Abstract
Virtual reality has been increasingly used in research on balance rehabilitation because it provides robust and novel sensory experiences in controlled environments. We studied 19 healthy young subjects performing a balance beam walking task in two virtual reality conditions and with unaltered view (15 minutes each) to determine if virtual reality high heights exposure induced stress. We recorded number of steps off the beam, heart rate, electrodermal activity, response time to an auditory cue, and high-density electroencephalography (EEG). We hypothesized that virtual high heights exposure would increase measures of physiological stress compared to unaltered viewing at low heights. We found that the virtual high height condition increased heart rate variability and heart rate frequency power relative to virtual low heights. Virtual reality use resulted in increased number of step-offs, heart rate, electrodermal activity, and response time compared to the unaltered viewing at low heights condition. Our results indicated that virtual reality decreased dynamic balance performance and increased physical and cognitive loading compared to unaltered viewing at low heights. In virtual reality, we found significant decreases in source-localized EEG peak amplitude relative to unaltered viewing in the anterior cingulate, which is considered important in sensing loss of balance. Our findings indicate that virtual reality provides realistic experiences that can induce physiological stress in humans during dynamic balance tasks, but virtual reality use impairs physical and cognitive performance during balance.
1. Introduction
Humans regularly perform activities of daily living and tasks of mobility that require maintenance of dynamic balance. With human aging, balance control can deteriorate [1], leading to falls and other serious consequences [2]. In addition, falls can induce a fear of falling again, potentially leading to a loss of independence [3].
Balance training often reduces the risk of falling [4,5], even more than basic walking tasks [6]. Balance training equipment varies widely from wobble boards [7] to complex balance systems [8]. Because integrating balance training into everyday activities reduces falls [9], virtual reality has been used to motivate users to perform challenging balance tasks in realistic scenarios [10,11]. Dynamic training in virtual reality has improved walking speed for Parkinson’s patients [12] and walking stability for stroke patients [13]. However, many studies project virtual environments onto a screen, which does not move with the user and allows the user to look away [14,15]. Virtual reality presented using a head-mounted display may provide more effective immersion.
To test the realism of a head-mounted display virtual environment during dynamic balance, we exposed subjects to high heights in virtual reality while walking on a balance beam. High heights anxiety is both prevalent [16] and measurably affects dynamic and static stability [17,18]. Human physiological stress levels increase at higher heights and do not noticeably differ across age groups [19]. Immersive virtual reality can provide a cognitive sense of presence where the user feels that they are in a real environment [20,21]. Virtual reality heights exposure is comparable to real-world heights exposure [22], with virtual high heights increasing measures of fear and altering standing posture dynamics [23,24]. In addition, presence during virtual heights exposure can be enhanced by having subjects stand with their feet on a ledge raised only a few centimeters off the ground [25]. Given these results, virtual high heights should alter stress levels during a dynamic locomotor task.
Despite advances in physiological recording methods, stress remains challenging to quantify. Cortisol level is considered one of the best standards for stress detection because cortisol is generated by the hypothalamus-pituitary-adrenal axis directly in response to stress, but measuring cortisol from blood or urine is invasive. Salivary cortisol, while non-invasive, has less fine time resolution than blood cortisol, creating a time lag between stress and cortisol levels [26]. Heart rate variability is affected by both parasympathetic and sympathetic activity, which vary based on stress levels [27]. It has been generally thought that stress induces less heart rate variability [28,29], but results are conflicting and likely depend on the paradigm and stressor used [30]. Electrodermal activity may also indicate stress, as it is affected by sympathetic activity [31]. Electrodermal activity contains a tonic (slow) and phasic (fast) component [32], with increased phasic activity relating to increases in stress [33,34]. Other ways to quantify stress include cognitive task performance [35,36] and EEG activity [30]. Our primary outcome measures of stress were electrodermal activity and heart rate variability because of their direct connections to sympathetic and parasympathetic responses and the ease of recording them during a dynamic balance task.
In addition to stress, we wanted to quantify the physical and cognitive effects of virtual reality use. A head-mounted display moves with the user, which may be advantageous for dynamic balance training compared to screen displays, but immersive virtual reality may induce motion sickness. Motion sickness varies greatly across people and virtual reality setups [37], so it is important to limit and quantify its effects. To estimate cognitive loading, we measured response time to an auditory stimulus [38,39]. We also wanted to know where a potential change in cognitive load was occurring in the brain. For this, we used EEG source localization, which has revealed areas of the brain involved during balance beam walking [40]. This added complexity helped us determine which cognitive centers were most affected by virtual reality use during beam-walking. EEG has been used before to measure cognitive response to stimuli in virtual reality [41].
The purpose of this study was to determine if high height exposure in virtual reality induced stress and if virtual reality use affected physical and cognitive performance during a dynamic balance-beam walking task. Our hypotheses were: 1) subjects’ stress would increase at a high virtual height compared to a low virtual height, as measured by increases in heart rate variability and electrodermal activity, and 2) virtual reality use during beam-walking would increase cognitive load compared to no virtual reality use during the task, as measured by increased response time to an auditory stimulus and decreases in EEG event-related activity peak amplitude. We included a virtual reality low height condition, which matched the beam height of the unaltered view low height condition, for this second comparison. We found that high virtual heights induced stress, and virtual reality use at low heights increased cognitive loading compared to beam-walking without the headset, confirming both hypotheses.
2. Materials and methods
2.1. Subjects
Human subject research was approved by the University of Michigan Health Sciences and Behavioral Sciences Institutional Review Board (HUM00100932) for the protection of human subjects. All subjects provided written informed consent. Nineteen healthy subjects participated in the study (10 male, age 23±4 years old (mean±SD)). All subjects identified themselves as right hand and right foot dominant. Subjects were screened for any orthopedic, cardiac, or neurological conditions and injuries. Any subjects indicating they experienced acrophobia (fear of heights) were excluded from the study because we wanted all subjects to be able to complete the full experiment.
Prior to the main experiment session, we screened subjects for motion sickness in virtual reality. Subjects stood in place while wearing the headset (Oculus Rift DK2, Oculus VR, Irvine, CA) for 5 minutes. Subjects moved around a virtual environment using body gestures tracked by a Microsoft Kinect V2 (Microsoft, Redmond, WA). We intentionally included this disconnect between real and virtual movements to be more disorienting than the experiment. Subjects were allowed to participate in the main experiment if both the experimenter and subject agreed that the subject did not exhibit any symptoms of motion sickness. Two subjects exhibited symptoms of motion sickness and did not perform the experiment; 19 subjects passed this screening process.
2.2. Experiment setup
We tested subjects on a 3.8 cm-wide by 2.5 cm-tall by 3.05 meter-long wooden balance beam, similar to previous studies [42,43]. We attached the beam to a non-skid surface to prevent it from slipping. Subjects walked the entire length of the beam in one direction, referred to as a beam pass. After completing a beam pass, subjects walked over-ground back to their starting position. Subjects walked heel-to-toe with their arms crossed over their chest. We did not make subjects follow a specific gait speed to avoid any effects from attending to this speed. We demonstrated a desired pacing of 0.22 m/s and informed the subject if he or she was walking too fast or too slow. We chose this speed based on previous beam-walking experiments [40,42]. We also instructed subjects to look at their feet while balancing in all 3 conditions.
Subjects performed the same physical beam-walking task under 3 viewing conditions: unaltered view low, virtual reality low, and virtual reality high. Unaltered view low involved normal viewing without virtual reality. For virtual reality low and virtual reality high, subjects wore the Oculus virtual reality headset. Subjects viewed themselves 2.5 cm off the ground in virtual reality low, which agreed with the real-world balance-beam height, and 15 meters off the ground in virtual reality high (Fig 1). To enhance the effects in the virtual reality high condition, subjects “fell” 15 meters in the virtual environment when they stepped off the beam. Both virtual reality conditions contained a virtual beam that was aligned with the physical beam. In all 3 conditions, subjects performed the same balance task on the physical beam. Subjects took 10 minute breaks between each condition. We randomized the order of the virtual reality conditions, but all subjects performed the unaltered view low condition second to spread out virtual reality use during the experimental session.
Fig 1. Subject setup and virtual reality views.
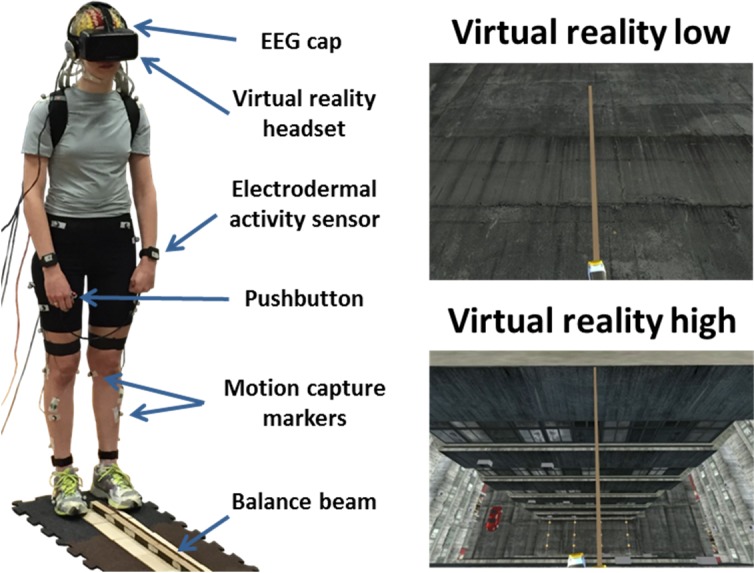
Typical subject setup is shown (left). All subjects walked on the same physical beam for all three conditions. In both virtual reality conditions, subjects saw a virtual beam that aligned with the physical beam. In virtual reality low, the virtual beam was the same height off the ground as in unaltered view low (top right), while the virtual beam was 15 meters off the ground in virtual reality high (bottom right).
The virtual environment was rendered using Unity 5 software (Unity Technologies, San Francisco, CA) and included a virtual avatar controlled by the Microsoft Kinect. This computer used a NVIDIA Titan X graphics card (NVIDIA, Santa Clara, CA) to avoid slow-downs in the virtual reality presentation. Because humans more reliably perceive heights when they have a body in virtual reality [44], each subject had a virtual avatar. This avatar mimicked the subject’s movements in the virtual environment, using the Kinect tracking with the ‘Kinect v2 Examples with MS-SDK’ Unity package. We did not have the Kinect control the avatar’s arms, hands, and toes because the Kinect could not reliably track them during the experiment. Because the Kinect can only reliably track a user that faces it, subjects made beam passes in one direction and walked over-ground in the other direction. Each condition ended after 15 minutes of forward beam passes. We choose a fixed time instead of a fixed number of passes so that subjects were not encouraged to walk faster.
2.3. Performance & physiological measures
While beam-walking, subjects wore several sensors to measure physiological and cognitive activity. To determine gait events, we placed 30 reflective motion capture markers placed on the feet and legs of each subject, sampled at 100 Hz (Vicon, Los Angeles, CA). A wearable device (Empatica E4) was placed on both wrists to record electrodermal activity (Empatica, Milan, Italy). We recorded from both wrists to average out any unreliable activity [45]. Fig 1 shows a representative subject during testing. Subjects also completed surveys after the experiment ended to assess motion sickness in virtual reality (Motion Sickness Assessment Questionnaire [46]) and high heights apprehension (Heights Interpretation Questionnaire [47]).
We analyzed beam-walking performance using motion capture markers at each foot. Marker traces were cleaned in Vicon Nexus and further processed in Visual3D (C-Motion, Germantown, MD). We implemented a similar algorithm as Zeni et al. to find gait events and manually inspected each trial to ensure accuracy [48]. We quantified balance performance by determining the number of times balance was lost divided by the total time spent on the beam. This metric is known as failures per minute and has previously assessed beam-walking performance [42,43]. By including the total time spent on the beam, faster walkers are not rewarded more than slower walkers for making fewer mistakes. In addition, we recorded the number of beam passes for each condition. We also estimated beam-walking speed by calculating the time subjects were on the beam and using the total number of passes as an estimate of distance.
We recorded heart rate via an electrode taped over the sternum. We reduced line noise using the Cleanline EEGLAB plugin and bandpass filtered between 5 and 20 Hz. Kubios HRV software found the R-peaks, which correspond to heartbeats [49]. We manually adjusted incorrectly labelled peaks. We determined heart rate variability as the standard deviation of the heart rate time series. We calculated heart rate frequency power by taking the fast Fourier transform power spectrum of the inter-beat intervals and determining the percent of total power not in the 0.04–0.15 Hz range. Kubios calculated all heart rate and heart rate variability metrics based on guidelines in the field [50].
Electrodermal activity data was processed using Ledalab [51,52], which uses deconvolution to separate the tonic and phasic components of the signal. We ran deconvolution with the default parameters. Electrodermal activity responses were calculated as differences in the deconvoluted phasic signal greater than 0.05μS [53]. We averaged responses across each condition and normalized by condition duration in minutes.
While beam-walking, subjects were instructed to listen for an auditory tone and press a pushbutton upon hearing the tone. We designed this secondary task to be simple because balance performance may worsen if it is too challenging [54,55]. Tones were spaced randomly 7–9 seconds apart, consistent with previous research [56]. We recorded the time subjects took to respond to the tone as an estimator of cognitive load during the beam-walking task. Increased cognitive load during beam-walking would be accompanied by decreased attention to the auditory tone task, resulting in increased response times.
2.4. Auxiliary experiment
To determine if any differences found in our measures were caused by simply wearing the headset, we performed an auxiliary experiment on 20 subjects (10 male, age 24±5 years old (mean±SD)). Four subjects participated in both the main and auxiliary experiments, but on separate days. All subjects gave written informed consent, and the protocol was approved by the University of Michigan Institutional Review Board for the protection of human subjects. Subjects performed 4 randomized 5-minute blocks of sitting and standing, both with and without the headset. Subjects were asked to stand and sit up straight while staring at a fixation cross displayed at eye level. We recorded the same electrodermal activity, response time, and heart rate metrics as the main experiment.
2.5. Fatigue assessment
Because we were concerned about fatigue, we quantified changes in failures per minute, heart rate, and response time during each condition. We chose these measures because they estimate motor performance, physical exertion, and cognitive loading, each of which can be affected by fatigue. We calculated percent change as the difference between the last and first 3 minutes of each condition, all divided by the first 3 minutes and converted to a percent. We divided the difference by the first 3 minutes because we wanted to see how each measure changed relative to its initial value during the first 3 minutes. If fatigue was present, we would expect to see a large percent change from the first 3 minutes to the last 3 minutes.
2.6. EEG data
In addition to response time, we recorded EEG to determine if specific brain areas showed increases in cognitive load during beam-walking. By comparing peak EEG activity following the tone, we can determine changes in electrocortical activity across conditions. Because an increase in cognitive loading during the main task likely results in less focus on the secondary task, we would expect a corresponding decrease in event-related peak amplitude [57,58]. We performed independent components analysis (ICA) to find brain source activity from the channel data [59]. We used ICA because event-related potentials show distinct activity from compact sources in the brain [60]. Unlike response time, EEG with source localization provides insight into cognitive loading differences in specific brain areas. We recorded EEG using a 136-channel BioSemi Active II system (BioSemi, Amsterdam, NL), sampled at 512 Hz. The EEG AD-box and battery were placed in a backpack worn by the subject [61].
EEG data was processed using custom scripts in EEGLAB [62]. We high-pass filtered the data at 1 Hz and removed noisy channels [63,64]. We removed 12±8 bad channels (mean±SD) and interpolated them to maintain a consistent montage across the head. We ran AMICA 15 on the data [65,66], using principal component analysis to reduce down to 100 principal components prior to ICA. This was less than the minimum number of channels remaining following bad channel removal (102 channels), ensuring that the data sent into ICA remained full rank.
After obtaining independent components, we fit the ICA scalp maps to equivalent current dipoles using DIPFIT2 [67]. Independent components with residual variance <15% were retained for further analysis. We manually rejected independent components with non-brain activity, using power spectra and dipole location. We manually rejected 17±4 dipoles and retained 7±3 (mean±SD) cortical dipoles per subject. Brain dipoles were grouped using k-means clustering, using weights of 10, 2, and 1 for dipole location, power spectra, and scalp maps, respectively.
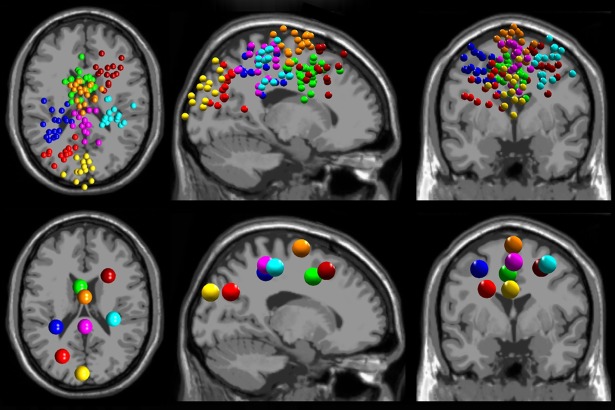
We grouped the 178 total dipoles into 11 clusters. We retained 8 clusters containing dipoles from more than half (>9) the total subjects (Fig 2): anterior parietal (12 subjects, 17 dipoles), left sensorimotor (11 subjects, 17 dipoles), right frontal (11 subjects, 14 dipoles), anterior cingulate (15 subjects, 27 dipoles), medial occipital (11 subjects, 13 dipoles), supplementary motor area (14 subjects, 21 dipoles), left posterior parietal (12 subjects, 13 dipoles), and right sensorimotor (13 subjects, 16 dipoles). We epoched the data from -300 to 800 ms around the auditory tone presentation, subtracted average activity across each epoch, and rejected epochs with amplitude outside ±75 μV to remove excessive artifact. We removed 1±1 trials for unaltered view low and 2±3 for virtual reality low, resulting in 102±4 trials for unaltered view low and 102±10 trials for virtual reality low (mean±SD). We only analyzed auditory events occurring while subjects were on the beam. We then calculated event-related potential activity time-locked to the auditory stimulus onset for each cluster. Auditory tone onset was set at time 0. We subtracted out 300 ms of average activity preceding the stimulus as baseline activity.
Fig 2. EEG source localization results.
EEG source localization results are shown for the 8 cortical clusters found across all subjects (n = 19). Dipole locations (top) and cluster centroids (bottom) are shown in transverse, sagittal, and coronal views (left to right). We found clusters in anterior parietal (purple), left sensorimotor (blue), right frontal (maroon), anterior cingulate (green), medial occipital (yellow), supplementary motor area (orange), left posterior parietal (red), and right sensorimotor (cyan).
2.7. Statistical analyses
For main experiment mean physiological and behavioral data, we used non-parametric Friedman and Wilcoxon signed-rank tests due to non-normal data. We were not interested in comparing unaltered view low and virtual reality high due to difficulty interpreting any differences between these two conditions. We used Bonferroni correction for multiple comparisons, except for electrodermal activity, failures per minute, heart rate, and response time because these were planned, a priori comparisons. For the auxiliary experiment and fatigue comparison of percent change, differences across conditions were determined using non-parametric Friedman and post-hoc Wilcoxon signed-rank tests with Bonferroni correction for multiple comparisons. Non-EEG statistical analyses were performed using SPSS Statistics 22 (IBM SPSS Statistics 22.0, Armonk, NY, United States). For EEG event-related activity, we used EEGLAB permutation statistics with 8000 permutations to calculate pairwise comparisons and false discovery rate to correct for multiple comparisons [68]. Significance was determined to be less than 0.05 for all statistical tests.
3. Results
3.1. Survey results
Subjects did not experience substantial motion sickness from participating in the study. Results from the motion sickness survey are shown in Table 1, using a normalized percentage scale (0–100%). 0% suggests motion sickness was completely absent during testing, while 100% indicates motion sickness was fully present across all subjects. Questions are also grouped into subsections referencing different factors of motion sickness. All subsection scores were less than 25% across subjects, with a score of 5.8% for feeling nauseous. We were primarily concerned with adverse effects from nausea and dizziness, but subjects reported minimal effects from these areas. Also, subjects scored 15.1%±14.9% (mean±SD) on the Heights Interpretation Questionnaire, with 0% indicating no high heights apprehension.
Table 1. Motion sickness assessment results.
| Feeling nauseous | 5.8% (7.5%) |
| Feeling dizzy | 12.0% (9.8%) |
| Feeling sweaty | 21.3% (22.0%) |
| Feeling tired | 20.2% (14.9%) |
| Total | 14.2% (10.3%) |
Mean motion sickness assessment scores are shown normalized from 0–100%, with standard deviation in parentheses (n = 19). The motion sickness assessment contains four subsections to analyze four factors of motion sickness: gastrointestinal (feeling nauseous), central (feeling dizzy), peripheral (feeling sweaty), and sopite (feeling tired).
3.2. Physiological and behavioral measures
We found significant increases in heart rate variability and heart rate frequency power in virtual reality high compared to virtual reality low (p = 0.015 and p = 0.006, respectively), as shown in Table 2. Both heart rate variability and heart rate frequency power did not significantly differ between unaltered view low and virtual reality low (p = 1.0 and p = 0.108). Electrodermal activity did not significantly differ between virtual reality conditions (p = 0.738), but did significantly increase in virtual reality low compared to unaltered view low (p = 0.009).
Table 2. Behavioral and physiological measures.
| Measure Value | P Value | ||||
|---|---|---|---|---|---|
| Measure | UVL | VRL | VRH | UVL|VRL | VRL|VRH |
| Heart Rate Variability (beats/min) | 6.6 (1.5) | 7.0 (1.6) | 8.3 (2.4) | 1.00 | 0.02* |
| Heart Rate Frequency Power (%) | 45.6 (12.5) | 51.8 (15.2) | 58.9 (14.0) | 0.11 | <0.01* |
| Electrodermal Activity (counts/min) | 8.2 (11.7) | 15.1 (14.7) | 13.7 (11.0) | <0.01* | 0.74 |
| Failures per Minute (counts/min) | 7.1 (2.5) | 26.1 (4.9) | 24.8 (5.6) | <0.01* | 1.00 |
| Heart Rate (beats/min) | 92.0 (7.9) | 97.0 (8.7) | 97.1 (10.6) | <0.01* | 1.00 |
| Response Time (s) | 0.76 (0.25) | 0.88 (0.25) | 0.94 (0.27) | 0.02* | 0.10 |
| Number of Beam Passes | 43.0 (15.7) | 26.6 (9.4) | 24.7 (7.6) | <0.01* | 0.38 |
| Estimated Gait Speed (m/s) | 0.18 (0.07) | 0.13 (0.04) | 0.13 (0.04) | 0.03* | 1.00 |
Mean behavioral and physiological measures are shown for unaltered view low (UVL), virtual reality low (VRL) and virtual reality high (VRH), with standard deviation in parentheses (n = 19 for each condition). The first 3 measures assessed stress induction. The other measures assessed cognitive and physical performance. All measures shown had significant Friedman test results across conditions. Pairwise comparison p-values are shown:
* denotes significant differences (p<0.05).
We only made two comparisons: 1) unaltered view low vs. virtual reality low and 2) virtual reality low vs. virtual reality high.
Subjects’ heart rate and response time significantly increased in virtual reality low compared to unaltered view low (p<0.001 and p = 0.018, respectively), indicating increased physical and cognitive exertion in virtual reality. We found no significant differences in heart rate (p = 1.0) and response time (p = 0.103) between virtual reality conditions.
Subjects’ balance performance significantly worsened in virtual reality low compared to unaltered view low (Table 2), as measured by failures per minute (p<0.001). There was no significant difference between virtual reality conditions (p = 1.0). In addition, subjects performed significantly more beam passes in unaltered view low compared to virtual reality low (p<0.001), likely because fewer step-offs occurred. We found no significant differences in beam passes between virtual reality conditions (p = 0.378). Subjects also beam-walked significantly faster in unaltered view low compared to virtual reality low (p = 0.027). We found no significant difference in gait speed between virtual reality conditions (p = 1.0). Gait speeds for all groups were lower than our desired speed of 0.22 m/s, potentially due to the difficulty of the beam-walking task.
3.3. Auxiliary experiment results
Our auxiliary experiment found few significant differences, and none of these differences appear to occur from wearing the virtual reality headset (Table 3). We only found significant effects for heart rate and heart rate variability (p<0.001 and p = 0.006). Heart rate increased when standing with the headset on and off compared to sitting with the headset on (p = 0.004 and p<0.001) and sitting with the headset off (p = 0.022 and p = 0.007). Comparisons within standing conditions and within sitting conditions had non-significant p-values (p = 1.0), suggesting that heart rate significantly changes due to alterations in physical task performance (sitting vs. standing), not from wearing the headset. While heart rate variability significantly differed across conditions, we did not find any significant pairwise comparisons. Sitting with the headset on decreased heart rate variability compared to standing with the headset off, but was not significant (p = 0.061). This difference may be caused by sitting vs. standing and has the opposite trend compared to the main experimental results. Electrodermal activity, response time, and heart rate frequency power did not significantly differ across conditions. Our auxiliary study of headset effects indicates that the significant differences found during beam-walking are likely not caused by just wearing the headset.
Table 3. Sitting/standing experiment results.
| Measure | Sit Headset Off | Sit Headset On | Stand Headset Off | Stand Headset On |
|---|---|---|---|---|
| Heart Rate Variability (beats/min) | 4.9 (1.5) | 4.5 (1.7) | 6.0 (1.5) | 5.6 (2.0) |
| Heart Rate Frequency Power (%) | 60.2 (15.5) | 59.7 (15.9) | 54.6 (16.5) | 50.8 (14.4) |
| Electrodermal Activity (counts/min) | 4.5 (9.9) | 2.8 (5.3) | 8.1 (12.7) | 6.9 (10.1) |
| Heart Rate (beats/min) | 72.7 (10.5)* | 70.7 (10.2)* | 88.4 (14.5)✝ | 86.0 (13.1)✝ |
| Response Time (s) | 0.63 (0.14) | 0.66 (0.14) | 0.62 (0.11) | 0.66 (0.13) |
* significantly different from standing conditions
✝ significantly different from sitting conditions
Mean physiological results are shown for the auxiliary headset experiment, with standard deviation in parentheses (n = 20). Pairwise significance was determined following a significant Friedman test (p<0.05). Heart rate and heart rate variability had significant Friedman test results, with significant pairwise differences in heart rate found between sitting and standing. No other significant differences were found.
3.4. Fatigue assessment results
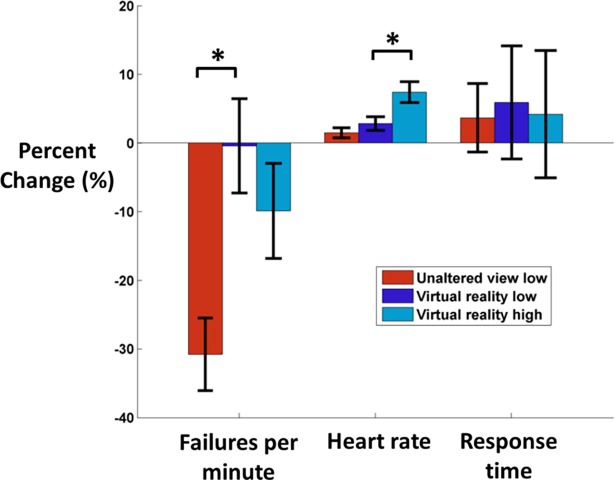
We found significant differences across conditions related to fatigue for heart rate and failures per minute, but not for response time (Fig 3). Subjects’ percent change in failures per minute significantly increased in virtual reality compared to unaltered view low (p = 0.004), suggesting that virtual reality impaired motor acquisition. Percent change in failures per minute did not significantly differ between virtual reality conditions (p = 0.703). Heart rate percent change did not significantly differ between virtual reality low and unaltered view low (p = 0.489). Heart rate percent change significantly increased in virtual reality high compared to virtual reality low (p = 0.021), which may have been induced by stress from virtual high heights. Response time percent change did not significantly differ between unaltered view low and virtual reality low (1.0) and between virtual reality conditions (1.0), suggesting that subjects did not experience significantly different cognitive fatigue across conditions.
Fig 3. Percent change in failures per minute, heart rate, and response time.
To assess fatigue effects, we calculated the percent change (mean±SE) between the first and last 3 minutes of each condition (n = 19). Failures per minute, heart rate, and response time are shown for unaltered view low (red), virtual reality low (dark blue), and virtual reality high (light blue). Negative percent change indicates that the value in the final 3 minutes decreased compared to the first 3 minutes. Failures per minute percent change significantly decreased in unaltered view low compared to virtual reality low. Heart rate percent change significantly increased in virtual reality high compared to virtual reality low. No other comparisons were significant between 1) unaltered view low vs. virtual reality low and 2) virtual reality low vs. virtual reality high.
3.5. EEG data
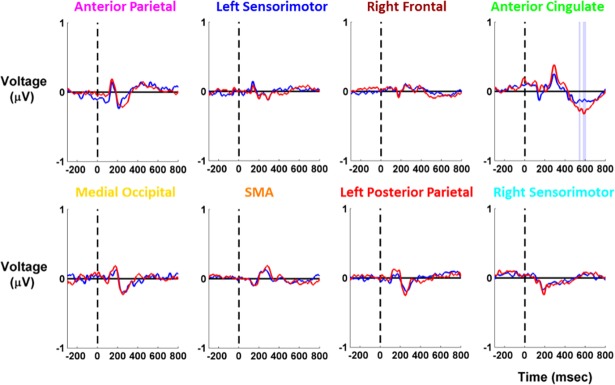
We found significant differences in EEG event-related activity following the tone for the anterior cingulate cluster only (Fig 4). Because response time significantly differed between unaltered view low and virtual reality high but not between virtual reality conditions, we only compared EEG activity between unaltered view low and virtual reality low. In the anterior cingulate, virtual reality low peak activity significantly increased from 500–600 ms after the tone compared to unaltered view low. We were not concerned about motion artifact in the EEG recordings due to time-locking to a cognitive event and the slow gait speeds during the task (Table 2).
Fig 4. EEG event-related activity for cortical clusters.
EEG event-related activity is shown for each cortical cluster (n = 19), with unaltered view low in red and virtual reality low in blue. Tone presentation occurred at 0 ms, preceded by 300 ms of baseline activity. We analyzed 800 ms following the tone presentation. Shading reflects the condition with significantly higher amplitude (red for unaltered view low, blue for virtual reality low). We found significant differences in the anterior cingulate cluster only.
4. Discussion
We found that heart rate variability indicated increased stress at virtual high heights, which agreed with our first hypothesis. Subjects’ response time also significantly increased in virtual reality low compared to unaltered view low, but this difference was not due to fatigue. Significantly decreased EEG event-related peak activity in anterior parietal and anterior cingulate areas further corroborated our response time findings, confirming our second hypothesis.
We also found increased heart rate variability during virtual high heights exposure compared to low virtual heights exposure (Table 2). Increased heart rate variability during stress runs contrary to some studies [28,29], but agrees with a recent study using an acute stressor [30]. Such a discrepancy may arise from the paradigm that induced stress. In addition, a faster heart rate makes it more difficult to have high heart rate variability because there would be less time in a heartbeat cycle for variation in timing [69]. Heart rate variability significantly increased in virtual reality high compared to virtual reality low, despite both conditions having similar heart rates.
Another measure of stress, heart rate frequency power, was also greater during virtual high heights exposure compared with virtual low heights exposure Table 2). Heart rate frequency power focused on the percent of total power at all frequencies except 0.04–0.15 Hz. This excluded band contains a mix of sympathetic and parasympathetic responses [27]. The frequency power in this band decreased with high heights exposure, despite an increased heart rate in virtual reality. This suggests that the 0.04–0.15 Hz frequency band primarily measured parasympathetic response during the task, which significantly decreased with virtual high heights exposure. It is worth noting that parasympathetic changes can occur rapidly (milliseconds) compared to the sympathetic response (seconds) [70]. The sympathetic response may have been masked by increased physical exertion. While many studies focus on the high frequency power band (0.15–0.4 Hz) or on the ratio of low to high frequency power [71,72], a decrease in low frequency power with stress has also been documented [28].
While both heart rate variability metrics supported our hypothesis that stress was induced during virtual high heights exposure, electrodermal activity was primarily affected by physical exertion, instead of stress (Table 2). Electrodermal activity only measures the sympathetic response and has been shown to increase under both stress and physical loading [73]. Our findings contrast with stationary studies that have found decreased electrodermal activity in virtual reality [24,74]. This suggests that physical exertion primarily affected electrodermal activity. This is an important consideration for future experiments and highlights the challenges of quantifying stress, particularly during paradigms with high physical exertion. While we presented the phasic results of electrodermal activity here, we found similar results for the slower tonic component as well.
Subjects performed worse on the beam-walking task in virtual reality, based on failures per minute (Table 2). In addition, we found that subjects significantly lowered their failures per minute in unaltered view low viewing compared to virtual reality low (Fig 3). This indicates that both motor performance and motor acquisition were impaired by virtual reality use. Virtual reality use has been shown to worsen balance performance, with comparable stability to being blindfolded [75,76]. Subjects may have had difficulty adapting to virtual reality, reflected by increased physical and cognitive exertion.
The cognitive load of the subjects was greater during virtual reality use than during the unaltered view low condition, as measured by significantly increased response time in virtual reality low (Table 2). Significantly decreased EEG peak amplitude also indicated increased cognitive loading in the anterior cingulate cluster during virtual reality use (Fig 4). Similar decreases in event-related activity have been seen for this type of secondary auditory task when subjects performed a more challenging cognitive task [77]. The anterior cingulate is important for maintaining balance [40] as it is thought to perform error-detection [78][79]. Bogost et al. also found that the activity of the anterior cingulate and somatosensory area weakened during a reactive balance task when performing a challenging secondary task [80]. Dual-task interference during balance also reduces activity in sensorimotor and sensory areas in parietal cortex [81]. Other studies have found strong EEG activity in these regions during balance control with eyes open [40,82] and eyes closed [83]. Increased cognitive loading in the anterior cingulate may affect error detection while balancing, which may help explain why balance performance significantly worsened during virtual reality viewing.
While virtual reality induces realistic stress during virtual high height exposure, virtual reality headsets leave something to be desired during postural control. Low latency and limited field of view may have affected balance performance. The latency of the headset was 60 frames per second, but the movement generated by the Kinect was approximately 30 frames per second, which was likely noticeable to the user. In addition, the Kinect may not have provided ideal body tracking, which could break a subject’s sense of presence in virtual reality. Such breaks in presence can alter cognitive processing [84] and may have affected how realistic the virtual reality high heights experience felt to each subject. The headset also had a 110 degree field of view. In contrast, humans have at least a 180 degree field of view [85], and peripheral vision plays a primary role in worsen postural control [86,87]. However, virtual reality can still impair stability even when controlling for latency and field of view [88]. Virtual reality headsets continue to improve, and other options such as augmented reality may improve balance without the limitations of virtual reality headsets. This experiment establishes useful measures for assessing future virtual reality headsets in a dynamic setting.
5. Conclusions
Dynamic virtual reality exposure to high heights induces stress, indicating that this setup could provide realistic scenarios during dynamic balance training. However, technological limitations of virtual reality headsets currently limit the efficacy of balancing with a virtual reality headset. Balance performance, physical exertion, and cognitive loading provided a comprehensive quantification of how virtual reality use affects healthy young adults. Virtual reality technology needs to facilitate comparable balance to real world use before assisting patients with a fear of falling. Virtual reality technology will continue to develop, and we expect that future virtual reality headsets will achieve comparable results to balancing without a headset on.
Acknowledgments
We would like to thank Zachary Ohs and Estefania Rios for their assistance with processing motion capture data, along with Jay Krembs for assisting in the development of our gait detector for the motion capture data. We would also like to thank members of the Human Neuromechanics Laboratory for their input regarding the experiment design and data processing.
Data Availability
All annotated EEG data, electrodermal activity, and timestamp data files are available from the figshare database (https://figshare.com/projects/Effects_of_virtual_reality_high_heights_exposure_during_beam-walking_on_physiological_stress_and_cognitive_loading/28545).
Funding Statement
This research was sponsored by a Graduate Research Fellowship Program grant from the National Science Foundation (DGE 1256260) to Steven M. Peterson: https://www.nsfgrfp.org/. It was also sponsored by the Army Research Laboratory under Cooperative Agreement Number W911NF-10-2-0022 (Cognition and Neuroergonomics Collaborative Technology Alliance) to Daniel P. Ferris: https://www.arl.army.mil/www/default.cfm?page=393. The views and conclusions contained in this research article are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Army Research Laboratory or the United States Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kanekar N, Aruin AS. The effect of aging on anticipatory postural control. Exp Brain Res. 2014;232: 1127–1136. doi: 10.1007/s00221-014-3822-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sterling DA, O’Connor JA, Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J Trauma. 2001;50: 116–119. [DOI] [PubMed] [Google Scholar]
- 3.Lach HW. Incidence and risk factors for developing fear of falling in older adults. Public Health Nurs. 2005;22: 45–52. doi: 10.1111/j.0737-1209.2005.22107.x [DOI] [PubMed] [Google Scholar]
- 4.Carter ND, Kannus P, Khan KM. Exercise in the prevention of falls in older people: a systematic literature review examining the rationale and the evidence. Sports Med. 2001;31: 427–438. [DOI] [PubMed] [Google Scholar]
- 5.Kannus P, Sievänen H, Palvanen M, Järvinen T, Parkkari J. Prevention of falls and consequent injuries in elderly people. Lancet. 2005;366: 1885–1893. doi: 10.1016/S0140-6736(05)67604-0 [DOI] [PubMed] [Google Scholar]
- 6.Sherrington C, Whitney JC, Lord SR, Herbert RD, Cumming RG, Close JCT. Effective Exercise for the Prevention of Falls: A Systematic Review and Meta-Analysis. J Am Geriatr Soc. 2008;56: 2234–2243. doi: 10.1111/j.1532-5415.2008.02014.x [DOI] [PubMed] [Google Scholar]
- 7.Ogaya S, Ikezoe T, Soda N, Ichihashi N. Effects of balance training using wobble boards in the elderly. J Strength Cond Res. 2011;25: 2616–2622. doi: 10.1519/JSC.0b013e31820019cf [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim MS, Mattar AG, Elhafez SM. Efficacy of virtual reality-based balance training versus the Biodex balance system training on the body balance of adults. J Phys Therapy Sci. 2016;28: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemson L, Fiatarone Singh MA, Bundy A, Cumming RG, Manollaras K, O’Loughlin P, et al. Integration of balance and strength training into daily life activity to reduce rate of falls in older people (the LiFE study): randomised parallel trial. BMJ. 2012;345: e4547 doi: 10.1136/bmj.e4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duque G, Boersma D, Loza-Diaz G, Hassan S, Suarez H, Geisinger D, et al. Effects of balance training using a virtual-reality system in older fallers. Clin Interv Aging. 2013;8: 257–263. doi: 10.2147/CIA.S41453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalron A, Fonkatz I, Frid L, Baransi H, Achiron A. The effect of balance training on postural control in people with multiple sclerosis using the CAREN virtual reality system: a pilot randomized controlled trial. J Neuroeng Rehabil. 2016;13: 13 doi: 10.1186/s12984-016-0124-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease? J Gerontol A Biol Sci Med Sci. 2011;66: 234–240. doi: 10.1093/gerona/glq201 [DOI] [PubMed] [Google Scholar]
- 13.Darekar A, McFadyen BJ, Lamontagne A, Fung J. Efficacy of virtual reality-based intervention on balance and mobility disorders post-stroke: a scoping review. J Neuroeng Rehabil. 2015;12: 46 doi: 10.1186/s12984-015-0035-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booth V, Masud T, Connell L, Bath-Hextall F. The effectiveness of virtual reality interventions in improving balance in adults with impaired balance compared with standard or no treatment: a systematic review and meta-analysis. Clin Rehabil. 2013;28: 419–431. doi: 10.1177/0269215513509389 [DOI] [PubMed] [Google Scholar]
- 15.Corbetta D, Imeri F, Gatti R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: a systematic review. J Physiother. 2015;61: 117–124. doi: 10.1016/j.jphys.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 16.Huppert D, Grill E, Brandt T. Down on heights? One in three has visual height intolerance. J Neurol. 2013;260: 597–604. doi: 10.1007/s00415-012-6685-1 [DOI] [PubMed] [Google Scholar]
- 17.Carpenter MG, Frank JS, Silcher CP. Surface height effects on postural control: a hypothesis for a stiffness strategy for stance. J Vestib Res. 1999;9: 277–286. [PubMed] [Google Scholar]
- 18.Schniepp R, Kugler G, Wuehr M, Eckl M, Huppert D, Huth S, et al. Quantification of gait changes in subjects with visual height intolerance when exposed to heights. Front Hum Neurosci. 2014;8: 963 doi: 10.3389/fnhum.2014.00963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown LA, Polych MA, Doan JB. The effect of anxiety on the regulation of upright standing among younger and older adults. Gait Posture. 2006;24: 397–405. doi: 10.1016/j.gaitpost.2005.04.013 [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Vives MV, Slater M. From presence to consciousness through virtual reality. Nat Rev Neurosci. 2005;6: 332–339. doi: 10.1038/nrn1651 [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Franco M, Lanier J. Model of Illusions and Virtual Reality. Front Psychol. 2017;8: 1125 doi: 10.3389/fpsyg.2017.01125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emmelkamp PM, Bruynzeel M, Drost L, van der Mast CA. Virtual reality treatment in acrophobia: a comparison with exposure in vivo. Cyberpsychol Behav. 2001;4: 335–339. doi: 10.1089/109493101300210222 [DOI] [PubMed] [Google Scholar]
- 23.Seinfeld S, Bergstrom I, Pomes A, Arroyo-Palacios J, Vico F, Slater M, et al. Influence of Music on Anxiety Induced by Fear of Heights in Virtual Reality. Front Psychol. 2015;6: 1969 doi: 10.3389/fpsyg.2015.01969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cleworth TW, Horslen BC, Carpenter MG. Influence of real and virtual heights on standing balance. Gait Posture. 2012;36: 172–176. doi: 10.1016/j.gaitpost.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 25.Meehan M, Insko B, Whitton M, Brooks FP. Physiological measures of presence in stressful virtual environments. Proceedings of the 29th annual conference on Computer graphics and interactive techniques—SIGGRAPH ‘02. 2002. doi: 10.1145/566570.566630
- 26.Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34: 163–171. doi: 10.1016/j.psyneuen.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 27.Thayer JF, Ahs F, Fredrikson M, Sollers JJ 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36: 747–756. doi: 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 28.Clays E, De Bacquer D, Crasset V, Kittel F, de Smet P, Kornitzer M, et al. The perception of work stressors is related to reduced parasympathetic activity. Int Arch Occup Environ Health. 2011;84: 185–191. doi: 10.1007/s00420-010-0537-z [DOI] [PubMed] [Google Scholar]
- 29.Boesch M, Sefidan S, Ehlert U, Annen H, Wyss T, Steptoe A, et al. Mood and autonomic responses to repeated exposure to the Trier Social Stress Test for Groups (TSST-G). Psychoneuroendocrinology. 2014;43: 41–51. doi: 10.1016/j.psyneuen.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 30.Schlink BR, Peterson SM, Hairston WD, König P, Kerick SE, Ferris DP. Independent Component Analysis and Source Localization on Mobile EEG Data Can Identify Increased Levels of Acute Stress. Front Hum Neurosci. 2017;11: 310 doi: 10.3389/fnhum.2017.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Critchley HD. Electrodermal responses: what happens in the brain. Neuroscientist. 2002;8: 132–142. doi: 10.1177/107385840200800209 [DOI] [PubMed] [Google Scholar]
- 32.Lim CL, Rennie C, Barry RJ, Bahramali H, Lazzaro I, Manor B, et al. Decomposing skin conductance into tonic and phasic components. Int J Psychophysiol. 1997;25: 97–109. [DOI] [PubMed] [Google Scholar]
- 33.Giromini L, Ando’ A, Morese R, Salatino A, Di Girolamo M, Viglione DJ, et al. Rorschach Performance Assessment System (R-PAS) and vulnerability to stress: A preliminary study on electrodermal activity during stress. Psychiatry Res. 2016;246: 166–172. doi: 10.1016/j.psychres.2016.09.036 [DOI] [PubMed] [Google Scholar]
- 34.Reinhardt T, Schmahl C, Wüst S, Bohus M. Salivary cortisol, heart rate, electrodermal activity and subjective stress responses to the Mannheim Multicomponent Stress Test (MMST). Psychiatry Res. 2012;198: 106–111. doi: 10.1016/j.psychres.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 35.Lasikiewicz N, Hendrickx H, Talbot D, Dye L. Exploring stress-induced cognitive impairment in middle aged, centrally obese adults. Stress. 2013;16: 44–53. doi: 10.3109/10253890.2012.682109 [DOI] [PubMed] [Google Scholar]
- 36.Munoz E, Sliwinski MJ, Scott SB, Hofer S. Global perceived stress predicts cognitive change among older adults. Psychol Aging. 2015;30: 487–499. doi: 10.1037/pag0000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolasinski EM, U.S. Army Research Institute for the Behavioral and Social Sciences. Simulator sickness in virtual environments. 1995.
- 38.Regenbogen C, De Vos M, Debener S, Turetsky BI, Mössnang C, Finkelmeyer A, et al. Auditory processing under cross-modal visual load investigated with simultaneous EEG-fMRI. PLoS One. 2012;7: e52267 doi: 10.1371/journal.pone.0052267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabri M, Humphries C, Verber M, Liebenthal E, Binder JR, Mangalathu J, et al. Neural effects of cognitive control load on auditory selective attention. Neuropsychologia. 2014;61: 269–279. doi: 10.1016/j.neuropsychologia.2014.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sipp AR, Gwin JT, Makeig S, Ferris DP. Loss of balance during balance beam walking elicits a multifocal theta band electrocortical response. J Neurophysiol. 2013;110: 2050–2060. doi: 10.1152/jn.00744.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.González-Franco M, Peck TC, Rodríguez-Fornells A, Slater M. A threat to a virtual hand elicits motor cortex activation. Exp Brain Res. 2014;232: 875–887. doi: 10.1007/s00221-013-3800-1 [DOI] [PubMed] [Google Scholar]
- 42.Domingo A, Ferris DP. Effects of physical guidance on short-term learning of walking on a narrow beam. Gait Posture. 2009;30: 464–468. doi: 10.1016/j.gaitpost.2009.07.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Domingo A, Ferris DP. The effects of error augmentation on learning to walk on a narrow balance beam. Exp Brain Res. 2010;206: 359–370. doi: 10.1007/s00221-010-2409-x [DOI] [PubMed] [Google Scholar]
- 44.Gutekunst M, Geuss M, Rauhoeft G, Stefanucci JK, Kloos U, Mohler B. Short Paper: A Video Self-avatar Influences the Perception of Heights in an Augmented Reality Oculus Rift. ICAT-EGVE 2014. 2014; 9–12. [Google Scholar]
- 45.Picard RW, Fedor S, Ayzenberg Y. Multiple Arousal Theory and Daily-Life Electrodermal Activity Asymmetry. Emot Rev. 2015;8: 62–75. [Google Scholar]
- 46.Gianaros PJ, Muth ER, Mordkoff JT, Levine ME, Stern RM. A questionnaire for the assessment of the multiple dimensions of motion sickness. Aviat Space Environ Med. 2001;72: 115–119. [PMC free article] [PubMed] [Google Scholar]
- 47.Steinman SA, Teachman BA. Cognitive processing and acrophobia: validating the Heights Interpretation Questionnaire. J Anxiety Disord. 2011;25: 896–902. doi: 10.1016/j.janxdis.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeni JA Jr, Richards JG, Higginson JS. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture. 2008;27: 710–714. doi: 10.1016/j.gaitpost.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarvainen MP, Niskanen J-P, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV—heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113: 210–220. doi: 10.1016/j.cmpb.2013.07.024 [DOI] [PubMed] [Google Scholar]
- 50.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93: 1043–1065. [PubMed] [Google Scholar]
- 51.Benedek M, Kaernbach C. A continuous measure of phasic electrodermal activity. J Neurosci Methods. 2010;190: 80–91. doi: 10.1016/j.jneumeth.2010.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benedek M, Kaernbach C. Decomposition of skin conductance data by means of nonnegative deconvolution. Psychophysiology. 2010;47: 647–658. doi: 10.1111/j.1469-8986.2009.00972.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt S, Walach H. Electrodermal activity (EDA)–state-of-the-art measurement and techniques for parapsychological purposes. J Parapsychol. 2000;64: 139–163. [Google Scholar]
- 54.Howell DR, Osternig LR, Chou L-S. Consistency and cost of dual-task gait balance measure in healthy adolescents and young adults. Gait Posture. 2016;49: 176–180. doi: 10.1016/j.gaitpost.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 55.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16: 1–14. [DOI] [PubMed] [Google Scholar]
- 56.Lawrence CA, Barry RJ. Cognitive processing effects on auditory event-related potentials and the evoked cardiac response. Int J Psychophysiol. 2010;78: 100–106. doi: 10.1016/j.ijpsycho.2010.06.027 [DOI] [PubMed] [Google Scholar]
- 57.Gentili RJ, Rietschel JC, Jaquess KJ, Lo L-C, Prevost M, Miller MW, et al. Brain biomarkers based assessment of cognitive workload in pilots under various task demands. Conf Proc IEEE Eng Med Biol Soc. 2014;2014: 5860–5863. doi: 10.1109/EMBC.2014.6944961 [DOI] [PubMed] [Google Scholar]
- 58.Neelon MF, Williams J, Garell PC. The effects of attentional load on auditory ERPs recorded from human cortex. Brain Res. 2006;1118: 94–105. doi: 10.1016/j.brainres.2006.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makeig S, Bell AJ, Jung TP, Sejnowski TJ. Independent component analysis of electroencephalographic data. Adv Neur In. 1996;8: 145–151. [Google Scholar]
- 60.Makeig S. Dynamic Brain Sources of Visual Evoked Responses. Science. 2002;295: 690–694. doi: 10.1126/science.1066168 [DOI] [PubMed] [Google Scholar]
- 61.Kline JE, Poggensee K, Ferris DP. Your brain on speed: cognitive performance of a spatial working memory task is not affected by walking speed. Front Hum Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134: 9–21. doi: 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- 63.Kline JE, Huang HJ, Snyder KL, Ferris DP. Isolating gait-related movement artifacts in electroencephalography during human walking. J Neural Eng. 2015;12: 046022 doi: 10.1088/1741-2560/12/4/046022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oliveira AS, Schlink BR, Hairston WD, König P, Ferris DP. Restricted vision increases sensorimotor cortex involvement in human walking. J Neurophysiol. 2017;118: 1943–1951. doi: 10.1152/jn.00926.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palmer JA, Kreutz-Delgado K, Makeig S. Super-Gaussian Mixture Source Model for ICA. Lecture Notes in Computer Science. 2006. pp. 854–861. [Google Scholar]
- 66.Palmer JA, Makeig S, Kreutz-Delgado K, Rao BD. Newton method for the ICA mixture model. 2008 IEEE International Conference on Acoustics, Speech and Signal Processing. 2008. doi: 10.1109/icassp.2008.4517982
- 67.Oostenveld R, Oostendorp TF. Validating the boundary element method for forward and inverse EEG computations in the presence of a hole in the skull. Hum Brain Mapp. 2002;17: 179–192. doi: 10.1002/hbm.10061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luck SJ. An Introduction to the Event-related Potential Technique. Bradford Book; 2014. [Google Scholar]
- 69.McCraty R, Shaffer F. Heart Rate Variability: New Perspectives on Physiological Mechanisms, Assessment of Self-regulatory Capacity, and Health risk. Glob Adv Health Med. 2015;4: 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thayer JF, Hansen AL, Johnsen BH. The Non-invasive Assessment of Autonomic Influences on the Heart Using Impedance Cardiography and Heart Rate Variability. Handbook of Behavioral Medicine. 2010. pp. 723–740. [Google Scholar]
- 71.Hjortskov N, Rissén D, Blangsted AK, Fallentin N, Lundberg U, Søgaard K. The effect of mental stress on heart rate variability and blood pressure during computer work. Eur J Appl Physiol. 2004;92: 84–89. doi: 10.1007/s00421-004-1055-z [DOI] [PubMed] [Google Scholar]
- 72.Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M. Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biol Psychiatry. 2004;55: 953–956. doi: 10.1016/j.biopsych.2003.12.018 [DOI] [PubMed] [Google Scholar]
- 73.Poh M-Z, Swenson NC, Picard RW. A wearable sensor for unobtrusive, long-term assessment of electrodermal activity. IEEE Trans Biomed Eng. 2010;57: 1243–1252. doi: 10.1109/TBME.2009.2038487 [DOI] [PubMed] [Google Scholar]
- 74.Simeonov PI, Hsiao H, Dotson BW, Ammons DE. Height effects in real and virtual environments. Hum Factors. 2005;47: 430–438. doi: 10.1518/0018720054679506 [DOI] [PubMed] [Google Scholar]
- 75.Akizuki H, Uno A, Arai K, Morioka S, Ohyama S, Nishiike S, et al. Effects of immersion in virtual reality on postural control. Neurosci Lett. 2005;379: 23–26. doi: 10.1016/j.neulet.2004.12.041 [DOI] [PubMed] [Google Scholar]
- 76.Horlings CGC, Carpenter MG, Küng UM, Honegger F, Wiederhold B, Allum JHJ. Influence of virtual reality on postural stability during movements of quiet stance. Neurosci Lett. 2009;451: 227–231. doi: 10.1016/j.neulet.2008.12.057 [DOI] [PubMed] [Google Scholar]
- 77.Shaw EP, Rietschel JC, Hendershot BD, Pruziner AL, Miller MW, Hatfield BD, et al. Measurement of attentional reserve and mental effort for cognitive workload assessment under various task demands during dual-task walking. Biol Psychol. 2018;134: 39–51. doi: 10.1016/j.biopsycho.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 78.Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3: 516–520. doi: 10.1038/74899 [DOI] [PubMed] [Google Scholar]
- 79.Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: the role of the human superior parietal lobe. Nat Neurosci. 1998;1: 529–533. doi: 10.1038/2245 [DOI] [PubMed] [Google Scholar]
- 80.Bogost MD, Burgos PI, Little CE, Woollacott MH, Dalton BH. Electrocortical Sources Related to Whole-Body Surface Translations during a Single- and Dual-Task Paradigm. Front Hum Neurosci. 2016;10: 524 doi: 10.3389/fnhum.2016.00524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Little CE, Woollacott M. EEG measures reveal dual-task interference in postural performance in young adults. Exp Brain Res. 2015;233: 27–37. doi: 10.1007/s00221-014-4111-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Slobounov S, Cao C, Jaiswal N, Newell KM. Neural basis of postural instability identified by VTC and EEG. Exp Brain Res. 2009;199: 1–16. doi: 10.1007/s00221-009-1956-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hülsdünker T, Mierau A, Strüder HK. Higher Balance Task Demands are Associated with an Increase in Individual Alpha Peak Frequency. Front Hum Neurosci. 2015;9: 695 doi: 10.3389/fnhum.2015.00695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Padrao G, Gonzalez-Franco M, Sanchez-Vives MV, Slater M, Rodriguez-Fornells A. Violating body movement semantics: Neural signatures of self-generated and external-generated errors. Neuroimage. 2016;124: 147–156. doi: 10.1016/j.neuroimage.2015.08.022 [DOI] [PubMed] [Google Scholar]
- 85.Walker HK, Hall WD, Hurst JW. Clinical methods: the history, physical, and laboratory examinations. 1976. [PubMed] [Google Scholar]
- 86.Amblard B, Carblanc A. Role of foveal and peripheral visual information in maintenance of postural equilibrium in man. Percept Mot Skills. 1980;51: 903–912. doi: 10.2466/pms.1980.51.3.903 [DOI] [PubMed] [Google Scholar]
- 87.Assaiante C, Marchand AR, Amblard B. Discrete visual samples may control locomotor equilibrium and foot positioning in man. J Mot Behav. 1989;21: 72–91. [DOI] [PubMed] [Google Scholar]
- 88.Kelly JW, Riecke B, Loomis JM, Beall AC. Visual control of posture in real and virtual environments. Percept Psychophys. 2008;70: 158–165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All annotated EEG data, electrodermal activity, and timestamp data files are available from the figshare database (https://figshare.com/projects/Effects_of_virtual_reality_high_heights_exposure_during_beam-walking_on_physiological_stress_and_cognitive_loading/28545).