Abstract
Post-weaning social isolation (PSI) has been shown to increase aggressive behavior and alter medial prefrontal cortex (mPFC) function in rats. The present study sought to determine whether this phenotype would be normalized by increasing levels of the endocannabinoid 2-arachidonoylglycerol (2-AG) using pharmacological inhibition of monoacylglycerol lipase (MAGL). Male and female Sprague-Dawley rats were exposed to either 4 weeks of PSI or social rearing (SR) starting on postnatal day 21, then underwent a 15 min trial of social interaction with a novel, same-sex juvenile rat. Rats were administered an acute injection of the MAGL inhibitor MJN110 or vehicle prior to the social interaction. Rats received either 0 mg/kg (vehicle), 1 mg/kg, or 5 mg/kg of MJN110. Both doses of MJN110 decreased aggressive grooming, a measure of agonistic behavior, in both males and females, largely driven by decreased aggressive grooming in PSI rats. There were no effects of MJN110 on overall social behavior or play behavior, while modest effects were observed on locomotor activity in SR rats only. While social interaction increased c-fos expression in the mPFC of both males and females, MJN110 reduced c-fos preferentially in females. These results suggest that 2-AG can modulate specific social behaviors during adolescence, and may affect mPFC function differentially in males and females.
1. Introduction
Adolescence is a critical period for the development of competent social behavior in gregarious species including rats and humans. Social isolation of rats during this period has long been known to produce persistent behavioral changes known as the isolation syndrome (Hatch et al. 1965). Outcomes such as increased anxiety-like behavior (Einon and Morgan 1977) and overreaction to novelty (Hall 1998) may be manifest as dysfunctional social interaction. Post-weaning social isolation (PSI), also known as isolation rearing, alters social behavior and increases unprovoked aggressive behavior in rats (Wongwitdecha and Marsden 1996; Ferdman et al. 2007; Toth et al. 2008, 2011, 2012; Zhao et al. 2009; Wall et al. 2012; Grotewold et al. 2014; Goodell et al. 2017, Biro et al., 2017). Although most investigations of the effects of PSI on aggression have focused exclusively on males, we have shown that 4 weeks of PSI alters social interaction and increases aggressive grooming in both male and female rats (Wall et al. 2012; Grotewold et al. 2014). Increased aggressive grooming has been observed by other groups after PSI (Vale and Montgomery 1997; Hurst et al. 1999) or maternal separation (Parent and Meaney, 2008) of rats and is considered an agonistic behavior (Grant and Mackintosh 1963; Luciano and Lore 1975; Stam et al. 1989; Vale and Montgomery 1997; Hurst et al. 1999; Parent and Meaney 2008), or as an “offensive threat” signal (Biro et al., 2017).
The medial prefrontal cortex (mPFC), which consists of the prelimbic (PL) and infralimbic (IL) subregions, is crucial for the regulation of emotion and undergoes considerable development during adolescence. The mPFC plays an important role in the processing of stressful or threatening stimuli as well as in determining the visceral, cognitive, and emotional responses to stressors (Vermetten and Bremner 2002). A number of cognitive and behavioral processes that are analogous to PFC-dependent executive functions in humans are mediated by the mPFC in rodents. Executive functions such as the ability to attend to or ignore stimuli, the control of inhibitory responses, and cognitive flexibility involve the PFC (Holmes and Wellman 2009). Furthermore, structural and functional abnormalities of the PFC have been linked to aggressive pathologies including antisocial personality disorder (Yang and Raine 2009). In rodents, PSI results in abnormalities in mPFC structure and function including decreased constitutive expression of immediate early genes (Day-Wilson et al. 2006; Levine et al. 2007) and synaptic-associated proteins (Hermes et al. 2011), reductions in mPFC volume (Day-Wilson et al. 2006), and changes in dendritic spine morphology (Ferdman et al. 2007). In studies assessing the protein product of the immediate early gene c-Fos, conflicting evidence exists regarding mPFC activation and aggression associated with PSI in rats. We have observed blunted c-Fos expression in the mPFC of male and female Sprague-Dawley rats after 4 weeks of PSI that was associated with increased aggression against a novel conspecific (Wall et al. 2012). However, Toth et al. (2012) have observed greater c-Fos activation in the anterior cingulate, but not in the IL or PL, of the mPFC of male rats after 8 weeks of PSI and a resident intruder test compared to socially reared (SR) rats. In another study, 8 weeks of PSI enhanced c-Fos activation in male rats after the resident intruder test in both the PL and IL (Biro et al. 2017). Thus, additional studies to determine effects of PSI on social interaction-induced c-fos are warranted. MAGL
The endocannabinoids anandamide (AEA) and 2-arachidonylglycerol (2-AG) have been implicated in emotionality (Wotjak 2005), anxiety regulation (Patel et al. 2017), social interaction (Manduca et al. 2014, 2015) and play behavior (Trezza and Vanderschuren 2008; Trezza et al. 2012; Vanderschuren et al. 2016). Endocannabinoids play a fundamental role in emotional homeostasis and dysfunctional endocannabinoid signaling has been observed in human and animal models of anxiety disorders. 2-AG is abundant in limbic regions including the mPFC (McLaughlin); it is produced on demand in postsynaptic neurons and acts in a retrograde manner on presynaptic cannabinoid type 1 receptors (CB1). The signaling action of 2-AG is terminated primarily via hydrolysis by the serine hydrolase monoacylglycerol lipase (MAGL) (Chanda et al. 2010), and inhibitors of MAGL have been shown to elevate 2-AG levels (Niphakis et al. 2013). MJN110, a selective inhibitor of MAGL, has been found to increase 2-AG levels while having no effect on AEA. CB1 receptor mRNA is dense in the mPFC, and preferentially expressed on GABAergic interneurons, although they are expressed on glutamatergic neurons as well (Marsicano and Lutz 1999); increased 2-AG in the mPFC may have complex effects in this brain region.
PSI for 8 weeks increased expression of mRNA transcripts of a number of several genes in the endocannabinoid system throughout the frontal cortex including the PFC (Robinson et al. 2010). In addition to increased expression of mRNA for CB1 receptors, expression of mRNA for both MAGL and the biosynthetic enzyme diacylglycerol lipase (DAGL) was increased, suggesting enhanced 2-AG turnover after PSI (Robinson et al. 2010). Thus, alterations in endocannabinoid function may be involved in the social deficits observed after PSI. To test this, male and female rats were subjected to either 4 weeks of PSI or SR beginning at postnatal day (P)21. Rats then received systemic injections of the MAGL inhibitor MJN110 prior to a single trial of social interaction with a novel same sex rat. In addition, we measured activation of the immediate early gene protein product c-Fos in the mPFC of the same rats.
2. Materials and methods
2.1. Animals
Male and female Sprague-Dawley rats were purchased (Harlan [now Envigo] Laboratories, Indianapolis IN) and shipped immediately after weaning at P21 (a period corresponding to the early adolescent period) and housed for 4 weeks either individually (post-weaning social isolation [PSI]) or in same-sex groups of 3 (socially reared [SR]) in standard Plexiglas cages before experimentation began. Experimentation took place at P50, a period corresponding to late adolescence (Tirelli et al. 2003; Spear 2013). Rats were on a 12 hr light/dark cycle and maintained at 23°C with food and water freely available in an AAALAC-accredited facility. Rats were weighed the week of the experiment but otherwise unhandled during the 4 weeks of group or isolation rearing. No effects of rearing condition were observed on animals’ weights. Vaginal smears were not performed on females because regular handling during the isolation period is known to reduce the effects of isolation rearing (Krebs-Thomson et al. 2001; Rosa et al. 2005) and the stress of monitoring cycle status has been shown to alter numerous responses to subsequent stressor exposure (Sfikakis et al. 1996). All procedures were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals with protocols approved by the University of Colorado Institutional Animal Care and Use Committee.
2.2. Drug preparation and administraton
MJN110 (Sigma-Aldrich item #SML0872) was dissolved in 200 proof ethanol (Sigma-Aldrich) and sonicated at 23°C for 60–90 min. Once in solution, the drug was diluted in 2% Cremaphore (Sigma-Aldrich) and 0.9% saline, constituting a 1:1:18 ratio. The drug was prepared fresh each day of the experiment. On PND 49 rats were randomly assigned a dose and social encounter condition; n = 8 animals/group. Rats were given an IP injection of 0 mg/kg, 1 mg/kg, or 5 mg/kg MJN110 and isolated in a clean fresh cage for 2 hr (File and Seth, 2003). Two hr was selected because it was the point of maximum 2-AG induction after an IP injection of JZL184 (Long et al. 2009) and antiallodynic effects of MJN110 were observed at 1 and 3 hr after MJN110 administration (Niphakis et al. 2013). The action of MJN110 is long lasting, as 2-AG was elevated 4 hr after MJN110 administration (Niphakis et al. 2013).
2.3. Behavioral testing
As mentioned above, rats were isolated for 2 hr in clean standard cages with fresh bedding before the social interaction trials. We reasoned that clean cages would be the least stressful environment for social testing, the clear cages make close-up video recording optimal, and social interaction is enhanced in group-reared rats after acute social isolation (Niesink and Van Ree 1989). Then, rats in their individual cages were placed behind a light-attenuating curtain. A novel, same-sex stimulus rat was introduced to the cage for 15 min and the interaction was recorded by webcam. Stimulus rats were 1 to 2 weeks younger than the experimental animals in order to be less threatening to the experimental rats. After the social encounter trial, rats were rehoused in their home cage for 100 min. We have previously observed increased social interaction and exaggerated levels of aggressive grooming in male and female rats after 4 weeks of PSI (Wall et al. 2012; Grotewold et al. 2014). Therefore, the following behaviors were scored by experimenters blinded to the treatment groups using JWatcher software (http://www.jwatcher.ucla.edu/). Aggressive Grooming: Vigorous grooming by the experimental rat of the novel conspecific when it is standing, crouching, supine, or trying to escape (Hurst et al. 1999). Aggressive grooming was first identified by Grant and Mackintosh (Grant and Mackintosh 1963), and is distinguished from normal social grooming by an increased use of the teeth, pulling of fur, vigorousness, and by targeting of the shoulder or back (Hurst et al., 1999). Total Social Interaction: The overall amount of time in seconds the experimental rat spends actively engaged in any type of social interaction (e.g. sniffing, following, grooming, pinning, pouncing) with the novel conspecific. We also assessed frequencies of pinning and pouncing, which are typical play behaviors (Panksepp et al. 1984). Pinning: Standing over/holding down the novel conspecific while it is in a supine posture. Pouncing: leaping at the nape of the conspecific with the forepaws. In order to account for the contribution of aggressive grooming on overall social interaction, we also calculated non-aggressive social interaction as follows: Total Social Interaction – Aggressive Grooming. Cage crossing: to assess locomotor activity we scored the number of times the experimental rat crossed from one side of the cage to the other.
Rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with cold 0.9% saline followed by 4% paraformaldehyde in 0.01 M PBS. Brains were postfixed for 4 h in the same paraformaldehyde solution, cryoprotected in 30% sucrose for three days, then rapidly frozen in −30 °C isopentane just prior to cryosectioning. Sections were taken (40 µm) through the prefrontal cortex using the atlas of Paxinos and Watson (2006) from 3.2 mm to 2.2 mm anterior to bregma. Sections were stored at 4 °C in cryoprotectant until immunohistochemistry was performed.
2.4. Immunohistochemistry
Free-floating sections were first washed 3 times in 0.01 M PBS and between each subsequent step except as noted. Sections were incubated in 1% hydrogen peroxide, followed by 5% normal goat serum (NGS) and 0.25% Triton X in PBS. Sections were incubated for 48 h at 4 °C in primary antibody directed against c-Fos (rabbit anti-Fos, 1:3,000, Santa Cruz Biotechnology) in PBS with 5% NGS and 0.25% Triton X. Sections were then incubated in biotinylated goat anti-rabbit secondary antibody (1:200, Jackson Labs) for 2 h, followed by incubation in avidin biotin complex (ABC kit, Vector Laboratories) for 2 h. Sections were washed 3 times in 0.1 M PB, then immunoreactivity was visualized with SG (SG substrate kit, Vector Laboratories). Sections were mounted on slides using a 0.0015% gelatin solution, dehydrated using a series of ethanol solution, defatted using Histoclear (Sigma-Aldrich), and coverslipped with Permount (Sigma-Aldrich).
2.5. Microscopy
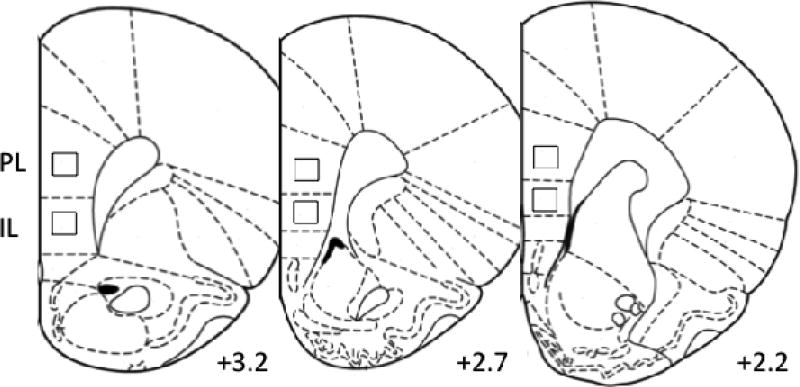
Analysis of c-Fos protein expression was performed using an Olympus BX51 microscope and VisioPharm software (VisioPharm, Hørsholm, Denmark). Immunolabeled cells in the prelimbic cortex (PL), and the infralimbic cortex (IL) were counted by centering the field within each subregion at 10× using the corpus callosum as a guide, then shifting to a 40× objective; counts were performed within a 22,406 µm2 counting frame centered within the 40× field. This ensured that the counting frame was within the subregion of interest. Placement of the field of view for each subregion is shown in Fig. 1. For each rat, 6–8 individual hemisphere measurements were assessed. A pilot experiment (data not shown) assessed c-Fos positive cells in deep, medium, and superficial layers; however, the magnitude of the effect did not differ between these regions. Thus, our counting frame was positioned between the corpus callosum and the midline of the mPFC in layers 4/5.
Figure 1.
Placement of counting frames in the PL and IL subregions of the mPFC shown at +2.2, +2.7, and +3.2 mm from bregma. Plate adapted from (Paxinos and Watson 2006).
2.6. Statistics
Behavioral data were analyzed using 2 × 2 × 3 factorial ANOVAs with rearing (SR or PSI), sex (male or female) and drug dose (0, 1, or 5 mg/kg) as between-group variables. Due to the large number of animals, c-Fos IHC assays were performed separately for males and females, thus statistics for c-Fos were performed separately within each sex. Analysis of c-Fos was performed using 2 × 2 × 3 factorial ANOVAs with rearing (group or ISO), social cue (no social or social) and drug dose (0, 1, or 5 mg/kg) as between-group variables. To determine constitutive sex differences in c-Fos, a separate IHC assay was performed with control male and female animals; these data were analyzed using a two-tailed Student’s t-test. When significant interactions were obtained, Fisher’s LSD post-hoc tests were performed to determine differences between groups. All statistical tests were conducted with an alpha set at .05.
3.0. Results
3.1. Aggressive grooming
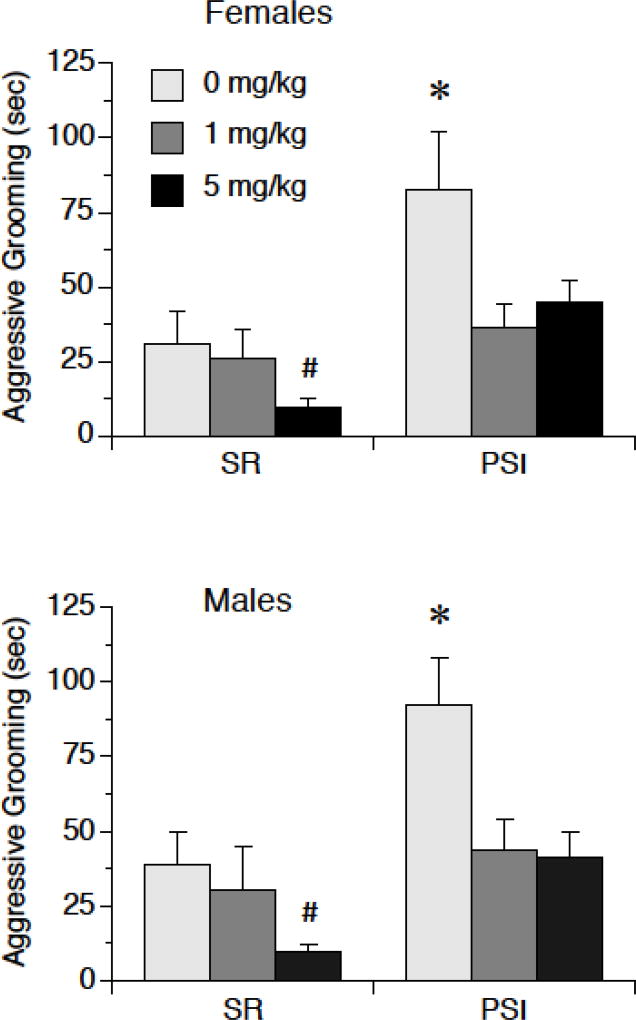
MAGL inhibition via MJN110 decreased aggressive grooming of a novel same-sex rat in both males and females, primarily in PSI rats, as shown in Figure 2. We observed a significant Rearing × Drug interaction, F (2, 85) = 3.31, p < 0.05. Post-hoc tests indicated that Vehicle (0 mg/kg) treated PSI rats spent more time engaged in aggressive grooming than all other groups, p < .05, and that SR rats at the 5 mg/kg dose spent less time engaged in aggressive grooming than SR vehicle rats and PSI rats at the 5 mg/kg dose, both p < 0.05. In addition, there was a significant main effect of Rearing, F (2, 85) = 24.99, p < 0.0001, indicating that PSI rats spent more time engaged in aggressive grooming than SR rats. There was also a significant main effect of Drug, F (2, 85) = 10.09, p < 0.001, indicating that overall, both doses of MJN110 decreased aggressive grooming. There was no main effect of Sex nor were there significant interactions involving Sex.
Figure 2.
MJN110 decreases aggressive grooming in male and female rats. Male and female rats were exposed to post-weaning social isolation (PSI) or social rearing (SR) for 4 weeks, then were injected with either 0 mg/kg (vehicle), 1 mg/kg, or 5 mg/kg MJN110. Two hr later rats were exposed to a novel same-sex rat for 15 min. PSI increased aggressive grooming in rats that received vehicle, and this was blunted in PSI rats that received either 1 mg/kg or 5 mg/kg MJN110. 5 mg/kg MJN110 also reduced aggressive grooming in rats that had experienced SR. * 0 mg/kg than all other groups, p < 0.05. # less than all other groups, p < 0.05. Data are means ± SEMs.
3.2. Total Social Interaction
MAGL inhibition had no impact on Total Social Interaction with a novel same-sex rat (Table 1). PSI rats engaged in more social interaction than SR rats, as revealed by a significant main effect of Rearing, F (2, 85) = 20.20, p < 0.001. In addition, females engaged in more social interaction than males, as revealed by a significant main effect of Sex, F (2, 85) = 96.21, p < 0.001. There was no effect of MJN110 dose on social interaction, F (2, 85) = 1.15, p =0.32, and there were no significant interactions involving Drug dose. However, there was a trend for a Housing by Dose interaction, F (2, 85) = 3.01, p = 0.06, which was likely attributable to the contribution of aggressive grooming to Total Social Interaction time.
Table 1.
Total social interaction (including aggressive grooming, seconds), pinning (frequency), pouncing (frequency), and cage crossings (frequency) during a 15 min social encounter with a novel same-sex rat. Experimental animals were either socially reared (SR) or had experienced 4 weeks of post-weaning social isolation (PSI) and acute treatment with vehicle (0 mg/kg) or MJN110. Values are means and SEMs of 8 rats/group.
| 0 mg/kg | 1 mg/kg | 5 mg/kg | ||||
|---|---|---|---|---|---|---|
| Total Social Interaction a, b | SR | PSI | SR | PSI | SR | PSI |
| Female | 531 (42.8) | 687 (31.4) | 581 (30.7) | 625 (49.1) | 503 (33.8) | 718 (24.8) |
| Male | 343 (96.2) | 465 (48.7) | 333 (31.9) | 353 (47.9) | 231 (31.8) | 380 (25.9) |
| Pinning a, b, c | ||||||
| Female | 5.87 (1.2) | 5.57 (1.6) | 6.55 (1.4) | 4.33 (0.9) | 4.28 (0.9) | 5.0 (1.5) |
| Male | 4.87 (1.5) | 13.37 (2.7) | 5.22 (1.9) | 9.25 (2.3) | 3.63 (1.5) | 11.38 (2.4) |
| Pouncing a, b | ||||||
| Female | 24.0 (3.3) | 36.3 (4.0) | 21.8 (3.2) | 34.7 (4.2) | 19.4 (3.0) | 34.3 (3.5) |
| Male | 10.8 (2.2) | 22.3 (3.8) | 12.7 (1.5) | 16.6 (1.0) | 10.9 (1.9) | 23.8 (3.4) |
| Cage crossings a, b, d | ||||||
| Female | 60.5 (4.97) | 77.4 (9.14) | 51.5 (3.38) | 73.1 (5.7) | 46.6 (4.0) | 82.5 (2.24) |
| Male | 41.7 (3.2) | 52.0 (3.6) | 43.1 (4.3) | 52.5 (6.2) | 33.8 (4.1) | 60.2(6.5) |
, main effect of Rearing, p < 0.01;
, main effect of Sex, p < 0.01;
, Rearing by Sex interaction, p < 0.01;
, Rearing by Dose interaction, p < .05.
3.3. Pinning
MAGL inhibition had no impact on Pinning (Table 1). PSI rats engaged in more Pinning than SR rats, as revealed by a significant main effect of Rearing, F (2, 85) = 9.32, p < 0.01. In addition, males engaged in more Pinning than females, as revealed by a significant main effect of Sex, F (2, 85) = 7.09, p < 0.01. Finally, PSI males Pinned more than SR males and both PSI and SR females, as revealed by a significant Rearing by Sex interaction, F (2, 85) = 13.34, p < 0.001. There was no effect of MJN110 dose on Pinning, F (2, 85) = 0.65, p = 0.52, and there were no significant interactions involving Drug dose.
3.4. Pouncing
MAGL inhibition had no impact on Pouncing (Table 1). PSI rats engaged in more Pouncing than SR rats, as revealed by a significant main effect of Rearing, F (2, 85) = 40.64, p < 0.001. In addition, females engaged in more Pouncing than males, as revealed by a significant main effect of Sex, F (2, 85) = 47.02, p < 0.001. There was no effect of MJN110 dose on Pouncing, F (2, 85) = 0.39, p =0.68, and there were no significant interactions involving Drug dose.
3.5. Non-aggressive social interaction
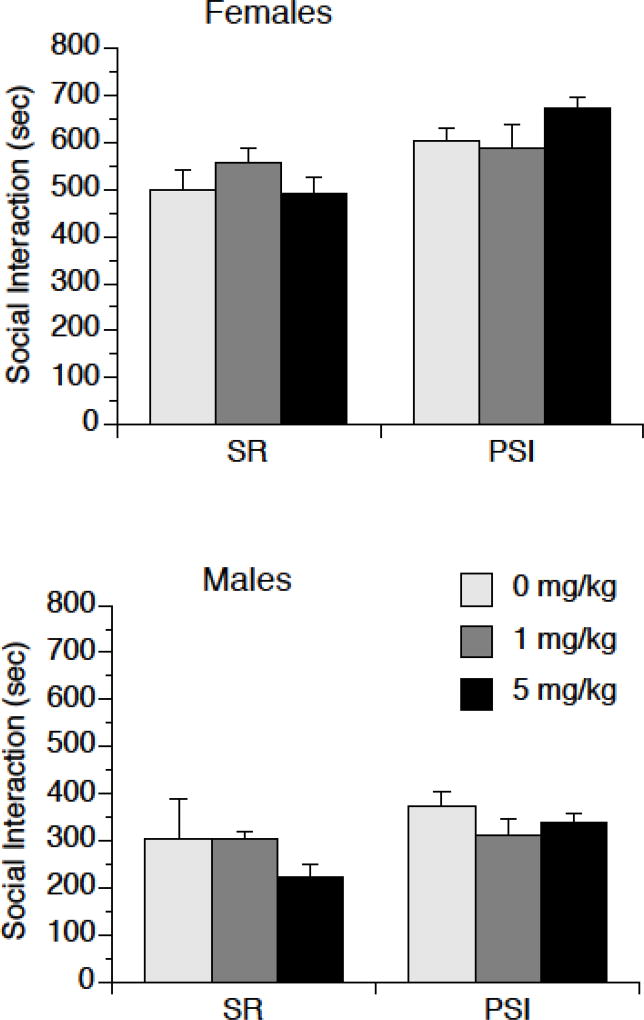
MAGL inhibition had no effect on non-aggressive social interaction (total time engaged in social interaction minus time spent engaged in aggressive grooming) with a novel same-sex rat, as shown in Figure 3. PSI rats engaged in more non-aggressive social interaction than SR rats, as revealed by a significant main effect of Rearing, F (2, 85) = 11.25, p < 0.01. In addition, females engaged in more non-aggressive social interaction than males, as revealed by a significant main effect of Sex, F (2, 85) = 105.79, p < 0.0001. There was no effect of MJN110 dose on non-aggressive social interaction, F (2, 85) = 0.09, p =0.91, and there were no significant interactions involving Drug dose.
Figure 3.
MJN110 does not affect non-aggressive social behavior in male or female rats. Male and female rats were exposed to post-weaning social isolation (PSI) or social rearing (SR) for 4 weeks, then were injected with either 0 mg/kg (vehicle), 1 mg/kg, or 5 mg/kg MJN110. Two hr later rats were exposed to a novel same-sex rat for 15 min. All social interactions were recorded, and aggressive social interaction was subtracted from this total. There was more non-aggressive social behavior in females than males (p < 0.05), and more non-aggressive social behavior in PSI rats than SR rats (p < 0.01). Data are means ± SEMs.
3.6. Cage crossings
Cage crossings were assessed as a measure of locomotor activation (Table 1). PSI rats engaged in more Cage crossings than SR rats, as revealed by a significant main effect of Rearing, F (2, 85) = 46.58, p < 0.001. In addition, females engaged in more Cage crossings than males, as revealed by a significant main effect of Sex, F (2, 85) = 39.53, p < 0.001. There was a significant Rearing × Drug interaction on Cage crossings, F (2, 85) = 3.63, p < 0.05. Post-hoc tests collapsed across Sex indicated that although SR and PSI differed significantly from each other at each of the MJN110 doses (all p < 0.05), within each Rearing group no doses differed significantly from any other doses. However, post-hoc tests revealed that within SR rats there was a trend for SR receiving 5 mg/kg to have fewer Cage crossings than SR rats receiving 0 mg/kg, p = 0.06. Furthermore, there was no main effect of Drug dose, F (2, 85) = 0.35, p = 0.70, and no other significant interactions.
3.7. c-Fos protein expression in the medial prefrontal cortex
Because of the large number of animals in the experiment we ran separate IHC assays for male and female rats. In order to determine if there were sex differences in baseline c-Fos we also assessed No Social control (SR) males and females side-by-side in another IHC assay. No differences in c-Fos were observed between males and females in the PL (males: 29.71 ± 5.7, females: 27.44 ± 6.0; p = .79) or IL (males: 43.50 ± 5.2, females: 41.50 ± 5.1; p =0.31; p = .78).
3.7.1. Females
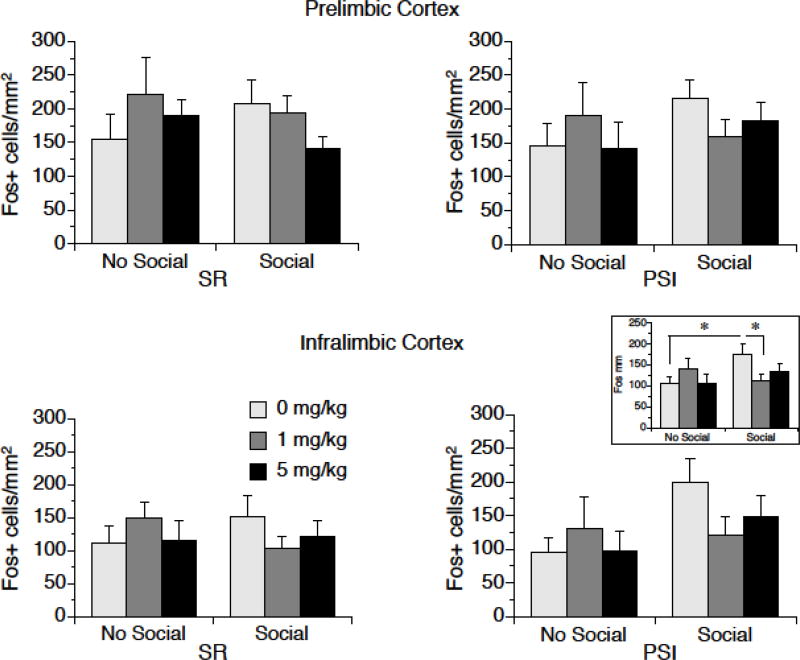
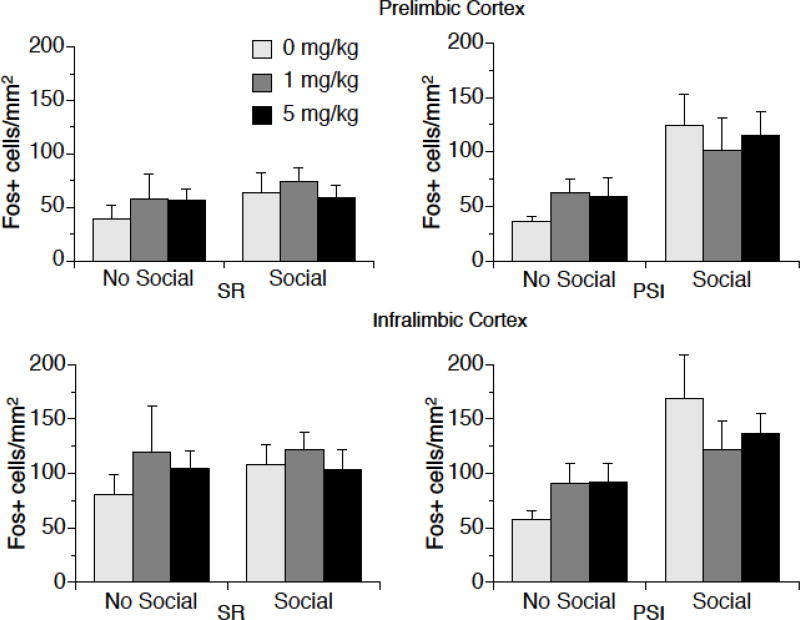
A low dose of MJN110 decreased c-Fos activation in the IL of female rats that were exposed to social interaction, as shown in Figure 4. There was a significant Social × Drug interaction in the IL of females; F (2, 82) = 3.15, p < 0.05. Post-hoc tests revealed that Social animals that received vehicle had greater numbers of c-Fos positive cells in the IL than No-Social Vehicle animals and Social animals that received 1 mg/kg MJN110; differences from 5 mg/kg were not statistically significant. There were no other significant main effects or interactions in the IL of females. Moreover, there were no significant effects of Social Interaction, Rearing, or Drug dose in the PL of females.
Figure 4.
c-Fos protein expression in the PL and IL of female rats that had been exposed to post-weaning social isolation (PSI) or social rearing (SR) for 4 weeks, then either 0 mg/kg (vehicle), 1 mg/kg, or 5 mg/kg MJN110. Two hr later rats were exposed to a novel same-sex rat for 15 min. There were no differences between groups in the PL (top). In the IL (bottom), there was no effect of rearing condition, but there was a significant Social × Drug effect. The inset shows c-fos in the IL collapsed over Rearing condition. * p < 0.05. Data are means ± SEMs.
3.7.2. Males
MJN110 had no significant effects on c-Fos activation in the mPFC of males, but Social interaction enhanced c-Fos in the mPFC, especially after PSI, as shown in Figure 5. Social interaction-induced c-Fos was enhanced in both the IL and PL of PSI male rats. There was a significant Rearing × Social interaction in the IL; F (2, 83) = 5.91, p < .05. Post-hoc tests revealed that Social animals that had experienced PSI had greater numbers of c-Fos positive cells in the IL than Social animals that had experienced SR, p < 0.05. In addition, there was a significant main effect of a Social encounter on c-Fos in the IL, F (2, 83) = 8.63, p < 0.01. A similar pattern of c-Fos expression was observed in the PL. In the PL, there was also a significant Rearing × Social interaction; F (2, 83) = 5.85, p < .05. Post-hoc tests revealed that Social animals that had experienced PSI had greater numbers of c-Fos positive cells in the PL than Social animals that had experienced SR, p < 0.05. In addition, there was a significant main effect of a Social encounter on c-Fos in the PL, F (2, 83) = 12.06, p < 0.001.
Figure 5.
c-Fos protein expression in the PL and IL of male rats. Rats were exposed to post-weaning social isolation (PSI) or social rearing (SR) for 4 weeks, then either 0 mg/kg (vehicle), 1 mg/kg, or 5 mg/kg MJN110. Two hr later rats were exposed to a novel same-sex rat for 15 min. There were no significant effects of MJN110 on c-fos expression in the PL or IL. However, in both the PL and IL, there were significant Social × Rearing interactions; PSI rats exposed to a novel same-sex rat had greater c-fos expression than SR rats exposed to a novel same-sex rat. Data are means ± SEMs.
4.0. Discussion
Our primary finding indicates that pharmacological inhibition of MAGL using a systemic injection of MJN110 decreased aggressive grooming towards a novel same-sex rat in both male and female rats. This decrease was primarily driven by an attenuation of the increase in aggressive grooming that was produced by 4 weeks of post-weaning social isolation. Importantly, MJN110 did not result in decreased non-aggressive social interactions, including play behaviors. These observations support a key role for 2-AG in the regulation of agonistic behavior. Effects of MJN110 on social interaction-induced c-Fos expression in the mPFC differed between males and females, with modest effects of MJN110 on social interaction-induced increases in c-fos protein expression in the IL of female rats regardless of their rearing condition, and no significant effects of MJN110 on c-fos protein expression in males.
Consistent with previous reports from our laboratory (Wall et al. 2012; Grotewold et al. 2014; Goodell et al. 2017) and others (Wongwitdecha and Marsden 1996; Ferdman et al. 2007; Toth et al. 2008; Zhao et al. 2009; Toth et al. 2011, 2012), PSI resulted in a marked increase in aggressive behavior and overall social interaction. In the present study, this increased aggression was normalized by treatment with MJN110; remarkably, PSI rats treated with either 1 mg/kg or 5 mg/kg of MJN110 exhibited levels of aggression that were no different from SR vehicle controls. In addition, 5 mg/kg of MJN110 reduced aggressive grooming in SR rats. Non-aggressive social interaction was not affected by MJN110 in any groups at the doses used here. Although MJN110 has not been reported to produce sedative effects, low doses of MJN110 were used in the present study in order to avoid the possibility of sedative effects at higher doses, as other MAGL inhibitors have been shown to produce sedation (Anderson et al. 2014; Adamson Barnes et al. 2016). Both 1 mg/kg and 5 mg/kg of MJN110 have been shown to significantly increase brain levels of 2-AG while having no effect on other endocannabinoids, including AEA (Niphakis et al. 2013); these effects may be due to effects on the serine hydrolase ABHD6, which also hydrolyzes 2-AG, in addition to its effects on MAGL (Niphakis et al. 2013). While highly specific to 2-AG hydrolases, MJN110 reduces levels of prostaglandin E2 as a result of decreased 2-AG hydrolysis (Burston et al. 2016); however, effects of reduced prostaglandin E2 on social behavior are unknown. The discriminative stimulus effects of MJN110 are blocked by the CB1 receptor antagonist rimonabant (Owens et al. 2017). Thus, one possibility is that enhanced 2-AG may have modulatory effects on aggressive behavior by producing decreases in anxiety produced by its actions on CB1 receptors. In a recent study by Bedse et al. (Bedse et al. 2017), increased anxiety in the light/dark box produced by restraint stress was reduced by treatment with the MAGL inhibitor JZL184. In accordance with this, disruption of 2-AG synthesis enhanced anxiety-like behavior in a battery of tasks including the light/dark box (Shonesy et al. 2014). Increases in both anxiety and aggression occur after adolescent challenges including stress (Walker et al. 2017) and PSI (Wright et al. 1991; Hall 1998; Weiss et al. 2004; Lukkes et al. 2009). Although social avoidance in the social interaction test is often used as a measure of anxiety (File and Seth 2003), after 4 weeks of PSI both male and female rats exhibit a strong motivation to interact with conspecifics in spite of an anxious-like phenotype. Additional evidence of anxiety-like behavior combined with high motivation to interact socially is provided by findings in adenosine receptor knockout mice (López-Cruz et al. 2017), suggesting that a simple interpretation of the social interaction test may not be possible when assessing only the amount of time spent in social interaction. Thus it is informative to assess specific social behaviors that the experimental rats engage in, such as aggressive grooming. It has been suggested that aggressive grooming may be related to a frustrated social response (Hurst et al. 1999), which is consistent with the anxiety-like and socially inept behaviors that are observed after PSI. In line with this idea, Parent and Meaney (2008) suggest that even if aggressive grooming is a component of play fighting, excessive levels of play fighting is indicative of agonistic behavior in juveniles and may be associated with anxiety. An alternative interpretation is that PSI produces socially immature rats that engage in inappropriate levels of play fighting; play behavior in normal rats decreases dramatically during late adolescence (Panksepp et al.1984). Panksepp et al. (1984) also note that play is more aggressive in males than females, but the present results indicate similar levels of aggressive grooming in males and females.
Effects of MJN110 on locomotor activity at the doses used here were minimal and dependent on rearing condition. A trend for a decrease in cage crossings in SR, but not PSI rats exposed to the 5 mg/kg dose of MJN110 suggests that isolation reared rats may be less sensitive to sedative effects of MAGL inhibition. A few studies have explored the effects of PSI on the cannabinoid system with mixed results. Expression of both CB1 receptor and MAGL mRNA was increased after 8 weeks of PSI, particularly in the mPFC (Robinson et al. 2010). However, 8 weeks of PSI had no effect on immunolabeling of presynaptic CB1 receptors in the mPFC in a recent study by Fitzgerald et al. (2013). The effects of 4 weeks of PSI on endocannabinoid signaling remain unknown. Regardless, locomotor effects of MJN110 were not observed after PSI in the present study, while aggressive grooming was decreased, suggesting that the effects of MJN110 on aggressive grooming are not mediated by alterations in behavioral activation.
The present c-fos results are dissimilar to a previous study of c-fos in the mPFC after differential rearing and acute social interaction from our laboratory. We have previously observed large increases in social interaction-induced c-Fos in the PL and IL of SR rats, especially males, but blunted responses in rats that had undergone PSI (Wall et al. 2012). A number of variables that differed between the two studies might explain this inconsistency. In the present study rats received an IP injection 2 hours prior to behavioral testing; moreover, all rats were placed in a fresh cage and moved to a different room in the animal facility for this 2 hour period. Testing occurred during the light phase but under dim red lighting conditions. Importantly, SR rats were isolated in the fresh cages during this 2 hr period. However, in our previous study, SR rats were never isolated, having been moved immediately before testing from their home cage to a fresh cage with a novel stimulus rat. Therefore, it is possible that the previous study the novelty of the new cage and the acute removal of cagemates in addition to the novel rat may have played a role in the large increase observed in SR rats. Alternatively, 2 hr of social isolation may have blunted the c-fos response in SR rats. In the study of Toth et al. (2012), fighting during the resident intruder task in males increased c-fos expression in the PL and IL of both SR and PSI rats in comparison to rats that did not fight, again regardless of rearing conditon. In contrast to the present study, in that study rats were tested in the dark phase and SR rats were isolated for 3 days prior to resident intruder testing, which took place in the home cage (Toth et al., 2012). Biro et al. (2017), using similar procedures as Toth et al. (2012) observed greater c-Fos expression produced by the resident-intruder task in the ventral PFC (combined PL and IL) in male PSI rats compared to SR; resident intruder testing did not increase c-Fos expression in the ventral PFC of male SR rats. The results of Biro et al. (2017) demonstrated that in isolates, both excitatory (CaMKII positive) and inhibitory (GABA positive) neurons were activated by resident intruder testing and thus we predict the same pattern would be evident here. Because CB1 receptors are presynaptic both excitatory and inhibitory neurons are likely affected by MJN110; future studies will be needed to determine the phenotype of c-Fos activation in our model.
Here, although both males and females exhibited increased social interaction-induced c-fos expression after PSI, MJN110 significantly reduced social interaction-induced c-fos only in females. It has previously been reported that females engage in more social interaction than males (Perkins et al. 2017), consistent with the current findings, and in that study social interaction-induced c-fos was greater in the mPFC of females than males (Perkins et al. 2017). Sex differences in the effects of cannabinoid manipulations have also been previously reported. For example, females are more susceptible to the memory impairing effects of THC (Cha et al. 2007). Whether sexual dimorphism exists with respect to the endocannabinoid system remains an open question as little research has been done on this topic. One group has reported that female mice have lower levels of circulati prostaglandin ng 2-AG than males (Thompson et al. 2017); however, in another study there was no apparent sex difference in plasma levels of 2-AG or of 2-AG and MAGL levels throughout the forebrain (Shonesy et al. 2014). The antidepressant effect of chronic administration of the CB1 agonist HU-210 was observed in both male and female rats (Morrish et al. 2009), suggesting that cannabinoid treatments for disorders of emotional regulation are effective for both males and females. However, it is possible that the mPFC may be affected more in females than males under the present conditions.
To the best of our knowledge only one other report exists of the effects of MJN110 on c-Fos expression in the cortex. In that study, MJN110 at a higher dose (10 mg/kg) attenuated lithium chloride-induced increases in c-Fos expression in the visceral insular cortex (Sticht et al. 2016). Consistent with the idea that endocannabinoids can decrease c-Fos expression in the cortex, administration of the CB1 receptor antagonist SR141716 (Rimonabant) increased the activation of c-Fos in the anterior cingulate cortex that was induced by stressor exposure (Patel et al. 2005). In the present study, MJN110 was administered systemically, therefore its effects on behavior may have been mediated by other brain regions important for emotion regulation. In an investigation of the dorsolateral periaqueductal gray (dlPAG) in panic, a type of anxiety disorder, the increases in panic-like defensive behavior and dlPAG c-fos produced by intra-dlPAG NMDA were blocked the MAGL-preferring 2-AG hydrolysis inhibitor URB602 (Gobira, 2016). The present results support the notion that regulation of 2-AG can be used therapeutically for anxiety disorders, including via drugs such as MJN110 that inhibit its metabolism. This strategy has promise as a treatment option for disorders that involve dysfunctional social behavior involving aggression and may be likely to have fewer side effects than CB1 agonists. An important benefit of MAGL inhibition is that because 2-AG is produced on demand, only the brain regions and circuits that are actively producing 2-AG when the drug is administered will be affected. In contrast, CB1 agonists act at all regions containing CB1 receptors, and may as a result produce unwanted side effects.
In conclusion, 4 weeks of PSI increased aggressive grooming of a novel same-sex conspecific in male and female rats. Treatment with the MAGL inhibitor MJN110 eliminated this increase in aggressive grooming in both males and females, without reducing non-aggressive social behavior. Activation of the mPFC, in particular the IL subdivision, was increased by social interaction but was blunted by MJN110 only in females. These results have implications for the treatment of social behavioral disorders involving aggression by reducing the breakdown of 2-AG.
Acknowledgments
This work was supported by NIH Grant R15MH102717. Jazmin Fontenot was supported by NIH BP-ENDURE undergraduate training grant R25GM097633. We also wish to thank the University of Colorado Denver Undergraduate Research Opportunity Program (UROP) for their support, and we thank Raleigh Jonscher and Emma Boxer for their contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
References
- Adamson Barnes NS, Mitchell VA, Kazantzis NP, Vaughan CW. Actions of the dual FAAH/MAGL inhibitor JZL195 in a murine neuropathic pain model. Br J Pharmacol. 2016;173:77–87. doi: 10.1111/bph.13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WB, Gould MJ, Torres RD, et al. Actions of the dual FAAH/MAGL inhibitor JZL195 in a murine inflammatory pain model. Neuropharmacology. 2014;81:224–230. doi: 10.1016/j.neuropharm.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Bedse G, Hartley ND, Neale E, et al. Functional Redundancy Between Canonical Endocannabinoid Signaling Systems in the Modulation of Anxiety. Biol Psychiatry. 2017;82:488–499. doi: 10.1016/j.biopsych.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro L, Toth M, Sipos E, et al. Structural and functional alterations in the prefrontal cortex after post-weaning social isolation: relationship with species-typical and deviant aggression. Brain Struct Funct. 2017;222:1861–1875. doi: 10.1007/s00429-016-1312-z. [DOI] [PubMed] [Google Scholar]
- Burston JJ, Mapp PI, Sarmad S, et al. Robust anti-nociceptive effects of monoacylglycerol lipase inhibition in a model of osteoarthritis pain. Br J Pharmacol. 2016;173:3134–3144. doi: 10.1111/bph.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YM, Jones KH, Kuhn CM, et al. Sex differences in the effects of delta9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav Pharmacol. 2007;18:563–569. doi: 10.1097/FBP.0b013e3282ee7b7e. [DOI] [PubMed] [Google Scholar]
- Chanda PK, Gao Y, Mark L, et al. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol. 2010;78:996–1003. doi: 10.1124/mol.110.068304. [DOI] [PubMed] [Google Scholar]
- Day-Wilson KM, Jones DNC, Southam E, et al. Medial prefrontal cortex volume loss in rats with isolation rearing-induced deficits in prepulse inhibition of acoustic startle. Neuroscience. 2006;141:1113–1121. doi: 10.1016/j.neuroscience.2006.04.048. [DOI] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ. A critical period for social isolation in the rat. Dev Psychobiol. 1977;10:123–132. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- Ferdman N, Murmu RP, Bock J, et al. Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behav Brain Res. 2007;180:174–182. doi: 10.1016/j.bbr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ML, Mackie K, Pickel VM. The impact of adolescent social isolation on dopamine D2 and cannabinoid CB1 receptors in the adult rat prefrontal cortex. Neuroscience. 2013;235:40–50. doi: 10.1016/j.neuroscience.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell DJ, Ahern MA, Baynard J, et al. A novel escapable social interaction test reveals that social behavior and mPFC activation during an escapable social encounter are altered by post-weaning social isolation and are dependent on the aggressiveness of the stimulus rat. Behav Brain Res. 2017;317:1–15. doi: 10.1016/j.bbr.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant EC, Mackintosh JH. A Comparison of the Social Postures of Some Common Laboratory Rodents. Behaviour. 1963;21:246–259. doi: 10.1163/156853963X00185. [DOI] [Google Scholar]
- Grotewold SK, Wall VL, Goodell DJ, et al. Effects of cocaine combined with a social cue on conditioned place preference and nucleus accumbens monoamines after isolation rearing in rats. Psychopharmacology (Berl) 2014;231:3041–3053. doi: 10.1007/s00213-014-3470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998a;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998b;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Hatch AM, Wiberg GS, Zawidzka Z, et al. Isolation syndrome in the rat. Toxicol Appl Pharmacol. 1965;7:737–745. doi: 10.1016/0041-008x(65)90132-8. [DOI] [PubMed] [Google Scholar]
- Hermes G, Li N, Duman C, Duman R. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol Behav. 2011;104:354–359. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst J, Barnard C, Tolladay U, et al. Housing and welfare in laboratory rats: effects of cage stocking density and behavioural predictors of welfare. Anim Behav. 1999;58:563–586. doi: 10.1006/anbe.1999.1165. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Giracello D, Solis A, Geyer MA. Post-weaning handling attenuates isolation-rearing induced disruptions of prepulse inhibition in rats. Behav Brain Res. 2001;120:221–224. doi: 10.1016/s0166-4328(00)00374-0. [DOI] [PubMed] [Google Scholar]
- Levine JB, Youngs RM, MacDonald ML, et al. Isolation rearing and hyperlocomotion are associated with reduced immediate early gene expression levels in the medial prefrontal cortex. Neuroscience. 2007;145:42–55. doi: 10.1016/j.neuroscience.2006.11.063. [DOI] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Cruz L, Carbó-Gas M, Pardo M, et al. Adenosine A2A receptor deletion affects social behaviors and anxiety in mice: Involvement of anterior cingulate cortex and amygdala. Behav Brain Res. 2017;321:8–17. doi: 10.1016/j.bbr.2016.12.020. [DOI] [PubMed] [Google Scholar]
- Luciano D, Lore R. Aggression and social experience in domesticated rats. J Comp Physiol Psychol. 1975;88:917–923. doi: 10.1037/h0076439. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav. 2009;55:248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Manduca A, Morena M, Campolongo P, et al. Distinct roles of the endocannabinoids anandamide and 2-arachidonoylglycerol in social behavior and emotionality at different developmental ages in rats. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol. 2015;25:1362–74. doi: 10.1016/j.euroneuro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Manduca A, Servadio M, Campolongo P, et al. Strain- and context-dependent effects of the anandamide hydrolysis inhibitor URB597 on social behavior in rats. Eur Neuropsychopharmacol. 2014:1–12. doi: 10.1016/j.euroneuro.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Morrish AC, Hill MN, Riebe CJN, Gorzalka BB. Protracted cannabinoid administration elicits antidepressant behavioral responses in rats: Role of gender and noradrenergic transmission. Physiol Behav. 2009;98:118–124. doi: 10.1016/j.physbeh.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Niesink RJ, Van Ree JM. Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology. 1989;28:411–418. doi: 10.1016/0028-3908(89)90038-5. [DOI] [PubMed] [Google Scholar]
- Niphakis MJ, Cognetta AB, III, Chang JW, et al. Evaluation of NHS Carbamates as a Potent and Selective Class of Endocannabinoid Hydrolase Inhibitors. ACS Chem Neurosci. 2013;4:1322–1332. doi: 10.1021/cn400116z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens RA, Mustafa MA, Ignatowska-Jankowska BM, et al. Inhibition of the endocannabinoid-regulating enzyme monoacylglycerol lipase elicits a CB1 receptor-mediated discriminative stimulus in mice. Neuropharmacology. 2017;125:80–86. doi: 10.1016/j.neuropharm.2017.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: Theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Parent CI, Meaney MJ. The influence of natural variations in maternal care on play fighting in the rat. Dev Psychobiol. 2008;50:767–776. doi: 10.1002/dev.20342. [DOI] [PubMed] [Google Scholar]
- Patel S, Hill MN, Cheer JF, et al. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev. 2017;76(Part A):56–66. doi: 10.1016/j.neubiorev.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition. Academic Press; 2006. [Google Scholar]
- Perkins AE, Woodruff ER, Chun LE, et al. Analysis of c-Fos induction in response to social interaction in male and female Fisher 344 rats. Brain Res. 2017;1672:113–121. doi: 10.1016/j.brainres.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SA, Loiacono RE, Christopoulos A, et al. The effect of social isolation on rat brain expression of genes associated with endocannabinoid signaling. Brain Res. 2010;1343:153–167. doi: 10.1016/j.brainres.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Rosa MLNM, Silva RCB, Moura-de-Carvalho FT, et al. Routine post-weaning handling of rats prevents isolation rearing-induced deficit in prepulse inhibition. Braz J Med Biol Res Rev Bras Pesqui Medicas E Biol. 2005;38:1691–1696. doi: 10.1590/s0100-879x2005001100018. doi: /S0100-879X2005001100018. [DOI] [PubMed] [Google Scholar]
- Sfikakis A, Galanopoulou P, Konstandi M, Tsakayannis D. Stress through handling for vaginal screening, serotonin, and ACTH response to ether. Pharmacol Biochem Behav. 1996;53:965–970. doi: 10.1016/0091-3057(95)02089-6. [DOI] [PubMed] [Google Scholar]
- Shonesy BC, Bluett RJ, Ramikie TS, et al. Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Rep. 2014;9:1644–1653. doi: 10.1016/j.celrep.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescent Neurodevelopment. J Adolesc Health. 2013;52:S7–S13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, de Bruin JP, van Haelst AM, et al. Influence of the mesocortical dopaminergic system on activity, food hoarding, social-agonistic behavior, and spatial delayed alternation in male rats. Behav Neurosci. 1989;103:24–35. doi: 10.1037//0735-7044.103.1.24. [DOI] [PubMed] [Google Scholar]
- Sticht MA, Limebeer CL, Rafla BR, et al. Endocannabinoid regulation of nausea is mediated by 2-arachidonoylglycerol (2-AG) in the rat visceral insular cortex. Neuropharmacology. 2016;102:92–102. doi: 10.1016/j.neuropharm.2015.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Z, Argueta D, Garland T, DiPatrizio N. Circulating levels of endocannabinoids respond acutely to voluntary exercise, are altered in mice selectively bred for high voluntary wheel running, and differ between the sexes. Physiol Behav. 2017;170:141–150. doi: 10.1016/j.physbeh.2016.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Toth M, Halász J, Mikics E, et al. Early social deprivation induces disturbed social communication and violent aggression in adulthood. Behav Neurosci. 2008;122:849–854. doi: 10.1037/0735-7044.122.4.849. [DOI] [PubMed] [Google Scholar]
- Toth M, Mikics E, Tulogdi A, et al. Post-weaning social isolation induces abnormal forms of aggression in conjunction with increased glucocorticoid and autonomic stress responses. Horm Behav. 2011;60:28–36. doi: 10.1016/j.yhbeh.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Toth M, Tulogdi A, Biro L, et al. The neural background of hyper-emotional aggression induced by post-weaning social isolation. Behav Brain Res. 2012;233:120–129. doi: 10.1016/j.bbr.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Manduca A, et al. Endocannabinoids in Amygdala and Nucleus Accumbens Mediate Social Play Reward in Adolescent Rats. J Neurosci. 2012;32:14899–14908. doi: 10.1523/JNEUROSCI.0114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJMJ. Cannabinoid and opioid modulation of social play behavior in adolescent rats: Differential behavioral mechanisms. Eur Neuropsychopharmacol. 2008;18:519–530. doi: 10.1016/j.euroneuro.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale AL, Montgomery AM. Social interaction: responses to chlordiazepoxide and the loss of isolation-reared effects with paired-housing. Psychopharmacology (Berl) 1997;133:127–132. doi: 10.1007/s002130050382. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Achterberg EJM, Trezza V. The neurobiology of social play and its rewarding value in rats. Neurosci Biobehav Rev. 2016;70:86–105. doi: 10.1016/j.neubiorev.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress. I. Preclinical studies. Depress Anxiety. 2002;15:126–147. doi: 10.1002/da.10016. [DOI] [PubMed] [Google Scholar]
- Walker SE, Zanoletti O, Guillot de Suduiraut I, Sandi C. Constitutive differences in glucocorticoid responsiveness to stress are related to variation in aggression and anxiety-related behaviors. Psychoneuroendocrinology. 2017;84:1–10. doi: 10.1016/j.psyneuen.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Wall VL, Fischer EK, Bland ST. Isolation rearing attenuates social interaction-induced expression of immediate early gene protein products in the medial prefrontal cortex of male and female rats. Physiol Behav. 2012;107:440–450. doi: 10.1016/j.physbeh.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Rêlo AL, et al. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Wongwitdecha N, Marsden CA. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav Brain Res. 1996;75:27–32. doi: 10.1016/0166-4328(96)00181-7. [DOI] [PubMed] [Google Scholar]
- Wotjak CT. Role of endogenous cannabinoids in cognition and emotionality. Mini Rev Med Chem. 2005;5:659–670. doi: 10.2174/1389557054368763. [DOI] [PubMed] [Google Scholar]
- Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol Behav. 1991;50:1129–1132. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiatry Res Neuroimaging. 2009;174:81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sun L, Jia H, et al. Isolation rearing induces social and emotional function abnormalities and alters glutamate and neurodevelopment-related gene expression in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1173–1177. doi: 10.1016/j.pnpbp.2009.06.016. [DOI] [PubMed] [Google Scholar]