Abstract
Background
A number of clinical systematic review and meta-analysis have been published on the use of tranexamic in the obstetric setting. The aim of this meta-analysis was to evaluate the safety and effectiveness of tranexamic acid in reducing blood loss when given prior to caesarean delivery.
Materials and methods
We searched the Cochrane Wounds Specialized Register, Cochrane Central, MEDLINE (through PUBMED), Embase, and SCOPUS electronic databases. We also searched clinical trials registries for ongoing and unpublished studies, and checked reference lists to identify additional studies. We used no restrictions with respect to language and date of publication. Two review authors independently performed study selection, “Risk of bias” assessment, and data extraction. Initial disagreements were resolved by discussion, or by including a third review author when necessary.
Results
We found 18 randomised controlled trials (RCTs) that met our inclusion criteria. Overall, 1,764 women receiving intravenous tranexamic acid for prevention of bleeding following caesarean sections and 1,793 controls receiving placebo were enrolled in the 18 RCTs evaluated. The use of tranexamic acid compared to controls (placebo or no intervention) reduces post-partum haemorrhage >400 mL (risk ratio [RR] 0.40, 95% confidence interval [CI] 0.24–0.65; 5 trials with a total of 786 participants), severe post-partum haemorrhage >1,000 mL (RR 0.32, 95% CI: 0.12–0.84; 5 trials with a total of 1,850 participants), and need for red blood cell transfusion (RR 0.30, 95% CI: 0.18–0.49; 10 trials with a total of 1,873 participants). No particular safety concerns on the use of this antifibrinolytic agent emerged from the analysis of the 18 RCTs included.
Discussion
Overall, the results of this meta-analysis support the evidence of a beneficial effect of tranexamic acid in reducing blood loss and need for blood transfusion in pregnant women undergoing caesarean section.
Keywords: obstetric haemorrhage, post-partum haemorrhage, tranexamic acid, bleeding, prevention
Introduction
Obstetric haemorrhage is a leading cause of premature maternal mortality, accounting for at least 100,000 deaths each year worldwide1–4. Although post-partum haemorrhage (PPH) may be unpredictable, the most common causes include uterine atony, abnormal placentation, retained placental tissue, and lacerations of the lower genital tract5. In addition, obesity, multiple pregnancies, and previous caesarean section have been recognised as risk factors for PPH6. Considering the health and social burden of PPH, it is not surprising that a number of recommendations and guidelines have been issued from national and international scientific societies and health authorities to optimise the use of obstetric interventions and uterotonic drugs in this critical clinical setting7–9.
As far as the pathophysiology is concerned, recent evidence has linked the activation of the fibrinolytic pathway with the onset of severe haemorrhage in different settings, including trauma, heart and orthopaedic surgery, and obstetrics10,11. Following these observations, several randomised controlled trials (RCTs) have assessed the impact of tranexamic acid (TA), a lysine analogue that inhibits plasmin-mediated fibrin degradation12, on decreasing bleeding complications and mortality in such clinical conditions at increased haemorrhagic risk13–21. The aim of this paper is to provide an up-dated review, through a systematic analysis of the existing literature on the published RCTs on the safety and efficacy of TA for prevention of postpartum blood loss.
Material and methods
This systematic review was conducted according to the recommended Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist guidelines22.
Search strategy
A computer-assisted literature search of the MEDLINE (through PUBMED), EMBASE, SCOPUS, OVID and Cochrane Library electronic databases was performed to identify RCTs on the use of TA for prevention and treatment of PPH. A combination of the following text words was used to maximise search specificity and sensitivity: “tranexamic acid” AND “TXA” AND “antifibrinolytic agent” AND “post-partum haemorrhage” AND “PPH” AND “obstetric haemorrhage” AND “caesarean” AND “vaginal” AND “randomized” AND “prevention” AND “treatment”. In addition, we checked the reference lists of the most relevant items (original studies and reviews) in order to identify potentially eligible studies not captured by the initial literature search. Abstracts from relevant conferences or scientific meetings were hand-searched for additional studies.
Study selection and inclusion criteria
Study selection was performed independently by two reviewers (MF and MC), with disagreements resolved through discussion and on the basis of the opinion of a third reviewer (CM). Eligibility assessment was based on the title or abstract and on the full text if required. Articles were eligible if they reported either in the title or in the abstract the use of TA for the prevention of PPH. Only RCTs published in full in English between January 1970 and December 2017 were included in this systematic review and meta-analysis. As we found only two RCTs evaluating the efficacy of TA for the prevention of PPH after vaginal delivery23,24, the present analysis was limited to RCTs evaluating this antifibrinolytic agent after caesarean delivery.
Data extraction and outcome analysis
For each study included in the systematic review, the following data were extracted by two reviewers (MF and MC) independently: publication date, sample size (TA and control groups), and protocol (TA dose administered). The primary outcome was the incidence of PPH (i.e. blood loss more than 400 mL) and severe PPH (i.e. blood loss >1,000 mL). Secondary outcomes included mean blood loss volume (mL), need for blood transfusion, and overall severe side effects related to TA (including thromboembolic events). Disagreement was resolved by consensus and by the opinion of a third reviewer, if necessary.
Assessment of risk of bias in included studies
Two review authors (MF and MC) independently assessed the risk of bias of each included study following the domain-based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions25. They discussed any discrepancies and achieved consensus on the final assessment. The Cochrane “Risk of bias” tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete data, selective outcome reporting, and other issues relating to bias. We have presented our assessment of risk of bias using two “Risk of bias” summary figures: 1) a summary of bias for each item across all studies; and 2) a cross tabulation of each trial by all of the “Risk of bias” items.
“Summary of findings” tables
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes, and constructed a “Summary of findings” table using REVMAN 526. These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of available data for the main outcomes27. The “Summary of findings” tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE approach, which defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest (see Online Supplementary Content, Table SI). The certainty of a body of evidence involves consideration of within-trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias28.
We have presented the following outcomes in the “Summary of findings” table: i) PPH; ii) severe PPH; iii) need for blood transfusion (Table SI).
When evaluating the “Risk of bias” domain, we down-graded the GRADE assessment only when we classified a study as being at high risk of bias for one or more of the following domains: selection, attrition, reporting, and other bias; or when the “Risk of bias” assessment for selection bias was unclear (this was classified as unclear for either the generation of the randomisation sequence or the allocation concealment domain). For the outcomes PPH, need for blood transfusion, and post-partum blood loss, we did not down-grade for high risk of bias in performance and detection domains since we judged that the outcomes considered are not likely to be influenced by lack of blinding, and for unclear “Risk of bias” assessments in other domains.
Data analysis
All calculations were made using Stata 15.1, R v.3.4.3, and REVMAN 5. The effect size measures were the risk ratio (RR) and the risk difference (RD) between the treated arm and the control study arm. The study weight was calculated using the Mantel-Haenszel method. The heterogeneity χ2 was calculated as the I2 for the variation due to heterogeneity25. If significant heterogeneity was detected, a random effect method of study weight calculation was followed (DerSimonian-Laird method)29; otherwise, the fixed effect procedure was used. We also calculated the number needed to treat (NNT), which is the average number of patients who need to be treated in order to avoid (or to harm) an event.
Results
Literature search and study characteristics
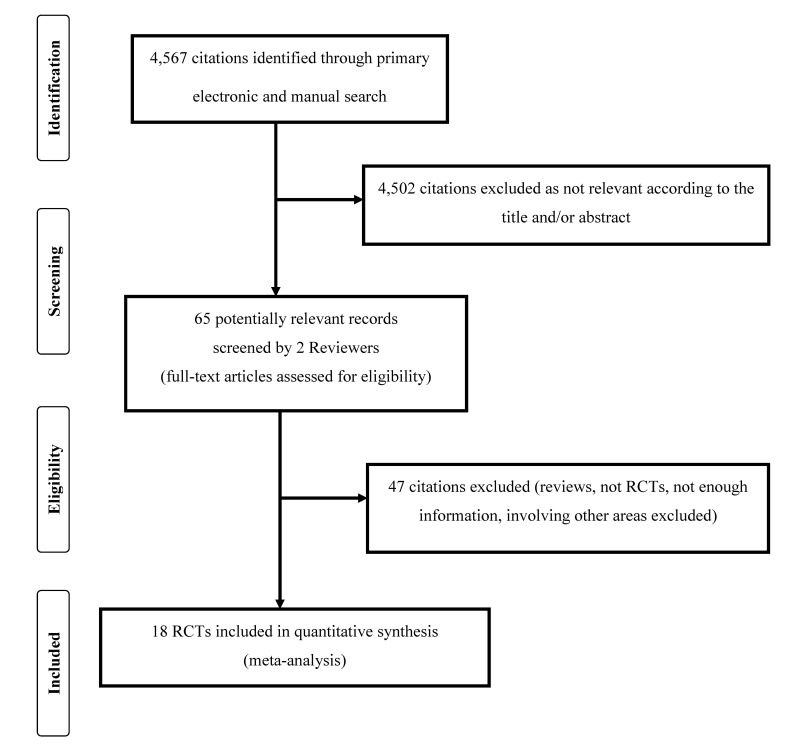
In total, 4,567 articles were initially identified after the initial electronic and manual search, which was concluded on 7 January 2018 (Figure 1). Of them, 4,502 were excluded because they were focusing on other topics. Thus, 65 potentially relevant articles were selected and the next screening led to the exclusion of 47 additional studies (reviews, protocols of RCTs, not RCTs, studies containing no informative data). The remaining 18 randomised studies30–47 were finally included in the systematic review and meta-analysis (see Table I for main characteristics and results of the included studies). Overall, 1,764 women receiving intravenous TA for prevention of bleeding following caesarean sections and 1,793 controls receiving placebo were enrolled in the 18 RCTs that went forward for evaluation.
Figure 1.
Flow chart of study inclusion criteria.
Table I.
Characteristics and main results of the 18 randomised controlled trials on the use of tranexamic acid for the prevention of obstetric haemorrhage.
| First Author yearref. | Cases (IVTA/C) | Age, years1 | IVTA dose | Controls | Post-partum blood loss, mL1 | PPH onset | RBC transfusion | TA-related SAEs |
|---|---|---|---|---|---|---|---|---|
| Gai 200425 | 91/89 | IVTA: 29.71 (4.18) | 1 g 10 min before CS | No treatment | IVTA: 359.29 (152.02)2 | IVTA: 22/913 | NR | IVTA: 0/91 |
| C: 29.75 (4.01) | C: 439.36 (191.48)2 | C: 35/893 | C: 0/89 | |||||
|
| ||||||||
| Sekhavat 200926 | 45/45 | IVTA: 26.2 (4.7) | 1 g 10 min before CS | 5% glucose | IVTA: 28.02 (5.53)4 | NR | NR | IVTA: 0/45 |
| C: 27.1 (4.1) | C: 37.12 (8.97)4 | C: 0/45 | ||||||
|
| ||||||||
| Gungorduk 201127 | 330/330 | IVTA: 26.3 (3.5) | 1 g 10 min before CS | 5% glucose | IVTA: 499.9 (206.4)5 | IVTA: 7/3306 | IVTA: 2/330 | IVTA: 0/330 |
| C: 26.6 (3.6) | C: 600.7 (215.7)5 | C: 19/3306 | C: 7/330 | C: 0/330 | ||||
|
| ||||||||
| Movafegh 201128 | 50/50 | IVTA: 27.0 (3.4) | 10 mg/kg 20 min before SA | Normal saline | IVTA: 262.5 (39.6)2 | NR | NR | IVTA: 0/50 |
| C: 27.6 (4.1) | C: 404.7 (94.4)2 | C: 0/50 | ||||||
|
| ||||||||
| Xu 201329 | 88/86 | IVTA: 26.7 (3.7) | 10 mg/kg 20 min before SA | Normal saline | IVTA: 379.2 (160.1)2 | IVTA: 19/887 | IVTA: 8/88 | IVTA: 2/88 |
| C: 27.1 (4.1) | C: 441.7 (189.5)2 | C: 28/867 | C: 19/86 | C: 2/86 | ||||
|
| ||||||||
| Shahid 201330 | 38/36 | IVTA: 24.18 (3.93) | 1 g 10 min before CS | Normal saline | IVTA: 356.44 (143.2)2 | NR | IVTA: 3/38 | IVTA: 0/38 |
| C: 24.89 (4.16) | C: 710.22 (216.72)2 | C: 12/36 | C: 0/36 | |||||
|
| ||||||||
| Goswami 201331 | 60/30 | IVTA: 23.6 (2.5) | 10 or 15 mg/kg 20 min before skin incision | 5% glucose | IVTA: 261.17 (56.78)–376.83 (31.96)8 | IVTA: 0/606 | IVTA: 0/60 | IVTA: 0/60 |
| C: 24.3 (2.6) | C: 527.17 (88.67)8 | C: 0/306 | C: 2/30 | C: 0/30 | ||||
|
| ||||||||
| Senturk 201332 | 101/122 | IVTA: 30.2 (6.83) | 1 g 10 min before CS | 5% glucose | IVTA: 272.05 (143.23) | NR | IVTA: 0/101 | IVTA: 0/101 |
| C: 29.22 (6.93) | C: 346.87 (189.49) | C: 0/122 | C: 0/122 | |||||
|
| ||||||||
| Abdel-Aleem 201333 | 373/367 | IVTA: 26.34 (5.16) | 1 g 10 min before CS | No treatment | IVTA: 241.61 (126.02)2 | IVTA: 2/3736 | NR | IVTA: 0/373 |
| C: 26.62 (5.05) | C: 510.66 (144.52)2 | C: 2/3676 | C: 0/367 | |||||
|
| ||||||||
| Ghosh 201434 | 70/70 | IVTA: 25.94 (3.78) | 1 g 10 min before CS | Normal saline | IVTA: 48.06 (8.20)4 | NR | IVTA: 0/70 | IVTA: 0/70 |
| C: 26.04 (3.39) | C: 76.01 (6.21)4 | C: 3/70 | C: 0/70 | |||||
|
| ||||||||
| Singh 201435 | 100/100 | IVTA: 25 (1.46) | 1 g 20 min before CS | No treatment | IVTA: 270.05 (30.88)9 | NR | NR | IVTA: 0/100 |
| C: 30 (1.24) | C: 510.45 (30.34)9 | C: 0/100 | ||||||
|
| ||||||||
| Yehia 201436 | 106/106 | IVTA: 28.4 (4.9) | 1 g 20 min before CS | Normal saline | IVTA: 369.5 (198.0)9 | IVTA: 33/1063 | IVTA: 0/106 | IVTA: 0/106 |
| C: 28.6 (4.7) | C: 606.8 (193.0)9 | C: 67/1063 | C: 2/106 | C: 0/106 | ||||
|
| ||||||||
| Gobbur 201437 | 50/50 | IVTA: 23.62 (3.43) | 1 g 20 min before CS | Normal saline | IVTA: 360.9 (110.3)2 | IVTA: 6/507 | NR | IVTA: 0/50 |
| C: 24.5 (3.98) | C: 443.0 (88.55)2 | C: 15/507 | C: 0/50 | |||||
|
| ||||||||
| Ramani 201438 | 60/60 | IVTA: 24.9 (3.9) | 1 g 10 min before CS | Normal saline | IVTA: 222.07 (97.02)2 | NR | IVTA: 2/60 | IVTA: 0/60 |
| C: 24.4 (3.7) | C: 274.5 (179.2)2 | C: 6/60 | C: 0/60 | |||||
|
| ||||||||
| Ahmed 201539 | 62/62 | IVTA: 28.6 (5.9) | 10 mg/kg 5 min before CS | Normal saline | IVTA: 391.0 (48.5)2 | NR | NR | IVTA: 0/62 |
| C: 26.9 (5.2) | C: 596.7 (38.02)2 | C: 0/62 | ||||||
|
| ||||||||
| Maged 201540 | 100/100 | IVTA: 24.9 (4.6) | 1 g 20 min before CS | Normal saline | IVTA: 459.4 (75.4) | IVTA: 0/1006 | NR | IVTA: 0/100 |
| C: 25.3 (4.7) | C: 700.3 (143.9) | C: 6/1006 | C: 0/100 | |||||
|
| ||||||||
| Lakshmi 201641 | 60/60 | IVTA: 26.77 (2.81) | 1 g 20 min before CS | No treatment | IVTA: 347.17 (108.6)9 | IVTA: 2/607 | IVTA: 0/60 | IVTA: 0/60 |
| C: 26.82 (2.8) | C: 517.72 (150.0)9 | C: 36/607 | C: 0/60 | C: 0/60 | ||||
|
| ||||||||
| Sujata 201642 | 30/30 | IVTA: 29.4 (4.16) | 1 g 15 min before CS | Normal saline | IVTA: 432 (337–497)5,10 | IVTA: 0/306 | IVTA: 1/30 | IVTA: 0/30 |
| C: 30.27 (4.31) | C: 819 (663–1001)5,10 | C: 7/306 | C: 4/30 | C: 0/30 | ||||
IVTA: intravenous tranexamic acid; C: controls; SD: standard deviation; min: minutes; CS: caesarean section; SAEs: severe adverse events; RBC: red blood cell transfusion; NR: not reported; PPH: post-partum haemorrhage; SA: spinal anaesthesia.
Mean (standard deviation);
measured from placental delivery to 2 hours post-partum;
defined as blood loss >400 mL;
measured from the end of cesarean section to 2 hours post-partum;
measured from the skin incision to 48 hours post-partum;
defined as blood loss >1,000 mL;
defined as blood loss >500 mL;
measured from placental delivery to 24 hours post-partum;
measured from placental delivery to the end of caesarean section;
median (interquartile range).
Risk of bias in included studies
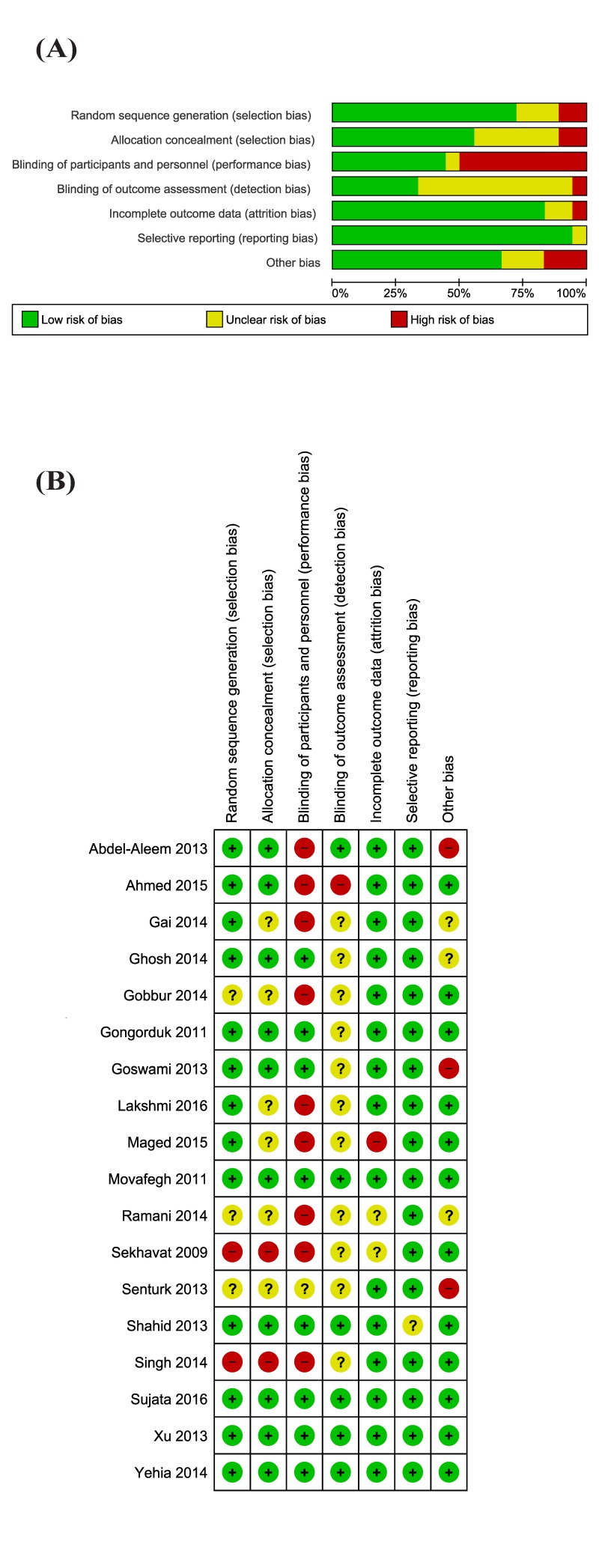
Eleven studies were at high risk of bias for one or more domains, and 12 studies were at unclear risk of bias for one or more domains (Figure 2 A and B).
Figure 2.
Risk of bias graph and summary.
(A) Review authors’ judgements about each risk of bias item presented as percentages across all included studies. (B) Review authors’ judgements about each risk of bias item for each included study.
Allocation
We assessed two studies as being at high risk of selection bias, as randomisation was by alternation of the two treatments, so the intervention allocations could have been foreseen in advance31,40. The reports of six studies were unclear for random sequence generation and/or allocation concealment, while ten studies were at low risk of selection biases.
Blinding
There were nine studies reported as open label, and they were graded as high risk of performance bias (blinding of participants and personnel). Eight studies were reported as double blind32–37,39,41,47, and one45 as single blind; one of these studies37 did not provide any information on the blinding procedures. Eleven studies were graded at unclear risk of detection bias due to the fact that it did not provide information to permit judgement about “high” or “low” risk of bias related to the blinding of outcome assessors; one study44 was graded at high risk of bias because it stated that the clinical care team was aware of the administered treatment.
Incomplete outcome data
One study45 was judged at high risk of attrition bias because it reported only per protocol analysis. Two studies31,44 were judged at unclear risk of bias. The remaining studies were judged at low risk of bias.
Selective reporting
Selective reporting was low in all included studies, and graded as “unclear” in one study35 where some data were not presented in detail (e.g. some side effects) although the report states that there were no differences between groups.
Other potential sources of bias
We judged three studies at high risk of other source of bias: two because of imbalance at baseline37,38, and one36 because it did not mention PPH, enrolled anaemic women, and because there was a significant difference in the duration of surgery between groups.
Effects of interventions
The overall incidence of PPH was 71 cases on 395 treated patients (17.9%) and 172 cases on 391 control patients (43.9%). Using the average treatment effect from a random-effects model, the use of TA reduces significantly the episodes of PPH when compared with control group: five trials, 786 patients; RR 0.40, 95% CI: 0.24–0.65; p=0.0003 for overall effect; I2=68% (Figure 3A). The overall incidence of severe PPH was 9 cases of 893 treated patients (1.0%) and 34 cases of 857 control patients (3.9%). Using the average treatment effect from a random-effects model, the use of TA significantly reduces the episodes of severe PPH when compared with control group: five trials, 1, 750 patients; RR 0.32, 95% CI: 0.12–0.84; p=0.02 for overall effect; I2=19% (Figure 3B).
Figure 3.
Forest plot demonstrating effects of tranexamic acid (TA) on the incidence of (A) post-partum haemorrhage and (B) severe post-partum haemorrhage.
M-H: Mantel-Haenszel; CI: confidence interval.
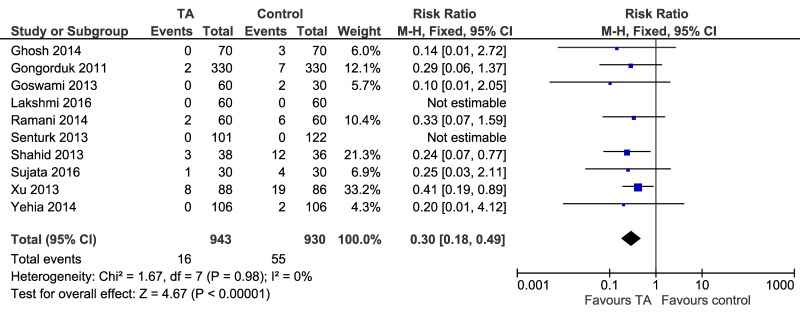
As far as secondary outcomes are concerned, using the average treatment effect from a fixed-effects model, the use of TA reduces the need of red blood cell transfusion compared to controls: ten trials, 1,873 patients; RR 0.30, 95% CI: 0.18–0.49; p=0.00001 for overall effect; I2=0% (Figure 4). The overall incidence for blood transfusion was 16 cases of 943 treated patients (1.69%) and 55 cases of 930 control patients (5.91%). The NNT was calculated as 25.6.
Figure 4.
Forest plot of the effect of tranexamic acid (TA) on red blood cell (RBC) transfusion need. M-H: Mantel-Haenszel; CI: confidence interval.
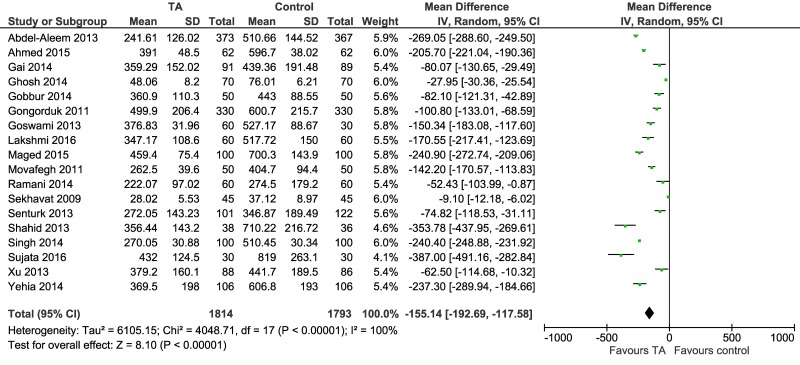
Tranexamic acid reduces the amount (mL) of post-partum blood loss: mean difference −155.14 (95% CI: −192.69 to −157.58; p=0.00001 for overall effect) (Figure 5). The direction of the effect was consistent across studies, with a beneficial effect of TA, but there was a considerable heterogeneity (I2=100%). The heterogeneity observed can be ascribed to different intervals of blood loss recorded (from placental delivery30,33–35,38,42–44 or caesarean section39 to two hours post-partum, from skin incision to 48 hours post-partum32,47, from placental delivery to 24 hours post-partum36, from placental delivery to the end of caesarean section40,41,46); to different populations of patients enrolled (e.g. several studies excluded anaemic women, but one study36 included only anaemic women); to different dosage of TA used; and also to methods used to quantify the loss (e.g. weight of gauze pads, and/or sponges, mops, drapes; bloods in suction bottles; pre- and post-intervention haemoglobin values).
Figure 5.
Forest plot of tranexamic acid (TA) on post-partum blood loss (mL).
CI: confidence interval.
One study34 reported severe side effects (deep vein thrombosis) in 2 of 88 TA recipients and in 2 of 86 controls (RR, 0.98, 95% CI: 0.14–6.78). The remaining 17 studies did not report severe adverse events, including thromboembolic events, in either group.
Based on GRADE assessment, all these comparisons were graded as moderate certainty evidence, and down-graded once due to inconsistency or to risk of biases (see Online Supplementary Content, Table SI).
Discussion
The antifibrinolytic agent TA is routinely used in cardiac, orthopaedic and oral surgeries to reduce perioperative blood loss13–15. Recent evidence from RCTs also indicate that TA usage results in a significant reduction of obstetric bleeding20,48,49. A recent randomised, placebo-controlled trial (WOMAN, WOrld Maternal ANtifibrinolytic) on 20,060 women with PPH found that TA reduces deaths due to bleeding (RR 0.81, 95% CI: 0.65–1.00; p=0.045) with no adverse effects, especially when given early after bleeding onset50. A Cochrane systematic review evaluating TA for preventing post-partum haemorrhage was recently published. After the analysis of 12 RCTs involving 3,285 women, the authors concluded that TA decreases postpartum blood loss and prevents PPH and blood transfusion requirements51. The results of our meta-analysis are in line with these observations and indicate that, in women undergoing caesarean delivery, the prophylactic use of TA significantly reduces the incidence of PPH, including severe PPH, total blood loss and transfusion requirements without increasing the risk of thromboembolic complications. The main strength of our study is that it represents a very large, up-to-date and comprehensive analysis which involved overall 4,557 women enrolled in 18 RCTs. In addition, most of the included studies are of high quality and with a low risk of bias according to the Cochrane risk of bias tools. Nevertheless, our systematic review has some limitations which are related to the different study designs of the RCTs evaluated that generate a substantial heterogeneity across studies. For instance, there were differences among the various RCTs in the definition of PPH (blood loss >400 or >500 mL) and post-partum blood loss assessment (2 hours, 24 hours or 48 hours post-partum). In particular, the abbreviated time for data collection may mean that total blood loss and the true incidence of PPH have been under-estimated. In addition, we were not able to detect the effect of TA on maternal death due to the lack of fatal events in the studies evaluated, which was probably related to the small size of the population of women enrolled. Larger RCTs on this clinical setting are, therefore, needed to assess this important outcome.
In summary, the results of our meta-analysis document the safety and efficacy of prophylactic administration of TA in reducing post-partum blood loss, PPH incidence and need for blood transfusion in women undergoing caesarean delivery. Therefore, given its efficacy in preventing one of the most common and serious complications of pregnancy, we recommend the use of TA in this clinical setting. The use of this drug in the framework of patient blood management (PBM) programmes52–55 can play a key role as a strategy to save blood loss but, to be really beneficial in improving patient outcome and efficient for health systems, it should be part of an adequate management of perioperative anaemia56, 57. In fact, the benefits of a PBM programme58 are greater when it includes optimisation of patient’s haemoglobin level59–65. This approach is very important also for the perinatal care of women, a setting where the management of anaemia and haematinic deficiencies should be obligatory both for clinicians and policymakers in charge of decision making processes aimed at up-dating clinical practice in health care66.
Online Supplementary Content
Footnotes
Disclosure of conflicts of interest
GML is the Editor-in-Chief of Blood Transfusion and this manuscript has undergone additional external review as a result. The other Authors declare no conflicts of interest.
References
- 1.Mehrabadi A, Hutcheon JA, Lee L, et al. Trends in postpartum hemorrhage from 2000 to 2009: a population-based study. BMC Pregnancy Childbirth. 2012;12:108. doi: 10.1186/1471-2393-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:980–1004. doi: 10.1016/S0140-6736(14)60696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdul-Kadir R, McLintock C, Ducloy AS, et al. Evaluation and management of postpartum hemorrhage: consensus from an international expert panel. Transfusion. 2014;54:1756–68. doi: 10.1111/trf.12550. [DOI] [PubMed] [Google Scholar]
- 4.Calvert C, Thomas SL, Ronsmans C, et al. Identifying regional variation in the prevalence of postpartum haemorrhage: a systematic review and meta-analysis. PLoS ONE. 2012;7:e41114. doi: 10.1371/journal.pone.0041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercier FJ, Van de Velde M. Major obstetric hemorrhage. Anesthesiol Clin. 2008;26:53–66. doi: 10.1016/j.anclin.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG Int J Obstet Gynaecol. 2008;115:1265–72. doi: 10.1111/j.1471-0528.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 7.Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30:270–382. doi: 10.1097/EJA.0b013e32835f4d5b. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Managing complications in pregnancy and childbirth. Geneva: WHO; 2007. [Google Scholar]
- 9.Istituto Superiore di Sanità. Emorragia post-partum: come prevenirla, come curarla. Roma: ISS; 2016. (Linea Guida 26). [Google Scholar]
- 10.Pabinger I, Fries D, Schochl H, et al. Tranexamic acid for treatment and prophylaxis of bleeding and hyperfibrinolysis. Wien Klin Wochenschr. 2017;129:303–16. doi: 10.1007/s00508-017-1194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen MJ, Christie SA. Coagulopathy of trauma. Crit Care Clin. 2017;33:101–18. doi: 10.1016/j.ccc.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Franchini M, Mannucci PM. Adjunct agents for bleeding. Curr Opin Hematol. 2014;21:503–8. doi: 10.1097/MOH.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 13.Franchini M, Mengoli C, Marietta M, et al. Safety of intravenous tranexamic acid in patients undergoing major orthopedic surgery: a meta-analysis of randomized controlled trial. Blood Transfus. 2018;16:36–43. doi: 10.2450//2017.0219-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ker K, Roberts I, Shakur H, Coats TJ. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev. 2015;5:CD004896. doi: 10.1002/14651858.CD004896.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerstein NS, Brierley JK, Windsor J, et al. Antifibrinolytic agents in cardiac and noncardiac surgery: a comprehensive overview and update. J Cardiothorac Vasc Anesth. 2017;31:2183–205. doi: 10.1053/j.jvca.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. WHO recommendation on tranexamic acid for the treatment of postpartum haemorrhage. Geneva: WHO; 2017. [PubMed] [Google Scholar]
- 17.Li C, Gong Y, Dong L, et al. Is prophylactic tranexamic acid administration effective and safe for postpartum hemorrhage? A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e5653. doi: 10.1097/MD.0000000000005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sentilhes L, Lasocki S, Ducloy-Bouthors AS, et al. Tranexamic acid for the prevention and treatment of postpartum hemorrhage. Br J Anaesth. 2015;114:576–87. doi: 10.1093/bja/aeu448. [DOI] [PubMed] [Google Scholar]
- 19.Simonazzi G, Bisulli M, Saccone G, et al. Tranexamic acid for preventing postpartum blood loss after cesarean delivery: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2016;95:28–37. doi: 10.1111/aogs.12798. [DOI] [PubMed] [Google Scholar]
- 20.Ker K, Shakur H, Roberts I. Does tranexamic acid prevent postpartum haemorrhage? A systematic review of randomized controlled trials. BJOG. 2016;123:1745–52. doi: 10.1111/1471-0528.14267. [DOI] [PubMed] [Google Scholar]
- 21.Alam A, Choi S. Prophylactic use of tranexamic acid for postpartum bleeding outcomes: a systematic review and meta-analysis of randomized controlled trials. Transfus Med Rev. 2015;29:231–41. doi: 10.1016/j.tmrv.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Gungorduk K, Asıcıoğlu O, Yıldırım G, et al. Can intravenous injection of tranexamic acid be used in routine practice with active management of the third stage of labor in vaginal delivery? A randomized controlled study. Am J Perinatol. 2013;30:407–13. doi: 10.1055/s-0032-1326986. [DOI] [PubMed] [Google Scholar]
- 24.Mirghafourvand M, Mohammed-Alizadeh S, Abbasalizadeh F, Shrdel M. The effect of prophylactic intravenous tranexamic acid on blood loss after vaginal delivery in women at low risk of postpartum hemorrhage. A double-blind randomized controlled trial. Aust NZJ Obstet Gynaecol. 2015;55:53–8. doi: 10.1111/ajo.12262. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions - Version 5.1.0 (updated March 2011) The Cochrane Collaboration; [Accessed on 31/01/2018]. Available at: http://www.cochranehandbook.org. [Google Scholar]
- 26.Guyatt GH, Oxman AD, Kunz R, et al. What is ‘quality of evidence’ and why is it important to clinicians? BMJ. 2008;336:995–8. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schünemann HJ, Oxman AD, Higgins JP, et al. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. [Accessed on 31/01/2018]. Available at: www.handbook.cochrane.org. [Google Scholar]
- 28.Schünemann HJ, Oxman AD, Higgins JP, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. [Accessed on 31/01/2018]. Available at: www.handbook.cochrane.org. [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Gai MY, Wu LF, Su QF, et al. Clinical observation of blood loss reduced by tranexamic acid during and after caesarian section: a multi-center, randomized trial. Euro J Obstet Gynecol Reprod Biol. 2004;112:154–7. doi: 10.1016/s0301-2115(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 31.Sekhavat L, Tabatabaii A, Dalili M, et al. Efficacy of tranexamic acid in reducing blood loss after cesarean section. J Matern Fetal Neonatal Med. 2009;22:72–5. doi: 10.1080/14767050802353580. [DOI] [PubMed] [Google Scholar]
- 32.Gungorduk K, Asıcıoğlu O, Yıldırım G, et al. Efficacy of intravenous tranexamic acid in reducing blood loss after elective cesarean section: a prospective, randomized, double-blind, placebo-controlled study. Am J Perinatol. 2011;28:233–40. doi: 10.1055/s-0030-1268238. [DOI] [PubMed] [Google Scholar]
- 33.Movafegh A, Eslamian L, Dorabadi A. Effect of intravenous tranexamic acid administration on blood loss during and after cesarean delivery. Int J Gynaecol Obstet. 2011;115:224–6. doi: 10.1016/j.ijgo.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Gao W, Ju Y. Tranexamic acid for the prevention of postpartum hemorrhage after cesarean section: a double-blind randomization trial. Arch Gynecol Obstet. 2013;287:463–8. doi: 10.1007/s00404-012-2593-y. [DOI] [PubMed] [Google Scholar]
- 35.Shahid A, Khan K. Tranexamic acid in decreasing blood loss during and after caesarean section. J Coll Physicians Surg Pak. 2013;23:459–62. [PubMed] [Google Scholar]
- 36.Goswami U, Sarangi S, Gupta S, et al. Comparative evaluation of two doses of tranexamic acid used prophylactically in anemic parturients for lower segment cesarean section: A double-blind randomized case control prospective trial. Saudi J Anaesth. 2013;7:427–31. doi: 10.4103/1658-354X.121077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sentürk MB, Cakmak Y, Yildiz G, Yildiz P. Tranexamic acid for cesarean section: a double-blind, placebo-controlled, randomized clinical trial. Arch Gynecol Obstet. 2013;287:641–5. doi: 10.1007/s00404-012-2624-8. [DOI] [PubMed] [Google Scholar]
- 38.Abdel-Aleem H, Alhusaini TK, Abdel-Aleem MA, et al. Effectiveness of tranexamic acid on blood loss in patients undergoing elective cesarean section: randomized clinical trial. J Matern Fetal Neonatal Med. 2013;26:1705–9. doi: 10.3109/14767058.2013.794210. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh A, Chaudhuri P, Muhuri B. Efficacy of intravenous tranexamic acid before cesarean section in preventing post-partum hemorrhage-a prospective randomized double blind placebo controlled study. Int J Bio Med Res. 2014;5:4461–4. [Google Scholar]
- 40.Singh T, Burute SB, Deshpande HG, et al. Efficacy of tranexamic acid in decreasing blood loss during and after caesarean section: a randomized case control prospective study. J Evolut Med Dent Sci. 2014;3:2780–8. [Google Scholar]
- 41.Yehia AH, Koleib MH, Abdelazim IA, et al. Tranexamic acid reduces blood loss during and after cesarean section: a double blinded, randomized, controlled trial. Asian Pac J Reprod. 2014;3:53–6. [Google Scholar]
- 42.Gobbur V, Shiragur S, Jhanwar U, et al. Efficacy of tranexamic acid in reducing blood loss during lower segment caesarean section. Int J Reprod Contracept Obstet Gynecol. 2014;3:414–7. [Google Scholar]
- 43.Ramani B, Nayak L. Intravenous 1 gram tranexamic acid for prevention of blood loss and blood transfusion during caesarean section: a randomized case control study. Int J Reprod Contracept Obstet Gynecol. 2014;3:366–9. [Google Scholar]
- 44.Ahmed MR, Sayed Ahmed WA, Madny EH, et al. Efficacy of tranexamic acid in decreasing blood loss in elective caesarean delivery. J Matern Fetal Neonatal Med. 2015;28:1014–8. doi: 10.3109/14767058.2014.941283. [DOI] [PubMed] [Google Scholar]
- 45.Maged AM, Helal OM, Elsherbini MM, et al. A randomized placebocontrolled trial of preoperative tranexamic acid among women undergoing elective cesarean delivery. Int J Gynaecol Obstet. 2015;131:265–8. doi: 10.1016/j.ijgo.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 46.Lakshmi SJD, Abraham R. Role of prophylactic tranexamic acid in reducing bloodloss during elective cesarean section: a randomized controlled study. J Clin Diagn Res. 2016;10:QC17–21. doi: 10.7860/JCDR/2016/21702.9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sujata N, Tobin R, Kaur R, et al. Randomized controlled trial of tranexamic acid among parturients at increased risk for postpartum hemorrhage undergoing cesarean delivery. Int J Gynaecol Obstet. 2016;133:312–5. doi: 10.1016/j.ijgo.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 48.Ducloy-Bouthors AS, Jude B, et al. EXADELI Study Group. Susen S. High-dose tranexamic acid reduces blood loss in postpartum haemorrhage. Crit Care. 2011;15:R117. doi: 10.1186/cc10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gayet-Ageron A, Prieto-Merino D, Ker K, et al. Antifibrinolytic Trials Collaboration. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: a meta-analysis of individual patient-level data from 40 138 bleeding patients. Lancet. 2018;391:125–32. doi: 10.1016/S0140-6736(17)32455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–16. doi: 10.1016/S0140-6736(17)30638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novikova N, Hofmeyr GJ, Cluver C. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2015;6:CD007872. doi: 10.1002/14651858.CD007872.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaglio S, Gentili S, Marano G, et al. The Italian Regulatory Guidelines for the implementation of Patient Blood Management. Blood Transfus. 2017;15:325–8. doi: 10.2450/2017.0060-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaglio S, Prisco D, Biancofiore G, et al. Recommendations for the implementation of a Patient Blood Management programme. Application to elective major orthopaedic surgery in adults. Blood Transfus. 2016;14:23–65. doi: 10.2450/2015.0172-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guerra R, Velati C, Liumbruno GM, Grazzini G. Patient Blood Management in Italy. Blood Transfus. 2016;14:1–2. doi: 10.2450/2015.0171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franchini M, Muñoz M. Towards the implementation of patient blood management across Europe. Blood Transfus. 2017;15:292–3. doi: 10.2450/2017.0078-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muñoz M, Gómez-Ramírez S, Kozek-Langeneker S, et al. ‘Fit to fly’: overcoming barriers to preoperative haemoglobin optimization in surgical patients. Br J Anaesth. 2015;115:15–24. doi: 10.1093/bja/aev165. [DOI] [PubMed] [Google Scholar]
- 57.Muñoz M, Gómez-Ramírez S, Campos A, et al. Pre-operative anaemia: prevalence, consequences and approaches to management. Blood Transfus. 2015;13:370–9. doi: 10.2450/2015.0014-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liumbruno GM, Vaglio S, Grazzini G, et al. Patient blood management: a fresh look at a fresh approach to blood transfusion. Minerva Anestesiol. 2015;81:1127–37. [PubMed] [Google Scholar]
- 59.Liumbruno GM, Vaglio S, Biancofiore G, et al. Transfusion thresholds and beyond. Blood Transfus. 2016;14:123–5. doi: 10.2450/2016.0008-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franchini M, Marano G, Mengoli C, et al. Red blood cell transfusion policy: a critical literature review. Blood Transfus. 2017;15:307–17. doi: 10.2450/2017.0059-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Girelli D, Marchi G, Busti F. Iron replacement therapy: entering the new era without misconceptions, but more research is needed. Blood Transfus. 2017;15:379–81. doi: 10.2450/2017.0152-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muñoz M, Gómez-Ramírez S, Besser M, et al. Current misconceptions in diagnosis and management of iron deficiency. Blood Transfus. 2017;15:422–37. doi: 10.2450/2017.0113-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muñoz M, Gómez-Ramírez S, Liumbruno GM. Peri-operative anaemia management in major orthopaedic surgery: the need to find a pathway. Blood Transfus. 2017;15:289–91. doi: 10.2450/2017.0296-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bisbe E, Basora M, Colomina MJ Spanish Best Practice in Peri-operative Anaemia Optimisation Panel. Peri-operative treatment of anaemia in major orthopaedic surgery: a practical approach from Spain. Blood Transfus. 2017;15:296–306. doi: 10.2450/2017.0177-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quintana-Díaz M, Muñoz-Romo R, Gómez-Ramírez S, et al. A fast-track anaemia clinic in the Emergency Department: cost-analysis of intravenous iron administration for treating iron-deficiency anaemia. Blood Transfus. 2017;15:438–46. doi: 10.2450/2017.0282-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muñoz M, Peña-Rosas JP, Robinson S, et al. Patient blood management in obstetrics: management of anaemia and haematinic deficiencies in pregnancy and in the post-partum period: NATA consensus statement. Transfus Med. 2018;28:22–39. doi: 10.1111/tme.12443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.