed. In particular, much evidence has been obtained from experiments using GF animals. For example, Wikoff et al165 first demonstrated that GF mice display lower levels of 5-HT than conventionally raised animals, suggesting that the presence of gut microbiota is essential for the production and release of 5-HT.165 In addition, Kashyap et al166 have clarified that colonization of GF mice with gut micro-biota from humans or mice can significantly shorten the GI transit time and that this effect is partially inhibited by 5-HT receptor antagonist.166 These findings strongly indicate that 5-HT induced by microbiota stimuli play a role in the acceleration of GI motility. Moreover, Yano et al167 have clarified that indigenous spore-forming bacteria from the gut microbiota promote tryptophan hydroxylase 1 expression and 5-HT biosynthesis in EC cells, thus modulating GI motility. Although the mechanism by which microbiota induce 5-HT expression is still unclear, SCFA and cytokines in minimal inflammation may be candidate stimuli for 5-HT-producing EC cells,168 and the microbiota themselves may also produce 5-HT.101 On the other hand, various types of 5-HT receptors are present on not only central and peripheral neural cells but also immune cells, smooth muscle, and enterocytes.168,169 Additionally, the SERT, which terminates the action of 5-HT, is expressed in epithelial cells, neural cells, and platelets.169 Accordingly, it is extremely difficult to determine how the gut microbiota/5-HT axis operates in GI motility and the development of symptoms in FGIDs.
Recently, not only 5-HT but also other gut hormones have been highlighted in relation to the gut microbiota/gut hormone axis in GI motility. As a result of their elegant work, Wichmann et al113 have proposed that the microbiota/GLP-1 axis is important for regulation of energy availability and GI motility. Specifically, enteroendocrine L cells sense the amount of bacteria-producing SCFA in the colon and secrete GLP-1 to allow greater nutrient absorption by inhibiting intestinal motility. Accordingly, it is tempting to speculate that GPR41 and GPR43 may play some roles in this process because these receptors are responsive to SCFA ligands. In support of this hypothesis, in vitro studies have shown that the SCFA affects GLP-1 secretion via GPR43 and/or GPR41 in L cells.111,170 Enteroendocrine cells are significant intermediates in facilitating communication between microbes and the ENS.171 By using GF with fecal transplantation animal models, we have recently demonstrated that gut microbiota accelerate GI motility while suppressing the expression of the GLP-1 receptor in myenteric neural cells throughout the GI tract.172 These findings suggest that the gut microbiota affects the expression of gut hormone receptors on the ENS in the GI muscle layer.
Except for 5-HT and GLP-1, there are few data on the role of the gut microbiota/gut hormone axis in GI motility. As PYY is produced in L cells, it may––like GLP-1––be a sensor for SCFA and inhibit GI motility. Interestingly, GPRs are widely expressed in enteroendocrine cells such as those producing ghrelin, CCK, GIP, PYY, or GLP-1.170 Moreover, since enteric neurons harbor the receptors for all of these gut hormones, a mechanism similar to that for GLP-1 or 5-HT may exist in the microbiota/gut hormone axis to mediate GI motility.
Summary and Conclusions
Gut microbiota have a pivotal influence on various functions including GI motility, metabolism, nutrition, immunity, and the neuroendocrine system in the host. This variety of bacterial actions is due to the production of SCFA and various mediators such as gut hormones and/or cytokines, which gut microbiota induce in enteroendocrine and immune cells. Moreover, the receptors for SCFA and gut hormones are widely distributed in neural cells, endocrine cells, immune cells and smooth muscle, further making it difficult to understand the role of gut microbiota in the pathophysiology of FGIDs. GI motility is well orchestrated by the ENS and hormonal networks, and its disturbance is closely associated with not only GI dysmotility but also visceral hypersensitivity and energy imbalance, that are significant abnormalities in the pathophysiology of FGIDs. In this article, we have mentioned that the gut microbiota may affect the ENS directly via the SCFA, or indirectly via gut hormones. Furthermore, we have described that gut microbiota elicit minimal inflammation by activation of the immune system and that mast cells and macrophages modify GI motility by acting on the ENS and smooth muscle. In this context, alteration of the gut microbiota/gut hormone axis significantly affects GI motility in the pathophysiology of FGIDs.
Although we have focused on GI motility in FGIDs, both the gut microbiota and gut hormones also play pivotal roles in metabolism, the brain-gut axis, and systemic immunity. Interestingly, the gut microbiota and gut hormones have been highlighted as targets of new therapeutic approaches for FGIDs, and indeed some trials using probiotics, antibiotics or gut hormone agonist/antagonists have been undertaken. Also, fecal microbiota transplantation, as applied to Clostridium difficile infection, may be attempted for FGIDs in the future. The research field focusing on interactions between gut microbiota and gut hormones may be referred to as “microbial endocrinology,” and is expected to become a focus of intense interest in the near future.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Hirokazu Fukui and Xin Xu drafted the manuscript and constructed Tables and a Figure; and Hiroto Miwa coordinated the study and performed critical revision of the manuscript.
References
- 1.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150:1262–1279. e2. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 3.Rehfeld JF. Beginnings: a reflection on the history of gastrointestinal endocrinology. Regul Pept. 2012;177(suppl):S1–S5. doi: 10.1016/j.regpep.2012.05.087. [DOI] [PubMed] [Google Scholar]
- 4.Peeters TL. New motilin agonists: a long and winding road. Neurogastroenterol Motil. 2006;18:1–5. doi: 10.1111/j.1365-2982.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 5.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 6.Müller TD, Nogueiras R, Andermann ML, et al. Ghrelin. Mol Metab. 2015;4:437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuda Y, Tanaka T, Inomata N, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 8.Tack J, Depoortere I, Bisschops R, et al. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–333. doi: 10.1136/gut.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550(pt 1):227–240. doi: 10.1113/jphysiol.2003.040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyano Y, Sakata I, Kuroda K, et al. The role of the vagus nerve in the migrating motor complex and ghrelin- and motilin-induced gastric contraction in suncus. PLoS One. 2013;8:e64777. doi: 10.1371/journal.pone.0064777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swartz EM, Browning KN, Travagli RA, Holmes GM. Ghrelin increases vagally mediated gastric activity by central sites of action. Neuro-gastroenterol Motil. 2014;26:272–282. doi: 10.1111/nmo.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edholm T, Levin F, Hellström PM, Schmidt PT. Ghrelin stimulates motility in the small intestine of rats through intrinsic cholinergic neurons. Regul Pept. 2004;121:25–30. doi: 10.1016/j.regpep.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Ivy AC, Oldberg E. A hormone mechanism for gallbladder contraction and evacuation. Am J Physiol. 1928;86:559–613. [Google Scholar]
- 14.Rehfeld JF. Cholecystokinin-from local gut hormone to ubiquitous messenger. Front Endocrinol. 2017;8:47. doi: 10.3389/fendo.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennan IM, Little TJ, Feltrin KL, et al. Dose-dependent effects of cholecystokinin-8 on antropyloroduodenal motility, gastrointestinal hormones, appetite, and energy intake in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E1487–E1494. doi: 10.1152/ajpendo.90791.2008. [DOI] [PubMed] [Google Scholar]
- 16.Little TJ, Gopinath A, Patel E, et al. Gastric emptying of hexose sugars: role of osmolality, molecular structure and the CCK receptor. Neurogastroenterol Motil. 2010;22:1183–1190. e314. doi: 10.1111/j.1365-2982.2010.01552.x. [DOI] [PubMed] [Google Scholar]
- 17.Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249(5 pt 2):R638–R641. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- 18.Forster ER, Green T, Elliot M, Bremner A, Dockray GJ. Gastric emptying in rats: role of afferent neurons and cholecystokinin. Am J Physiol. 1990;258(4 pt 1):G552–G556. doi: 10.1152/ajpgi.1990.258.4.G552. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Owyang C. Endogenous cholecystokinin stimulates pancreatic enzyme secretion via vagal afferent pathway in rats. Gastroenterology. 1994;107:525–531. doi: 10.1016/0016-5085(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 20.Lin HC, Zaidel O, Hum S. Intestinal transit of fat depends on accelerating effect of cholecystokinin and slowing effect of an opioid pathway. Dig Dis Sci. 2002;47:2217–2221. doi: 10.1023/A:1020179009559. [DOI] [PubMed] [Google Scholar]
- 21.van der Schaar PJ, van Hoboken E, Ludidi S, Masclee AA. Effect of cholecystokinin on rectal motor and sensory function in patients with irritable bowel syndrome and healthy controls. Colorectal Dis. 2013;15:e29–e34. doi: 10.1111/codi.12034. [DOI] [PubMed] [Google Scholar]
- 22.McIntosh CH, Widenmaier S, Kim SJ. Glucose-dependent insulinotropic polypeptide (gastric inhibitory polypeptide; GIP) Vitam Horm. 2009;80:409–471. doi: 10.1016/S0083-6729(08)00615-8. [DOI] [PubMed] [Google Scholar]
- 23.Morgan LM. The metabolic role of GIP: physiology and pathology. Biochem Soc Trans. 1996;24:585–591. doi: 10.1042/bst0240585. [DOI] [PubMed] [Google Scholar]
- 24.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 25.Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 26.Marathe CS, Rayner CK, Jones KL, Horowitz M. Effects of GLP-1 and incretin-based therapies on gastrointestinal motor function. Exp Diabetes Res. 2011;2011:279530. doi: 10.1155/2011/279530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amato A, Cinci L, Rotondo A, et al. Peripheral motor action of glucagon-like peptide-1 through enteric neuronal receptors. Neurogastroenterol Motil. 2010;22:664–e203. doi: 10.1111/j.1365-2982.2010.01476.x. [DOI] [PubMed] [Google Scholar]
- 28.Halim MA, Degerblad M, Sundbom M, et al. Glucagon-like peptide-1 inhibits prandial gastrointestinal motility through myenteric neuronal mechanisms in humans. J Clin Endocrinol Metab. 2018;103:575–585. doi: 10.1210/jc.2017-02006. [DOI] [PubMed] [Google Scholar]
- 29.Spreckley E, Murphy KG. The L-Cell in nutritional sensing and the regulation of appetite. Front Nutr. 2015;2:23. doi: 10.3389/fnut.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witte AB, Grybäck P, Holst JJ, et al. Differential effect of PYY1-36 and PYY3-36 on gastric emptying in man. Regul Pept. 2009;158:57–62. doi: 10.1016/j.regpep.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Sanger GJ, Lee K. Hormones of the gut-brain axis as targets for the treatment of upper gastrointestinal disorders. Nat Rev Drug Discov. 2008;7:241–254. doi: 10.1038/nrd2444. [DOI] [PubMed] [Google Scholar]
- 32.Tough IR, Forbes S, Tolhurst R, et al. Endogenous peptide YY and neuropeptide Y inhibit colonic ion transport, contractility and transit differentially via Y1 and Y2 receptors. Br J Pharmacol. 2011;164:471–484. doi: 10.1111/j.1476-5381.2011.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 34.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kendig DM, Grider JR. Serotonin and colonic motility. Neurogastroenterol Motil. 2015;27:899–905. doi: 10.1111/nmo.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tack J, Lee KJ. Pathophysiology and treatment of functional dyspepsia. J Clin Gastroenterol. 2005;39(5 suppl 3):S211–S216. doi: 10.1097/01.mcg.0000156109.97999.d1. [DOI] [PubMed] [Google Scholar]
- 37.McCallum RW, Cynshi O Investigative Team. Clinical trial: effect of mitemcinal (a motilin agonist) on gastric emptying in patients with gastroparesis – a randomized, multicentre, placebo-controlled study. Aliment Pharmacol Ther. 2007;26:1121–1130. doi: 10.1111/j.1365-2036.2007.03461.x. [DOI] [PubMed] [Google Scholar]
- 38.Talley NJ, Verlinden M, Snape W, et al. Failure of a motilin receptor antagonist (ABT-229) to relieve the symptoms of functional dyspepsia in patients with and without delayed gastric emptying: a randomized double-blind placebo-controlled trial. Aliment Pharmacol Ther. 2000;14:1653–1661. doi: 10.1046/j.1365-2036.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 39.Chapman MJ, Deane AM, O’Connor SL, et al. The effect of camicinal (GSK962040), a motilin agonist, on gastric emptying and glucose absorption in feed-intolerant critically ill patients: a randomized, blinded, placebo-controlled, clinical trial. Crit Care. 2016;20:232. doi: 10.1186/s13054-016-1420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desachy A, Clavel M, Vuagnat A, Normand S, Gissot V, François B. Initial efficacy and tolerability of early enteral nutrition with immediate or gradual introduction in intubated patients. Intensive Care Med. 2008;34:1054–1059. doi: 10.1007/s00134-007-0983-6. [DOI] [PubMed] [Google Scholar]
- 41.Takamori K, Mizuta Y, Takeshima F, et al. Relation among plasma ghrelin level, gastric emptying, and psychologic condition in patients with functional dyspepsia. J Clin Gastroenterol. 2007;41:477–483. doi: 10.1097/01.mcg.0000225614.94470.47. [DOI] [PubMed] [Google Scholar]
- 42.Lee KJ, Cha DY, Cheon SJ, Yeo M, Cho SW. Plasma ghrelin levels and their relationship with gastric emptying in patients with dysmotility-like functional dyspepsia. Digestion. 2009;80:58–63. doi: 10.1159/000215389. [DOI] [PubMed] [Google Scholar]
- 43.Shinomiya T, Fukunaga M, Akamizu T, et al. Plasma acylated ghrelin levels correlate with subjective symptoms of functional dyspepsia in female patients. Scand J Gastroenterol. 2005;40:648–653. doi: 10.1080/00365520510015403. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki H, Nishizawa T, Hibi T. Therapeutic strategies for functional dyspepsia and the introduction of the Rome III classification. J Gastroenterol. 2006;41:513–523. doi: 10.1007/s00535-006-1847-5. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki H, Masaoka T, Hosoda H, et al. Helicobacter pylori infection modifies gastric and plasma ghrelin dynamics in Mongolian gerbils. Gut. 2004;53:187–194. doi: 10.1136/gut.2003.021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akamizu T, Iwakura H, Ariyasu H, et al. Repeated administration of ghrelin to patients with functional dyspepsia: its effects on food intake and appetite. Eur J Endocrinol. 2008;158:491–498. doi: 10.1530/EJE-07-0768. [DOI] [PubMed] [Google Scholar]
- 47.Pilichiewicz AN, Feltrin KL, Horowitz M, et al. Functional dyspepsia is associated with a greater symptomatic response to fat but not carbohydrate, increased fasting and postprandial CCK, and diminished PYY. Am J Gastroenterol. 2008;103:2613–2623. doi: 10.1111/j.1572-0241.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- 48.Chua AS, Dinan TG, Rovati LC, Keeling PW. Cholecystokinin hyper-responsiveness in dysmotilitytype nonulcer dyspepsia. Ann N Y Acad Sci. 1994;713:298–299. doi: 10.1111/j.1749-6632.1994.tb44077.x. [DOI] [PubMed] [Google Scholar]
- 49.Kendrick K, Leng G, Higuchi T. Noradrenaline, dopamine and serotonin release in the paraventricular and supraoptic nuclei of the rat in response to intravenous cholecystokinin injections. J Neuroendocrinol. 1991;3:139–144. doi: 10.1111/j.1365-2826.1991.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 50.Chua AS, Keeling PW, Dinan TG. Role of cholecystokinin and central serotonergic receptors in functional dyspepsia. World J Gastroenterol. 2006;12:1329–1335. doi: 10.3748/wjg.v12.i9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bharucha AE, Camilleri M, Burton DD, et al. Increased nutrient sensitivity and plasma concentrations of enteral hormones during duodenal nutrient infusion in functional dyspepsia. Am J Gastroenterol. 2014;109:1910–1920. doi: 10.1038/ajg.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witte AB, Hilsted L, Holst JJ, Schmidt PT. Peptide YY3-36 and glucagon-like peptide-1 in functional dyspepsia. Secretion and role in symptom generation. Scand J Gastroenterol. 2016;51:400–409. doi: 10.3109/00365521.2015.1101780. [DOI] [PubMed] [Google Scholar]
- 53.Serra J. [Levosulpiride in the management of functional dyspepsia and delayed gastric emptying]. Gastroenterol Hepatol. 2010;33:586–590. doi: 10.1016/j.gastrohep.2010.07.002. [Spanish] [DOI] [PubMed] [Google Scholar]
- 54.Mujakovic S, ter Linde JJ, de Wit NJ, et al. Serotonin receptor 3A polymorphism c.−42C > T is associated with severe dyspepsia. BMC Med Genet. 2011;12:140. doi: 10.1186/1471-2350-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toyoshima F, Oshima T, Nakajima S, et al. Serotonin transporter gene polymorphism may be associated with functional dyspepsia in a Japanese population. BMC Med Genet. 2011;12:88. doi: 10.1186/1471-2350-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheung CK, Lee YY, Chan Y, et al. Decreased basal and postprandial plasma serotonin levels in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2013;11:1125–1129. doi: 10.1016/j.cgh.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 57.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 58.Ohe K, Sumii K, Sano K, Kishimoto S, Miyoshi A. Serum motilin in gastrointestinal diseases. Endocrinol Jpn. 1980;27(suppl 1):167–172. doi: 10.1507/endocrj1954.27.Supplement_167. [DOI] [PubMed] [Google Scholar]
- 59.Preston DM, Adrian TE, Christofides ND, Lennard-Jones JE, Bloom SR. Positive correlation between symptoms and circulating motilin, pancreatic polypeptide and gastrin concentrations in functional bowel disorders. Gut. 1985;26:1059–1064. doi: 10.1136/gut.26.10.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sjölund K, Ekman R, Lindgren S, Rehfeld JF. Disturbed motilin and cholecystokinin release in the irritable bowel syndrome. Scand J Gastroenterol. 1996;31:1110–1114. doi: 10.3109/00365529609036895. [DOI] [PubMed] [Google Scholar]
- 61.Simrén M, Björnsson ES, Abrahamsson H. High interdigestive and postprandial motilin levels in patients with the irritable bowel syndrome. Neurogastroenterol Motil. 2005;17:51–57. doi: 10.1111/j.1365-2982.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 62.Kamerling IM, Burggraaf J, van Haarst AD, et al. The effect of motilin on the rectum in healthy volunteers. Br J Clin Pharmacol. 2003;55:538–543. doi: 10.1046/j.1365-2125.2003.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lehtola J, Jauhonen P, Kesäniemi A, Wikberg R, Gordin A. Effect of erythromycin on the orocaecal transit time in man. Eur J Clin Pharmacol. 1990;39:555–558. doi: 10.1007/BF00316094. [DOI] [PubMed] [Google Scholar]
- 64.Landry C, Vidon N, Sogni P, et al. Effects of erythromycin on gastric emptying, duodenocaecal transit time, gastric and biliopancreatic secretion during continuous gastric infusion of a liquid diet in healthy volunteers. Eur J Gastroenterol Hepatol. 1995;7:797–802. [PubMed] [Google Scholar]
- 65.Minocha A, Gallo SH. Effect of erythromycin on orocecal transit time in normal healthy male subjects: a double-blind placebo controlled study. Can J Gastroenterol. 1995;9:195–198. doi: 10.1155/1995/125298. [DOI] [Google Scholar]
- 66.El-Salhy M, Lillebø E, Reinemo A, Salmelid L. Ghrelin in patients with irritable bowel syndrome. Int J Mol Med. 2009;23:703–707. doi: 10.3892/ijmm_00000183. [DOI] [PubMed] [Google Scholar]
- 67.Charles F, Phillips SF, Camilleri M, Thomforde GM. Rapid gastric emptying in patients with functional diarrhea. Mayo Clin Proc. 1997;72:323–328. doi: 10.4065/72.4.323. [DOI] [PubMed] [Google Scholar]
- 68.Lee OY. Asian motility studies in irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:120–130. doi: 10.5056/jnm.2010.16.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El-Salhy M, Hatlebakk JG, Hausken T. Reduction in duodenal endocrine cells in irritable bowel syndrome is associated with stem cell abnormalities. World J Gastroenterol. 2015;21:9577–9587. doi: 10.3748/wjg.v21.i32.9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dizdar V, Spiller R, Singh G, et al. Relative importance of abnormalities of CCK and 5-HT (serotonin) in giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:883–891. doi: 10.1111/j.1365-2036.2010.04251.x. [DOI] [PubMed] [Google Scholar]
- 71.Li ZY, Zhang N, Wen S, et al. Decreased glucagon-like peptide-1 correlates with abdominal pain in patients with constipation-predominant irritable bowel syndrome. Clin Res Hepatol Gastroenterol. 2017;41:459–465. doi: 10.1016/j.clinre.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 72.Amato A, Baldassano S, Liotta R, Serio R, Mulè F. Exogenous glucagon-like peptide1 reduces contractions in human colon circular muscle. J Endocrinol. 2014;221:29–37. doi: 10.1530/JOE-13-0525. [DOI] [PubMed] [Google Scholar]
- 73.Gulpinar MA, Bozkurt A, Coskun T, Ulusoy NB, Yegen BC. Glucagon-like peptide (GLP-1) is involved in the central modulation of fecal output in rats. Am J Physiol Gastrointest Liver Physiol. 2000;278:G924–G929. doi: 10.1152/ajpgi.2000.278.6.G924. [DOI] [PubMed] [Google Scholar]
- 74.Ayachi SE, Borie F, Magous R, et al. Contraction induced by glicentin on smooth muscle cells from the human colon is abolished by exendin (9–39) Neurogastroenterol Motil. 2005;17:302–309. doi: 10.1111/j.1365-2982.2004.00628.x. [DOI] [PubMed] [Google Scholar]
- 75.Camilleri M, Vazquez-Roque M, Iturrino J, et al. Effect of a glucagon-like peptide 1 analog, ROSE-010, on GI motor functions in female patients with constipation-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G120–G128. doi: 10.1152/ajpgi.00076.2012. [DOI] [PubMed] [Google Scholar]
- 76.Hellström PM, Hein J, Bytzer P, Björnssön E, Kristensen J, Schambye H. Clinical trial: the glucagon-like peptide-1 analogue ROSE-010 for management of acute pain in patients with irritable bowel syndrome: a randomized, placebo-controlled, double-blind study. Aliment Pharmacol Ther. 2009;29:198–206. doi: 10.1111/j.1365-2036.2008.03870.x. [DOI] [PubMed] [Google Scholar]
- 77.Yang Y, Cui X, Chen Y, et al. Exendin-4, an analogue of glucagon-like peptide-1, attenuates hyperalgesia through serotonergic pathways in rats with neonatal colonic sensitivity. J Physiol Pharmacol. 2014;65:349–357. [PubMed] [Google Scholar]
- 78.Nakade Y, Tsukamoto K, Iwa M, Pappas TN, Takahashi T. Glucagon-like peptide-1 accelerates colonic transit via central CRF and peripheral vagal pathways in conscious rats. Auton Neurosci. 2007;131:50–56. doi: 10.1016/j.autneu.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 79.El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Endocrine cells in the ileum of patients with irritable bowel syndrome. World J Gastroenterol. 2014;20:2383–2391. doi: 10.3748/wjg.v20.i9.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manabe N, Wong BS, Camilleri M, Burton D, McKinzie S, Zinsmeister AR. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22:282–e83. doi: 10.1111/j.1365-2982.2009.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El-Salhy M, Gundersen D, Hatlebakk JG, Gilja OH, Hausken T. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul Pept. 2014;188:60–65. doi: 10.1016/j.regpep.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 82.El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014;20:384–400. doi: 10.3748/wjg.v20.i2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mearin F, Balboa A, Badia X, et al. Irritable bowel syndrome subtypes according to bowel habit: revisiting the alternating subtype. Eur J Gastroenterol Hepatol. 2003;15:165–172. doi: 10.1097/00042737-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 84.Baró E, Roset M, Badía X, Zárate N, Pérez I. Clinical patterns over time in irritable bowel syndrome: symptom instability and severity variability. Am J Gastroenterol. 2004;99:113–121. doi: 10.1046/j.1572-0241.2003.04023.x. [DOI] [PubMed] [Google Scholar]
- 85.Stasi C, Bellini M, Gambaccini D, et al. Neuroendocrine dysregulation in irritable bowel syndrome patients: a pilot study. J Neurogastroenterol Motil. 2017;23:428–434. doi: 10.5056/jnm16155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park SY, Park MH, Yoon KW, et al. Plasma 5-hydroxytryptamine concentration and its correlation with psychopathology in patients with irritable bowel syndrome. Gut Liver. 2009;3:26–30. doi: 10.5009/gnl.2009.3.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Houghton LA, Atkinson W, Lockhart C, Whorwell PJ, Keevil B. Sigmoid-colonic motility in health and irritable bowel syndrome: a role for 5-hydroxytryptamine. Neurogastroenterol Motil. 2007;19:724–731. doi: 10.1111/j.1365-2982.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 88.Thijssen AY, Mujagic Z, Jonkers DM, et al. Alterations in serotonin metabolism in the irritable bowel syndrome. Aliment Pharmacol Ther. 2016;43:272–282. doi: 10.1111/apt.13459. [DOI] [PubMed] [Google Scholar]
- 89.Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/S1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 90.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 91.Houghton LA, Atkinson W, Whitaker RP, Whorwell PJ, Rimmer MJ. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52:663–670. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cremon C, Carini G, Wang B, et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290–1298. doi: 10.1038/ajg.2011.86. [DOI] [PubMed] [Google Scholar]
- 93.El-Salhy M, Gilja OH, Hatlebakk JG, Hausken T. Stomach antral endocrine cells in patients with irritable bowel syndrome. Int J Mol Med. 2014;34:967–974. doi: 10.3892/ijmm.2014.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.El-Salhy M, Gilja OH. Abnormalities in ileal stem, neurogenin 3, and enteroendocrine cells in patients with irritable bowel syndrome. BMC Gastroenterol. 2017;17:90. doi: 10.1186/s12876-017-0643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park MI, Camilleri M. Genetics and genotypes in irritable bowel syndrome: implications for diagnosis and treatment. Gastroenterol Clin North Am. 2005;34:305–317. doi: 10.1016/j.gtc.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 96.Yeo A, Boyd P, Lumsden S, et al. Association between a functional polymorphism in the serotonin transporter gene and diarrhoea predominant irritable bowel syndrome in women. Gut. 2004;53:1452–1458. doi: 10.1136/gut.2003.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Camilleri M, Atanasova E, Carlson PJ, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–432. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- 98.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simrén M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV. Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004;20:292–299. doi: 10.1016/j.tig.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 101.Neuman H, Debelius JW, Knight R, Koren O. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol Rev. 2015;39:509–521. doi: 10.1093/femsre/fuu010. [DOI] [PubMed] [Google Scholar]
- 102.Lyte M. The role of microbial endocrinology in infectious disease. J Endocrinol. 1993;137:343–345. doi: 10.1677/joe.0.1370343. [DOI] [PubMed] [Google Scholar]
- 103.Cook SI, Sellin JH. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther. 1998;12:499–507. doi: 10.1046/j.1365-2036.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 104.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL., Jr Short chain fatty acids and their receptors: new metabolic targets. Transl Res. 2013;161:131–140. doi: 10.1016/j.trsl.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 107.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 108.Le Poul E, Loison C, Struyf S, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 109.Greiner TU, Bäckhed F. Microbial regulation of GLP-1 and L-cell biology. Mol Metab. 2016;5:753–758. doi: 10.1016/j.molmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaji I, Karaki S, Kuwahara A. Short-chain fatty acid receptor and its contribution to glucagon-like peptide-1 release. Digestion. 2014;89:31–36. doi: 10.1159/000356211. [DOI] [PubMed] [Google Scholar]
- 111.Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Larraufie P, Martin-Gallausiaux C, Lapaque N, et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8:74. doi: 10.1038/s41598-017-18259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wichmann A, Allahyar A, Greiner TU, et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. 2013;14:582–590. doi: 10.1016/j.chom.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 114.Karaki S, Mitsui R, Hayashi H, et al. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–360. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- 115.Fukumoto S, Tatewaki M, Yamada T, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 116.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009;52:289–298. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liou AP, Lu X, Sei Y, et al. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology. 2011;140:903–912. doi: 10.1053/j.gastro.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15:421–431. doi: 10.1016/j.cmet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 120.Barbara G, Feinle-Bisset C, Ghoshal UC, et al. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology. 2016;150:1305–1318. e8. doi: 10.1053/j.gastro.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 121.Stanghellini V, Chan FK, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology. 2016;150:1380–1392. doi: 10.1053/j.gastro.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 122.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the maastricht v/florence consensus report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 123.Nakae H, Tsuda A, Matsuoka T, Mine T, Koga Y. Gastric microbiota in the functional dyspepsia patients treated with probiotic yogurt. BMJ Open Gastroenterol. 2016;3:e000109. doi: 10.1136/bmjgast-2016-000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Igarashi M, Nakae H, Matsuoka T, et al. Alteration in the gastric microbiota and its restoration by probiotics in patients with functional dyspepsia. BMJ Open Gastroenterol. 2017;4:e000144. doi: 10.1136/bmjgast-2017-000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McKendrick MW, Read NW. Irritable bowel syndrome--post salmonella infection. J Infect. 1994;29:1–3. doi: 10.1016/S0163-4453(94)94871-2. [DOI] [PubMed] [Google Scholar]
- 126.Futagami S, Itoh T, Sakamoto C. Systematic review with meta-analysis: post-infectious functional dyspepsia. Aliment Pharmacol Ther. 2015;41:177–188. doi: 10.1111/apt.13006. [DOI] [PubMed] [Google Scholar]
- 127.Beatty JK, Bhargava A, Buret AG. Post-infectious irritable bowel syndrome: mechanistic insights into chronic disturbances following enteric infection. World J Gastroenterol. 2014;20:3976–3985. doi: 10.3748/wjg.v20.i14.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 129.Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rajilić-Stojanović M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 131.Crouzet L, Gaultier E, Del’Homme C, et al. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil. 2013;25:e272–282. doi: 10.1111/nmo.12103. [DOI] [PubMed] [Google Scholar]
- 132.De Palma G, Lynch MD, Lu J, et al. Tu1797 the adoptive transfer of anxiety and gut dysfunction from IBS patients to axenic mice through microbiota transplantation. Gastroenterology. 2014;146(suppl 1):S–845. doi: 10.1016/S0016-5085(14)63073-0. [DOI] [Google Scholar]
- 133.Mendall MA, Kumar D. Antibiotic use, childhood affluence and irritable bowel syndrome (IBS) Eur J Gastroenterol Hepatol. 1998;10:59–62. doi: 10.1097/00042737-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 134.Sansone RA, Sansone LA. Irritable bowel syndrome: relationships with abuse in childhood. Innov Clin Neurosci. 2015;12:34–37. [PMC free article] [PubMed] [Google Scholar]
- 135.Uusijärvi A, Bergström A, Simrén M, et al. Use of antibiotics in infancy and childhood and risk of recurrent abdominal pain--a Swedish birth cohort study. Neurogastroenterol Motil. 2014;26:841–850. doi: 10.1111/nmo.12340. [DOI] [PubMed] [Google Scholar]
- 136.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 137.Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 138.Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547–1561. doi: 10.1038/ajg.2014.202. [DOI] [PubMed] [Google Scholar]
- 139.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 140.Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology. 2012;143:1006–1016. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kabouridis PS, Lasrado R, McCallum S, et al. Gut microbial products regulate murine gastrointestinal motility via toll-like receptor 4 signaling. Gastroenterology. 2012;143:1006–1016. e4. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koppel N, Balskus EP. Exploring and Understanding the Biochemical Diversity of the Human Microbiota. Cell Chem Biol. 2016;23:18–30. doi: 10.1016/j.chembiol.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 143.Brun P, Giron MC, Qesari M, et al. Exploring and understanding the biochemical diversity of the human microbiota. Cell Chem Biol. 2016;23:18–30. doi: 10.1016/j.chembiol.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 144.Soret R, Chevalier J, De Coppet P, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–1782. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 145.Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr. 2008;100:297–305. doi: 10.1017/S0007114508888733. [DOI] [PubMed] [Google Scholar]
- 146.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in caco-2 cell monolayers. J Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Plöger S, Stumpff F, Penner GB, et al. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012;1258:52–59. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- 148.Pozuelo M, Panda S, Santiago A, et al. Reduction of butyrate- and methane-producing microorganisms in patients with irritable bowel syndrome. Sci Rep. 2015;5:12693. doi: 10.1038/srep12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:545–552. doi: 10.1111/j.1365-2982.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 150.Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–1294. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 151.Hughes PA, Zola H, Penttila IA, Blackshaw LA, Andrews JM, Krumbiegel D. Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? Am J Gastroenterol. 2013;108:1066–1074. doi: 10.1038/ajg.2013.120. [DOI] [PubMed] [Google Scholar]
- 152.Cipriani G, Gibbons SJ, Kashyap PC, Farrugia G. Intrinsic gastrointestinal macrophages: their phenotype and role in gastrointestinal motility. Cell Mol Gastroenterol Hepatol. 2016;2:120–130. e1. doi: 10.1016/j.jcmgh.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Türler A, Schwarz NT, Türler E, Kalff JC, Bauer AJ. MCP-1 causes leukocyte recruitment and subsequently endotoxemic ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G145–G155. doi: 10.1152/ajpgi.00263.2001. [DOI] [PubMed] [Google Scholar]
- 154.Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012;189:3669–3680. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155–168. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 156.Barbara G, Cremon C, Carini G, et al. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349–359. doi: 10.5056/jnm.2011.17.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shea-Donohue T, Notari L, Sun R, Zhao A. Mechanisms of smooth muscle responses to inflammation. Neurogastroenterol Motil. 2012;24:802–811. doi: 10.1111/j.1365-2982.2012.01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao A, Urban JF, Jr, Anthony RM, et al. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology. 2008;135:217–225. e1. doi: 10.1053/j.gastro.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boyer J, Saint-Paul MC, Dadone B, et al. Inflammatory cell distribution in colon mucosa as a new tool for diagnosis of irritable bowel syndrome: a promising pilot study. Neurogastroenterol Motil. 2018;30 doi: 10.1111/nmo.13223. [DOI] [PubMed] [Google Scholar]
- 160.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 161.Novoselov VV, Sazonova MA, Ivanova EA, Orekhov AN. Study of the activated macrophage transcriptome. Exp Mol Pathol. 2015;99:575–580. doi: 10.1016/j.yexmp.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 162.de las Casas-Engel M, Domínguez-Soto A, Sierra-Filardi E, et al. Serotonin skews human macrophage polarization through HTR2B and HTR7. J Immunol. 2013;190:2301–2310. doi: 10.4049/jimmunol.1201133. [DOI] [PubMed] [Google Scholar]
- 163.Yang M, Fukui H, Eda H, et al. Involvement of gut microbiota in the association between gastrointestinal motility and 5-HT expression/M2 macrophage abundance in the gastrointestinal tract. Mol Med Rep. 2017;16:3482–3488. doi: 10.3892/mmr.2017.6955. [DOI] [PubMed] [Google Scholar]
- 164.Muller PA, Koscsó B, Rajani GM, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kashyap PC, Marcobal A, Ursell LK, et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology. 2013;144:967–977. doi: 10.1053/j.gastro.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Manocha M, Khan WI. Serotonin and GI disorders: an update on clinical and experimental studies. Clin Transl Gastroenterol. 2012;3:e13. doi: 10.1038/ctg.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.De Ponti F. Pharmacology of serotonin: what a clinician should know. Gut. 2004;53:1520–1535. doi: 10.1136/gut.2003.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nøhr MK, Pedersen MH, Gille A, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 171.Ray K. Gut microbiota. Host-microbe interactions and the enteric nervous system: a new connection? Nat Rev Gastroenterol Hepatol. 2015;12:311. doi: 10.1038/nrgastro.2015.69. [DOI] [PubMed] [Google Scholar]
- 172.Yang M, Fukui H, Eda H, et al. Involvement of gut microbiota in association between GLP-1/GLP-1 receptor expression and gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol. 2017;312:G367–G373. doi: 10.1152/ajpgi.00232.2016. [DOI] [PubMed] [Google Scholar]
- 173.Shindo T, Futagami S, Hiratsuka T, et al. Comparison of gastric emptying and plasma ghrelin levels in patients with functional dyspepsia and non-erosive reflux disease. Digestion. 2009;79:65–72. doi: 10.1159/000205740. [DOI] [PubMed] [Google Scholar]
- 174.Futagami S, Shimpuku M, Kawagoe T, et al. The preproghrelin 3056 TT genotype is associated with the feeling of hunger and low acylated ghrelin levels in Japanese patients with Helicobacter pylori-negative functional dyspepsia. Intern Med. 2013;52:1155–1163. doi: 10.2169/internalmedicine.52.8662. [DOI] [PubMed] [Google Scholar]
- 175.Kazemi M, Eshraghian A, Hamidpour L, Taghavi S. Changes in serum ghrelin level in relation to meal-time in patients with functional dyspepsia. United European Gastroenterol J. 2015;3:11–16. doi: 10.1177/2050640614563373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hijaz NM, Friesen CA, Schurman JV, Pearce RE, Abdel-Rahman SM. Plasma ghrelin and liquid gastric emptying in children with functional dyspepsia consistent with post-prandial distress syndrome. Neurogastroenterol Motil. 2015;27:1120–1126. doi: 10.1111/nmo.12591. [DOI] [PubMed] [Google Scholar]
- 177.van Boxel OS, ter Linde JJ, Oors J, et al. Functional dyspepsia patients have lower mucosal cholecystokinin concentrations in response to duodenal lipid. Eur J Gastroenterol Hepatol. 2014;26:205–212. doi: 10.1097/MEG.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 178.Van Der Veek PP, Biemond I, Masclee AA. Proximal and distal gut hormone secretion in irritable bowel syndrome. Scand J Gastroenterol. 2006;41:170–177. doi: 10.1080/00365520500206210. [DOI] [PubMed] [Google Scholar]
- 179.Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249–258. doi: 10.1053/j.gastro.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kumar S, Ranjan P, Mittal B, Ghoshal UC. Serotonin transporter gene (SLC6A4) polymorphism in patients with irritable bowel syndrome and healthy controls. J Gastrointestin Liver Dis. 2012;21:31–38. [PubMed] [Google Scholar]
- 181.El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. 2012;57:873–878. doi: 10.1007/s10620-011-1948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wendelbo I, Mazzawi T, El-Salhy M. Increased serotonin transporter immunoreactivity intensity in the ileum of patients with irritable bowel disease. Mol Med Rep. 2014;9:180–184. doi: 10.3892/mmr.2013.1784. [DOI] [PubMed] [Google Scholar]
- 183.Malinen E, Rinttilä T, Kajander K, et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 184.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, et al. The fecal micro-biota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 185.Krogius-Kurikka L, Lyra A, Malinen E, et al. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–519. e114–e115. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 187.Codling C, O’Mahony L, Shanahan F, Quigley EM, Marchesi JR. A molecular analysis of fecal and mucosal bacterial communities in irritable bowel syndrome. Dig Dis Sci. 2010;55:392–397. doi: 10.1007/s10620-009-0934-x. [DOI] [PubMed] [Google Scholar]
- 188.Carroll IM, Chang YH, Park J, Sartor RB, Ringel Y. Luminal and mucosal-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Gut Pathog. 2010;2:19. doi: 10.1186/1757-4749-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kerckhoffs AP, Ben-Amor K, Samsom M, et al. Molecular analysis of faecal and duodenal samples reveals significantly higher prevalence and numbers of Pseudomonas aeruginosa in irritable bowel syndrome. J Med Microbiol. 2011;60(pt 2):236–245. doi: 10.1099/jmm.0.022848-0. [DOI] [PubMed] [Google Scholar]
- 190.Saulnier DM, Riehle K, Mistretta TA, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141:1782–1791. doi: 10.1053/j.gastro.2011.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Carroll IM, Ringel-Kulka T, Keku TO, et al. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011;301:G799–G807. doi: 10.1152/ajpgi.00154.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Maccaferri S, Candela M, Turroni S, et al. IBS-associated phylogenetic unbalances of the intestinal microbiota are not reverted by probiotic supplementation. Gut Microbes. 2012;3:406–413. doi: 10.4161/gmic.21009. [DOI] [PubMed] [Google Scholar]
- 193.Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:521–530. e248. doi: 10.1111/j.1365-2982.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jeffery IB, O’Toole PW, Öhman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 195.Parkes GC, Rayment NB, Hudspith BN, et al. Distinct microbial populations exist in the mucosa-associated microbiota of sub-groups of irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:31–39. doi: 10.1111/j.1365-2982.2011.01803.x. [DOI] [PubMed] [Google Scholar]
- 196.Jalanka-Tuovinen J, Salojärvi J, Salonen A, et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. 2014;63:1737–1745. doi: 10.1136/gutjnl-2013-305994. [DOI] [PubMed] [Google Scholar]
- 197.Rangel I, Sundin J, Fuentes S, Repsilber D, de Vos WM, Brummer RJ. The relationship between faecal-associated and mucosal-associated micro-biota in irritable bowel syndrome patients and healthy subjects. Aliment Pharmacol Ther. 2015;42:1211–1221. doi: 10.1111/apt.13399. [DOI] [PubMed] [Google Scholar]
- 198.Casén C, Vebø HC, Sekelja M, et al. Deviations in human gut micro-biota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther. 2015;42:71–83. doi: 10.1111/apt.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Giamarellos-Bourboulis E, Tang J, Pyleris E, et al. Molecular assessment of differences in the duodenal microbiome in subjects with irritable bowel syndrome. Scand J Gastroenterol. 2015;50:1076–1087. doi: 10.3109/00365521.2015.1027261. [DOI] [PubMed] [Google Scholar]
- 200.Shukla R, Ghoshal U, Dhole TN, Ghoshal UC. Fecal microbiota in patients with irritable bowel syndrome compared with healthy controls using real-time polymerase chain reaction: an evidence of dysbiosis. Dig Dis Sci. 2015;60:2953–2962. doi: 10.1007/s10620-015-3607-y. [DOI] [PubMed] [Google Scholar]
- 201.Sundin J, Rangel I, Fuentes S, et al. Altered faecal and mucosal microbial composition in post-infectious irritable bowel syndrome patients correlates with mucosal lymphocyte phenotypes and psychological distress. Aliment Pharmacol Ther. 2015;41:342–351. doi: 10.1111/apt.13055. [DOI] [PubMed] [Google Scholar]
- 202.Chung CS, Chang PF, Liao CH, et al. Differences of microbiota in small bowel and faeces between irritable bowel syndrome patients and healthy subjects. Scand J Gastroenterol. 2016;51:410–419. doi: 10.3109/00365521.2015.1116107. [DOI] [PubMed] [Google Scholar]
- 203.Zeber-Lubecka N, Kulecka M, Ambrozkiewicz F, et al. Limited prolonged effects of rifaximin treatment on irritable bowel syndrome-related differences in the fecal microbiome and metabolome. Gut Microbes. 2016;7:397–413. doi: 10.1080/19490976.2016.1215805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gobert AP, Sagrestani G, Delmas E, et al. The human intestinal microbiota of constipated-predominant irritable bowel syndrome patients exhibits anti-inflammatory properties. Sci Rep. 2016;6:39399. doi: 10.1038/srep39399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.De Palma G, Lynch MD, Lu J, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017;9:eaaf6397. doi: 10.1126/scitranslmed.aaf6397. [DOI] [PubMed] [Google Scholar]
- 206.Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ, Liu TT. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: a systematic review and meta-analysis. Dig Liver Dis. 2017;49:331–337. doi: 10.1016/j.dld.2017.01.142. [DOI] [PubMed] [Google Scholar]
- 207.Tap J, Derrien M, Törnblom H, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology. 2017;152:111–123. e8. doi: 10.1053/j.gastro.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 208.Zhuang X, Xiong L, Li L, Li M, Chen M. Alterations of gut microbiota in patients with irritable bowel syndrome: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:28–38. doi: 10.1111/jgh.13471. [DOI] [PubMed] [Google Scholar]
- 209.Yusof N, Hamid N, Ma ZF, et al. Exposure to environmental microbiota explains persistent abdominal pain and irritable bowel syndrome after a major flood. Gut Pathog. 2017;9:75. doi: 10.1186/s13099-017-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Aasbrenn M, Valeur J, Farup PG. Evaluation of a faecal dysbiosis test for irritable bowel syndrome in subjects with and without obesity. Scand J Clin Lab Invest. 2018;78:109–113. doi: 10.1080/00365513.2017.1419372. [DOI] [PubMed] [Google Scholar]
- 211.Johnsen PH, Hilpüsch F, Cavanagh JP, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018;3:17–24. doi: 10.1016/S2468-1253(17)30338-2. [DOI] [PubMed] [Google Scholar]
- 212.Lopetuso LR, Petito V, Graziani C, et al. Gut microbiota in health, diverticular disease, irritable bowel syndrome, and inflammatory bowel diseases: time for microbial marker of gastrointestinal disorders. Dig Dis. 2018;36:56–65. doi: 10.1159/000477205. [DOI] [PubMed] [Google Scholar]