Abstract
BACKGROUND
Antimicrobial stewardship programs (ASPs) effectively optimize antibiotic use for inpatients; however, the extent of emergency department (ED) involvement in ASPs has not been described.
OBJECTIVE
To determine current ED involvement in children’s hospital ASPs and to assess beliefs and preferred methods of implementation for ED-based ASPs.
METHODS
A cross-sectional survey of 37 children’s hospitals participating in the Sharing Antimicrobial Resistance Practices collaboration was conducted. Surveys were distributed to ASP leaders and ED medical directors at each institution. Items assessed included beliefs regarding ED antibiotic prescribing, ED prescribing resources, ASP methods used in the ED such as clinical decision support and clinical care guidelines, ED participation in ASP activities, and preferred methods for ED-based ASP implementation.
RESULTS
A total of 36 ASP leaders (97.3%) and 32 ED directors (86.5%) responded; the overall response rate was 91.9%. Most ASP leaders (97.8%) and ED directors (93.7%) agreed that creation of ED-based ASPs was necessary. ED resources for antibiotic prescribing were obtained via the Internet or electronic health records (EHRs) for 29 hospitals (81.3%). The main ASP activities for the ED included production of antibiograms (77.8%) and creation of clinical care guidelines for pneumonia (83.3%). The ED was represented on 3 hospital ASP committees (8.3%). No hospital ASPs actively monitored outpatient ED prescribing. Most ASP leaders (77.8%) and ED directors (81.3%) preferred implementation of ED-based ASPs using clinical decision support integrated into the EHR.
CONCLUSIONS
Although ED involvement in ASPs is limited, both ASP and ED leaders believe that ED-based ASPs are necessary. Many children’s hospitals have the capability to implement ED-based ASPs via the preferred method: EHR clinical decision support.
Antibiotic resistance has been identified as one of the greatest threats to public health, resulting in 2 million illnesses and 23,000 deaths each year.1,2 Both the Centers for Disease Control and Prevention and the Infectious Diseases Society of America have emphasized the importance of improved antibiotic prescription practices to combat antimicrobial resistance.2,3 The National Action Plan for Combating Antibiotic-Resistant Bacteria proposed goals to reduce infections and adverse events caused by resistant organisms.4 In response, many pediatric hospitals across the country have implemented antimicrobial stewardship programs (ASPs) to minimize inappropriate and unnecessary antibiotic prescribing.5,6 Using strategies such as guideline development, formulary restriction, provider education, and audit and feedback, ASPs have led to increased guideline adherence, have reduced the incidence of antibiotic-resistant bacterial infections, and have produced substantial institutional cost savings.6–11
Traditionally, antimicrobial stewardship committees have been composed of specialists in infectious diseases, clinical pharmacology, and clinical providers from inpatient settings.6 Therefore, ASP activities have typically been limited to inpatient services, intensive care units, and surgical settings. Methods of antimicrobial stewardship, including audit and feedback, have been evaluated in the pediatric office setting, with encouraging results.12 However, of the antibiotic prescriptions given to 49 million children from ambulatory settings each year, approximately 10 million prescriptions are written in emergency departments (EDs).13–15 However, methods of antimicrobial stewardship in the ED setting have not been adequately studied. ASP implementation for EDs can be challenging because of the unique approach to antibiotic prescribing in this fast-paced clinical setting, where need for empiric antibiotic therapy in the absence of a confirmatory diagnosis is common.16
To better understand the need and potential for ASP implementation in the pediatric ED setting, we aimed to determine the current involvement of EDs within children’s hospitals in ASPs and the resources available for appropriate antibiotic prescribing in EDs. We also aimed to identify beliefs regarding ED-based ASPs and to determine preferred methods for ASP implementation in the ED.
METHODS
Study Design, Subjects, and Setting
We conducted a cross-sectional survey of institutions belonging to the Sharing Antimicrobial Reports for Pediatric Stewardship (SHARPS) collaborative.17 The SHARPS collaborative comprises 37 children’s hospitals across the United States that share best practices and relevant data for pediatric antimicrobial stewardship. SHARPS institutions include free-standing pediatric hospitals and designated children’s hospitals within general hospitals. Cumulatively, these hospitals receive an estimated 2.2 million ED visits annually, in which cared is provided by more than 900 certified practitioners in pediatric emergency medicine.
We solicited participation from 2 respondents in each SHARPS institution: 1 hospital ASP leader and 1 clinical or medical director within the institution’s ED. Because ASPs often involve joint leadership from infectious diseases specialists and clinical pharmacists, either was permitted to serve as the hospital ASP respondent. There were no specific exclusion criteria. Consent for participation was implied via completion of the survey. The study was granted exemption from written informed consent by our hospital’s institutional review board.
Survey Items
The survey items assessed antimicrobial stewardship activities and ED involvement within the ASPs. Accordingly, we generated several items specifically for hospital ASP leaders and others for ED medical directors. We used branching logic at the start of the survey to direct respondents to the appropriate set of questions for their positions. Specific aspects of ASPs addressed included specialty member composition, current infections and clinical care settings monitored, and currently available ED antibiotic prescribing resources. ED medical directors were asked to report on the presence of current prescribing guidelines as well as capabilities for delivery of prescribing recommendations (eg, ability to embed recommendations into the electronic health record [EHR]).
For both ASP leaders and ED directors, we used 5-point Likert scales (1 to 5; 5 = strongly agree) to assess the level of agreement with statements regarding ED-based antibiotic prescribing practices and potential antimicrobial stewardship. Responses of 4 or 5 on the Likert scale were considered “in agreement.” ASP leaders and ED directors also ranked, in order of preference, 4 common methods for ED implementation of antimicrobial stewardship: EHR clinical decision support (EHR CDS), posting of clinical care guidelines, direct provider education, and provider audit and feedback.
Survey Development
The principal investigator (R.D.M.) developed survey items in conjunction with experts in the field of pediatric infectious diseases (J.G.N., J.S.G., A.L.H.), pediatric emergency medicine (R.D.M., P.S.D., N.K.), and general emergency medicine (L.M., S.M.P.). The initial iteration of the survey consisted of 47 items. Study investigators first assessed each item for importance and applicability. Based on this assessment, the items were then revised. Following item generation and reduction, each remaining survey item was rated for usefulness using a modified Delphi method. An independent, non-investigator panel of 3 experts in pediatric infectious diseases and 2 in pediatric emergency medicine rated each survey item on 5-point scale (1 to 5; 5 = very useful). We retained items rated as useful or very useful (4 or 5) by all panelists. Any item rated as a 3 was revised and retained based on feedback; items with a score below 3 were removed.
The final survey consisted of 41 items. The survey was then adapted for electronic administration using REDCap software (Vanderbilt University Medical Center, Nashville, TN). After electronic development, the survey was piloted for functionality and then finalized for distribution.
Data Analysis
Study data were summarized using standard descriptive statistics: continuous variables were described according to their parametric distributions, while categorical variables were described using frequencies and proportions. We performed all data analyses using the Statistical Package for the Social Sciences (SPSS) version 23.0 (College Station, TX).
RESULTS
SHARPS Hospital Characteristics
All 37 SHARPS hospitals had at least 1 of the 2 potential respondents complete a survey. Of the 74 surveys distributed, 68 (91.9%) were completed, including 36 of 37 (97.3%) from hospital ASP leaders and 32 of 37 (86.5%) from ED medical directors. All participating SHARPS hospitals were tertiary referral centers and most were in urban settings, were free-standing pediatric hospitals, and were academic and/or university affiliated (Table 1).
TABLE 1.
Participating Hospital Characteristics (N = 37)
| Characteristic | No. (%) |
|---|---|
| Hospital Type | |
| Freestanding pediatric | 29 (78.4) |
| Children's within general hospital | 8 (21.6) |
| Location | |
| Urban | 34 (91.9) |
| Suburban | 3 (8.1) |
| Academic/University affiliated | 33 (89.2) |
| Tertiary-care referral | 37 (100) |
| Region | |
| East | 6 (16.2) |
| South | 12 (32.5) |
| Midwest | 10 (27.0) |
| West | 9 (24.3) |
| Estimated annual ED visits, median (range) | 60,000 (13,000–130,000) |
| ED admission rate, median (range) | 13% (7–28) |
| No. of PEM faculty, median (range) | 25 (7–80) |
NOTE. ED, emergency department; PEM, pediatric emergency medicine.
Beliefs and Methods of ED Antimicrobial Stewardship
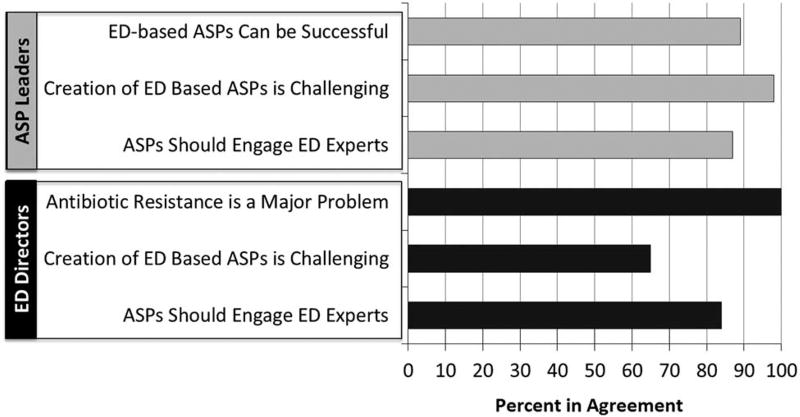
Although ASP leaders and ED directors agreed that implementation of ED-based antimicrobial stewardship is challenging, there was a consistent belief that antimicrobial resistance represents a threat to public health and that ED involvement in ASPs is necessary (Figure 1). Both ED and ASP respondents believed that ED experts should be included on ASP committees. Furthermore, 28 of 32 ED medical directors (87.5%) believed that implementation of ASPs are cost-effective for their healthcare institutions.
FIGURE 1.
Beliefs regarding ED antimicrobial stewardship by ED medical directors and ASP leaders. Bars represent proportion in agreement; agreement defined as response of 4 or 5 of 5 on 5-point Likert scale. ASP, antimicrobial stewardship program; ED, emergency department.
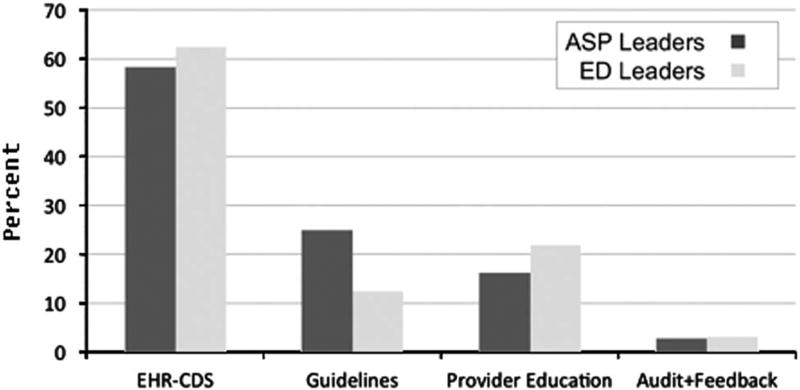
Figure 2 presents the preferred methods of implementation of ED-based antimicrobial stewardship. Both ED medical directors and ASP leaders favored the use of EHR CDS over other methods. More than 80% of respondents ranked EHR CDS first or second, compared to alternative methods such as guideline development and enforcement, direct provider education, and audited ED prescribing.
FIGURE 2.
Preferred methods for ED-based antimicrobial stewardship as ranked by ED and ASP leadership. Bars represent proportion who ranked that method first. ED, emergency department; ASP, antimicrobial stewardship.
Current ASP Activities in SHARPS Institutions
Among the 37 SHARPS hospitals, 36 (97.3%) had functional ASPs; 1 hospital had yet to develop their ASP and was excluded from the remaining analyses. Data were, therefore, available for 36 institutions. Among SHARPS hospital ASPs, 19 ASPs (52.8%) were in the first 3 years of activity, 8 ASPs (22.2%) had been active between 3 and 5 years, and 9 ASPs (25.0%) had been active for >5 years.
The specialty composition of the 36 ASPs varied. Within the SHARPS collaborative, outpatient pediatric specialists were infrequently represented in ASPs: the ED was only represented in only 3 ASPs (8.3%) and outpatient pediatricians in just 1 ASP (2.8%). Representation of other specialties on ASPs were as follows: pediatric infectious diseases were represented in 34 ASPs (94.4%), microbiology laboratory specialists in 20 ASPs (55.6%), clinical pharmacology and/or pharmacists in 15 ASPs (41.7%), inpatient pediatricians in 9 ASPs (25.0%), and pediatric critical care specialists in 11 ASPs (30.6%).
Among current ASP activities, 35 programs (97.2%) created general institutional clinical care guidelines for antibiotic prescribing. Table 2 lists the ASP guidelines created for common outpatient infections. Guidelines for community-acquired pneumonia (83.3%) were most frequently created among participating institutions, followed by urinary tract infections (33.3%) and skin infections (33.3%). Table 2 also demonstrates the infrequent involvement of ED specialists in clinical care guideline development. No ASPs monitored outpatient antibiotic prescribing for children discharged from the ED. Active monitoring of antibiotic prescribing in outpatient clinical settings was uncommon among ASPs; only 3 hospitals (8.3%) reported monitoring antibiotic use in the outpatient general pediatric clinic setting.
TABLE 2.
Frequency and Characteristics of Antimicrobial Stewardship Program (ASP) Clinical Care Guidelines Developed and Proportion of ASPs Monitoring Prescribing at SHARPS Hospitals (n = 36)
| Outpatient Infection | CCG, No. (%) |
ED Specialist Involved in CCG Development, No. (%)a |
Prescription Monitoring, No. (%) |
|---|---|---|---|
| Community-acquired pneumonia | 30 (83.3) | 15 (50.0) | 0 (0.0) |
| Otitis media | 5 (13.8) | 1 (20.0) | 0 (0.0) |
| Pharyngitis | 1 (2.8) | 0 (0.0) | 2 (5.6) |
| Sexually transmitted infections | 6 (16.7) | 5 (83.3) | 0 (0.0) |
| Skin and soft-tissue infections | 12 (33.3) | 9 (75.0) | 0 (0.0) |
| Urinary tract infections | 12 (33.3) | 9 (75.0) | 0 (0.0) |
| Upper respiratory infections | 4 (11.1) | 3 (75.0) | 1 (2.8) |
NOTE. CCG, clinical care guideline; ED, emergency department.
Expressed as percentage of those with CCG for each infection, as reported in column 1.
Integration of ASP Recommendations into ED Practice
Among the 32 responding SHARPS hospital EDs, access to institutional clinical care guideline recommendations occurred via several methods (Table 3). Overall, 29 of 32 EDs (90.6%) reported current use of computerized resources and/or EHRs to access local antibiotic prescribing recommendations. Furthermore, 7 ASPs (21.8%) incorporated formulary restrictions to common antimicrobials in EDs using order sets or limiting medication availability to providers by hospital pharmacists. The use of ED provider feedback for prescribing was uncommon; only 1 site (2.8%) reported this ASP mechanism currently being used at their institution. Methods to optimize antibiotic use through rapid diagnostic testing at the point-of-care were also limited at participating SHARPS EDs (Table 4).
TABLE 3.
Access to Clinical Care Guidelines and Resources for Emergency Department Antibiotic Prescribing in SHARPS Institutions (n = 32)
| Variable | No. (%) |
|---|---|
| Access to Clinical Care Guidelines | |
| Local hospital intranet | 23 (71.9) |
| Electronic health record embedded weblink | 12 (37.5) |
| Posted paper recommendations | 11 (34.3) |
| Institutional share drive | 8 (25.0) |
| ASP Methods and Resources for Antibiotic Prescribing | |
| Hospital antimicrobiograms | 28 (87.5) |
| Emergency department-based pharmacist | 22 (68.8) |
| Formulary restriction | 7 (21.9) |
| Provider feedback on prescribing | 1 (3.1) |
TABLE 4.
Other Methods of Antimicrobial Stewardship Currently Present in SHARPS Institution Emergency Departments (n = 32)
| Variable | No. (%) |
|---|---|
| Point-of-care diagnostic tests | |
| Rapid streptococcal antigen | 22 (68.8) |
| Bedside urinalysis | 17 (53.1) |
| Rapid testing for respiratory viruses | 10 (31.3) |
| Culture call-back processes | |
| Institution of treatment for positive cultures | 32 (100) |
| Antibiotics changed based on results of antibiotic sensitivities | 29 (90.6) |
| Discontinuation of antibiotics after discharge if urine cultures were negative | 7 (21.9) |
| Discontinuation of antibiotics after discharge if throat cultures were negative | 1 (3.1) |
Many participating EDs (26; 81.3%) had ongoing internal quality improvement (QI) activities related to antibiotic prescribing. For outpatient infections, 22 EDs (68.8%) had ongoing QI projects related to community-acquired pneumonia, and 12 EDs (37.5%) had QI activities regarding both skin and soft-tissue infections and urinary tract infections. Pediatric ED experts were involved in more than half of ongoing QI activities (18; 56.2%) related to antibiotic prescribing in SHARPS hospitals.
DISCUSSION
Our findings demonstrate that programmatic involvement of the ED in ASPs was very limited in this sample of pediatric hospitals. Pediatric emergency medicine specialists were rarely engaged in ASP committees, and resources to enhance ED antibiotic prescribing, such as the development of institutional clinical care guidelines and prescription monitoring, were infrequent. Nonetheless, leaders from both hospital ASPs and EDs consistently recognized a need for ED engagement in and implementation of ED-based antimicrobial stewardship. Each year, approximately 250 million antibiotic prescriptions are written from the ED; as many as 30% of antibiotics prescribed in the ED are considered either inappropriate or unnecessary.13,18 Therefore, the pediatric ED setting represents a great opportunity for antibiotic stewardship.13,19 Our results suggest that initial efforts should include instituting stewardship through EHR-CDS, which is well-suited for the ED clinical environment. Moreover, we found that pediatric EDs were well equipped for incorporation into ASP activities, as most institutions already utilized typical tools for antibiotic stewardship, such as clinical care guidelines and QI processes.
We found that the components of preferred and previously successful ASPs existed in most children’s hospitals. Prior studies have noted that EHRs, the preferred vehicle for ASP implementation in EDs, are present in nearly all US hospitals.20,21 Furthermore, there are precedents for effective CDS interventions for antibiotic prescribing CDS in UTI and viral respiratory infections using varying forms of delivery (eg, paper, posters).10,22–29 However, several studies have noted that presentation of antibiotic prescribing recommendations at the point-of-care is possible using the EHR.15,30,31 Integration of the EHR carries great potential for intervention on outpatient antibiotic prescribing.20,31,32 In a promising trial, Gerber et al12 implemented EHR-based automated provider audit and feedback for office pediatricians, demonstrating reductions in prescribing broad-spectrum antimicrobials over time. However, when feedback mechanisms were removed after the completion of this trial, effectiveness was not sustained.33 Therefore, leveraging prescription monitoring and provider feedback capabilities in the ED setting hold promise for effective antimicrobial stewardship.
Although we found that several additional methods are being used to regulate antibiotic prescribing in pediatric EDs, including review by ED-based pharmacists, use of hospital antibiograms, and development of clinical care guidelines, the effectiveness of these ASP interventions in the pediatric ED remains unclear.16 Moreover, each of these methods poses limitations for ED ASP implementation. While ED-based pharmacists have the ability to recommend antibiotic choices and provide dosing recommendations, the personnel costs are substantial for around-the-clock coverage. Although hospital antibiograms may be relatively easy to produce, they are rarely ED specific and they require active interpretation by the ED provider. Nonetheless, creation of ED-specific antibiograms is important in the creation of accurate ED-specific guidelines. ED guideline uptake appears to be limited if it is not tailored to the unique ED clinical setting.15,34
Accurate identification of specific infections via ED point-of-care testing has also been suggested to reduce antibiotic use.16 Although point-of-care testing has proven effective for numerous outpatient infections, education regarding proper use of point-of-care testing in the ED is necessary, and cost-effectiveness must be considered.35–39 In the present study, we identified variable use of point-of-care testing in the SHARPS EDs.
Finally, culture call-back systems were common among the EDs studied; typically, they were used to initiate antibiotics for missed infections in the ED. Discontinuation of antibiotics when cultures are negative represents an important opportunity for reducing unnecessary antibiotic exposure; however, discontinuation rarely occurred in the institutions surveyed.40,41
The results of our study have implications for the creation of ED-based ASPs. The need for novel and unique approaches to antimicrobial stewardship tailored for the ED is clear. Our findings are also consistent with the guiding principles of “Core Elements of Outpatient Antibiotic Stewardship”42 produced by the Centers for Disease Control and Prevention. These include tailored antibiotic treatment recommendations, use of clinical decision support, and prescription tracking and reporting; many of these proposed methods can be implemented using EHRs. Moreover, the widespread implementation of EHRs in EDs facilitates the generalizability of EHR-CDS for ED-based ASPs. These methods for ED stewardship can be applied to other high-priority outpatient infectious diseases, including pharyngitis, otitis media, pneumonia, and upper respiratory tract infections.42
Our study has several limitations. First, we sampled only participants in the SHARPS collaborative, which is restricted to academic pediatric hospitals. Therefore, the data collected and opinions expressed by respondents may not be representative of hospitals in other settings. However, querying this group allowed for a cohesive ASP leader–ED medical director dyad for this study. In addition, the relative lack of ED involvement at hospitals with substantial ASP investment is quite striking. Second, use of a survey mechanism for data collection assumes that responses are accurate, without verification. Finally, we were unable to obtain responses from 5 ED medical directors, though we were able to achieve an overall high response rate.
In conclusion, despite the large number of antibiotic prescriptions written each year by ED providers, antimicrobial stewardship efforts in pediatric EDs are limited. Although ASP and ED leaders agree that implementation of antimicrobial stewardship in the ED setting is feasible and necessary, there is a clear lack of ED presence in pediatric hospital–based ASPs with respect to the creation of recommendations, prescription monitoring, and regulation of ED antibiotic prescribing. The single most preferred method for ED ASP implementation was via the EHR using clinical decision support.
Acknowledgments
Financial support: No financial support was provided relevant to this article.
Footnotes
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
References
- 1.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368:299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Threat Report 2013. [Accessed December 31, 2014];Antimicrobial resistance. Centers for Disease Control and Prevention website. http://www.cdc.gov/drugresistance/threat-report-2013/. Published 2013.
- 3.Infectious Diseases Society of America (IDSA) Spellberg B, Blaser M, et al. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52:S397–S428. doi: 10.1093/cid/cir153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National action plan for combating antibiotic-resistant bacteria. [Accessed May 26, 2016];The White House website. https://www.whitehouse.gov/sites/default/files/docs/national_action_plan_for_combating_antiboticresistant_bacteria.pdf. Published 2015.
- 5.Hersh AL, Lurgio SAD, Thurm C, et al. Antimicrobial stewardship programs in freestanding children’s hospitals. Pediatrics. 2014 Dec; doi: 10.1542/peds.2014-2579. peds.2014–2579. [DOI] [PubMed] [Google Scholar]
- 6.Newland JG, Gerber JS, Weissman SJ, et al. Prevalence and characteristics of antimicrobial stewardship programs at freestanding children’s hospitals in the United States. Infect Control Hosp Epidemiol. 2014;35:265–271. doi: 10.1086/675277. [DOI] [PubMed] [Google Scholar]
- 7.Carling P, Fung T, Killion A, et al. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol. 2003;24:699–706. doi: 10.1086/502278. [DOI] [PubMed] [Google Scholar]
- 8.Ohl CA, Luther VP. Antimicrobial stewardship for inpatient facilities. J Hosp Med. 2011;6:S4–S15. doi: 10.1002/jhm.881. [DOI] [PubMed] [Google Scholar]
- 9.Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS) Infect ControlHosp Epidemiol. 2012;33:322–327. doi: 10.1086/665010. [DOI] [PubMed] [Google Scholar]
- 10.Lutters M, Harbarth S, Janssens J-P, et al. Effect of a comprehensive, multidisciplinary, educational program on the use of antibiotics in a geriatric university hospital. J Am Geriatr Soc. 2004;52:112–116. doi: 10.1111/j.1532-5415.2004.52019.x. [DOI] [PubMed] [Google Scholar]
- 11.Fishman N. Antimicrobial stewardship. Am J Infect Control. 2006;34:S55–S73. doi: 10.1016/j.ajic.2006.05.237. [DOI] [PubMed] [Google Scholar]
- 12.Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA J Am Med Assoc. 2013;309:2345–2352. doi: 10.1001/jama.2013.6287. [DOI] [PubMed] [Google Scholar]
- 13.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA. 2016;315:1864–1873. doi: 10.1001/jama.2016.4151. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother. 2014;69:234–240. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 15.Mistry RD, Dayan PS, Kuppermann N. The battle against antimicrobial resistance: time for the emergency department to join the fight. JAMA Pediatr. 2015;169:421–422. doi: 10.1001/jamapediatrics.2015.79. [DOI] [PubMed] [Google Scholar]
- 16.May L, Cosgrove S, L’Archeveque M, et al. A call to action for antimicrobial stewardship in the emergency department: approaches and strategies. Ann Emerg Med. 2013;62:69–77. doi: 10.1016/j.annemergmed.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zachariah P, Newland JG, Gerber JS, et al. Costs of antimicrobial stewardship programs at US children’s hospitals. Infect Control Hosp Epidemiol. Mar;2016:1–3. doi: 10.1017/ice.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis Off Publ Infect Dis Soc Am. 2015;60:1308–1316. doi: 10.1093/cid/civ076. [DOI] [PubMed] [Google Scholar]
- 19.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053–1061. doi: 10.1542/peds.2011-1337. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Medicare and Medicaid Services (CMS), HHS. Medicare and Medicaid Programs; Electronic Health Record Incentive Program—Stage 3 and modifications to meaningful use in 2015 through 2017. Final rules with comment period. Fed Regist. 2015;80:62761–62955. [PubMed] [Google Scholar]
- 21.Wright A, Henkin S, Feblowitz J, et al. Early results of the meaningful use program for electronic health records. N Engl J Med. 2013;368:779–780. doi: 10.1056/NEJMc1213481. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins TC, Knepper BC, Sabel AL, et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med. 2011;171:1072–1079. doi: 10.1001/archinternmed.2011.29. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins TC, Irwin A, Coombs L, et al. Effects of clinical pathways for common outpatient infections on antibiotic prescribing. Am J Med. 2013;126:327–335. doi: 10.1016/j.amjmed.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metlay JP, Camargo CA, Jr, MacKenzie T, et al. Cluster-randomized trial to improve antibiotic use for adults with acute respiratory infections treated in emergency departments. Ann Emerg Med. 2007;50:221–230. doi: 10.1016/j.annemergmed.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Roque F, Herdeiro MT, Soares S, Teixeira Rodrigues A, Breitenfeld L, Figueiras A. Educational interventions to improve prescription and dispensing of antibiotics: a systematic review. BMC Public Health. 2014;14:1276. doi: 10.1186/1471-2458-14-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris RH, MacKenzie TD, Leeman-Castillo B, et al. Optimizing antibiotic prescribing for acute respiratory tract infections in an urban urgent care clinic. J Gen Intern Med. 2003;18:326–334. doi: 10.1046/j.1525-1497.2003.20410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demonchy E, Dufour J-C, Gaudart J, et al. Impact of a computerized decision support system on compliance with guidelines on antibiotics prescribed for urinary tract infections in emergency departments: a multicentre prospective before-and-after controlled interventional study. J Antimicrob Chemother. 2014;69:2857–2863. doi: 10.1093/jac/dku191. [DOI] [PubMed] [Google Scholar]
- 28.Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315:562–570. doi: 10.1001/jama.2016.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecker MT, Fox CJ, Son AH, et al. Effect of a stewardship intervention on adherence to uncomplicated cystitis and pyelonephritis guidelines in an emergency department setting. PloS One. 2014;9:e87899. doi: 10.1371/journal.pone.0087899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kullar R, Goff DA, Schulz LT, et al. The “epic” challenge of optimizing antimicrobial stewardship: the role of electronic medical records and technology. Clin Infect Dis. 2013;57:1005–1013. doi: 10.1093/cid/cit318. [DOI] [PubMed] [Google Scholar]
- 31.Sintchenko V, Coiera E, Gilbert GL. Decision support systems for antibiotic prescribing. Curr Opin Infect Dis. 2008;21:573–579. doi: 10.1097/qco.0b013e3283118932. [DOI] [PubMed] [Google Scholar]
- 32.Shebl NA, Franklin BD, Barber N. Clinical decision support systems and antibiotic use. Pharm World Sci PWS. 2007;29:342–349. doi: 10.1007/s11096-007-9113-3. [DOI] [PubMed] [Google Scholar]
- 33.Gerber JS, Prasad PA, Fiks AG, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA. 2014;312:2569–2570. doi: 10.1001/jama.2014.14042. [DOI] [PubMed] [Google Scholar]
- 34.Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 35.Bonner AB, Monroe KW, Talley LI, et al. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003;112:363–367. doi: 10.1542/peds.112.2.363. [DOI] [PubMed] [Google Scholar]
- 36.Iyer SB, Gerber MA, Pomerantz WJ, et al. Effect of point-of-care influenza testing on management of febrile children. Acad Emerg Med Off J Soc Acad Emerg Med. 2006;13:1259–1268. doi: 10.1197/j.aem.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 37.Sharma V, Dowd MD, Slaughter AJ, Simon SD. Effect of rapid diagnosis of influenza virus type A on the emergency department management of febrile infants and toddlers. Arch Pediatr Adolesc Med. 2002;156:41–43. doi: 10.1001/archpedi.156.1.41. [DOI] [PubMed] [Google Scholar]
- 38.Ozkaya E, Cambaz N, Coşkun Y, et al. The effect of rapid diagnostic testing for influenza on the reduction of antibiotic use in paediatric emergency department. Acta Paediatr Oslo Nor 1992. 2009;98:1589–1592. doi: 10.1111/j.1651-2227.2009.01384.x. [DOI] [PubMed] [Google Scholar]
- 39.Shaw KN, McGowan KL, Gorelick MH, Schwartz JS. Screening for urinary tract infection in infants in the emergency department: Which test is best? Pediatrics. 1998;101:E1. doi: 10.1542/peds.101.6.e1. [DOI] [PubMed] [Google Scholar]
- 40.Burchett P, Harpin S, Petersen-Smith A, Emery K. Improving a urine culture callback follow-up system in a pediatric emergency department. J Pediatr Health. 2015;29:518–525. doi: 10.1016/j.pedhc.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management. Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595–610. doi: 10.1542/peds.2011-1330. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65:1–12. doi: 10.15585/mmwr.rr6506a1. [DOI] [PubMed] [Google Scholar]