Abstract
Background
Substance use disorders (SUDs) are a leading cause of disability worldwide. While several pharmacological and behavioral treatments for SUDs are available, these may not be effective for all patients. Recent studies using non-invasive neuromodulation techniques including Repetitive Transcranial Magnetic Stimulation (rTMS), Transcranial Direct Current Stimulation (tDCS), and Deep Brain Stimulation (DBS) have shown promise for SUD treatment.
Objective
Multiple studies were evaluated investigating the therapeutic potential of non-invasive brain stimulation techniques in treatment of SUDs.
Method
Through literature searches (eg, PubMed, Google Scholar), 60 studies (2000–2017) were identified examining the effect of rTMS, tDCS, or DBS on cravings and consumption of SUDs, including tobacco, alcohol, cannabis, opioids, and stimulants.
Results
rTMS and tDCS demonstrated decreases in drug craving and consumption, while early studies with DBS suggest similar results. Results are most encouraging when stimulation is targeted to the Dorsolateral Prefrontal Cortex (DLPFC).
Conclusions
Short-term treatment with rTMS and tDCS may have beneficial effects on drug craving and consumption. Future studies should focus on extending therapeutic benefits by increasing stimulation frequency and duration of treatment.
Scientific Significance
The utility of these methods in SUD treatment and prevention are unclear, and warrants further study using randomized, controlled designs.
INTRODUCTION
Substance use disorders (SUDs) are a major contributor to morbidity and mortality worldwide.1 According to the United Nations, more than 200,000 deaths were attributable to SUDs in 2014.2 Moreover, in 2015 over 20 million people in the U.S. had an SUD, with <20% seeking treatment.2 While several treatments are available (eg, behavioral and pharmacological therapies), relapse rates continue to be as high as 60%.1 This suggests a need for further research to develop novel and more effective treatments. Neuromodulation techniques such as repetitive transcranial magnetic stimulation (rTMS), trans-cranial direct current stimulation (tDCS), and deep brain stimulation (DBS) have been investigated as possible treatments for SUDs and may have promise in comparison to conventional pharmacotherapy and behavioral intervention strategies, and there have been several more selective reviews on this topic.3–5 The purpose of this article is to provide a broad and critical review of currently available brain stimulation techniques (rTMS, tDCS, DBS) as treatment for SUDs, including a comparison of effect sizes for the various brain stimulation methods across SUD diagnoses.
Description of Contemporary Brain Stimulation Methods
Repetitive Transcranial Magnetic Stimulation (rTMS)
rTMS is a non-invasive brain stimulation method used to treat various neurological and psychiatric disorders, including Parkinson’s disease, schizophrenia, obsessive compulsive disorder, and chronic pain.6 In 2008, rTMS was Food and Drug Administration (FDA) approved for the treatment of major depression in human subjects, offering a potential alternative to traditional pharmacotherapies, which may not be effective or well-tolerated.6 rTMS involves the use of an electromagnetic coil held against the scalp, producing repetitive trains of magnetic pulses, resulting in a temporary magnetic field pulse in the coil that can be targeted to specific brain regions.7,8 This process has been shown to induce temporary electrical currents in localized cortical tissue, which can modulate cortical excitability. Studies have also demonstrated its ability to produce clinically significant and lasting neuroplasticity changes in targeted brain regions.7,8 rTMS stimulation parameters can vary significantly with respect to stimulus intensity, total number of pulses, and pulse frequencies.9 However, ultimately these variations aim to personalize rTMS parameters and may improve inhibitory processes, which may be abnormal in SUDs (ie, impulsivity). As such, many investigators have used cortical inhibition paradigms with single or paired pulse TMS to index cortical inhibition and excitability effects.10,11 This review will focus on low frequency (LF) and high frequency (HF) rTMS, both of which provide therapeutic applications. LF rTMS (<1 Hz) has been shown to reduce neuronal firing rates and cortical excitability, whereas HF rTMS (10–20 Hz) has demonstrated opposing effects.7,8 Furthermore, two robust rTMS adaptations compared to the conventional protocols have been emerging which use a theta burst stimulation (TBS) pattern.12 This involves bursts of three pulses of stimulation at 50 Hz frequencies repeated every 200 ms. The first method is intermittent bursting frequency, also known as intermittent that burst stimulation (iTBS), which is applied to the cortex resulting in facilitating effects. The parameters involve a 2 second train of TBS repeated every 10 seconds for a total of 190 seconds (600 pulses).12,13 The other technique using continuous bursting frequency, also known as continuous theta burst stimulation (cTBS), induces transient long-term depression of behavior and inhibitory effects. This technique involves a 40 second train of uninterrupted TBS (600 pulses).12,14
rTMS is a promising treatment for neurological and psychiatric disorders, as this treatment method has minor side effects (ie, headache, dizziness) and is pain free, making it well-tolerated by most patients.6 Additionally, rTMS is a cost-effective alternative to other more expensive treatment methods (ie, electroconvulsive therapy). rTMS is a promising potential treatment for SUDs that is currently being investigated, although available research is preliminary. Twenty-eight studies were identified for this review, using rTMS as a potential treatment for SUDs, with a total of 788 participants receiving active or sham rTMS stimulation treatment. See Table 1 and Figure 1A.
TABLE 1.
Repetitive transcranial magnetic stimulation (rTMS) (total N = 788; total stuclies = 28)
| Author | Sample | Study design | # of sessions and targeted brain region | Stimulation intensity | Stimulation frequency | Coil type | Primary outcomes and effect size (Cohen’s d) | Secondary outcomes and effect size (Cohen’s d) | Results |
|---|---|---|---|---|---|---|---|---|---|
| Alcohol: single active stimulation sessions (total N = 95; four studies) | |||||||||
|
| |||||||||
| Addolorato et al.28 | N = 11 | A randomized, between subjects, sham-controlled study with alcohol-dependent participants | 1 active or 1 sham session of dTMS Left and right DLPFC |
100% | 10 Hz | H-coil |
Consumption Sham: −0.60 Active: 1.33 |
Craving Sham: −0.72 Active: −0.70 |
A significant ↓ in alcohol consumption was observed in the active group compared to sham No significant differences in craving scores between groups |
| Herremans et al.29 | N = 29 | A sham controlled, crossover study with recently detoxified, alcohol-dependent participants | 1 active or 1 sham session Right DLPFC |
110% | 20 Hz | Figure 8 coil |
Executive functioning NA |
Craving Sham: 0.13 Active: 0.11 |
No significant effect on alcohol craving was found post-treatment in either sham or active groups |
| Herremans et al.30 | N = 36 | A prospective, sham controlled study with recently detoxified alcohol-dependent inpatients | 1 active or 1 sham session Right DLPFC |
110% | 20 Hz | Figure 8 coil |
Craving ES: −0.14 |
None | No significant effect on alcohol craving were observed post- treatment compared to sham |
| Hoppner et al.31 | N = 19 | A sham controlled study with alcohol-dependent patients | 1 active or 1 sham session Left DLPFC |
90% | 20 Hz | Figure 8 coil |
Craving Active vs. sham: 0.05 |
Depressive symptoms Active vs. sham: 0.13 |
No significant effects on alcohol craving or mood were observed post-treatment |
|
| |||||||||
| Alcohol: multiple active stimulation sessions (total N = 133; five studies) | |||||||||
|
| |||||||||
| Ceccanti et al.32 | N = 18 | A placebo controlled pilot study with alcohol-dependent participants with concurrent dysthymic disorder | 10 active or 10 sham sessions of dTMS MPFC |
120% | 20 Hz | H-coil |
Cortisol blood levels Active vs. sham: 2.08 |
Consumption Active pre vs. post: 1.90 Craving Active pre vs. post: 0.46 |
Significant ↓ in cortisol blood levels, alcohol craving, and consumption were observed in the active group compared to control |
| Girardi et al.33 | N = 24 | A double-blind study with alcohol-dependent participants with MDD or bipolar disorder | 10 active dTMS sessions with SDT or SDT alone MPFC |
120% | 18 Hz | H-coil |
Craving Active vs. sham: 1.94 |
Depressive symptoms Active vs. sham: 1.98 |
Deep TMS combined with pharmacotherapy treatment produced significant ↓ in alcohol cravings across participants |
| Herremans et al.34 | N = 26 | An open label, accelerated design with alcohol-dependent participants | 15 active sessions R DLPFC |
110% | 20 Hz | Figure 8 coil |
Craving (after 1 session) Active vs. sham: 0.00 |
Craving (after 15 sessions) Active vs. sham: 0.54 |
A significant ↓ in general alcohol cravings post 15 sessions of rTMS No difference between sham and active groups after 1 session of treatment |
| Mishra et al.35 | N = 20 | A prospective, parallel group study with alcohol-dependent participants | 10 active sessions Left or right DLPFC |
110% | 10 Hz | Figure 8 coil |
Craving Left: 3.93 Right: 4.31 Left vs. right: 0.05 |
None | A significant ↓ in alcohol-related cravings in both left and right DLPFC stimulation groups with no significant difference between stimulation sites |
| Mishra et al.36 | N = 45 | A sham controlled, prospective study with alcohol-dependent patients | 10 active or 10 sham sessions Right DLPFC |
110% | 10 Hz | Figure 8 coil |
Craving Active vs. sham: 2.99 |
None | Active rTMS significantly ↓ alcohol cravings, compared to sham |
|
| |||||||||
| Tobacco: single active stimulation sessions (total N = 54; four studies) | |||||||||
|
| |||||||||
| Li et al.37 | N = 10 | A sham-controlled, counterbalanced, crossover study design with nicotine-dependent participants | 1 active and 1 sham session Left DLPFC |
100% | 10 Hz | Figure 8 coil |
fFLAFF Right insula Active vs. sham: 4.00 Right thalamus Active vs. sham: 2.97 |
Craving Active vs. sham: −0.47 |
Active rTMS significantly ↓ activity in the Insula and thalamus regions in fFLAFF No differences in craving scores were observed in active compared to sham |
| Pripfl et al.38 | N = 14 | A within subjects, sham controlled study with nicotine-dependent participants | 1 active and 1 sham session Left DLPFC |
90% | 10 Hz | Figure 8 coil |
EEG Delta Power Active vs. sham: 0.69 |
Craving Active vs. sham: −0.26 |
A significant reduction in EEG delta power was observed after active treatment compared to sham Nicotine craving significantly ↓ after rTMS treatment compared to sham |
| Li et al.39 | N = 16 | A sham-controlled cross over study with treatment seeking nicotine-dependent participants | 1 active and 1 sham session Left DLPFC |
100% | 10 Hz | Figure 8 coil |
Cue-induced craving Active pre vs. post: 0.77 Active vs. sham: 0.45 |
None | Active rTMS significantly ↓ cravings of tobacco compared to sham and baseline scores |
| Eichammer et al.40 | N = 14 | A sham controlled, crossover study with treatment seeking, nicotine-dependent participants | 1 active and 1 sham session Left DLPFC |
90% | 20 Hz | Figure 8 coil |
Consumption Active vs. sham: 0.27 |
Craving NA |
Significant ↓ in cigarette consumption was observed in the active group compared to sham No differences in post-stimulation craving scores were observed between groups |
|
| |||||||||
| Tobacco: multiple active stimulation studies (total N = 339; seven studies) | |||||||||
|
| |||||||||
| Trojak et al.41 | N = 37 | A prospective, clinical trial with nicotine-dependent participants | 10 active or 10 sham sessions Right DLPFC |
120% | 1 Hz | Figure 8 coil |
Abstinence Active vs. sham: 4.42 |
Craving Active vs. sham: 0.12 |
Combination treatment of NRT and active rTMS produced significantly ↑ abstinent participants (n=16) but results not maintained at follow up (12 weeks) No lasting effects on craving were observed |
| Dieler et al.42 | N = 74 | A between groups, sham controlled study with nicotine-dependent participants | 4 active or 4 sham sessions of iTBS with CBT Right DLPFC |
80% | 50 Hz | Figure 8 coil |
Craving Active vs. sham: −0.33 |
Abstinence NA |
iTBS with adjunct CBT produced ↑ abstinence rates at 3 months compared to sham Craving was not significantly affected |
| Prikryl et al.43 | N = 35 | A prospective, sham controlled, study with male patients with SCZ and concurrent nicotine dependence | 21 active or 21 sham sessions Left DLPFC |
110% | 10 Hz | Figure 8 coil |
Consumption Active vs. sham: 0.41 |
None | Active rTMS significantly ↓ cigarette consumption in patients with SCZ |
| Dinur-Klein et al.44 | N = 115 | A prospective, placebo controlled, clinical trial with nicotine-dependent participants (>20 CPD) | 13 active or 13 sham sessions of dTMS Lateral PFC and insula |
120% | LF: 1 Hz HF: 10 Hz |
H-coil |
Consumption Sham: 0.64 1 Hz: 1.41 a10 Hz: 3.17 |
Abstinence NA |
10 Hz dTMS significantly ↓ cigarette consumption and compared to low frequency and sham conditions No differences in abstinence rates between groups |
| Wing et al.45 | N = 15 | A sham controlled, counterbalanced rTMS study with nicotine-dependent participants with concurrent SCZ | 20 active or 20 sham sessions Left and right DLPFC |
90% | 20 Hz | Figure 8 coil |
Craving Active vs. sham: 1.28 |
None | Participants who received active rTMS showed a significant ↓ in cravings for tobacco |
| Rose et al.46 | N = 15 | A repeated measures, counterbalanced design with nicotine-dependent participants (>15 CPD) | 1 active (1 Hz) and 1 active (10 Hz) and 1 sham session MOC, SFG |
90% | HF: 10 Hz LF: 1 Hz |
Figure 8 coil |
Cue-induced craving Sham vs. 1 Hz: −0.38 Sham vs. 10 Hz: −0.29 |
Craving Sham vs. 10 Hz: 0.96 1 Hz vs. 10 Hz: 1.02 |
SFG specific rTMS significantly ↓ cigarette cravings after neutral cue presentations Conversely, 10 Hz stimulation with smoking cues caused a significant ↑ in craving |
| Amiaz et al.47 | N = 48 | An experimental, counterbalanced design with nicotine-dependent participants | 10 active +3 maintenance sessions or 10 sham sessions Left DLPFC |
100% | 10 Hz | Figure 8 coil |
Consumption Active pre/post smoking cues: 1.53 |
Dependence Active pre vs. post: 5.63 |
Significant ↓ in cigarette consumption and nicotine dependence following the active rTMS (not maintained until the 6-month follow-up) |
|
| |||||||||
| Cocaine: single active stimulation studies (total N = 6; one study) | |||||||||
|
| |||||||||
| Camprodon et al.51 | N = 6 | A counterbalanced, crossover design with cocaine dependent, rTMS naïve participants | 1 active right or 1 active left session Left or right DLPFC |
90% | 10 Hz | Figure 8 coil |
Craving Right: 2.19 Left: −0.22 |
None | Right, but not left DLPFC stimulation significantly ↓ cocaine cravings temporarily (<4 hours) |
|
| |||||||||
| Cocaine: multiple active stimulation sessions (total N = 49; three studies) | |||||||||
|
| |||||||||
| Bolloni et al.48 | N = 10 | A double blind, experimental design with cocaine-dependent participants | 12 active or 12 sham sessions Left and right DLPFC |
100% | 10 Hz | Figure 8 coil |
Consumption Active pre/post: −1.82 Active post vs. follow-up: 0.93 |
None | No significant change in cocaine consumption were observed post-treatment However, a long-term ↓ in amount of cocaine consumed was demonstrated when considering time as a factor in the active group |
| Rapinesi et al.49 | N = 7 | A within subjects, double-blind study in cocaine-dependent male participants | 12 active sessions Left DLPFC |
100% | 20 Hz | H-coil |
Craving Active pre vs. post: 3.58 |
None | A significant ↓ in craving scores were observed after dTMS to the DLPFC, although maintenance sessions were needed to extend results |
| Terraneo et al.50 | N = 32 | A between-subject open-label, clinical trial with cocaine-dependent participants | 8 active sessions or SDT only Left DLPFC |
110% | 15 Hz | Figure 8 coil |
Consumption Active vs. sham: 1.78 |
None | Active rTMS treatment produced significantly ↑ clean urine screens and a ↓ in cravings for cocaine for active stimulation group |
|
| |||||||||
| Methamphetamine: single active stimulation sessions (total N = 18; one studies) | |||||||||
|
| |||||||||
| Li et al.,53 | N = 18 | A sham-controlled crossover study with MA dependent participants and matched controls | 1 active and 1 sham session Left DLPFC |
100% | 1 Hz | Figure 8 coil |
Craving Active vs. sham in MA group: −1.99 |
None | Active rTMS ↑ self-reported craving in MA users compared to sham stimulation This effect was not observed in healthy controls |
|
| |||||||||
| Methamphetamine: Multiple active stimulation sessions (total N = 80; two studies) | |||||||||
|
| |||||||||
| Su et al.52 | N = 30 | A sham-controlled clinical trial with male MA-dependent participants | 5 active or 5 sham sessions Left DLPFC |
80% | 10 Hz | Figure 8 coil |
Cue-induced craving Active vs. sham: 2.24 |
Cognitive function Active pre/post: 0.24 |
Active rTMS significantly ↓ cravings for MA compared to sham |
| Liu et al.54 | N = 50 | A sham-controlled study in MA-dependent males | 5 active sessions of left DLPFC (1 Hz) or left DLPFC (10 Hz) or right DLPFC (1 Hz) or right DLPFC (10 Hz) or 5 sham sessions |
100% | 1 Hz or 10 Hz | Round coil |
Cue-induced craving R 10 Hz pre/post:2.78 L10 Hz pre/post:1.73 R 1 Hz pre/post:2.11 L 1 Hz pre/post:1.47 |
None | All active stimulation conditions significantly ↓ cue-induced MA craving compared to the sham condition |
|
| |||||||||
| Cannabis: multiple active stimulation sessions (total N = 14; one study) | |||||||||
|
| |||||||||
| Sahlem et al.55 | N = 14 | A crossover, sham-controlled study in cannabis-dependent participants | 1 active and 1 sham session Left DLPFC |
100% | 10 Hz | Figure 8 coil |
Safety NA |
Craving Active vs. sham: −0.41 |
rTMS can be safely administered to cannabis-dependent participants No significant changes in craving were observed compared to sham |
rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; DBS, deep brain stimulation; dTMS, deep transcranial magnetic stimulation; iTBS, intermittent theta burst stimulation; cTBS, continuous theta burst stimulation; dTMS, deep transcranial magnetic stimulation; RMT, resting motor threshold; Hz, hertz; Tx, treatment; DLPFC, dorsolateral prefrontal cortex; MOC, motor cortex; IFG, inferior frontal gyrus; SFG, superior frontal gyrus; PFC, prefrontal cortex; MPFC, medial prefrontal cortex; FTP, fronto-temporal parietal area; SOA, supra-orbital area; CSOA, contralateral supraorbital area; ACC, anterior cingulate cortex; NAc, nucleus accumbens; An+, anodal; Ca−, cathodal; MDD, Major Depressive Disorder; SCZ, schizophrenia; OCD, obsessive compulsive disorder; mA, milliamp; CPD, cigarettes per day; NRT, nicotine replacement therapy; SDT, standard treatment; ↑, increase; ↓, decrease; NA, not assessed/not available.
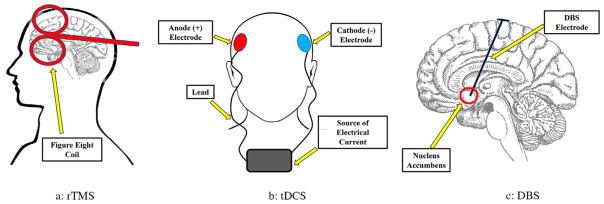
FIGURE 1.
Diagrams to illustrate the three brain stimulation techniques: (a) rTMS, (b) tDCS, and (c) DBS.
Transcranial Direct Current Stimulation (tDCS)
tDCS is another non-invasive brain stimulation method involving two or more electrodes (ie, anodal, cathodal) placed on the scalp.15 These electrodes facilitate delivery of a low intensity direct current at a constant rate to a targeted area of the brain.16 tDCS protocols vary with respect to the current size and strength, electrode size and number, amount of contact medium used, and stimulation durations.17 Similarly, as with the differing rTMS parameters, these factors alter the distribution of current crossing the scalp eventually to the brain.
tDCS stimulation produces a low intensity current (between 0.5 and 2.0 milliamps [mA]) that allows for the modulation of resting membrane potential and cortical excitability in targeted brain regions through mechanisms of depolarization or hyperpolarization, depending on stimulation parameters.18 The anodal electrode has been shown to increase cortical excitability, whereas the cathodal electrode has opposing effects. tDCS is a low cost, easily accessible, pain free stimulation method with minor side effects such as scalp irritation and itchiness and no recovery time requirement, thus making it well-tolerated treatment across patients.15
Similar to rTMS, tDCS has been used to treat various neurological and psychiatric conditions such as Parkinson’s disease, chronic pain, and major depression.15 Although its mechanisms of action are not fully understood, tDCS may induce neurochemical changes in the targeted brain tissue, which extend beyond active stimulation periods.18 tDCS is also currently being investigated as a potential treatment for SUD’s in human participants. Twenty-three studies have been identified in which tDCS has been explored as a viable treatment option, with 677 participants having been exposed to active or sham stimulation. See Table 2 and Figure 1B.
TABLE 2.
transcranial direct current stimulation (tDCS) (total N =677; total studies =23)
| Author | Sample | Study design | # of sessions and targeted brain region | Active stimulation intensity and duration | Primary outcome and effect size (Cohen’s d) | Secondary outcome and effect size (Cohen’s d) | Results |
|---|---|---|---|---|---|---|---|
| Alcohol: single active stimulation sessions (total N =120; three studies) | |||||||
|
| |||||||
| Wietschorke et al.56 | N =30 | A double-blind, placebo controlled trial with alcohol-dependent outpatients | 1 session of An+ right, Ca− left DLPFC or 1 session of sham |
1 mA for 20 minutes |
Alcohol cue reactivity Active vs. sham: 0.64 |
Craving Active vs. sham: 0.53 |
A significant ↓ in both alcohol cue reactivity and alcohol cravings were observed in alcohol-dependent participants who received active tDCS stimulation |
| Den Uyl et al.57 | N =41 | A sham controlled study with alcohol-dependent participants | 1 session of An+ left DLPFC, Ca− CSOA or l session of An+ IFG (F7 ×Cz), Ca− IFG (Fz ×T3) or 1 session of sham |
1 mA for 10 minutes |
Craving DLPFC vs. sham: 0.18 DLPFC vs. IFG: 0.12 |
Response bias DLPFC pre/post: 0.10 IFG pre/post: −0.09 |
Active tDCS over the left DLPFC significantly ↓ alcohol cravings, compared to sham and IFG stimulation No changes were observed in response bias variable |
| Nakamura-Palacios et al.58 | N =49 | A sham-controlled, crossover design with alcohol-dependent participants | 1 session of An+ left DLPFC, Ca−
CSOA or 1 session of sham |
1 mA for 10 minutes |
P3 amplitude NA |
Craving Active vs. sham: −0.18 |
Results showed an ↑ in P3 amplitude to alcohol-related sounds in the active tDCS group compared to sham (not seen in neutral sounds) No significant changes in craving were observed between groups |
|
| |||||||
| Alcohol: multiple active stimulation sessions (total N =150; four studies) | |||||||
|
| |||||||
| Den Uyl et al.59 | N =91 | A double-blind, parallel design with alcohol-dependent participants | 4 sessions An+ left, Ca− right DLPFC with concurrent CBM or 4 sessions of An+ left, Ca− right DLPFC before CBM or 4 sessions of sham |
2 mA for 20 minutes |
Abstinence Active with CBM vs. sham: 0.27 Active with CBM vs. active without CBM: 0.04 |
Craving Active with CBM vs. sham: 1.22 Active with CBM vs. active without CBM: 1.53 |
No effect on abstinence at 3 or 6 months was observed across groups Relapse ↓ in active condition, only when compared to sham control Craving scores in all conditions ↓ overtime |
| Klauss et al.60 | N =33 | A prospective, sham controlled study with alcohol-dependent participants | 5 sessions An+ right, Ca− left DLPFC or 5 sessions of sham |
2 mA for 13 minutes |
Relapse Active vs. sham: 2.20 |
Craving Active pre/post: 1.24 Active vs. sham: 0.17 |
tDCS stimulation had no effect on alcohol related cravings, but did ↑ abstinent rates significantly at 6 months follow-up |
| Da Silva et al.61 | N =13 | A sham controlled, experimental design with alcohol-dependent male participants | 5 sessions of An+ left, Ca− right DLPFC or 5 sessions of sham stimulation |
2 mA for 20 minutes |
Relapse NA |
Craving Active vs. sham: 2.18 |
A significant ↑ in relapse rates in active tDCS condition (66.7%) were observed compared to sham (14.3%) Conversely, a significant ↓ in alcohol-related cravings in the active tDCS group compared to sham |
| Boggio et al.62 | N =13 | A sham-controlled, crossover study with alcohol-dependent participants (abstinent for 10 days) | 1 session of An+ right, Ca− left DLPFC and 1 session of An+ left, Ca− right DLPFC and 1 session of sham |
2 mA for 20 minutes |
Craving Left: 4.25 Right: 1.9 |
None | Both active tDCS conditions significantly ↓ alcohol craving compared to sham Results were maintained with presentation of alcohol cues |
|
| |||||||
| Tobacco: single active stimulation sessions (total N =108; four studies) | |||||||
|
| |||||||
| Falcone et al.70 | N =25 | A within subjects, counterbalanced study with nicotine-dependent participants | 1 session of An+ left DLPFC, Ca−
SOA and 1 session of sham |
1 mA for 20 minutes |
Time to first cigarette Active vs. sham: 0.29 |
Cigarette consumption Active vs. sham: 0.20 |
Active tDCS significantly ↑ time to first cigarette and ↓ total cigarettes smoked compared to sham with the presentation of smoking-related cues |
| Krozec et al.69 | N =29 | A sham-controlled study with nicotine-dependent participants | 1 session of An+ left DLPFC, Ca−
OFC or 1 session of sham |
2 mA for 15 minutes |
Functional cortical connectivity NA |
Craving Active vs. sham: −0.39 |
Active tDCS significantly ↑ functional cortical connectivity in the active group compared to sham No differences in craving scores between groups were observed |
| Meng et al.68 | N =30 | A sham-controlled, counterbalanced study with nicotine-dependent participants | 1 session of bilateral Ca− left, Ca− right FTP or 1 session of Ca− right DLPFC, An+ occipital lobe or 1 session of sham |
2 mA for 20 minutes |
Attention bias Bilateral pre/post: 0.42 Ca− right: −0.33 |
Consumption Single vs. bilateral: 1.22 Sham vs. bilateral: 2.16 |
A significant ↓ in cigarette consumption was observed post-bilateral tDCS condition only, compared to sham and compared to single stimulation Attention bias showed a ↓ trend in bilateral tDCS condition but results were not significant |
| Xu et al.67 | N =24 | A counterbalanced, sham controlled study with nicotine-dependent participants (abstinent >10 hours) | 1 session of An+ left DLPFC, Ca−
SOA and 1 session of sham |
2 mA for 20 minutes |
Affect modulation Active vs. sham: −0.10 |
Craving Active vs. sham: 0.05 |
Active tDCS stimulation had a significant ↓ in negative affect (positively correlated with dependence level) but no effect on cravings compared to sham post-treatment |
|
| |||||||
| Tobacco: multiple active stimulation sessions (total N = 113; four studies) | |||||||
|
| |||||||
| Smith et al.63 | N =37 | A sham controlled, parallel group study with nicotine-dependent participants with concurrent SCZ | 5 sessions of An+ left DLPFC, Ca−
CSOA or 5 sessions of sham |
2 mA for 20 minutes |
Cognitive performance Active vs. sham: 0.16 |
Craving NA |
Participants who received active tDCS saw a significant ↑ in cognitive performance, as indexed by the MCCB composite score There was no significant effect of active tDCS on craving or consumption |
| Fecteau et al.64 | N =12 | A crossover, sham controlled design with nicotine-dependent participants | 5 sessions of An+ right, Ca− left DLPFC and 5 sessions of sham |
2 mA for 30 minutes |
Consumption Active vs. sham day 4: 0.35 |
Risk taking NA |
A significant ↓ in number of cigarettes smoked in participants who received active tDCS compared to sham No difference in risk taking between sham and active conditions |
| Boggio et al.65 | N =27 | A sham controlled, parallel, crossover study with nicotine-dependent participants | 5 sessions of An+ right, Ca− left DLPFC stimulation or 5 sessions of sham |
2 mA for 20 minutes |
Craving Day 4 sham vs. active: 0.27 Day 5 active vs. sham: 0.61 |
Consumption NA |
Active tDCS significantly ↓ cue-induced nicotine craving compared to sham A significant ↓ in cigarette consumption was also observed in the active group (30%) compared to sham (10%) |
| Fregni et al.66 | N =24 | A sham controlled, crossover design with nicotine-dependent participants | 1 session of An+ right, Ca− left DLPFC and 1 session of An+ left, Ca− right DLPFC and 1 session of sham |
2 mA for 20 minutes |
Craving Left: 2.00 Right: 1.30 |
None | Active tDCS of both the right and left DLPFC significantly ↓ cue-induced cravings compared to sham |
|
| |||||||
| Cocaine: single active stimulation session (total N = 13; one study) | |||||||
|
| |||||||
| Conti and Nakamura- Palacios71 | N =13 | A between subjects sham-controlled design with 13 crack cocaine-dependent participants | 1 session of Ca− left, An+ right DLPFC or 1 session of sham |
2 mA for 20 minutes |
ACC activity During srug cues Active vs. sham: 2.36 |
None | Active tDCS ↓ activity in the ACC during drug cue exposure, while sham tDCS activity in the same region |
|
| |||||||
| Cocaine: multiple active stimulation sessions (total N = 96; four studies) | |||||||
|
| |||||||
| De Almeida Ramos et al.73 | N =11 | An open label design with cocaine-dependent participants (abstinent for 3 weeks) | 10 sessions of An+ left, Ca− right DLPFC stimulation | 2 mA for 20 minutes |
Craving Active pre/post: 0.59 |
Anxiety NA |
A significant ↓ in cravings for crack cocaine compared to baseline scores, maintained with the presentation of crack-cocaine related cues No changes in anxiety or cognitive performance were observed |
| Batista et al.74 | N =36 | A sham controlled clinical trial with crack-cocaine-dependent male participants | 5 sessions of Ca− left, An+ right DLPFC or 5 sessions of sham |
2 mA for 20 minutes |
Craving Active pre/post: 3.15 Active vs. sham: 2.97 |
Depressive symptoms Active pre/post: 0.60 Sham pre/post: 0.09 |
A significant ↓ in cravings for crack cocaine in the active tDCS group compared to baseline and sham No significant differences between active and sham groups in depressive symptoms, post-treatment |
| Conti et al.72 | N =13 | A between subject, sham controlled study in crack cocaine-dependent participants | Pt 1: 1 session of bilateral An+ right, Ca− left DLPFC or 1 session of sham Pt 2: 5 sessions of bilateral An+ right, Ca− left DLPFC or 5 sessions of sham |
2 mA for 20 minutes |
P3 current density Pt 1 left: 0.47 Pt 1 right: −0.9 Pt 2 left: 0.68 Pt 2 right: 0.55 |
None | After a single session of active tDCS, P3 current density ↑ with neutral cues and ↓ with crack cues in the left DLPFC only, compared to sham After 5 sessions of active tDCS, P3 current density for both neutral and crack cues in both the left and right hemispheres |
| Gorini et al.75 | N =36 | A within subjects, sham-controlled study in 18 cocaine-dependent participants and 18 healthy controls | 1 session of An+ left, Ca− right DLPFC and 1 session of An+ right, Ca− left DLPFC and 1 session of sham |
1.5 mA for 20 minutes |
Risk taking Right An+ pre/post: 0.40 Left An+ pre/post: 0.76 |
None | Left and right DLPFC stimulation resulted in a ↓ in risk taking behaviors for both cocaine-dependent participants and healthy controls There was an ↑ in safety behaviors after right An+ stimulation and an ↑ in risk taking behaviors after left An+ stimulation These results were not observed in healthy controls |
|
| |||||||
| Methamphetamine: single active stimulation sessions (total N =32; one study) | |||||||
|
| |||||||
| Shahbabaie et al.76 | N =32 | A sham controlled, crossover study with male, MA-dependent participants (abstinent for >7 days) | 1 session of An+ right DLPFC, Ca−
SOA and 1 session of sham |
2 mA for 20 minutes |
Craving (acute) Sham vs. active: 0.22 |
Craving (cue-induced) Active vs. sham: −0.07 |
A significant ↓ in acute MA cravings after active tDCS and a significant ↑ in cue-induced MA cravings were observed post-tDCS compared to sham |
|
| |||||||
| Opioids: single active stimulation sessions (total N =20; one study) | |||||||
|
| |||||||
| Wang et al.77 | N =20 | A sham controlled study with heroin-dependent participants | 1 session of bilateral Ca−
FTP or 1 session of sham |
1.5 mA for 20 minutes |
Cue-induced craving Active pre/post: 3.29 Active vs. sham: 3.02 |
None | A significant ↓ in heroin cravings were observed, maintained with presentation of heroin related cues when compared to baseline scores and sham group |
|
| |||||||
| Cannabis: single active stimulation sessions (total N =25; one study) | |||||||
|
| |||||||
| Boggio et al.78 | N =25 | A sham controlled study with cannabis using participants (abstinent 24 hours) | 1 session of An+ right, Ca− left DLPFC or 1 session of An+ left, Ca− right DLPFC or 1 session of sham |
2 mA for 10 minutes |
Risk taking Right An+ vs. sham: 4.45 Left An+ vs. sham: 3.33 |
Craving Right: 1.89 Left: −0.75 |
A significant ↑ in risk taking propensity was observed in both active tDCS stimulation sessions Active right An+ tDCS (but not left) was associated with significant ↓ in cannabis cravings |
rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; DBS, deep brain stimulation; dTMS, deep transcranial magnetic stimulation; iTBS, intermittent theta burst stimulation; cTBS, continuous theta burst stimulation; dTMS, deep transcranial magnetic stimulation; RMT, resting motor threshold; Hz, hertz; Tx, treatment; DLPFC, dorsolateral prefrontal cortex; MOC, motor cortex; IFG, inferior frontal gyrus; SFG, superior frontal gyrus; PFC, prefrontal cortex; MPFC, medial prefrontal cortex; FTP, fronto-temporal parietal area; SOA, supra-orbital area; CSOA, contralateral supraorbital area; ACC, anterior cingulate cortex; NAc, nucleus accumbens; An+, anodal; Ca−, cathodal; MDD, Major Depressive Disorder; SCZ, schizophrenia; OCD, obsessive compulsive disorder; mA, milliamp; CPD, cigarettes per day; NRT, nicotine replacement therapy; SDT, standard treatment; ↑, increase; ↓, decrease; NA, not assessed/ not available.
Deep Brain Stimulation (DBS)
DBS is a third neuromodulation technique which has been used to treat disorders such as Alzheimer’s disease, obsessive-compulsive disorder (OCD), and chronic pain. Unlike rTMS and tDCS, DBS involves an invasive surgical procedure, whereby varying numbers of electrodes are implanted directly into the brain, enabling continuous modulation of brain activity and subsequent changes in neuroplasticity.19 It has also demonstrated the ability to regulate abnormal brain impulses occurring in specified cortical regions. DBS may be able to trigger neurotransmitter release in the brain, depending on the regions of electrode implantation. In comparison to TBS which activates or inhibits neurons with lower frequencies to targeted areas, DBS uses very high frequencies to block neural transmissions.14 Once implanted into the individual’s brain, DBS electrodes are connected to an implantable pulse generator (IPG), typically inserted in the chest wall, allowing for easy modulation of parameters and continuous stimulation at a designated frequency (>130 Hz).19 Furthermore, due to its invasive nature, DBS may cause serious side effects associated with surgical procedures, such as infection, seizure, and stroke.19 Notably, once a patient has recovered from the original surgical procedure, DBS seems to be well-tolerated. The exact mechanisms by which DBS exerts its clinical effects remain unclear, however, the ability of DBS to directly manipulate neural circuits in reward pathways may target addictive behaviors. As such, DBS is being investigated as a possible treatment for SUDs. Nine studies investigating the use of DBS as treatment for SUDs have been identified, with 25 participants having received active or sham stimulation. See Table 3 and Figure 1C.
TABLE 3.
Deep brain stimulation (DBS) (total N = 25; total stuclies = 9)
| Author | Sample | Study design | Targeted region | # of treatments | Primary outcome measure and effect size | Secondary outcome measure and effect size | Results |
|---|---|---|---|---|---|---|---|
| Alcohol: continuous active stimulation session (total N = 9; three studies) | |||||||
|
| |||||||
| Muller et al.80 | N = 3 | Case reports with three alcohol-dependent male participants | NAc | Continuous |
Abstinence NA |
None | At 1 year follow-up post DBS stimulation, two of three participants remained abstinent, while the third decreased number drinking days |
| Voges et al.81 | N = 5 | Case reports with male alcohol-dependent participants | NAc | Continuous |
Craving 4.57 |
Abstinence NA | A significant decrease in alcohol cravings were observed across participants. 2/5 participants became abstinent from alcohol |
| Kuhn et al.83 | N = 1 | Case report with an alcohol-dependent participant | NAc | Continuous |
Craving NA |
Cognitive control NA | DBS led to a decrease in alcohol consumption post-treatment |
|
| |||||||
| Tobacco: continuous active stimulation session (total N = 11; two studies) | |||||||
|
| |||||||
| Mantione et al.82 | N = 1 | Case report with a nicotine-dependent female participant with concurrent OCD | NAc | Continuous |
OCD symptoms NA |
Craving NA |
Participant originally underwent DBS procedure for symptoms of OCD A decrease in both nicotine craving and cigarette consumption was also observed |
| Kuhn et al.83 | N = 10 | A retrospective, self-report, longitudinal study with nicotine-dependent participants | NAc | Continuous |
Dependence Active pre vs. post: 0.43 |
None | 3/10 participants quit smoking post-treatment |
|
| |||||||
| Cocaine: continuous stimulation session (total N = 1; one study) | |||||||
|
| |||||||
| Goncalves-Ferreira et al.84 | N = 1 | A longitudinal, crossover case study with a 36-year-old cocaine-dependent male participant | NAc | Continuous |
Craving NA |
Consumption NA |
Active DBS decreased craving and consumption of cocaine |
|
| |||||||
| Opioids: continuous active stimulation session (total N = 4; three studies) | |||||||
|
| |||||||
| Kuhn et al.85 | N = 2 | Case report with heroin-dependent participants | NAc | Continuous |
Craving NA |
Depressive symptoms NA |
DBS led to a decrease in opioid use and a significant decline in depressive symptoms in both participants |
| Valencia Alfonso et al.86 | N = 1 | Case report of a 47-year-old male with refractory heroin dependence | NAc | Continuous |
Consumption NA |
Desire to use NA |
A decrease in desire to use and consumption of heroin was observed with active DBS treatment |
| Zhou et al.87 | N = 1 | Case report with a heroin-dependent participant | NAc | Continuous |
Abstinence NA |
Relapse NA |
Patient became abstinent from heroin use for 5 years without relapse, and also decreased cigarette consumption significantly (from 40 to 10 CPD) |
rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; DBS, deep brain stimulation; dTMS, deep transcranial magnetic stimulation; iTBS, intermittent theta burst stimulation; cTBS, continuous theta burst stimulation; dTMS, deep transcranial magnetic stimulation; RMT, resting motor threshold; Hz, hertz; Tx, treatment; DLPFC, dorsolateral prefrontal cortex; MOC, motor cortex; IFG, inferior frontal gyrus; SFG, superior frontal gyrus; PFC, prefrontal cortex; MPFC, medial prefrontal cortex; FTP, fronto-temporal parietal area; SOA, supra-orbital area; CSOA, contralateral supra-orbital area; ACC, anterior cingulate cortex; NAc, nucleus accumbens; An,+, anodal; Ca−, cathodal; MDD, Major Depressive Disorder; SCZ, schizophrenia; OCD, obsessive compulsive disorder; mA, milliamp; CPD, cigarettes per day; NRT, nicotine replacement therapy; SDT, standard treatment; ↑, increase; ↓, decrease; NA, not assessed/not available.
METHODS
A comprehensive literature search (by A.C. and K.K.) was conducted using PubMed and Google Scholar. Articles published after 2000 in peer-reviewed academic journals were included. A combination of key search terms was used to locate the articles for this review, including: non-invasive brain stimulation, repetitive transcranial magnetic stimulation, rTMS, transcranial direct current stimulation, tDCS, deep brain stimulation, DBS, addiction, substance use disorder, abuse, dependence, alcohol, tobacco, smoking, nicotine, cocaine, cannabis, amphetamines, opioids, treatment, therapy, craving, and consumption. Inclusion criteria also consisted of participants who met DSM-IV criteria for substance abuse or dependence, or DSM-5 criteria for substance use disorders. Only studies whose primary and/or secondary outcomes were related to substance use outcomes (ie, consumption, abstinence, craving, and withdrawal) or drug cue-induced neurophysiological/functional imaging changes were included in this review. This process yielded 69 studies, of which nine were excluded14,20–27 that did not meet these criteria.
RESULTS
Repetitive Transcranial Magnetic Stimulation (rTMS)
Alcohol
To date, nine studies28–36 have investigated the potential efficacy of rTMS in the treatment of alcohol use disorder, with mixed results. Six studies28,32–36 examined the effects of multiple sessions of rTMS (10–20 sessions) targeting the dorsolateral prefrontal cortex (DLPFC) or medial prefrontal cortex (mPFC), using a randomized, sham controlled study design. Findings from all six studies demonstrated a significant decrease in alcohol-related cravings or consumption post-active rTMS treatment. However, three other studies29–31 found opposing results: rTMS to the DLPFC did not have significant effects on alcohol craving or consumption. Notably, these studies involved fewer total rTMS treatment sessions, which may explain the lack of efficacy in these trials (Table 1).
Tobacco
Eleven studies37–47 were conducted to investigate the use of rTMS on tobacco use disorder (nicotine dependence). Apart from Li et al.,37 all studies demonstrated positive effects of rTMS, via reduction in nicotine cravings and/or overall cigarette consumption post-active stimulation compared to baseline and sham data. Nine studies37–43,45,47 directed stimulation at either the left or the right DLPFC with a stimulation frequency of 1–20 Hz.
Rose et al.46 applied 1 and 10 Hz rTMS stimulation to the superior frontal gyrus and motor cortex (sham condition), which demonstrated differential effects after smoking cue presentation. In the neutral cue condition, 10 Hz stimulation reduced craving significantly compared to both 1 Hz and sham conditions. Notably, craving was significantly increased in the 10 Hz condition with the presentation of smoking cues.46 In contrast, Dinur Klein et al.44 applied 1 and 10 Hz stimulation to the lateral prefrontal cortex and insula, resulting in a significant decline in consumption in the 10 Hz condition only.
Cocaine
Four studies48–51 investigated the effects of rTMS on craving for cocaine in dependent adult participants. The number of treatment sessions ranged from 1 to 12, with a frequency range between 5 and 20 Hz. Three49–51 of these studies demonstrated positive results, with a significant reduction in craving for cocaine, with the exception of Bolloni et al.48 who found no significant change in craving compared to sham, although long-term reduction in consumption was significant when considering time as a factor.
Methamphetamine
Thus far, there have been only three studies investigating rTMS for methamphetamine (MA) dependence.52–54 Su et al.52 applied five sessions of 10 Hz rTMS to the left DLPFC. Results indicated a significant reduction in cravings for MA compared to sham stimulation. Conversely, Li et al.53 studied the effects of two sessions of rTMS in MA-dependent participants and demonstrated no significant impact of rTMS on craving or consumption of MA, compared to sham.53 A third study by Liu et al.54 studied the effects of five sessions of rTMS to the left or right DLPFC in both 1 and 10 Hz conditions compared to sham, with a significant reduction in cue-induced craving across all four stimulation conditions compared to sham.
Cannabis
A single study55 investigated the use of rTMS on cannabis craving in a crossover, sham-controlled study, using a single session of 10 Hz stimulation to the left DLPFC in cannabis-dependent participants. There were no significant reductions in craving scores between active and sham stimulation groups.55
Transcranial Direct Current Stimulation (tDCS)
Alcohol
Seven studies56–62 have been conducted examining the effects of tDCS as a possible treatment for alcohol use disorder. Of these studies, six56,57,59–62 have demonstrated positive effects of tDCS on alcohol craving and/or consumption. Notably, in a study by Da Silva et al,61 although craving was significantly attenuated in the active group, active tDCS resulted in a significant increase in relapse rates. Furthermore, one study60 showed that although tDCS had no significant effect on attenuation of alcohol cravings or acute consumption, abstinence rates improved significantly at 6-month follow-up. Finally, a study by Nakamura-Palacios et al.58 investigated the effects of neutral versus active cues on P3 amplitudes, with positive results. All studies varied on stimulation parameters, with no apparent pattern associated with either positive or non-significant results (Table 2).
Tobacco
Eight studies63–70 have examined the effects of tDCS on nicotine craving in dependent participants. All studies applied 2.0 mA stimulation for 15–30 minutes, except one by Falcone et al.,70 who used a stimulation intensity of 1.0 mA for 20 minutes. Five64–66,68,70 of the aforementioned studies demonstrated positive effects on nicotine cravings and/or consumption, with significant reductions seen across participants. In contrast, three additional studies63,67,69 observed no significant effect on craving or consumption of tobacco; two of these studies67,69 used a single stimulation session of tDCS, and the third63 used a sample that consisted of patients with a diagnosis of schizophrenia or schizoaffective disorder, which may have contributed to the negative findings, as this population tends to be more highly nicotine dependent than non-psychiatric populations.
Cocaine
Five studies71–75 have been conducted to understand the effects of tDCS on cocaine use disorder. Two studies73,74 examined the effects of tDCS on craving in this population using 2 mA stimulation for 20 minutes with positive results (Table 2). Gorini et al.75 applied contralateral stimulation of 1.5 mA to participants with cocaine dependence and controls, finding significant decreases in indices of risk-taking behavior. Finally, tDCS to the bilateral DLPFC reduced anterior cingulate activation71 and increased P3 physiological responses.72
Methamphetamine
Shahbabaie et al.76 conducted a study using one session of 2.0 mA tDCS to the right DLPFC over 20 minutes in MA-dependent participants. While a significant reduction in acute MA cravings was observed at rest, an increase in cue-induced MA cravings was reported 20 minutes post-treatment.
Opioids
A single study conducted by Wang et al.77 investigated the effects of tDCS on craving scores of 20 heroin-dependent participants. In contrast to other tDCS research in SUD’s, this bilateral stimulation was applied to the fronto–temporal–parietal area (FTP). Despite this difference, investigators observed a significant decline in heroin craving, which persisted with the presentation of heroin-related cues.77
Cannabis
A study by Boggio et al.78 investigated the use of tDCS as a treatment for cannabis use disorder. Twenty-five chronic cannabis users received 10 Hz stimulation to either the left or right DLPFC. Results from this study showed that stimulation of the right DLPFC, but not the left, was associated with a decline in cannabis-related craving.78
Deep Brain Stimulation (DBS)
Alcohol
Three studies79–81 investigated the effects of DBS on alcohol use disorder, applying active stimulation to the nucleus accumbens, showing a decrease in alcohol consumption or craving levels across studies (Table 3).
Tobacco
There have been two studies82,83 investigating the use of DBS on cigarette smoking and nicotine-dependence targeting the nucleus accumbens. Kuhn et al.83 conducted a study in nicotine craving and cigarette consumption. Of 10 nicotine-dependent participants, three quit smoking altogether, while the remaining seven participants demonstrated a significant decline in cravings and consumption. In another study,82 active DBS significantly reduced craving and consumption of cigarettes in a single subject who originally underwent DBS treatment for refractory OCD.
Cocaine
A single study84 investigated the effects of DBS on cocaine use in a single participant. Active DBS targeting the nucleus accumbens significantly reduced both craving and consumption of cocaine in this dependent participant.
Opioids
Recently, three studies85–87 have examined the effects of active DBS on opioid consumption or craving in heroin-dependent participants, targeting the nucleus accumbens. All three studies demonstrated a significant decline in consumption and/or cravings and an increase in abstinent participants.
DISCUSSION
This article reviews current research on available neuromodulation techniques (rTMS, tDCS, DBS), and their potential safety and efficacy across a broad range of SUDs. Findings were mixed across all three stimulation methods, which may in part, be due to the variation in stimulation parameters (ie, frequency, intensity, duration of treatment, brain regions stimulated), differences in SUDs, the severity of these SUDs between participants in each study, as well as heterogeneity in the populations studied, including the presence of co-occurring psychiatric disorders.
With respect to effects of brain stimulation observed between SUDs, commonalities between rTMS and tDCS emerged. Studies utilizing rTMS observed promising findings in trials conducted in nicotine or stimulant (cocaine, methamphetamine) dependent samples, with 10/1137–45,47 and 5/749–52,54 positive studies, respectively in domains of craving and/or consumption. Moreover, in alcohol-dependent participants, 6/9 studies28,32–36 suggested reductions in alcohol craving and/or consumption after active rTMS treatment. Effect sizes (Cohen’s d) for rTMS on alcohol (−0.07–2.99), tobacco (−0.40–4.42), methamphetamine (−0.20–2.78), and cocaine (−1.82–3.58) were promising but highly variable, consistent with the (methodological) heterogeneity of the published studies (Table 1). Similarly, tDCS demonstrated comparable efficacy to rTMS in the treatment of nicotine and stimulant dependence: 5/8 studies64–66,68,70 in nicotine dependence and 3/6 studies73,74,76 conducted in stimulant dependence (cocaine, methamphetamine) had positive study findings in measures of craving and/or consumption. This was further supported with medium to large effect sizes (Table 2). Specifically, in studies of tDCS on tobacco craving and consumption, there were variable effects (Cohen’s d’s −0.39–5.63), with similar variability observed for alcohol studies (Cohen’s d −0.18–4.25): Five of seven studies56,57,59,61,62 conducted in alcohol-dependent participants resulted in positive outcomes in domains of craving and/or consumption. Effect sizes for tDCS and cocaine (−0.90–3.15) were highly variable (N = 5), and for methamphetamine one study76 suggested a small effect size with acute treatment (d = 0.22). Finally, tDCS also showed promise in both cannabis and opioid use disorders, demonstrating positive results on craving after active treatment, as evidenced by a large effect for 1/1 study in opioids77 (d = 3.29), and 1/1 study in cannabis78 (d = −0.75–1.89).77,78
Finally, DBS trials were promising with positive results across all nine reviewed studies that used this technique in alcohol, nicotine, stimulant, and opioid-dependent samples. Nonetheless, available data with DBS is limited to case series so the ability to calculate resultant effect sizes is limited (Table 3), and further research is required to validate these findings, using larger sample sizes. Sample sizes of the DBS studies are very low (ranging 1–10; averaging 3.3 per study). These studies were primarily case series, and caution should be used when interpreting this data.
Findings Based on Stimulation Parameters
Stimulation parameters (ie, frequency, duration, brain region, and sample size/demographics) between and within the three brain stimulation methods also warrants comment. First, both rTMS and tDCS appear to be most effective when delivered using repeated versus single administration sessions, and these effects may vary as a function of treatment duration (including number of pulses, inter-stimulus intervals, and frequency of stimulation sessions). Further, the tDCS studies across the various SUDs, demonstrated that longer duration of treatment sessions (>10 minutes) had the most promising results. Moreover, the majority of positive studies utilized high-frequency (HF; >10 Hz) rTMS stimulation parameters (>10 Hz) in SUDs, while those using low-frequency (LF; <10 Hz) rTMS stimulation were associated with less promising outcomes.
Second, the effect of bilateral versus unilateral stimulation, as well as which brain region was targeted, appeared to have differential and distinctive effects. In rTMS and tDCS, nearly all studies conducted in treatment of SUDs targeted the left or right DLPFC. Exceptions to these brain targets for stimulation included two rTMS studies targeting the mPFC.32,33 Furthermore, Dinur-Klein et al.44 used rTMS to target the lateral prefrontal cortex and insula, while Rose et al.46 targeted the motor cortex and superior frontal gyrus. Two tDCS studies68,77 targeted the bilateral fronto–temporal–parietal area (FTP), with positive findings post-treatment, while a third study targeted the IFG.57 Despite variation in brain region targets, these studies all had positive outcomes for drug craving and/or consumption. However, based on both the number of studies conducted and the magnitude of effect sizes calculated, specifically targeting the right (vs. left) DLPFC was associated with the most promising treatment outcomes. With regards to DBS, all studies to date have targeted the nucleus accumbens (NAc), which is also an area of the brain associated with reward pathway in SUDs. Therefore, the importance of targeting regions of the brain relating to the reward pathway is supported with the most efficacious results on cravings/consumptions. Nonetheless, more systematic and comparative studies are needed to determine dose effects of stimulus intensity, duration, and laterality. Moreover, few of the studies have followed subjects in the long-term (eg, 3–6 months post-treatment) to determine durability of neuromodulation effects. Additionally, comparative studies between brain regions and between treatment modalities have generally not been performed in a rigorous manner, and warrant further research.
Finally, since most of the published studies were preliminary (with total sample sizes <40), further research in larger patient samples using randomized, sham-controlled designs are necessary to validate these brain stimulation methods for the treatment of SUDs. Notably, four studies involved the use of participants with a comorbid psychiatric condition.32,45,63,82 For instance, Wing et al.45 used rTMS in a sample consisting of 15 participants with a primary diagnosis of schizophrenia. Despite this comorbidity, cravings for nicotine decreased significantly across participants. As this vulnerable population is 2–3 times more likely to smoke cigarettes and tend to be more highly nicotine dependent, rTMS may be a promising treatment option for patients with schizophrenia and comorbid tobacco use disorder.45 Another study by Smith63 used tDCS in a sample of patients with schizophrenia and concurrent nicotine dependence, but found that five sessions of stimulation had no significant effect on craving or consumption in this group. Furthermore, a study by Ceccanti et al.32 studied participants with co-occurring dysthymic disorder and alcohol use disorder, with positive results indicating a significant decline in consumption after ten sessions of active rTMS. Finally, Mantione82 implemented DBS in a woman with treatment refractory OCD. While this effect was unintended, the participant saw a significant decline in craving and consumption of cigarettes after DBS stimulation. Therefore, co-occurring disorders (ie, schizophrenia, mood, and anxiety) may be important determinants of the efficacy of these stimulation techniques. Further studies are warranted to understand the potential differential effects of brain stimulation in co-occurring disorders.
Other Neuromodulation Modalities
While other neuromodulation methods such as Electroconvulsive therapy (ECT), Magnetic Seizure Therapy (MST), and Transcranial Electric Stimulation (TES) are used, studies examining their effects on SUDs in human subjects are extremely limited. A single study88 has investigated the effects of ECT on methamphetamine use disorders in a single participant, with positive results on withdrawal induced delirium and craving scores.88
Directions for Future Research
While research on brain stimulation methods for the treatment of SUDs has shown considerable promise,3 are preliminary and further research is needed. Future research should aim to identify optimal stimulation parameters for all three stimulation methods (rTMS, tDCS, DBS) with specific regard to duration and total number of stimulation treatments, targeted brain region, stimulation frequency or intensity, and proximity between treatments (ie, four treatments over 2 days versus four treatments over 4 days). Additionally, investigations to understand the lasting effects of each stimulation treatment method and the potential necessity of maintenance treatment sessions are critical for prolonging treatment effects. Studies examining the effects of brain stimulation while participants are undergoing fMRI scanning may be beneficial to understand the changes in targeted brain regions during stimulation sessions.
Additionally, studies comparing brain stimulation methods in SUDs may be beneficial to understand differences in efficacy between techniques. Furthermore, studies investigating patients with polysubstance use disorders and co-occurring psychiatric disorders are warranted, as some 7.9 million individuals in 2015 had co-occurring SUDs and mental illness in the U.S.1 Moreover, studies investigating the effects of brain stimulation in participants with polysubstance use may also be beneficial, as the use of only one substance is relatively uncommon. Finally, studies to understand the potential benefits of concurrent therapies (pharmacological, behavioral) used in combination with brain stimulation may increase a participant’s chances of becoming abstinent. For example, Trojak41 used rTMS in combination with nicotine replacement therapy (NRT) in the form of a patch, with positive results: 16 of 37 participants became abstinent from cigarettes after treatment.
We believe this article is a unique contribution to the literature on neuromodulation and SUDs for the following reasons: (1) We calculate effect sizes of individual studies across modalities and SUDs in order to identify areas of promise and gaps in the field; (2) We attempt to provide a systematic review of this topic, with clear inclusion and exclusion criteria for the reviewed studies, and; (3) we included several new studies on brain stimulation and SUDs which have been published since the review by Salling and Martinez.4
Limitations of the Current Review
There are certain limitations of the current review. First, the total number of studies examining the effects of brain stimulation techniques within each SUD is highly variable. Accordingly, the effectiveness of different neuromodulation techniques on certain SUDs should be considered preliminary. Second, there are several gaps in the literature that are worth noting, including: the lack of systematic study of optimal brain stimulation parameters in SUDs, lack of combination studies with approved pharmacological, and behavioral treatments, and a paucity of studies in co-morbid psychiatric and medical disorders.
CONCLUSIONS
In conclusion, the use of neuromodulation techniques (rTMS, tDCS, DBS) may be a promising treatment option for SUDs. Nonetheless, there is a need for further empirical data in this emerging field, which is particularly important given the high rates for relapse and polysubstance use in this vulnerable population.
Acknowledgments
This work was supported in part by Canadian Institutes of Health Research (CIHR) operating grant MOP#115145, the Chair in Addiction Psychiatry at the University of Toronto, and NIDA grant 1R21-DA-043949 to Dr. George. We gratefully acknowledge the comments of Mera S. Barr, PhD, and Dr. Sarah S. Dermody on an earlier version of the manuscript.
References
- 1.Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. U.S. Department of Health and Human Services (HHS); Washington, DC: 2016. [PubMed] [Google Scholar]
- 2.World Drug Report 2014. Vienna: United Nations Publication; 2014. [Google Scholar]
- 3.Wing VC, Barr MS, Wass CE, et al. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 2013;6:221–230. doi: 10.1016/j.brs.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Salling MC, Martinez D. Brain stimulation in addiction. Neuropsychopharmacology. 2016;41:2798–2809. doi: 10.1038/npp.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barr MS, Farzan F, Wing VC, et al. Repetitive transcranial magnetic stimulation and drug addiction. Int Rev Psychiatry (Abingdon, England) 2011;23:454–466. doi: 10.3109/09540261.2011.618827. [DOI] [PubMed] [Google Scholar]
- 6.Bellamoli E, Manganotti P, Schwartz RP, et al. RTMS in the treatment of drug addiction: An update about human studies. Behav Neurol. 2014;2014:815215. doi: 10.1155/2014/815215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barr MS, Farzan F, Rusjan PM, et al. Potentiation of gamma oscillatory activity through repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex. Neuropsychopharmacology. 2009;34:2359–2367. doi: 10.1038/npp.2009.79. [DOI] [PubMed] [Google Scholar]
- 8.Barr MS, Farzan F, Rajji TK, et al. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry. 2013;73:510–517. doi: 10.1016/j.biopsych.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 9.de Jesus DR, Favalli GP, Hoppenbrouwers SS, et al. Determining optimal rTMS parameters through changes in cortical inhibition. Clin Neurophysiol. 2014;125:755–762. doi: 10.1016/j.clinph.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Daskalakis ZJ, Moller B, Christensen BK, et al. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174:403–412. doi: 10.1007/s00221-006-0472-0. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald PB, Brown TL, Daskalakis ZJ. The application of transcranial magnetic stimulation in psychiatry and neurosciences research. Acta Psychiatr Scand. 2002;105:324–340. doi: 10.1034/j.1600-0447.2002.1r179.x. [DOI] [PubMed] [Google Scholar]
- 12.Huang YZ, Edwards MJ, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 13.Bulteau S, Sebille V, Fayet G, et al. Efficacy of intermittent Theta Burst Stimulation (iTBS) and 10-Hz high-frequency repetitive transcranial magnetic stimulation (rTMS) in treatment-resistant unipolar depression: Study protocol for a randomised controlled trial. Trials. 2017;18:17. doi: 10.1186/s13063-016-1764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanlon CA, Dowdle LT, Austelle CW, et al. What goes up, can come down: Novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Res. 2015;1628:199–209. doi: 10.1016/j.brainres.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods AJ, Antal A, Bikson M, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127:1031–1048. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Kronberg G, Bikson M. Electrode assembly design for transcranial direct current stimulation: A FEM modeling study. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:891–895. doi: 10.1109/EMBC.2012.6346075. [DOI] [PubMed] [Google Scholar]
- 18.Utz KS, Dimova V, Oppenlander K, et al. Electrified minds: Transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology—A review of current data and future implications. Neuropsychologia. 2010;48:2789–2810. doi: 10.1016/j.neuropsychologia.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald PB, Segrave RA. Deep brain stimulation in mental health: Review of evidence for clinical efficacy. Aust N Z J Psychiatry. 2015;49:979–993. doi: 10.1177/0004867415598011. [DOI] [PubMed] [Google Scholar]
- 20.Contia CL. Dorsolateral prefrontal cortex activity and neuromodulation in crack-cocaine dependents during early abstinence. J Neurol Neurophysiol. 2016;7:374. [Google Scholar]
- 21.Politi E, Fauci E, Santoro A, et al. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am J Addict. 2008;17:345–346. doi: 10.1080/10550490802139283. [DOI] [PubMed] [Google Scholar]
- 22.Del Felice A, Bellamoli E, Formaggio E, et al. Neurophysiological, psychological and behavioural correlates of rTMS treatment in alcohol dependence. Drug Alcohol Depend. 2016;158:147–153. doi: 10.1016/j.drugalcdep.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 23.De Ridder D, Vanneste S, Kovacs S, et al. Transient alcohol craving suppression by rTMS of dorsal anterior cingulate: An fMRI and LORETA EEG study. Neurosci Lett. 2011;496:5–10. doi: 10.1016/j.neulet.2011.03.074. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Ko JH, Strafella AP, et al. Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc Natl Acad Sci U S A. 2013;110:4422–4427. doi: 10.1073/pnas.1212185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen JM, van Wingen G, van den Brink W, et al. Resting state connectivity in alcohol dependent patients and the effect of repetitive transcranial magnetic stimulation. Eur Neuropsychopharmacol. 2015;25:2230–2239. doi: 10.1016/j.euroneuro.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Heldmann M, Berding G, Voges J, et al. Deep brain stimulation of nucleus accumbens region in alcoholism affects reward processing. PLoS ONE. 2012;7:e36572. doi: 10.1371/journal.pone.0036572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shariatirad S, Vaziri A, Hassani-Abharian P, et al. Cumulative and booster effects of tdcs sessions on drug cravings, lapse, and cognitive impairment in methamphetamine use disorder: A case study report. Am J Addict. 2016;25:264–266. doi: 10.1111/ajad.12373. [DOI] [PubMed] [Google Scholar]
- 28.Addolorato G, Antonelli M, Cocciolillo F, et al. Deep transcranial magnetic stimulation of the dorsolateral prefrontal cortex in alcohol use disorder patients: Effects on dopamine transporter availability and alcohol intake. Eur Neuropsychopharmacol. 2017;27:450–461. doi: 10.1016/j.euroneuro.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Herremans SC, Vanderhasselt MA, De Raedt R, et al. Reduced intra-individual reaction time variability during a Go-NoGo task in detoxified alcohol-dependent patients after one right-sided dorsolateral prefrontal HF-rTMS session. Alcohol Alcohol (Oxford, Oxfordshire) 2013;48:552–557. doi: 10.1093/alcalc/agt054. [DOI] [PubMed] [Google Scholar]
- 30.Herremans SC, Baeken C, Vanderbruggen N, et al. No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: Results of a naturalistic study. Drug Alcohol Depend. 2012;120:209–213. doi: 10.1016/j.drugalcdep.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Hoppner J, Broese T, Wendler L, et al. Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. World J Biol Psychiatry. 2011;12:57–62. doi: 10.3109/15622975.2011.598383. [DOI] [PubMed] [Google Scholar]
- 32.Ceccanti M, Inghilleri M, Attilia ML, et al. Deep TMS on alcoholics: Effects on cortisolemia and dopamine pathway modulation. A pilot study. Can J Physiol Pharmacol. 2015;93:283–290. doi: 10.1139/cjpp-2014-0188. [DOI] [PubMed] [Google Scholar]
- 33.Girardi P, Rapinesi C, Chiarotti F, et al. Add-on deep transcranial magnetic stimulation (dTMS) in patients with dysthymic disorder comorbid with alcohol use disorder: A comparison with standard treatment. World J Biol Psychiatry. 2015;16:66–73. doi: 10.3109/15622975.2014.925583. [DOI] [PubMed] [Google Scholar]
- 34.Herremans SC, Van Schuerbeek P, De Raedt R, et al. The impact of accelerated right prefrontal high-frequency repetitive transcranial magnetic stimulation (rTMS) on cue-reactivity: An fMRI study on craving in recently detoxified alcohol-dependent patients. PLoS ONE. 2015;10:e0136182. doi: 10.1371/journal.pone.0136182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mishra BR, Praharaj SK, Katshu MZ, et al. Comparison of anticraving efficacy of right and left repetitive transcranial magnetic stimulation in alcohol dependence: A randomized double-blind study. J Neuropsychiatry Clin Neurosci. 2015;27:e54–e59. doi: 10.1176/appi.neuropsych.13010013. [DOI] [PubMed] [Google Scholar]
- 36.Mishra BR, Nizamie SH, Das B, et al. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: A sham-controlled study. Addiction (Abingdon, England) 2010;105:49–55. doi: 10.1111/j.1360-0443.2009.02777.x. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Du L, Sahlem GL, et al. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex reduces resting-state insula activity and modulates functional connectivity of the orbitofrontal cortex in cigarette smokers. Drug Alcohol Depend. 2017;174:98–105. doi: 10.1016/j.drugalcdep.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pripfl J, Tomova L, Riecansky I, et al. Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul. 2014;7:226–233. doi: 10.1016/j.brs.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Hartwell KJ, Owens M, et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry. 2013;73:714–720. doi: 10.1016/j.biopsych.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eichhammer P, Johann M, Kharraz A, et al. High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J Clin Psychiatry. 2003;64:951–953. doi: 10.4088/jcp.v64n0815. [DOI] [PubMed] [Google Scholar]
- 41.Trojak B, Meille V, Achab S, et al. Transcranial magnetic stimulation combined with nicotine replacement therapy for smoking cessation: A randomized controlled trial. Brain Stimul. 2015;8:1168–1174. doi: 10.1016/j.brs.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Dieler AC, Dresler T, Joachim K, et al. Can intermittent theta burst stimulation as add-on to psychotherapy improve nicotine abstinence? Results from a pilot study. Eur Addict Res. 2014;20:248–253. doi: 10.1159/000357941. [DOI] [PubMed] [Google Scholar]
- 43.Prikryl R, Ustohal L, Kucerova HP, et al. Repetitive transcranial magnetic stimulation reduces cigarette consumption in schizophrenia patients. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;49:30–35. doi: 10.1016/j.pnpbp.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Dinur-Klein L, Dannon P, Hadar A, et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: A prospective, randomized controlled trial. Biol Psychiatry. 2014;76:742–749. doi: 10.1016/j.biopsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Wing VC, Bacher I, Wu BS, et al. High frequency repetitive transcranial magnetic stimulation reduces tobacco craving in schizophrenia. Schizophr Res. 2012;139:264–266. doi: 10.1016/j.schres.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Rose JE, McClernon FJ, Froeliger B, et al. Repetitive transcranial magnetic stimulation of the superior frontal gyrus modulates craving for cigarettes. Biol Psychiatry. 2011;70:794–799. doi: 10.1016/j.biopsych.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 47.Amiaz R, Levy D, Vainiger D, et al. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction (Abingdon, England) 2009;104:653–660. doi: 10.1111/j.1360-0443.2008.02448.x. [DOI] [PubMed] [Google Scholar]
- 48.Bolloni C, Panella R, Pedetti M, et al. Bilateral transcranial magnetic stimulation of the prefrontal cortex reduces cocaine intake: A pilot study. Front Psychiatry. 2016;7:133. doi: 10.3389/fpsyt.2016.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rapinesi C, Del Casale A, Di Pietro S, et al. Add-on high frequency deep transcranial magnetic stimulation (dTMS) to bilateral prefrontal cortex reduces cocaine craving in patients with cocaine use disorder. Neurosci Lett. 2016;629:43–47. doi: 10.1016/j.neulet.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 50.Terraneo A, Leggio L, Saladini M, et al. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. Eur Neuropsychopharmacol. 2016;26:37–44. doi: 10.1016/j.euroneuro.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camprodon JA, Martinez-Raga J, Alonso-Alonso M, et al. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 2007;86:91–94. doi: 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Su H, Zhong N, Gan H, et al. High frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex for methamphetamine use disorders: A randomised clinical trial. Drug Alcohol Depend. 2017;175:84–91. doi: 10.1016/j.drugalcdep.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Malcolm RJ, Huebner K, et al. Low frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex transiently increases cue-induced craving for methamphetamine: A preliminary study. Drug Alcohol Depend. 2013;133:641–646. doi: 10.1016/j.drugalcdep.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Q, Shen Y, Cao X, et al. Either at left or right, both high and low frequency rTMS of dorsolateral prefrontal cortex decreases cue induced craving for methamphetamine. Am J Addict. 2017;26:776–779. doi: 10.1111/ajad.12638. [DOI] [PubMed] [Google Scholar]
- 55.Sahlem GL, Baker NL, George MS, et al. Repetitive transcranial magnetic stimulation (rTMS) administration to heavy cannabis users. Am J Drug Alcohol Abuse. 2017;44:47–55. doi: 10.1080/00952990.2017.1355920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wietschorke K, Lippold J, Jacob C, et al. Transcranial direct current stimulation of the prefrontal cortex reduces cue-reactivity in alcohol-dependent patients. J Neural Transm (Vienna, Austria: 1996) 2016;123:1173–1178. doi: 10.1007/s00702-016-1541-6. [DOI] [PubMed] [Google Scholar]
- 57.Den Uyl TE, Gladwin TE, Wiers RW. Transcranial direct current stimulation, implicit alcohol associations and craving. Biol Psychol. 2015;105:37–42. doi: 10.1016/j.biopsycho.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura-Palacios EM, de Almeida Benevides MC, da Penha Zago-Gomes M, et al. Auditory event-related potentials (P3) and cognitive changes induced by frontal direct current stimulation in alcoholics according to Lesch alcoholism typology. Int J Neuropsychopharmacol. 2012;15:601–616. doi: 10.1017/S1461145711001040. [DOI] [PubMed] [Google Scholar]
- 59.Den Uyl TE, Gladwin TE, Rinck M, et al. A clinical trial with combined transcranial direct current stimulation and alcohol approach bias retraining. Addict Biol. 2016;22:1632–1640. doi: 10.1111/adb.12463. [DOI] [PubMed] [Google Scholar]
- 60.Klauss J, Penido Pinheiro LC, Silva Merlo BL, et al. A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int J Neuropsychopharmacol. 2014;17:1793–1803. doi: 10.1017/S1461145714000984. [DOI] [PubMed] [Google Scholar]
- 61.Da Silva MC, Conti CL, Klauss J, et al. Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J Physiol Paris. 2013;107:493–502. doi: 10.1016/j.jphysparis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Boggio PS, Sultani N, Fecteau S, et al. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: A double-blind, sham-controlled study. Drug Alcohol Depend. 2008;92:55–60. doi: 10.1016/j.drugalcdep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 63.Smith RC, Boules S, Mattiuz S, et al. Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: A randomized controlled study. Schizophr Res. 2015;168:260–266. doi: 10.1016/j.schres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 64.Fecteau S, Agosta S, Hone-Blanchet A, et al. Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: A preliminary study. Drug Alcohol Depend. 2014;140:78–84. doi: 10.1016/j.drugalcdep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boggio PS, Liguori P, Sultani N, et al. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett. 2009;463:82–86. doi: 10.1016/j.neulet.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 66.Fregni F, Liguori P, Fecteau S, et al. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: A randomized, sham-controlled study. J Clin Psychiatry. 2008;69:32–40. doi: 10.4088/jcp.v69n0105. [DOI] [PubMed] [Google Scholar]
- 67.Xu J, Fregni F, Brody AL, et al. Transcranial direct current stimulation reduces negative affect but not cigarette craving in overnight abstinent smokers. Front Psychiatry. 2013;4:112. doi: 10.3389/fpsyt.2013.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meng Z, Liu C, Yu C, et al. Transcranial direct current stimulation of the frontal-parietal-temporal area attenuates smoking behavior. J Psychiatric Res. 2014;54:19–25. doi: 10.1016/j.jpsychires.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Kroczek AM, Haussinger FB, Rohe T, et al. Effects of transcranial direct current stimulation on craving, heart-rate variability and prefrontal hemodynamics during smoking cue exposure. Drug Alcohol Depend. 2016;168:123–127. doi: 10.1016/j.drugalcdep.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 70.Falcone M, Bernardo L, Ashare RL, et al. Transcranial direct current brain stimulation increases ability to resist smoking. Brain Stimul. 2016;9:191–196. doi: 10.1016/j.brs.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conti CL, Nakamura-Palacios EM. Bilateral transcranial direct current stimulation over dorsolateral prefrontal cortex changes the drug-cued reactivity in the anterior cingulate cortex of crack-cocaine addicts. Brain Stimul. 2014;7:130–132. doi: 10.1016/j.brs.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 72.Conti CL, Moscon JA, Fregni F, et al. Cognitive related electrophysiological changes induced by non-invasive cortical electrical stimulation in crack-cocaine addiction. Int J Neuropsychopharmacol. 2014;17:1465–1475. doi: 10.1017/S1461145714000522. [DOI] [PubMed] [Google Scholar]
- 73.De Almeida Ramos R, Taiar I, Trevizol AP, et al. Effect of a ten-day prefrontal transcranial direct current stimulation protocol for crack craving: A proof-of-concept trial. J ECT. 2016;32:e8–e9. doi: 10.1097/YCT.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 74.Batista EK, Klauss J, Fregni F, et al. A randomized placebo-controlled trial of targeted prefrontal cortex modulation with bilateral tDCS in patients with crack-cocaine dependence. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyv066. pii: pyv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorini A, Lucchiari C, Russell-Edu W, et al. Modulation of risky choices in recently abstinent dependent cocaine users: A trans-cranial direct-current stimulation study. Front Human Neurosci. 2014;8:661. doi: 10.3389/fnhum.2014.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shahbabaie A, Golesorkhi M, Zamanian B, et al. State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. Int J Neuropsychopharmacol. 2014;17:1591–1598. doi: 10.1017/S1461145714000686. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, Shen Y, Cao X, et al. Transcranial direct current stimulation of the frontal-parietal-temporal area attenuates cue-induced craving for heroin. J Psychiatric Res. 2016;79:1–3. doi: 10.1016/j.jpsychires.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 78.Boggio PS, Zaghi S, Villani AB, et al. Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC) Drug Alcohol Depend. 2010;112:220–225. doi: 10.1016/j.drugalcdep.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 79.Kuhn J, Grundler TO, Bauer R, et al. Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addict Biol. 2011;16:620–623. doi: 10.1111/j.1369-1600.2011.00337.x. [DOI] [PubMed] [Google Scholar]
- 80.Muller UJ, Sturm V, Voges J, et al. Nucleus accumbens deep brain stimulation for alcohol addiction—Safety and clinical long-term results of a pilot trial. Pharmacopsychiatry. 2016;49:170–173. doi: 10.1055/s-0042-104507. [DOI] [PubMed] [Google Scholar]
- 81.Voges J, Muller U, Bogerts B, et al. Deep brain stimulation surgery for alcohol addiction. World Neurosurg. 2013;80:S28.e21–S28.e31. doi: 10.1016/j.wneu.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 82.Mantione M, Nieman D, Figee M, et al. Cognitive effects of deep brain stimulation in patients with obsessive-compulsive disorder. J Psychiatry Neurosci: JPN. 2015;40:378–386. doi: 10.1503/jpn.140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuhn J, Bauer R, Pohl S, et al. Observations on unaided smoking cessation after deep brain stimulation of the nucleus accumbens. Eur Addict Res. 2009;15:196–201. doi: 10.1159/000228930. [DOI] [PubMed] [Google Scholar]
- 84.Goncalves-Ferreira A, do Couto FS, Rainha Campos A, et al. Deep brain stimulation for refractory cocaine dependence. Biol Psychiatry. 2016;79:e87–e89. doi: 10.1016/j.biopsych.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 85.Kuhn J, Moller M, Treppmann JF, et al. Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction. Mol Psychiatry. 2014;19:145–146. doi: 10.1038/mp.2012.196. [DOI] [PubMed] [Google Scholar]
- 86.Valencia-Alfonso CE, Luigjes J, Smolders R, et al. Effective deep brain stimulation in heroin addiction: A case report with complementary intracranial electroencephalogram. Biol Psychiatry. 2012;71:e35–e37. doi: 10.1016/j.biopsych.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 87.Zhou H, Xu J, Jiang J. Deep brain stimulation of nucleus accumbens on heroin-seeking behaviors: A case report. Biol Psychiatry. 2011;69:e41–e42. doi: 10.1016/j.biopsych.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 88.Ahmadi J, Ekramzadeh S, Pridmore S. Remission of methamphetamine-induced withdrawal delirium and craving after electroconvulsive therapy. Iran J Psychiatry Behav Sci. 2015;9:e1793. doi: 10.17795/ijpbs-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]