Abstract
Hepatitis B virus (HBV) infection is a major cause of hepatic inflammation. Successful HBV clearance in patients is associated with sustained viral control by effector T cells. Compared with acute hepatitis B, chronic HBV infection is associated with the depletion of T cells, resulting in weak or absent virus-specific T cells reactivity, which is described as 'exhaustion'. This exhaustion is characterized by impaired cytokine production and sustained expression of multiple coinhibitory molecules. Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) is one of many coinhibitory molecules that can attenuate T cell activation by inhibiting costimulation and transmitting inhibitory signals to T cells. Persistent HBV infection results in the upregulation of CTLA-4 on hepatic CD8+ T cells. This prompts CD8+ T cell apoptosis, and the activation of cytotoxic T lymphocytes is blocked. Similar to CD8+ T cells, CD4+ T helper (Th) cell proliferation is hindered following CTLA-4 upregulation. In addition, the differentiation of CD4+ Th is polarized toward the Th2/peripherally-inducible T regulatory cell types, increasing the levels of anti-inflammatory cytokines. Conversely, the activation of proinflammatory cells (Th1 and follicular helper T) is blocked, and the levels of proinflammatory cytokines decline. This review summarizes the current literature relevant to T cell exhaustion in patients with HBV-related chronic hepatitis, and discusses the roles of CTLA-4 in T cell exhaustion.
Keywords: cytotoxic T lymphocyte-associated antigen-4, CD8+ T cells, CD4+ T helper cells, T cell subsets, cytokines
1. Introduction
Infectious viral hepatitis is a major public health problem worldwide. The number of global viral hepatitis deaths has recently increased. More than half of these viral hepatitis-related deaths are associated with chronic hepatitis B virus (HBV) infection (1). China has made tremendous progress in controlling HBV infection, largely owing to the enactment of a universal hepatitis B vaccination program and implementation of effective health management programs (2). However, the prevalence of the HBV surface antigen (HBsAg) (7.18%) is still high in Chinese adults (3). HBV infection is a major cause of inflammatory liver disease. Of note, HBV is considered to be a 'mysterious' virus that is not readily sensed by the host immune system (4). A large number of studies have demonstrated that effective antiviral therapy and successful HBV clearance in patients is associated with the breadth and depth of the proliferation and responses of HBV-specific T cells (5). However, compelling evidence has suggested that the host immune system often exhibits weak or absent virus-specific T-cell reactivity following chronic HBV infection. This is referred to as T cell 'exhaustion', which is characterized by poor effector cytotoxic responses, decreased cytokine production and upregulated expression of multiple inhibitory molecules, including programmed cell death-1 (PD-1), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and lymphocyte activation gene-3 (6).
The human CTLA4 gene has an exon and intron structure similar to human CD28, it exhibits extensive homology at the nucleotide level, and it encodes a 233 amino-acid protein (CTLA-4) belonging to the immunoglobulin superfamily (7). CTLA-4 consists of one V-like domain flanked by two hydrophobic regions, one of which has a structure suggesting that it may be anchored to the membrane (8). It binds to CD80/CD86 with an affinity 20-fold higher than CD28, and functions to attenuate T cell activation by inhibiting costimulation and transmitting inhibitory signals to T cells (9,10). Polymorphisms in CTLA4 have been associated with susceptibility to multiple diseases, including type I diabetes (11), primary biliary cirrhosis (12) and Graves' disease (13). However, they are assumed to confer a higher risk for persistent HBV infection. A recent meta-analysis study demonstrated that CTLA4+49A/G polymorphisms were associated with the ability to resist HBV infection in the Asian population (14).
2. Impact of CTLA-4 on HBV-specific CD8+ T cells
CD8+ T cells circulating in the blood migrate to the liver, where they initially arrest in liver sinusoids by preferentially docking onto platelets that have previously adhered to the liver sinusoids. Upon detachment from platelets, the effector CD8+ T cells crawl intrasinusoidally, irrespective of the direction of the blood flow, and probe the underlying hepatocytes for the presence of antigen by extending filopodia-like protrusions through the sinusoidal fenestrae (15). Effector CD8+ T cells recognize hepatocellular antigens and perform effector functions, while still in the intravascular space, and only later extravasate into the parenchyma (16). Under conditions of chronic antigen stimulation, the CD8+ T cells exhibit dysfunctional responses, characterized by low cytokine secretion and increased apoptosis. Multiple inhibitory pathways have been implicated in this exhaustion of CD8+ T cells (17). The relationship between coinhibitory receptors and CD8+ T cell immune functions has been previously elucidated in a murine model of lymphocytic choriomeningitis virus (LCMV) infection (18). However, Legat et al (19) found no major impairment of cytokine production in CD8+ T cells positive for a broad array of inhibitory receptors following chronic antigen stimulation. Furthermore, when they analyzed CD8+ T cells from blood, metastatic lymph nodes (LNs) and normal LNs from melanoma patients, the results demonstrated that altered expression of inhibitory receptors was not associated with cytokine production, but was strongly correlated with T cell differentiation or T cell activation state.
In a previous study, programmed death-1 (PD-1) pathway-mediated inhibitory signals were demonstrated to serve a key role in CD8+ T cell exhaustion during persistent viral infection (18). However, the exhaustion could not be completely reversed by PD-1 blockade alone, and full restoration required a combined PD-1/CTLA-4 blockade (20). The critical immunoregulatory role of CTLA-4 in induced peripheral immune tolerance is illustrated by the massive and fatal lymphoproliferation that occurs in CTLA-4-deficient mice (21). During the symptomatic phase of acute Hepatitis A (AHA), CTLA-4 is highly expressed on virus-specific CD8+ T cells, and functions as an inhibitory molecule that suppresses cytotoxic T-cells and prevents the destruction of virus-infected hepatocytes to avoid the occurrence of severe acute hepatitis (22). However, during hepatitis C virus (HCV) infection, high expression of CTLA-4 on CD8+ T cells lead to increased susceptibility of the cells to spontaneous apoptosis (23). By contrast, functional skewing of the global CD8+ T cell population led to impairment in their ability to produce cytokines [interleukin (IL)-2, interferon (IFN)-γ and tumor necrosis factor (TNF) α] and to proliferate in cells with chronic hepatitis B virus (CHB) infection (24). Similar findings have been reported by Wongjitrat et al (25); CD8+ expressing CTLA-4 molecules in CHB-infected patients were significantly higher compared with healthy controls, and CD8+ T cells presenting CTLA-4 might contribute to the impaired immune response and the failure of immunological control of the persisting pathogens. However, it is astounding that children and young adults with CHB infection in the period of immune tolerance (IT) are not associated with an immune profile of T cell tolerance, but have an HBV-specific immune profile (26). In addition, the expression of CTLA-4 and other inhibitory receptors (such as lymphocyte activating 3, hepatitis A virus cellular receptor 2, and leukocyte associated immunoglobulin like receptor 1) was not increased on HBV-specific CD8+ T cells from peripheral blood mononuclear cells (PBMCs). This may seem controversial to the viewpoint that immunity is not activated in younger CHB patients. Velazquez et al (27) may propose a possible explanation, as this review considered that the CD8+ T cells expression of the C-C motif chemokine ligand 3 (CCL3), which is involved in migration, was impaired in the immune tolerant cohort, compared with healthy controls and immune active CHB patients. The absence of CCL3 may prompt a potential migratory defect that could hamper functional HBV-specific CD8+ T cells from gaining access to virus-infected hepatocytes. Under these conditions, immune tolerance would be largely a matter of sequestration.
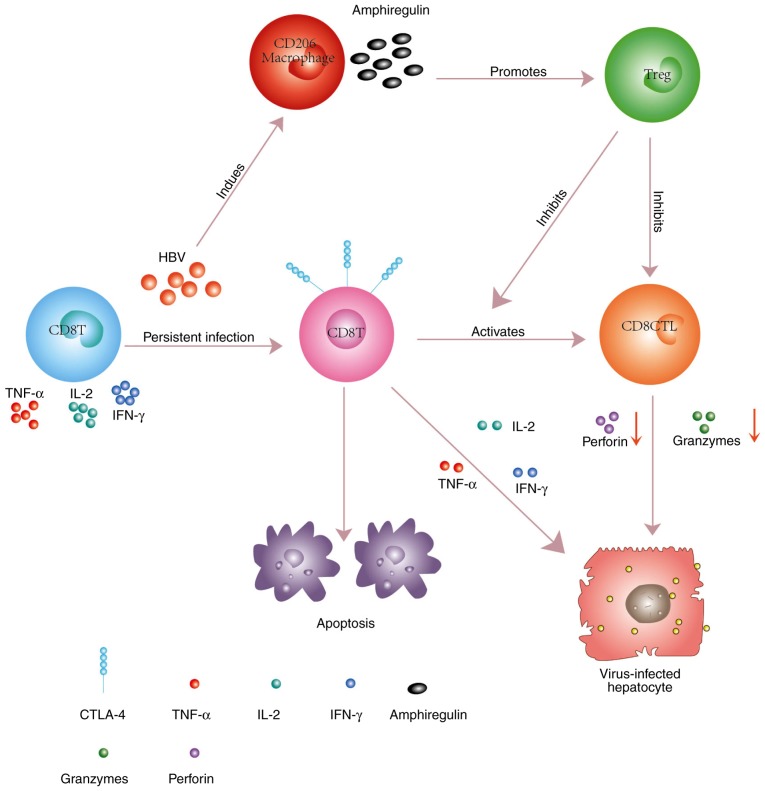
However, continuous exposure to high concentrations of HBV antigens (HBeAg, HBsAg, HBx) exhausts a large proportion or the majority of CD8+ T cells, which is associated with gradual upregulation of CTLA-4 expression (6). There is insufficient information to explain the relationship between the upregulated expression of CTLA-4 on CD8+ T cells and chronic HBV infection. Recent findings by Peng et al confirming that HBeAg may increase the expression of CTLA-4 on CD8+ T cells and that this was associated with a high HBV DNA load, may lead to some breakthroughs, but the pathway underlying this effect remains unclear (28). It is possible that research focusing on other diseases may provide clues into these mechanisms. Recently, it was demonstrated in tumor models that the mannose receptor (MR) antigens were internalized and processed specifically for cross-presentation, which may have resulted in upregulated expression of CTLA-4 on CD8+ T cells. The regulatory effect of the MR was mediated by a direct interaction with CD45 on the CD8+ T cells, inhibiting its phosphatase activity, which prevented the expression of B-cell lymphoma 6 (Bcl-6), a transcriptional inhibitor that directly binds the CTLA-4 promoter and regulates its activity (29). This pathway may be one reason for the immune escape of tumor cells that induces the development and metastasis of tumors. However, the upregulation of CTLA-4 is accompanied by a high level of BCL2 interacting mediator of cell death (Bim) on CD8+ T cells following HBV infection. Bim is a proapoptotic member of the BCL2 family, a protein important for regulating apoptosis (30). The activation of Bim can induce BCL2 associated X (Bax) expression, thus increasing cell apoptosis via the mitochondrial pathways (31). In addition, it was demonstrated that CD206+ macrophage-derived amphiregulin promoted the immunosuppressive activity of intrahepatic regulatory T cells (Treg) by inducing mammalian target of rapamycin (mTOR) activation, upregulating CTLA-4 expression on Treg cells, and subsequently restraining the antiviral activity of CD8+ T cells during chronic HBV infection (32). As a result, the proliferation of CD8+ T cells and their activation into CD8+ cytotoxic T cells (CTL) was blocked (Fig. 1). Encouragingly, the CD8+ T cells could be rescued by reducing the HBV DNA load, and the immune response to the HBV protein was enhanced (33). Schurich et al (30) similarly demonstrated that the function of IFN-γ-secreting HBV-specific CD8+ T cells could improve after viral load reduction, but without any effect on the CTLA-4 or Bim levels. It should be noted that the latter study was based on a cohort of CHB patients commencing antiviral therapy, all of whom had been treated for 18 months or less. Long-term antiviral therapy may be necessary to change the CTLA-4 and Bim levels and their impact on CD8+ T cells.
Figure 1.
Persistent HBV infection results in the upregulation of CTLA-4 and Bim on hepatic CD8+ T cells. Subsequently, the cytokine secretion by CD8+ T cells decreases. Increased expression of Bim promotes CD8+ T cell apoptosis. Furthermore, the levels of granzyme and perforin are decreased, leading to intrahepatic cccDNA not being removed, because the activation of CD8+ T cells to generate CTLs is blocked. In addition, the CD206+ macrophage-derived amphiregulin promotes the immunosuppressive activity of the Treg cells by inducing mTOR activation and by upregulating the expression of CTLA-4 on Treg cells following HBV persistent infection. HBV, hepatitis B virus; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; Bim, BCL2 interacting mediator of cell death; cccDNA, covalently closed circular DNA; CTL, cytotoxic T cell; Treg, regulatory T cells; mTOR, mammalian target of rapamycin; TNF, tumor necrosis factor; IL, interleukin; IFN, interferon.
3. CTLA-4 mediates HBV-specific dysfunctions in CD4+ Th cell differentiation
Naïve T cells may differentiate into CD4+ T helper (Th) cells following antigen stimulation. A variety of CD4+ Th cell subsets has been confirmed, including Th1, Th2, peripherally-inducible Treg (iTreg), and follicular helper T (TFH) (34). HBV infection can induce a variety of inhibitory molecules and upregulate their expression on CD4+ Th cells. For example, HBV core protein (HBc) induces PD-1 upregulation through activation of the c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K)/AKT pathways (35). HBV surface protein (HBs) enhances transcription of human protein inhibitor of activated STAT1 (hPIAS1) dependent on the activation of P38MAPK and ERK signal pathways, which might contribute to the ineffectiveness of treatment in CHB patients (36). Recently, Raziorrouh et al (37) analyzed the HBV-specific CD4+ T cells from the peripheral blood of CHB patients using a newly-established DRB1*01-restricted MHC class II tetramer, and demonstrated that the frequencies and functions of CD4+ T cells were diminished in these patients compared with healthy controls. When the levels of multiple inhibitory receptors on CD4+ T cells were examined, it was noted that PD-1 was strongly upregulated. However, the levels of CTLA-4 were similar to those in healthy controls, suggesting that CD4+ T cell dysfunction that occurs during chronic HBV infection is associated with strong PD-1 upregulation, but not associated with CTLA-4. However, other studies have demonstrated that the cytoplasmic tail of CTLA-4 is associated with the medium chain of the clathrin adaptor, AP-50, which correlates with CTLA-4 endocytosis, so the majority of CTLA-4 is rapidly endocytosed away from the cell surface and is localized in intracellular stores (38). Thus, it is difficult to detect CTLA-4 on the surface of activated CD4+ T cells.
There are a variety of ways in which CTLA-4 may interfere with CD4+ Th cells. First, CTLA-4 may scavenge B7 ligands, rendering them unable to bind CD28 (39). Second, it may disrupt the localization of CD28 in the immunological synapse (40), thus altering the immune responses, because CTLA-4 can bind the lipid kinase PI3K, and the protein tyrosine phosphatase non-receptor type (PTPN) 11 (also known as SHP2) (41). In addition, CTLA-4 may recruit protein tyrosine phosphatases to induce the negative feedback regulation of T cell receptor (TCR) signaling, perhaps via PTPN6 (also known as SHP1), by dephosphorylating the site of the immunoreceptor tyrosine-based activation motif in CD3, and via PTPN22 (also known as Lyp), by dephosphorylating the CD3ζchain, zeta chain of T cell receptor associated protein kinase 70 (ZAP-70), and lymphocyte cytosolic protein 2 (LCP2, also known as SLP-76) (42).
4. Effects of CTLA-4 on the Th1, Th2 and TFH subtypes of CD4+ Th cells
Depending on the cell environment, CD4+ Th cells can differentiate into different subtypes (43). Several factors likely contribute to CD4+ Th cell dysfunction in chronic HBV infection, including the action of Treg cells (which secrete large quantities of IL-10 and TGF-β), the depletion of arginine in the inflamed liver microenvironment, and enhanced CD4+ Th cell apoptosis in the tolerogenic liver environment (5,17). Notably, these effects are amplified by the upregulation of CTLA-4 expression on CD4+ Th cells (Fig. 2). Tang et al (44) demonstrated that the expression levels of the CD28 family receptors (PD-1 and CTLA-4) in the CD4+ Th cells were increased in CHB patients compared with healthy controls. Additionally, the frequency of Th1 cells was lower compared with Th2 cells in the peripheral blood of these patients. In a subsequent experiment, the authors cultured PBMCs pulsed with anti-CTLA-4 in vitro, and demonstrated that the frequency of Th1 cells increased, while the frequency of Th2 cells decreased. The mechanism by which CTLA-4 affects the polarization of CD4+ T cells toward a Th2 phenotype is not entirely clear, but it has been confirmed that CTLA-4 can change the structure of immune synapses, which serve a pivotal role in T cell proliferation and differentiation (45).
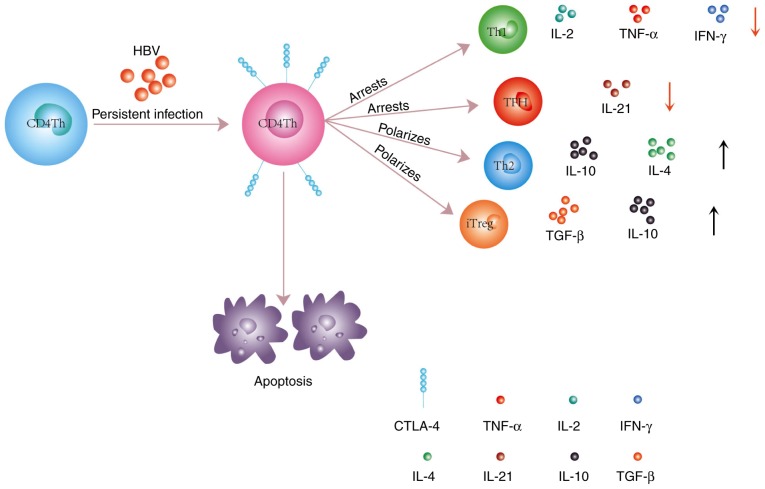
Figure 2.
CD4+ Th cells have similar susceptibility to apoptosis to CD8+ T cells following persistent HBV infection. Upregulated expression of CTLA-4 on CD4+ Th cells polarizes them toward the Th2 and iTreg phenotypes, which results in an increase of anti-inflammatory cytokine (TGF-β, IL-10, and IL-4) levels. Conversely, the differentiation of proinflammatory cells, such as Th1, TFH and CD4+ Th cells, is blocked, and the levels of proinflammatory cytokines (IL-2, IL-21, and IFN-γ) is decreased. Th, T helper cells; HBV, hepatitis B virus; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; iTreg, peripherally-inducible Treg; TGF, transforming growth factor; IL, interleukin; TFH, follicular helper T cells; IFN, interferon.
The classical immunological synapse is described as a central supramolecular activation cluster, which involves molecules, such as the TCR, CD28, protein kinase Cθ (PCKθ), and lymphocyte-specific protein tyrosine kinase (Lck) (46). In the presence of CTLA-4, CD4+ T cells fail to form this cluster of TCR, Lck and PCKθ. Lck binds the cytoplasmic domain of CD4 and CD8 molecules in T cells, which are continuously phosphorylated in resting T cells. After being recruited to the TCR-pMHC-complex with CD4+ or CD8+ T cells, Lck phos-phorylates the tyrosine in the immunoreceptor tyrosine-based activation (ITAM) motif of CD3's cytoplasmic domain, which is responsible for TCR-induced intracellular signal initiation from tyrosine phosphorylation (47). Therefore, the absence of Lck blocks intracellular signal transduction. A lack of TCR clustering in the immune synapse affects antigen presentation via the MHCII receptor and the TCR receptor (signal 1). The nature of activation (signal 1), defined by the strength of the TCR stimulation, can affect the polarization of T helper cells towards Th1 or Th2, in which a high affinity interaction favors Th1 development and a low affinity interaction drives Th2 development (48). Smeets et al (49) recently confirmed that phorbol 12-myristate 13-acetate (PMA)/CD3 stimulation enhances the Th1-like response in a Lck- and PKC-dependent manner, whereas PMA/CD28 stimulation results in a Th2-like phenotype, independent of the proximal TCR-tyrosine kinase Lck. It is intriguing to speculate that a lack of Lck and PKCθ in the immune synapses of CD4+ Th cells may polarize them towards a Th2 phenotype.
Regardless of the mechanism, it has been clearly demonstrated that CTLA-4 induces the differentiation of CD4+ Th cells toward the Th2 cell phenotype, and increases the frequencies of these cells in the liver and peripheral blood. However, higher expression of CTLA-4 might be a consequence of a low responsiveness by Th2 cells to CTLA-4 function, and a lack of the TCR cluster in the immune synapse hinders the proliferation of these cell and their synthesis of cytokines (IL-3, IL-4 and IL-5) (47). It is possible that CTLA-4 may play a coordinating role with Th2 cells through humoral immunity. Yin et al (50) immunized mice with DNA plasmids (HBcAg fused to the extracellular domain of CTLA-4, termed pCTLA-4-HBc) prior to challenging by hydrodynamic injection (HI) of pAAV/HBV1.2, and demonstrated that the clearance of HBsAg was completed more quickly (within 16 days) in the immunized mice compared with the control mice. Of note, >50% of the control mice were still positive for HBsAg on day 22. Meanwhile, the Th1/Th2 bias (the Th2 type cells were increased) and anti-HBs antibody response developed rapidly in the mice immunized with pCTLA-4-HBc. Similarly, Zhou et al demonstrated that a CTLA4-fused DNA vaccine, which was constructed by linking the extracellular domain of CTLA-4 with HBsAg, led to breakdown of IT to viral infection in HBV transgenic mice (51). The underlying mechanism may be an increase of Th, Th2 and HBsAg-specific CD8+ T cell responses, as well as CTL, following vaccination with this vaccine. These findings underline the complex impact of CTLA-4 on the immune response.
TFH cells represent a class of effector CD4+ Th cells that regulate the stepwise development of antigen-specific B cell immunity and memory B cell responses by deploying C-X-C motif chemokine receptor 5 (CXCR5)+ TFH cells (52). However, effective immunologic homeostasis appears to be reliant on maintaining an appropriate level of CD28 engagement. The CD80/CD86-CD28/CTLA-4 costimulatory pathway can provide a pivotal signal for T cells activation. In the absence of CTLA-4, excessive CD28 engagement with CD80/CD86 can activate peripheral T cells, and leads to their spontaneous differentiation, resulting in TFH-mediated lethal tissue injury. Recent research has confirmed that CTLA-4-deficient mice die at a young age, which has been suggested to be due to a rapid development of multiorgan lymphocytic infiltration and tissue destruction (53). Remarkably, short-term blockade with an anti-CTLA-4 antibody is sufficient to elicit TFH generation in wild-type mice. CTLA-4 acts by downregulating CD80 and CD86 on antigen-presenting cells (APCs), thereby altering the level of CD28 engagement, resulting in tailored modulation of the TFH response (54). It is clear that the expression of costimulatory ligands on APCs fluctuates in response to environmental stimuli, with their expression being upregulated by inflammatory cytokines and TLR agonists and downregulated by CTLA-4 expression on Treg cells (55). Therefore, during HBV infection, CTLA-4 may downregulate costimulatory ligands to block TFH differentiation and effector responses. The effector responses of TFH, via production of IL-21, appear to have a significant role in facilitating HBeAg seroconversion (56).
5. Upregulating the expression of CTLA-4 may improve the function of Treg cells
Treg cells are broadly subdivided into two major subtypes: Thymus-derived natural Treg (nTreg) cells and peripherally-inducible Treg (iTreg) cells (57). iTreg cells have a large repertoire of self-specific and non-self-specific T-cell receptors and have been detected in several human infectious diseases (58). Highlighting the importance of CTLA-4 in the suppression of the immune response, CTLA-4 expression and signaling are essential for Treg cells to execute their suppressive function (59). CTLA-4 can induce a costimulatory blockade either by sequestering or removing costimulatory ligands from the surface of APCs (perhaps via transendocytosis), and might also stimulate these cells to secrete indoleamine 2,3-dioxygenase (IDO), which limits the availability of tryptophan and metabolically suppresses the activation of naïve T cells (60). Direct mutation of CTLA-4 or CTLA-4 deficiency leads to defective Treg cell function, which is associated with an impaired ability to control the levels of the CTLA-4 ligands, CD80 and CD86 (61). In addition, CTLA-4 may strengthen the secretion of cytokines, such as TGF-β and IL-10, by the Treg cells, to suppress a wide range of cells and cytokines (62). Yang et al (63), Stoop et al (64) and Xu et al (65) have demonstrated that patients with CHB infection have a higher frequency of Treg cells in peripheral blood and liver compared with healthy controls. A recent study concluded that there were increased levels of Treg cells accompanied by CTLA-4 during CHB infection, and that these cells were associated with the antiviral immune response and disease progression (66). Previously, Feng et al (67) have demonstrated that HBcAg peptide-specific Treg cells from liver and peripheral blood of CHB patients express CTLA-4 and have a pivotal role in modulating IT. Enhanced HBcAg peptide-specific CTL frequencies by blocking of CTLA-4 may account for spontaneous Acute exacerbations (AEs) in the process of CHB infection. One possible mechanism for the change in the natural history of CHB infection by blocking CTLA-4 may be the inhibition of TGF-β secretion, which functions as a mediator of Treg cell suppressor effectors (68). Recently, Zhang et al (69) reported similar findings; they demonstrated that CTLA-4 expression levels were significantly higher on Treg cells from patients with HBsAg-positive hepatocellular carcinoma compared with healthy controls, whereas no difference was observed in HBsAg-negative HCC patients. Furthermore, when the human hepatoma cell line HepG2.2.15 (transfected with HBV) and its parental cell line HepG2 were co-cultured with healthy donor PBMCs, the results demonstrated that HepG2.2.15 cells strongly increased the frequencies of Treg cells and CTLA-4 expression compared with the HepG2 cells. Notably, the Treg cells from CHB patients can help HCC tumor antigens escape tumor immunosurveillance. It appears that high expression of CTLA-4 on Treg cells is a risk factor in HBsAg-positive HCC. Inhibition of Treg cells and CTLA-4 may therefore represent a novel therapeutic approach for CHB patients. However, these studies have failed to explain the mechanism by which CHB infection upregulated CTLA-4 on Treg cells. Liu et al (70) may have provided evidence in that direction, by demonstrating that IL-10-producing regulatory B-cells, which are a new subset of B-cells that exert immunosuppressive functions in autoimmunity and infections, can enhance Treg cells in chronic HBV infection and upregulate the expression of CTLA-4. Therefore, it can be speculated that an imbalance of cytokines in the microenvironment of the liver may be one of the reasons. A recent study indicated that total Treg cell frequencies were affected by IL-2, and the strongest Treg cell suppressive phenotype was observed under low-dose IL-2. It was hypothesized that low-dose IL-2 promotes STAT5 phosphorylation and subsequently upregulates CTLA-4 (71). Nevertheless, the relationship between the proteins of HBV and CTLA-4 expression following CHB infection remain unclear.
There have recently been conflicting findings regarding the function of Treg cells (Table I), suggesting that their activation may not be entirely dependent on CTLA-4. Treg activation can be mediated by forkhead box P3 (Foxp3), as it serves a central role in directing the regulatory program (72). Foxp3 expression is essentially confined to CD4+CD25+ cells and is responsible for the regulatory activity of this subset of cells. Accordingly, the adoptive transfer of CD4+CD25+ T cells from wild type mice can rescue scurfy mice, which are deficient in Treg cells, from lymphoproliferative syndrome. Retroviral expression and transgenic expression of Foxp3 in CD25− T cells have been demonstrated to endow them with regulatory functions, and even induce regulatory activity in CD8+ T cells (73). Consistent with the large body of evidence obtained in mouse models, mutations in the Foxp3 gene in humans are associated with defective immune regulation, manifesting as a syndrome that has been termed 'immune dysregulation polyendocrinopathy enteropathy Xlinked' (IPEX) (74). Together, these findings suggest that Foxp3 and CTLA-4 exhibit some redundant functions and can function independently of one another. More recently, Paterson et al (75) have put forward new evidence that Treg cells remain functionally suppressive and produce an overabundance of IL-10 following the deletion of CTLA-4. The authors demonstrated that the deletion of CTLA-4 in mice during adulthood did not precipitate systemic autoimmunity, but surprisingly conferred protection against experimental autoimmune encephalomyelitis (EAE). Furthermore, they demonstrated that the deletion of CTLA-4 was accompanied by the activation and expansion of both conventional CD4+ T cells and Treg cell subsets. Notwithstanding, Fontenot et al reported that all mice receiving a mix of scurfy and CTLA-4−/− bone marrow died, and while transgenic overexpression of Foxp3 could delay the lethality in CTLA-4−/− mice, it could not completely rescue the mice (73). Hence, effective immune regulation by Treg cells requires the co-expression of Foxp3 and CTLA-4. In conclusion, these findings suggest that upregulation of CTLA-4 in Treg cells in HBV infection patients is involved in the immunopathogenesis of acute Hepatitis B (AHB) to CHB.
Table I.
Importance of CTLA-4 on Treg cells.
| Status of CTLA-4 | Changes in Treg functions | Effects on mouse model |
|---|---|---|
| Present | Cell cycle arrest, but resistance against AICD (58); Suppresses Tcon cell activity and proliferation, controls cytokine production (58,59) | Inhibits AMA production, intrahepatic T-cell infiltration, and bile duct damage (80), and may relieve autoimmunity and inflammatory bowel disease (59) |
| Absent | Increases the frequencies of Foxp3+T cells, leads to Treg expansion but the cells were prone to apoptosis (58,74); remains functionally suppressive and produces an overabundance of IL-10 (74) | Lethal lymphoproliferative disease (73) Protection from EAE (58) |
CTLA-4, cytotoxic T lymphocyte-associated antigen-4; Treg, regulatory T cells; ACID, activation-induced cell death; Tcon cells, conventional CD4+ T cells; AMA, anti-mitochondrial antibodies; Foxp3, forkhead box P3; IL, interleukin; EAE, experimental autoimmune encephalomyelitis.
6. CTLA-4 downregulates an array of cytokines
Cytokines mediate the non-cytolytic clearance of HBV, and have been demonstrated to control HBV replication and to contribute to curing HBV in several disease models (76). IFN-γ restricts HBV entry by inducing soluble factors that bind to heparan sulfate proteoglycan (HSPG) and block HBV attachment (77). IL-1β regulates sodium taurocholate cotransporting polypeptide (NTCP) expression to inhibit HBV infection (78). IFN-γ and TNF-α interfere with covalently closed circular DNA (cccDNA) integrity and stability by inducing cccDNA deamination and subsequent degradation in HBV-infected primary human hepatocytes and HepaRG cells (79). IL-6 may reduce cccDNA and HBsAg secretion (80). CTLA-4 has been demonstrated to be a major and specific regulator of the production of both pro- and anti-inflammatory cytokines. In the dominant negative transforming growth factor β receptor II (dnTGFβRII) mouse model, CTLA-4 Ig quickly reduced the level of intrahepatic proinflammatory cytokines, thereby reducing the bile duct damage (81). Blockade of CTLA-4 resulted in increased secretion of granulocyte-macrophage colony stimulating factor (GM-CSF), IL-1, IL-2 and IFN-γ by CD4+ Th cells (82). Similar to CD4+ Th cells, CD8+ T cells exhibited an enhanced ability to control HBV infection following inhibition of CTLA-4. Virus-specific CD8+ T cells can function in two different ways during an interaction with HBV-producing hepatocytes. When they are cytolytic, they can induce the clearance of intrahepatic cccDNA by killing a fraction of the infected cells. The noncytolytic activity is mediated by an array of cytokines, including IFN-γ, TNF-α and IL-2 (83). When CTLA-4 is silenced by RNA interference in in vitro cultures of PBMCs derived from CHB-infected patients, an enhanced secretion of IFN-γ and IL-2 were observed. Furthermore, when the CD8+ T cells from CHB patients were treated with anti-CTLA-4 antibodies, the levels of IFN-γ, TNF-α and IL-2 were significantly increased following stimulation with an HBV peptide (28,84). Finally, Pedicord et al (85) confirmed that anti-CTLA-4 antibodies could enhance the memory formation, function, and maintenance of CD8+ T cells. The above studies confirmed that CTLA-4 decreases the synthesis of many proinflammatory cytokines. However, further studies are required to determine the specific mechanisms involved.
7. Conclusion
This review summarized the current literature highlighting the negative effects of CTLA-4 in the context of chronic HBV infection (Table II). Although the specific mechanisms leading to the upregulation of CTLA-4 on T cells remain unclear, CTLA-4 has been demonstrated to inhibit HBV-specific T cell immune responses. Anti-CTLA-4 antibodies have exhibited immunomodulatory effects in patients with hepatocellular carcinoma and chronic hepatitis C (86). The mechanism underlying the antitumor and antiviral activity of these antibodies may be associated with a recovery of the function of the host T cells, as well as decreased deletion of T cells by blockade of CTLA-4. However, severe immune-mediated adverse events may limit the clinical potential of such treatments. Therefore, future studies are needed to explore the specific effector T-cell response that may be regulated by CTLA-4, as well as potential T cell-independent mechanisms.
Table II.
Immunological characteristics and clinical manifestations following upregulation of CTLA-4.
| Status | Immunological characteristics
|
Compared with healthy controls | Clinical manifestations | (Refs.) | ||
|---|---|---|---|---|---|---|
| Cells | Cytokines | Molecules | ||||
| Immune clearance | CD8+T, Th1 CD8+ CTL, TFH |
IL-2, IFN-γ, TNF-α, GM-CS, IL-1β, IL-21, perforin, granzyme, CCL3 | CD28, CD80/CD86 Lck, TCR, PCKθ |
Decreased | Mild liver damage, but poor response to HBV proteins | (6,22,25,27,28,39,43, 48,55,81,83,84) |
| Immune tolerance | Treg, Th2 | IL-10, TGF-β | PD-1, CTLA-4, Bim | Increased | Low-grade inflammation, but led to T cell exhaustion and apoptosis | (29,32,62,63, 64,66,70) |
CTLA-4, cytotoxic T lymphocyte-associated antigen-4; Th, T helper cells; CTL, cytotoxic T cell; TFH, follicular helper T cells; Treg, regulatory T cell; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor; GM-CSF, granulocyte-macrophage colony stimulating factor; CCL3, C-C motif chemokine ligand 3; TGF, transforming growth factor; Lck, transforming growth factor; TCR, T cell receptor; PCKθ, protein kinase Cθ; PD-1, programmed cell death-1; HBV, hepatitis B virus.
Acknowledgments
The authors thank Ying Yu Wang for assistance with figure production.
Funding
This study was funded by the National Natural Science Foundation of China (grant no. 81373860).
Availability of data and materials
The analyzed datasets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
HC wrote the manuscript. WZ edited and proofread this manuscript. RWZ was a major contributor in commenting and revising the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B, et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the global burden of disease study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu FM, Li T, Liu S, Zhuang H. Epidemiology and prevention of hepatitis B virus infection in China. J Viral Hepat. 2010;17(Suppl 1):S4–S9. doi: 10.1111/j.1365-2893.2010.01266.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Zhang S, Wang Q, Shen H, Zhang M, Zhang Y, Yan D, Liu M. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21-49 years in rural China: A population-based, cross-sectional study. Lancet Infect Dis. 2016;16:80–86. doi: 10.1016/S1473-3099(15)00218-2. [DOI] [PubMed] [Google Scholar]
- 4.Blendis L, Lurie Y, Oren R. Occult HBV infection-both hidden and mysterious. Gastroenterology. 2003;125:1903–1905. doi: 10.1053/j.gastro.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Park JJ, Wong DK, Wahed AS, Lee WM, Feld JJ, Terrault N, Khalili M, Sterling RK, Kowdley KV, Bzowej N, et al. Hepatitis B virus - specific and global T-cell dysfunction in chronic hepatitis B. Gastroenterology. 2016;150:684–695.e5. doi: 10.1053/j.gastro.2015.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: Current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. doi: 10.1038/cddis.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling V, Wu PW, Finnerty HF, Sharpe AH, Gray GS, Collins M. Complete sequence determination of the mouse and human CTLA4 gene loci: Cross-species DNA sequence similarity beyond exon borders. Genomics. 1999;60:341–355. doi: 10.1006/geno.1999.5930. [DOI] [PubMed] [Google Scholar]
- 8.Lindsten T, Lee KP, Harris ES, Petryniak B, Craighead N, Reynolds PJ, Lombard DB, Freeman GJ, Nadler LM, Gray GS, et al. Characterization of CTLA-4 structure and expression on human T cells. J Immunol. 1993;151:3489–3499. [PubMed] [Google Scholar]
- 9.Adams AB, Ford ML, Larsen CP. Costimulation blockade in autoimmunity and transplantation: The CD 28 pathway. J Immunol. 2016;197:2045–2050. doi: 10.4049/jimmunol.1601135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily - CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 11.Tang ST, Tang HQ, Zhang Q, Wang CJ, Wang YM, Peng WJ. Association of cytotoxic T-lymphocyte associated antigen 4 gene polymorphism with type 1 diabetes mellitus: A meta-analysis. Gene. 2012;508:165–187. doi: 10.1016/j.gene.2012.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Wang B, Pan F, Zhang R, Xiao L, Guo H, Ma S, Zhou C. Association between cytotoxic T-lymphocyte antigen 4 gene polymorphisms and primary biliary cirrhosis in Chinese population: Data from a multicenter study. J Gastroenterol Hepatol. 2013;28:1397–1402. doi: 10.1111/jgh.12165. [DOI] [PubMed] [Google Scholar]
- 13.Du L, Yang J, Huang J, Ma Y, Wang H, Xiong T, Xiang Z, Zhang Y, Huang J. The associations between the polymorphisms in the CTLA-4 gene and the risk of Graves' disease in the Chinese population. BMC Med Genet. 2013;14:46. doi: 10.1186/1471-2350-14-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang R, Hao Y, Fan Y, Yang C, Wu K, Cao S, Wu C. Association between cytotoxic T-lymphocyte-associated antigen 4+49A/G polymorphism and persistent hepatitis B virus infection in the Asian population: Evidence from the current studies. Genet Test Mol Biomarkers. 2013;17:601–606. doi: 10.1089/gtmb.2013.0069. [DOI] [PubMed] [Google Scholar]
- 15.Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8+ T cell effector functions after antigen recognition in the liver. Immunity. 2005;23:53–63. doi: 10.1016/j.immuni.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Benechet AP, Iannacone M. Determinants of hepatic effector CD8+ T cell dynamics. J Hepatol. 2017;66:228–233. doi: 10.1016/j.jhep.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Bengsch B, Martin B, Thimme R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol. 2014;61:1212–1219. doi: 10.1016/j.jhep.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 19.Legat A, Speiser DE, Pircher H, Zehn D, Fuertes Marraco SA. Inhibitory receptor expression depends more dominantly on differentiation and activation than 'Exhaustion' of human CD8 T cells. Front Immunol. 2013;4:455. doi: 10.3389/fimmu.2013.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 22.Cho H, Kang H, Kim CW, Kim HY, Jang JW, Yoon SK, Lee CD. Phenotypic characteristics of PD-1 and CTLA-4 expression in symptomatic acute hepatitis A. Gut Liver. 2016;10:288–294. doi: 10.5009/gnl14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radziewicz H, Ibegbu CC, Hon H, Osborn MK, Obideen K, Wehbi M, Freeman GJ, Lennox JL, Workowski KA, Hanson HL, Grakoui A. Impaired hepatitis C virus (HCV)-specific effector CD8+ T cells undergo massive apoptosis in the peripheral blood during acute HCV infection and in the liver during the chronic phase of infection. J Virol. 2008;82:9808–9822. doi: 10.1128/JVI.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das A, Hoare M, Davies N, Lopes AR, Dunn C, Kennedy PT, Alexander G, Finney H, Lawson A, Plunkett FJ, et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med. 2008;205:2111–2124. doi: 10.1084/jem.20072076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wongjitrat C, Sukwit S, Chuenchitra T, Seangjaruk P, Rojanasang P, Romputtan P, Srisurapanon S. CTLA-4 and its ligands on the surface of T- and B-lymphocyte subsets in chronic hepatitis B virus infection. J Med Assoc Thai. 2013;96(Suppl 1):S54–S59. [PubMed] [Google Scholar]
- 26.Kennedy PTF, Sandalova E, Jo J, Gill U, Ushiro-Lumb I, Tan AT, Naik S, Foster GR, Bertoletti A. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology. 2012;143:637–645. doi: 10.1053/j.gastro.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Velazquez VM, Grakoui A. Immune quiescence and hepatitis B virus: Tolerance has its limits. Gastroenterology. 2012;143:529–532. doi: 10.1053/j.gastro.2012.07.089. [DOI] [PubMed] [Google Scholar]
- 28.Peng G, Luo B, Li J, Zhao D, Wu W, Chen F, Chen Z. Hepatitis B e-antigen persistency is associated with the properties of HBV-specific CD8 T cells in CHB patients. J Clin Immunol. 2011;31:195–204. doi: 10.1007/s10875-010-9483-5. [DOI] [PubMed] [Google Scholar]
- 29.Schuette V, Embgenbroich M, Ulas T, Welz M, Schulte- Schrepping J, Draffehn AM, Quast T, Koch K, Nehring M, König J, et al. Mannose receptor induces T-cell tolerance via inhibition of CD45 and up-regulation of CTLA-4. Proc Natl Acad Sci USA. 2016;113:10649–10654. doi: 10.1073/pnas.1605885113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, Nebbia G, Kennedy PT, Geretti AM, Dusheiko G, Maini MK. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology. 2011;53:1494–1503. doi: 10.1002/hep.24249. [DOI] [PubMed] [Google Scholar]
- 31.Wang YM, Zhang GY, Wang Y, Hu M, Zhou JJ, Sawyer A, Cao Q, Wang Y, Zheng G, Lee VW, et al. Exacerbation of spontaneous autoimmune nephritis following regulatory T cell depletion in B cell lymphoma 2-interacting mediator knock-out mice. Clin Exp Immunol. 2017;188:195–207. doi: 10.1111/cei.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai K, Huang L, Sun X, Yang L, Gong Z. Hepatic CD206-positive macrophages express amphiregulin to promote the immunosuppressive activity of regulatory T cells in HBV infection. J Leukoc Biol. 2015;98:1071–1080. doi: 10.1189/jlb.4A0415-152R. [DOI] [PubMed] [Google Scholar]
- 33.Nitschke K, Luxenburger H, Kiraithe MM, Thimme R, Neumann-Haefelin C. CD8+ T-cell responses in hepatitis B and C: The (HLA-) A, B, and C of hepatitis B and C. Dig Dis. 2016;34:396–409. doi: 10.1159/000444555. [DOI] [PubMed] [Google Scholar]
- 34.Farhan RK, Vickers MA, Ghaemmaghami AM, Hall AM, Barker RN, Walsh GM. Effective antigen presentation to helper T cells by human eosinophils. Immunology. 2016;149:413–422. doi: 10.1111/imm.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li M, Sun XH, Zhu XJ, Jin SG, Zeng ZJ, Zhou ZH, Yu Z, Gao YQ. HBcAg induces PD-1 upregulation on CD4+ T cells through activation of JNK, ERK and PI3K/AKT pathways in chronic hepatitis-B-infected patients. Lab Invest. 2012;92:295–304. doi: 10.1038/labinvest.2011.157. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Wu D, Wang X, Chen G, Zhang Y, Yan W, Luo X, Han M, Ning Q. Hepatitis B virus surface protein-induced hPIAS1 transcription requires TAL1, E47, MYOG, NFI, and MAPK signal pathways. Biol Chem. 2016;397:1173–1185. doi: 10.1515/hsz-2015-0290. [DOI] [PubMed] [Google Scholar]
- 37.Raziorrouh B, Heeg M, Kurktschiev P, Schraut W, Zachoval R, Wendtner C, Wächtler M, Spannagl M, Denk G, Ulsenheimer A, et al. Inhibitory phenotype of HBV-specific CD4+ T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS One. 2014;9:e105703. doi: 10.1371/journal.pone.0105703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 39.Halpert MM, Konduri V, Liang D, Chen Y, Wing JB, Paust S, Levitt JM, Decker WK. Dendritic cell-secreted cytotoxic T-lymphocyte-associated protein-4 regulates the T-cell response by downmodulating bystander surface B7. Stem Cells Dev. 2016;25:774–787. doi: 10.1089/scd.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, et al. Basis of CTLA-4 function in regulatory and conventional CD4+ T cells. Blood. 2012;119:5155–5163. doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ville S, Poirier N, Blancho G, Vanhove B. Co-stimulatory blockade of the CD28/CD80-86/CTLA-4 balance in transplantation: Impact on memory T cells? Front Immunol. 2015;6:411. doi: 10.3389/fimmu.2015.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cloutier JF, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med. 1999;189:111–121. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang ZS, Hao YH, Zhang EJ, Xu CL, Zhou Y, Zheng X, Yang DL. CD28 family of receptors on T cells in chronic HBV infection: Expression characteristics, clinical significance and correlations with PD-1 blockade. Mol Med Rep. 2016;14:1107–1116. doi: 10.3892/mmr.2016.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dilek N, Poirier N, Hulin P, Coulon F, Mary C, Ville S, Vie H, Clémenceau B, Blancho G, Vanhove B. Targeting CD28, CTLA-4 and PD-L1 costimulation differentially controls immune synapses and function of human regulatory and conventional T-cells. PLoS One. 2013;8:e83139. doi: 10.1371/journal.pone.0083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: A molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 47.Alegre ML, Shiels H, Thompson CB, Gajewski TF. Expression and function of CTLA-4 in Th1 and Th2 cells. J Immunol. 1998;161:3347–3356. [PubMed] [Google Scholar]
- 48.Turner MS, Isse K, Fischer DK, Turnquist HR, Morel PA. Low TCR signal strength induces combined expansion of Th2 and regulatory T cell populations that protect mice from the development of type 1 diabetes. Diabetologia. 2014;57:1428–1436. doi: 10.1007/s00125-014-3233-9. [DOI] [PubMed] [Google Scholar]
- 49.Smeets RL, Fleuren WW, He X, Vink PM, Wijnands F, Gorecka M, Klop H, Bauerschmidt S, Garritsen A, Koenen HJ, et al. Molecular pathway profiling of T lymphocyte signal transduction pathways; Th1 and Th2 genomic fingerprints are defined by TCR and CD28-mediated signaling. BMC Immunol. 2012;13:12. doi: 10.1186/1471-2172-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin Y, Wu C, Song J, Wang J, Zhang E, Liu H, Yang D, Chen X, Lu M, Xu Y. DNA immunization with fusion of CTLA-4 to hepatitis B virus (HBV) core protein enhanced Th2 type responses and cleared HBV with an accelerated kinetic. PLoS One. 2011;6:e22524. doi: 10.1371/journal.pone.0022524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou C, Peng G, Jin X, Tang J, Chen Z. Vaccination with a fusion DNA vaccine encoding hepatitis B surface antigen fused to the extracellular domain of CTLA4 enhances HBV-specific immune responses in mice: Implication of its potential use as a therapeutic vaccine. Clin Immunol. 2010;137:190–198. doi: 10.1016/j.clim.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer- Williams MG. Follicular helper T cells: Lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 54.Wang CJ, Heuts F, Ovcinnikovs V, Wardzinski L, Bowers C, Schmidt EM, Kogimtzis A, Kenefeck R, Sansom DM, Walker LS. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci USA. 2015;112:524–529. doi: 10.1073/pnas.1414576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y, Ma S, Tang L, Li Y, Wang W, Huang X, Lai Q, Zhang M, Sun J, Li CK, et al. Circulating chemokine (C-X-C Motif) receptor 5(+) CD4(+) T cells benefit hepatitis B e antigen seroconversion through IL-21 in patients with chronic hepatitis B virus infection. Hepatology. 2013;58:1277–1286. doi: 10.1002/hep.26489. [DOI] [PubMed] [Google Scholar]
- 57.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, et al. Regulatory T cells: Recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 58.Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: Similarities and differences. Immunol Rev. 2014;259:88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kolar P, Knieke K, Hegel JK, Quandt D, Burmester GR, Hoff H, Brunner-Weinzierl MC. CTLA-4 (CD152) controls homeostasis and suppressive capacity of regulatory T cells in mice. Arthritis Rheum. 2009;60:123–132. doi: 10.1002/art.24181. [DOI] [PubMed] [Google Scholar]
- 60.Guntermann C, Alexander DR. CTLA-4 suppresses proximal TCR signaling in resting human CD4+ T cells by inhibiting ZAP-70 Tyr319 phosphorylation: A potential role for tyrosine phosphatases. J Immunol. 2002;168:4420–4429. doi: 10.4049/jimmunol.168.9.4420. [DOI] [PubMed] [Google Scholar]
- 61.Hou TZ, Verma N, Wanders J, Kennedy A, Soskic B, Janman D, Halliday N, Rowshanravan B, Worth A, Qasim W, et al. Identifying functional defects in patients with immune dysregulation due to LRBA and CTLA-4 mutations. Blood. 2017;129:1458–1468. doi: 10.1182/blood-2016-10-745174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mellor AL, Munn DH. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 63.Yang G, Liu A, Xie Q, Guo TB, Wan B, Zhou B, Zhang JZ. Association of CD4+CD25+Foxp3+ regulatory T cells with chronic activity and viral clearance in patients with hepatitis B. Int Immunol. 2007;19:133–140. doi: 10.1093/intimm/dxl130. [DOI] [PubMed] [Google Scholar]
- 64.Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771–778. doi: 10.1002/hep.20649. [DOI] [PubMed] [Google Scholar]
- 65.Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, Zhang B, Shi M, Ding X, et al. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739–747. doi: 10.4049/jimmunol.177.1.739. [DOI] [PubMed] [Google Scholar]
- 66.Tavakolpour S, Alavian SM, Sali S. Manipulation of regulatory cells' responses to treatments for chronic hepatitis B virus infection. Hepat Mon. 2016;16:e37927. doi: 10.5812/hepatmon.37927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng IC, Koay LB, Sheu MJ, Kuo HT, Sun CS, Lee C, Chuang WL, Liao SK, Wang SL, Tang LY, et al. HBcAg-specific CD4+CD25+ regulatory T cells modulate immune tolerance and acute exacerbation on the natural history of chronic hepatitis B virus infection. J Biomed Sci. 2007;14:43–57. doi: 10.1007/s11373-006-9129-z. [DOI] [PubMed] [Google Scholar]
- 68.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4+ T cells. J Exp Med. 1998;188:1849–1857. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP+CTLA-4+Foxp3+ T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435–2444. doi: 10.1158/0008-5472.CAN-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y, Cheng LS, Wu SD, Wang SQ, Li L, She WM, Li J, Wang JY, Jiang W. IL-10-producing regulatory B-cells suppressed effector T-cells but enhanced regulatory T-cells in chronic HBV infection. Clin Sci. 2016;130:907–919. doi: 10.1042/CS20160069. [DOI] [PubMed] [Google Scholar]
- 71.Jeffery HC, Jeffery LE, Lutz P, Corrigan M, Webb GJ, Hirschfield GM, Adams DH, Oo YH. Low-dose interleukin-2 promotes STAT-5 phosphorylation, Treg survival and CTLA-4-dependent function in autoimmune liver diseases. Clin Exp Immunol. 2017;188:394–411. doi: 10.1111/cei.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker LS. Treg and CTLA-4: Two intertwining pathways to immune tolerance. J Autoimmun. 2013;45:49–57. doi: 10.1016/j.jaut.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 74.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 75.Paterson AM, Lovitch SB, Sage PT, Juneja VR, Lee Y, Trombley JD, Arancibia-Cárcamo CV, Sobel RA, Rudensky AY, Kuchroo VK, et al. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J Exp Med. 2015;212:1603–1621. doi: 10.1084/jem.20141030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xia Y, Protzer U. Control of hepatitis B virus by cytokines. Viruses. 2017;9:pii: E18. doi: 10.3390/v9010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xia Y, Cheng X. Secreted interferon-inducible factors restrict hepatitis B and C virus entry in vitro. J Immunol Res. 2017;2017:4828936. doi: 10.1155/2017/4828936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Le Vee M, Gripon P, Stieger B, Fardel O. Down-regulation of organic anion transporter expression in human hepatocytes exposed to the proinflammatory cytokine interleukin 1beta. Drug Metab Dispos. 2008;36:217–222. doi: 10.1124/dmd.107.016907. [DOI] [PubMed] [Google Scholar]
- 79.Xia Y, Stadler D, Lucifora J, Reisinger F, Webb D, Hösel M, Michler T, Wisskirchen K, Cheng X, Zhang K, et al. Interferon-γ and tumor necrosis Factor-α produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology. 2016;150:194–205. doi: 10.1053/j.gastro.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 80.Bouezzedine F, Fardel O, Gripon P. Interleukin 6 inhibits HBV entry through NTCP down regulation. Virology. 2015;481:34–42. doi: 10.1016/j.virol.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 81.Dhirapong A, Yang GX, Nadler S, Zhang W, Tsuneyama K, Leung P, Knechtle S, Ansari AA, Coppel RL, Liu FT, et al. Therapeutic effect of cytotoxic T lymphocyte antigen 4/immunoglobulin on a murine model of primary biliary cirrhosis. Hepatology. 2013;57:708–715. doi: 10.1002/hep.26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kolar P, Hoff H, Maschmeyer P, Burmester GR, Brunner-Weinzierl MC. CTLA-4 (CD152) blockade does not cause a pro-inflammatory cytokine profile in regulatory T cells. Clin Exp Rheumatol. 2011;29:254–260. [PubMed] [Google Scholar]
- 83.Phillips S, Chokshi S, Riva A, Evans A, Williams R, Naoumov NV. CD8+ T cell control of hepatitis B virus replication: Direct comparison between cytolytic and noncytolytic functions. J Immunol. 2010;184:287–295. doi: 10.4049/jimmunol.0902761. [DOI] [PubMed] [Google Scholar]
- 84.Yu Y, Wu H, Tang Z, Zang G. CTLA4 silencing with siRNA promotes deviation of Th1/Th2 in chronic hepatitis B patients. Cell Mol Immunol. 2009;6:123–127. doi: 10.1038/cmi.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pedicord VA, Montalvo W, Leiner IM, Allison JP. Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci USA. 2011;108:266–271. doi: 10.1073/pnas.1016791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed datasets generated during the study are available from the corresponding author on reasonable request.