Abstract
Background
Scrub typhus is a potentially life-threatening vector-borne infection caused by Orientia species. It occurs mainly in the Asian-Pacific region, where it causes significant morbidity and mortality. Recently, an endemic focus of scrub typhus has been described in South America, on Chiloé Island in southern Chile. Dogs have been used as sentinel hosts to determine the presence and spatial distribution of various vector-borne infections. Their suitability to gain insight into human exposure to Orientia tsutsugamushi has been suggested in studies from Asia.
Methodology
In January 2016, we conducted a cross-sectional study, which included the two main cities on Chiloé Island. Canine blood samples were obtained in households, chosen by double stratified random sampling in urban and by convenience in rural locations. Specimens were tested by ELISA for IgG antibodies against whole-cell antigen preparations from three strains of O. tsutsugamushi. Data were further analyzed for factors associated with seropositivity including spatial clustering.
Results
Serum samples from 202 dogs (104 urban, 98 rural) were tested for IgG against O. tsutsugamushi, of which 43 (21.3%) were positive. Seroprevalence rates were higher in rural than in urban settings (p<0.01) and in older compared to younger dogs (p<0.01). Spatial analysis by LISA indicated the presence of four localities of highly grouped cases.
Conclusions
The detected seroprevalence supports the endemicity of scrub typhus in southern Chile and suggests a wide exposure of household dogs to the infected, yet unknown vector(s). The spatial data will be used for future research identifying further human cases as well as the local vector(s)/reservoirs for scrub typhus in southern Chile. The study reinforces that dogs are useful sentinels for Orientia spp. in regions of uncertain endemicity and distribution.
Introduction
Scrub typhus is a vector-borne zoonosis caused by Orientia species that typically manifests as a febrile disease with or without eschar and/or rash and has a potentially severe outcome [1]. Although widely under-recognized and under-diagnosed, it is considered the most important rickettsial infection worldwide [2]. Until recently, scrub typhus was associated with a single species, Orientia tsutsugamushi, which exclusively occurred within the so-called ‘tsutsugamushi triangle’ ranging from Pakistan in the West, far-eastern Russia in the East to northern Australia in the South [1]. But now with two single cases of the disease observed in 2006, this epidemiological paradigm has been reevaluated. One of the patients was infected in the Middle East by a new Orientia pathogen, named Candidatus Orientia chuto [3], the second case was observed in a Chilean traveler returning from the Chiloé Archipelago in southern Chile [4]. In 2015 and 2016, our group was able to prove further autochthonous scrub typhus cases in the same region [5]. Until now, many aspects of this new infectious disease in South America including the spectrum of causative Orientia species/strains as well as the vectors and zoonotic reservoirs are unknown; still, this finding has important global implications suggesting a much wider geographic distribution of scrub typhus than previously known [6,7].
Since dogs share the same environment and are co-exposed to the same arthropod vectors as their human owners, they are useful sentinel hosts for human diseases [8,9]. This “One Health” principle has been applied in various seroepidemiological surveys to analyze spatial and temporal aspects of tick-borne and other zoonotic pathogens. Dogs are also susceptible to O. tsutsugamushi infection and their suitability to gain insight into human exposure has been suggested in studies from endemic areas in Asia [10–12]. Our study aimed to analyze the prevalence, spatial distribution, and associated factors of seropositivity to Orientia antigens in household dogs from the Chiloé Island in southern Chile.
Methods
A cross-sectional study was conducted in January 2016 in urban and rural areas of Ancud and Castro, the two main cities of the Chiloé Island. Households were chosen by double stratified random sampling per building block in urban and by convenience in rural locations, as described previously [13]. After owners signed informed consent, one dog per household was examined by a veterinarian and blood samples were obtained. Demographic and health information on dogs and their owners were collected by a standardized questionnaire [14] and household locations were recorded using a GPS device. Serum samples were separated from clotted blood and kept at -20°C until shipment to the Naval Medical Research Center (Silver Spring, MD, USA), where they were processed in a blinded manner. Specimens were assessed at 1:100, 1:400, 1:1600, and 1:6400 dilutions for IgG against a mixture of whole-cell antigen preparations from O. tsutsugamushi Karp, Kato and Gilliam strains in an ELISA as described previously [15], except that goat-anti-dog IgG HRP (KPL, Gaithersburg, MD, USA) was used as secondary antibody; this assay has been shown previously to be specific for Orientia species [3, 4, 16]. Samples with a total net absorbance ≥1.000 were considered positive with the titer defined as the inverse of the highest dilution with an OD of ≥0.2 [17]. Serum samples from dogs (n = 5; 3 negative and 2 spotted fever group rickettsia positive samples with antibody titers of 1600 and 6400) of a non-endemic region (USA) were tested to assure the level of non-specific seroreactivity for dog sera. All 5 samples were negative (total net absorbance <1.000) by the Orientia-specific ELISA (data not shown).
To assess factors associated to seropositivity, unconditional logistic regressions analyses of variables of household (education, number of persons, number of dogs, husbandry practices), location (city, setting), and dog (sex, breed, age, anti-parasitic treatment, presence of ticks or fleas) were carried out, followed by a multivariable GLM model with binomial errors. Factors with a likelihood-ratio test p-value <0.15 were used for a multivariable logistic regression. The fit of the fixed-effect model was assessed using the area under the curve (AUC) of the receiver-operating characteristic (ROC) and Hosmer-Lemeshow goodness-of-fit test [18]. Regression analysis to identify influential covariate patterns was carried out by plotting the Pearson’s residual squared (Δχ2), the influence (Δβ), and delta D (ΔD) against the predicted probabilities of being seropositive as suggested by Hosmer and Lemeshow [18]. The diagnostic parameters ΔD and Δχ2 determine the effect of each covariate pattern on the fit of the model by measuring the change in the deviance or χ2 residual, while Δβ measures the effect of each covariate pattern on the value of the estimated parameters. All statistical analyses were carried out in R version 3.4.1 [19]. Additionally, we assessed clustering of seropositive dogs using Nearest Neighbor test, Moran test, and Local indicators of spatial association (LISA test) in ArcGis 10.1. Finally, clustering was further investigated by Cuzick and Edwards' test for inhomogeneous populations. In this analysis, binary data (seropositive, negative) and up to the 6th nearest neighbor were considered. The significance of spatial clustering was assessed by calculating a z-statistic [20].
Ethics statement
The study was approved by the Ethics Committee on Animal Welfare in Research, Faculty of Medicine, Pontificia Universidad Católica de Chile (Protocol #12–033), in accordance with the Terrestrial Animal Health Code of the World Organisation for Animal Health (OIE, 24th Edition, 2015), the Directive 2010/63/EU on the protection of animals used for scientific purposes, and the Chilean Law 20.380 on Animal Protection (2009).
Results
A total of 202 dogs were included, 104 from urban and 98 from rural areas. Most dogs were infested with fleas, whereas ticks and mites were detected in five and two dogs, respectively. A total of 43 dogs (21.3%) were seropositive for Orientia tsutsugamushi with titers from 400 to 1600. Seroprevalence rates were similar in the two cities studied, but higher in rural than in urban areas (Table 1). Univariable logistic regression analysis, which included 12 variables, demonstrated that “Rural Setting” (vs. “Urban Setting”) and “Age ≥ 24 months” (vs. “Age <24 months”) were associated with seropositivity (Table 2). Final analysis by the multivariate model using these two categorical variables confirmed that dogs from rural areas and those older than 24 months were 3.1- and 3.4-times more likely to be seropositive, respectively (Table 3). The Hosmer-Lemeshow test indicated adequate regression model fit (p>0.05).
Table 1. Canine seropositivity rates to Orientia–specific antigens in study cites on Chiloé Island and in previous studies from Asia.
| Study region | N | Positive | Prevalence | 95% CI |
|---|---|---|---|---|
| Chiloé | ||||
| Ancud | 100 | 22 | 22.0% | 15.0–31.1 |
| Rural | 48 | 13 | 27.1% | 16.6–41.0 |
| Urban | 52 | 9 | 17.3% | 9.4–29.7 |
| Castro | 102 | 21 | 20.6% | 13.9–29.4 |
| Rural | 50 | 15 | 30.0% | 19.1–43.8 |
| Urban | 52 | 6 | 11.5% | 5.4–23.0 |
| Rural (all) | 98 | 28 | 28.6% | 20.6–38.2 |
| Urban (all) | 104 | 15 | 14.4% | 8.9–22.4 |
| Total | 202 | 43 | 21.3% | 16.2–27.4 |
| Vietnam [10] | ||||
| Total | 64 | 29 | 45.3% | 33.7–57.4 |
| Malaysia [11] | ||||
| Rural | 97 | 31 | 32.0% | 23.5–41.8 |
| Urban | 97 | 0 | 0% | 0–3.8 |
| Total | 194 | 31 | 16.0% | 11.5–22.8 |
| Sri Lanka [12] | ||||
| Total | 123 | 29 | 23.6% | 16.6–32.3 |
95% CI, 95% confidence interval without continuity correction
Table 2. Univariable Generalized Lineal Model with binomial error indicating the factors associated with Orientia seropositivity in dogs (n = 202) on Chiloé Island.
| Factor | Positives | Negatives | OR | CI95% | p |
|---|---|---|---|---|---|
| City | |||||
| Ancud | 22 | 78 | 1.00 | ||
| Castro | 21 | 81 | 0.92 | 0.47–1.81 | 0.81 |
| Setting | |||||
| Urban | 15 | 89 | 1.00 | ||
| Rural | 28 | 70 | 2.37 | 1.19–4.88 | 0.02* |
| Owner’s education | |||||
| Primary | 15 | 38 | 1.00 | ||
| >Primary | 28 | 117 | 0.61 | 0.29–1.27 | 0.18 |
| Sex | |||||
| Female | 14 | 67 | 1.00 | ||
| Male | 29 | 91 | 1.53 | 0.76–3.18 | 0.25 |
| Age | |||||
| <24 months | 9 | 64 | 1.00 | ||
| ≥ 24 months | 34 | 94 | 2.57 | 1.19–6.04 | 0.02* |
| Pure breed | |||||
| No | 31 | 116 | 1.00 | ||
| Yes | 12 | 42 | 1.07 | 0.49–2.23 | 0.86 |
| Free-roaming | |||||
| No | 6 | 22 | 1.00 | ||
| Yes | 37 | 136 | 0.99 | 0.40–2.87 | 0.99 |
| Antiparasitic treatment | |||||
| No | 26 | 105 | 1.00 | ||
| Yes | 15 | 53 | 1.14 | 0.55–2.32 | 0.72 |
| Presence of ticks | |||||
| No | 41 | 155 | 1.00 | ||
| Yes | 2 | 3 | 2.52 | 0.32–15.8 | 0.32 |
| Presence of fleas | |||||
| No | 10 | 32 | 1.00 | ||
| Yes | 33 | 126 | 0.84 | 0.38–1.96 | 0.67 |
| No of person per household | 1.06 | 0.84–1.33 | 0.61 | ||
| No of dogs per household | 1.20 | 0.87–1.63 | 0.25 |
*Variables used for multivariable analysis
Table 3. Multivariable Generalized Lineal Model with binomial error indicating the factors associated with Orientia seropositivity in dogs (n = 202) on Chiloé Island.
| Risk Factor | OR | 95% CI | P |
|---|---|---|---|
| Site | |||
| Urban | 1.00 | ||
| Rural | 3.10 | 1.51–6.61 | <0.01 |
| Age | |||
| <24 months | 1.00 | ||
| >24 months | 3.38 | 1.52–8.23 | <0.01 |
OR, odd ratio
Hosmer-Lemeshow test: AUC = 0.68; χ2 = 5.34, p = 0.07
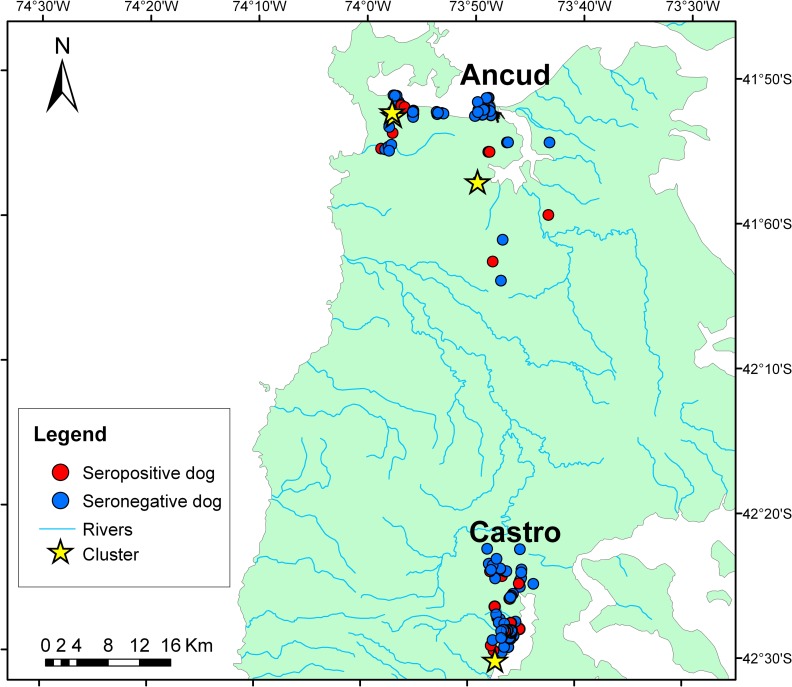
Spatial analysis of positive cases was negative for global clustering (Moran’s Index = –0.24, p = 0.24), but positive for local clusters (Nearest Neighbor testing, z = –6.96, p < 0.0001). The latter test, however, showed also clusters of negative cases (z = –17.5, p < 0.0001), indicating an inhomogeneous dog population in the area. Further analysis by Cuzick and Edwards’ test did not detect clustering (p > 0.05). Calculation of “Local indicators of spatial association” (LISA), however, indicated four highly grouped cases, three in rural areas of Ancud and one in rural Castro (Fig 1).
Fig 1. Geographical distribution of seropositive and -negative dogs and location of grouped cases by LISA analysis on Chiloé Island (Open source maps from Albers C. (2012): Coberturas SIG para la enseñanza de la Geografía en Chile.
Universidad de La Frontera. Temuco. Available at: www.rulamahue.cl/mapoteca.
Discussion
Sentinel surveillance of animals is an established method to detect and/or monitor human environmental and biological threats [21]. Among such sentinel animals, domestic dogs have various advantages. They are almost ubiquitous, live in close vicinity to people, are identifiable by name and owner, easily accessible and safe to sample, and can repeatedly be located for follow-up studies [21]. Canine seroprevalence studies were applied to detect zoonotic pathogens such as Yersinia pestis and Francisella tularensis [22], Trypanosoma cruzi [23], and various tick-borne diseases such as Lyme borreliosis [24]. Similar studies also served to monitor the spatio-temporal epidemiology of rickettsioses in endemic areas [25,26] as well as in regions of uncertain epidemiology, e.g. Germany [27], Sri Lanka [12], Brazil [28] or Australia [29]. Regarding the epidemiology of scrub typhus, dogs have only sporadically been studied, although early Japanese researchers reported them as hosts of “Akamushi” (chigger mites) and susceptible to O. tsutsugamushi infection [30–32]. Dogs have experimentally been infected with O. tsutsugamushi and strain- and dose-dependent clinical signs have been observed in the 1970s [32]. A recent report from Japan detected for the first time O. tsutsugamushi DNA in blood samples of a sick and several asymptomatic dogs [33], data which await further confirmation. Trombiculiasis (infestation with chigger mites) is a known ectoparasitosis in dogs, but the clinical relevance and range of species is uncertain since taxonomical identification of mites is challenging [34]. The concept of using dogs as sentinel hosts for scrub typhus has been applied in three surveys in Asia (Table 1). The first showed that military scout dogs in Vietnam were frequently exposed to O. tsutsugamushi, and that after 6 months of service, their seroprevalence reached >50%. Interestingly, this exposure occurred despite the regular treatment with insecticides, effectively controlling flea infestation and Rickettsia typhi infection [10]. Dogs from rural areas in Malaysia were seroreactive to O. tsutsugamushi in 32% of cases, with geographical variations ranging from 0% to 81%, while dogs from urban study sites were all negative [11]. The third work from Sri Lanka revealed an overall canine seroprevalence of 23.6% [12]; regions of high seropositivity were in accordance with high risk areas for human scrub typhus reported elsewhere [35]. The authors of all three studies proposed that dogs were suitable indicators for the presence of scrub typhus and useful for the surveillance of human exposure.
Dogs might also directly influence human exposure to chigger mites. A survey in Malaysia demonstrated that close contact with dogs and other pets was associated with higher rates of O. tsutsugamushi exposure. The authors proposed that dogs can serve as transport hosts for infected chigger mites, thus increasing the scrub typhus risk for their owners [36]. In our study, mite infestation was reported in two dogs. This number has to be interpreted with caution since, in contrast to larger ectoparasites such as ticks and fleas, a reliable detection and sampling of the fragile chigger mites in larger animals is difficult (without anesthesia). Furthermore, the veterinarians performing the examination in our study were not specifically trained to detect and identify parasitic mites. Vector studies of scrub typhus generally focus on smaller vertebrates such as rodents, partly because they are abundant and can be trapped, euthanized, and examined. This methodological preference, however, might bias our understanding of the complete host spectrum of trombiculid mites [37].
Although Chiloé Island has been identified as a focus of autochthonous scrub typhus [5], our understanding of this new infection in South America is only sketchy. We observed a high seroprevalence against O. tsutsugamushi antigens in dog populations of two study sites in Chiloé suggesting that the infection is endemic in the northern (Ancud) and central part (Castro) of the island. This is in accordance with the diagnosed scrub typhus cases diagnosed by our group during the last three years ([5] and unpublished data). The association of our results to the observed clinical infections suggests that seroprevalence studies in dogs are a useful surveillance tool for Orientia spp. in other regions of uncertain endemicity, e.g. in Chile or other countries in South America. Interestingly, the seroprevalence rates in the studies dog populations were within the same range as those reported in Sri Lanka and Malaysia [11,12]. In accordance with the latter study [11], we detected a higher exposure in dogs from rural areas. This matches the clinical experience in Asia, where the disease is also named “rural typhus” [38] and the cases in Chiloé, who to date were exclusively acquired in rural sites ([5] and unpublished data). The results of our spatial analysis of positive cases were used to determine geographical sites for ongoing studies such as rodent trapping to identify the yet unknown vectors and zoonotic reservoirs in Chile. Interestingly, one of the clusters (south of Ancud) was in very close vicinity to one of the reported human cases [5]. The recognition of hot spots is of particular relevance, since the O. tsutsugamushi infected mites commonly occurs in well-defined foci of “mite islands” [1]. Hence, our work reinforces that dog surveillance is useful to screen for the existence and spatial distribution of Orientia spp. in regions of uncertain epidemiology. Compared to rodents, which are usually used in non-human seroepidemiological surveys, dogs have the advantage, that they are also more convenient to sample than rodents, especially in regions with a risk of Hantavirus Cardiopulmonary Syndrome, and that they are closer related to human environments.
In conclusion, our seroepidemiological study proved the wide and cumulative exposure of household dogs to Orientia spp. in southern Chile, which will contribute to our knowledge of this newly discovered pathogen and its clinical and public health relevance in South America.
Acknowledgments
We thank Heidi St. John for her help with the bibliographic search.
Preliminary results from this study were presented at the 9th Tick and Tick Borne Pathogen Conference & 1st Asia Pacific Rickettsia Conference, August 27–September 1, 2017, Cairns, Australia.
Disclaimers
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense nor the U.S. Government. ALR is an employee of the U.S. Government. This work was prepared as a part of his official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. JJ and ALR tested the supplied sera and provided consultation, but did not perform any animal work at Naval Medical Research Center, USA.
Data Availability
All relevant data are within the paper.
Funding Statement
The work was funded by the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT N° 1130817 and N° 1170810) and by the Global Emerging Infections Surveillance System, a Division of the Armed Forces Health Surveillance Branch, Defense Health Agency, U.S. Department of Defense; work unit #A1402. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kim IS, Walker DH. Scrub Typhus In: Guerrant RL, Walker DH, Weller PF, editors. Tropical Infectious Diseases: Principles, Pathogens and Practice. Third Edition New York: Elsevier Inc.; 2011. p. 334–8. [Google Scholar]
- 2.Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg. 2013;89:301–7. doi: 10.4269/ajtmh.13-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izzard L, Fuller A, Blacksell SD, Paris DH, Richards AL, Aukkanit N, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404–9. doi: 10.1128/JCM.01526-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balcells ME, Rabagliati R, García P, Poggi H, Oddó D, Concha M, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659–63. doi: 10.3201/eid1709.100960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weitzel T, Dittrich S, López J, Phuklia W, Martinez-Valdebenito C, Velásquez K, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954–61. doi: 10.1056/NEJMoa1603657 [DOI] [PubMed] [Google Scholar]
- 6.Walker DH. Scrub Typhus—Scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913–5. doi: 10.1056/NEJMp1608499 [DOI] [PubMed] [Google Scholar]
- 7.Xu G, Walker DH, Jupiter D, Melby PC, Arcari CM. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis. 2017;11:e0006062 doi: 10.1371/journal.pntd.0006062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day MJ. One health: the importance of companion animal vector-borne diseases. Parasit Vectors. 2011;4:49 doi: 10.1186/1756-3305-4-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleaveland S, Meslin FX, Breiman R. Dogs can play useful role as sentinel hosts for disease. Nature. 2006;440:605. [DOI] [PubMed] [Google Scholar]
- 10.Alexander AD, Binn LN, Elisberg B, Husted P, Huxsoll DL, Marshall JD Jr, et al. Zoonotic infections in military scout and tracker dogs in Vietnam. Infect Immun. 1972;5:745–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huxsoll DL, Shirai A, Robinson DM, Yap LF, Lim BL. Presence of antibodies to scrub typhus and murine typhus in dogs from Selangor, Peninsular, Malaysia. Southeast Asian J Trop Med Public Health. 1977;8:232–5. [PubMed] [Google Scholar]
- 12.Nanayakkara DM, Rajapakse RP, Wickramasinghe S, Kularatne SA. Serological evidence for exposure of dogs to Rickettsia conorii, Rickettsia typhi, and Orientia tsutsugamushi in Sri Lanka. Vector Borne Zoonotic Dis. 2013;13:545–9. doi: 10.1089/vbz.2012.1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weitzel T, López J, Acosta-Jamett G, Edouard S, Parola P, Abarca K. Absence of convincing evidence of Coxiella burnetii infection in Chile: a cross-sectional serosurvey among healthy adults in four different regions. BMC Infect Dis. 2016;16:541 doi: 10.1186/s12879-016-1880-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acosta-Jamett G, Cleaveland S, Cunningham AA, Bronsvoort BM. Demography of domestic dogs in rural and urban areas of the Coquimbo region of Chile and implications for disease transmission. Prev Vet Med. 2010;94:272–81. doi: 10.1016/j.prevetmed.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 15.Jiang J, Marienau KJ, May LA, Beecham HJ, Wilkinson R, Ching W-M, et al. Laboratory diagnosis of two scrub typhus outbreaks at Camp Fuji, Japan in 2000 and 2001 by enzyme-linked immunosorbent assay, rapid flow assay, and Western blot assay using outer membrane 56 kDa recombinant proteins. Am J Trop Med Hyg. 2003;69:60–6. [PubMed] [Google Scholar]
- 16.Suwanabun N, Chouriyagune C, Eamsila C, Watcharapichat P, Dasch GA, Howard RS, et al. Evaluation of an enzyme-linked immunosorbent assay in Thai scrub typhus patients. Am J Trop Med Hyg 1997;56:38–43. [DOI] [PubMed] [Google Scholar]
- 17.Jiang J, Myers TE, Rozmajzl PJ, Graf PC, Chretien JP, Gaydos JC, et al. Seroconversions to rickettsiae in US military personnel in South Korea. Emerg Infect Dis 2015;21:1073–4. doi: 10.3201/eid2106.141487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons Inc; 2000. [Google Scholar]
- 19.R-Development-Core-Team R. A Language and Environment for Statistical Computing. Vienna, Austria: the R Foundation for Statistical Computing; Available from: http://www.R-project.org. [Google Scholar]
- 20.Ward MP, Carpenter TE. Techniques for analysis of disease clustering in space and in time in veterinary epidemiology. Prev Vet Med. 2000;45:257–84. [DOI] [PubMed] [Google Scholar]
- 21.Halliday JEB. Animal sentinel surveillance: Evaluating domestic dogs as sentinels for zoonotic pathogen surveillance. University of Edinburg, PhD Thesis, 2010. Available at https://www.era.lib.ed.ac.uk/handle/1842/4794 (accessed 4 May 2018)
- 22.Leighton FA, Artsob HA, Chu MC, Olson JG. A serological survey of rural dogs and cats on the southwestern Canadian prairie for zoonotic pathogens.Can J Public Health. 2001;92:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castañera M, Lauricella M, Chuit R, Gürtler R. Evaluation of dogs as sentinels of the transmission of Trypanosoma cruzi in a rural area of north-western Argentina. Ann Trop Med Parasitol. 1998;92:671–83. [DOI] [PubMed] [Google Scholar]
- 24.Duncan AW, Correa MT, Levine JF, Breitschwerdt EB. The dog as a sentinel for human infection: prevalence of Borrelia burgdorferi C6 antibodies in dogs from southeastern and mid-Atlantic States. Vector Borne Zoonotic Dis. 2005;5:101–9. doi: 10.1089/vbz.2005.5.101 [DOI] [PubMed] [Google Scholar]
- 25.Ortuño A, Pons I, Nogueras MM, Castellà J, Segura F. The dog as an epidemiological marker of Rickettsia conorii infection. Clin Microbiol Infect. 2009;15(Suppl 2):241–2. [DOI] [PubMed] [Google Scholar]
- 26.Yancey CB, Hegarty BC, Qurollo BA, Levy MG, Birkenheuer AJ, Weber DJ, et al. Regional seroreactivity and vector-borne disease co-exposures in dogs in the United States from 2004–2010: utility of canine surveillance. Vector Borne Zoonotic Dis. 2014;14:724–32. doi: 10.1089/vbz.2014.1592 [DOI] [PubMed] [Google Scholar]
- 27.Wächter M, Pfeffer M, Schulz N, Balling A, Chirek A, Bach JP, et al. Seroprevalence of spotted fever group Rickettsiae in dogs in Germany. Vector Borne Zoonotic Dis. 2015;15:191–4. doi: 10.1089/vbz.2014.1715 [DOI] [PubMed] [Google Scholar]
- 28.Batista FG, Silva DM, Green KT, Tezza LB, Vasconcelos SP, Carvalho SG, et al. Serological survey of Rickettsia sp. in horses and dogs in a non-endemic area in Brazil. Rev Bras Parasitol Vet. 2010;19:205–9. [DOI] [PubMed] [Google Scholar]
- 29.Bennett MD, Abdad MY, Stenos J. Serological Evidence of Rickettsia spp. in Western Australian Dogs. Am J Trop Med Hyg. 2017;97:407–12. doi: 10.4269/ajtmh.16-0998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blake FG, Maxcy KF, Sadusk JF, Kohls GM, Bell EJ. Tsutsugamushi Disease (Scrub Typhus, Mite-borne Typhus) in New Guinea. Am J Public Health Nations Health. 1945;35:1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison JL, Audy JR. Hosts of the mite vector of scrub typhus. I. A check list of the recorded hosts. Ann Trop Med Parasitol. 1951;45:171–85. [DOI] [PubMed] [Google Scholar]
- 32.Shirai A, Huxsoll DL, Montrey RD, Werner RM, Arimbalam S. Experimental Rickettsia tsutsugamushi infections in dogs. Jpn J Med Sci Biol. 1979;32:175–8. [DOI] [PubMed] [Google Scholar]
- 33.Namikawa K, Tanabe A, Satake S, Enishi H, Kusaka H, Ide N, et al. Canine Orientia tsutsugamushi infection: report of a case and its epidemicity. Southeast Asian J Trop Med Public Health. 2014;45:395–401. [PubMed] [Google Scholar]
- 34.Stekolnikov AA, Waap H, Gomes J, Antunes T. Chigger mites of the genus Ericotrombidium (Acariformes: Trombiculidae) attacking pets in Europe. Vet Parasitol. 2016;221:60–3. doi: 10.1016/j.vetpar.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 35.Liyanapathirana VC, Thevanesam V. Seroepidemiology of rickettsioses in Sri Lanka: a patient based study. BMC Infect Dis. 2011;11:328 doi: 10.1186/1471-2334-11-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tay ST, Mohamed Zan HA, Lim YA, Ngui R. Antibody prevalence and factors associated with exposure to Orientia tsutsugamushi in different aboriginal subgroups in West Malaysia. PLOS Negl Trop Dis. 2013;7:e2341 doi: 10.1371/journal.pntd.0002341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison JL, Audy JR. Hosts of the mite vector of scrub typhus. II. An analysis of the list of recorded hosts. Ann Trop Med Parasitol. 1951;45:186–94. [DOI] [PubMed] [Google Scholar]
- 38.Kelly DJ, Richards AL, Temenak J, Strickman D, Dasch GA. The past and present threat of rickettsial diseases to military medicine and international public health. Clin Infect Dis. 2002;34(Suppl 4):S145–69. doi: 10.1086/339908 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.