Abstract
Background
Evidence on the role of intravenous iron (IVI) supplementation after colorectal cancer (CRC) surgery is rather scant. This study was aimed at assessing the benefit of post-operative IVI administration after elective CRC surgery at our institution.
Materials and methods
This was a single-centre, retrospective observational study including all patients who underwent CRC surgery during 2014. Anaemia was defined as a haemoglobin (Hb) <13 g/dL, regardless of gender. Anaemic patients received 200 mg IVI up to three times a week to cover iron deficiency (IVI group). Those who did not receive IVI were placed on standard care (NIVI group). The primary outcome was the proportion of anaemic patients on post-operative day (POD)1 and POD30. Secondary outcomes included Hb changes from POD1 to POD30, transfusion requirements and complication rates.
Results
Of the 159 patients studied, 139 (87%) presented with anaemia: 47 (34%) of these received post-operative IVI and 92 (66%) did not. Patients in the IVI group had lower POD1 Hb levels compared to those in the NIVI group (p=0.001). On POD30, only 103 had their Hb measured (34 IVI, 69 NIVI). Anaemia was more prevalent and more severe among the patients in the IVI group (p=0.027), despite their greater increment in Hb (2.0±1.5 g/dL vs 1.1±1.2 g/dL; p=0.001). Eleven patients needed post-operative transfusions (7 IVI, 4 NIVI; p=0.044). There were no differences in post-operative complication rates between the groups. No IVI-related adverse events were recorded
Discussion
Compared with standard care, post-operative IVI administration to anaemic patients improved the recovery of Hb levels at POD30, without increasing post-operative complications.
Keywords: colorectal cancer, post-operative anaemia, intravenous iron
Introduction
Post-operative anaemia following resection of colorectal cancer (CRC) results from a variety of factors but is heavily influenced by pre-operative haemoglobin (Hb) levels and surgical blood loss and is further aggravated by the negative effects of surgery-induced inflammation on erythropoiesis1,2.
Pre-operative anaemia in CRC is a common phenomenon, mainly as a consequence of chronic blood loss1. Some studies have assessed the efficacy of intravenous iron (IVI) administration for treating CRC-associated pre-operative anaemia3–7. However, the evidence of the role of IVI supplementation after CRC surgery is rather scant8,9, thus limiting recommendations by guidelines until its benefits are confirmed10,11. In contrast, guidelines do not recommend oral iron administration during the immediate post-operative period due to lack of efficacy10,12,13.
The implementation of a CRC fast diagnosis programme in 2012 in our hospital provided the opportunity to assess patients’ status pre-operatively and to initiate treatment of anaemia if indicated7. A multidisciplinary team for the management of peri-operative anaemia in CRC surgery later included the recommendation to administer IVI post-operatively to anaemic patients, to fulfil the requirements of the first pillar of Patient Blood Management. However, whether this strategy further improves post-operative patients’ outcomes at our centre has never been studied. The aim of the present study was, therefore, to assess the prevalence and severity of post-operative anaemia, adherence to the anaemia management, and the benefit from post-operative IVI administration after elective CRC surgery at our institution.
Methods
Design
We performed a single-centre, retrospective observational study retrieving data from the medical records of all patients who underwent elective CRC surgery during 2014.
The study protocol was approved by an independent Ethics Committee (CEIC Corporació Sanitaria Parc Taulí, Sabadell, Spain), which waived the requirement for written informed consent since only non-identifiable, disaggregated data were used, which maintained confidentiality.
The study population included patients aged 18 years or over, who were diagnosed with colorectal adenocarcinoma and underwent elective surgery with curative purposes following the CRC fast diagnosis programme. Patients who underwent emergency or palliative surgery were excluded.
Outcomes
The primary end-point was the prevalence and severity of anaemia on post-operative day 1 (POD1) and 30 (POD30). Secondary study end-points included changes in haemoglobin (Hb) concentration from the day of surgery (DOS) to POD30, the proportion of patients who received post-operative IVI treatment, post-operative red blood cell (RBC) transfusion requirements, and post-operative complication rates.
Data collection and definitions
A set of medical and laboratory data was retrieved from medical records, including gender, age, risk classification (according to the American Society of Anesthesiologists) and type of surgery, Hb level at diagnosis, DOS, POD1 and POD30, prescription of oral iron or IVI, RBC transfusion rate (%) and index (units per patient), post-operative complications (e.g., haemorrhage and infection) and IVI-related adverse events.
Anaemia was defined as Hb <13g/dL regardless of gender14 and was categorised according to the World Health Organization classification as mild (Hb 11–12.9 g/dL), moderate (Hb 8–10.9 g/dL) or severe (Hb <8 g/dL)15.
Post-operative iron supplementation
According to our institutional protocol, anaemic patients should receive post-operative IVI infusions. The cumulative IVI dose (mg) was calculated according to the formula:
For patients who had not received IVI pre-operatively, 500 mg IVI were added for deposits. Iron sucrose (different brand names) was given at doses of 200 mg in 100 mL saline over 30–60 min, up to three times a week during hospitalisation. Patients who did not receive the total IVI calculated dose during hospitalisation, were given oral iron on discharge (generally 100 mg elemental iron per day). Those who did not receive IVI were placed on standard care (oral iron or no iron).
Transfusion protocol
According to transfusion criteria at our centre, patients are given a RBC transfusion when: (i) their Hb levels are <7 g/dL; (ii) their Hb levels are 7–9 g/dL and they have haemodynamic instability or decompensated cardiovascular disease; or (iii) their Hb levels are >9 g/dL and they have acute haemorrhage.
Statistics
Standard descriptive statistics were used to summarise the study findings. Categorical variables were described by numbers of patients and percentages, and continuous variables by means and standard deviations. The numbers and percentages of missing values for analytical measures are also provided. Differences in outcome variables between anaemic patients who received post-operative IVI and those who did not were assessed by Pearson’s chi-square test for categorical variables and Student’s t-test for continuous variables. A two-sided p-value <0.05 was considered statistically significant.
Results
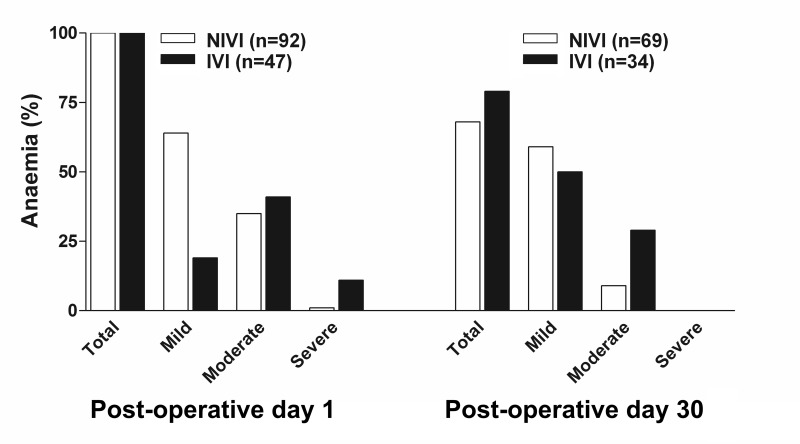
Between January and December 2014, 159 patients underwent elective surgery following the CRC fast diagnosis programme. On POD1, 139 (87%) out of the 159 presented with anaemia (68 mild, 65 moderate, 6 severe), and were considered as the study population. Of these, 47 (34%) received post-operative IVI according to the hospital’s protocol (467±337 mg/patient) (IVI group) and 92 (66%) did not (NIVI group). As presented in Table I, there were no differences in clinical characteristics between the two groups of anaemic patients, except for lower DOS and POD1 Hb levels in patients in the IVI group, among whom moderate-to-severe post-operative anaemia was also more frequent (Figure 1; p=0.001). Compared to those in the NIVI group, patients in the IVI group also more frequently presented with cancer localization in the right colon (28 vs 47%, respectively: p=0.038), underwent open laparotomy surgery (32 vs 51%, respectively; p=0.028), and anaemia at diagnosis (60 vs 83%, respectively; p=0.01), though there were not significant differences in the proportion of patients who received pre-operative IVI (47 vs 60%, respectively; p=NS),
Table I.
Clinical and laboratory data of the patients who underwent colorectal cancer surgery and were anaemic on post-operative day 1.
| IVI group (n=47) | NIVI group (n=92) | p | |
|---|---|---|---|
| Male gender, n (%) | 31 (66) | 57 (62) | 0.712 |
|
| |||
| Age, years | 72±10.2 | 69±11 | 0.094 |
|
| |||
| ASA classification, n (%) | |||
| I–II | 32 (68) | 71 (77) | 0.672 |
| III–IV | 15 (32) | 21 (23) | |
|
| |||
| Type of surgery, n (%) | 0.290 | ||
| Right hemicolectomy | 22 (47) | 26 (28) | |
| Left hemicolectomy | 5 (11) | 9 (10) | |
| Sigmoidectomy | 12 (25) | 28 (30) | |
| Rectum resection | 6 (13) | 19 (21) | |
| Miles | 2 (4) | 9 (10) | |
| Subtotal colectomy | 0 (0) | 1 (1) | |
|
| |||
| Laparoscopic surgery, n (%) | 23 (49) | 63 (68) | 0.028 |
|
| |||
| Hb DOS, g/dL | 11.2±1.3 | 12.3±1.4 | 0.001 |
|
| |||
| Hb POD1, g/dL | 9.9±1.4 | 11.3±1.2 | 0.001 |
|
| |||
| Hb POD30, g/dL (n) | 11.9±1.5 (34) | 12.4±1.2 (69) | 0.038 |
|
| |||
| PostOP RBCT rate, n (%) | 7 (15) | 4 (4) | 0.044 |
|
| |||
| PostOP RBCT index, units/patient | 1.6±0.5 | 1.8±1.0 | 0.695 |
|
| |||
| Infections, n (%) | |||
|
| |||
| Surgical wound infection | 4 (8.5) | 3 (3.3) | 0.096 |
|
| |||
| Suture dehiscence and intra-abdominal abscess | 7 (14.9) | 8 (8.7) | 0.266 |
|
| |||
| Urinary tract infection | 2 (4.3) | 3 (3.3) | 1.000 |
|
| |||
| Respiratory infection | 3 (6.4) | 1 (1.1) | 0.112 |
|
| |||
| Haemorrhagic complications, n (%) | |||
|
| |||
| Surgical wound haematoma | 0 (0) | 6 (6.5) | 0.096 |
|
| |||
| Rectal bleeding | 5 (10.6) | 6 (6.5) | 0.508 |
|
| |||
| Haemoperitoneum | 0 (0) | 1 (1.1) | 1.000 |
|
| |||
| Paralytic Ileus, n (%) | 5 (10.6%) | 6 (6.5%) | 0.508 |
|
| |||
| Cardiovascular complications, n (%) | 1 (2.1%) | 1 (1.1%) | 1.000 |
|
| |||
| Length of hospital stay, days | 10±6 | 9±6 | 0.125 |
ASA: American Society of Anesthesiologists; DOS: day of surgery; IVI: intravenous iron; NIVI. no intravenous iron; POD1: post-operative day 1; POD30: post-operative day 30; RBCT: red blood cell transfusion.
Figure 1.
Presence and severity of anaemia at post-operative days 1 and 30 in patients with colorectal cancer, according to whether they received postoperative intravenous iron (IVI) supplementation or not (NIVI).
Only 103 had their Hb measured (34 IVI, 69 NIVI) on POD30, of whom 74 (72%) remained anaemic (58 mild, 16 moderate). Again, anaemia was more prevalent and more severe among patients in the IVI group (Figure 1; p=0.027), despite their greater increment in Hb from POD1 to POD30 (2.0±1.5 g/dL for the IVI group, and 1.1±1.2 g/dL for the NIVI group; p=0.001).
Eleven patients from the entire cohort needed post-operative RBC transfusions (7 in the IVI group, 4 in the NIVI group; p=0.044) (Table I). Overall, five patients needed one unit, five patients two units, and one patient three units, without differences in RBC transfusion index between groups (Table I). Two patients from each group were transfused in a haemorrhagic context.
There were no differences in post-operative complication rates between groups (Table I). Only one patient given a RBC transfusion developed a post-operative infection. No IVI-related adverse events were witnessed.
Discussion
The aim of this retrospective study was to describe the prevalence and management of post-operative anaemia after elective CRC surgery in our centre, in which a protocol for pre-operative optimisation of anaemic patients has been established, although underutilised7.
We found that anaemia was present in 87% of patients on POD1, being mild-to-moderate in most cases. However, only one third of anaemic patients received post-operative IVI (iron sucrose 467±337 mg/patient). Anaemic patients in the IVI group presented with lower Hb levels on the DOS and had more severe anaemia on POD1 which conferred them a higher transfusion risk compared to those in the NIVI group (Table I). Consequently, RBC transfusions were more frequent among the IVI group (15 vs 4%, respectively; p=0.040), despite IVI being superior to standard care in improving the recovery from post-operative anaemia, as reflected by the greater Hb increment from POD1 to POD30 (2.0±1.5 g/dL for the IVI group, and 1.1±1.2 g/dL for the NIVI group; p=0.001). Importantly, as in previous studies7–9, neither differences in post-operative complication rates between groups (Table I) nor IVI-related adverse effects were witnessed.
Titos-Arcos et al.8 performed a case-control study of 52 CRC patients who received post-operative IVI (iron sucrose 592±445 mg/patient) vs 52 controls. Mean Hb concentration on POD1 for the case group was 10.6±1.2 g/dL vs 10.2±1.4 g/dL in the controls (p=0.121), and mean decreases in Hb from POD1 to discharge were 0.82±0.74 g/dL and 0.82±0.69 g/dL, respectively (p=0.806). They found no differences in either RBC transfusion rates (28.8 vs 30.8 %, respectively; p=0.830) or RBC transfusion index (3.0±1.6 units/patient vs 3.3±3.0 units/patient, respectively; p=0.682). These figures are significantly higher than those observed in our series, despite similar Hb levels on DOS, and could have been influenced by a higher incidence of rectal localisation of malignancy, reduced use of laparoscopic surgery and a more liberal transfusion threshold.
In contrast, Khallafallah et al.9 randomised 201 patients with similar post-operative Hb (10.5–10.6 g/dL) to either a single post-operative dose of intravenous 1,000 mg ferric carboxymaltose (intervention group) or standard care, consisting of observation (control group). At 4 weeks, Hb improved to 12.2 g/dL in the standard group and 13.0 g/dL in the ferric carboxymaltose group (mean difference 0.8 g/dL, 95% CI: 0.38–1.19; p<0.0001). In addition, fewer patients in the ferric carboxymaltose group than in the standard care group received RBC transfusions (1 vs 5%, respectively; p=0.035). However, it should be noted that the IVI dose administered was significantly higher than in our series and that the patients in mainly underwent elective major orthopaedic surgery.
In a more recent study of 454 adults with isovolaemic anaemia following radical gastrectomy (Hb 7–10 g/dL), the use of ferric carboxymaltose (500–1,500 mg) compared with placebo was more likely to result in an improved Hb response at 3 weeks (2.6 g/dL vs 1.4 g/dL, respectively; p<0.001) and at 12 weeks (3.3 g/dL vs 1.6 g/dL, respectively; p<0.001)16. No significant difference was observed in the global health status/quality of life scale at weeks 3 and 12. Nevertheless, it should be noted that patients with gastric cancer who undergo gastrectomy are particularly affected by acute anaemia because of their decreased ability to absorb iron17.
As most RBC transfusions were given early after surgery, our data suggest that the effect of post-operative IVI on erythropoiesis is not fast enough to reduce/avoid RBC transfusion requirements, despite our restrictive transfusion criteria18. Whether the greater Hb increment observed in the IVI group would further benefit patients receiving adjuvant post-operative chemotherapy or radiotherapy remains to be determined19,20.
The use of iron sucrose, instead of ferric carboxymaltose or iron isomaltoside which allows high-dose IVI administration in a single infusion21, may have resulted in an IVI dose which was insufficient to normalise Hb by POD30, especially in patients with more severe anaemia on POD1. However, in-hospital use of carboxymaltose iron was restricted in our centre for economic reasons, and this should be considered as a study limitation.
In addition, as previously seen for the pre-operative Hb optimisation programme7, our data suggest that surgeons were more prone to prescribe IVI to patients with lower postoperative Hb, while targeted those with milder anaemia to standard care (Table I and Figure 1). This should be considered as another study limitation. Reinforced training, education and awareness among the surgical staff would be useful to increase adherence to the peri-operative anaemia management protocol, thus limiting the exposure of anaemic CRC patients to RBC transfusions and their related risks7,22,23.
Conclusions
Compared with standard care, post-operative IVI administration to anaemic patients improves the recovery of Hb levels at POD30, without increasing post-operative complications. A prospective, randomised study to provide convincing evidence of the benefits of post-operative IVI therapy and to determine the potential for adverse effects in subjects undergoing CRC surgery is ongoing (Eudra-CT: 2015-001005-13).
Acknowledgements
This study is part of a PhD thesis completed by María Jesús Laso Morales.
The Authors would like to thank Dr. José Antonio García-Erce, Director of the Regional Blood and Tissue Bank of Navarra, Osansubidea, Pamplona (Spain), for his invaluable input and critical comments on this work.
Footnotes
Authorship contributions
All Authors contributed, making critical revisions related to important intellectual content of the manuscript, and gave final approval of the version of the article to be published.
The Authors declare no conflicts of interest.
References
- 1.Muñoz M, García-Erce JA, Remacha AF. Disorders of iron metabolism. Part II: iron deficiency and iron overload. J Clin Pathol. 2011;64:287–96. doi: 10.1136/jcp.2010.086991. [DOI] [PubMed] [Google Scholar]
- 2.Muñoz M, Gómez-Ramírez S, Martín-Montañez E, Auerbach M. Perioperative anemia management in colorectal cancer patients: a pragmatic approach. World J Gastroenterol. 2014;20:1972–85. doi: 10.3748/wjg.v20.i8.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisbe E, García-Erce JA, Díez-Lobo AI, Muñoz M Anaemia Working Group España. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth. 2011;107:477–8. doi: 10.1093/bja/aer242. [DOI] [PubMed] [Google Scholar]
- 4.Hernández-Cera C, Cantero C, Rivas Ferreira E, et al. Efficacy of protocol implementation based on intravenous iron treatment in gastrointestinal cancer surgery: 1AP3–8 [Abstract] Eur J Anesth. 2011;28(suppl 48):13–4. [Google Scholar]
- 5.Calleja JL, Delgado S, del Val A, et al. Colon Cancer Study Group. Ferric carboxymaltose reduces transfusions and hospital stay in patients with colon cancer and anemia. Inter J Colorectal Dis. 2016;3:543–51. doi: 10.1007/s00384-015-2461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keeler BD, Simpson JA, Ng O, et al. IVICA Trial Group. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br J Surg. 2017;104:214–21. doi: 10.1002/bjs.10328. [DOI] [PubMed] [Google Scholar]
- 7.Laso-Morales M, Jericó C, Gómez-Ramírez S, et al. Preoperative management of colorectal cancer-induced iron deficiency anemia in clinical practice: data from a large observational cohort. Transfusion. 2017;57:3040–8. doi: 10.1111/trf.14278. [DOI] [PubMed] [Google Scholar]
- 8.Titos-Arcos JC, Soria-Aledo V, Carrillo-Alcaraz A, et al. Is intravenous iron useful for reducing transfusions in surgically treated colorectal cancer patients? World J Surg. 2012;36:1893–7. doi: 10.1007/s00268-012-1589-x. [DOI] [PubMed] [Google Scholar]
- 9.Khalafallah AA, Yan C, Al-Badri R, et al. Intravenous ferric carboxymaltose versus standard care in the management of postoperative anaemia: a prospective, open-label, randomised controlled trial. Lancet Haematol. 2016;3:e415–25. doi: 10.1016/S2352-3026(16)30078-3. [DOI] [PubMed] [Google Scholar]
- 10.Leal-Noval SR, Muñoz M, Asuero M, et al. Spanish Consensus Statement on alternatives to allogeneic blood transfusion: the 2013 update of the “Seville Document”. Blood Transfus. 2013;11:585–610. doi: 10.2450/2013.0029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. First update 2016. Eur J Anaesthesiol. 2017;43:332–95. doi: 10.1097/EJA.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 12.Vaglio S, Prisco D, Biancofiore G, et al. Recommendations for the implementation of a Patient Blood Management programme. Application to elective major orthopaedic surgery in adults. Blood Transfus. 2016;14:23–65. doi: 10.2450/2015.0172-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaglio S, Gentili S, Marano G, et al. The Italian Regulatory Guidelines for the implementation of Patient Blood Management. Blood Transfus. 2017;15:325–8. doi: 10.2450/2017.0060-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia. 2017;72:233–47. doi: 10.1111/anae.13773. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Geneva: WHO; 2011. [Google Scholar]
- 16.Kim YW, Bae JM, Park YK, et al. Effect of intravenous ferric carboxymaltose on hemoglobin response among patients with acute isovolemic anemia following gastrectomy: the FAIRY randomized clinical trial. JAMA. 2017;317:2097–104. doi: 10.1001/jama.2017.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim CH, Kim SW, Kim WC, et al. Anemia after gastrectomy for early gastric cancer: long-term follow-up observational study. World J Gastroenterol. 2012;18:6114–9. doi: 10.3748/wjg.v18.i42.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franchini M, Marano G, Mengoli C, et al. Red blood cell transfusion policy: a critical literature review. Blood Transfus. 2017;15:307–17. doi: 10.2450/2017.0059-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin JS, Tut TG, Ho V, Lee CS. Predictive markers of radiotherapy-induced rectal cancer regression. J Clin Pathol. 2014;67:859–64. doi: 10.1136/jclinpath-2014-202494. [DOI] [PubMed] [Google Scholar]
- 20.Mao Q, Zhang Y, Fu X, et al. A tumor hypoxic niche protects human colon cancer stem cells from chemotherapy. J Cancer Res Clin Oncol. 2013;139:211–22. doi: 10.1007/s00432-012-1310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muñoz M, Gómez-Ramírez S, Besser M, et al. Current misconceptions in diagnosis and management of iron deficiency. Blood Transfus. 2017;15:422–37. doi: 10.2450/2017.0113-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auerbach M, Muñoz M, Macdougall IC. Intravenous iron: out of sight, out of mind. Lancet Haematol. 2018;5:e10–2. doi: 10.1016/S2352-3026(17)30230-2. [DOI] [PubMed] [Google Scholar]
- 23.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery. Ann Surg. 2012;256:235–44. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]