Abstract
Background
Chronic alcohol intake leads to long lasting changes in reward- and stress-related neuronal circuitry. The nucleus accumbens (NAc) is an integral component of this circuitry. Here, we investigate the effects of DREADDs (Designer Receptors Exclusively Activated by Designer Drugs) on neuronal activity in the NAc and binge-like drinking.
Methods
C57BL/6J mice were stereotaxically injected with AAV2 hSyn-HA hM3Dq, -hM4Di, or -eGFP bilaterally into NAc [core+shell, core or shell]. We measured CNO (clozapine-n-oxide) induced changes in NAc activity and assessed binge-like ethanol or tastant/fluid intake in a limited access Drinking in the Dark (DID) schedule.
Results
We found that CNO increased NAc firing in hM3Dq positive cells and decreased firing in hM4Di cells, confirming the efficacy of these channels to alter neuronal activity both spatially and temporally. Increasing NAc core+shell activity decreased binge-like drinking without altering intake of other tastants. Increasing activity specifically in the NAc core reduced binge-like drinking, and decreasing activity in the NAc core increased drinking. Manipulation of NAc shell activity did not alter DID. Thus, we find that increasing activity in the entire NAc, or just the NAc core is sufficient to decrease binge drinking.
Conclusions
We conclude that the reduction in ethanol drinking is not due to general malaise, altered perception of taste, or reduced calorie-seeking. Furthermore, we provide the first evidence for bidirectional control of NAc core and binge-like drinking. These findings could have promising implications for treatment.
Keywords: alcohol, binge-drinking, nucleus accumbens shell or core, DREADDs
Introduction
Alcohol use disorders (AUDs) are devastating to individuals and their families, with substantial medical and societal impact. There exists a serious public health need for 1) better understanding of disease mechanisms and 2) improved treatments. Koob and Volkow (2010) reviewed decades of clinical and pre-clinical studies that address the neural circuitry associated with three stages of the addiction cycle: binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation. Chronic alcohol abuse leads to long lasting changes in reward- and stress-related neuronal circuitry (Koob and Volkow 2010). A significant point of convergence for this circuitry is the nucleus accumbens (NAc). There are two sub-regions of the NAc, the shell (medial and ventral aspect) and core (lateral and dorsal aspect). There are also two subtypes of GABAergic medium spiny neurons (MSNs) in the NAc, differentiated by expression of dopamine receptor D1 (Gs coupled receptor) or D2 (Gi coupled receptor). Human and animal studies thus far have indicated that both sub-regions and both MSN subtypes are important for drug-related behaviors, although they may contribute to different behaviors and during different stages of dependence (Thanos, Taintor et al. 2004, Chaudhri, Sahuque et al. 2010, Koob and Volkow 2010, Ambroggi, Ghazizadeh et al. 2011, Voges, Muller et al. 2013). Previous studies show that binge use increases dopamine release in the NAc shell, and withdrawal/negative affect recruits activity in the extended amygdala (including the NAc shell), whereas regions involved during the preoccupation/anticipation stage project (Glu) to the NAc core (among other brain regions) (Koob and Volkow 2010).
Over the last several years, evidence has emerged showing that manipulation of the NAc [via lesion, deep brain stimulation (DBS), or pharmacological inactivation] reduces alcohol- and drug-related behaviors (Ozburn, Janowsky et al. 2015). For instance, in humans, NAc lesions reduce relapse rates in alcohol dependent patients (but may cause irreversible cognitive deficits) (He, Guan et al. 2008, Wu, Wang et al. 2010). There is also evidence that stimulating or increasing activity in the NAc reduces alcohol drinking, craving, and relapse in humans (Pierce and Vassoler 2013). To further study the therapeutic potential of changing NAc activity, clinical studies have applied DBS to the NAc and found that electrical stimulation dramatically reduced alcohol craving and consumption (Heinze, Heldmann et al. 2009, Kuhn, Grundler et al. 2011, Voges, Muller et al. 2013).
A number of studies implicate both the NAc core and shell sub-regions as important for alcohol drinking and alcohol craving. Previous animal studies have found that lesioning the NAc core or shell reduced limited access drinking in mice (Dhaher, Finn et al. 2009, Cassataro, Bergfeldt et al. 2014). Knapp et al. (2009) found that DBS of either the NAc core or shell reduces alcohol drinking in rats (using a continuous access paradigm), and Wilden et al. (2014) found that DBS of the NAc shell reduced ethanol consumption in alcohol-preferring (P) rats (Knapp, Tozier et al. 2009, Wilden, Qing et al. 2014). The mechanisms by which stimulation exerts its behavioral effects are unknown. Although DBS of other brain regions can result in inactivation, this does not appear to be the case for the NAc. DBS of NAc core or shell increased c-Fos immunoreactivity in these nuclei, as well as the mPFC (Vassoler, White et al. 2013). Further, distal effects of acute NAc core DBS (for 2 hr) in rats include increases in dopamine and serotonin in the mPFC and dopamine and norepinephrine in the orbital frontal cortex (van Dijk, Klompmakers et al. 2012). Notably, reductions in alcohol craving (human studies) and drinking (animal studies) occur only during stimulation. Thus, DBS effects on craving and drinking are not lasting.
These DBS studies provide a solid basis for moving forward with the development of similar, and possibly more selective and long lasting treatments. Another strategy to increase activity is through cell-signaling cascades associated with G-protein coupled receptors (GPCRs). Stimulation of GPCRs invokes cell-signaling mechanisms that underlie plasticity, including transient and long term potentiation of neurons (Carr, Day et al. 2003, Anisuzzaman, Uwada et al. 2013). DREADDs (Designer Receptors Exclusively Activated by Designer Drugs) are mutagenized M3 (Gq-coupled; excitatory) or M4 (Gi-coupled; inhibitory) muscarinic GPCRs selectively activated by CNO (clozapine-n-oxide) that can be used to specifically manipulate neuronal firing in an acute or chronic manner (Rogan and Roth 2011). We focused here on determining whether CNO/DREADD mediated changes in NAc neuronal activity alters binge-like drinking in mice. The National Institute on Alcohol Abuse and Alcoholism defines binge drinking as a pattern of drinking in a short period of time that results in a blood ethanol level > 0.08 mg% or above. Binge-like drinking can be modeled in mice using limited access paradigms, such as Drinking in the Dark (DID, where mice are offered ethanol early in the dark phase of their circadian cycle) (Rhodes, Best et al. 2005). In the present set of studies, we tested the hypotheses that increasing NAc (core and/or shell sub-regions) activity via CNO/DREADDs would reduce binge-like drinking, whereas decreasing activity would increase drinking. Changes in ethanol drinking can sometimes be generalized to other tastants or fluids, thus we further investigated whether altering NAc activity changed tastant or fluid intake.
Methods
Animals
Adult C57BL/6J (The Jackson Laboratory, Bar Harbor, Maine, USA) female mice aged 2–5 months old were used for all experiments. C57BL/6J mice are genetically predisposed to high ethanol drinking, as shown by several inbred strain studies revealing that C57BL/6J female mice exhibit high alcohol preferring/drinking behaviors in DID and two bottle choice paradigms (Belknap, Crabbe et al. 1993, Rhodes, Ford et al. 2007, Yoneyama, Crabbe et al. 2008). We opted to perform these studies in female mice because females often consume higher levels of alcohol and are understudied. All mice were group housed in a reverse 12:12 light/dark (LD) cycle with food and water ad libitum unless otherwise specified. All procedures were approved by local Institutional Animal Care and Use Committees and were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Stereotaxic Surgeries
All mice received stereotaxic surgery (performed as in (Ozburn, Falcon et al. 2015)). Mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) in saline (0.9% NaCl). Bilateral stereotaxic injections of 1 µl of purified high titer AAV2-hSyn-HA-hM3D(Gq)-IRES-mCitrine (hM3Dq), AAV2-hSyn-HA-hM4D(Gi)-IRES-mCitrine (hM4Di), or AAV2-hSyn-eGFP was injected into the NAc core+shell (from bregma, in mm: angle 10°, AP +1.5, Lat +1.5, DV −4.4), NAc core (from bregma: AP +1.34, Lat +1.0, DV −4.0 and −4.5; where 0.5 ul was infused at both DV −4.0 and −4.5 to infuse above and below the anterior commissure), or NAc shell (from bregma: AP +1.46, Lat +0.5, DV −4.75) using a 33 gauge Hamilton syringe (Hamilton, Reno, NV). AAV titers were 10E11–10E12 vg and were produced by Dr. R. Jude Samulski at University of North Carolina Viral Vector Core. Injection speed was 0.1 µl/minute, and the needle was kept in place for an additional 5 minutes before it was slowly withdrawn. Mice recovered for 2–3 weeks in their home cage to allow for full viral expression prior to experimental testing.
Electrophysiology
Two weeks after viral infusions of AAV vectors into the NAc, animals (n=4–6/group) were sacrificed and 200µm coronal brain slices were taken. Slices were maintained at 30°–32°C in aCSF (in mM: 119 NaCl, 2.5 KCl, 26 NaHCO3, 1 NaH2PO4, 2.5 CaCl2, 1.3 MgCl2, 11 D-Glucose 290–300 mOsm; 95% O2/ 5% CO2) and standard whole-cell current clamp recordings were made from infected MSNs in the NAc identified by differential interference contrast (DIC) optics using the anterior commissure as a landmark. Evoked action potential firing was measured with resting membrane potential adjusted to −80mV and current steps from 100–450 pA. A current step sufficient to evoke spikes was delivered every 10 seconds and spike numbers were averaged from stable baseline recordings (5–10 sweeps) and following 5 minutes of CNO application (10uM). A K+ based internal solution was used (in mM: 119 K-MeSO4, 2 KCl, 1 MgCl2, 1 EGTA, 0.1 CaCl2, 10 HEPES, 2 Mg-ATP, 0.4 Na-GTP 275–285 mOsm; pH 7.2–7.4). Series resistance for all recordings was monitored continuously. Cells with a change in series resistance beyond 20% were excluded from data analysis. Evoked action potentials were recorded with a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Signals were filtered at 2.6–3 kHz and amplified 10 times, then digitized at 20 kHz with a Digidata 1322A analog-to-digital converter (Molecular Devices). Data were analyzed using pClamp 10 software (Molecular Devices).
Drinking in the Dark (DID)
Ethanol consumption is determined by many factors, including palatability; therefore, we tested the effects of altering NAc activity on intake of ethanol, as well as other tastants and water using a modified DID schedule. As in Rhodes et al. (2005), mice were habituated to individual housing and a new sipper bottle for one week prior to testing (Rhodes, Best et al. 2005). For the ethanol DID assay, mice were then offered a single bottle of 20% ethanol (v/v, in tap water; Decon Laboratories, Inc., Pennsylvania), starting 3 hours after lights off. For tastant and fluid DID assays, mice were offered a single bottle containing a solution of quinine hemisulfate salt monohydrate (0.03 mM or 0.06 mM in tap water), sucrose (2.5% or 5% in tap water), or tap water. Fluid intake was measured for either 2 or 4 hrs; see specific experiment details below. Water was then restored for the remainder of the day (unmeasured). Mice were injected (IP) daily with vehicle (1%DMSO in saline) or CNO (0.5 or 1mg/kg; RTI International, North Carolina) 15–30 min prior to measured fluid access. Details for each study follow below.
Acute Study - NAc core + shell (excitatory DREADD vs. GFP)
Groups of mice were injected IP with CNO (0.5 mg/kg on days 1,2,3,4) 15–30 min before DID. The 0.5mg/kg dose of CNO was chosen based on a study showing this dose increased neuronal activity in mice expressing hM3Dq (Alexander et al., 2009). The water tube was removed and a 20% ethanol solution was presented for two hours (3 hours after lights off) on days 1, 2, 3, and for four hours on day 4. This typical DID paradigm was then modified by adding one day of abstinence with no treatment (day 5) followed by administration of CNO (1mg/kg) before 2 hr of ethanol availability on day 6. We administered a higher dose of CNO on day 6 because we anticipated employing the inhibitory DREADD (hM4Di) in our next experiment and CNO has a slightly lower affinity for this receptor, requiring the 1 mg/kg dose to be administered. Further, we wanted to determine whether the 1mg/kg CNO dose produced a more robust effect. n=7–8/group.
Chronic Study - NAc core + shell (excitatory or inhibitory DREADD, or GFP)
DID was carried out as in Rhodes et al. (2005), with a modification to limit access to 2 hr/day. Groups of mice were habituated to individual housing and a new sipper bottle one week prior to testing. There were four periods of ethanol testing with 2 weeks of recovery between each period. This schedule was designed to assess the effect of changing NAc activity on binge-like (1) ethanol drinking (14 days: vehicle pretreatment daily for 7 days, 1 mg/kg CNO pretreatment daily for 7 days), (2) intake of a bitter solution (4 days: quinine, 2 days each concentration), (3) a sweet, caloric solution (4 days: sucrose, 2 days each concentration), and (4) water (four days). Prior to each 4 day period of tastant/fluid testing, all mice were injected with CNO (1 mg/kg) each day. Each measured solution was presented (alone, no choice) for two hours. n=8–9/ group.
Chronic Study - NAc core or medial shell (excitatory or inhibitory DREADD, or GFP)
In order to determine whether increasing or decreasing NAc activity chronically and specifically in core or medial shell could alter binge-like alcohol drinking, we assayed the effects of CNO/DREADDs on binge-like drinking using a two week DID paradigm (2 hr/day ethanol access). During the first week of DID access to 20% ethanol, groups of mice were pre-treated with vehicle to establish baseline levels of intake, and during the second week, mice were administered CNO (1mg/kg) prior to the drinking session. n=11–13/group (core); n=9–14/group (shell).
Immunohistochemistry
After the completion of behavioral studies, mice were deeply anesthetized with 250 mg/kg ketamine and 25 mg/kg xylazine and transcardially perfused with PBS and 4% paraformaldehyde in PBS. The brains were allowed to post-fix in 4% paraformaldehyde in PBS for 24 hours and then placed in 30% glycerol in PBS for an additional 24 hours before being stored in PBS-0.01% sodium azide. Using a freezing microtome (Leica, Wetzlar, Germany), 30 µm brain sections were obtained and immunohistochemical staining was carried out using standard procedures. Antibodies used include rabbit anti-GFP (1:20,000; AbCam, cat#ab290) or rabbit anti-HA (1:1000; Cell Signaling, cat#3724) and anti-rabbit conjugated with Alexa 488 (1:500; Molecular Probes, Carlsbad, CA). Brain sections were mounted using Vectashield with DAPI (Vector Labs, Burlingame, CA) and observed using a fluorescence microscope. Animals were excluded from our study if expression was not localized to the NAc.
Statistics
Data are presented as the mean +/− SEM. Data with normal distributions were analyzed using Student’s t-test, or one- or two-way ANOVAs, followed by Bonferroni’s post-hoc tests; data with non-normal distributions were analyzed using Mann-Whitney tests (data presented in Figure 2). Data analysis was performed and graphs were generated using GraphPad Prism.
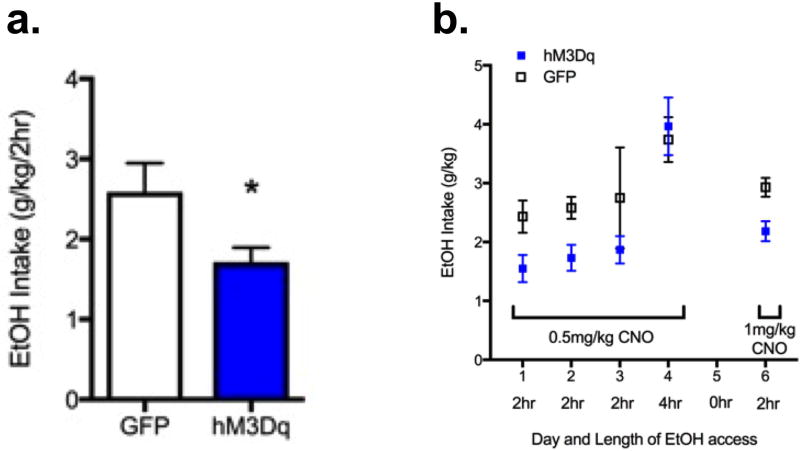
Figure 2. Increasing NAc core and shell activity decreased acute binge-like ethanol drinking.
a) Average 2 hr intake for individuals on DID days 1,2,3: Mann-Whitney test, Median ethanol intake for GFP and hM3Dq mice were 2.33g/kg (n=8) and 1.79 g/kg (n=7); the distributions in the two groups differed significantly (Mann–Whitney U = 7, p = 0.01, two-tailed). b) Increasing NAc core+shell activity decreased acute binge-like ethanol drinking on Days 1,2,3 and 6. Two way ANOVA (only includes data from 2 hr drinking days 1, 2, 3, and 6) revealed main effect of AAV group F(1,52)=9.70, p < 0.01). The effect of CNO on mice expressing hM3Dq was noticeably absent on day 4, when ethanol access was 4 hr. There was not a significant difference in intake between 0.5 and 1 mg/kg CNO. Because the half-life of CNO is ~2 hours, the length of access to ethanol drinking was adjusted to 2 hours in subsequent studies. n=7–8/group.
Results
Pharmacogenetic manipulation of neuronal activity in the NAc
To assess the effect of CNO on hM3Dq, hM4Di, or GFP expressing neurons, we performed standard whole-cell current clamp recordings from virally transduced MSNs in the NAc. Evoked action potential firing was measured with resting membrane potential adjusted to −80mV and current steps from 100–450 pA.
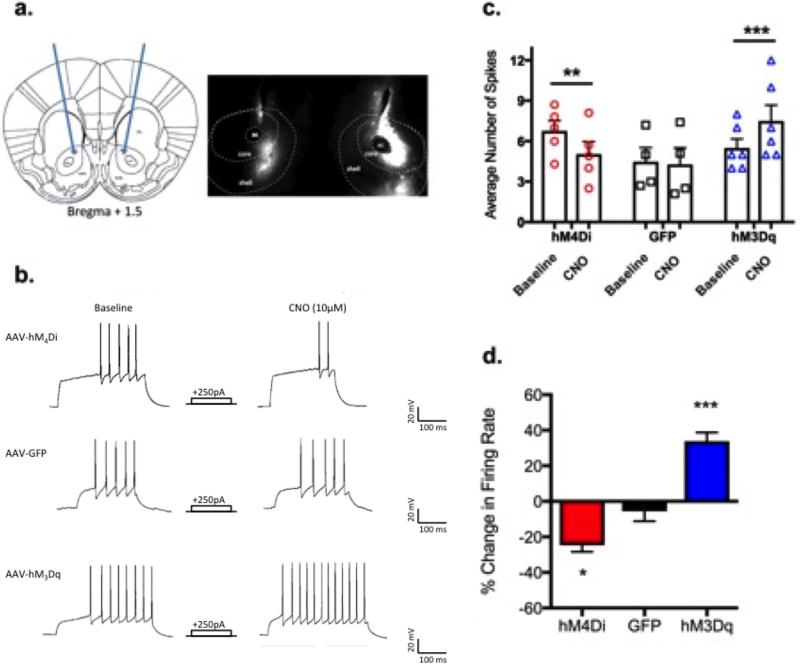
Figure 1a shows an example of viral placement in core+shell and Figure 1b shows representative traces from recordings, where CNO application decreased the number of evoked action potentials in hM4Di (top) expressing MSNs, did not alter firing in GFP (middle) expressing MSNs, and increased evoked action potentials in hM3Dq (bottom) expressing MSNs. Data showing individual and average (+/− SEM) responses to successive current injections at baseline and following CNO application are shown in Figure 1c, where a treatment × group interaction was identified [F(2,12)=22.8, p<0.0001]. Bonferroni post-hoc testing revealed CNO reduced firing for cells expressing hM4Di (p<0.01) and significantly increased firing in cells expressing hM3Dq (p<0.001).
Figure 1. Pharmacogenetic manipulation of neuronal activity in the NAc.
a.) Targeted region for stereotaxic delivery of AAV2 hM3Dq, hM4Di, or GFP to the NAc core+shell (left) and representative 2× image showing viral expression in the NAc core and shell. b.) Representative traces show CNO application reduced the number of evoked action potentials in hM4Di expressing neurons (top), did not change firing rates in GFP expressing neurons (middle), and increased firing in hM3Dq neurons. c.) individual and mean firing rates for each group at baseline and after CNO (n=4–6/group; Two-way ANOVA treatment×group interaction, F(2,12)=22.8, p<0.0001). Bonferroni post-hoc **=p<0.01, ***p<0.001 (CNO vs.baseline). d.) Percent change in firing rate after CNO application (One-way ANOVA F(2,12)=42.8, p<0.0001); Bonferroni post-hoc *=p<0.05 (hM4Di vs GFP), ***p<0.001 (hM3Dq vs. GFP).
Percent change in firing rate after CNO application is shown in Figure 1d (One-way ANOVA F(2,12)=42.8, p<0.0001); Bonferroni post-hoc *=p<0.05 (hM4Di vs GFP), ***p<0.001 (hM3Dq vs. GFP). CNO 1) significantly decreased evoked firing rates by approximately 25% in hM4Di expressing MSNs, 2) increased evoked firing rates by approximately 35% in hM3Dq expressing MSNs, and 3) produced no significant changes in firing rates in MSNs expressing GFP.
Increasing NAc (core + shell) activity decreased binge-like ethanol drinking in an acute DID schedule
Results from this study indicate that acute and repeated stimulation of NAc activity (via CNO/DREADDs) significantly reduced ethanol intake in the DID paradigm for 3 days (Figure 2a; average 2 hr intake for individuals on DID days 1,2,3: Mann-Whitney test, Median ethanol intake was 2.33g/kg for GFP mice (n=8) and 1.79 g/kg for hM3Dq mice (n=7); the distributions in the two groups differed significantly (Mann–Whitney U = 7, p = 0.01, two-tailed). However, when the DID access was increased to 4 hours on day 4, the effect was noticeably absent (Figure 2b). Due to the short half-life of CNO (<2 hrs; see discussion), and because mice were injected with CNO 15–30 minutes prior to DID, we presume that there was no bioavailable CNO during the last 2 hours of the session on day 4. After a one-day break, we assayed the effects of 1 mg/kg CNO on 2 hr DID and again found that it reduced intake in mice expressing the excitatory DREADD, hM3Dq (Figure 2b). We did not expect the 1mg/kg dose to have a more robust effect of DID intake than the 0.5 mg/kg CNO dose, specifically because there is limited expression of DREADDs in the membrane for the CNO to bind to and exert an effect (notably, these doses are in the low range of published studies with DREADDs). Results from this study show that increasing activity in the NAc reduced binge-like drinking by ~34%. However, one caveat to this experimental design is that it lacked a vehicle control (all mice were given CNO), which we addressed in the next experiment.
Increasing NAc (core + shell) activity reduced chronic binge-like ethanol drinking
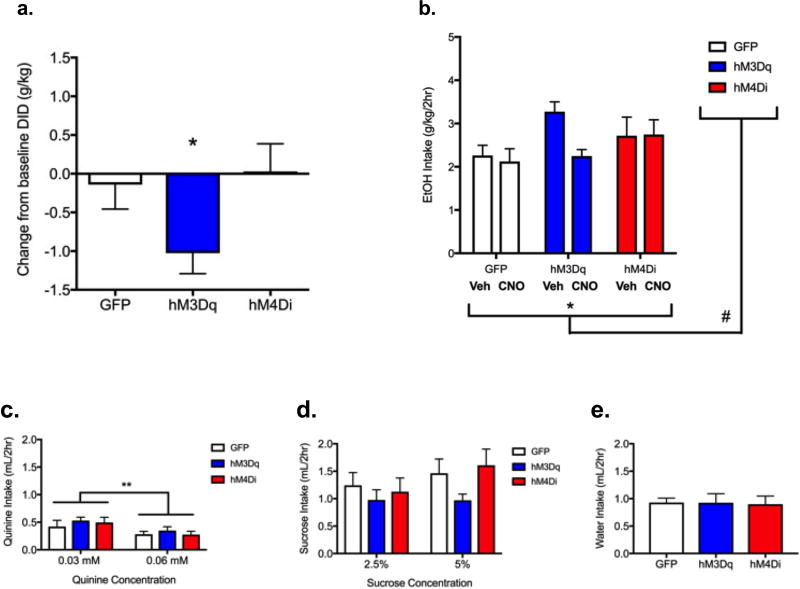
In order to determine whether increasing or decreasing NAc (core and shell) activity could more chronically alter binge-like alcohol drinking, we used a two-week DID paradigm. Mice were pre-treated with vehicle to establish baseline levels of intake during the first week, and then in the second week, mice were treated daily with CNO prior to DID. Average daily ethanol intakes for each treatment week (vehicle and CNO) data are presented in Figure 3b. Repeated-measures two-way ANOVA revealed a significant main effect of CNO treatment to reduce drinking (F(1,22)=4.29, p=0.05) and a trend toward a group×treatment interaction (F(2,22)=3.11, p=0.06). To pursue the interaction, we expressed the response of each animal during CNO treatment as a change from its own individual average baseline intake score. These difference scores are shown in Figure 3a. One way ANOVA revealed a trend toward significant group differences (F(2,22)=3.112, p=0.06). We hypothesized that increasing activity would reduce binge-like drinking, and a direct test of this hypothesis revealed that mice in the hM3Dq group showed a significant reduction as compared to mice in the GFP group (t=2.114, df=15, p< 0.05). Chronically increasing activity in the NAc thus reduced binge-like drinking by ~29%, similar to the acute effects (Figure 2).
Figure 3. Increasing NAc core+shell activity decreased chronic binge-like ethanol drinking without altering intake of other fluids.
a) Ethanol intake presented as average individual difference scores (intake during treatment with CNO – intake during treatment with vehicle). As in previous experiment (see Fig. 2), mice in the hM3Dq group showed a significant reduction as compared to mice in the GFP group (Student’s t-test, one tailed, t=2.114, df=15, p< 0.05). b) Average weekly ethanol intake after treatment with vehicle or CNO. Two-way ANOVA revealed a significant main effect of CNO treatment [F(1,22)=4.29, p=0.05 (*)] and a nonsignificant trend toward a group × treatment interaction [F(2,22)=3.11, p=0.06 (#)]. c) Altering NAc activity does not change intake of a bitter solution. There was a significant decrease in quinine intake at the higher concentration for all groups [F(1,23)=13.54, **p<0.01]. Mice consumed high levels of sucrose solutions (d) and moderate levels of the neutral solution, water (e). Separate ANOVAs were carried out for d) and e), where no trends or significant effects of treatment were observed for either fluid. n=8–9/group.
After the alcohol DID and a period of abstinence, we assessed whether changing NAc MSN activity altered intake of specific tastants or fluid more generally by measuring intake of a bitter quinine solution (0.03 and then 0.06 mM quinine), a sweet sucrose solution (2.5% and then 5%, w/v), and tap water. Separate ANOVAs were carried out for each solution. As expected, mice consumed low levels of the quinine solutions. There was a significant decrease in intake at the higher concentration for all groups [F(1,23)=13.54, p<0.01] (Figure 3c). Mice consumed high levels of sucrose solutions and moderate levels of the neutral solution (water). While CNO appeared to reduce consumption of 5% sucrose in hM3Dq mice, no significant effects of treatment were observed for either sucrose or water (Figures 3d,e). These results suggest altering NAc activity did not alter tastant or fluid intake.
Altering NAc core activity bidirectionally altered chronic binge-like ethanol drinking
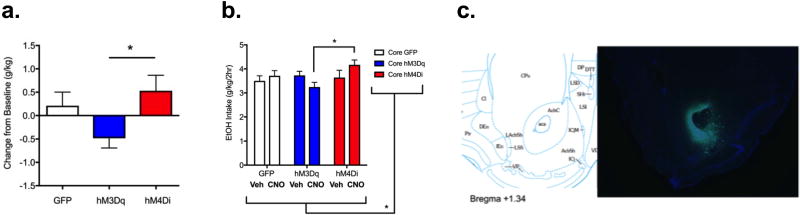
In order to determine whether chronically increasing or decreasing activity specifically in the core sub-region of the NAc could alter binge-like alcohol drinking, we used drinking in a two week DID paradigm. During the first week of DID, mice were pre-treated with vehicle to establish baseline levels of intake prior to the CNO pre-treatment during the second week. Mean individual difference scores (intake after CNO treatment as a change from the baseline intake score) are shown in Figure 4a. One way ANOVA revealed a significant effect of CNO treatment (F(2,33)=3.582, p<0.05). This appeared to result from differentially altering NAc core activity: although post-hoc testing revealed that neither DREADD group differed significantly from the GFP group, they differed significantly from each other (p<0.05, hM3Dq versus hM4Di). Average ethanol intakes for each treatment week (vehicle and CNO) are presented in Figure 4b. Representative image from immunofluorescent verification of AAV injection placement is shown in Figure 4c.
Figure 4. Bi-directional modulation of NAc core activity and chronic binge-like ethanol drinking.
a) Ethanol intake presented as average individual difference scores (intake during treatment with CNO – intake during treatment with vehicle) suggests bidirectional modulation of binge-like drinking in the mice treated with hM3Dq as compared with hM4Di (One way ANOVA, F(2,33)=3.6, p<0.05). Bonferroni post-hoc *=p<0.05. b) Two-way ANOVA identified a significant group × treatment interaction (F(2,33)=3.57, * p<0.05), with a Bonferroni post-hoc test revealing significant differences between hM3Dq and hM4Di groups in response to CNO treatment (* p<0.05). c) Diagram (left; from Franklin and Paxinos, 2007) and 4× image (right) of coronal section showing targeting of NAc core (where green indicates viral transduction and blue indicates DAPI stain). n=11–13/group.
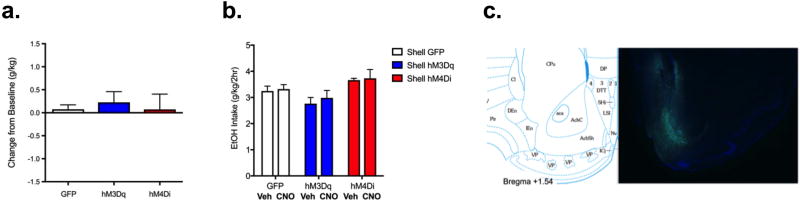
Altering NAc shell activity did not affect chronic binge-like ethanol drinking
The difference scores for DREADD groups are presented in Figure 5a, where we observed no significant effects, suggesting that changing NAc medial shell activity did not affect binge-like drinking. Average ethanol intakes for each treatment week (vehicle and CNO) are presented in Figure 5b. Representative image from immunofluorescent verification of AAV injection placement is shown in Figure 5c.
Figure 5. Altering NAc shell activity did not alter chronic binge-like ethanol drinking.
a) Ethanol intake presented as difference score (individual intake during treatment with CNO). One way ANOVA N/S. b) Two-way ANOVA identified a significant main effect of group (F(2,30)=3.97, p<0.05), but not treatment or treatment × group interaction (suggesting changing NAc shell activity did not affect binge-like drinking). c) Diagram (left; from Franklin and Paxinos, 2007) and 4× image (right) of coronal section showing targeting of NAc shell (where green indicates viral transduction and blue indicates DAPI stain). n=9–14/group.
Discussion
These experiments demonstrate that neuronal activity can be controlled in a spatial and temporal manner using CNO/DREADDs. Increasing activity in the entire NAc, or just the NAc core was sufficient to decrease binge-like drinking. The ability to change brain activity with spatial and temporal precision has opened up new avenues for therapeutics, as well as for basic studies in neuroscience, where the NAc is currently a promising target for addiction (Luigjes, van den Brink et al. 2012). Acute and chronic alcohol alters electrical membrane properties of MSNs, and produces a dysregulation of neurotransmission in the NAc (Volkow, Fowler et al. 2004, Marty and Spigelman 2012, Marty and Spigelman 2012). Individuals with substance use disorders exhibit hypoactive mesolimbic/cortical circuitry (Volkow, Fowler et al. 2004). Stimulation of the NAc may mitigate hypoactive circuitry in the brain of individuals with AUD, acting as a substitute for the effects of drug action.
Clinical studies in treatment resistant alcoholics suggest deep brain stimulation (DBS) of the NAc shell relieves symptoms of craving and reduced relapse (Heinze, Heldmann et al. 2009). Additionally, several pre-clinical studies found that stimulating the NAc reduced alcohol intake and relapse-like drinking (Knapp, Tozier et al. 2009, Henderson, Green et al. 2010, Wilden, Qing et al. 2014). van Dijk et al. (2012) evaluated the effects of DBS in the NAc core in rats on monoamine neurotransmitter concentrations in cortical regions (van Dijk, Klompmakers et al. 2012). Acute stimulation (2 hr) significantly increased dopamine and serotonin in the mPFC, and dopamine and noradrenaline in the orbital frontal cortex (van Dijk, Klompmakers et al. 2012). This important study provides further evidence for the distal effects of increasing brain activity via DBS. Stimulating the NAc has also been shown to effectively reduce craving for morphine, heroin, and cocaine (Guo, Zhou et al. 2013, Ma, Chen et al. 2013, Vassoler, White et al. 2013). One potential therapeutic caveat to DBS is that its effects can be short lasting. The studies we report here with DREADDs offer the potential for longer lasting modulation of neuroplasticity via G-protein coupled receptor manipulations. It will require further studies for us to determine how long the increased NAc activity can be maintained, and whether its effect to reduce binge-like drinking will persist.
Studies report that stimulation of either the NAc core or shell (or both) is effective in reducing drug-related behaviors. The core-shell-dichotomy has been elaborated mainly in rodents, whereas in humans, these sub-regions are less well characterized. Here, we report sub-region specific effects of manipulating activity via CNO/DREADDs on binge-like drinking in C57BL/6J mice. We found that increasing activity in the NAc core reduced binge-like drinking, whereas decreasing activity appeared to increase drinking. These “bi-directional” effects were significant, but modest. It is possible that the modest effect size could be attributed to the short duration of action of CNO, which limits the time of the scheduled alcohol access period, and makes it difficult to observe large increases or decreases in alcohol intake. The inhibotry DREADD effects were not observed when manipulating activity either in both the core and shell of the NAc or in the NAc shell alone. This phenomenon could be due to the role of the NAc core sub-region in specific neurocircuitry engaged in binge-like drinking. For example, the core has reciprocal projections with other regions known to be important for motivated behaviors, as well as alcohol behaviors (e.g. prelimibic and infralimbic cortex, ventral pallidum, and basolateral amygdala) (Ozburn, Janowsky et al. 2015). The effects appeared more robust for mice from the NAc (core+shell) hM3Dq group. It is not clear if this is because viral transduction was also present in the core and shell border region. The core-shell border region is not well-studied in binge-like drinking. Howard et al. (20XX) reported increases in DA in the border area between the core and shell when male rats are exposed to alcohol self-administration cues and during self-administration, whereas increases in DA were observed in the core and shell only during cue exposures. As discussed in Namburi et al. (2016), there exist specific populations of neurons which have been found to differentially respond to or impact appetitive and aversive behaviors. It is important to note there exists functional, anatomical, and genetic characteristics which all contribute to the representation of valence in the NAc shell. Perhaps the reason we did not observe effects of medial shell manipulation on binge-like drinking is because our manipulations spanned rostral to caudal regions of the medial shell (generally, rostral medial shell has been associated with positive valence and caudal medial shell is associated with negative valence). Together, these findings suggest further study of the organization of the NAc as it relates to binge-like drinking is warranted.
An additional consideration in the interpretation of these findings is that we used CNO/DREADDs, which are GPCRs, to produce physiologically relevant changes in activity. Recent work has shown that lesions, pharmacological inactivation, and DBS of the NAc decrease alcohol intake in animal models (Dhaher, Finn et al. 2009, Cassataro, Bergfeldt et al. 2014, Wilden, Qing et al. 2014). These manipulations likely have much more robust consequences on activity and circuits than CNO/DREADD-induced alterations in neuronal activity (which result in approximately a 30% increase or decrease in activity in the accumbens, which may only last for a few hours). Thus, we propose the effects of CNO/DREADDs on DID we observed here are mediated via more subtle effects on cell signaling and circuit level. Cassataro et al. (2013) reported that chronic NAc inhibition (using CNO stimulation of inhibitory DREADDs) reduced limited access drinking in male mice (Cassataro, Bergfeldt et al. 2014). It is possible that there exist sex differences in response to CNO/DREADDs effects on binge-like drinking since the results of our experiments in female mice are the opposite of those reported by Cassataro et al. (2014). Future work will focus on carrying out these experiments in both male and female mice. Further differences between these studies include CNO dose (not reported by Cassataro et al.) and routes of administration. Cassataro et al. administered CNO via unmeasured tap water (not during ethanol drinking), thus it is possible NAc activity was not inhibited during the ethanol access (CNO plasma levels are not detectable 2 hours after injection; Guettier et al., 2009).
Another possible interpretation of these findings is that removal of the CNO/DREADD-induced inhibition of the NAc resulted in a rebound firing effect (consistent with findings that increased firing decreases alcohol intake). In studies where DREADDs are expressed in specific brain regions via viral-mediated gene transfer (e.g. Navarro et al., 2016; Pleil et al., 2016; Rinker et al., 2017; Vardy et al., 2015; Zhu et al., 2014), behavioral effects of CNO (administered via intra-peritoneal or intra-cranial injection) are observed for periods of time ranging from 30 minutes to 4 hours. Notably, Navarro et al. (2016) observed CNO-induced reductions in alcohol drinking (and other consummatory behaviors) for a period of 2, but not 4 hours (where hM4Di was expressed in the lateral hypothalamus). Rinker et al. (2017) observed a reduction in 2-hour alcohol intake when hM4Di was expressed in the bed nucleus of the stria terminalis (BNST) and CNO was administered directly into the ventral tegmental area (VTA). Additionally, Pleil et al. (2015) observed that CNO reduced alcohol intake for 4 hours in mice expressing hM4Di in the BNST. However, in studies where transgenic mice express DREADDs, the behavioral effects of CNO can last for several hours (Alexander et al., 2009; Guettier et al., 2009). Thus, studies to date indicate that the effects of changing activity using CNO and DREADDs may depend a number of factors, including route of CNO administration, expression levels of DREADDs, and connectivity with other regions. Additionally, sex as a biological variable is often overlooked. Many of these types of studies have only been performed in males. We know very little about the effects of sex on these types of manipulations. The inclusion of sex as a biological variable in preclinical alcohol research will increase scientific rigor, and lead to a better understanding of alcohol-related brain circuitry, pharmacology and drug discovery, and improve translation to the clinic. And, a final caveat is the recent report (MacLaren et al 2016) that CNO may have active metabolites. Addressing this possibility was beyond the scope of the current studies, but should be evaluated in the future.
We have long known that the NAc plays an important role in reward-related processes (Everitt, Morris et al. 1991). Thus, manipulating neuronal activity of this region may be rewarding, or alter the perceived rewarding effects of other stimuli. Reduced drinking of ethanol might result from a general reduction in interest in any rewarding stimuli. Alternatively, CNO treatment could have altered taste thresholds for sweet or bitter, or could have reduced thirst in general or induced a state of general malaise. Bull et al. (2014) addressed the effects of CNO/DREADDs on both reward and ethanol drinking in a recent study (Bull, Freitas et al. 2014). They expressed excitatory DREADDs selectively in NAc core astrocytes and found that CNO increased cytosolic calcium in primary astrocytes, modestly facilitated responding for rewarding brain stimulation, and reduced the motivation for ethanol (Bull, Freitas et al. 2014). These results suggest that NAc core astrocytes can modulate sensitivity to rewarding stimulation. We assessed whether the DREADD-induced changes in neuronal activity were accompanied by altered tastant or fluid intake and found no significant effects of treatment. Since CNO treatment failed to reduce sucrose intake significantly, we tentatively propose that CNO/DREADDs do not alter the reinforcing effects of this natural reward. That intake of the highest concentration of sucrose appeared to be lower, however, means that we cannot entirely rule out an effect based on calories or sweet taste. Due to the challenging nature of these experiments, and the need to analyze data from only those animals with verified placements, our sample sizes were also fairly small, so increased numbers of subjects might have detected a reduction in 5% sucrose drinking. Our data show definitively that CNO did not alter aversion for the bitter taste of quinine, or produce malaise or general thirst reduction, as neither quinine nor water intake were affected.
These studies are the first to systematically study alterations in neuronal activity in the NAc and its sub-regions, the core and shell, using CNO/DREADDs and measuring alcohol-related behaviors. There are strengths and caveats to the studies presented here. Our approach included replication: increasing NAc activity reduced binge-like drinking across three separate experiments. Importantly, we did not observe sensitization or tolerance to repeated CNO administration (data not shown). In addition to the interpretational caveats discussed above, we did not measure blood ethanol levels, as we would have needed to perform repeated blood sampling and this would have been stressful to the animals and possibly have interfered with behavior. Although the reductions in binge drinking were significant, but modest, it is likely that they are pharmacologically relevant. Notably, even modest reductions in alcohol drinking can robustly decrease achieved blood alcohol levels (see Fig. 3a from Rhodes et al., 2005 reporting correlation between achieved blood alcohol concentrations and ethanol intake for C57BL/6J mice). Lastly, another caveat is that we were unable to pseudorandomize animals to groups since all mice underwent surgery and were injected with different AAVs (hM3Dq, hM4Di, or GFP). This can sometimes lead to variable ethanol intake between groups (e.g. Figure 5) and experiments (e.g. Figures 2 and 4). However, only mice with confirmed placements were included in the analyses. Therefore, we could not a priori pseudo-randomize individuals to specific groups prior to the beginning of treatment to minimize group differences, which can lead to group differences in ethanol intake (at baseline). Conducting these studies with specific injections of an AAV into a sub-region, especially in mice, is very challenging. Including baseline (vehicle) and CNO (treatment) periods, as well as always including an AAV-GFP group allows us to focus on changes within groups in response to treatment. We replicated these findings in 3 different experiments carried out at two different universities, as well as in 2 experiments carried out another strain of mice (Ozburn et al, in preparation).
Summary
Increasing NAc core + shell medium spiny neuron firing decreased binge-like alcohol drinking (acutely and chronically), but not tastant or fluid intake. Further, we found that manipulating activity in the NAc core can bi-directionally regulate binge-like drinking. Moving forward, we are using DREADD viral approaches in combination with the Cre/loxP system to further pursue cell-type and circuit characterization of binge-like drinking. Lastly, it will be important to determine whether chronically altering NAc activity induces plasticity on a behavioral, neuronal, transcriptome, and/or morphological level.
Acknowledgments
We thank the following individuals for their intellectual contributions and/or technical assistance: Bryan Roth, Hu Zhu, Tanvi Batish, Snigdha Kanadibhotla, Gian Greenberg, Amanda Barkley-Levenson, Jason Schlumbohm, Larry Huang, Stephanie Spence, Robert Hitzemann, and University of North Carolina at Chapel Hill Viral Vector Core.
Funding: Supported by NIH grants [AA020452 (ARO), AA10760 and AA13519 (JCC, ARO), R24 AA020245 (JCC), DA039865 and DA023988 (CAM), DA038654 (RWL)], US Department of Veterans Affairs Awards [IK2 BX002488 (ARO) and 101BX000313 (JC)], and funds from a BBRF NARSAD Young Investigator Award (ARO) and the Andrews Genomics Fund (ARO).
Footnotes
Conflict of Interest Disclosures: The authors have nothing to disclose.
References
- Ambroggi F, Ghazizadeh A, Nicola SM, Fields HL. Roles of nucleus accumbens core and shell in incentive-cue responding and behavioral inhibition. J Neurosci. 2011;31(18):6820–6830. doi: 10.1523/JNEUROSCI.6491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisuzzaman AS, Uwada J, Masuoka T, Yoshiki H, Nishio M, Ikegaya Y, Takahashi N, Matsuki N, Fujibayashi Y, Yonekura Y, Momiyama T, Muramatsu I. Novel contribution of cell surface and intracellular M1-muscarinic acetylcholine receptors to synaptic plasticity in hippocampus. J Neurochem. 2013;126(3):360–371. doi: 10.1111/jnc.12306. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112(4):503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bull C, Freitas KC, Zou S, Poland RS, Syed WA, Urban DJ, Minter SC, Shelton KL, Hauser KF, Negus SS, Knapp PE, Bowers MS. Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology. 2014;39(12):2835–2845. doi: 10.1038/npp.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Transmitter Modulation of Slow, Activity-Dependent Alterations in Sodium Channel Availability Endows Neurons with a Novel Form of Cellular Plasticity. Neuron. 2003;39(5):793–806. doi: 10.1016/s0896-6273(03)00531-2. [DOI] [PubMed] [Google Scholar]
- Cassataro D, Bergfeldt D, Malekian C, Van Snellenberg JX, Thanos PK, Fishell G, Sjulson L. Reverse pharmacogenetic modulation of the nucleus accumbens reduces ethanol consumption in a limited access paradigm. Neuropsychopharmacology. 2014;39(2):283–290. doi: 10.1038/npp.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35(3):783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, Finn DA, Oberbeck DL, Yoneyama N, Snelling CC, Wu W, Hitzemann RJ. Electrolytic lesions of the medial nucleus accumbens shell selectively decrease ethanol consumption without altering preference in a limited access procedure in C57BL/6J mice. Pharmacol Biochem Behav. 2009;92(2):335–342. doi: 10.1016/j.pbb.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O'Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42(1):1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- Guo L, Zhou H, Wang R, Xu J, Zhou W, Zhang F, Tang S, Liu H, Jiang J. DBS of nucleus accumbens on heroin seeking behaviors in self-administering rats. Drug Alcohol Depend. 2013;129(1–2):70–81. doi: 10.1016/j.drugalcdep.2012.09.012. [DOI] [PubMed] [Google Scholar]
- He F, Guan H, Zhao Z, Miao X, Zhou Q, Li L, Huang D, Liu A, Miao D. Evaluation of short-term psychological functions in opiate addicts after ablating the nucleus accumbens via stereotactic surgery. Stereotact Funct Neurosurg. 2008;86(5):320–329. doi: 10.1159/000160155. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Heldmann M, Voges J, Hinrichs H, Marco-Pallares J, Hopf JM, Muller UJ, Galazky I, Sturm V, Bogerts B, Munte TF. Counteracting incentive sensitization in severe alcohol dependence using deep brain stimulation of the nucleus accumbens: clinical and basic science aspects. Front Hum Neurosci. 2009;3:22. doi: 10.3389/neuro.09.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MB, Green AI, Bradford PS, Chau DT, Roberts DW, Leiter JC. Deep brain stimulation of the nucleus accumbens reduces alcohol intake in alcohol-preferring rats. Neurosurg Focus. 2010;29(2):E12. doi: 10.3171/2010.4.FOCUS10105. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Tozier L, Pak A, Ciraulo DA, Kornetsky C. Deep brain stimulation of the nucleus accumbens reduces ethanol consumption in rats. Pharmacol Biochem Behav. 2009;92(3):474–479. doi: 10.1016/j.pbb.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J, Grundler TO, Bauer R, Huff W, Fischer AG, Lenartz D, Maarouf M, Buhrle C, Klosterkotter J, Ullsperger M, Sturm V. Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addict Biol. 2011;16(4):620–623. doi: 10.1111/j.1369-1600.2011.00337.x. [DOI] [PubMed] [Google Scholar]
- Luigjes J, van den Brink W, Feenstra M, van den Munckhof P, Schuurman PR, Schippers R, Mazaheri A, De Vries TJ, Denys D. Deep brain stimulation in addiction: a review of potential brain targets. Mol Psychiatry. 2012;17(6):572–583. doi: 10.1038/mp.2011.114. [DOI] [PubMed] [Google Scholar]
- Ma Y, Chen N, Wang HM, Meng FG, Zhang JG. Inhibition of the reinstatement of morphine-induced place preference in rats by high-frequency stimulation of the bilateral nucleus accumbens. Chin Med J (Engl) 2013;126(10):1939–1943. [PubMed] [Google Scholar]
- Marty VN, Spigelman I. Effects of alcohol on the membrane excitability and synaptic transmission of medium spiny neurons in the nucleus accumbens. Alcohol. 2012;46(4):317–327. doi: 10.1016/j.alcohol.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty VN, Spigelman I. Long-lasting alterations in membrane properties, k(+) currents, and glutamatergic synaptic currents of nucleus accumbens medium spiny neurons in a rat model of alcohol dependence. Front Neurosci. 2012;6:86. doi: 10.3389/fnins.2012.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Falcon E, Twaddle A, Nugent AL, Gillman AG, Spencer SM, Arey RN, Mukherjee S, Lyons-Weiler J, Self DW, McClung CA. Direct regulation of diurnal Drd3 expression and cocaine reward by NPAS2. Biol Psychiatry. 2015;77(5):425–433. doi: 10.1016/j.biopsych.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Janowsky AJ, Crabbe JC. Commonalities and Distinctions Among Mechanisms of Addiction to Alcohol and Other Drugs. Alcohol Clin Exp Res. 2015;39(10):1863–1877. doi: 10.1111/acer.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Vassoler FM. Deep brain stimulation for the treatment of addiction: basic and clinical studies and potential mechanisms of action. Psychopharmacology (Berl) 2013;229(3):487–491. doi: 10.1007/s00213-013-3214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6(1):1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63(2):291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Taintor NB, Rivera SN, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R, Fowler JS, Gatley SJ, Wang GJ, Volkow ND. DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol Clin Exp Res. 2004;28(5):720–728. doi: 10.1097/01.alc.0000125270.30501.08. [DOI] [PubMed] [Google Scholar]
- van Dijk A, Klompmakers AA, Feenstra MG, Denys D. Deep brain stimulation of the accumbens increases dopamine, serotonin, and noradrenaline in the prefrontal cortex. J Neurochem. 2012;123(6):897–903. doi: 10.1111/jnc.12054. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Hopkins TJ, Guercio LA, Espallergues J, Berton O, Schmidt HD, Pierce RC. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine reinstatement through local and antidromic activation. J Neurosci. 2013;33(36):14446–14454. doi: 10.1523/JNEUROSCI.4804-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges J, Muller U, Bogerts B, Munte T, Heinze HJ. Deep brain stimulation surgery for alcohol addiction. World Neurosurg. 2013;80(3–4):S28, e21–31. doi: 10.1016/j.wneu.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Wilden JA, Qing KY, Hauser SR, McBride WJ, Irazoqui PP, Rodd ZA. Reduced ethanol consumption by alcohol-preferring (P) rats following pharmacological silencing and deep brain stimulation of the nucleus accumbens shell. J Neurosurg. 2014;120(4):997–1005. doi: 10.3171/2013.12.JNS13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HM, Wang XL, Chang CW, Li N, Gao L, Geng N, Ma JH, Zhao W, Gao GD. Preliminary findings in ablating the nucleus accumbens using stereotactic surgery for alleviating psychological dependence on alcohol. Neurosci Lett. 2010;473(2):77–81. doi: 10.1016/j.neulet.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42(3):149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]