Abstract
Although interferon (IFN)-based treatment of patients with chronic hepatitis C virus (HCV) infection is widely applied, treatment resistance is often observed in patients with advanced liver fibrosis. Given that the molecular mechanisms of IFN resistance in liver fibrosis remain elusive, the present study investigated the effects of extracellular matrix (ECM) on IFN signaling in hepatic cells. The native HuH-7 human hepatoma cell line and HuH-7 cells were stably transfected with full-length HCV-RNA fused with Renilla luciferase (OR6 cells) were cultured on ECM-coated dishes or non-coated plastic dishes (NDs), and treated with human IFN-α. In Huh-7 cells cultured on coated dishes, the IFN-stimulated response element (ISRE) luciferase activity was measured following ISRE plasmid transfection and the expression of IFN-stimulated genes (ISG) were significantly lower than those in cells cultured on NDs. In addition, after IFN-α treatment, the amount of HCV-RNA and viral protein produced by OR6 cells cultured on coated dishes was higher than that produced by cells cultured on NDs. When cells were treated with β1-integrin-blocking antibody to disrupt the cell-matrix interaction, the ISRE luciferase activity was restored, and the protein expression of ISG was increased, while that of HCV proteins was suppressed. Treatment of cells with integrin-linked kinase (ILK) inhibitor or focal adhesion kinase (FAK) inhibitor restored the ISRE luciferase activity and expression of ISG proteins. These results suggested that β1-integrin-mediated signals affected the IFN signaling and promoted HCV replication. Therefore, the accumulation of ECM in liver fibrosis may impair IFN signaling through β1-integrin-mediated signaling involving ILK and FAK.
Keywords: extracellular matrix, β1-integrin, hepatitis C virus, interferon, interferon-stimulated genes
Introduction
Interferon (IFN) has important roles in innate immunity to fight off viral infections and has been widely used for the treatment of patients with hepatitis C virus (HCV) infection (1-3). Secreted and/or exogenously administered IFN-α/β binds to the IFN-α/β receptors and activates the Janus kinase (Jak)/signal transducer and activator of transcription (STAT) pathway, in which the receptor-associated protein kinases Jak1 and tyrosine kinase 2 cause the phosphorylation of STAT proteins on critical serine and tyrosine residues. The activated STATs associate with IFN-stimulated gene factor (IRF)9 to form an IRF3 transcription factor complex, and then stimulate the expression of IFN-stimulated genes (ISGs) through the IFN-stimulated response element (ISRE) by interacting with its promoter/enhancer region. Hundreds of ISGs induced by IFN act as effectors of the host defense against viruses, including HCV.
Polyethylene glycol-conjugated IFN (PEG-IFN) and ribavirin therapy for HCV achieves a sustained viral response (SVR) of >70% in genotype 2/3- and 40-60% in genotype 1-infected patients (4,5). Numerous host and viral factors have been associated with resistance to IFN-based anti-HCV treatment, and the presence of liver fibrosis remains an important factor influencing the failure of HCV clearance (6-10). Combination treatment of PEG-IFN and ribavirin with recently developed direct-acting antivirals (DAAs) further increases the response rate to 80-90% SVR for HCV genotype 1. However, the response rates of patients with advanced liver fibrosis and cirrhosis are still lower (11,12). More recently, DAAs have also made it possible to treat HCV infection without IFN. DAA combination therapies for HCV are well-tolerated and effective for viral suppression, reaching a probable SVR of >90% in clinical trials (13-15). However, it has been reported that even with IFN-free DAA regimens, an endogenous, intrahepatic type I IFN response may be important for achieving SVR (16). Therefore, investigating the factors that antagonize IFN signaling in HCV treatments may be important, irrespective of whether or not the treatment itself involves IFN.
Excessive accumulation of extracellular matrix (ECM) components, including fibrillar type I and III collagens, fibronectin and laminin, is a feature of liver fibrosis (17,18), and these fibrotic ECM components provoke diverse cellular responses, mainly through the integrin family transmembrane receptors (19). Increased ECM stimulates integrin receptor-mediated signaling in hepatocytes and promotes the growth and survival of cells through the activation of several signaling cascades, including the phosphoinositide-3 kinase (PI3K), mitogen-activated protein kinase (MAPK) and transforming growth factor (TGF)-β/Smad signaling pathways (20-22).
Although the presence of liver fibrosis remains an important factor influencing the response to IFN-based anti-HCV therapy, the molecular mechanisms by which liver fibrosis prevents IFN from eliminating HCV have remained to be fully elucidated, and the direct roles of ECMs in the IFN signaling pathway in hepatic cells remain unknown. The present study investigated the effects of ECMs on the IFN signaling cascade in hepatic cells and indicated that the presence of ECMs, including fibrotic collagen, attenuated IFN-mediated signaling in a β1-integrin-dependent manner and inhibited the effects of IFN on HCV replication.
Materials and methods
Cells and cell culture
The human hepatoma-derived cell line HuH-7 was obtained from the Japanese Cancer Research Resources Bank (Osaka, Japan). OR6 cells derived from HuH-7 cells with the stable transfection of the full-length genotype 1 replicon containing the Renilla luciferase gene were selected by neomycin, ORN/C-5B/KE (23), were used to examine the anti-HCV effect of IFN-α. The cells were cultured and maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1% antibiotics (ampicillin/streptomycin) in 5% CO2 at 37°C. ECM (type I collagen, laminin, type IV collagen or fibronectin)-coated dishes (Cosmo Bio, Tokyo, Japan) were used for cell culture to investigate the differences in cell signaling between cells cultured on ECM-coated dishes and those cultured on non-ECM-coated dishes, which had hydroxyl and carboxyl groups on the surface to facilitate cell adhesion (cat. no. 150687; Thermo Fisher Scientific, Inc.).
Reagents and antibodies
Human IFN-α was obtained from Merck KGaA. The β1-integrin function-blocking antibody was purchased from EMD Millipore (Billerica, MA, USA; cat. no. MABT821). The rabbit polyclonal anti-IFN-stimulated gene (ISG) 15 (cat. no. 2743S) and anti-protein kinase R (PKR; cat. no. 3072S) antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). The antibody against the HCV core protein (cat. no. ab2740) and HCV nonstructural protein (NS) 5A (cat. no. ab13833) were purchased from Abcam (Cambridge, UK). The rabbit polyclonal anti-β-actin antibody (Cell Signaling Technology, Inc.; cat. no. 4967) was used as a control. Anti-rabbit horseradish peroxidase (HRP) conjugated IgG (cat. no. 7074; Cell Signaling Technology, Inc.) was used as the secondary antibody. The integrin-linked kinase (ILK) inhibitor Cpd 22 was purchased from EMD Millipore, and the focal adhesion kinase (FAK) inhibitor PF 573228 was from Sigma-Aldrich (Merck KGaA).
Plasmids and luciferase assays
The ISRE-inducible lucif-erase reporter plasmid (p-ISRE-Luc) was from Invitrogen (Thermo Fisher Scientific, Inc.). The ISRE-dependent transcriptions were detected by a luciferase assay performed with the Dual-Luciferase Reporter Assay System (Promega Corporation, Madison, WI, USA) according to the manufacturer's protocol. Values were normalized to the luciferase activity of the co-transfected pGL4.75 Renilla luciferase-expressing plasmid (Promega Corp.). HCV-RNA replication in OR6 cells was also detected with the Renilla luciferase assay system (Promega Corp.).
HuH-7 cells or OR6 cells were seeded onto 48-well plates with hydroxyl and carboxyl groups on the surface to facilitate cell adhesion and 48-well type I collagen-coated plates (cat. no. 354505; Cosmo Bio) at 1×104 cells per well. After culture for 48 h, HuH-7 cells were transfected with p-ISRE-Luc, a luciferase reporter plasmid driven by the promoter region of ISRE (Clontech Laboratories, Inc., Mountainview, CA, USA) and co-transfected with pGL4.75, a plasmid that encodes the Renilla luciferase reporter gene (Promega Corporation), using Lipofectamine™ LTX and PLUS ligand (Thermo Fisher Scientific, Inc.) in accordance with the manufacturer's protocols. Following incubation for 6 h, the medium was changed to serum- and antibiotic-free medium. The cells were then treated with IFN-α at the indicated concentrations for 12 h. OR6 cells were cultured for 48 h and subsequently treated with IFN-α at the indicated concentrations for 12 h. Following the IFN-α treatment, HuH-7 and OR6 cells were washed twice with PBS and lysed. The cell extracts were immediately assayed for luciferase activity using a Multi-label plate reader (Wallac 1420 ARVOsx; PerkinElmer, Inc., Waltham, MA, USA).
Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR)
The total RNA was extracted from the cultured hepatocellular carcinoma cells using ISOGEN (Nippon Gene, Tokyo, Japan) in accordance with the manufacturer's protocol. The concentration of RNA was determined with a spectrophotometer, and the integrity of the samples was confirmed by visualizing 28S and 18S ribosomal RNA bands under ultraviolet light after gel electrophoresis. RT-PCR was performed as described previously (24). The primers used in the experiments were as follows: ISG15 sense, 5′-GCAGCGAACTCATCTTTG-3′ and antisense, 5′-GCCCTTGTTATTCCTCACC-3′; PKR sense, 5′-GTCCTCTGGTTCTTTTGCTAC-3′ and antisense, 5′-TCCCAACAGCCATTGTAG-3′; GAPDH sense, 5′-ACGCATTTGGTCGTATTGGG-3′ and antisense 5′-TGATTTTGGAGGGATCTCGC-3′.
Western blot analysis
HuH-7 or OR6 cells were seeded on 35-mm plastic dishes or 35-mm type I collagen-coated dishes at 2×105 cells per dish and cultured under various conditions for 48 h. They were collected and lysed with extraction buffer containing 50 mM Tris (pH 7.5), 0.1% SDS and 1 mM phenylmethylsulfonylfluoride. The lysate was sonicated for 5 min (sonication for 10 sec, pause for 20 sec, repeated 10 times) at 4°C and clarified by centrifugation at 12,000 × g for 10 min, and the supernatant was then collected. After measuring the protein concentration using a protein assay kit (cat. no. 5000113-5000115; Bio-Rad Laboratories, Hercules, CA, USA), 30 µg of protein was mixed with SDS sample buffer, separated by SDS-PAGE (6% acrylamide for β1-integrin, 10% for PKR, HCV-NS5A, HCV core protein and β-actin, 15% for ISG15), transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories) and blocked with 0.1% Tween-20 and 5% skimmed milk overnight at 4°C. The membranes were incubated with the primary antibodies described above in Tris-buffered saline with 1% skimmed milk at a dilution of 1:1,000 overnight at 4°C. The specific bands were visualized by further incubation with anti-rabbit HRP-conjugated secondary antibodies at 1:1,000 dilution in Tris-buffered saline with 1% skimmed milk for 1 h at room temperature, followed by a chemiluminescence reaction using Amersham ECL Prime (GE Healthcare, Little Chalfont, UK) in accordance with the manufacturer's protocol.
Treatment of cells with anti-β1-integrin antibody
To investigate whether the ECM affects IFN signaling via β1-integrin, cells were treated with β1-integrin function-blocking antibody. HuH-7 cells were cultured as described above and treated with β1-integrin function-blocking antibody at 1 µg/l in DMEM for 6 h at 37°C prior to IFN-α treatment.
Treatment of cells with inhibitors
To investigate whether the effect of ECM on IFN signaling proceeds via ILK or FAK, cells were treated with ILK inhibitor or FAK inhibitor. HuH-7 cells were cultured as described above and treated with FAK or ILK inhibitor at 0.1 and 1 µM, respectively, prior to IFN-α treatment.
Statistical analyses
Differences between two groups were analyzed using Student's t-test, and P<0.05 was considered to indicate a statistically significant difference. All experiments were performed at least three times. Values are expressed as the mean ± standard deviation. Analysis of variance followed by a post-hoc multiple comparisons test was performed to compare multiple groups. Tukey's test was used when all pairwise comparisons were performed and Dunnett's test was used when one control was compared to all other experiment groups. We used JMP® version 12 (SAS Institute, Inc., Cary, NC, USA) software for statistical analysis.
Results
Impairment of IFN-α signaling in HuH-7 on ECM-coated dishes
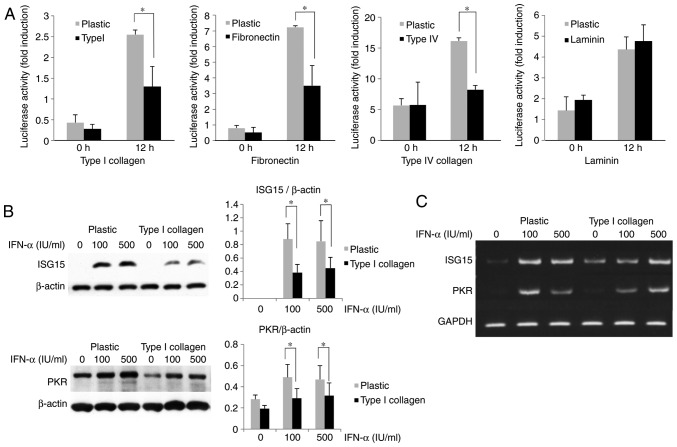
With the progression of liver fibrosis, the levels of ECM components are increased in the liver. To evaluate the effects of ECM on IFN signaling, HuH-7 cells were cultured on ECM (type I collagen, fibronectin, type IV collagen and laminin)-coated dishes and compared to cells cultured on non-coated plastic dishes. The cells were transfected with ISRE-luciferase plasmids, and the luciferase activities were measured after IFN-α treatment. As presented in Fig. 1A, the ISRE luciferase activities of HuH-7 cells induced by IFN-α were significantly reduced when they were cultured on plates coated with ECM components, except for laminin, compared with those cultured on normal plastic plates. The IFN-α-induced mRNA or protein expression of ISGs, including ISG15 and PKR, in HuH-7 cells cultured on type I collagen-coated dishes was compared with those in cells cultured on non-coated plastic dishes. As displayed in Fig. 1B and C, the expression of ISG15 and PKR was decreased at the mRNA and protein level, indicating that IFN-α signaling was attenuated in cells grown on the type I collagen-coated dishes.
Figure 1.
Extracellular matrix attenuates IFN-α-induced ISRE activity of HuH-7. (A) Inhibition of IFN-α-induced ISRE luciferase activity in cells grown on ECM-coated dishes. HuH-7 cells were cultured on plastic dishes or type I collagen-coated dishes for 2 days and subsequently treated with 100 U/ml IFN-α for 12 h. (B) Attenuation of IFN-α-induced ISG (ISG15 and PKR) expression in HuH-7 cells grown on type I collagen-coated dishes. HuH-7 cells were cultured on plastic dishes or type I collagen-coated dishes for 3 days. The ISG15 and PKR expression was measured by western blot analysis after IFN-α treatment for 12 h. β-actin was used as the control. Values are expressed as the mean ± standard deviation (n=3). *P<0.05. (C) Reverse transcription polymerase chain reaction analysis of ISG15 and PKR mRNA expression in HuH-7 cells cultured in the same manner as in B. GAPDH was used as a control. IFN, interferon; ISRE, IFN-stimulated response element; ISG, IFN-stimulated gene; PKR, protein kinase R.
Type I collagen inhibits the IFN-α-associated suppression of HCV-RNA replication in OR6 cells
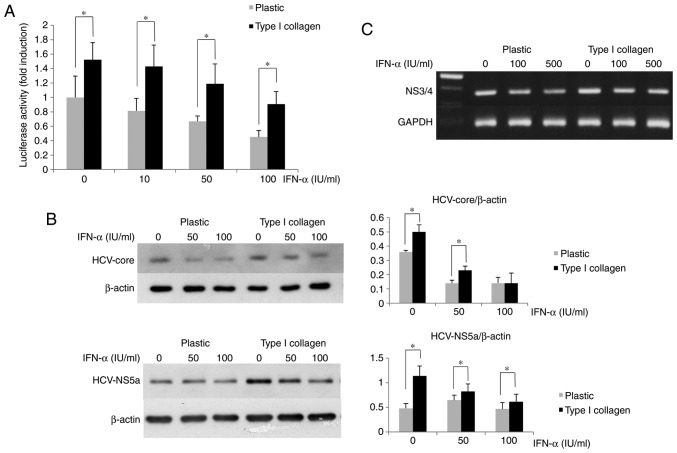
The suppressive effect of IFN-α on HCV replication was assessed using OR6 cells cultured on type I collagen-coated dishes to determine the effects of ECM on HCV replication. In the type I collagen-coated plates, the luciferase activity of OR6 cells was higher than that in the cells cultured on normal plates (Fig. 2A). The amounts of HCV core and NS5A protein expression in OR6 cells grown on type I collagen-coated dishes were measured by western blot analysis, revealing that their expression in the cells cultured on the coated dishes was higher than that in the cells cultured on the normal plastic dishes (Fig. 2B). The HCV-RNA expression in OR6 cells grown on type I collagen-coated dishes measured by RT-PCR was higher than that in cells cultured on normal dishes (Fig. 2C). These results indicated that the inhibitory effect of IFN-α on HCV replication was impaired in OR6 cells cultured on type I collagen-coated dishes compared with the effect in those cultured on normal plastic dishes.
Figure 2.
Extracellular matrix attenuates the suppressive effect of IFN-α on HCV replication in OR6 cells. (A) The luciferase activity of OR6 cells cultured on type I collagen-coated dishes was higher than that in OR6 cells cultured on normal plastic dishes. Cells (1×104 per well) were cultured on 48-well plates coated with type I collagen or on non-coated plates for 2 days. They were treated with 50 or 100 U/ml IFN-α for 12 h, and the Renilla luciferase activity was then measured. (B) Inhibition of the suppressive effect of IFN-α on the HCV protein expression in OR6 cells grown on type I collagen-coated dishes. The OR6 cells were cultured on plastic dishes or type I collagen-coated dishes for 3 days. The HCV core protein and HCV NS5a protein expression was measured by western blot analysis after IFN-α treatment for 12 h. β-actin was used as a control. Values are expressed as the mean ± standard deviation (n=3). *P<0.05. (C) Reverse transcription polymerase chain reaction analysis of the expression of the HCV NS3/4 coding region in OR6 cells cultured as in B. GAPDH was used as a control. IFN, interferon; OR6 cells, HuH-7 cells stably transfected with full-length HCV-RNA fused with Renilla luciferase HCV, hepatitis C virus; NS, nonstructural protein.
Inhibition of β1-integrin function restores IFN-α-induced signaling
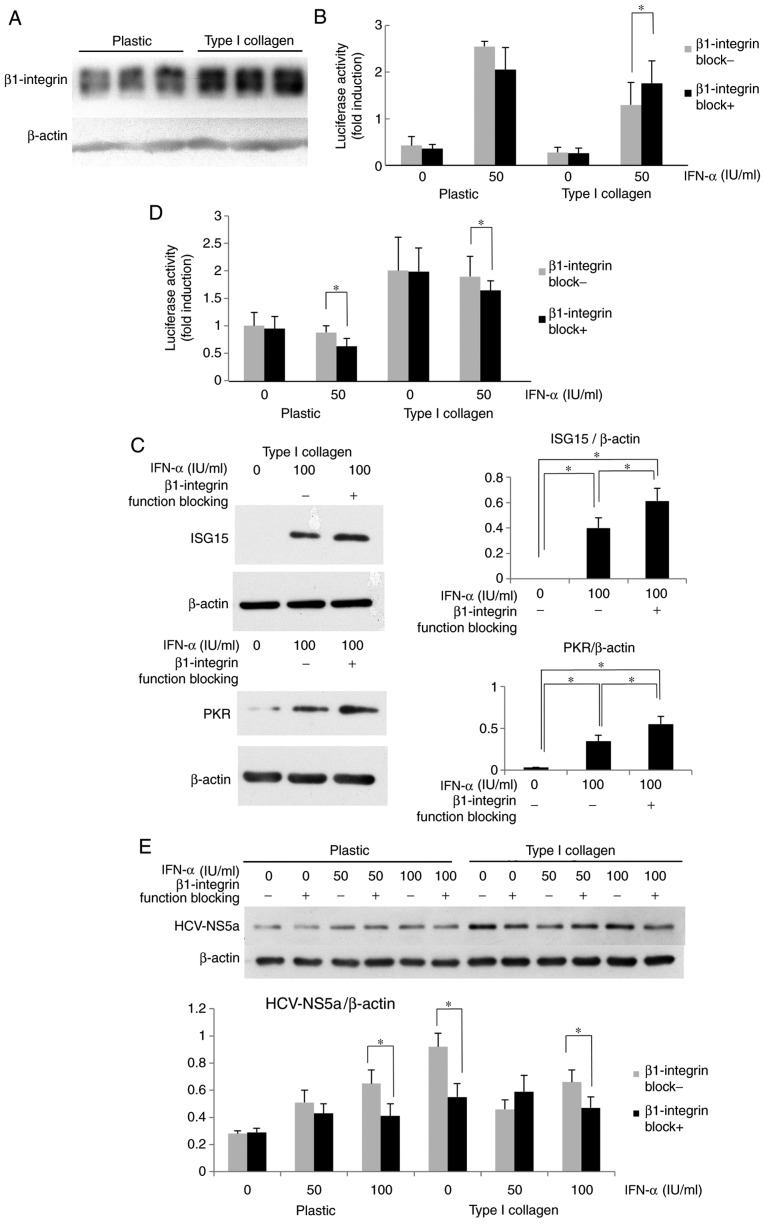
The mechanisms of the ECM-mediated inhibition of IFN-α signaling were then examined. Since β1-integrin is a major subunit of ECM receptors and is known to influence diverse signaling pathways in adherent cells, the expression and role of β1-integrin in HuH-7 cells was examined. As presented in Fig. 3A, the β1-integrin expression of cells on type I collagen-coated dishes was increased compared with that in cells cultured on plastic dishes. It was then investigated whether β1-integrin, which was highly expressed in HuH-7 cells cultured on type I collagen-coated dishes, affected IFN-α signaling. The cells were optionally pre-treated with β1-integrin function-blocking antibody, and after optional IFN-α treatment for 12 h, the ISRE luciferase activity was measured. The ISRE luciferase activity of HuH-7 activated by IFN-α was clearly higher in the plastic dishes compared with the collagen-coated dishes. When β1-integrin function blocking antibody was administered, there was a significant elevation of ISRE luciferase activity observed in the collagen coated dish, but not in the plastic dish (Fig. 3B). In HuH-7 cells treated with β1-integrin function-blocking antibody, the IFN-α-induced ISG protein expression was also increased compared with that in cells without blocking treatment (Fig. 3C). The effects of β1-integrin-blocking antibody on HCV-RNA replication in OR6 cells were then evaluated. As presented in Fig. 3D, when OR6 cells were treated with β1-integrin blocking antibody prior to IFN-α treatment, the Renilla luciferase activity was reduced compared with that in cells without β1-integrin blocking. As displayed in Fig. 3E, when cells were cultured on type I collagen-coated dishes, β1-integrin function-blocking antibody reduced the expression of HCV-NS5a, although no clear differences were seen between the groups treated with or without β1-blocking antibody when cells were cultured on plastic. It appeared that β1-integrin blocking affects endogenous IFN-α signaling and improves the effect of IFN treatment on HCV-RNA replication. These results suggest that type I collagen may support HCV replication via attenuation of IFN signaling in a β1-integrin-dependent manner, and that the ECM-stimulated integrin signal may promote HCV replication in an IFN-dependent and -independent manner.
Figure 3.
Attenuation of IFN-α signaling by type I collagen is β1-integrin-dependent. (A) β1-integrin was overexpressed in Huh7 cells grown on type I collagen-coated dishes. (B) Improvement in the ISRE luciferase activity after treatment with β1-integrin function-blocking antibody in HuH-7 cells cultured on type I collagen-coated dishes. Cells were treated with β1-integrin function-blocking antibody (1 µg/ml) for 6 h. The ISRE-luciferase activity was measured after IFN-α treatment for 12 h. The results are presented as the mean fold induction of the controls. (C) Improvement in the ISG protein expression by treatment with β1-integrin function-blocking antibody in HuH-7 cells grown on type I collagen-coated dishes. The cells were cultured on type I collagen-coated dishes for 3 days and then treated with β1-integrin function-blocking antibody for 6 h, followed by treatment with IFN-α for 12 h. The ISG15 and PKR expression was measured by western blot analysis with β-actin used as a control. (D) Improvement in the suppressive effect of IFN-α on HCV replication in OR6 cells cultured on type I collagen-coated dishes. After 6-h treatment with β1-integrin function-blocking antibody (1 µg/ml), the cells were treated with IFN-α for 12 h, and the Renilla luciferase activity was then measured. (E) Improvement in the suppressive effect of IFN-α on HCV protein expression. HCV-NS5a expression was suppressed by co-treatment with β1-integrin function-blocking antibody in OR6 cells grown on type I collagen-coated dishes. The OR6 cells were cultured on type I collagen-coated dishes for 3 days and then treated with β1-integrin function-blocking antibody for 6 h, followed by treatment with IFN-α for 12 h. The HCV-NS5a expression was measured by western blot analysis with β-actin used as a control. Values are expressed as the mean ± standard deviation (n=3). *P<0.05. IFN, interferon; OR6 cells, HuH-7 cells stably transfected with full-length HCV-RNA fused with Renilla luciferase HCV, hepatitis C virus; NS, nonstructural protein; ISRE, IFN-stimulated response element; ISG, IFN-stimulated gene; PKR, protein kinase R.
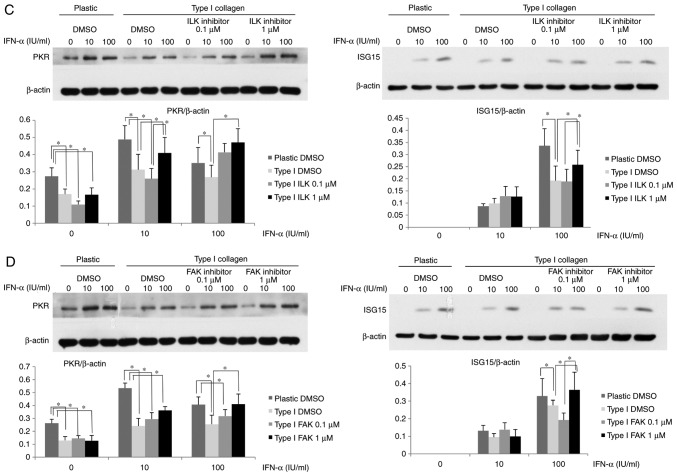
Attenuation of IFN-α signaling via β1-integrin involves ILK and FAK
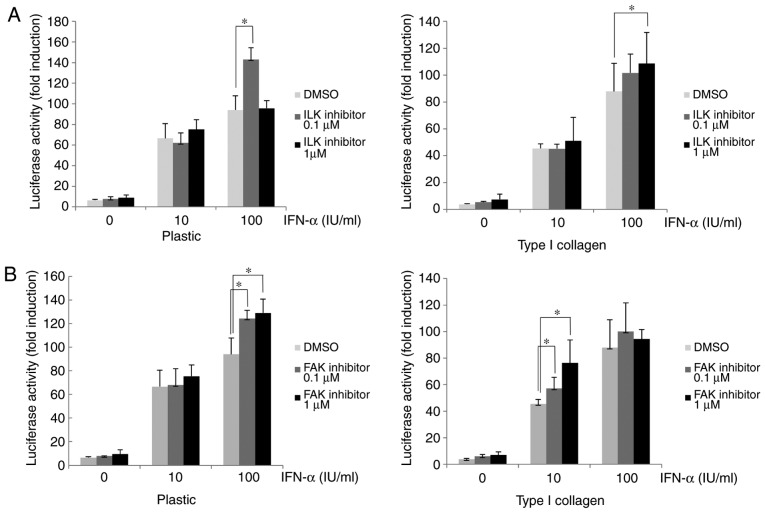
Various proteins associated with integrin α/β heterodimers, including FAK and ILK, are known be involved in the activation of diverse cellular signaling pathways (19-21). In the present study, cells were treated with ILK or FAK inhibitor to determine the roles of ILK and FAK in the β1-integrin-mediated attenuation of IFN-α signaling. After treatment with ILK or FAK inhibitor, the IFN-α-induced ISRE-luciferase activity in Huh-7 cells cultured on type I collagen-coated dishes was higher than that in dimethyl-sulfoxide-treated control cells (Fig. 4A and B). In the plastic dishes in the ILK 0.1 µM with IFN-α 100 IU/ml group, the ISRE-luciferase activity was significantly increased compared with the DMSO control, while in the ILK 1 µM with IFN-α 100 IU/ml group an increase in ISRE-luciferase activity was not observed. In the collagen-coated dishes in the ILK 1 µM with IFN-α 100 IU/ml group the ISRE-luciferase activity was significantly increased compared with the DMSO group, whereas it was not significantly increased in the ILK 0.1 µM with IFN-α 100 IU/ml group. Although these results suggested that ISRE-luciferase activity was increased when ILK was inhibited, the effect of the ILK inhibitor was matrix-dependent and an excessive inhibition of ILK in a plastic dish may cancel the effects and reverse the increase in ISRE-luciferase activity. The ISG15 and PKR expression in HuH-7 cells treated with ILK or FAK inhibitor was then examined. As presented in Fig. 4C, the cells cultured on type I collagen-coated dishes had a lower expression of PKR and ISG15 after IFN-α treatment compared with the plastic dishes, and subsequent treatment with ILK inhibitor restored their expression. Similarly, treatment with FAK inhibitor restored the reduced PKR and ISG15 expression induced by IFN-α in cells cultured on type I collagen-coated dishes (Fig. 4D). These results suggested that the IFN-α-induced attenuation of ISRE luciferase activity and ISG expression in cells cultured on type I collagen-coated dishes were mediated by ILK and FAK downstream of β1-integrin.
Figure 4.
Attenuation of IFN-α-signaling in HuH-7 cells by type I collagen involves ILK and FAK. (A and B) Improvement in the ISRE-luciferase activity after treatment with (A) ILK inhibitor or (B) FAK inhibitor in HuH-7 cells cultured on normal plastic dishes or type I collagen-coated dishes. Cells were treated with ILK or FAK inhibitor for 6 h. The ISRE-luciferase activity was measured after IFN-α treatment for 12 h. (C and D) Improvement in the ISG protein expression by treatment of (C) ILK inhibitor or (D) FAK inhibitor in OR6 cells grown on normal plastic dishes or type I collagen-coated dishes. OR6 cells were cultured for 3 days and then treated with ILK or FAK inhibitor for 6 h, followed by treatment with IFN-α for 12 h. The expression of ISG15 and PKR was measured by western blot analysis with β-actin used as a control. Values are expressed as the mean ± standard deviation (n=3). *P<0.05. DMSO, dimethyl sulfoxide; ILK, integrin-linked kinase; FAK, focal adhesion kinase; IFN, interferon; OR6 cells, HuH-7 cells stably transfected with full-length HCV-RNA fused with Renilla luciferase ISRE, IFN-stimulated response element; ISG, IFN-stimulated gene; PKR, protein kinase R.
Discussion
Although the presence of advanced liver fibrosis or cirrhosis in patients with chronic HCV is a major predictive factor for the failure of IFN-based antiviral therapy (6-10), the molecular mechanisms of the resistance to IFN action by fibrosis remain elusive. The recent development of IFN-free oral DAA regimens has markedly increased the rate of SVR in HCV-infected patients (13-15). However, even in patients treated with DAA without IFN, an altered IFN response in the liver tissue was observed to be associated with the failure of DAA treatment, suggesting the importance of an adequate host IFN response for the eradication of HCV (16). The present study investigated whether ECM components that are increased in the fibrotic liver directly affect IFN signaling in vitro. The results indicated that ECM components, e.g. type I collagen, attenuate the IFN-α-induced ISRE-mediated transcriptional activity and the expression of a subset of ISGs in a β1-integrin-dependent manner, and that the presence of ECM components decreased the inhibitory effects of IFN-α on HCV replication in HCV replicon cells.
Hepatic stellate cells (HSCs) are a major ECM-producing cell type that are activated after exposure to liver-injurious stimuli, including HCV, and have central roles in the development of liver fibrosis (17,18). Several studies have investigated the roles of HSCs on HCV replication in hepatocytes and revealed that the activation of HSCs via the innate immune system produced antiviral cytokines, including IFN-β and IFN-λ, and inhibited the replication of HCV in hepatocytes. Therefore, activated HSCs appear to have an anti-viral phenotype against HCV infection, while conversely producing various ECM components to thereby contribute to the development of liver fibrosis (25-27).
The present results indicated that culture of cells on type I collagen-coated plates resulted in an increased expression of β1-integrin and the attenuation of IFN-stimulated ISRE activity. Integrins, a heterodimer complex consisting of an α and β subunit, serve as major receptors for ECM (19-21). Integrins have been reported to interact with a wide variety of cytokine receptor-mediated signals, including the MAPK (28-30), PI3K/mammalian target of rapamycin (mTOR) (29,31,32) and TGF-β/Smad pathways (30,33,34). However, the interaction between integrins and IFN receptor-mediated signals has remained to be fully elucidated. TGF-β/Smad signaling was reported to be involved in IFN resistance in HCV patients with advanced liver fibrosis (35). TGF-β is a potent stimulator of the production of various ECM components, including collagen (17,18), and is also known to increase the expression of integrins (36). Shirasaki et al (35) demonstrated that TGF-β1 inhibited the IFN-induced expression of ISG and the IFN-mediated suppression of HCV replication in HCV-transfected HuH-7 cells. They also reported that TGF-β1 impaired the mTOR activation required for IFN-induced ISG expression (37,38) by downregulating the expression of Ras homolog enriched in brain and increasing that of suppresser of cytokine signaling 3 under conditions of reduced amino acid levels. Furthermore, replacement of branched-chain amino acids, which are reduced in patients with liver cirrhosis and known to activate mTOR signaling, restored the effects of DAAs. Since integrins activate the PI3K/mTOR pathway (29,31,32,39), the ECM-induced inhibition of IFN signaling does not appear to be due to the inhibition of the mTOR pathway that was observed under conditions of reduced amino acid concentrations.
Among various proteins associated with integrin-α/β heterodimers, kinases including FAK and ILK are involved in the activation of diverse cellular signaling pathways, including the MAPK (extracellular signal-regulated kinase, p38 and c-Jun N-terminal kinase), PI3K/Akt/mTOR/ribosomal protein S6 kinase β-1 pathway, TGF-β/Smad pathway and PKCs (19-21,40,41). The present study demonstrated that the IFN-stimulated ISRE activity was attenuated via β1-integrin, indicating the presence of an interaction between integrins and IFN signaling. The results also suggested a possible involvement of ILK and FAK in the ECM-mediated attenuation of IFN signaling and ISG expression, since the inhibition of ILK or FAK partially restored the ISRE-luciferase activity and ISG expression. Since integrin-associated proteins, including ILK and FAK, have significant roles in the modulation of growth factor/cytokine receptor-mediated signaling, further investigation is required to identify the key molecules involved in the integrin-mediated inhibition of IFN signaling.
Another possible role of IFN resistance in liver fibrosis may be linked to hepatocarcinogenesis. Liver cancer develops more often from the fibrotic liver than the normal liver, even after the achievement of SVR in patients with HCV treated with IFN-based therapy (17,18,42,43). Accumulated ECM in the fibrotic liver has been suggested to contribute to hepatocarcinogenesis via a wide variety of signaling pathways that promote hepatocyte proliferation and survival, including integrin-mediated signaling (34,44). While IFNs have been reported to exert diverse antiviral effects, type I IFNs, including IFN-α, have been used for the treatment of numerous human cancer types, and the type I IFN status is associated with the outcome of anti-cancer therapy (45). Indeed, IFN is known to suppress liver cancer growth via the induction of apoptosis in vitro and in vivo (46,47). Therefore, the attenuation of IFN-signaling by ECM may contribute to the frequent development of liver cancer from the fibrotic liver.
In conclusion, the present study indicated that ECM inhibited IFN signaling in a β1-integrin-dependent manner, possibly involving FAK and ILK, and impairment of antiviral activity by IFN-α in HCV-replicon cells. These results may provide a mechanism for the role of fibrosis in IFN resistance, which may be harnessed for the treatment of cirrhotic patients with HCV infection.
Acknowledgments
Not applicable.
Funding
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 16590606 to TM and IO).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
IO and TM devised the study and designed the experiments. TK, SI, XJ, SM and IO performed the experiments. NS, MI and NK established and provided the replicon cells. TK, SM, NK, YE, KA, KF and IO analyzed the data. TK and IO drafted the manuscript. All authors have read and approved the final manuscript.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gale M, Jr, Foy EM. Evasion of intracellular host defense by hepatitis C virus. Nature. 2005;436:939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- 2.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark G. Interferons at age 50: Past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber F. Interaction of hepatitis C virus with type I interferon system. World J Gastroenterol. 2007;13:4818–4823. doi: 10.3748/wjg.v13.i36.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 5.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/S0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 6.Marrache F, Consigny Y, Ripault MP, Cazals-Hatem D, Martinot M, Boyer N, Degott C, Valla D, Marcellin P. Safety and efficacy of peginterferon plus ribavirin in patients with chronic hepatitis C and bridging fibrosis or cirrhosis. J Viral Hepat. 2005;12:421–428. doi: 10.1111/j.1365-2893.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 7.Everson GT, Hoefs JC, Seeff LB, Bonkovsky HL, Naishadham D, Schiffman ML, Kahn JA, Lok ASF, Di Bisceglie AM, Lee WM, et al. Impact of disease severity on outcome of antiviral therapy for chronic hepatitis C: Lessons from the HALT-C trial. Hepatology. 2006;44:1675–1684. doi: 10.1002/hep.21440. [DOI] [PubMed] [Google Scholar]
- 8.Bruno S, Shiffman ML, Roberts SK, Gane EJ, Messinger D, Hadziyannis SJ, Marcellin P. Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology. 2010;51:388–397. doi: 10.1002/hep.23340. [DOI] [PubMed] [Google Scholar]
- 9.Cheng WS, Roberts SK, McCaughan G, Sievert W, Weltman M, Crawford D, Rawlinson W, Marks PS, Thommes J, Rizkalla B, et al. Low virological response and high relapse rates in hepatitis C genotype 1 patients with advanced fibrosis despite adequate therapeutic dosing. J Hepatol. 2010;53:616–623. doi: 10.1016/j.jhep.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Asselah T, Estrabaud E, Bieche I, Lapalus M, De Muynck S, Vidaud M, Saadoun D, Soumelis V, Marcellin P. Hepatitis C: Viral and host factors associated with non-response to pegylated interferon plus ribavirin. Liver Int. 2010;30:1259–1269. doi: 10.1111/j.1478-3231.2010.02283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 12.Bruno S, Vierling JM, Esteban R, Nyberg LM, Tanno H, Goodman Z, Poordad F, Bacon B, Gottesdiener K, Pedicone LD, et al. Efficacy and safety of boceprevir plus peginterferon-ribavirin in patients with HCV G1 infection and advanced fibrosis/cirrhosis. J Hepatol. 2013;58:479–487. doi: 10.1016/j.jhep.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–1917. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey LC, Lee WM. Hepatitis C virus therapy update 2013. Curr Opin Gastroenterol. 2013;29:243–249. doi: 10.1097/MOG.0b013e32835ff972. [DOI] [PubMed] [Google Scholar]
- 15.Shah N, Pierce T, Kowdley KV. Review of directacting antiviral agents for the treatment of chronic hepatitis C. Expert Opin Investig Drugs. 2013;22:1107–1121. doi: 10.1517/13543784.2013.806482. [DOI] [PubMed] [Google Scholar]
- 16.Meissner EG, Wu D, Osinusi A, Bon D, Virtaneva K, Sturdevant D, Porcella S, Wang H, Herrmann E, McHutchison J, et al. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J Clin Invest. 2014;124:3352–3363. doi: 10.1172/JCI75938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman SL. Mechanisms of hepatic fibrosis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inagaki Y, Okazaki I. Emerging insights into transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 20.Hehlgans S, Hasse M, Cordes N. Signalling via integrins: Implications for cell survival and anticancer strategies. Biochem Biophys Acta. 2017;1775:163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Millard M, Odde S, Neamati N. Integrin targeted therapeutics. Thearanostics. 2011;1:154–188. doi: 10.7150/thno/v01p0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashida T. Integrins modulate cellular fibrogenesis at multiple levels; Regulation of TGF-β signaling. Endocr Metab Immune Disord Drug Targets. 2010;10:302–319. doi: 10.2174/1871530311006040302. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda M, Abe K, Dansako H, Nakamura T, Naka K, Kato N. Efficient replication of a full-length hepatitis C virus genome, strain O, in cell culture, and development of a luciferase reporter system. Biochem Biophys Res Commun. 2005;329:1350–1359. doi: 10.1016/j.bbrc.2005.02.138. [DOI] [PubMed] [Google Scholar]
- 24.Ozaki I, Zhang H, Mizuta T, Ide Y, Eguchi Y, Yasutake T, Sakamaki T, Pestell RG, Yamamoto K. Menatetrenone, a vitamin K2 analogue, inhibits hepatocellular carcinoma cell growth by suppressing cyclin D1 expression through inhibition of nuclear factor kappaB activation. Clin Cancer Res. 2007;13:2236–2245. doi: 10.1158/1078-0432.CCR-06-2308. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Trippler M, Pei R, Lu M, Broering R, Gerken G, Schlaak JF. Toll-like receptor activated human and murine hepatic stellate cells are potent regulators of hepatitis C virus replication. J Hepatol. 2009;51:1037–1045. doi: 10.1016/j.jhep.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Li J, Wang X, Ye L, Zhou Y, Ho W. Induction of interferon-λ contributes to Toll-like receptor-3-activated hepatic stellate cell-mediated hepatitis C virus inhibition in hepatocytes. J Viral Hepat. 2013;20:385–394. doi: 10.1111/jvh.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alisi A, Arciello M, Petrini S, Conti B, Missale G, Balsano C. Focal adhesion kinase (FAK) mediates the induction of pro-oncogenic and fibrogenic phenotypes in hepatitis C virus (HCV)-infected cells. PLoS One. 2012;7:e44147. doi: 10.1371/journal.pone.0044147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu X, Assoian RK. Integrin-dependent activation of MAP kinase: A link to shape-dependent cell proliferation. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khwaja A, Rodriguez-Viciana P, Wennström S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH-kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Ozaki I, Mizuta T, Yoshimura T, Matsuhashi S, Eguchi Y, Yasutake T, Hisatomi A, Sakai T, Yamamoto K. Transforming growth factor-beta 1-induced apoptosis is blocked by beta 1-integrin-mediated mitogen-activated protein kinase activation in human hepatoma cells. Cancer Sci. 2004;95:878–886. doi: 10.1111/j.1349-7006.2004.tb02197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yau CY, Wheeler JJ, Sutton KL, Hedley DW. Inhibition of integrin-linked kinase by a selective small molecule inhibitor, QLT0254, inhibits the PI3K/PKB/mTOR, Stat3, and FKHR pathways and tumor growth, and enhances gemcitabine-induced apoptosis in human orthotopic primary pancreatic cancer xenografts. Cancer Res. 2005;65:1497–1504. doi: 10.1158/0008-5472.CAN-04-2940. [DOI] [PubMed] [Google Scholar]
- 32.Riaz A, Ilan N, Vlodavsky I, Li JP, Johansson S. Characterization of heparanase-induced phosphatidylinositol 3-kinase-AKT activation and its integrin dependence. J Biol Chem. 2013;288:12366–12375. doi: 10.1074/jbc.M112.435172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garamszegi N, Garamszegi SP, Samavarchi-Tehrani P, Walford E, Schneiderbauer MM, Wrana JL, Scully SP. Extracellular matrix-induced transforming growth factor-beta receptor signaling dynamics. Oncogene. 2010;29:2368–2380. doi: 10.1038/onc.2009.514. [DOI] [PubMed] [Google Scholar]
- 34.Ozaki I, Hamajima H, Matsuhashi S, Mizuta T. Regulation of TGF-β1-induced pro-apoptotic signaling by growth factor receptors and extracellular matrix receptor integrins in the liver. Front Physiol. 2011;2:78. doi: 10.3389/fphys.2011.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shirasaki T, Honda M, Shimakami T, Murai K, Shiomoto T, Okada H, Takabatake A, Tokumaru A, Sakai Y, Yamashita T, et al. Impaired interferon signaling in chronic hepatitis C patients with advanced fibrosis via the transforming growth factor beta signaling pathway. Hepatology. 2014;60:1519–1530. doi: 10.1002/hep.27277. [DOI] [PubMed] [Google Scholar]
- 36.Wang D, Zhou GH, Birkenmeier TM, Gong J, Sun L, Brattain MG. Autocrine transforming growth factor beta-1 modulates the expression of integrin alpha 5 beta1 in human colon carcinoma FET cells. J Biol Chem. 1995;270:14154–14159. doi: 10.1074/jbc.270.23.14154. [DOI] [PubMed] [Google Scholar]
- 37.Kaur S, Sassano A, Dolniak B, Joshi S, Majchrzak-Kita B, Baker DP, Hay N, Fish EN, Platanias LC. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc Natl Acad Sci USA. 2008;105:4808–4813. doi: 10.1073/pnas.0710907105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lekmine F, Uddin S, Sassano A, Parmar S, Brachmann SM, Majchrzak B, Sonenberg N, Hay N, Fish EN, Platanias LC. Activation of the p70 S6 kinase and phosphorylation of the 4E-BP1 repressor of mRNA translation by type I interferons. J Biol Chem. 2003;278:27772–27780. doi: 10.1074/jbc.M301364200. [DOI] [PubMed] [Google Scholar]
- 39.Zeller KS, Idevall-Hagren O, Stefansson A, Velling T, Jackson SP, Downward J, Tengholm A, Johansson S. PI3-kinase 110α mediates β1 integrin-induced Akt activation and membrane protrusion during cell attachment and initial spreading. Cell Signal. 2010;22:1838–1848. doi: 10.1016/j.cellsig.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Tang Q, Zhao S, Wu J, Zheng F, Yang L, Hu J, Hann SS. Inhibition of integrin-linked kinase expression by emodin through crosstalk of AMPKα and ERK1/2 signaling and reciprocal interplay of Sp1 and c-Jun. Cell signaling. 2015;27:1469–1477. doi: 10.1016/j.cellsig.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, Larkin J, Marais R, Sahai E. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin β1/FAK signaling. Cancer Cell. 2015;27:574–588. doi: 10.1016/j.ccell.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asahina Y, Tsuchiya K, Tamaki N, Hirayama I, Tanaka T, Sato M, Yasui Y, Hosokawa T, Ueda K, Kuzuya T, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52:518–527. doi: 10.1002/hep.23691. [DOI] [PubMed] [Google Scholar]
- 43.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: A meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 44.Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–775. doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15:405–414. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 46.Yano H, Iemura A, Haramaki M, Ogasawara S, Takayama A, Akiba J, Kojiro M. Interferon alfa receptor expression and growth inhibition by interferon alfa in human liver cancer cell lines. Hepatology. 1999;29:1708–1717. doi: 10.1002/hep.510290624. [DOI] [PubMed] [Google Scholar]
- 47.Kusano H, Akiba J, Ogasawara S, Sanada S, Yasumoto M, Nakayama M, Ueda K, Ueda K, Kurita T, Todoroki K, et al. Pegylated interferon-α2a inhibits proliferation of human liver cancer cells in vitro and in vivo. PLoS One. 2013;8:e83195. doi: 10.1371/journal.pone.0083195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.