Abstract
Strategies are needed to identify at-risk patients for adverse events associated with prescription opioids. This study identified prescription opioid misuse in an integrated health system using electronic health record (EHR) data, and examined predictors of misuse and overdose. The sample included patients from an EHR-based registry of adults who used prescription opioids in 2011 in Kaiser Permanente Northern California, a large integrated health care system. We characterized time-at-risk for opioid misuse and overdose, and used Cox proportional hazard models to model predictors of these events from 2011–2014. Among 396,452 patients, 2.7% were identified with opioid misuse and 1,044 had an overdose event. Older patients were less likely to meet misuse criteria or have an overdose. Whites were more likely to be identified with misuse, but not to have an overdose. Alcohol and drug disorders were related to higher risk of misuse and overdose, with the exception that marijuana disorder was not related to opioid misuse. Higher daily opioid dosages and benzodiazepine use increased the risk of both opioid misuse and overdose. We characterized several risk factors associated with misuse and overdose using EHR-based data, which can be leveraged relatively quickly to inform preventive strategies to address the opioid crisis.
Keywords: opioid overdose, opioid misuse, registry, electronic health record
1. Introduction
The misuse and abuse of prescription opioid medications and related overdose is a critical U.S. public health issue. While opioid prescribing has decreased nationally since 2012,1,2 opioid misuse and overdose continue to increase.3 In 2016, nearly half of all U.S. opioid overdose deaths involve a prescription opioid.3 Approximately 2 million people had a prescription opioid use disorder in 2015,4 and more than 15,000 people had a fatal overdose related to prescription opioids,5 higher than in 2014.6 In addition, misuse of prescription opioids is a risk factor for heroin use, which is a key contributor to the increasing rate of overdoses.7 It is essential that policymakers and healthcare providers can identify factors that predispose some individuals to misuse of prescribed opioids and overdose in order to address the opioid crisis.8
Data from large health systems allow for optimal study of opioid misuse, abuse, and related overdose, given that these events are relatively infrequent. While much of this research has been conducted with administrative data and has used varying definitions and algorithms to identify prescription opioid misuse,9 similar risk factors have emerged (i.e., male sex, younger age, substance use disorder, medical and psychiatric comorbidities, and using opioids >100 milligrams/day in morphine equivalents).10–13 Individuals at risk for opioid misuse are also likely to have multiple complex health needs, which not only increase the risk of medical harms associated with opioid misuse but also result in substantial burdens on society and health systems.9
Several studies have shown opioid-related overdose deaths increase proportionally with the prescribed dose, with significant increases at doses >100 mg/day in morphine equivalents.14,15 Other factors placing patients at high risk of overdose include long-term opioid use, concurrent benzodiazepine use,16,17 depression, substance use diagnoses, and poor overall health.11–13
Understanding predictors of misuse and overdose is critical so that health policy and healthcare systems can effectively target high risk opioid prescribing practices, and identify at-risk patients, as recommended by the Centers for Disease Control Guidelines.18 Using electronic health record (EHR) data can provide clinicians and health systems with timely clinical information and drive health system prevention strategies, such prescribing initiatives,7 naloxone distribution, surveillance, and disease management approaches to prevent misuse and overdose.
We build on previous literature by developing an EHR-based prescription opioid registry to examine misuse and overdose within a large, integrated health care system. EHR data based on encounters have been found to have more comprehensive diagnostic data relative to claims data,19–22 particularly in integrated systems where specialty care is provided internally. In addition, some data elements available in EHR data are not available in claims data (e.g. smoking status). We characterize individuals at risk of misuse and overdose among patients prescribed opioid medications from 2011 to 2014. Specifically, we (1) identify opioid misuse using EHR data; (2) describe time-at-risk for patients identified with misuse and overdose; and (3) examine the socio-demographic, clinical (e.g. medical and mood/anxiety comorbidity, alcohol and other drug use disorder), and pharmacological risk factors (e.g. concurrent benzodiazepine use) associated with misuse and overdose (non-fatal and fatal).
2. Methods
2.1. Study setting
Kaiser Permanente Northern California (KPNC) is a nonprofit, integrated healthcare system with approximately 4 million members. The population represents the region; however, as an insured population, it underrepresents those with low levels of education and income.23 All patients were selected from the KPNC membership, and approval was obtained from the Kaiser Foundation Research Institute Institutional Review Board.
2.2. Data sources and study population
The study population was adult members from a prescription opioid registry developed using KPNC’s EHR data. The registry represents all patients with an opioid fill from a KPNC outpatient pharmacy during 2011 (n=396,452), with follow-up data through 2014.24 The patient’s index date was the first opioid fill made during 2011. Encounters outside of KPNC were captured through claims. More than 90% of enrollees obtain their prescription medications through KPNC pharmacies.25 We excluded patients diagnosed with cancer. The detailed methodology used to create the opioid registry has been reported elsewhere.24
2.3. Data elements
The registry contains patient demographics (age, sex, race/ethnicity: Asian, African American, Hispanic, Native American, Multi-racial, white, and other/unknown), KPNC membership status, health service utilization, clinical International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnoses, pharmacy data, and mortality. As a proxy for socio-economic status (SES), it includes a neighborhood deprivation index (NDI) based on home address and census tract variables (e.g. households in poverty) from the U.S. Census Bureau’s 2006–2010 American Community Survey.26,27
2.3.1. Comorbid health conditions and mortality
The registry contains baseline comorbidities for each patient, based on all ICD-9-CM diagnoses documented during healthcare encounters. As in prior research with complex patient populations,28 we identified whether persons received a diagnosis for any of the following chronic medical conditions in the year prior to their index date: arthritis; hypertension; chronic pain; diabetes mellitus; asthma; ischemic heart disease; congestive heart failure; stroke/cerebrovascular accident; epilepsy; Parkinson’s Disease; end-stage renal disease; osteoporosis; chronic obstructive pulmonary disease.24 We categorized the number of conditions into 4 groups: 0, 1–2, 3–4, and >5. Based on their prevalence in this population,29,30 we also created a combined dichotomous indicator for mood/anxiety disorder diagnoses representing depression, anxiety, and bipolar disorder.28 Substance use disorder measures were dichotomous, reflecting ICD-9-CM abuse/dependence diagnoses for: alcohol, opioid, marijuana, and other non-opioid drug disorders (e.g. cocaine, methamphetamine). Smoking status indicated current tobacco smoker, former, or neither, gathered through annual universal screening in primary care.
Non-fatal opioid overdoses were identified by ICD-9-CM codes of opioid overdose or poisoning (Supplemental Table 1). Fatal overdoses were identified by state death certificates (underlying cause of death was listed as opioid overdose or poisoning).
2.3.2. Daily opioid and benzodiazepine use
All outpatient prescriptions of opioid and benzodiazepine (Supplemental Tables 2 and 3) fills from 1/1/11–12/31/14 are included. The registry contains one record/person/day from 1/1/11–12/31/14. For each day, variables indicate: 1) whether an opioid prescription was filled, 2) days supply; 3) the prescribing provider; 4) whether the person was assumed to be using opioids that day; 5) short- or long-acting opioid, and; 6) the morphine daily dose equivalent (MDDE) categories of: 0, 1–<20, 20–<50, 50–<100, 100+ milligrams. We assumed persons used according to provider instructions (for additional detail, see Ray, 2016).24 If a person had ≥7 days remaining on a prior fill at dispensation time, the new fill was assumed to be used concurrently, otherwise, the new fill was assumed to be used consecutively. We also created number of days of benzodiazepine use in six groups: 0, 1–<30, 30–<60, 60–<120, 120–<180, and 180 days.
2.3.3. Opioid misuse algorithm and score
Our measure of opioid misuse followed the method developed by Sullivan and Edlund.12 First, opioid misuse scores were computed using three variables: days supplied of short-acting opioids, days supplied of long-acting opioids, and number of prescribers. (We did not include the number of different pharmacies, as originally done by Sullivan and Edlund,12 since within the closed KPNC system, pharmacies are electronically connected and all dispensations are observable across the system. In addition, most patients fill their prescriptions at 1–2 KPNC pharmacies and would have received a “0” for this component). Scores were computed for each 180-day period between the patient’s index date and 12/31/2014.12,31 For long-acting and short-acting days supplied, 186–240 days = “1”, and >240 days = “2” for 6-month periods. Prescribers were categorized as: 3–4 prescribers = ‘1’, and > 4 prescribers = ‘2’; Scores for days supplied of short- and long-acting opioids, and number of prescribers were summed, with a possible range from 0–6. Higher misuse scores reflect a greater probability of misuse. Similarly, for each 180-day period, we created an indicator for whether the person received an ICD-9 opioid use disorder diagnosis (Supplemental Table 1). A period was classified as a misuse period if the misuse score was ≥3, (suggesting “possible” misuse)12 or if the person had an ICD-9 opioid use disorder diagnosis during that 180-day period. Patients were censored at the time of their first misuse period.
2.4. Data analysis
2.4.1. Descriptive analyses
We examined demographics, medical and mood/anxiety comorbidities, and substance use disorders (alcohol, opioid-, marijuana-, non-opioid drug disorder, smoking status) for patients taking prescription opioids in 2011. We also examined time-at-risk for opioid misuse by dose level for: person years, average days of opioid use/180-day period, average days of benzodiazepine use/180-day period, and percent of 180-day periods classified as misuse periods. Similarly, we examined time-at-risk for opioid overdose by dose level for: person years, average days of benzodiazepine use in prior 90 days, and overdoses/1000 person-years.
2.4.2. Predictors of opioid misuse
We identified characteristics from a prior 180-day period that predicted opioid misuse in the subsequent 180-day period. Given the length of follow-up, each person could have up to eight 180-day periods. Only periods during which the person died or had continuous KPNC membership were retained. Individuals whose first 180-day period met misuse criteria were excluded (since there were no “prior period” characteristics to use as predictors).
We conducted an extended Cox hazard model (SAS PHREG, version 9.3) with the dichotomous misuse indicator for the 180-day period as the dependent variable, and days since index date as the time scale. The models included time-invariant independent variables for each 180-day period (age at index date; sex; race; NDI in quartiles; number of chronic medical conditions; and psychiatric-, alcohol-, marijuana-, or non-opioid drug disorders; smoking status in year prior to the index date), and time-changing variables (MDDE and days of benzodiazepine use) from the prior 180-day period.
2.5. Predictors of opioid overdose
We predicted overdose using an extended Cox hazard model, with days since index date as the time scale. Patients were followed from their index date until any of the following occurred: 1) fatal or non-fatal overdose or poisoning; 2) death from other causes; 3) disenrollment from KPNC; or 4) December 31, 2014 (the last three events were treated as censoring events.). Fatal and non-fatal overdoses were analyzed together; there were not enough fatal overdoses (n=37) to be analyzed separately. Baseline time-invariant measures were the same as those in the misuse model. The two time-varying variables were the mean MDDE and number of days of benzodiazepine use, both measured in the prior 90 days. The start and stop variables in the SAS PHREG procedure represented days since index date.
3. Results
3.1. Opioid registry characteristics
The registry was 59% female, 57% white, and on average 52 years old (Table 1). A majority had at least 1 medical condition, and 23% had a mood/anxiety condition. Two percent had an alcohol, 0.8% had an opioid, 0.6% marijuana, and 1.3% had a non-opioid drug disorder diagnosis. About three percent (2.7%) of patients met the criteria for opioid misuse (not shown), and there were 1,044 overdoses (0.28%) in the study period.
Table 1.
Characteristics of Patients on Prescription Opioids, 2011, Kaiser Permanente Northern California
| Characteristic | Patients on Opioid Medication (n=396,452) |
|---|---|
| Gender, n (%) | |
| Female | 234,104 (59.0) |
| Male | 162,348 (41.0) |
| Age, mean (median) | 51.83 (51.8) |
| Age Group, n (%) | |
| 19 – <40 | 107,668 (27.2) |
| 40 – <65 | 199,297 (50.3) |
| 65 – <80 | 65,164 (16.4) |
| 80+ | 24,323 (6.1) |
| Race/Ethnicity, n (%) | |
| Asian | 38,428 (9.7) |
| Black | 37,161 (9.4) |
| Hispanic | 68,206 (17.2) |
| Native American | 2,400 (0.6) |
| Multi-racial | 17,700 (4.5) |
| Other or unknown | 7,333(1.8) |
| White | 225,224 (56.8) |
| Neighborhood deprivation, n (%) | |
| Quartile 1, least deprived | 90,382 (22.8) |
| Quartile 2 | 116,275 (29.3) |
| Quartile 3 | 110,873 (28.0) |
| Quartile 4, most deprived | 73,444 (18.5) |
| No. of chronic medical conditions at baseline, n (%)a | |
| None | 141,736 (35.8) |
| 1 to 2 conditions | 176,492 (44.5) |
| 3 to 4 conditions | 63,599 (16.0) |
| 5+ conditions | 14,625 (3.7) |
| Mood/anxiety disorder at baseline, n (%)a | 91,008 (23.0) |
| Opioid disorder at baseline, n (%)a | 3,367 (0.8) |
| Alcohol disorder at baseline, n (%)a | 8,448 (2.1) |
| Marijuana disorder at baseline, n (%)a | 2,537 (0.6) |
| Other non-opioid drug disorder at baseline, n (%)a | 5,230 (1.3) |
| Smoking status at baseline, n (%) | |
| Current smoker | 41,134 (10.4) |
| Former smoker | 81,052 (20.4) |
| Unknown smoking status | 97,585 (24.6) |
| Never smoked | 176,681 (44.6) |
Person was considered to have condition if they received a diagnosis code for it in the year prior to their index date (first opioid fill in 2011).
3.2. Time-at-risk for misuse and overdose
Time-at-risk for misuse analyses demonstrated there were fewer person years at higher opioid dose levels; more days of opioid use in each period at higher dosages; and a higher percent of misuse periods at the 100+mg level (7.4%) (Table 2). More days of benzodiazepine use occurred at the highest dose level (43 days).
Table 2.
Time-at-risk for opioid misuse and overdose analyses by Morphine Daily Dose Equivalent, 2011–2014.a
| Misuse analysis | Overdose analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Mean MDDE mgb | # Unique personsc |
Person- years |
Mean days opioid use per period |
Mean days BZD use per period |
% periods that were misuse |
# Unique personsc |
Person- years |
Mean days BZD use prior 90 daysd |
Total # opioid overdoses |
Overdoses per 1k person- years |
| 0 | 270,028 | 482,380 | 0 | 5 | 0.06 | 336,375 | 764,497 | 2 | 161 | 0.21 |
| 1 – <20 | 338,356 | 402,795 | 30 | 12 | 0.30 | 378,923 | 346,530 | 7 | 267 | 0.77 |
| 20 – < 50 | 37,509 | 53,709 | 131 | 31 | 2.27 | 72,884 | 71,005 | 16 | 190 | 2.68 |
| 50 – < 100 | 13,958 | 19,858 | 157 | 39 | 4.60 | 30,344 | 29,439 | 20 | 163 | 5.54 |
| 100+ | 7,825 | 14,124 | 168 | 43 | 7.43 | 15,217 | 26,465 | 24 | 263 | 9.94 |
| All dose levels | 361,785 | 972,866 | 25 | 10 | 0.48 | 396,168 | 1,237,936 | 5 | 1044 | 0.84 |
All patients had at least one opioid fill in year 2011 in Kaiser Permanente Northern California. BZD: benzodiazepines; MDDE: morphine daily dose equivalent.
For misuse analysis, daily dose is across 180-day periods. For overdose analysis daily dose is in the prior 90 days.
Persons could contribute time to one or more of the opioid dose categories. This sample is reduced due to exclusion of persons who had misuse in their first 180-day period (as there would be no prior period to use for prediction.)
On each day a patient was at risk for an overdose, we measured the number of days the patient used benzodiazepines in the prior 90 days
Time-at-risk for overdose analyses (Table 2) showed fewer person years at higher opioid dosages. Average days of benzodiazepine use in the prior 90 days was higher at higher opioid dosages, as were overdoses/1000 person years. There were 161 overdoses without a dispensed opioid in the prior 90 days.
3.3. Opioid misuse model
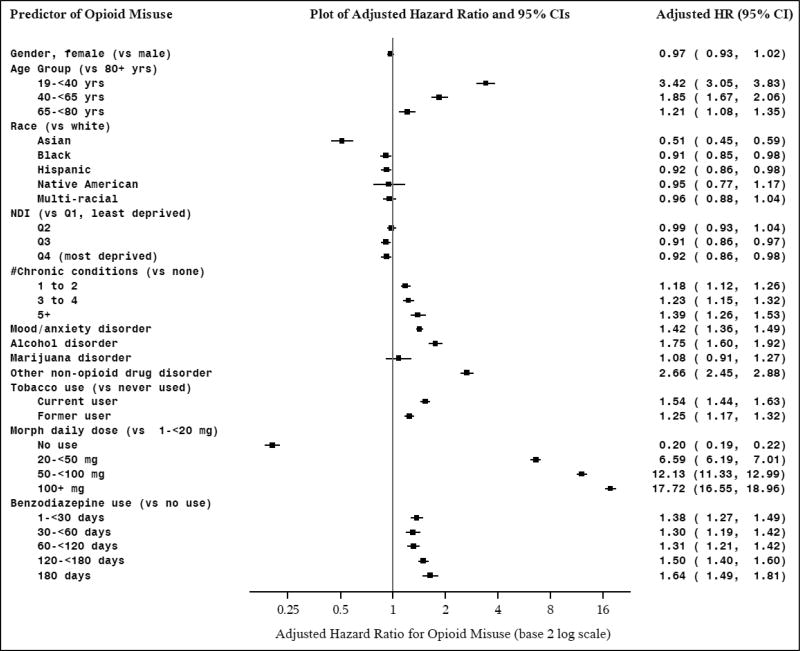
In the misuse hazard model, women were as likely as men to meet misuse criteria (Figure 1). Younger patients had higher risk of misuse than older patients (HRs from: 1.21–3.42). Asian (HR: 0.51, 95%CI: 0.45–0.59), African-American (HR: 0.91, 95%CI: 0.85–0.98), and Hispanic (HR: 0.92, 95%CI: 0.86–0.98) patients were less likely than Whites to have misuse, with no difference for Native American and Multi-racial groups. Patients with more chronic medical conditions (HRs: 1.18–1.39), and patients with a mood/anxiety disorder (HR: 1.42, 95%CI: 1.36–1.49) were more likely to meet misuse criteria. Patients in lower SES neighborhoods had less risk of misuse (HR: 0.92, 95%CI: 0.86–0.98). Those with alcohol (HR: 1.75, 95%CI: 1.60–1.92) and non-opioid drug disorders (HR: 2.66, 95%CI: 2.45–2.88) were more likely to have misuse, as were former (HR: 1.25, 95%CI: 1.17–1.32) and current smokers (HR: 1.54, 95%CI: 1.44–1.63) than never smokers. No association was found for marijuana disorder diagnoses and misuse. Opioid misuse risk increased with higher MDDE, with >100mg having the highest risk (HR: 17.72, 95%CI: 16.55–18.96) compared to the lowest dose group, although even those on lower dosages had increased risk. Benzodiazepine use increased risk of opioid misuse, even at 1–30 days (HR: 1.38, 95%CI: 1.27–1.49).
Figure 1.
Hazard Models of Predictors of Prescription Opioid Misuse
Notes: Persons with a prescription opioid fill in 2011, who were continuous KPNC members in the year prior to their first opioid fill in 2011, and who did not have an opioid overdose in the year prior to their first fill in 2011 (n=396,168); MDDE: morphine daily dose equivalent; NDI: neighborhood deprivation index.
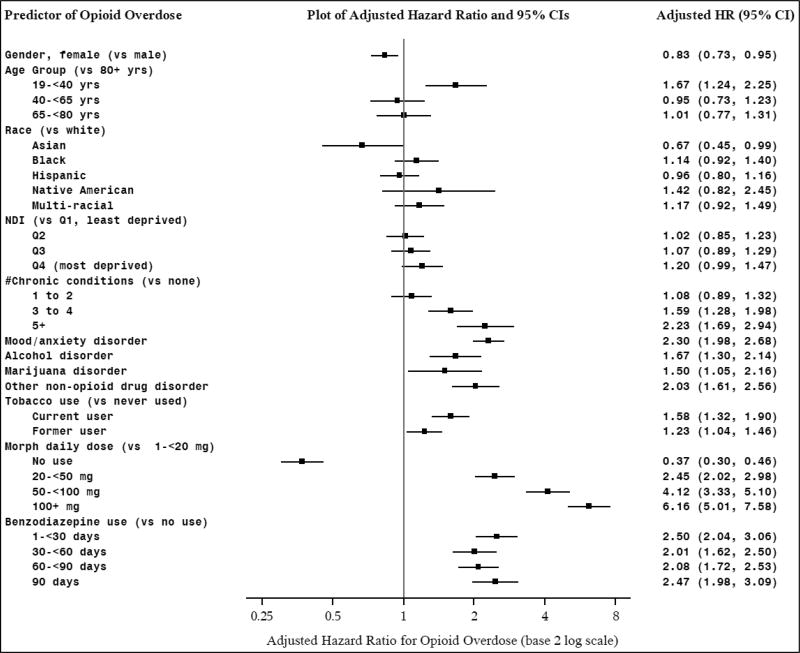
3.4. Opioid overdose model
Results of the overdose hazard model indicated that women were less likely (HR: 0.83, 95%CI: 0.73–0.95), and the youngest patients were more likely (HR: 1.67, 95%CI: 1.24–2.25), to experience an overdose (Figure 2). We found no differences by race/ethnicity or SES. Those with more chronic medical conditions (HR: 2.23, 95%CI: 1.69–2.94), or a mood/anxiety condition (HR: 2.30, 95%CI: 1.98–2.68) had higher risk. Patients with an alcohol, opioid, marijuana, and/or other non-opioid drug use disorder were more likely to have an overdose than those without each disorder (HRs from 1.50–2.03), as were current (HR: 1.58, 95%CI: 1.32–1.90) or former smokers (HR: 1.23, 95%CI: 1.04–1.46) (vs. never smokers). Increasing risk of overdose was related to higher MDDE (>100mg) (HR: 6.16, 95%CI: 5.01–7.58) compared to the lowest dose. Concurrent benzodiazepine use in the prior 180 days predicted roughly twice the overdose risk across all categories of use.
Figure 2.
Hazard Models of Predictors of Opioid Overdose
Notes: Persons with a prescription opioid fill in 2011, who were continuous KPNC members in the year prior to their first opioid fill in 2011, and who did not have an opioid overdose in the year prior to their first fill in 2011 (n=396,168); MDDE: morphine daily dose equivalent; NDI: neighborhood deprivation index.
4. Discussion
The current U.S. policy and clinical context regarding opioid prescribing is evolving rapidly. The prescribing environment has grown increasingly conservative in response to the ‘opioid epidemic.’18 While the use of prescription opioids has leveled off,32 opioid abuse and related deaths continue to increase.33,34 We used EHR data to examine detailed time-at-risk for opioid misuse and overdose, and predictors of these events. This has not been done before in an integrated health care delivery system to our knowledge, as previous efforts have focused on Medicaid and claims data.
Our estimate of patients with opioid misuse (2.7%) is in the range of recent studies,9,35,36 although lower than the commercially insured sample from Sullivan et al.12 Due to pharmacy data being integrated with the EHR across different pharmacies, prescribing may have been more tightly controlled, resulting in less opportunity for misuse behavior. Our use of more recent data may also reflect a more conservative prescribing environment.18 Our time-at-risk investigation showed that while relatively few individuals are at high dosages, they had the most days of opioid and benzodiazepine use, and the most periods identified with misuse and overdose.
Our findings for misuse suggest that younger patients and White patients may benefit from close monitoring and education about the risks of long-term opioid use. Similarly, the youngest age group had the highest risk of overdose, while women were less likely to experience an overdose, consistent with studies in other populations.14,15 However, we found no association for race/ethnicity, which differs from national data that have shown White individuals at higher risk of overdose than other race/ethnic groups.34 It could be that disparities in overdose risk are mitigated by access to health services in an integrated healthcare system. Findings may also differ in an insured population. We conducted ad hoc analyses of race/ethnicity by daily opioid dosage (results not shown). Daily dose prior to overdose event was similar for White patients relative to African American and Hispanic patients, which also may explain the lack of race/ethnic disparities. In an integrated system with potentially greater access to care, patients may have more opportunity to receive intervention (e.g. substance use or psychiatric treatment), and problems may be identified before adverse events occur. Opioid prescribing may be more amenable to standardizing and guidelines easier to enforce, with a mature EHR that can be leveraged to manage patient care (e.g. alerts for non-guideline concordant care).
Our investigation suggested patients residing in deprived areas were less likely to have opioid misuse, although residents in lower SES environments can be at higher risk of using chronic opioid therapy,24 which is often a risk factor for misuse. Even in an insured population, we cannot rule out that unobserved factors may preclude these patients from engaging with healthcare resources, thus limiting opportunities to identify misuse. Additionally, patients with insurance from lower SES environments may not be representative of those environments. However, studying an insured population removes a key barrier, insurance status, from confounding analyses, providing a more complete picture of care-seeking by the patient and of adverse health events. While other insurance characteristics (e.g. insurance type, benefit design) and barriers to care (e.g. transportation) exist, we nonetheless believe that the integrated health system provides an important laboratory to study these issues. At the same time, future work will be needed to explore the SES environments, to understand how related factors (e.g. type or lack of insurance) are associated with opioid misuse.
We observed the same dose-response relationship of increasing risk of misuse and overdose with higher MDDE that has been observed in other research, including our own.14,15,37 MDDE has been a robust predictor of misuse and overdose, and although earlier research has focused on high dosages (>100 MDDE), risk is also evident at low dosages. Our findings suggest even dosages of 20–50 MDDE signal risk, reinforcing federal guidelines on opioid prescribing that advise caution at >50 MDDE.18
All categories of days of benzodiazepine use demonstrated increased risk of opioid misuse, even <30 days supply, suggesting comparable risk between sporadic and more regular use, and this was similar for overdose, supporting the high level of concern surrounding concurrent benzodiazepine and opioid use. Concurrent drug use continues to increase,38 despite guidelines advising against concomitant prescribing.18,39 If, as our findings suggest, any concurrent benzodiazepine and opioid use raises risk of opioid misuse and overdose, clinicians need to be aware such adverse events can occur even with a low days supply. Our findings differ from a recent study of U.S. veterans which found no evidence of a relationship between benzodiazepine dosing schedule (i.e. as needed and regular) and opioid overdose.17 Differences in measurement, design, and sample may explain this difference, but both studies underscore the need to evaluate the risk-benefits of prescribing benzodiazepines at even low levels. Future investigations are needed on patterns of risky benzodiazepine use and the relationship to overdose risk to inform policies on prescribing benzodiazepines to patients taking prescription opioids.
Patients with medical and psychiatric comorbidities were at higher risk of misuse and overdose, highlighting a range of complex health needs, in addition to potential opioid problems. Our large sample also allowed us to examine different types of substance use problems. Alcohol and non-opioid drug use disorder raised the risk for misuse and overdose, as did smoking, with even former smokers having higher risk than those who had never smoked. Given growing interest in the relationship of marijuana use to opioid problems,40,41 we specifically examined marijuana use disorder. Interestingly, we found no association with opioid misuse, but higher risk of overdose. Clearly, this study is very different from state-level analyses of medical marijuana and opioid overdose rates, but to the extent that these patients may represent those using marijuana for pain, there was no evidence that it lessens the risk for overdose.
Our finding that 161 (15%) overdoses occurred with no documented dispensed opioids in the prior 90 days is concerning. There could be several reasons: patients received opioids from external prescribers, from friends or family, illegally from other sources, or had stockpiled opioids from previously dispensed prescriptions. These patients may be more difficult to identify in clinical settings through pharmacy strategies since they do not exhibit the same high dosage characteristics immediately prior to the overdose event.
4.1. Limitations
Pharmacy data represent dispensations, not actual consumption, although they are commonly used in prescription opioid studies.17,42,43 Pharmacy data also do not reflect prescriptions filled at external pharmacies, and may underestimate opioid and benzodiazepine consumption, and also our misuse indicator. Our misuse algorithm relies in part on clinical diagnoses of substance use, which likely represents a lower boundary of identified misuse, although our estimate is similar to studies using administrative or EHR data.9 Indication for type of pain related to opioid use was not available for most of the sample. ICD-9 codes for opioid abuse/dependence do not distinguish between type of opioid, thus we cannot examine problems with prescribed vs. illicit opioids (e.g. heroin). As with all observational data, associations do not indicate cause-and-effect relationships, and may be related to unobserved population factors. The generalizability of our data is limited to similar settings and populations, although enrollment is increasing in such health systems.
4.2. Conclusions and implications
EHR data can be used to identify several risk factors associated with misuse and overdose, including comorbidities and benzodiazepine use, critical information for identifying at-risk patients. Findings can inform targeted patient screening and intervention, prescribing of medications such as buprenorphine for opioid dependence or naloxone for opioid reversal, disease management approaches for this patient population, and guide appropriate opioid prescribing policies which are becoming increasingly conservative and risk being applied without an individualized approach.
Supplementary Material
Highlights.
-
-
Electronic health record data were used to create a prescription opioid registry.
-
-
Patients with chronic conditions were at higher risk for opioid misuse and overdose.
-
-
Younger patients and those with chronic conditions were more likely to have an overdose.
-
-
Even low opioid dose and benzodiazepine use increases misuse and overdose risk.
Acknowledgments
We thank Agatha Hinman, B.S., for her assistance with preparing the manuscript.
Funding source
This study was supported by the National Institute on Drug Abuse, Clinical Trials Network (1UG1DA040314).
G. Thomas Ray has received research support in the past three years through his institution from Pfizer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The remaining authors have no conflicts of interest to disclose.
References
- 1.Guy GP, Jr, Zhang K, Bohm MK, et al. Vital Signs: Changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697–704. doi: 10.15585/mmwr.mm6626a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuchat A, Houry D, Guy GP. New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425–426. doi: 10.1001/jama.2017.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration. NSDUH Data review: Prescription Drug Use and Misuse in the United States: Results from the 2015 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; Sep, 2016. [Google Scholar]
- 5.Centers for Diease Control and Prevention. [last updated August 1, 2017];Prescription opioid overdose data. 2017 https://www.cdc.gov/drugoverdose/data/overdose.html.
- 6.Centers for Diease Control and Prevention. [last updated February 9, 2017];Opioid data analysis. 2017 https://www.cdc.gov/drugoverdose/data/analysis.html.
- 7.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescriptionopioid use and heroin use. N Engl J Med. 2016;374(2):154–163. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Diease Control and Prevention. [last updated July 31, 2017];U.S. Prescribing rate maps. 2017 https://www.cdc.gov/drugoverdose/maps/rxrate-maps.html.
- 9.Cochran G, Woo B, Lo-Ciganic WH, Gordon AJ, Donohue JM, Gellad WF. Defining nonmedical use of prescription opioids within health care claims: a systematic review. Subst Abus. 2015;36(2):192–202. doi: 10.1080/08897077.2014.993491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic noncancer pain. Pain. 2007;129(3):355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Rice JB, White AG, Birnbaum HG, Schiller M, Brown DA, Roland CL. A model to identify patients at risk for prescription opioid abuse, dependence, and misuse. Pain Med. 2012;13(9):1162–1173. doi: 10.1111/j.1526-4637.2012.01450.x. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150(2):332–339. doi: 10.1016/j.pain.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White AG, Birnbaum HG, Schiller M, Tang J, Katz NP. Analytic models to identify patients at risk for prescription opioid abuse. Am J Manag Care. 2009;15(12):897–906. [PubMed] [Google Scholar]
- 14.Bohnert AS, Roeder K, Ilgen MA. Unintentional overdose and suicide among substance users: a review of overlap and risk factors. Drug Alcohol Depend. 2010;110(3):183–192. doi: 10.1016/j.drugalcdep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Annals of Internal Medicine. 2010;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brady KT, McCauley JL, Back SE. Prescription opioid misuse, abuse and treatment in the United States: an update. Am J Psychiatry. 2016;173(1):18–26. doi: 10.1176/appi.ajp.2015.15020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 19.Devoe JE, Gold R, McIntire P, Puro J, Chauvie S, Gallia CA. Electronic health records vs Medicaid claims: completeness of diabetes preventive care data in community health centers. Ann Fam Med. 2011;9(4):351–358. doi: 10.1370/afm.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heintzman J, Bailey SR, Hoopes MJ, et al. Agreement of Medicaid claims and electronic health records for assessing preventive care quality among adults. J Am Med Inform Assoc. 2014;21(4):720–724. doi: 10.1136/amiajnl-2013-002333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angier H, Gold R, Gallia C, et al. Variation in outcomes of quality measurement by data source. Pediatrics. 2014;133(6):e1676–1682. doi: 10.1542/peds.2013-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey SR, Heintzman JD, Marino M, et al. Measuring preventive care delivery: Comparing rates across three data sources. Am J Prev Med. 2016;51(5):752–761. doi: 10.1016/j.amepre.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon NP. Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: statistics from the 2009 California Health Interview Survey. Kaiser Permanente Northern California Division of Research. 2012 http://www.dor.kaiser.org/external/chis_non_kp_2009/
- 24.Ray GT, Bahorik AL, VanVeldhuisen PC, Weisner CM, Rubinstein AL, Campbell CI. Prescription opioid registry protocol in an integrated health system. Am J Manag Care. 2017;23(5):e146–e155. [PMC free article] [PubMed] [Google Scholar]
- 25.Selby JV, Smith DH, Johnson ES, Raebel MA, Friedman GD, McFarland BH. Kaiser Permanente Medical Care Program. In: Strom BL, editor. Pharmacoepidemiology. 4. New York: Wiley; 2005. pp. 241–259. [Google Scholar]
- 26.U.S. Census Bureau. A compass for understanding and using American Community Survey data: what general data users need to know. Washington, DC: U.S. Government Printing Office; 2008. [Google Scholar]
- 27.Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83(6):1041–1062. doi: 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young JQ, Kline-Simon AH, Mordecai DJ, Weisner C. Prevalence of behavioral health disorders and associated chronic disease burden in a commercially insured health system: findings of a case-control study. Gen Hosp Psychiatry. 2015;37(2):101–108. doi: 10.1016/j.genhosppsych.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Scherrer JF, Svrakic DM, Freedland KE, et al. Prescription opioid analgesics increase the risk of depression. J Gen Intern Med. 2014;29(3):491–499. doi: 10.1007/s11606-013-2648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barry DT, Cutter CJ, Beitel M, Kerns RD, Liong C, Schottenfeld RS. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(10):1413–1419. doi: 10.4088/JCP.15m09963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155(11):2337–2343. doi: 10.1016/j.pain.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 33.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths - United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 34.Han B, Compton WM, Jones CM, Cai R. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003-2013. JAMA. 2015;314(14):1468–1478. doi: 10.1001/jama.2015.11859. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19(8):648–665. [PubMed] [Google Scholar]
- 36.Cochran G, Gordon AJ, Lo-Ciganic WH, et al. An examination of claims-based predictors of overdose from a large Medicaid program. Med Care. 2017;55(3):291–298. doi: 10.1097/MLR.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weisner CM, Campbell CI, Ray GT, et al. Trends in prescribed opioid therapy for noncancer pain for individuals with prior substance use disorders. Pain. 2009;145(3):287–293. doi: 10.1016/j.pain.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang CS, Kang EM, Kornegay CJ, Staffa JA, Jones CM, McAninch JK. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002-2014. Am J Prev Med. 2016;51(2):151–160. doi: 10.1016/j.amepre.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Washington State Agency Medical Directors’ Group (AMDG) Interagency Guideline on Prescribing Opioids for Pain. 3. Olympia, Wa: Agency Medical Directors Group; Jun, 2015. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. [Google Scholar]
- 40.Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999-2010. JAMA Intern Med. 2014;174(10):1668–1673. doi: 10.1001/jamainternmed.2014.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bahorik A. Marijuana: It’s adverse impact on mental health outcomes in depression. Invited lecture at: University of California San Francisco Alliance Health Project. San Francisco, CA: Jun, 2016. [Google Scholar]
- 42.Campbell CI, Weisner C, Leresche L, et al. Age and gender trends in long-term opioid analgesic use for non-cancer pain. American Journal of Public Health. 2010;100(12):2541–2547. doi: 10.2105/AJPH.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiology and Drug Safety. 2009;18(12):1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.