Introduction
Pathogen reduction technologies have been developed to reduce the risk of transmission of infectious agents through blood transfusion1. The Mirasol® Pathogen Reduction Technology (PRT) System from Terumo BCT (Lakewood, CO, USA) uses ultraviolet light and riboflavin (vitamin B2) to reduce the load of active pathogens and to inactivate residual white blood cells in blood components used for transfusion2. A CE mark was granted for the Mirasol® PRT System in 2007 for use with apheresis and buffy coat platelets stored in 100% plasma, and subsequently CE marks were granted in 2008 and 2009 for the system’s use in the treatment of plasma and platelets stored in platelet additive solution, respectively.
Haemovigilance of blood components is required by European legislation (Directive 2005/61/EC) and is also named by the World Health Organization (WHO) as an integral part of the quality management in a blood system, being required for continual improvement of the quality and safety of blood components and the transfusion process3,4. Different haemovigilance programmes have different scopes, but in essence they should be able to collect and assess information on unexpected or undesirable effects resulting from the therapeutic use of labile blood components5,6.
The collection of passive haemovigilance data on Mirasol®-treated components in multiple blood transfusion centres in Europe started in 2010. By 2011 the number of collected reports (n>50,000) and sites (n=6) permitted the first statistical analysis of the data. The objective of this study is to report the cumulative haemovigilance data on Mirasol®-treated blood components in routine use in Europe for the last 6 years.
Materials and methods
Sites using the Mirasol® PRT System were invited to participate in the Mirasol® haemovigilance programme at the time of implementation of the technology and were reminded regularly of the opportunity to do so during the course of the programme. Participation was on a voluntary basis. A questionnaire (Table I) was sent to interested blood transfusion centres and was filled out at regular intervals (from monthly to yearly, depending on the sites’ preferences). In this questionnaire users were asked to record data on platelet and plasma transfusions, as well as any reported transfusion reactions related to the components. Additional information about the production process - apheresis or whole blood-derived production - was also requested. Sites produced blood components according to Standard Operating Procedures following site-specific Quality Management System programmes and in accordance with European guidelines7. The compiled data were regularly updated and analysed, enabling identification of trends. The data presented in this manuscript include the cumulative data from sites in Austria, Greece, Lithuania, Luxembourg, Poland and Spain. None of the six sites used other safety measures, e.g. bacterial screening, to reduce bacterial contamination of platelet concentrates. Gamma-irradiation of platelet concentrates upon medical indication has been discontinued in four out of the six sites. According to the European Commission Directive 2005/61, severe adverse reactions and severe adverse events must be reported annually by the national competent authority to the European Commission3. Participating sites followed this directive at a centralised, national level. For this study all adverse reactions reported to the blood production sites are included independently of grade of severity and imputability. Adverse reaction criteria and grading follow established definitions by European and national guidelines7.
Table I.
Questionnaire sent to participants of the Mirasol® haemovigilance programme.
| Name of Institution, Department: | ||||
|---|---|---|---|---|
| Period of report: | ||||
| Blood component | Platelets | Plasma | ||
| Apheresis | Whole blood | Apheresis | Whole blood | |
| Total produced | ||||
| Total Mirasol®-treated | ||||
| Total issued to hospitals | ||||
| Total Mirasol® products issued to hospitals | ||||
| Total expired Mirasol® products | ||||
| Total transfused Mirasol® products | ||||
| Total n. of patients transfused with the products | ||||
| Total n. of adverse event reports, with grades of severity (I, II, III, IV) | ||||
| N. of adverse events reports for Mirasol®-treated products, with grades of severity (I, II, III, IV) | ||||
| Imputability levels: definite, probable, unlikely, excluded | ||||
| Patient’s disease | ||||
| Previous history of transfusion (yes/no) | ||||
| Presence of allo-antibodies before transfusion (yes/no) | ||||
Descriptive statistics were performed using Microsoft Office Excel 2007, version (12.0.6747.5000) SP 3 MSO (12.0.6743.5000), which is part of Microsoft Office Professional Plus 2007 (Redmond, WA, USA).
Results
Site participation in the Mirasol® haemovigilance programme fluctuated during the 6-year period reported here. Six sites contributed to the programme in the first 2 years until 2011 reporting data on 20,095 and 33,303 platelet and plasma transfusions, respectively. Participation decreased in 2012 to four sites, which contributed to the accumulated 37,323 platelet and 47,663 plasma transfusion reports. In 2013 the number of reports reached 53,023 and 66,115 for platelet and plasma transfusions, respectively. In 2014 two new sites joined the programme and the number of reports rose to 71,626 and 77,243 for platelets and plasma, respectively. Finally, by the end of 2015, reports of 91,954 platelet and 96,115 plasma transfusions had been recorded in the programme. Seventy-one percent of platelets were collected by apheresis (n=64,890) and 29% were produced from whole blood-derived pools of buffy-coat and interim platelet units (n=27,064).
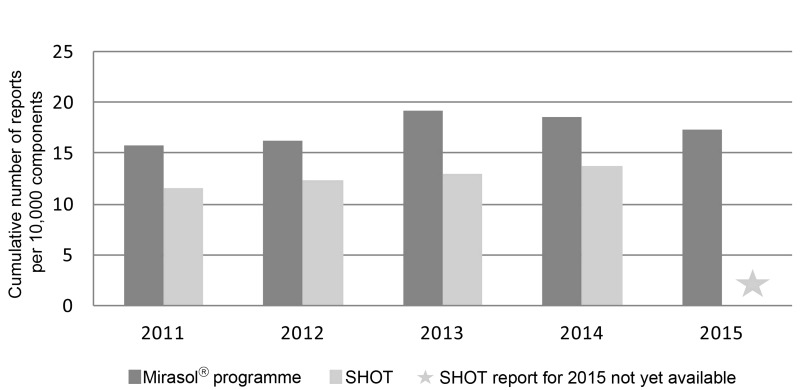
Reasons given for not participating or terminating participation were mostly related to scarcity of resources. Nevertheless, the number of transfusion reports was directly proportional to the number of Mirasol® treatment kits sold. In the first 3 years a constant yearly addition of approximately 30,000 transfusion reports to the database was observed, which increased to 40,000 transfusion reports in 2015. The number of adverse reactions per 10,000 transfused components started at 15.7, peaked in 2013 at 19.2 and decreased to 17.5 in 2015. The number of adverse reactions reported per treated platelet and plasma components is comparable to the number reported by the Serious Hazards Of Transfusion (SHOT) haemovigilance system (Figure 1)8.
Figure 1.
Reports of adverse reactions in the Mirasol® haemovigilance programme and the SHOT (Serious Hazards Of Transfusion) haemovigilance system.
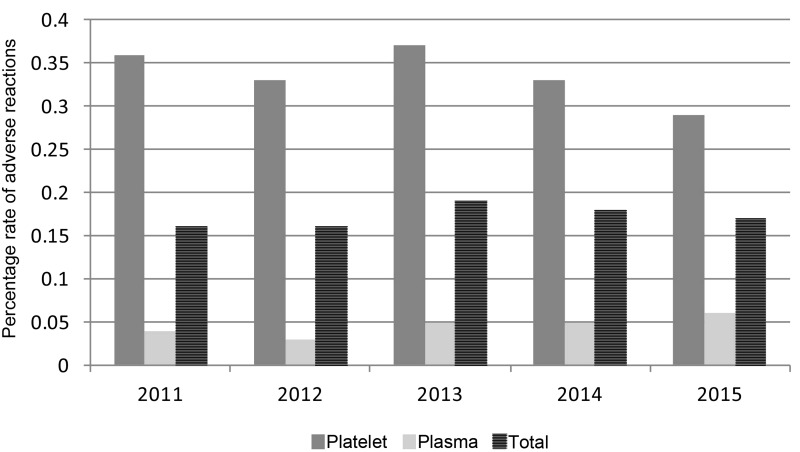
Over the entire observation period, the average rate of adverse reactions was 0.34 and 0.05% for platelets and plasma, respectively. Seventy-five percent of the reactions to transfused Mirasol®-treated platelets reported to the programme involved platelets stored in plasma and 25% involved platelets stored in platelet additive solution. Adverse reaction rates to platelet components peaked at 0.37% in 2013 with four sites participating in the programme and decreased to 0.29% by 2015 after two new sites joined the programme (Figure 2). Reaction rates to fresh-frozen plasma remained constant at 0.05%, but reports are limited to three sites and over 99% of the data came from a single site. Overall, the rate of adverse reactions to Mirasol®-treated platelets and plasma was 0.17%.
Figure 2.
Rates (%) of adverse reaction reports regarding Mirasol®-treated platelets and plasma.
Most of the reported adverse reactions were of grade I severity: fever, rash and chills. Over 99.9 % of the reports classified the imputability of the reactions as unlikely to be linked to the device. Very few reports considered observed reactions definitely related to the transfusion of Mirasol®-treated components (0.01%). Similarly, very few cases of grade II reactions, all with low imputability, were observed. These reached rates of 0.02 and 0.008% for platelets and plasma, respectively, at the end of 2015. No septic transfusion reactions, virus transmission or severe adverse reactions (grades III or IV) have been reported to date since the inception of the Mirasol® haemovigilance programme.
Discussion
Cumulative reporting shows an average of 17.5 adverse reactions per 10,000 Mirasol®-treated transfused blood components, which is the same magnitude as the level of 13.8 reported for the year 2014 by the SHOT, a passive haemovigilance programme including data on the transfusion of more than 2.6×106 blood components, such as platelets, plasma (pathogen-inactivated or not), red blood cells and cryoprecipitate, within the United Kingdom8. This finding suggests that the reporting rate of the Mirasol® programme is at an acceptable level for a passive haemovigilance system. Moreover, the rates of adverse reactions remained very stable through the years for both components suggesting consistency of the data. The fact that reporting levels increased over time by regularly participating sites and decreased as soon as new sites joined the programme implies a learning curve for reporting adherence. It is well known that the consistency and quality of reporting increase with time and upon continuous education of professionals in the transfusion chain6. The reduction in adverse reactions might also be associated with the use of platelets stored in platelet additive solution by the last two sites that joined the programme, as has already been reported by others9. Future analyses comparing rates of adverse reactions to platelets stored in plasma with those to platelets stored in platelet additive solution should be performed, once numbers for both types of products are equally reported.
The average rate of 0.34% for adverse reactions to Mirasol®-treated platelets seems to be very similar to the rate reported for platelet components by the Swiss haemovigilance system, which is also based on passive monitoring. It is worth noting that in Switzerland 100% of transfused platelets are treated with an alternative pathogen reduction technology and rates of adverse reactions reached 0.29% for high imputability cases in the year 201410. Both rates are slightly lower than the 0.6% reported recently from a prospective, one-arm, observational haemovigilance study with the same alternative pathogen reduction technology11. The explanation for the differences in rates might lie in the nature of the monitoring system, i.e. passive vs active.
The Mirasol® haemovigilance programme described in this manuscript is based on passive surveillance by professionals involved in the transfusion chain at hospitals and blood banks. Passive haemovigilance by its nature tends to underreport adverse reactions, mostly because patients receiving blood components are generally in poor health and undergoing treatment with multiple drugs, hence symptoms are often confounded. Other possible reasons for underestimation of adverse events include the difficulty of performing post-transfusion surveillance of outpatients and the possibility that delayed transfusion reactions may remain undetected10,12. Nevertheless, even if the numbers reported are regarded as minimal, assuming a relatively constant level of underreporting allows for a surveillance system to identify deviations, investigate risks and perform improvements within the blood transfusion setting8,10.
The system described in this manuscript collects data from collaborating sites that participate on a voluntary basis. This programme included all reports of adverse reactions, since investigation of imputability was not disclosed by all participating sites. Data are cumulative and were analysed at regular intervals in an effort to identify trends. Resource constraints were described as the major reason for a site deciding not to participate or terminating participation in this programme. Although all participating sites are located in the European region, compliance with the European Directive requires only reporting of severe adverse reactions and events. As the Mirasol® programme demands more comprehensive data collection and recording than the minimal requirement of the European Union legislation, adoption of the programme by additional sites has been limited3.
Although over 99% of the plasma transfusion data in the Mirasol® programme originated from a single site, this site supplies blood components to 140 hospitals in the region. The average adverse reaction rate of 0.05%, summing all reports from these hospitals, coincides closely with the adverse reaction rate of 0.06% for high imputability cases reported in Switzerland for fresh-frozen plasma (a mix of solvent/detergent-treated and quarantine plasma).
Finally, it is important to highlight that no severe adverse reactions (grade III or IV) were reported to the Mirasol® haemovigilance programme. In spite of the limitations of the reported haemovigilance system, the consistency of numbers and rates validate the programme’s observations.
Conclusions
In conclusion, constant low adverse reaction rates were observed during the 6 years of passive surveillance of the use of a riboflavin-based pathogen reduction system in multiple sites. The data are consistent with an acceptable safety profile for the Mirasol® PRT system in routine use. Transfusion of Mirasol®-treated components appears to be safe for patients, as indicated by the consistently low rates and severity of reported adverse reactions. Future efforts to collect more exhaustive passive haemovigilance data are being discussed: this would enable a more comprehensive analysis, reinforcing the results presented in this study.
Footnotes
Authorship contributions
DP, ZP-B, TJ-M, LK, AP, AM, PH and SC-DW contributed to the acquisition, reporting, analysis and revision of the manuscript. MC drafted reporting forms, maintained the database, analysed data and drafted the manuscript.
Disclosure of conflicts of interest
DP, ZP-B, TJ-M, LK, AP, AM, PH and SC-DW have no conflicts of interest to disclose. MC is an employee of Terumo BCT.
References
- 1.Klein HG, Anderson D, Bernardi MJ, et al. Pathogen inactivation: making decisions about new technologies. Report of a consensus conference. Transfusion. 2007;47:2338–47. doi: 10.1111/j.1537-2995.2007.01512.x. [DOI] [PubMed] [Google Scholar]
- 2.Mundt JM, Rouse L, Van den Bossche J, Goodrich RP. Chemical and biological mechanisms of pathogen reduction technologies. Photochem Photobiol. 2014;90:957–64. doi: 10.1111/php.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Official Journal of the European Union. Brussels: [Accessed on 26/08/2016]. Commission Directive 2005/61/EC. Available at: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32005L0061&from=EN. [Google Scholar]
- 4.World Health Organization. Global Consultation on Haemovigilance. [Accessed on 10/11/2016]. Available at: http://www.who.int/bloodsafety/haemovigilance/global_consultation/en/
- 5.Jain A, Kaur R. Hemovigilance and blood safety. Asian J Transfus Sci. 2012;6:137–8. doi: 10.4103/0973-6247.98911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries RRP, Faber JC, Strengers PF, et al. Hemovigilance: an effective tool for improving transfusion practice. Vox Sang. 2011;100:60–7. doi: 10.1111/j.1423-0410.2010.01442.x. [DOI] [PubMed] [Google Scholar]
- 7.Council of Europe, EDQM. Guide to the Preparation, Use and Quality Assurance of Blood Components. 18th ed. Strasbourg: Council of Europe Publishing; 2015. [Google Scholar]
- 8.Serious Hazards Of Transfusion (SHOT) Steering Group. Annual SHOT report 2014. [Accessed on 26/08/2016]. Available at: http://www.shotuk.org/wp-content/uploads/a.pdf.
- 9.Cohn CS, Stubbs J, Schwartz J, et al. A comparison of adverse reaction rates for PAS C versus plasma platelet units. Transfusion. 2014;55:1927–34. doi: 10.1111/trf.12597. [DOI] [PubMed] [Google Scholar]
- 10.Swissmedic (Swiss Agency for Therapeutic Products) Haemovigilance Annual Report 2014. [Accessed on 26/08/2016]. Available at: https://www.swissmedic.ch/marktueberwachung/00138/00188/index.html?lang=en.
- 11.Knutson F, Osselaer J, Pierelli L, et al. A prospective, active haemovigilance study with combined cohort analysis of 19,175 transfusions of platelet components prepared with amotosalen-UVA photochemical treatment. Vox Sang. 2015;109:343–52. doi: 10.1111/vox.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong H, Xiao W, Lazarus HM, et al. Detection of septic transfusion reactions to platelet transfusions by active and passive surveillance. Blood. 2016;127:496–502. doi: 10.1182/blood-2015-07-655944. [DOI] [PubMed] [Google Scholar]