Abstract
Effective treatment of Alzheimer’s disease (AD) remains a critical unmet need in medicine. The lack of useful treatment for AD led to an intense search for novel therapies based on the amyloid hypothesis, which states that amyloid β-42 (Aβ42) plays an early and crucial role in all cases of AD. β-Secretase (also known as BACE-1 β-site APP-cleaving enzyme, Asp-2 or memapsin-2) is an aspartyl protease representing the rate limiting step in the generation of Aβ peptide fragments, therefore it could represent an important target in the steady hunt for a disease-modifying treatment. Generally, β-secretase inhibitors are grouped into two families: peptidomimetic and nonpeptidomimetic inhibitors. However, irrespective of the class, serious challenges with respect to blood–brain barrier (BBB) penetration and selectivity still remain. Discovering a small molecule inhibitor of β-secretase represents an unnerving challenge but, due to its significant potential as a therapeutic target, growing efforts in this task are evident from both academic and industrial laboratories. In this frame, the rising availability of crystal structures of β-secretase-inhibitor complexes represents an invaluable opportunity for optimization. Nevertheless, beyond the inhibitory activity, the major issue of the current research approaches is about problems associated with BBB penetration and pharmacokinetic properties. This review follows the structural evolution of the early β-secretase inhibitors and gives a snap-shot of the hottest chemical templates in the literature of the last five years, showing research progress in this field.
Keywords: Alzheimer’s disease, BACE-1, β-secretase, peptidomimetic inhibitors, protease inhibitors, small-molecule inhibitors
1. INTRODUCTION
The development of effective therapies for Alzheimer’s Disease (AD) is a formidable challenge in medicine. AD is characterized by a progressive slow decline in cognitive function that leaves the end-stage patient dependent on custodial care with death occurring on the average of 9 years after diagnosis [1,2]. The lack of an effective treatment for AD has stimulated an intense search for novel therapies based on the amyloid hypothesis. The amyloid β-42 (Aβ42), a proteolytic derivative of the large transmembrane amyloid precursor protein (APP), plays an early and crucial role in all cases of AD [3]. These secreted amyloid peptides form neurotoxic oligomers that disrupt neuronal function and lead to cell death and memory loss that is phenotypical of AD. Consequently, blocking the production of Aβ42 by specific inhibition of the key proteases required for Aβ42 generation represents a major focus of research in AD therapy. In addition, treatments that address the amyloid cascade would offer the advantage of disease modification [2, 4, 5]. However, a considerable debate about the molecules downstream from the Aβ42 monomer and their relative contributions to the overall disease process has resulted from the plausible risk of choosing an irrelevant target.
β-Secretase (also known as BACE-1, β-site APP-cleaving enzyme, Asp-2 or memapsin-2) is an aspartyl protease representing the rate limiting step in the generation of these Aβ peptide fragments. The release of Aβ from its precursor APP requires two sequential proteolytic cleavages by β-secretase and γ-secretase. β-Secretase sheds the ectodomain of APP creating a C99 membrane-bound C-terminal fragment that is further processed by γ-secretase to release Aβ peptides [5,6]. The finding that β-secretase knockout mice are deficient in Aβ production provided in vivo validation of the β-secretase role. This has also demonstrated that no compensatory mechanism for β-secretase cleavage exists in mice [7,8]. From a therapeutic perspective, β-secretase garnered further interest as a pharmaceutically suitable target since it was reported that mice genetically deficient in β-secretase were viable, displaying a minimally altered phenotype [9].
Although β-secretase is an attractive target, it has been quite challenging from a drug discovery point of view. The difficulties arise from its belonging to aspartyl protease class and, most importantly, from its brain localization. Most of the aspartyl protease inhibitors (such as those of HIV protease and renin), that have been reported in the literature so far, contain a transition-state (TS) isostere as the key binding element [10–12]. Since aspartyl proteases generally have large active sites, substrates typically require 6–10 amino acids for attaining selectivity [13, 14]. Inhibitors of these enzymes have been large sized as well. As a consequence, these inhibitor classes exhibit poor pharmacokinetic properties. Beyond their size, multiple hydrogen bond donor and acceptor sites also impart poor properties to these types of compounds to cross the blood-brain barrier (BBB), a necessity for an AD drug candidate. β-Secretase represents a further challenge over other aspartyl proteases since its active site is larger (>1,000 Å) and less hydrophobic suggesting that balancing hydrophilic interaction with central nervous system (CNS) penetration is of critical importance [14].
Lately novel structural templates have been surfacing in the literature showing the potential for drug advancement [11]. This review will outline the structural evolution of the β-secretase inhibitors from the typical peptidomimetic inhibitors to the latest structural classes discovered to date. Particularly, the development of chemical entities bearing heterocyclic scaffolds will be examined in detail as well as the current outlooks in the inhibitor design strategies.
2. β-SECRETASE INHIBITORS: A MEANDERING PATH FOR GAINING EFFICACY
Inhibitors based on the peptidomimetic strategy suffer from predictable difficulties associated with peptides, such as BBB crossing, poor oral bioavailability, and P-glycoprotein (P-gp) liability. An ideal β-secretase inhibitor should be 700 kDa or smaller and possess high lipophilicity, in order to penetrate the BBB and to access neuronal membranes, in particular those of subcellular organelles where β-secretase is located. Toward this end, a number of publications report reductions in brain Aβ with β-secretase inhibitors. In one study, a β-secretase inhibitor, fused to a carrier peptide to facilitate transport across the BBB, caused a significant reduction in brain Aβ in Tg2576 mice [15]. In another study it was explored the potential of an inhibitor with a penetratin sequence added at its N-terminus [16]. In three other studies, β-secretase inhibitors intracranially delivered reduced brain Aβ in transgenic and wild-type mice [17, 18, 19]. Modest but significant reductions in brain Aβ were observed in APP-transgenic mice treated with BACE inhibitors delivered i.v., but only at high doses (50–100 mg/kg) [20, 21]. Finally, compound GSK188909 induced robust reductions in brain Aβ in a transgenic line after a single dose co-administered with a P-gp inhibitor [22]. A subsequent study, performed on three potent in vitro β-secretase inhibitors, showed that all the three compounds decreased brain Aβ in P-gp knock-out mice, demonstrating that P-gp is a major limitation for development of centrally active inhibitors [23]. However, in the same study a comparison of plasma Aβ and brain Aβ dose responses for these three compounds revealed differences in relative ED50 values, indicating that factors other than P-gp may also contribute to modest brain activity by β-secretase inhibitors [23].
A further challenge for β-secretase inhibitors is represented by the selectivity towards other aspartic proteases, in particular towards β–site APP-cleaving enzyme 2 (BACE-2), for its close similarity to β-secretase, and cathepsin D (CatD), for its ubiquitous presence in nearly all the cells. β-secretase and BACE-2 are members of the A1 aspartic protease family, commonly known as the pepsin family. Human aspartic proteases of this family include pepsin, cathepsin E (CatE), CatD, renin, pepsinogen-C and napsin. β-secretase and BACE-2 represent a novel subgroup of this family, being the first reported aspartic proteases to contain a transmembrane domain and carboxyl terminal extension [24], and also possessing unique disulphide bridge distribution [25, 26]. The eight known functional human A1 aspartic proteases vary in genomic structure. Main features of A1 aspartic proteases are their bilobar structure, with an essential catalytic Asp dyad located at the interface of the homologous N- and C-terminal lobes. These Asp residues activate water molecules to mediate nucleophilic attack on the substrate peptide bond. Both β-secretase and BACE-2 can process APP, but BACE-2 has a preference to cleave between aminoacids 19 and 20 of the Aβ sequence, thus precluding Aβ formation. In addition, although the general organization of the subsites belonging to the active site is similar to other aspartic proteases, their specificity and conformation display key differences, which may be exploited in selective inhibitors design [6, 27, 28]. Moreover, the active site of β-secretase is larger, having additional subsites, and although it works well with the eight substrate residues as is normal for other aspartic proteases, it can also accommodate a greater number of substrate residues [28, 29]. CatD is a soluble lysosomal aspartic endopeptidase. Numerous physiological functions of CatD have been suggested, based on its ability to cleave structural and functional proteins and peptides. These include metabolic degradation of intracellular proteins, activation and degradation of polypeptide hormones and growth factors, activation of enzymatic precursors, processing of enzyme activators and inhibitors, brain antigen processing and regulation of programmed cell death [30, 31, 32]. CatD is abundant in various cells, especially in those belonging to CNS. Furthermore CatD gene knockout mice showed marked phenotypic response including high mortality rate [33, 34]. Compared with other mammalian aspartyl proteases, β-secretase is more similar to CatD (relatively unspecific) than renin (highly specific) in terms of substrate specificity. However, β-secretase clearly has a different substrate selectivity from CatD, preferring polar or acidic amino acids in the P2 and P1′ positions (Fig. 1) [35]. CatD and indeed all other mammalian aspartyl proteases prefer hydrophobic amino acids in these positions, thus offering a glimmer for effectively targeting selective β-secretase inhibition.
Fig. 1.
Progressive truncation of early peptidomimetic inhibitors.
3. CURTAILING TRANSITION-STATE ANALOGUE INHIBITORS: FROM LARGE TO SMALLER PEPTIDES
The strong similarities in the catalytic apparatus between β-secretase and other already well-known proteases [e.g. renin and HIV protease] have been used as the logical starting point for the search of novel β-secretase inhibitors [11]. The generation of the early β-secretase inhibitors using peptidomimetics based on APPSWE (a double mutated variant of APP more favorable to β-secretase cleavage [36]) bearing non-cleavable isosteres, has been employed to define the crystal structure of the enzyme and to investigate substrate and inhibitor interactions at the active site level.
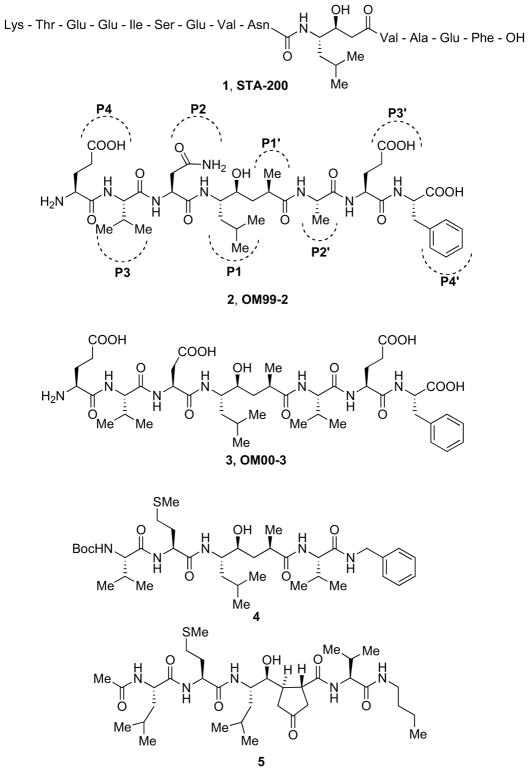
The progenitor was represented by the tetradecapeptide Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val-Asn-LeuStatine-Val-Ala-Glu-Phe-OH, named STA-200 (1, Fig. 1) corresponding to residues 588–599 of the human APPSWE, used to purify and isolate the enzyme. The cleavage site was modified by the introduction of a statine TS isostere. The incorporation of statine increased binding affinity of the peptide for β-secretase by three orders of magnitude. Replacing of APPSWE Asp596 by Val resulted in a 30 nM tetradecapeptide inhibitor [37].
The X-ray structure of β-secretase protease domain was achieved for the first time at 1.9 Å resolution (PDB ID: 1FKN), with the enzyme bound to the P4–P4′ octapeptide TS analogue inhibitor Glu-Val-Asn-Leu*Ala-Ala-Glu-Phe, named OM99-2 (2, Fig. 1). This inhibitor incorporated a non-cleavable hydroxyethylene (HE) isostere (*) at the cleavage site. This compound displayed a Ki value of 1.6 nM, thus highlighting the strong potential of HE isostere for designing effective inhibitors [6]. Subsequent structural fine-tuning provided the octapeptide Glu-Leu-Asp-Leu*Ala-Val-Glu-Phe, named OM00-3 (3, Fig. 1) with a Ki value of 0.3 nM [13, 38].
Structural adjustment carried out onto original peptidomimetic inhibitor 2 led to new perceptions. Size reduction of this inhibitor revealed that the removal of the four outside residues P4, P3, P3′ and P4′ resulted in greatly reduced potency. The inclusion of P3 Val, combined with the optimization of P2 and C-terminal blocking group, brought the Ki back to the nanomolar level, as represented by compound 4. Evidence from these studies suggested that, from the structural template derived from inhibitor 2, high potency could be attained by inhibitors with five subsites, from P3 to P2′, resulting in a molecular size of approximately 700 Da [39].
Molecular modeling based on the X-ray crystal structure of inhibitor 2-bound β-secretase also led to the design and synthesis of a series of constrained P1′ analogues. Progressive truncation at the P2′–P4′ sites accompanied by incorporation of cyclopentane, cyclopentanone, tetrahydrofuran, pyrrolidine, and pyrrolidinone spanning the P1′ and replacing the Ala and the adjacent methylene carbon atom of the TS mimetic group led to potent truncated analogs. The most representative compound 5 (Fig. 1) displayed an IC50 = 10 nM in enzyme assays and a notable selectivity over CatD [40].
4. BIOISOSTERIC MORPHING OF EARLY TRANSITION STATE ISOSTERE-CONTAINING PEPTIDOMIMETIC INHIBITORS
Although peptidomimetic inhibitors with potent in vitro properties have been developed, their large size and their predominantly aminoacidic composition seriously impair their use in vivo. Therefore, the exploitation of the classical bioisosterism principles has been the leading early design strategy adopted for modifying all the TS isostere containing peptidomimetic inhibitors.
4.1. Inhibitors Bearing Phenylnorstatine and Tert-Hydroxyl
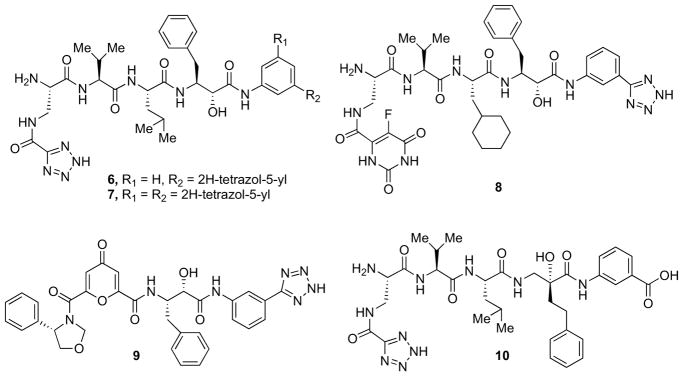
Kimura et al. [41, 42] reported a series of inhibitors (compounds 6 and 7, Fig. 2) in which a phenylnorstatine isostere was accompanied by tetrazole rings as suitable bioisosteric replacement of the carboxyl group at P4 and P1′ (6, IC50 = 4.8 nM; 7, IC50 = 1.2 nM). With the aim of improving the druggability profile, they subsequently investigated the likelihood of a 5-fluoroorotyl group at the P4 as bioisosteric replacement of tetrazole, followed by a L-cyclohexylalanine residue at P2. The resulting compound displayed an optimal inhibitory activity of β-secretase (8, IC50 = 5.6 nM, Fig. 2) while displaying 84% β-secretase inhibition in cultured cells at a concentration of 100 μM [43]. In order to improve cellular inhibitory activity, a series of β-secretase inhibitors possessing a heterocyclic ring at the P2 position and a 5- membered ring at the P3 position, were developed. Compound 9 showed the best inhibition profile (IC50 = 50 nM) [44].
Fig. 2.
Phenylnorstatine and tert-hydroxy-containing inhibitors.
More recently, Larhed et al. complemented a P4 tetrazole with a tert-hydroxy central core. The most potent analogue (10, IC50 = 0.19 μM, Fig. 2), co-crystallized with the enzyme showed a novel binding mode, with the N-terminal amine working as the TS-isostere in place of the tert-hydroxyl group [45].
4.2. Hydroxyethylene-Containing Inhibitors
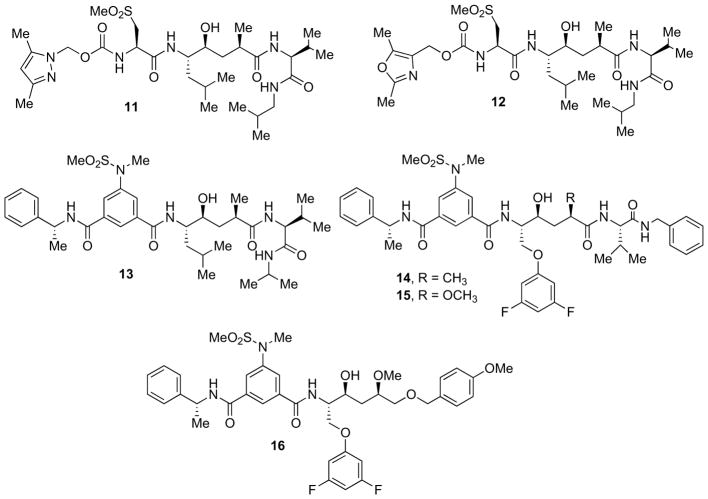
Structure-based design by Ghosh and co-workers led to the development of very potent and highly selective β-secretase inhibitors (e.g. 11, Fig. 3) [46]. To gain further molecular insights, the 11-bound β-secretase X-ray structure was determined at 1.8 Å resolution (PDB ID: 2G94). As it appeared from the structure, one of the P3-pyrazole nitrogens was hydrogen bonded to Thr232 with one of the dimethyl groups effectively filling in the shallow hydrophobic pocket in the S3 site and the other occupying the hydrophobic S3 subsite. Moreover, the P2-sulfone functionality was found within hydrogen-bonding distance to Arg-235 as well as with a tightly bound water molecule in the S2-site. These interactions of the P2 and P3 ligands are likely responsible for the observed enhanced selectivity of 11.
Fig. 3.
Hydroxyethylene-containing inhibitors.
Based upon this molecular insights, the same authors designed the oxazolylmethyl P3-ligand 12 (Fig. 3) [46]. This provided an extremely potent (Ki = 0.12 nM) and selective inhibitor (>3800-fold over BACE-2 and >2500-fold over CatD). Also the cellular inhibition profile of β-secretase by inhibitors 11 and 12 in Chinese hamster ovary cells was favorable (IC50 values of respectively 1.4 μM and 1.7 μM).
Further evolution of the inhibitor structures along this line and focused on increasing cell permeability, gave rise to isophthalamide containing compounds (13–16, Fig. 3). The tri-substituted orientation of the isophthalamide ring allows P2 and P3 residues to mimic the zig-zag sidechain disposition of a peptide backbone [14]. The optimum was represented by i) the (R)-α-methylbenzamide occupying the S3 subsite (R-stereochemistry at the methyl is preferred), ii) the sulfonamide occupying the S2 subsite with the sulfone oxygen involved in H-bonding interactions with the enzyme, iii) the alkyl group on the sulfonamide nitrogen orienting the binding conformation [47, 48]. Accordingly, structure-based design and energy minimizations boosted Ghosh et al. to develop compound 13 (Fig. 3) [49], which contained a substituted isophthalamide ring at P2 and optimized side chains. Compound 13 was a potent inhibitor (Ki = 1.1 nM) with moderately good selectivity towards BACE-2 and CatD (Ki values of 31 nM and 41 nM, respectively). Compound 13 well penetrated cell membrane and inhibited Aβ production in cultured cells with an IC50 of 39 nM. The authors reported that this compound is active in vivo, showing 30% reduction of Aβ40 production in Tg2576 transgenic mice after a single intraperitoneal administration (8 mg/kg).
Samuelson and co-workers explored the insertion of an oxygen atom at P1 position of the originally described HE-based peptidomimetic inhibitors [50]. The most potent inhibitor 14 (Fig. 3) exhibited a good β-secretase inhibition profile (IC50 = 3.1 nM) and an IC50 value of 160 nM in the cell-based assays. Compound 15 (Fig. 3) showed to be potent (IC50 = 0.32 nM) and selective toward CatD. Subsequently, inhibitor 16 (Fig. 3) displayed an IC50 value of 140 nM showing that the interactions in the S2′ and S3′ pockets as well as the amide linkage are of key importance for potency [51].
4.3. Hydroxyethylamine-Containing Inhibitors
The introduction of the hydroxyethylamine (HEA) scaffolds successfully provided very potent β-secretase inhibitors. The HEA TS-analogues inhibit aspartic proteases with both the secondary amine and the secondary alcohol interacting with the two catalytic aspartates. The hydroxyethyl secondary amine isostere was an alternative to HE because of the strict preferences of β-secretase for acyclic residues at the S1′ subsite [52].
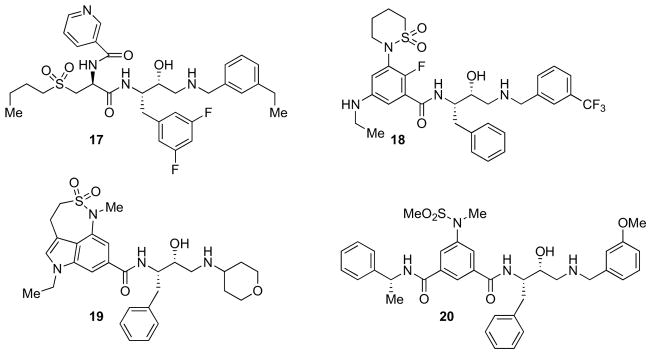
Maillard and Kortum developed a series of HEA-based inhibitors combining the isophthalamide moiety with an HEA isostere bearing R stereochemistry at the TS-hydroxyl and a 3,5-difluorophenyl fragment as the P1 aryl group [52, 53]. However, a drawback of this class of compounds was their poor metabolic stability. Replacing the isophthalate Nterminus by an acyclic sulfone turned out as a suitable strategy for circumventing this liability. Subsequent structure-based design provided derivative 17 (Fig. 4) with high inhibitory activity (IC50 = 2 nM) and cellular potency (IC50 = 1 nM) [54]. The X-ray crystal structure of 17-bound β-secretase (PDB ID: 2HM1) highlighted a close association between the pyridyl nitrogen and Arg235 in the S2 site. The authors ascribed the improved selectivity profile (CatD, IC50 = 474 nM) to the higher lipophilicity of the CatD S2 pocket which is less tolerant to the pyridyl nitrogen of the compound [54].
Fig. 4.
Hydroxyethylamine-based inhibitors containing sulfone and sulfonamide functionalities.
Inhibitor 18 (Fig. 4) (GSK188909) is an orally bioavailable β-secretase inhibitor capable of lowering brain Aβ in APP transgenic mice [22, 55–57]. Compound 18 possesses an IC50 of 4 nM, and shows good selectivity with respect to BACE-2, renin and CatD. Further optimization, driven by molecular modeling, led to the discovery of 19 [58] (Fig. 4) which incorporates a tricyclic nonprime side and a truncated prime side residues. This compound displayed good potency in a cell-based assay (IC50 = 19 nM). Moreover, 19 has shown good oral bioavailability.
Ghosh et al. developed inhibitor 20 [59] (Fig. 4) which displayed excellent inhibitory activity (Ki = 1.8 nM) and, most importantly, showed an impressive cellular potency (IC50 = 1 nM), which might be due to its optimal balance of lipophilic and basic amine properties. An i.p. administration of 20 at a dose of 8 mg/kg to Tg2576 mice resulted in 65% reduction of Aβ production in plasma after 3 h. Furthermore, 20 was recently shown to rescue age-related cognitive decline in APP transgenic mice [60].
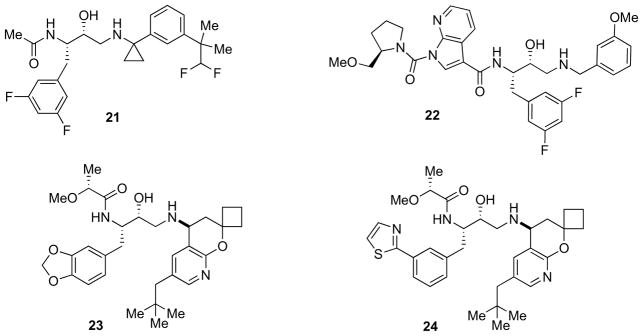
Truong et al. developed 21 [61] (Fig. 5) as a highly permeable compound with very low levels of P-gp liability. Compound 21 is an orally efficacious inhibitor in the wildtype pre-clinical guinea pig animal model and showed a cell IC50 of 26 nM but only a 230 nM value in the biochemical assays.
Fig. 5.
Diverse hydroxylamine-containing inhibitors.
More recently, Marcin et al. discovered indole- and 7- azaindole-1,3-dicarboxamide HEA-containing compounds exhibiting potent and selective inhibition of β-secretase [62]. Analog 22 (Fig. 5, IC50 = 10 nM) demonstrated robust reductions in rat plasma Aβ levels, but did not lower rat brain Aβ due to poor central exposure. The same analog exhibited a high efflux ratio in a bidirectional Caco-2 assay and was likely a substrate of the efflux transporter P-gp. Weiss and co-workers highlighted the importance of optimizing pharmacokinetic properties, although they experienced a drop of potency, when trying to identify centrally active agents [63]. These authors tried to optimize a series of potent inhibitors for improving pharmacokinetic properties and enhancing penetration into the CNS. Their work began by introducing polar functionalities to improve metabolic stability and they identified a series of benzodioxolanes with improved properties in terms of clearance and bioavailability. Improving brain uptake was successfully accomplished by introducing functional elements to mitigate P-gp-mediated efflux. The best compound (23, Fig. 5, IC50 = 16.8 nM and cell IC50 = 26.0 nM) demonstrated robust and dose dependent CNS Aβ40 reduction [63]. Subsequent work by the same authors was focused at improving the cardiovascular safety margin of the inhibitors [64]. α-Alkoxy acetamides bearing 2- thiazolyl or ethynyl groups on the P1 phenyl ring provided the best balance of functional potency, intrinsic metabolic stability, high passive permeability, and low efflux ratios. By virtue of their CYP 3A4-inhibiting properties, these compounds exhibited good pharmacokinetic profiles in both rats and dogs. Compound 24 (Fig. 5) displayed good inhibition profile (IC50 = 5.5 nM and IC50 = 9.9 nM in HEK cells) and reduced cerebrospinal fluid (CSF) Aβ40 levels by 50% at effective unbound plasma concentrations in the range of 6–13 nM. Compound 24 was further profiled in an anesthetized dog model for cardiovascular hazard identification and was found to produce no significant changes in cardiovascular functions at concentrations exceeding 16-fold the effective unbound plasma concentration required for 50% reduction of CSF Aβ40 levels in rats [64].
4.4. Constrained Hydroxyethylamine-Containing Inhibitors
Structure-based design of a series of cyclic HEA β-secretase inhibitors allowed the incorporation of prime- and nonprime-side fragments to a central core template without any amide functionality. The core scaffold selection and the structure–activity relationships were supported by molecular modelling studies and by X-ray analysis of β-secretase complexes with various ligands to expedite their optimization in terms of BBB permeability.
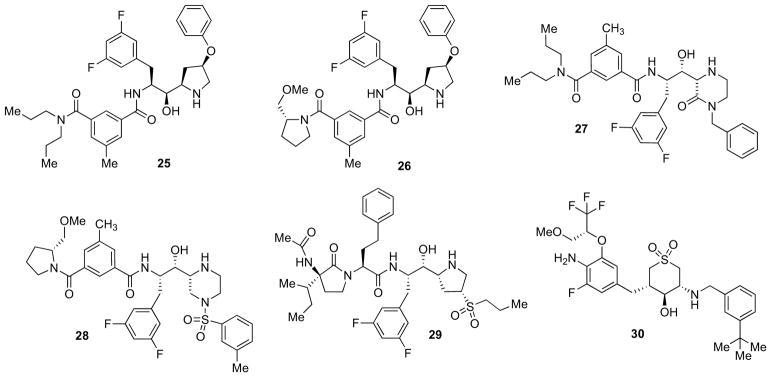
Iserloh and co-workers successfully demonstrated the potential of novel pyrrolidine-based β-secretase inhibitors [65]. As conformationally constrained versions of HEA-type peptidomimetics, their cyclic amine core allowed the specific targeting of the S2′ subsite. Compound 25 (Fig. 6) displayed the best profile of the series showing IC50 of 3 nM, cell IC50 (HEK 293 cells) of 165 nM and good selectivity over CatD (Ki = 291 nM) [65]. In a subsequent paper, the same authors reported various isophthalamide replacements to improve the potency, the pharmacokinetic parameters as well as the ancillary profile of their cyclic amine β-secretase inhibitors [66]. Replacement of the N,N-dipropylamide present in 25 with a 2-(R)-methoxymethylpyrrolidine amide resulted in compound 26 (Fig. 6) with a marked increase in cellular potency (Ki = 0.7 nM, cell IC50 = 21 nM), while maintaining good selectivity over related human aspartyl proteases such as CatD, CatE, and renin. Although moderate permeability was observed in the Caco-2 P-gp assay, the efflux ratio was high indicating that 26 is most likely a substrate for the P-gp transporter. This may account for the low brain/plasma ratio observed in the rat PK studies. Dose-dependent reduction of plasma Aβ40 was observed both upon oral and subcutaneous dosing, demonstrating the utility of 26 as a tool for in vivo studies [66].
Fig. 6.
Constrained hydroxylamine-containing inhibitors.
X-ray crystallography and molecular modeling led to the design of a series of novel, potent piperazinone and imidazolidinone- containing β-secretase inhibitors [67]. Piperazinones showed to tolerate wider modifications to their non-prime side. Compound 27 (Fig. 6, IC50 = 3 nM, cell IC50 = 300 nM), produced modest inhibition of peripheral Aβ40 in a transgenic mouse model with a single dose.
Combination of the m-tolyl sulfonamide moiety with the optimal methoxymethyl pyrrolidine isophthalamide group on the non-prime side, led to the extremely potent inhibitor 28 (Fig. 6, Ki = 0.18 nM, cell IC50 = 7 nM) which induced a robust and persistent lowering of peripheral Aβ in a transgenic mouse model following a single subcutaneous dose. However, P-gp liability limited its central efficacy [68].
Thompson and co-workers developed a series of cyclic diaminopropane isosteres combined with a number of different P2–P3 headgroups to provide potent β-secretase inhibitors [69]. Combinations of the pyrrolidine-based isostere substituted with a 4-sulfone group provided compound 29 (Fig. 6) that was highly potent in both enzyme and cellular assays (IC50 = 5 nM in HEK 293 cells). The peripheral Aβ40 lowering effect was also evaluated. Compound 29 showed to be effective in lowering plasma Aβ in normal rodents and both plasma and brain Aβ in P-gp deficient mice, thus demonstrating its P-gp liability [69].
Guided by structure-based optimization, Rueeger et al. identified the highly potent 30 (Fig. 6) with enzymatic and cellular IC50 values of 2 and 50 nM, respectively, and with >200-fold selectivity over CatD. Pharmacodynamic studies in APP51/16 transgenic mice at oral doses of 180 μmol/kg demonstrated a significant reduction of brain Aβ levels [70, 71].
4.5. Carbinamine- and Reduced Amide-Containing Inhibitors
Rajapakse et al. developed an intriguing class of small-molecule inhibitors [72]. In particular, the discovery and optimization of tertiary carbinamine-derived inhibitors provided novel promising ligands incorporating a single primary amine to interact with the catalytic aspartates of the target enzyme.
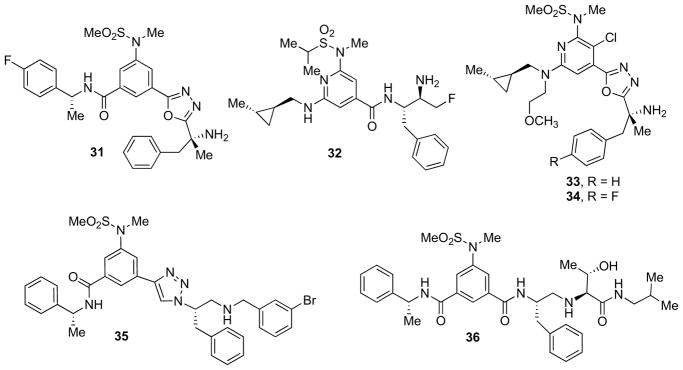
Inhibitor 31 (Fig. 7) displayed high potency in enzymatic and cellular assays (IC50 = 12 and 65 nM, respectively) and good selectivity toward both renin and CatD, but only moderate selectivity towards BACE-2 (IC50 = 620 nM). A series of interesting inhibitors based on a 2,6-diamino-isonicotinamide core coupled to a truncated reduced amide isostere as the aspartate binding element was developed [21]. Compound 32 (Fig. 7) displayed a cellular IC50 of 49 nM and in vivo activity in transgenic mice expressing human wildtype APP. After i.v. administration of a 50 mg/kg dose of inhibitor 32, a maximal reduction of Aβ40 (34%) at 3 h from dosing was observed and the concentration of drug in the brain was 1.9 μM [73].
Fig. 7.
Carbinamine- and reduced amide-containing inhibitors.
Optimization of this series provided inhibitors with intrinsic and functional potency comparable to evolved TS isostere derived inhibitors of β-secretase. Particularly, the combination of the isonicotinic core containing a P3 methylcyclopropyl group with the oxadiazolyl tertiary carbinamine resulted in compound 33 (Fig. 7) [74]. Although it displayed very good potency (IC50 = 0.4 nM; sAPPβ_NF IC50 = 40 nM) and minimal P-gp liability, a major drawback was represented by the poor pharmacokinetic profile and the limited bioavailability in multiple species [74,75]. Further SAR studies, driven by the evidence that 33 occupies the S1–S3 sites, led to the identification of its 4-fluoro substituted analog 34 (Fig. 7) [76]. A 2-fold more potent inhibitor of the enzyme was identified from this effort and its efficacy in the rhesus CSF Aβ lowering was evaluated. Unfortunately, the modest improvement of in vitro potency did not result in superior pharmacodynamic response for compound 34 [76].
Based upon the first reduced amide inhibitors reported by Coburn et al. at Merck [77], Monceaux and co-workers sought to further reduce the peptidic nature by replacing the P2 amide with an anti-1,2,3-triazole unit (35)[78]. Unfortunately, this replacement resulted in a 1000-fold decrease in potency. Docking studies of 35 (Fig. 7) and of 31 within the β-secretase binding site suggest that the docking poses of the two inhibitors in the active sites are quite similar, with one exception. In the docked structures the placement of the protonated amine that engages catalytic Asp228 differs considerably between the two inhibitors. This difference could account for the lower β-secretase inhibition potency of 35 [78].
Very recently, Ghosh and co-workers reported the structure- based design, synthesis, and X-ray structure of protein–ligand complexes of exceptionally potent and selective β-secretase reduced-amide inhibitors containing isosteres [79]. The inhibitors were specifically designed to interact with S1′ active site residues to provide selectivity over BACE-2 and CatD. Inhibitor 36 (Fig. 7) exhibited exceedingly potent inhibitory activity (Ki = 17 pM) and high selectivity over BACE-2 (>7000-fold) and CatD (>250000-fold). A protein–ligand crystal structure (PDB ID: 4GID) revealed important molecular insights about the selectivity thus representing an important guide to design selective inhibitors over the physiologically relevant aspartic acid proteases [79].
4.6. Macrocyclic Inhibitors
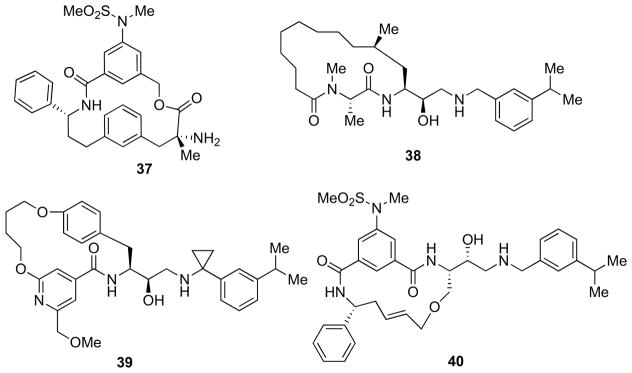
The close spatial proximity between the P1 aryl group and the P3 methyl of carbinamine-based inhibitors suggested the possibility of increasing potency by linking these two portions with the aim of both stabilizing the bioactive conformation and improving the physicochemical liabilities of the acyclic counterparts with the preparation of macrocyclic ethers and macrolactones [80]. Macrolactone 37 (Fig. 8), developed by Lindsley and co-workers [81], displayed good potency (IC50 = 2 nM; sAPPβ_NF IC50 = 5 nM), and was found to be hydrolytically stable at physiological pH and stable in rat and human plasma and in microsomal preparations in the absence of nicotinamide adenine dinucleotide phosphate (NADPH).
Fig. 8.
Macrocyclic inhibitors.
In an effort of improving their previously developed macrocyclic inhibitors, displaying moderate enzymatic and cellular activity [82], Machauer et al. exchanged the HE with an ethanolamine-TS-mimetic thus reducing the peptidic character and providing the highly potent and selective inhibitor 38 (Fig. 8, IC50 = 0.022 μM on β-secretase and 1.4 μM on CatD) [83]. In a subsequent paper, Lerchner et al. designed another series of macrocyclic peptidic β-secretase inhibitors [84]. Representative compound 39 (Fig. 8) showed an IC50 of 9 nM. The introduction of the cyclopropyl into HEA-bearing β-secretase inhibitors led to a loss of selectivity over the closely related aspartyl proteases CatD and E.
Also Sandgren and co-workers reported a series of macrocyclic inhibitors containing a HEA isostere [85]. The most potent inhibitor 40 (Fig. 8) displayed an IC50 value of 3.2 nM in the enzymatic assay and 0.15 nM in the cell-based assays. Furthermore, good selectivity over CatD could generally be observed for all the inhibitors of this series. X-ray crystallography studies concluded that the macrocycle-attached phenyl group of inhibitor 40 did not fully reach into the S3 sub-pocket of β-secretase, indicating room for further potency optimization. However, all the compounds synthesized suffered from inadequate permeability, tentatively preventing them from reaching the CNS [85].
5. SMALL-MOLECULE β-SECRETASE INHIBITORS
5.1. Acylguanidines, 2-Aminopyridines and Aminoimidazoles
In recent years, β-secretase inhibitors with alternative chemotypes such as acylguanidines and amino heterocycles have been discovered. High-throughput screening (HTS) of Wyeth’s compound library by means of a FRET assay identified acylguanidine as a prototype of a new class of β-secretase inhibitors [86]. Acylguanidines directly bind to the catalytic aspartic acids (Asp32 and Asp228) in β-secretase by displacing the tightly bound catalytic water. The third nitrogen faces away from the catalytic residues toward the S1′ pocket.
Optimization of the hit using structure-based design led to compound 41 (Fig. 9, IC50 = 110 nM). The p-propyloxyphenyl group of this compound extends from the S1 to the S3 pocket with minimal strain and the pyrrole ring forms a π-stacking interaction with the flap Tyr71. In contrast to complexes with peptidomimetic inhibitors, the flap region adopts an “open conformation” that hosts the diarylpyrrole portion of the inhibitor (such a conformation would be stabilized by π-stacking interactions between the pyrrole and the phenyl ring of Tyr71) [87, 88].
Fig. 9.
Acylguanidines, 2-aminopyridines and aminoimidazoles.
Preliminary structure–activity relationship (SAR) investigations as well as the bioisosteric replacement of the guanidyl functionality did not provide better performing inhibitors. Furthermore, poor selectivity over BACE-2 enzyme and poor permeability are limiting liabilities which affected further investigation of this class of compounds [87, 88].
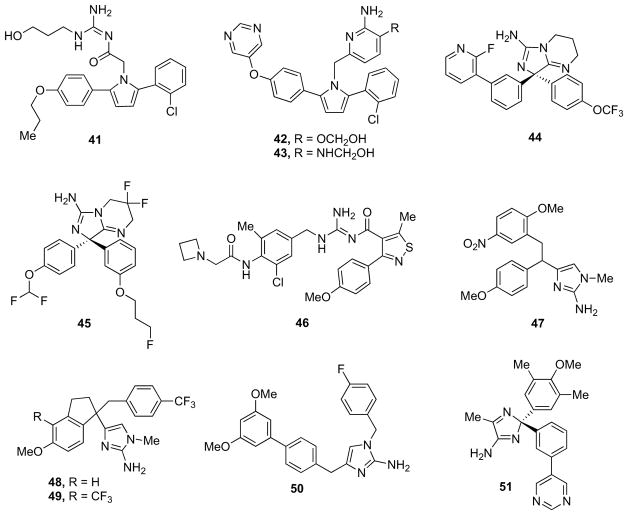
The acylguanidine inhibitors are polar compounds, particularly due to the acylguanidine moiety, as suggested by the high total polar surface area (TPSA), which was the main determinant for the observed poor BBB permeation (<5%). Malamas and co-workers modified the acylguanidine moiety with the aim of effectively reduce TPSA thus improving compound permeability [89]. Molecular modeling studies suggested that the aminopyridine moiety could replace the acylguanidine moiety, bind satisfactorily to the bis-Asp war-head, and also decrease the TPSA of the molecule. Further-more, potentiometric pKa measurements suggested that the ideal pKa range for the acylguanidine mimetic would be 6–7, making 2-aminopyridine (pKa = 6.86) to fall within the appropriate range. Analogs 42 and 43 (Fig. 9) exhibited IC50 values of 40 and 70 nM, respectively, for β-secretase. Moreover, their cell-based activity also improved well with their increased binding. The aminopyridine inhibitors were also evaluated against BACE-2 and catD. The BACE-2 selectivity ranged between 10 to 130 times, while they were even more selective against CatD (>100-fold), with the most potent inhibitor 42 showing >100-fold selectivity against BACE-2, and >500-fold against CatD [89]. Addition of either a pyridine or a pyrimidine ring on a lead previously identified at Wyeth allowed the extension deep into the S3 region of the β-secretase binding pocket and enhanced the ligand’s potency. Compound 44 (Fig. 9) displayed an IC50 value for β-secretase of 20 nM, cellular activity of 90 nM, and >100-fold selectivity over related aspartyl proteases. Acute oral administration of 44 at 30 mg/kg resulted in a significant 71% reduction of plasma Aβ40 measured at the 6 h time point in a Tg2576 mouse model [90]. Later on, Swanh and collaborators discussed the important properties for in vivo brain efficacy and described the effort to improve bicyclic aminoimidazole derivatives towards achieving in vivo brain efficacy. Compound 45 (Fig. 9) (pIC50 = 7.50) was selected for evaluation in vivo and, 1.5 h after oral co-administration of 300 μmol/kg 45 and efflux inhibitor GF120918, the brain Aβ40 level was reduced by 17% and the plasma Aβ40 level by 76% [91].
Very recently, Gerritz and co-workers described the discovery and optimization of a β-secretase inhibitor series containing an unusual acyl guanidine chemotype that was originally synthesized as part of a 6041-membered solid-phase library [92]. The synthesis of multiple follow-up solid- and solution-phase libraries facilitated the optimization of the original micromolar hit into a single-digit nanomolar inhibitor in both radioligand binding and cell-based functional assay formats. The X-ray structure of a very close analog of the best inhibitor 46 (Fig. 9) bound to β-secretase (PDB ID: 4FSL) revealed a number of key ligand-protein interactions, including a hydrogen bond between the side chain amide of flap residue Gln73 and the acyl guanidine carbonyl group, and a cation-π interaction between Arg235 and the isothiazole 4-methoxyphenyl substituent. Inhibitor 46 displayed a Ki value of 5 nM and a cell IC50 of 5 nM, afforded good plasma exposures and a dose-dependent reduction in plasma Aβ levels, but poor brain exposure was observed (likely due to P-gp-mediated efflux) and significant reductions in brain Aβ levels were not obtained [92].
Stachel and collaborators published an interesting study in which they developed a series of aromatic heterocycles as β-secretase inhibitors [93]. The potency of the weak initial lead structure was dramatically enhanced using a traditional medicinal chemistry approach. These inhibitors were shown to bind the enzyme in a non-traditional fashion. Compound 47 (Fig. 9) was active in the enzymatic assay (IC50 = 0.47 μM) at pH 6.5 and more importantly demonstrated low micromolar activity in cell-based assay (IC50 = 1.8 μM). The authors delineated subtleties in pH dependent binding in a modified HEK-293 cell line, in which there was a pronounced pH dependence demonstrating that β-secretase assay at pH 6.5 rather than pH 4.5 was more predictive of the measured cellular potency. Taken together, the enzymatic and cellular data tend to support the notion that APP cleavage is occurring in less acidic cellular components than previously hypothesized [93].
As a further development of these aminoheterocyclic β-secretase inhibitors, focusing on simultaneously enhancing potency and maintaining or lowered P-gp efflux, Hills et al. introduced a conformationally constrained inhibitor inspired by a crystal structure [94]. Constrained compound 48 (Fig. 9) was even more active than potent non brain penetrant inhibitor 47, bearing the 2-methoxy-5-nitro substituted benzyl subunit (IC50 = 0.35 μM versus IC50 = 0.47 μM). Additional optimization led to inhibitor 49 (Fig. 9), a highly active β-secretase inhibitor with a relatively low P-gp efflux ratio (IC50 = 0.063 μM; P-gp (BA/AB) = 3.6). However, computational studies unexpectedly suggested that the constrained inhibitors show enhanced potency via additional favorable hydrophobic interactions, rather than conformational restriction [94].
Chiriano and co-workers described a rational structure-based approach, integrated with a synthetic protocol amenable to parallel synthesis, aimed at the discovery of new 2-aminoimidazole derivatives as β-secretase inhibitors [95]. Among ten novel derivatives, 50 (Fig. 9) emerged as a promising β-secretase inhibitor. The good chemical accessibility allowed to carry out extensive SAR studies. A low micromolar inhibitory profile (IC50 = 7.40 μM) was assessed by enzymatic and cellular assays. Inhibitor 50 represented the starting point for an extensive campaign of hit-to-lead and eventually lead optimization [95].
Gravenfors et al. described amino-2H-imidazole-containing compounds as β-secretase inhibitors [96]. Compound 51 (Fig. 9) was one of the most promising derivatives, with an IC50 value of 24 nM and a good overall profile. When guinea pigs were treated with compound 51, a concentration and time dependent decrease in Aβ40 and Aβ42 levels in plasma, brain, and CSF was observed. The maximum reduction of brain Aβ was 40–50%, 1.5 h after oral dosing (100 μmol/kg). The results presented highlight the potential of this new class of β-secretase inhibitors with good target potency and with low effect on hERG, in combination with a fair CNS exposure in vivo [96].
5.2. Aminohydantoins, Iminohydantoins and other Amino/Imino-Heterocycles
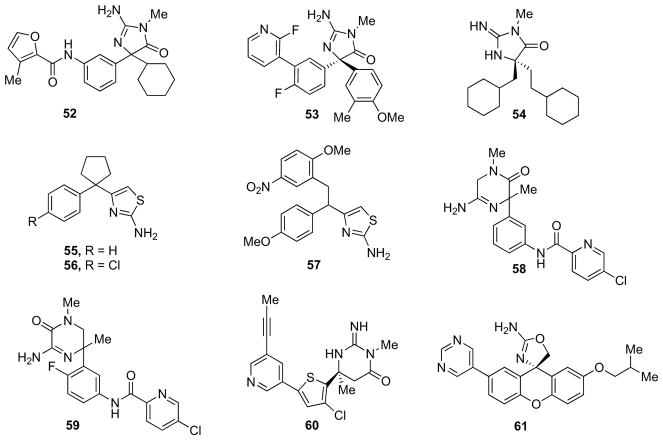
The identification of small molecule aminohydantoins as potent and selective human β-secretase inhibitors evolved based upon the assumption that removal of tetrahydro-pyrimidine ring of compounds like 44 (Fig. 9), should have little effect on the binding affinity since the resulting diphenylhydantoins or their partially or fully saturated analogs should retain all the key interactions with the protein [97].
Furthermore, Nowak et al. envisaged that the potency of aminohydantoin-containing β-secretase inhibitors could be improved by introducing substituents extending from the S1 to the S3 pocket [97]. This strategy was already shown to be successful on the acylguanidine series inhibitors. Overlaying the two scaffolds in the β-secretase binding site indicated that the meta-position of the phenyl ring of aminohydantoin is ‘equivalent’ to para-position of phenyl ring of aminoguanidine and it should allow for introduction of substituents extending into the deeper S3 pocket. Accordingly, the possibility of reaching further into the S1–S3 pocket was explored with a set of amides. The best performing compound 52 (Fig. 10), containing a 3-methyl-2-furoic amide, is a 40 nM β-secretase inhibitor with an EC50 of 180 nM in the Aβ cellular ELISA assay. Although these compounds were selective over CatD, further optimization was necessary for improving BACE-2 selectivity, physical and pharmaceutical properties [97].
Fig. 10.
Aminohydantoins, iminohydantoins and other amino/imino heterocycles.
Later on, Malamas and co-workers developed analogues exhibiting low nanomolar potency for β-secretase, with comparable activity in ELISA assay, and demonstrated >100-fold selectivity for the other structurally related aspartyl proteases BACE-2, CatD, renin, and pepsin [98]. On the basis of the cocrystal structure of a HTS-hit in the β-secretase active site (PDB ID: 3IGB [90]) and by use of a structure-based drug design approach, the authors identified key interactions between the ligand and the protein that contributed to the affinity. One of the most potent compounds, 53 (Fig. 10), displayed an IC50 value of 10 nM and exhibited comparable cellular activity (EC50 = 20 nM) in the ELISA assay. Acute oral administration of 53 at 100 mg/kg resulted in a 69% reduction of plasma Aβ40 after 8 h in a Tg2576 mouse (p < 0.001) [98].
Zhu and collaborators reported a number of novel β-secretase inhibitors with amidine-containing heterocycles. These compounds were designed to reproduce the unique interaction pattern, revealed by X-ray crystallography, between the β-secretase catalytic diad and a weak hit derived from NMR screening. Further optimization was carried out by maintaining the appropriate basicity and limiting the number of H-bonding donors of these scaffolds. Compound 54 (Fig. 10) was identified, with an IC50 of 605 nM, a molecular weight around 300 Da, two hydrogen bond donors, two hydrogen bond acceptors and high ligand efficiency. Although compound 54 had a modest pharmacokinetic profile in rat, it was brain penetrant with a 6 h brain/plasma ratio of 1 and a brain concentration at 6 h of over 100 nM. Therefore, iminohydantoin 54 offered an excellent starting point for further development of CNS penetrant aspartyl protease inhibitors [99].
Stachel et al. identified 55 (Fig. 10), a weak inhibitor of β-secretase (IC50 = 217 μM) from a HTS effort [93]. While only exhibiting meager activity against β-secretase, its compact size represented an alluring ligand efficiency value of 0.3 and since 55 comprised a novel structural motif it was hoped that it would provide a foray into inhibitor series lacking the liabilities of traditional TS-isosteres. SAR around the aromatic ring of lead structure 55 provided compound 56 (Fig. 10), in which a para-chloro substituent was found to impart a 10-fold increase in intrinsic potency (IC50 = 38 μM). More importantly when compound 56 was administered to mice at a 20 mg/kg i.v. dose the brain to plasma ratio was determined to be 3.9 (t = 30 min; brain concentration = 15 μM). Additional SAR studies provided compound 57 (Fig. 10, racemic mixture, IC50 = 1.1 μM) where the spirocyclopentyl ring was replaced with a 2-methoxy-5-nitro benzyl group [93].
Aminopiperazinone inhibitors of β-secretase were identified by rational design by Tresadern and collaborators [100]. The initial racemic hit 58 (Fig. 10) showed good activity (IC50 = 447 nM). The crystal structure of 58 (PDB ID: 3UA6) showed a binding mode driven by interaction with the catalytic aspartate dyad and distribution of the biaryl amide towards S1 and S3 pockets. Modification and chiral separation resulted in the 40 nM inhibitor (−)−59 (Fig. 10), with improved cellular activity (cell IC50 = 0.025 μM). (−)−59 was also tested for its ability to reduce Aβ peptides in vivo. At 60 mg/kg subcutaneous dose it was showed a reduction of Aβ by 48%, 67% and 73% at 1, 2 and 4 h respectively. However, these molecules showed a rapid decrease in plasma concentration levels, likely due to low metabolic stability [100].
Stamford and co-workers described structure-based optimization of a series of brain-penetrant β-secretase inhibitors derived from an iminopyrimidinone scaffold. Application of structure-based design and optimization of physicochemical properties culminated in the discovery of compound 60 (Fig. 10, Ki = 1.7 nM, cell IC50 = 11 nM), which potently reduced cortex (ED50 = 6 mg/kg) and CSF Aβ40 (ED50 = 4 mg/kg) levels when administered orally to rats [101].
Very recently, Huang et al. proposed an interesting structure- and property-based drug design approach employed to identify aminooxazoline xanthenes as potent and selective human β-secretase inhibitors [102]. Starting from an early series of racemic biaryl spiro-aminooxazolines, a key insight was the realization that rotation of the right-most phenylring by ~90° would allow direct access to S2′ by a meta substituent. To lock this conformation, the authors designed a xanthene-based 2-aminooxazoline core. Compound 61 (Fig. 10), the best performing of the series, displayed an IC50 value of 8 nM and a cell IC50 value of 36 nM with a 560-fold selectivity over CatD. Compound 61 exhibited a significant reduction of Aβ40 in the CNS. The brain–blood ratios of 61, 1.1 at 30 mg/kg and 1.6 at 100 mg/kg, suggest that the compound readily passes the BBB. 61 significantly inhibited Aβ40 production in both CSF (76%) and brain (45%) at 30 mg/kg relative to the vehicle-treated animals. At 100 mg/kg, the inhibition of Aβ40 production was slightly improved in CSF (81%) and a more robust Aβ40 reduction was seen in brain (63%). The degree of Aβ40 reduction in the CSF and brain improved with the increased exposure in the corresponding compartments [102].
5.3. Dihydroquinazolines and Aminoquinolines
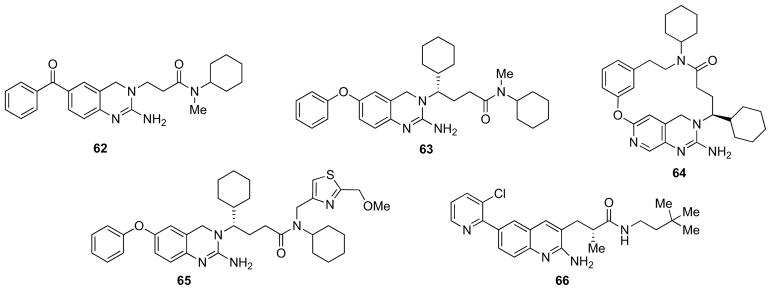
A new aspartic protease inhibitory chemotype bearing a 2-amino-3,4-dihydroquinazoline ring was identified by HTS for the inhibition of β-secretase (compound 62, Fig. 11) [103]. X-ray crystallography (PDB ID: 2Q11) revealed that the exocyclic amino group participated in a hydrogen bonding array with the two catalytic aspartic acids of β-secretase (Asp32, Asp228). β-secretase inhibitory potency was increased from a Ki = 0.9 μM to a Ki = 11 nM Ki by substitution into the unoccupied S1′ pocket (compound 63, Fig. 11; PDB ID: 2Q15). This inhibitor exhibited a moderate selectivity over CatD and renin and lowered Aβ levels in plasma by 40–70% in rats after oral administration (30 mg/kg). However, it was a substrate for P-gp, as indirectly evaluated by the efflux ratio calculations in the Caco-2 model [103].
Fig. 11.
Dihydroquinazolines and aminoquinolines.
X-ray structure also suggested that the two ends of the hit compound could be connected to form a macrocycle. A variety of prospective macrocycles were evaluated computationally by Huang and collaborators and, subsequently, the authors embarked on a SAR study on the best performing calculation outcomes. Analog 64 (Fig. 11) showed impressive potency (Ki = 5 nM), and cellular activity (cell IC50 = 7 nM). However, upon i.v, i.p. or oral administration, only trace levels of compound were detected in the brain, and no lowering of Aβ levels in the brain was observed. Preliminary experiments suggested that P-gp efflux might prevent brain penetration of these compounds [104].
Ghosh et al. carried out structure-based modifications of 2-amino-3,4-dihydroquinazoline-derived β-secretase inhibitors. In particular, they incorporated thiazole and pyrazole-based P2-ligands to make specific interactions in the S2-subsite. Inhibitor 65 (Fig. 11) showed enhanced enzyme inhibitory activity (Ki = 13 nM) as well as very good cellular inhibitory potency in neuroblastoma cells (IC50 = 21 nM). Furthermore, a protein-ligand X-ray structure-based model of 65-bound β-secretase provided important molecular insight into the ligand-binding site interactions [105].
Using fragment-based screening of a focused fragment library, Cheng and co-workers identified 2-aminoquinoline as an initial hit for β-secretase [106]. Further SAR development was supported by X-ray structures of β-secretase cocrystallized with various ligands and molecular modeling studies expedited the discovery of potent compounds. These strategies enabled the authors to integrate the C-3 side chain on 2-aminoquinoline extending deep into the P2′ binding pocket of β-secretase enhancing the ligand’s potency. Further optimization of the lead compounds for improving BBB permeability led to inhibitors with greatly improved cellular activity and permeability. Compound 66 (Fig. 11) showed an IC50 value of 11 nM on β-secretase and cellular activity of 80 nM. This compound was advanced into rat pharmacokinetic and pharmacodynamic studies and demonstrated significant reduction of Aβ levels in CSF [106].
5.4. Pyrrolidine-Based Scaffolds
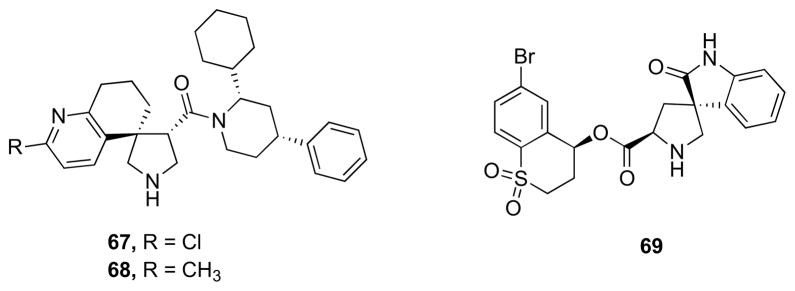
Stachel et al. developed a novel series of pyrrolidine derived β-secretase inhibitors [107]. The potency of the weak initial lead structure was enhanced using library-based SAR methods. The series was then further advanced by rational design while maintaining a minimal ligand binding efficiency threshold. Ultimately, the co-crystal structure was obtained revealing that these inhibitors interacted with the enzyme in a unique fashion. The potency of the series was enhanced by 4 orders of magnitude from the HTS lead with concomitant increases in physical properties needed for series advancement. Compound 67 (Fig. 12) displayed significant potency (IC50 = 29 nM) but modest cellular potency (cell IC50 = 570 nM). This compound also displayed selectivity toward other related aspartyl proteases, modest in vivo clearance in rats (15 mL/min/kg) and a respectable oral bioavailability of 44%. Replacement of the chloride with a 2-methyl substituent provided the more basic pyridyl derivative 68 (Fig. 12), which again retained a favorable enzymatic potency with an IC50 = 36 nM, but with improved cellular potency (IC50 = 100 nM) [107]. Efremov and co-workers performed an X-ray-based fragment screen of Pfizer’s pro-prietary fragment collection which resulted in the identification of a novel β-secretase binder featuring spiropyrrolidine framework [108]. Although exhibiting only weak inhibitory activity against the β-secretase enzyme (IC50 = 1100 μM), the small compound was verified by biophysical and NMR-based methods as a bona fide β-secretase inhibitor. Subsequent optimization of the lead compound, relying heavily on structure-based drug design and computational prediction of physiochemical properties, resulted in a nearly 1000-fold improvement in potency with compound 69 (Fig. 12, IC50 = 1 μM) while maintaining ligand efficiency and properties predictive of good permeability and low P-gp liability [108].
Fig. 12.
Pyrrolidine-based scaffolds.
5.5. Miscellaneous Small-Molecule Inhibitors
A quickly growing number of papers involve the identification of small molecules β-secretase inhibitors, bearing a variety of structural templates. We included here a selection of the most significant chemical templates riding off from peptide-like structures.
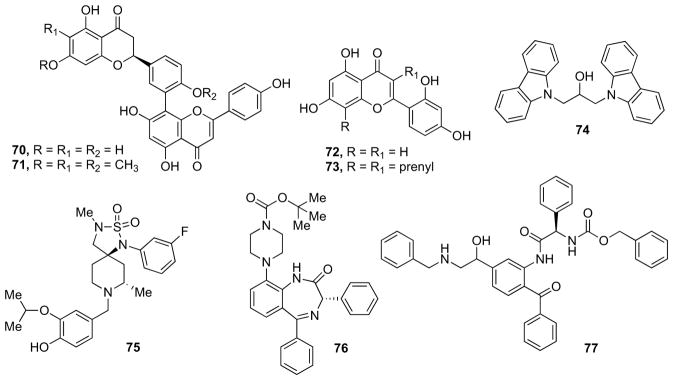
Sasaki et al. found that amentoflavone-type biflavonoids have significant neuroprotective effects and β-secretase inhibitory activity. Among these compounds, 2,3-dihydroamentoflavone 70 (Fig. 13) and 2,3-dihydro-6-methylginkgetin 71 (Fig. 13) exhibited potent inhibitory effects with IC50 values of 0.75 and 0.35 μM, respectively [109].
Fig. 13.
Miscellaneous small-molecule inhibitors.
Later on, Cho and co-workers showed that flavones from Morus lhou potently inhibit β-secretase [110]. The authors aimed at exploring the inhibitory kinetics of natural compounds and developing a pharmacophore model in which are highlighted the critical features responsible for inhibitory activity. Prenylated flavone 73 (Fig. 13, IC50 = 3.4 μM) was 20 times more effective than its parent compound, norato-carpetin 72 (Fig. 13, IC50 = 60.6 μM). Enhanced activity was related with isoprenyl functionality at C-3. Kinetic analysis showed that the compounds have a noncompetitive mode of action. The binding affinity of flavones for β-secretase calculated using in silico docking experiments correlated well with their IC50 values and noncompetitive inhibition modes [110].
Kumar et al., inspired by previous work by Macchia and co-workers [111], conducted a study on a compound series of 1,3-disubstituted 2-propanols for the inhibition of β-secretase. A selected set of 121 compounds was combinatorially prepared and tested for inhibition. The screening results including the detailed SAR studies revealed that best β-secretase inhibition was observed if i) a naphthyl or carbazole ring in combination with an extended aromatic ring system such as a carbazole or a 2-phenyl-indole was present to occupy the S1, S2, S1′, and S2′ subpockets, ii) the linker had four methylene units, iii) the linker was sterically compact for accommodating only one hydroxyl group. Further optimization efforts ultimately led to the most potent and selective compound, bis-carbazole 74 (Fig. 13), with an IC50 of 710 nM against β-secretase and a selectivity of 422 times over CatD [112].
Brodney et al. described the identification and optimization of a novel series of spirocyclic sulfamide β-secretase inhibitors binding to the catalytic aspartic acids via water mediated hydrogen bond [113]. A combination of structure-based drug design and computational modeling guided the rational design of new analogues and explained the obtained SAR. Excellent in vitro potency was achieved in the series by combining a substituent in the S3 pocket with a phenol, resulting in favorable interactions with the protein. Improved brain penetration was achieved by reducing the number of hydrogen bonds, lipophilicity, and molecular weight. Resulting compound 75 (Fig. 13) was a potent inhibitor of β-secretase (IC50 = 0.10 μM) with excellent permeability and a moderate P-gp liability. Administration of 75 to mice produced a significant, dose-dependent reduction in central Aβ40 levels at a free drug exposure equivalent to the whole cell IC50 (100 nM). Furthermore, studies of the P-gp knock-out mouse provided evidence that efflux transporters affected the amount of Aβ lowering versus that observed in wild-type mouse at an equivalent dose [113].
Recently, Butini et al. investigated a series of novel peptidomimetics based on the druggable 1,4-benzodiazepine (BDZ) core and their seco-analogues [114, 115]. The secoanalogues proved to be slightly more potent than BDZs (Fig. 13, 76, IC50 = 35 μM, and 77 IC50 = 2.6 μM represent the best compounds for each series) and through docking studies the authors were able to explain the improvement of the inhibitory potency due to the opening of the BDZ system. The compounds were tested to determine their IC50 against the generation of sAPPβ in HEK293 cells and in SHSY5Y cells. 77 was found the most interesting compound of the series showing an IC50 values in both cell lines ranging from 1.2 μM to 2.6 μM [115].
5.6. Recent Small-Molecule Templates from Computational Approaches
Since its discovery, β-secretase has been subjected to a steadily growing number of computational studies in order to improve the activity of existing compounds and to explore the enzyme for unveiling structural portions to be exploited for the binding of novel structural templates.
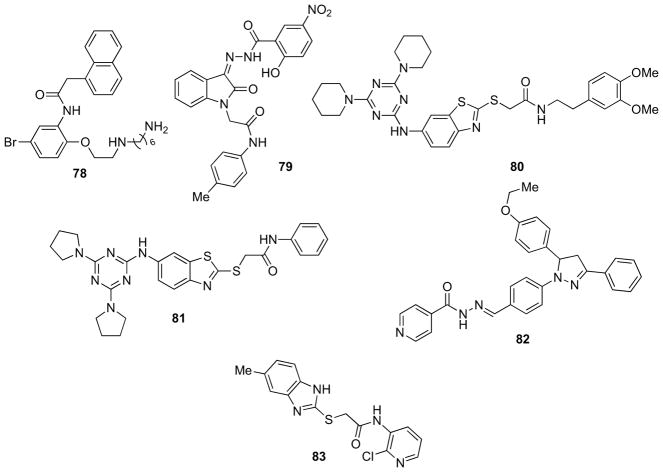
Huang et al. performed a study for identifying the key features of arylpiperazine amide derivatives for interactions with enzyme by pharmacophore models using Discovery Studio 2.0 (Accelrys, Inc., San Diego, CA) software package [116]. The validation of the best pharmacophore model (Hypo 1) was ascertained by Enrichment and receiver operating curve (ROC) method. The Hypo 1 was used for in silico screening of a designed database. As a result, eleven novel N-phenyl-1-arylamide, N-phenylbenzenesulfonamide derivatives were selected and synthesized. β-Secretase inhibitory activities of these compounds were subsequently evaluated experimentally and their theoretical results were in good agreement with the experimental values. Compound 78 (Fig. 14), which displayed the highest β-secretase activity (18.33 μmol/L) among the two series, was chosen to study the protein binding pattern and the result showed that it was in close contact with the two essential catalytic aspartates (Asp32 and Asp228) of the enzyme [116].
Fig. 14.
Recent small-molecule templates from computational approaches.
Using the virtual HTS software eHiTS, Mok and co-workers discovered a novel non-peptidic inhibitor of β-secretase based on an isatin motif (compound 79, Fig. 14, IC50 = 2.4 μM) [117]. Studies on the biological activity of structural variants in combination with in silico docking suggested that the inhibitor adopts a planar conformation, which is stabilized by intramolecular H-bonding from the phenolic moiety. Additionally, binding to β-secretase appeared to involve H-bonding interactions between the p-tolylamide and the catalytic residue Asp228. Although the relatively poor solubility of the present series of compounds (e.g., clogP values for compound 79 of 3.97), coupled with the presence of the nitro and phenolic moieties make them challenging candidates for further development. The authors demonstrated that eHiTS is a powerful screening tool to identify biologically active compounds quickly and efficiently [117].
Xu and collaborators described an efficient approach by integrating virtual screening with bioassay technology for finding small inhibitors targeting β-secretase [118]. Fifteen hits with inhibitory potencies (IC50) ranging from 2.8 to 118 μM were successfully identified. Compound 80 (Fig. 14) with IC50 of 2.8 μM represented the most potent hit. Docking simulation performed by GOLD 3.0 (CCDC, Cambridge, UK) software suggested putative binding mode of 80 into β-secretase and potential key pharmacophore groups for further design of non-peptide inhibitors [118].
Subsequent work was represented by a similarity search on the structural analogs of inhibitor 80. By using this approach compound 81 (Fig. 14, IC50 = 0.12 μM) stood out and improved the inhibitory potency by 24 folds. These results suggest that a pyrrolidinyl side group at the P3′ and P4′ of the inhibitors are favored for strong inhibition and a small aromatic group at the P4 position is also essential to the potency [119].
In a subsequent paper, the same authors reported the discovery of two novel β-secretase small organic inhibitors via a receptor-based virtual screening followed by compound drug-likeness analysis, cytotoxicity prediction, and bioassays [120]. The SPECS chemical library (about 280,000 compounds) was screened using GOLD 3.0.1 (CCDC, Cambridge, UK) with default parameters. To select which of the in silico compounds would be purchased and tested in vitro, several factors were taken account in this step: i) information on molecular weight, predicted LogP, LogS and toxicity values of the compounds, toxicity prediction by Pallas (Compudrug Pty Ltd) software; ii) knowledge of the molecular interactions between β-secretase and its substrate, together with the predicted putative H-bonds formed by the hits and active site residues, potential hydrophobic interaction and aromatic–aromatic interactions. Based on these factors, the consensus judgment from both of the selection criteria led to purchase 20 compounds for enzymatic FRET assays and methylthiazoletetrazolium (MTT) cytotoxicity experiment. Among them, compound 82 (Fig. 14) emerged as a sub-micromolar inhibitor (IC50 = 0.53 μM) with low cytotoxicity. Docking of compound 82 in the enzyme active site revealed that a central hydrophobic benzene ring occupied the aspartic acid catalytic holes and also suggested the putative binding and interactions with other subsites [120].
More recently, Chiriano et al. reported the sequential application of virtual screening approaches to identify novel scaffolds toward the discovery of new β-secretase inhibitors [121]. As a prerequisite for the BBB permeability, they searched for molecules matching the following physico-chemical properties: molecular weight < 450 Da, number of H-bonds < 8, logP from 2 to 5; and polar surface area (PSA) < 100 Å. Seven hits were retrieved by this procedure, and one of them, 83 (Fig. 14) showed an IC50 = 3.5 μM. Furthermore, 83 had a low molecular weight and was compliant with both the requirements of BBB permeability and the Lipinski rule [121].
CONCLUSION AND PERSPECTIVES
β-Secretase represents an important disease-modifying target for drug development against Alzheimer’s disease. As a consequence, during the last decade, many research efforts have focused on the identification of new β-secretase inhibitors as drug candidates. However, serious challenges with respect to BBB penetration and selectivity are still around the corner, irrespective of their chemical class. An inhibitor with the appropriate pharmacokinetic and pharmacodynamic profiles will ultimately validate β-secretase as feasible drug target. Discovering a small molecule inhibitor of β-secretase still represents an unnerving challenge. However, due to its significant potential as a therapeutic target, growing efforts in this task are evident from both academic and industrial laboratories. The availability of crystal structures of β-secretase-inhibitor complexes represents an invaluable opportunity for structure-based design. Nevertheless, beyond the inhibitory activity, the common denominator of the current research approaches is the big attention at addressing the problems associated with BBB penetration and pharmacokinetics.
This review outlined the structural evolution of the early β-secretase inhibitors and covered the hottest chemical templates in literature, showing how research in this field is making strides in the right direction.
Inhibitor design usually focuses on active-site binding, neglecting the subcellular localization of active enzyme. Recently, this issue has been addressed by synthesizing a membrane-anchored version of a β-secretase TS-inhibitor linked to a sterol moiety. This strategy allowed the targeting of active β-secretase found in endosomes increasing its local membrane concentration. This inhibitor reduced enzyme activity much more efficiently than did the free inhibitor in cultured cells and in vivo [122].
In parallel with basic research providing a better under-standing of the diseases, future directions might shift to the exploitation of β-secretase inhibition in multi-target approaches, by single molecules or suitable combinations. Such multi-directed interventions could have the potential for slowing or modifying even more effectively the neurodegenerative processes [123, 124].
Acknowledgments
Financial support by the National Institutes of Health (AKG) is gratefully acknowledged.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
References
- 1.Stachel SJ, Coburn CA, Rush D, Jones KL, Zhu H, Rajapakse H, Graham SL, Simon A, Katharine Holloway M, Allison TJ, Munshi SK, Espeseth AS, Zuck P, Colussi D, Wolfe A, Pietrak BL, Lai MT, Vacca JP. Discovery of aminoheterocycles as a novel beta-secretase inhibitor class: pH dependence on binding activity part 1. Bioorg Med Chem Lett. 2009;19(11):2977–2980. doi: 10.1016/j.bmcl.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 2.Citron M. Beta-secretase inhibition for the treatment of Alzheimer’s disease--promise and challenge. Trends Pharmacol Sci. 2004;25(2):92–97. doi: 10.1016/j.tips.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ, Schenk D. Alzheimer’s disease: Molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- 4.Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer’s disease: regulation, cell biology, function, and therapeutic potential. J Neurosci. 2009;29(41):12787–12794. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evin G, Barakat A, Masters CL. BACE: Therapeutic target and potential biomarker for Alzheimer’s disease. Int J Biochem Cell Biol. 2010;42(12):1923–1926. doi: 10.1016/j.biocel.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Hong L, Koelsch G, Lin X, Wu S, Terzyan S, Ghosh AK, Zhang XC, Tang J. Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science. 2000;290(5489):150–153. doi: 10.1126/science.290.5489.150. [DOI] [PubMed] [Google Scholar]
- 7.Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4(3):231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 8.Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4(3):233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 9.Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, Freedman SB, Frigon NL, Games D, Hu K, Johnson-Wood K, Kappenman KE, Kawabe TT, Kola I, Kuehn R, Lee M, Liu W, Motter R, Nichols NF, Power M, Robertson DW, Schenk D, Schoor M, Shopp GM, Shuck ME, Sinha S, Svensson KA, Tatsuno G, Tintrup H, Wijsman J, Wright S, McConlogue L. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer’s disease therapeutics. Hum Mol Genet. 2001;10(12):1317–1324. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh AK, Gemma S, Tang J. beta-Secretase as a therapeutic target for Alzheimer’s disease. Neurotherapeutics. 2008;5(3):399–408. doi: 10.1016/j.nurt.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh AK, Brindisi M, Tang J. Developing beta-secretase inhibitors for treatment of Alzheimer’s disease. J Neurochem. 2012;120(Suppl 1):71–83. doi: 10.1111/j.1471-4159.2011.07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan J, Venkatraman S, Zheng YJ, McKeever BM, Dillard LW, Singh SB. Structure-based design of beta-site APP cleaving enzyme 1 (BACE1) inhibitors for the treatment of Alzheimer’s disease. J Med Chem. 2013;56(11):4156–4180. doi: 10.1021/jm301659n. [DOI] [PubMed] [Google Scholar]
- 13.Turner RT, 3rd, Koelsch G, Hong L, Castanheira P, Ermolieff J, Ghosh AK, Tang J. Subsite specificity of memapsin 2 (beta-secretase): implications for inhibitor design. Biochemistry. 2001;40(34):10001–10006. doi: 10.1021/bi015546s. [DOI] [PubMed] [Google Scholar]
- 14.Stachel SJ. Progress Toward the Development of a Viable BACE-1 Inhibitor. Drug Dev Res. 2009;70(2):101–110. [Google Scholar]
- 15.Chang WP, Koelsch G, Wong S, Downs D, Da H, Weerasena V, Gordon B, Devasamudram T, Bilcer G, Ghosh AK, Tang J. In vivo inhibition of Abeta production by memapsin 2 (beta-secretase) inhibitors. J Neurochem. 2004;89(6):1409–1416. doi: 10.1111/j.1471-4159.2004.02452.x. [DOI] [PubMed] [Google Scholar]
- 16.Lefranc-Jullien S, Lisowski V, Hernandez JF, Martinez J, Checler F. Design and characterization of a new cell-permeant inhibitor of the beta-secretase BACE1. Br J Pharmacol. 2005;145(2):228–235. doi: 10.1038/sj.bjp.0706183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asai M, Hattori C, Iwata N, Saido TC, Sasagawa N, Szabo B, Hashimoto Y, Maruyama K, Tanuma S, Kiso Y, Ishiura S. The novel beta-secretase inhibitor KMI-429 reduces amyloid beta peptide production in amyloid precursor protein transgenic and wild-type mice. J Neurochem. 2006;96(2):533–540. doi: 10.1111/j.1471-4159.2005.03576.x. [DOI] [PubMed] [Google Scholar]
- 18.Nishitomi K, Sakaguchi G, Horikoshi Y, Gray AJ, Maeda M, Hirata-Fukae C, Becker AG, Hosono M, Sakaguchi I, Minami SS, Nakajima Y, Li HF, Takeyama C, Kihara T, Ota A, Wong PC, Aisen PS, Kato A, Kinoshita N, Matsuoka Y. BACE1 inhibition reduces endogenous Abeta and alters APP processing in wild-type mice. J Neurochem. 2006;99(6):1555–1563. doi: 10.1111/j.1471-4159.2006.04178.x. [DOI] [PubMed] [Google Scholar]
- 19.Sankaranarayanan S, Price EA, Wu G, Crouthamel MC, Shi XP, Tugusheva K, Tyler KX, Kahana J, Ellis J, Jin L, Steele T, Stachel S, Coburn C, Simon AJ. In vivo beta-secretase 1 inhibition leads to brain Abeta lowering and increased alpha-secretase processing of amyloid precursor protein without effect on neuregulin-1. J Pharmacol Exp Ther. 2008;324(3):957–969. doi: 10.1124/jpet.107.130039. [DOI] [PubMed] [Google Scholar]
- 20.Stachel SJ, Coburn CA, Sankaranarayanan S, Price EA, Wu G, Crouthamel M, Pietrak BL, Huang Q, Lineberger J, Espeseth AS, Jin L, Ellis J, Holloway MK, Munshi S, Allison T, Hazuda D, Simon AJ, Graham SL, Vacca JP. Macrocyclic inhibitors of beta-secretase: functional activity in an animal model. J Med Chem. 2006;49(21):6147–6150. doi: 10.1021/jm060884i. [DOI] [PubMed] [Google Scholar]
- 21.Stauffer SR, Stanton MG, Gregro AR, Steinbeiser MA, Shaffer JR, Nantermet PG, Barrow JC, Rittle KE, Collusi D, Espeseth AS, Lai MT, Pietrak BL, Holloway MK, McGaughey GB, Munshi SK, Hochman JH, Simon AJ, Selnick HG, Graham SL, Vacca JP. Discovery and SAR of isonicotinamide BACE-1 inhibitors that bind beta-secretase in a N-terminal 10s-loop down conformation. Bioorg Med Chem Lett. 2007;17(6):1788–1792. doi: 10.1016/j.bmcl.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 22.Hussain I, Hawkins J, Harrison D, Hille C, Wayne G, Cutler L, Buck T, Walter D, Demont E, Howes C, Naylor A, Jeffrey P, Gonzalez MI, Dingwall C, Michel A, Redshaw S, Davis JB. Oral administration of a potent and selective non-peptidic BACE-1 inhibitor decreases beta-cleavage of amyloid precursor protein and amyloid-beta production. in vivo J Neurochem. 2007;100(3):802–809. doi: 10.1111/j.1471-4159.2006.04260.x. [DOI] [PubMed] [Google Scholar]
- 23.Meredith JE, Jr, Thompson LA, Toyn JH, Marcin L, Barten DM, Marcinkeviciene J, Kopcho L, Kim Y, Lin A, Guss V, Burton C, Iben L, Polson C, Cantone J, Ford M, Drexler D, Fiedler T, Lentz KA, Grace JE, Jr, Kolb J, Corsa J, Pierdomenico M, Jones K, Olson RE, Macor JE, Albright CF. P-glycoprotein efflux and other factors limit brain amyloid beta reduction by beta-site amyloid precursor protein-cleaving enzyme 1 inhibitors in mice. J Pharmacol Exp Ther. 2008;326(2):502–513. doi: 10.1124/jpet.108.138974. [DOI] [PubMed] [Google Scholar]
- 24.Bennett BD, Babu-Khan S, Loeloff R, Louis JC, Curran E, Citron M, Vassar R. Expression analysis of BACE2 in brain and peripheral tissues. J Biol Chem. 2000;275(27):20647–20651. doi: 10.1074/jbc.M002688200. [DOI] [PubMed] [Google Scholar]
- 25.Fischer F, Molinari M, Bodendorf U, Paganetti P. The disulphide bonds in the catalytic domain of BACE are critical but not essential for amyloid precursor protein processing activity. J Neurochem. 2002;80(6):1079–1088. doi: 10.1046/j.0022-3042.2002.00806.x. [DOI] [PubMed] [Google Scholar]
- 26.Haniu M, Denis P, Young Y, Mendiaz EA, Fuller J, Hui JO, Bennett BD, Kahn S, Ross S, Burgess T, Katta V, Rogers G, Vassar R, Citron M. Characterization of Alzheimer’s beta-secretase protein BACE. A pepsin family member with unusual properties. J Biol Chem. 2000;275(28):21099–21106. doi: 10.1074/jbc.M002095200. [DOI] [PubMed] [Google Scholar]
- 27.Hong L, Tang J. Flap position of free memapsin 2 (beta-secretase), a model for flap opening in aspartic protease catalysis. Biochemistry. 2004;43(16):4689–4695. doi: 10.1021/bi0498252. [DOI] [PubMed] [Google Scholar]
- 28.Hong L, Turner RT, 3rd, Koelsch G, Shin D, Ghosh AK, Tang J. Crystal structure of memapsin 2 (beta-secretase) in complex with an inhibitor OM00-3. Biochemistry. 2002;41(36):10963–10967. doi: 10.1021/bi026232n. [DOI] [PubMed] [Google Scholar]
- 29.Turner RT, 3rd, Hong L, Koelsch G, Ghosh AK, Tang J. Structural locations and functional roles of new subsites S5, S6, and S7 in memapsin 2 (beta-secretase) Biochemistry. 2005;44(1):105–112. doi: 10.1021/bi048106k. [DOI] [PubMed] [Google Scholar]
- 30.Baechle D, Flad T, Cansier A, Steffen H, Schittek B, Tolson J, Herrmann T, Dihazi H, Beck A, Mueller GA, Mueller M, Stevanovic S, Garbe C, Mueller CA, Kalbacher H. Cathepsin D is present in human eccrine sweat and involved in the postsecretory processing of the antimicrobial peptide DCD-1L. J Biol Chem. 2006;281(9):5406–5415. doi: 10.1074/jbc.M504670200. [DOI] [PubMed] [Google Scholar]
- 31.Diment S, Leech MS, Stahl PD. Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem. 1988;263(14):6901–6907. [PubMed] [Google Scholar]
- 32.Bankowska A, Gacko M, Chyczewska E, Worowska A. Biological and diagnostic role of cathepsin D. Rocz Akad Med Bialymst. 1997;42(Suppl 1):79–85. [PubMed] [Google Scholar]
- 33.Koike M, Nakanishi H, Saftig P, Ezaki J, Isahara K, Ohsawa Y, Schulz-Schaeffer W, Watanabe T, Waguri S, Kametaka S, Shibata M, Yamamoto K, Kominami E, Peters C, von Figura K, Uchiyama Y. Cathepsin D deficiency induces lysosomal storage with ceroid lipofuscin in mouse CNS neurons. J Neurosci. 2000;20(18):6898–6906. doi: 10.1523/JNEUROSCI.20-18-06898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benes P, Vetvicka V, Fusek M. Cathepsin D--many functions of one aspartic protease. Crit Rev Oncol Hematol. 2008;68(1):12–28. doi: 10.1016/j.critrevonc.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gruninger-Leitch F, Schlatter D, Kung E, Nelbock P, Dobeli H. Substrate and inhibitor profile of BACE (beta-secretase) and comparison with other mammalian aspartic proteases. J Biol Chem. 2002;277(7):4687–4693. doi: 10.1074/jbc.M109266200. [DOI] [PubMed] [Google Scholar]
- 36.Haass C, Lemere CA, Capell A, Citron M, Seubert P, Schenk D, Lannfelt L, Selkoe DJ. The Swedish mutation causes early-onset Alzheimer’s disease by beta-secretase cleavage within the secretory pathway. Nat Med. 1995;1(12):1291–1296. doi: 10.1038/nm1295-1291. [DOI] [PubMed] [Google Scholar]
- 37.Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402(6761):537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh AK, Shin DW, Downs D, Koelsch G, Lin XL, Ermolieff J, Tang J. Design of potent inhibitors for human brain memapsin 2 (beta-secretase) J Am Chem Soc. 2000;122(14):3522–3523. doi: 10.1021/ja000300g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh AK, Bilcer G, Harwood C, Kawahama R, Shin D, Hussain KA, Hong L, Loy JA, Nguyen C, Koelsch G, Ermolieff J, Tang J. Structure-based design: potent inhibitors of human brain memapsin 2 (beta-secretase) J Med Chem. 2001;44(18):2865–2868. doi: 10.1021/jm0101803. [DOI] [PubMed] [Google Scholar]
- 40.Hanessian S, Yun H, Hou Y, Yang G, Bayrakdarian M, Therrien E, Moitessier N, Roggo S, Veenstra S, Tintelnot-Blomley M, Rondeau JM, Ostermeier C, Strauss A, Ramage P, Paganetti P, Neumann U, Betschart C. Structure-based design, synthesis, and memapsin 2 (BACE) inhibitory activity of carbocyclic and heterocyclic peptidomimetics. J Med Chem. 2005;48(16):5175–5190. doi: 10.1021/jm050142+. [DOI] [PubMed] [Google Scholar]
- 41.Kimura T, Shuto D, Hamada Y, Igawa N, Kasai S, Liu P, Hidaka K, Hamada T, Hayashi Y, Kiso Y. Design and synthesis of highly active Alzheimer’s beta-secretase (BACE1) inhibitors, KMI-420 and KMI-429, with enhanced chemical stability. Bioorg Med Chem Lett. 2005;15(1):211–215. doi: 10.1016/j.bmcl.2004.09.090. [DOI] [PubMed] [Google Scholar]
- 42.Kimura T, Hamada Y, Stochaj M, Ikari H, Nagamine A, Abdel-Rahman H, Igawa N, Hidaka K, Nguyen JT, Saito K, Hayashi Y, Kiso Y. Design and synthesis of potent beta-secretase (BACE1) inhibitors with P1′ carboxylic acid bioisosteres. Bioorg Med Chem Lett. 2006;16(9):2380–2386. doi: 10.1016/j.bmcl.2006.01.108. [DOI] [PubMed] [Google Scholar]
- 43.Hamada Y, Igawa N, Ikari H, Ziora Z, Nguyen JT, Yamani A, Hidaka K, Kimura T, Saito K, Hayashi Y, Ebina M, Ishiura S, Kiso Y. beta-Secretase inhibitors: modification at the P4 position and improvement of inhibitory activity in cultured cells. Bioorg Med Chem Lett. 2006;16(16):4354–4359. doi: 10.1016/j.bmcl.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 44.Hamada Y, Ohta H, Miyamoto N, Yamaguchi R, Yamani A, Hidaka K, Kimura T, Saito K, Hayashi Y, Ishiura S, Kiso Y. Novel non-peptidic and small-sized BACE1 inhibitors. Bioorg Med Chem Lett. 2008;18(5):1654–1658. doi: 10.1016/j.bmcl.2008.01.056. [DOI] [PubMed] [Google Scholar]
- 45.Wangsell F, Nordeman P, Savmarker J, Emanuelsson R, Jansson K, Lindberg J, Rosenquist S, Samuelsson B, Larhed M. Investigation of alpha-phenylnorstatine and alpha-benzylnorstatine as transition state isostere motifs in the search for new BACE-1 inhibitors. Bioorg Med Chem. 2011;19(1):145–155. doi: 10.1016/j.bmc.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh AK, Kumaragurubaran N, Hong L, Lei H, Hussain KA, Liu CF, Devasamudram T, Weerasena V, Turner R, Koelsch G, Bilcer G, Tang J. Design, synthesis and X-ray structure of protein-ligand complexes: important insight into selectivity of memapsin 2 (beta-secretase) inhibitors. J Am Chem Soc. 2006;128(16):5310–5311. doi: 10.1021/ja058636j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hom RK, Gailunas AF, Mamo S, Fang LY, Tung JS, Walker DE, Davis D, Thorsett ED, Jewett NE, Moon JB, John V. Design and synthesis of hydroxyethylene-based peptidomimetic inhibitors of human beta-secretase. J Med Chem. 2004;47(1):158–164. doi: 10.1021/jm0304008. [DOI] [PubMed] [Google Scholar]
- 48.Stachel SJ, Coburn CA, Steele TG, Jones KG, Loutzenhiser EF, Gregro AR, Rajapakse HA, Lai MT, Crouthamel MC, Xu M, Tugusheva K, Lineberger JE, Pietrak BL, Espeseth AS, Shi XP, Chen-Dodson E, Holloway MK, Munshi S, Simon AJ, Kuo L, Vacca JP. Structure-based design of potent and selective cell-permeable inhibitors of human beta-secretase (BACE-1) J Med Chem. 2004;47(26):6447–6450. doi: 10.1021/jm049379g. [DOI] [PubMed] [Google Scholar]
- 49.Ghosh AK, Kumaragurubaran N, Hong L, Kulkarni SS, Xu X, Chang W, Weerasena V, Turner R, Koelsch G, Bilcer G, Tang J. Design, synthesis, and X-ray structure of potent memapsin 2 (beta-secretase) inhibitors with isophthalamide derivatives as the P2-P3-ligands. J Med Chem. 2007;50(10):2399–2407. doi: 10.1021/jm061338s. [DOI] [PubMed] [Google Scholar]
- 50.Bjorklund C, Oscarson S, Benkestock K, Borkakoti N, Jansson K, Lindberg J, Vrang L, Hallberg A, Rosenquist A, Samuelsson B. Design and synthesis of potent and selective BACE-1 inhibitors. J Med Chem. 2010;53(4):1458–1464. doi: 10.1021/jm901168f. [DOI] [PubMed] [Google Scholar]
- 51.Meredith JA, Bjorklund C, Adolfsson H, Jansson K, Hallberg A, Rosenquist A, Samuelsson B. P2′-truncated BACE-1 inhibitors with a novel hydroxethylene-like core. Eur J Med Chem. 2010;45(2):542–554. doi: 10.1016/j.ejmech.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 52.Maillard MC, Hom RK, Benson TE, Moon JB, Mamo S, Bienkowski M, Tomasselli AG, Woods DD, Prince DB, Paddock DJ, Emmons TL, Tucker JA, Dappen MS, Brogley L, Thorsett ED, Jewett N, Sinha S, John V. Design, synthesis, and crystal structure of hydroxyethyl secondary amine-based peptidomimetic inhibitors of human beta-secretase. J Med Chem. 2007;50(4):776–781. doi: 10.1021/jm061242y. [DOI] [PubMed] [Google Scholar]
- 53.Kortum SW, Benson TE, Bienkowski MJ, Emmons TL, Prince DB, Paddock DJ, Tomasselli AG, Moon JB, LaBorde A, TenBrink RE. Potent and selective isophthalamide S2 hydroxyethylamine inhibitors of BACE1. Bioorg Med Chem Lett. 2007;17(12):3378–3383. doi: 10.1016/j.bmcl.2007.03.096. [DOI] [PubMed] [Google Scholar]
- 54.Freskos JN, Fobian YM, Benson TE, Moon JB, Bienkowski MJ, Brown DL, Emmons TL, Heintz R, Laborde A, McDonald JJ, Mischke BV, Molyneaux JM, Mullins PB, Bryan Prince D, Paddock DJ, Tomasselli AG, Winterrowd G. Design of potent inhibitors of human beta-secretase. Part 2. Bioorg Med Chem Lett. 2007;17(1):78–81. doi: 10.1016/j.bmcl.2006.09.091. [DOI] [PubMed] [Google Scholar]
- 55.Beswick P, Charrier N, Clarke B, Demont E, Dingwall C, Dunsdon R, Faller A, Gleave R, Hawkins J, Hussain I, Johnson CN, MacPherson D, Maile G, Matico R, Milner P, Mosley J, Naylor A, O’Brien A, Redshaw S, Riddell D, Rowland P, Skidmore J, Soleil V, Smith KJ, Stanway S, Stemp G, Stuart A, Sweitzer S, Theobald P, Vesey D, Walter DS, Ward J, Wayne G. BACE-1 inhibitors part 3: identification of hydroxy ethylamines (HEAs) with nanomolar potency in cells. Bioorg Med Chem Lett. 2008;18(3):1022–1026. doi: 10.1016/j.bmcl.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 56.Clarke B, Demont E, Dingwall C, Dunsdon R, Faller A, Hawkins J, Hussain I, MacPherson D, Maile G, Matico R, Milner P, Mosley J, Naylor A, O’Brien A, Redshaw S, Riddell D, Rowland P, Soleil V, Smith KJ, Stanway S, Stemp G, Sweitzer S, Theobald P, Vesey D, Walter DS, Ward J, Wayne G. BACE-1 inhibitors part 1: identification of novel hydroxy ethylamines (HEAs) Bioorg Med Chem Lett. 2008;18(3):1011–1016. doi: 10.1016/j.bmcl.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 57.Clarke B, Demont E, Dingwall C, Dunsdon R, Faller A, Hawkins J, Hussain I, MacPherson D, Maile G, Matico R, Milner P, Mosley J, Naylor A, O’Brien A, Redshaw S, Riddell D, Rowland P, Soleil V, Smith KJ, Stanway S, Stemp G, Sweitzer S, Theobald P, Vesey D, Walter DS, Ward J, Wayne G. BACE-1 inhibitors part 2: identification of hydroxy ethylamines (HEAs) with reduced peptidic character. Bioorg Med Chem Lett. 2008;18(3):1017–1021. doi: 10.1016/j.bmcl.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 58.Charrier N, Clarke B, Cutler L, Demont E, Dingwall C, Dunsdon R, East P, Hawkins J, Howes C, Hussain I, Jeffrey P, Maile G, Matico R, Mosley J, Naylor A, O’Brien A, Redshaw S, Rowland P, Soleil V, Smith KJ, Sweitzer S, Theobald P, Vesey D, Walter DS, Wayne G. Second generation of hydroxyethylamine BACE-1 inhibitors: optimizing potency and oral bioavailability. J Med Chem. 2008;51(11):3313–3317. doi: 10.1021/jm800138h. [DOI] [PubMed] [Google Scholar]
- 59.Ghosh AK, Kumaragurubaran N, Hong L, Kulkarni S, Xu X, Miller HB, Reddy DS, Weerasena V, Turner R, Chang W, Koelsch G, Tang J. Potent memapsin 2 (beta-secretase) inhibitors: design, synthesis, protein-ligand X-ray structure, and in vivo evaluation. Bioorg Med Chem Lett. 2008;18(3):1031–1036. doi: 10.1016/j.bmcl.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang WP, Huang X, Downs D, Cirrito JR, Koelsch G, Holtzman DM, Ghosh AK, Tang J. Beta-secretase inhibitor GRL-8234 rescues age-related cognitive decline in APP transgenic mice. FASEB J. 2011;25(2):775–784. doi: 10.1096/fj.10-167213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Truong AP, Toth G, Probst GD, Sealy JM, Bowers S, Wone DW, Dressen D, Hom RK, Konradi AW, Sham HL, Wu J, Peterson BT, Ruslim L, Bova MP, Kholodenko D, Motter RN, Bard F, Santiago P, Ni H, Chian D, Soriano F, Cole T, Brigham EF, Wong K, Zmolek W, Goldbach E, Samant B, Chen L, Zhang H, Nakamura DF, Quinn KP, Yednock TA, Sauer JM. Design of an orally efficacious hydroxyethylamine (HEA) BACE-1 inhibitor in a preclinical animal model. Bioorg Med Chem Lett. 2010;20(21):6231–6236. doi: 10.1016/j.bmcl.2010.08.102. [DOI] [PubMed] [Google Scholar]
- 62.Marcin LR, Higgins MA, Zusi FC, Zhang Y, Dee MF, Parker MF, Muckelbauer JK, Camac DM, Morin PE, Ramamurthy V, Tebben AJ, Lentz KA, Grace JE, Marcinkeviciene JA, Kopcho LM, Burton CR, Barten DM, Toyn JH, Meredith JE, Albright CF, Bronson JJ, Macor JE, Thompson LA. Synthesis and SAR of indole-and 7-azaindole-1,3-dicarboxamide hydroxyethylamine inhibitors of BACE-1. Bioorg Med Chem Lett. 2011;21(1):537–541. doi: 10.1016/j.bmcl.2010.10.079. [DOI] [PubMed] [Google Scholar]
- 63.Weiss MM, Williamson T, Babu-Khan S, Bartberger MD, Brown J, Chen K, Cheng Y, Citron M, Croghan MD, Dineen TA, Esmay J, Graceffa RF, Harried SS, Hickman D, Hitchcock SA, Horne DB, Huang H, Imbeah-Ampiah R, Judd T, Kaller MR, Kreiman CR, La DS, Li V, Lopez P, Louie S, Monenschein H, Nguyen TT, Pennington LD, Rattan C, San Miguel T, Sickmier EA, Wahl RC, Wen PH, Wood S, Xue Q, Yang BH, Patel VF, Zhong W. Design and preparation of a potent series of hydroxyethylamine containing beta-secretase inhibitors that demonstrate robust reduction of central beta-amyloid. J Med Chem. 2012;55(21):9009–9024. doi: 10.1021/jm300119p. [DOI] [PubMed] [Google Scholar]
- 64.Dineen TA, Weiss MM, Williamson T, Acton P, Babu-Khan S, Bartberger MD, Brown J, Chen K, Cheng Y, Citron M, Croghan MD, Dunn RT, 2nd, Esmay J, Graceffa RF, Harried SS, Hickman D, Hitchcock SA, Horne DB, Huang H, Imbeah-Ampiah R, Judd T, Kaller MR, Kreiman CR, La DS, Li V, Lopez P, Louie S, Monenschein H, Nguyen TT, Pennington LD, San Miguel T, Sickmier EA, Vargas HM, Wahl RC, Wen PH, Whittington DA, Wood S, Xue Q, Yang BH, Patel VF, Zhong W. Design and synthesis of potent, orally efficacious hydroxyethylamine derived beta-site amyloid precursor protein cleaving enzyme (BACE1) inhibitors. J Med Chem. 2012;55(21):9025–9044. doi: 10.1021/jm300118s. [DOI] [PubMed] [Google Scholar]
- 65.Iserloh U, Wu Y, Cumming JN, Pan J, Wang LY, Stamford AW, Kennedy ME, Kuvelkar R, Chen X, Parker EM, Strickland C, Voigt J. Potent pyrrolidine- and piperidine-based BACE-1 inhibitors. Bioorg Med Chem Lett. 2008;18(1):414–417. doi: 10.1016/j.bmcl.2007.10.116. [DOI] [PubMed] [Google Scholar]
- 66.Iserloh U, Pan J, Stamford AW, Kennedy ME, Zhang Q, Zhang L, Parker EM, McHugh NA, Favreau L, Strickland C, Voigt J. Discovery of an orally efficaceous 4-phenoxypyrrolidine-based BACE-1 inhibitor. Bioorg Med Chem Lett. 2008;18(1):418–422. doi: 10.1016/j.bmcl.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 67.Cumming JN, Le TX, Babu S, Carroll C, Chen X, Favreau L, Gaspari P, Guo T, Hobbs DW, Huang Y, Iserloh U, Kennedy ME, Kuvelkar R, Li G, Lowrie J, McHugh NA, Ozgur L, Pan J, Parker EM, Saionz K, Stamford AW, Strickland C, Tadesse D, Voigt J, Wang L, Wu Y, Zhang L, Zhang Q. Rational design of novel, potent piperazinone and imidazolidinone BACE1 inhibitors. Bioorg Med Chem Lett. 2008;18(11):3236–3241. doi: 10.1016/j.bmcl.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 68.Cumming J, Babu S, Huang Y, Carrol C, Chen X, Favreau L, Greenlee W, Guo T, Kennedy M, Kuvelkar R, Le T, Li G, McHugh N, Orth P, Ozgur L, Parker E, Saionz K, Stamford A, Strickland C, Tadesse D, Voigt J, Zhang L, Zhang Q. Piperazine sulfonamide BACE1 inhibitors: design, synthesis, and in vivo characterization. Bioorg Med Chem Lett. 2010;20(9):2837–2842. doi: 10.1016/j.bmcl.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 69.Thompson LA, Shi J, Decicco CP, Tebben AJ, Olson RE, Boy KM, Guernon JM, Good AC, Liauw A, Zheng C, Copeland RA, Combs AP, Trainor GL, Camac DM, Muckelbauer JK, Lentz KA, Grace JE, Burton CR, Toyn JH, Barten DM, Marcinkeviciene J, Meredith JE, Albright CF, Macor JE. Synthesis and in vivo evaluation of cyclic diaminopropane BACE-1 inhibitors. Bioorg Med Chem Lett. 2011;21(22):6909–6915. doi: 10.1016/j.bmcl.2011.06.116. [DOI] [PubMed] [Google Scholar]
- 70.Rueeger H, Rondeau JM, McCarthy C, Mobitz H, Tintelnot-Blomley M, Neumann U, Desrayaud S. Structure based design, synthesis and SAR of cyclic hydroxyethylamine (HEA) BACE-1 inhibitors. Bioorg Med Chem Lett. 2011;21(7):1942–1947. doi: 10.1016/j.bmcl.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 71.Rueeger H, Lueoend R, Rogel O, Rondeau JM, Mobitz H, Machauer R, Jacobson L, Staufenbiel M, Desrayaud S, Neumann U. Discovery of cyclic sulfone hydroxyethylamines as potent and selective beta-site APP-cleaving enzyme 1 (BACE1) inhibitors: structure-based design and in vivo reduction of amyloid beta-peptides. J Med Chem. 2012;55(7):3364–3386. doi: 10.1021/jm300069y. [DOI] [PubMed] [Google Scholar]
- 72.Rajapakse HA, Nantermet PG, Selnick HG, Munshi S, McGaughey GB, Lindsley SR, Young MB, Lai MT, Espeseth AS, Shi XP, Colussi D, Pietrak B, Crouthamel MC, Tugusheva K, Huang Q, Xu M, Simon AJ, Kuo L, Hazuda DJ, Graham S, Vacca JP. Discovery of oxadiazoyl tertiary carbinamine inhibitors of beta-secretase (BACE-1) J Med Chem. 2006;49(25):7270–7273. doi: 10.1021/jm061046r. [DOI] [PubMed] [Google Scholar]
- 73.Stanton MG, Stauffer SR, Gregro AR, Steinbeiser M, Nantermet P, Sankaranarayanan S, Price EA, Wu G, Crouthamel MC, Ellis J, Lai MT, Espeseth AS, Shi XP, Jin L, Colussi D, Pietrak B, Huang Q, Xu M, Simon AJ, Graham SL, Vacca JP, Selnick H. Discovery of isonicotinamide derived beta-secretase inhibitors: in vivo reduction of beta-amyloid. J Med Chem. 2007;50(15):3431–3433. doi: 10.1021/jm070272d. [DOI] [PubMed] [Google Scholar]
- 74.Nantermet PG, Rajapakse HA, Stanton MG, Stauffer SR, Barrow JC, Gregro AR, Moore KP, Steinbeiser MA, Swestock J, Selnick HG, Graham SL, McGaughey GB, Colussi D, Lai MT, Sankaranarayanan S, Simon AJ, Munshi S, Cook JJ, Holahan MA, Michener MS, Vacca JP. Evolution of tertiary carbinamine BACE-1 inhibitors: Abeta reduction in rhesus CSF upon oral dosing. ChemMedChem. 2009;4(1):37–40. doi: 10.1002/cmdc.200800308. [DOI] [PubMed] [Google Scholar]
- 75.Sankaranarayanan S, Holahan MA, Colussi D, Crouthamel MC, Devanarayan V, Ellis J, Espeseth A, Gates AT, Graham SL, Gregro AR, Hazuda D, Hochman JH, Holloway K, Jin L, Kahana J, Lai MT, Lineberger J, McGaughey G, Moore KP, Nantermet P, Pietrak B, Price EA, Rajapakse H, Stauffer S, Steinbeiser MA, Seabrook G, Selnick HG, Shi XP, Stanton MG, Swestock J, Tugusheva K, Tyler KX, Vacca JP, Wong J, Wu G, Xu M, Cook JJ, Simon AJ. First demonstration of cerebrospinal fluid and plasma A beta lowering with oral administration of a beta-site amyloid precursor protein-cleaving enzyme 1 inhibitor in nonhuman primates. J Pharmacol Exp Ther. 2009;328(1):131–140. doi: 10.1124/jpet.108.143628. [DOI] [PubMed] [Google Scholar]
- 76.Zhu H, Young MB, Nantermet PG, Graham SL, Colussi D, Lai MT, Pietrak B, Price EA, Sankaranarayanan S, Shi XP, Tugusheva K, Holahan MA, Michener MS, Cook JJ, Simon A, Hazuda DJ, Vacca JP, Rajapakse HA. Rapid P1 SAR of brain penetrant tertiary carbinamine derived BACE inhibitors. Bioorg Med Chem Lett. 2010;20(5):1779–1782. doi: 10.1016/j.bmcl.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Coburn CA, Stachel SJ, Jones KG, Steele TG, Rush DM, DiMuzio J, Pietrak BL, Lai MT, Huang Q, Lineberger J, Jin L, Munshi S, Katharine Holloway M, Espeseth A, Simon A, Hazuda D, Graham SL, Vacca JP. BACE-1 inhibition by a series of psi[CH2NH] reduced amide isosteres. Bioorg Med Chem Lett. 2006;16(14):3635–3638. doi: 10.1016/j.bmcl.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 78.Monceaux CJ, Hirata-Fukae C, Lam PC, Totrov MM, Matsuoka Y, Carlier PR. Triazole-linked reduced amide isosteres: an approach for the fragment-based drug discovery of anti-Alzheimer’s BACE1 inhibitors. Bioorg Med Chem Lett. 2011;21(13):3992–3996. doi: 10.1016/j.bmcl.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Ghosh AK, Venkateswara Rao K, Yadav ND, Anderson DD, Gavande N, Huang X, Terzyan S, Tang J. Structure-based design of highly selective beta-secretase inhibitors: synthesis, biological evaluation, and protein-ligand X-ray crystal structure. J Med Chem. 2012;55(21):9195–9207. doi: 10.1021/jm3008823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silvestri R. Boom in the development of non-peptidic beta-secretase (BACE1) inhibitors for the treatment of Alzheimer’s disease. Med Res Rev. 2009;29(2):295–338. doi: 10.1002/med.20132. [DOI] [PubMed] [Google Scholar]
- 81.Lindsley SR, Moore KP, Rajapakse HA, Selnick HG, Young MB, Zhu H, Munshi S, Kuo L, McGaughey GB, Colussi D, Crouthamel MC, Lai MT, Pietrak B, Price EA, Sankaranarayanan S, Simon AJ, Seabrook GR, Hazuda DJ, Pudvah NT, Hochman JH, Graham SL, Vacca JP, Nantermet PG. Design, synthesis, and SAR of macrocyclic tertiary carbinamine BACE-1 inhibitors. Bioorg Med Chem Lett. 2007;17(14):4057–4061. doi: 10.1016/j.bmcl.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 82.Machauer R, Veenstra S, Rondeau JM, Tintelnot-Blomley M, Betschart C, Neumann U, Paganetti P. Structure-based design and synthesis of macrocyclic peptidomimetic beta-secretase (BACE-1) inhibitors. Bioorg Med Chem Lett. 2009;19(5):1361–1365. doi: 10.1016/j.bmcl.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 83.Machauer R, Laumen K, Veenstra S, Rondeau JM, Tintelnot-Blomley M, Betschart C, Jaton AL, Desrayaud S, Staufenbiel M, Rabe S, Paganetti P, Neumann U. Macrocyclic peptidomimetic beta-secretase (BACE-1) inhibitors with activity. in vivo Bioorg Med Chem Lett. 2009;19(5):1366–1370. doi: 10.1016/j.bmcl.2009.01.055. [DOI] [PubMed] [Google Scholar]
- 84.Lerchner A, Machauer R, Betschart C, Veenstra S, Rueeger H, McCarthy C, Tintelnot-Blomley M, Jaton AL, Rabe S, Desrayaud S, Enz A, Staufenbiel M, Paganetti P, Rondeau JM, Neumann U. Macrocyclic BACE-1 inhibitors acutely reduce Abeta in brain after po application. Bioorg Med Chem Lett. 2010;20(2):603–607. doi: 10.1016/j.bmcl.2009.11.092. [DOI] [PubMed] [Google Scholar]
- 85.Sandgren V, Agback T, Johansson PO, Lindberg J, Kvarnstrom I, Samuelsson B, Belda O, Dahlgren A. Highly potent macrocyclic BACE-1 inhibitors incorporating a hydroxyethylamine core: design, synthesis and X-ray crystal structures of enzyme inhibitor complexes. Bioorg Med Chem. 2012;20(14):4377–4389. doi: 10.1016/j.bmc.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 86.Cole DC, Manas ES, Stock JR, Condon JS, Jennings LD, Aulabaugh A, Chopra R, Cowling R, Ellingboe JW, Fan KY, Harrison BL, Hu Y, Jacobsen S, Jin G, Lin L, Lovering FE, Malamas MS, Stahl ML, Strand J, Sukhdeo MN, Svenson K, Turner MJ, Wagner E, Wu J, Zhou P, Bard J. Acylguanidines as small-molecule beta-secretase inhibitors. J Med Chem. 2006;49(21):6158–6161. doi: 10.1021/jm0607451. [DOI] [PubMed] [Google Scholar]
- 87.Jennings LD, Cole DC, Stock JR, Sukhdeo MN, Ellingboe JW, Cowling R, Jin G, Manas ES, Fan KY, Malamas MS, Harrison BL, Jacobsen S, Chopra R, Lohse PA, Moore WJ, O’Donnell MM, Hu Y, Robichaud AJ, Turner MJ, Wagner E, Bard J. Acylguanidine inhibitors of beta-secretase: optimization of the pyrrole ring substituents extending into the S1′ substrate binding pocket. Bioorg Med Chem Lett. 2008;18(2):767–771. doi: 10.1016/j.bmcl.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 88.Cole DC, Stock JR, Chopra R, Cowling R, Ellingboe JW, Fan KY, Harrison BL, Hu Y, Jacobsen S, Jennings LD, Jin G, Lohse PA, Malamas MS, Manas ES, Moore WJ, O’Donnell MM, Olland AM, Robichaud AJ, Svenson K, Wu J, Wagner E, Bard J. Acylguanidine inhibitors of beta-secretase: optimization of the pyrrole ring substituents extending into the S1 and S3 substrate binding pockets. Bioorg Med Chem Lett. 2008;18(3):1063–1066. doi: 10.1016/j.bmcl.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 89.Malamas MS, Barnes K, Hui Y, Johnson M, Lovering F, Condon J, Fobare W, Solvibile W, Turner J, Hu Y, Manas ES, Fan K, Olland A, Chopra R, Bard J, Pangalos MN, Reinhart P, Robichaud AJ. Novel pyrrolyl 2-aminopyridines as potent and selective human beta-secretase (BACE1) inhibitors. Bioorg Med Chem Lett. 2010;20(7):2068–2073. doi: 10.1016/j.bmcl.2010.02.075. [DOI] [PubMed] [Google Scholar]
- 90.Malamas MS, Erdei J, Gunawan I, Barnes K, Johnson M, Hui Y, Turner J, Hu Y, Wagner E, Fan K, Olland A, Bard J, Robichaud AJ. Aminoimidazoles as potent and selective human beta-secretase (BACE1) inhibitors. J Med Chem. 2009;52(20):6314–6323. doi: 10.1021/jm9006752. [DOI] [PubMed] [Google Scholar]
- 91.Swahn BM, Holenz J, Kihlstrom J, Kolmodin K, Lindstrom J, Plobeck N, Rotticci D, Sehgelmeble F, Sundstrom M, Berg S, Falting J, Georgievska B, Gustavsson S, Neelissen J, Ek M, Olsson LL, Berg S. Aminoimidazoles as BACE-1 inhibitors: the challenge to achieve in vivo brain efficacy. Bioorg Med Chem Lett. 2012;22(5):1854–1859. doi: 10.1016/j.bmcl.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 92.Gerritz SW, Zhai W, Shi S, Zhu S, Toyn JH, Meredith JE, Jr, Iben LG, Burton CR, Albright CF, Good AC, Tebben AJ, Muckelbauer JK, Camac DM, Metzler W, Cook LS, Padmanabha R, Lentz KA, Sofia MJ, Poss MA, Macor JE, Thompson LA., 3rd Acyl guanidine inhibitors of beta-secretase (BACE-1): optimization of a micromolar hit to a nanomolar lead via iterative solid- and solution-phase library synthesis. J Med Chem. 2012;55(21):9208–9223. doi: 10.1021/jm300931y. [DOI] [PubMed] [Google Scholar]
- 93.Stachel SJ, Coburn CA, Rush D, Jones KL, Zhu H, Rajapakse H, Graham SL, Simon A, Katharine Holloway M, Allison TJ, Munshi SK, Espeseth AS, Zuck P, Colussi D, Wolfe A, Pietrak BL, Lai MT, Vacca JP. Discovery of aminoheterocycles as a novel beta-secretase inhibitor class: pH dependence on binding activity part 1. Bioorg Med Chem Lett. 2009;19(11):2977–2980. doi: 10.1016/j.bmcl.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 94.Hills ID, Holloway MK, de Leon P, Nomland A, Zhu H, Rajapakse H, Allison TJ, Munshi SK, Colussi D, Pietrak BL, Toolan D, Haugabook SJ, Graham SL, Stachel SJ. A conformational constraint improves a beta-secretase inhibitor but for an unexpected reason. Bioorg Med Chem Lett. 2009;19(17):4993–4995. doi: 10.1016/j.bmcl.2009.07.071. [DOI] [PubMed] [Google Scholar]
- 95.Chiriano G, De Simone A, Mancini F, Perez DI, Cavalli A, Bolognesi ML, Legname G, Martinez A, Andrisano V, Carloni P, Roberti M. A small chemical library of 2-aminoimidazole derivatives as BACE-1 inhibitors: Structure-based design, synthesis, and biological evaluation. Eur J Med Chem. 2012;48:206–213. doi: 10.1016/j.ejmech.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 96.Gravenfors Y, Viklund J, Blid J, Ginman T, Karlstrom S, Kihlstrom J, Kolmodin K, Lindstrom J, von Berg S, von Kieseritzky F, Slivo C, Swahn BM, Olsson LL, Johansson P, Eketjall S, Falting J, Jeppsson F, Stromberg K, Janson J, Rahm F. New aminoimidazoles as beta-secretase (BACE-1) inhibitors showing amyloid-beta (Abeta) lowering in brain. J Med Chem. 2012;55(21):9297–9311. doi: 10.1021/jm300991n. [DOI] [PubMed] [Google Scholar]
- 97.Nowak P, Cole DC, Aulabaugh A, Bard J, Chopra R, Cowling R, Fan KY, Hu B, Jacobsen S, Jani M, Jin G, Lo MC, Malamas MS, Manas ES, Narasimhan R, Reinhart P, Robichaud AJ, Stock JR, Subrath J, Svenson K, Turner J, Wagner E, Zhou P, Ellingboe JW. Discovery and initial optimization of 5,5′-disubstituted aminohydantoins as potent beta-secretase (BACE1) inhibitors. Bioorg Med Chem Lett. 2010;20(2):632–635. doi: 10.1016/j.bmcl.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 98.Malamas MS, Erdei J, Gunawan I, Turner J, Hu Y, Wagner E, Fan K, Chopra R, Olland A, Bard J, Jacobsen S, Magolda RL, Pangalos M, Robichaud AJ. Design and synthesis of 5,5′-disubstituted aminohydantoins as potent and selective human beta-secretase (BACE1) inhibitors. J Med Chem. 2010;53(3):1146–1158. doi: 10.1021/jm901414e. [DOI] [PubMed] [Google Scholar]
- 99.Zhu Z, Sun ZY, Ye Y, Voigt J, Strickland C, Smith EM, Cumming J, Wang L, Wong J, Wang YS, Wyss DF, Chen X, Kuvelkar R, Kennedy ME, Favreau L, Parker E, McKittrick BA, Stamford A, Czarniecki M, Greenlee W, Hunter JC. Discovery of cyclic acylguanidines as highly potent and selective beta-site amyloid cleaving enzyme (BACE) inhibitors: Part I--inhibitor design and validation. J Med Chem. 2010;53(3):951–965. doi: 10.1021/jm901408p. [DOI] [PubMed] [Google Scholar]
- 100.Tresadern G, Delgado F, Delgado O, Gijsen H, Macdonald GJ, Moechars D, Rombouts F, Alexander R, Spurlino J, Van Gool M, Vega JA, Trabanco AA. Rational design and synthesis of aminopiperazinones as beta-secretase (BACE) inhibitors. Bioorg Med Chem Lett. 2011;21(24):7255–7260. doi: 10.1016/j.bmcl.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 101.Stamford AW, Scott JD, Li SW, Babu S, Tadesse D, Hunter R, Wu YS, Misiaszek J, Cumming JN, Gilbert EJ, Huang CL, McKittrick BA, Hong LW, Guo T, Zhu ZN, Strickland C, Orth P, Voigt JH, Kennedy ME, Chen X, Kuvelkar R, Hodgson R, Hyde LA, Cox K, Favreau L, Parker EM, Greenlee WJ. Discovery of an Orally Available, Brain Penetrant BACE1 Inhibitor That Affords Robust CNS A beta Reduction. ACS Med Chem Lett. 2012;3(11):897–902. doi: 10.1021/ml3001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang H, La DS, Cheng AC, Whittington DA, Patel VF, Chen K, Dineen TA, Epstein O, Graceffa R, Hickman D, Kiang YH, Louie S, Luo Y, Wahl RC, Wen PH, Wood S, Fremeau RT., Jr Structure- and property-based design of aminooxazoline Xanthenes as selective, orally efficacious, and CNS penetrable BACE inhibitors for the treatment of Alzheimer’s disease. J Med Chem. 2012;55(21):9156–9169. doi: 10.1021/jm300598e. [DOI] [PubMed] [Google Scholar]
- 103.Baxter EW, Conway KA, Kennis L, Bischoff F, Mercken MH, Winter HL, Reynolds CH, Tounge BA, Luo C, Scott MK, Huang Y, Braeken M, Pieters SM, Berthelot DJ, Masure S, Bruinzeel WD, Jordan AD, Parker MH, Boyd RE, Qu J, Alexander RS, Brenneman DE, Reitz AB. 2-Amino-3,4-dihydroquinazolines as inhibitors of BACE-1 (beta-site APP cleaving enzyme): Use of structure based design to convert a micromolar hit into a nanomolar lead. J Med Chem. 2007;50(18):4261–4264. doi: 10.1021/jm0705408. [DOI] [PubMed] [Google Scholar]
- 104.Huang Y, Strobel ED, Ho CY, Reynolds CH, Conway KA, Piesvaux JA, Brenneman DE, Yohrling GJ, Moore Arnold H, Rosenthal D, Alexander RS, Tounge BA, Mercken M, Vandermeeren M, Parker MH, Reitz AB, Baxter EW. Macrocyclic BACE inhibitors: Optimization of a micromolar hit to nanomolar leads. Bioorg Med Chem Lett. 2010;20(10):3158–3160. doi: 10.1016/j.bmcl.2010.03.097. [DOI] [PubMed] [Google Scholar]
- 105.Ghosh AK, Pandey S, Gangarajula S, Kulkarni S, Xu X, Rao KV, Huang X, Tang J. Structure-based design, synthesis, and biological evaluation of dihydroquinazoline-derived potent beta-secretase inhibitors. Bioorg Med Chem Lett. 2012;22(17):5460–5465. doi: 10.1016/j.bmcl.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng Y, Judd TC, Bartberger MD, Brown J, Chen K, Fremeau RT, Jr, Hickman D, Hitchcock SA, Jordan B, Li V, Lopez P, Louie SW, Luo Y, Michelsen K, Nixey T, Powers TS, Rattan C, Sickmier EA, St Jean DJ, Jr, Wahl RC, Wen PH, Wood S. From fragment screening to in vivo efficacy: optimization of a series of 2-aminoquinolines as potent inhibitors of beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) J Med Chem. 2011;54(16):5836–5857. doi: 10.1021/jm200544q. [DOI] [PubMed] [Google Scholar]
- 107.Stachel SJ, Steele TG, Petrocchi A, Haugabook SJ, McGaughey G, Katharine Holloway M, Allison T, Munshi S, Zuck P, Colussi D, Tugasheva K, Wolfe A, Graham SL, Vacca JP. Discovery of pyrrolidine-based beta-secretase inhibitors: lead advancement through conformational design for maintenance of ligand binding efficiency. Bioorg Med Chem Lett. 2012;22(1):240–244. doi: 10.1016/j.bmcl.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 108.Efremov IV, Vajdos FF, Borzilleri KA, Capetta S, Chen H, Dorff PH, Dutra JK, Goldstein SW, Mansour M, McColl A, Noell S, Oborski CE, O’Connell TN, O’Sullivan TJ, Pandit J, Wang H, Wei B, Withka JM. Discovery and optimization of a novel spiropyrrolidine inhibitor of beta-secretase (BACE1) through fragment-based drug design. J Med Chem. 2012;55(21):9069–9088. doi: 10.1021/jm201715d. [DOI] [PubMed] [Google Scholar]
- 109.Sasaki H, Miki K, Kinoshita K, Koyama K, Juliawaty LD, Achmad SA, Hakim EH, Kaneda M, Takahashi K. beta-Secretase (BACE-1) inhibitory effect of biflavonoids. Bioorg Med Chem Lett. 2010;20(15):4558–4560. doi: 10.1016/j.bmcl.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 110.Cho JK, Ryu YB, Curtis-Long MJ, Kim JY, Kim D, Lee S, Lee WS, Park KH. Inhibition and structural reliability of prenylated flavones from the stem bark of Morus lhou on beta-secretase (BACE-1) Bioorg Med Chem Lett. 2011;21(10):2945–2948. doi: 10.1016/j.bmcl.2011.03.060. [DOI] [PubMed] [Google Scholar]
- 111.Asso V, Ghilardi E, Bertini S, Digiacomo M, Granchi C, Minutolo F, Rapposelli S, Bortolato A, Moro S, Macchia M. alpha-Naphthylaminopropan-2-ol Derivatives as BACE1 Inhibitors. ChemMedChem. 2008;3(10):1530–1534. doi: 10.1002/cmdc.200800162. [DOI] [PubMed] [Google Scholar]
- 112.Kumar AB, Anderson JM, Melendez AL, Manetsch R. Synthesis and structure-activity relationship studies of 1,3-disubstituted 2-propanols as BACE-1 inhibitors. Bioorg Med Chem Lett. 2012;22(14):4740–4744. doi: 10.1016/j.bmcl.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 113.Brodney MA, Barreiro G, Ogilvie K, Hajos-Korcsok E, Murray J, Vajdos F, Ambroise C, Christoffersen C, Fisher K, Lanyon L, Liu J, Nolan CE, Withka JM, Borzilleri KA, Efremov I, Oborski CE, Varghese A, O’Neill BT. Spirocyclic sulfamides as beta-secretase 1 (BACE-1) inhibitors for the treatment of Alzheimer’s disease: utilization of structure based drug design, WaterMap, and CNS penetration studies to identify centrally efficacious inhibitors. J Med Chem. 2012;55(21):9224–9239. doi: 10.1021/jm3009426. [DOI] [PubMed] [Google Scholar]
- 114.Butini S, Gabellieri E, Huleatt PB, Campiani G, Franceschini S, Brindisi M, Ros S, Coccone SS, Fiorini I, Novellino E, Giorgi G, Gemma S. An efficient approach to chiral C8/C9-piperazino-substituted 1,4-benzodiazepin-2-ones as peptidomimetic scaffolds. J Org Chem. 2008;73(21):8458–8468. doi: 10.1021/jo8015456. [DOI] [PubMed] [Google Scholar]
- 115.Butini S, Gabellieri E, Brindisi M, Casagni A, Guarino E, Huleatt PB, Relitti N, La Pietra V, Marinelli L, Giustiniano M, Novellino E, Campiani G, Gemma S. Novel peptidomimetics as BACE-1 inhibitors: synthesis, molecular modeling, and biological studies. Bioorg Med Chem Lett. 2013;23(1):85–89. doi: 10.1016/j.bmcl.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 116.Huang W, Yu H, Sheng R, Li J, Hu Y. Identification of pharmacophore model, synthesis and biological evaluation of N-phenyl-1-arylamide and N-phenylbenzenesulfonamide derivatives as BACE 1 inhibitors. Bioorg Med Chem. 2008;16(24):10190–10197. doi: 10.1016/j.bmc.2008.10.059. [DOI] [PubMed] [Google Scholar]
- 117.Yi Mok N, Chadwick J, Kellett KA, Hooper NM, Johnson AP, Fishwick CW. Discovery of novel non-peptide inhibitors of BACE-1 using virtual high-throughput screening. Bioorg Med Chem Lett. 2009;19(23):6770–6774. doi: 10.1016/j.bmcl.2009.09.103. [DOI] [PubMed] [Google Scholar]
- 118.Xu W, Chen G, Liew OW, Zuo Z, Jiang H, Zhu W. Novel non-peptide beta-secretase inhibitors derived from structure-based virtual screening and bioassay. Bioorg Med Chem Lett. 2009;19(12):3188–3192. doi: 10.1016/j.bmcl.2009.04.113. [DOI] [PubMed] [Google Scholar]
- 119.Xu W, Chen G, Zhu W, Zuo Z. Molecular docking and structure-activity relationship studies on benzothiazole based non-peptidic BACE-1 inhibitors. Bioorg Med Chem Lett. 2010;20(21):6203–6207. doi: 10.1016/j.bmcl.2010.08.111. [DOI] [PubMed] [Google Scholar]
- 120.Xu W, Chen G, Zhu W, Zuo Z. Identification of a sub-micromolar, non-peptide inhibitor of beta-secretase with low neural cytotoxicity through in silico screening. Bioorg Med Chem Lett. 2010;20(19):5763–5766. doi: 10.1016/j.bmcl.2010.07.140. [DOI] [PubMed] [Google Scholar]
- 121.Chiriano G, Sartini A, Mancini F, Andrisano V, Bolognesi ML, Roberti M, Recanatini M, Carloni P, Cavalli A. Sequential virtual screening approach to the identification of small organic molecules as potential BACE-1 inhibitors. Chem Biol Drug Des. 2011;77(4):268–271. doi: 10.1111/j.1747-0285.2011.01087.x. [DOI] [PubMed] [Google Scholar]
- 122.Rajendran L, Schneider A, Schlechtingen G, Weidlich S, Ries J, Braxmeier T, Schwille P, Schulz JB, Schroeder C, Simons M, Jennings G, Knolker HJ, Simons K. Efficient inhibition of the Alzheimer’s disease beta-secretase by membrane targeting. Science. 2008;320(5875):520–523. doi: 10.1126/science.1156609. [DOI] [PubMed] [Google Scholar]
- 123.Bajda M, Guzior N, Ignasik M, Malawska B. Multi-target-directed ligands in Alzheimer’s disease treatment. Curr Med Chem. 2011;18(32):4949–4975. doi: 10.2174/092986711797535245. [DOI] [PubMed] [Google Scholar]
- 124.Rampa A, Belluti F, Gobbi S, Bisi A. Hybrid-Based Multi-Target Ligands for the Treatment of Alzheimer’s Disease. Curr Top Med Chem. 2011;11(22):2716–2730. doi: 10.2174/156802611798184409. [DOI] [PubMed] [Google Scholar]