Abstract
Background and aims
Given the similarities in clinical symptoms, Internet gaming disorder (IGD) is thought to be diagnostically similar to Internet-based gambling disorder (ibGD). However, cognitive enhancement and educational use of Internet gaming suggest that the two disorders derive from different neurobiological mechanisms. The goal of this study was to compare subjects with ibGD to those with IGD.
Methods
Fifteen patients with IGD, 14 patients with ibGD, and 15 healthy control subjects were included in this study. Resting-state functional magnetic resonance imaging data for all participants were acquired using a 3.0 Tesla MRI scanner (Philips, Eindhoven, The Netherlands). Seed-based analyses, the three brain networks of default mode, cognitive control, and reward circuitry, were performed.
Results
Both IGD and ibGD groups demonstrated decreased functional connectivity (FC) within the default-mode network (DMN) (family-wise error p < .001) compared with healthy control subjects. However, the IGD group demonstrated increased FC within the cognitive network compared with both the ibGD (p < .01) and healthy control groups (p < .01). In contrast, the ibGD group demonstrated increased FC within the reward circuitry compared with both IGD (p < .01) and healthy control subjects (p < .01).
Discussion and conclusions
The IGD and ibGD groups shared the characteristic of decreased FC in the DMN. However, the IGD group demonstrated increased FC within the cognitive network compared with both ibGD and healthy comparison groups.
Keywords: Internet gaming disorder, Internet gambling disorder, magnetic resonance imaging, resting-state functional magnetic resonance imaging, functional connectivity
Introduction
Diagnostic classification
The Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) classifies gambling disorder within substance use and addictive disorders (American Psychiatric Association [APA], 2013). Substance addiction and gambling disorder are thought to share the core components of addictive behaviors, including neurobiological mechanisms (Grant, Potenza, Weinstein, & Gorelick, 2010) and immediate reward-seeking behavior without accomplishment of long-term goals (Diekhof & Gruber, 2010). Internet gambling is defined to consist of wagering and gambling through Internet-enabled devices, such as computers, mobile phones, and digital television (Gainsbury et al., 2015; Monaghan, 2009). It is a modified form of gambling that provides rapid feedback and easy access to a number of betting options (Gainsbury et al., 2015; Monaghan, 2009). Given the similarities in clinical symptoms between excessive use of both Internet gambling and Internet gaming devices and the potential harm induced by excessive Internet use, several researchers have posited that Internet gaming disorder (IGD) might be diagnostically similar to Internet-based gambling disorder (ibGD; Dowling, 2014).
However, Internet game play has the potential to induce positive effects, including cognitive enhancement, and can be used in educational settings. In contrast, Internet gambling does not have these benefits (Li, Chau, Wong, Lai, & Yip, 2013; van Muijden, Band, & Hommel, 2012). Moreover, the diagnostic classification of IGD has been controversial. Several authors have suggested that IGD, with its characteristic urge for game play, extensive playing time, and harmful side effects, should be classified as a form of addiction (Carbonell, Guardiola, Fuster, Gil, & Panova, 2016). Other investigators have suggested that problematic Internet use with the characteristics of compulsive game play should be classified as an impulse control disorder (Shapira, Goldsmith, Keck, Khosla, & McElroy, 2000). The DSM-5 did not include IGD as a formal diagnosis, but suggests the need for further research (APA, 2013). For these reasons, further investigation into the similarities and differences between IGD and ibGD is warranted.
Brain networks involved in addiction and impulse control disorder
To investigate the biological difference between IGD and ibGD, we compared brain connectivity within the default-mode network (DMN), reward circuitry, and the cognitive control network (CCN). The DMN consists of the precuneus, the posterior cingulate cortex (PCC), the medial prefrontal cortex, and the dorsal anterior cingulate cortex (Inuggi et al., 2014; Raichle et al., 2001). The DMN has shown higher activity at resting baseline and decreased activity during externally oriented performance of cognitive tasks (Fox et al., 2005; Uddin, Clare Kelly, Biswal, Xavier Castellanos, & Milham, 2009). Recent investigations showed that the DMN is related to episodic memory, memory consolidation, self-related processes, and stimulus-independent thought (Uddin et al., 2009). Jung et al. (2014) reported decreased FC of default-mode connectivity among the left superior frontal gyrus, right middle temporal gyrus, and precuneus in a pathological gambling group. They also found that the severity of pathological gambling was negatively correlated with FC in the seeds of the PCC and precuneus (Jung et al., 2014). The DMN is also known to be associated with impulsive traits in tobacco smokers (Tang, Razi, Friston, & Tang, 2016), impulsive violence, and a socially deviant life style (Juarez, Kiehl, & Calhoun, 2013).
The reward circuitry is related to a strong desire for incentive-based learning and the growth of goal-directed behaviors (Haber & Knutson, 2010). Traditionally, the striatum is considered to be the core region of reward processing. However, the amygdala and the orbitofrontal cortex are also regarded as part of the reward circuit (Haber & Knutson, 2010; McClure, York, & Montague, 2004; Schultz, 2006), especially in terms of reward-predicting stimulus (Schultz, 2006).
The CCN consists of the dorsolateral prefrontal cortex (DLPFC), the presupplementary motor area, the inferior frontal junction, the anterior insular cortex, the dorsal premotor cortex, and the posterior parietal cortices. Because it is associated with goal-directed decision-making (Cole & Schneider, 2007), some studies of gambling and Internet gaming have reported an association between these areas (Sohrabi, Smith, West, & Cameron, 2015). Yuan et al. (2017) showed decreased FC in the DLPFC-caudate in an IGD group and a correlation between FC in the DLPFC-caudate and cognitive control assessed by the Stroop task. During a gambling task, conflict and uncertainty resulting from risky decision-making have been associated with activation within the DLPFC (Li et al., 2013; Sohrabi et al., 2015). Several reports have also suggested that online game play might enhance cognition in young people (Li et al., 2013). On the whole, these findings indicate that comparing brain connectivity between these three networks could differentiate between biological mechanisms underlying IGD and those of ibGD.
Hypothesis
This study aimed to provide biological evidence to differentiate ibGD and IGD. Based on prior studies, we hypothesized that both Internet gambling and Internet gaming individuals would have decreased connectivity within the DMN (impulse control dysfunction) compared with healthy control subjects. In contrast, we anticipated that the IGD group would have increased functional connectivity (FC) within the cognitive network compared with the ibGD group, due to the prefrontal cognitive enhancement associated with online game play. Finally, due to intense stimulation with rapid and instant feedback, as well as easy access to a number of betting options, we further expected that the ibGD group would have increased connectivity within the reward circuitry compared with the IGD group. To summarize, we hypothesized that IGD participants would show fewer addictive features in terms of brain connectivity compared with ibGD participants.
Methods
Participants
Twenty-two male patients with IGD group and 20 male patients with ibGD group who visited the outpatient department of a university hospital were screened for this study. Through advertisements posted within the university hospital, 15 age-matched, male, healthy control participants were also recruited. Four IGD patients with major depressive disorder (MDD), three IGD patients with attention-deficit hyperactivity disorder (ADHD), two ibGD patients with MDD, three ibGD patients with ADHD, and one ibGD patient with bipolar disorder were excluded. No patients were addicted to other substances. Ultimately, 15 patients with IGD, 14 patients with ibGD, and 15 healthy comparison subjects (HC) were included in the analysis. Seven IGD patients (32% of the total recruited IGD) and six ibGD patients (30% of total recruited ibGD) were excluded due to comorbid psychiatric diseases. The inclusion criteria were a DSM-5 diagnosis (research criteria) of IGD or ibGD, >18 years, male, and psychiatric medication-naive. In addition, these conditions were added to the inclusion criteria for IGD: (a) excessive Internet game play time (more than 4 hr per day/30 hr per week), (b) Young Internet Addiction Scale (YIAS) score (Young, 1996) >50, (c) irritable, anxious, and aggressive behavior when forced to stop online game play, (d) impaired behaviors or distress, economic crisis, and maladaptive regular life patterns including disrupted diurnal rhythms (sleeping during the day due to gaming at night), irregular meals, failure to maintain personal hygiene, and (e) job loss or absence of school. We used the diagnostic criteria of gambling disorders for additional inclusion criteria for ibGD but changed problematic gambling to Internet-based gambling. The exclusion criteria were low intelligence quotient (IQ) (<80), other comorbid medical or psychiatric diseases, and history of substance abuse except for social alcohol drinking and smoking.
Procedure
All participants were assessed with the DSM-IV (First, Spitzer, Gibbon, & Williams, 1996) structural clinical interview. In addition, all participants were asked to complete a battery of clinical scales. The symptom severity of ibGD was assessed with the Yale–Brown Obsessive Compulsive Scale for pathologic gambling (PG-YBOCS; Pallanti, DeCaria, Grant, Urpe, & Hollander, 2005). The symptom severity of IGD was assessed with the YIAS score. The Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and the Korean ADHD Rating Scale (K-ARS; So, Noh, Kim, Ko, & Koh, 2002) were administered to assess depression and attention symptom severity, respectively. The Behavioral Inhibition System (BIS)/Behavioral Activation System (BAS) Scale was used (Carver & White, 1994; Kim & Kim, 2001) to evaluate inhibitory and excitatory personal traits for aversive or appetitive motivation in behavior. The Korean-Wechsler Adult Intelligence Scale was used to assess the IQ of all subjects (Kim, Yum, Oh, Park, & Lee, 1992).
Brain analysis
Resting-state functional magnetic resonance imaging (fMRI) data for all participants were acquired using a 3.0 Tesla MRI scanner (Philips). All functional images were acquired with the following parameters: 240 volumes/participant during 720 s, axially with an echo-planar imaging sequence, repetition time/echo time = 3,000/40 ms, 40 slices, 64 × 64 matrix, 90° flip angle, 230 mm field of view, and 3-mm section thickness without a gap. Data processing was performed using the Data Processing Assistant for Resting-State fMRI (http://www.restfmri.net), which is a plug-in that operates with Statistical Parametric Mapping (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) and the Resting-State fMRI Data Analysis Toolbox (REST, http://resting-fmri.sourceforge.net). The data processing for resting-state connectivity was similar to that described in a previous study (Kim et al., 2016). In addition, motion correction was conducted as detailed in a study by Karalunas et al. (2014). To correct the possibility of head movement affecting FC, censoring of time points with head motion >0.03 mm was performed (Karalunas et al., 2014). No subjects were screened out due to excessive head motion (>2-mm translation and >2° rotation). The normalized data were smoothed with a 4-mm full width at half-maximum 3D Gaussian kernel. Based on previous resting-state FC studies of brain networks (McCarthy et al., 2013; Sheline, Price, Yan, & Mintun, 2010), we assessed connectivity in the DMN, the CCN, and the reward circuitry. FC analysis was applied to assess functional brain activity using REST software (the seed-base approach). Seed-based analysis involves selecting an a priori region to serve as a seed by which to estimate connectivity with other brain regions. Increased connectivity between the seed region and other regions indicates that the seed region and the other region are simultaneously activated. As suggested in previous studies, we selected seeds located within the left/right PCC for the DMN (Juarez et al., 2013; Tang et al., 2016), the left/right DLPFC for the CCN (McCarthy et al., 2013; Sheline et al., 2010), and the left/right amygdala for the reward circuitry (Haber & Knutson, 2010; McClure et al., 2004; Schultz, 2006). Each seed region of interest was predefined using automated anatomical labeling through the WFU PickAtlas software (Maldjian, Laurienti, Kraft, & Burdette, 2003; Tzourio-Mazoyer et al., 2002). To analyze the effect of the psychological parameters on FC, we performed Pearson’s correlations as a second-level analysis instead of psychophysiological interaction analysis.
Statistical analysis
Demographic and clinical characteristics were analyzed using analysis of variance (ANOVA) tests with significance set at p < .05. In the first-level analysis comparing the IGD, the ibGD, and the healthy control groups, seed-based FC analyses were performed using ANOVA. To correct for multiple comparisons in the cluster analysis, the resulting maps were set to a threshold of p < .01, with a family-wise corrected type I error rate using Monte Carlo simulation (uncorrected p < .0005 and 10 extended voxels) (AlphaSim, Cox, 1996). The threshold for significance was reduced to p < .01 from p < .05 due to the assessment of six different seeds. We included the BDI and BIS/BAS scores as covariate variables for all brain analyses, because these two clinical values exhibited difference among the three groups. We extracted the signal from significant regions using 3-level tests and then performed an analysis of covariance controlling for BDI, K-ARS, and BIS/BAS scores using SPSS 18.0 (SPSS Inc., Chicago, IL, USA).
Ethics
The study procedures were performed in accordance with the Declaration of Helsinki. The Chung-Ang University Hospital Institutional Review Board approved the research protocol for this study. All participants were informed about the procedures of this study and provided written informed consent.
Results
Demographic characteristics
There were no significant differences in age, gender, education, IQ, alcohol consumption, or smoking between the three groups (Table 1). The K-ARS scores in the ibGD group were higher than those observed in the IGD and HC groups (F = 21.82, p < .01). The YIAS scores in the IGD group were higher than those observed in the ibGD and HC groups. The PG-YBOCS scores in the ibGD group were higher than those observed in the IGD and HC groups. The BDI (F = 5.55, p < .01) and the BIS/BAS scores (F = 6.99, p < .01) were higher in the IGD and ibGD groups than those observed in the HC group.
Table 1.
Demographic characteristics
| IGD (n = 15) | ibGD (n = 14) | HC (n = 15) | Statistics | |
|---|---|---|---|---|
| Age | 25.7 ± 5.5 | 26.0 ± 5.5 | 25.7 ± 4.7 | F = 0.02, p = .98 |
| Education (years) | 13.3 ± 2.5 | 12.5 ± 2.4 | 13.1 ± 2.3 | F = 0.44, p = .65 |
| IQ | 98.3 ± 12.1 | 96.7 ± 15.3 | 103.8 ± 9.9 | F = 1.32, p = .28 |
| Alcohol (yes/no) | 10/5 | 11/3 | 12/3 | χ2 = 0.85, p = .40 |
| Smoking (yes/no) | 8/7 | 9/5 | 8/7 | χ2 = 0.47, p = .79 |
| YIAS | 69.6 ± 8.9 | 39.4 ± 7.7 | 37.6 ± 6.6 | F = 79.7, p < .01a |
| PG-YBOCS | 5.5 ± 2.4 | 16.7 ± 4.2 | 4.1 ± 1.8 | F = 78.81, p < .01b |
| K-ARS | 12.7 ± 4.7 | 18.8 ± 7.7 | 5.4 ± 3.4 | F = 21.82, p < .01c |
| BDI | 9.5 ± 6.3 | 14.3 ± 8.4 | 6.1 ± 4.2 | F = 5.55, p < .01d |
| BIS/BAS | 56.3 ± 4.8 | 59.1 ± 9.2 | 49.0 ± 8.1 | F = 6.99, p < .01e |
Note. IGD: patients with Internet gaming disorder; ibGD: patient with Internet-based gambling disorder; HC: healthy comparison subjects; IQ: intelligence quotient; YIAS: Young Internet Addiction Scale; PG-YBOCS: Yale–Brown Obsessive Compulsive Scale for pathologic gambling; K-ARS: Korean ADHD Rating Scale; BDI: Beck Depression Inventory; BIS/BAS: behavioral inhibition system/behavioral activation system.
Post-hoc test: aIGD > ibGD = HC; bibGD > IGD = HC; cibGD > IGD > HC; dibGD = IGD > HC; eibGD = IGD > HC.
Resting-state FC among the three groups
Assessment of the DMN with left and right PCC seeds
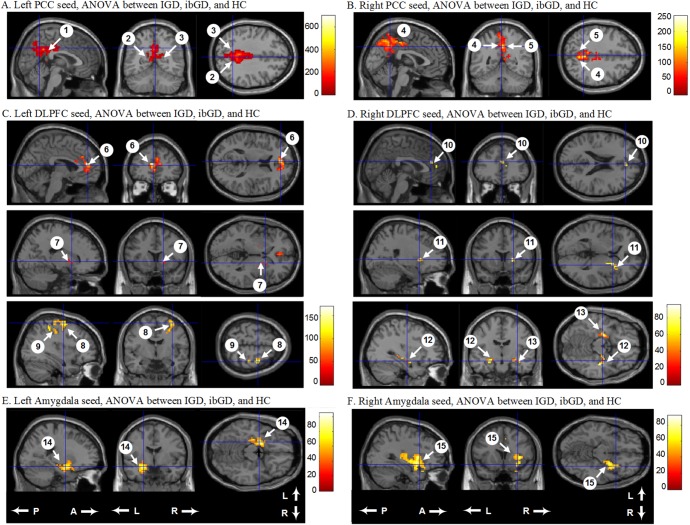
For the left PCC seed-based analysis, FC was significantly different among the three groups from the left PCC to the left cingulate gyrus, left precuneus, and right precuneus. For the right PCC seed-based analysis, there were significant FC differences between three groups from the right PCC to the left and right precuneus [family-wise error (FWE), p < .001] (Figure 1; Table 2). Post-hoc analysis showed that the IGD and ibGD groups had decreased FC from the left PCC to the left and right precuneus as well as from the right PCC to the left and right precuneus compared with healthy control subjects (p < .01). There was no significant difference in FC between the IGD and ibGD groups within the DMN.
Figure 1.
Comparison of functional connectivity from the left and right PCC with other brain regions among the IGD, ibGD, and HC groups. A and B: The IGD and ibGD groups had decreased FC in the default-mode network compared with HC; C and D: The IGD group had increased FC in the cognitive network compared with the ibGD and HC groups; E and F: The ibGD group had increased FC in the reward circuit compared with the IGD and HC groups. PCC: posterior cingulate cortex; FC: functional connectivity; DLPFC: dorsolateral prefrontal cortex; IGD: Internet gaming disorder; ibGD: Internet-based gambling disorder; HC: healthy comparison subjects; A: anterior; P: posterior; L: left; R: right. The numbers observed in Table 2 are the same as those in this figure
Table 2.
Comparison of functional connectivity between three groups
| Talairach coordinates | No. of voxels | p, FEW corrected | Brain regions | |||
|---|---|---|---|---|---|---|
| No. | x | y | z | |||
| Left PCC seed, ANOVA between IGD, ibGD, and HC | ||||||
| 1 | −3 | −42 | 33 | 796 | <.001 | Left cingulate gyrus, BA 31 |
| 2 | 3 | −48 | 33 | <.001 | Right parietal precuneus, BA 31 | |
| 3 | −1 | −50 | 33 | <.001 | Left parietal precuneus, BA 31 | |
| Right PCC seed, ANOVA between IGD, ibGD, and HC | ||||||
| 4 | 3 | −51 | 39 | 787 | <.001 | Right parietal precuneus, BA 7 |
| 5 | −3 | −50 | 51 | <.001 | Left parietal precuneus, BA 7 | |
| Left DLPFC seed, ANOVA between IGD, ibGD, and HC | ||||||
| 6 | −3 | 45 | 12 | 160 | <.001 | Left medial frontal gyrus, BA 10 |
| 7 | 18 | 12 | 0 | 25 | <.001 | Right lentiform nucleus, putamen |
| 8 | −36 | −6 | 63 | 101 | .007 | Right middle frontal gyrus, BA 6 |
| 9 | −51 | −30 | 57 | 40 | .004 | Right postcentral gyrus, BA 2 |
| Right DLPFC seed, ANOVA between IGD, ibGD, and HC | ||||||
| 10 | 3 | 39 | 21 | 27 | <.001 | Right medial frontal gyrus, BA 9 |
| 11 | 24 | 12 | 3 | 36 | <.001 | Right lentiform nucleus, putamen |
| 12 | 33 | −12 | −15 | 64 | .009 | Right hippocampus |
| 13 | −23 | −10 | −15 | 51 | .009 | Left amygdala |
| Left amygdala seed, ANOVA between IGD, ibGD, and HC | ||||||
| 14 | −21 | −3 | −15 | 163 | .007 | Left parahippocampal gyrus |
| Right amygdala seed, ANOVA between IGD, ibGD, and HC | ||||||
| 15 | 30 | 3 | −9 | 198 | .006 | Right sub-lobar insular |
Note. FWE: family-wise error; PCC: posterior cingulate cortex; ANOVA: analysis of variance; DLPFC: dorsolateral prefrontal cortex; IGD: patient with Internet gaming disorder; ibGD: patient with Internet-based gambling disorder; HC: healthy comparison subjects; BA: Brodmann area.
Assessment of the cognitive network with left and right DLPFC seeds
For the left DLPFC seed-based analysis, FC was significantly different among the three groups from the left DLPFC to the left medial frontal gyrus, right lentiform nucleus, right middle frontal gyrus, and right postcentral gyrus (FWE, p < .001). Post-hoc analysis showed that the IGD and ibGD groups demonstrated increased FC from the left DLPFC to the left medial frontal gyrus and right lentiform nucleus relative to the healthy control group (p < .01) (Figure 1; Table 2). The IGD group also showed increased FC from the left DLPFC to the right middle frontal gyrus and right postcentral gyrus compared with the ibGD (p < .01) and healthy control groups (p < .01).
For the right DLPFC seed-based analysis, FC was significantly different among the three groups from the right DLPFC to the right medial frontal gyrus, right lentiform nucleus, right hippocampus, and left amygdala (FWE, p < .001) (Figure 1; Table 2). Post-hoc analysis showed that the IGD and ibGD groups had increased FC from the right DLPFC to the right medial frontal gyrus and right lentiform nucleus (p = .01). The ibGD group had increased FC from the right DLPFC to right hippocampus and left amygdala compared with the IGD (p < .01) and healthy control groups (p < .01).
Assessment of the reward circuit with left and right amygdala seeds
For the left amygdala seed-based analysis, FC was significantly different among the three groups from the left amygdala to the left parahippocampal gyrus (FWE, p < .001). Post-hoc analysis showed that the IGD and the ibGD groups demonstrated increased FC from the left amygdala to the left parahippocampal gyrus (p < .01) (Figure 1; Table 2). The ibGD group had increased FC from the left amygdala to the left parahippocampal compared with the IGD group (p < .01).
For the right amygdala seed-based analysis, FC was significantly different among the three groups from the right amygdala to the right insular (FWE, p < .001) (Figure 1; Table 2). Post-hoc analysis showed that the IGD and ibGD groups had increased FC from the right amygdala to the right insular (p = .01) (Figure 2). The ibGD group had increased FC from the right amygdala to the right insular compared with the IGD group (p < .01).
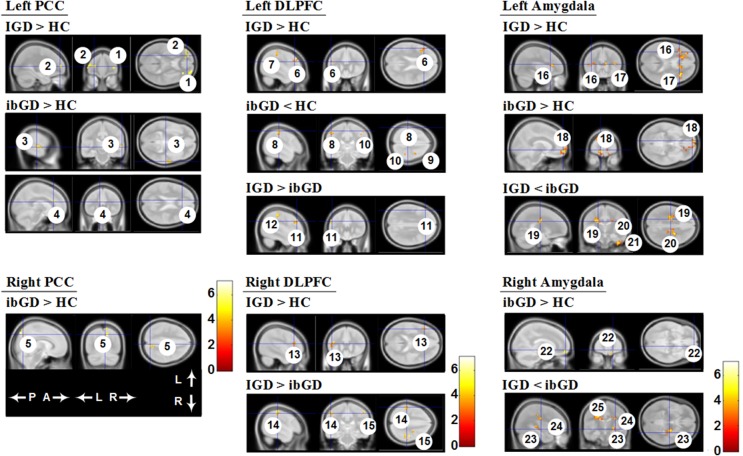
Figure 2.
Three group direct comparison of functional connectivity. The IGD group had increased FC in the cognitive network compared with the ibGD and HC groups. The ibGD group had increased FC in the reward circuit compared with the IGD and HC groups. FC: functional connectivity; DLPFC: dorsolateral prefrontal cortex; IGD: Internet gaming disorder; ibGD: Internet-based gambling disorder; HC: healthy comparison subjects; A: anterior; P: posterior; L: left; R: right. The numbers observed in Table 3 are the same as those in this figure
Three group direct comparison of FC
For the left PCC seed-based analyses, the IGD group had increased FC in the bilateral middle frontal gyrus compared with the HC group. The right superior temporal gyrus and left anterior cingulate gyrus showed increased FC in the ibGD group compared with the HC group. In the right PCC analyses, the right precuneus showed increased FC in the ibGD group compared with the HC group (Figure 2; Table 3).
Table 3.
Comparison of functional connectivity between three groups
| Talairach coordinates | No. of voxels | p, uncorrected | Brain regions | |||
|---|---|---|---|---|---|---|
| No. | x | y | z | |||
| Left PCC seed, t-tests between IGD, ibGD, and HC | ||||||
| 1 | 24 | 66 | 9 | 61 | <.001 | [IGD > HC] right middle frontal gyrus, BA 10 |
| 2 | −27 | 51 | 9 | 58 | <.001 | [IGD > HC] left middle frontal gyrus, BA 10 |
| 3 | 63 | −24 | 0 | 71 | <.001 | [ibGD > HC] right superior temporal gyrus, BA 22 |
| 4 | −3 | 30 | 21 | 45 | <.001 | [ibGD > HC] left anterior cingulate gyrus, BA 24 |
| Right PCC seed, t-tests between IGD, ibGD, and HC | ||||||
| 5 | 6 | −81 | 48 | 63 | <.001 | [ibGD > HC] right precuneus, BA 7 |
| Left DLPFC seed, t-tests between IGD, ibGD, and HC | ||||||
| 6 | −48 | 30 | 33 | 44 | <.001 | [IGD > HC] left middle frontal gyrus, BA 9 |
| 7 | −51 | −30 | 57 | 43 | <.001 | [IGD > HC] left parietal postcentral gyrus, BA 2 |
| 8 | −50 | −20 | 45 | 58 | <.001 | [ibGD < HC] left parietal postcentral gyrus, BA 2 |
| 9 | 27 | −11 | 67 | 41 | <.001 | [ibGD < HC] right frontal precentral gyrus, BA 6 |
| 10 | 47 | −27 | 59 | 43 | <.001 | [ibGD < HC] right parietal postcentral gyrus, BA 1 |
| 11 | −43 | 23 | 41 | 42 | <.001 | [IGD > ibGD] left middle frontal gyrus, BA 8 |
| 12 | −43 | −32 | 55 | 47 | <.001 | [IGD > ibGD] left parietal postcentral gyrus, BA 40 |
| Right DLPFC seed, t-tests between IGD, ibGD, and HC | ||||||
| 13 | −45 | 13 | 41 | 55 | <.001 | [IGD > HC] left middle frontal gyrus, BA 8 |
| 14 | −56 | −21 | 54 | 53 | <.001 | [IGD > ibGD] left postcentral gyrus, BA 2 |
| 15 | 55 | −28 | 51 | 87 | <.001 | [IGD > ibGD] right postcentral gyrus, BA 2 |
| Left amygdala seed, t-tests between IGD, ibGD, and HC | ||||||
| 16 | −36 | 21 | 15 | 106 | <.001 | [IGD > HC] left insular, BA 13 |
| 17 | 39 | 21 | 15 | 121 | <.001 | [IGD > HC] right insular, BA 13 |
| 18 | 33 | 39 | 6 | 114 | <.001 | [ibGD > HC] right middle frontal gyrus, BA 10 |
| 19 | −21 | −25 | 35 | 92 | <.001 | [IGD < ibGD] left cingulate gyrus, BA31 |
| 20 | 21 | −24 | 33 | 87 | <.001 | [IGD < ibGD] right cingulate gyrus, BA 31 |
| 21 | 45 | −15 | −42 | 86 | <.001 | [IGD < ibGD] right inferior temporal gyrus BA 20 |
| Right amygdala seed, t-tests between IGD, ibGD, and HC | ||||||
| 22 | 15 | 60 | −9 | 45 | <.001 | [ibGD > HC] right superior frontal gyrus, BA11 |
| 23 | 36 | −33 | 3 | 122 | <.001 | [IGD < ibGD] right caudate |
| 24 | 24 | −24 | 36 | 141 | <.001 | [IGD < ibGD] right cingulate gyrus, BA 31 |
| 25 | −15 | −9 | 33 | 183 | <.001 | [IGD < ibGD] left cingulate gyrus, BA 24 |
Note. PCC: posterior cingulate cortex; DLPFC: dorsolateral prefrontal cortex; IGD: patient with Internet gaming disorder; ibGD: patient with Internet-based gambling disorder; HC: healthy comparison subjects; BA: Brodmann area.
For left DLPFC seed-based analysis, the IGD group had increased FC in the left middle frontal gyrus and left postcentral gyrus compared with the HC group. The ibGD group showed decreased FC compared with the HC group in the left postcentral gyrus, right precentral gyrus, and right postcentral gyrus. The IGD group had increased FC in the left middle frontal gyrus and left precentral gyrus compared with the ibGD group. In the right DLPFC seed-based analysis, the IGD group showed increased FC in the left middle frontal gyrus compared with the HC group. The IGD group also exhibited increased FC in the bilateral postcentral gyrus compared with the ibGD group (Figure 2; Table 3).
In the left amygdala, the IGD group showed increased FC in the bilateral insula compared with the HC group. The ibGD group exhibited increased FC in the middle frontal gyrus compared with the HC group. The IGD group showed decreased FC in the bilateral cingulate gyrus and right inferior temporal gyrus compared with the ibGD group. For right amygdala seed-based analyses, the ibGD group had increased FC in the right superior frontal gyrus compared with the HC group. The IGD group showed decreased FC in the bilateral cingulate gyrus and right caudate compared with the ibGD group (Figure 2; Table 3).
Correlations between FC coefficient value and clinical scales
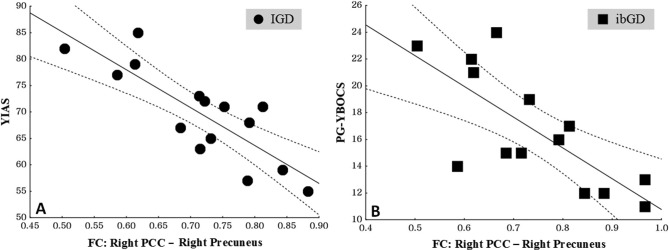
The FC coefficient values from the right PCC to the right precuneus in IGD patients were negatively correlated with YIAS score (r = −.53, p = .02) (Figure 3). In addition, the FC coefficient values from the right PCC to the right precuneus in ibGD patients were negatively correlated with PG-YBOCS score (r = −.56, p = .03) (Figure 3). However, there were no significant correlations between FC coefficient values and clinical scales in other regions.
Figure 3.
A: The correlations between functional connectivity coefficient values from the right PCC to the right precuneus and YIAS scores in IGD patients (r = −.53, p = .02); B: The correlations between functional connectivity coefficient values from the right PCC to the right precuneus and PG-YBOCS scores in ibGD patients (r = −.56, p = .03). The dashed lines represent 95% confidence intervals. PCC: posterior cingulate cortex; YIAS: Young Internet Addiction Scale; PG-YBOCS: Yale–Brown Obsessive Compulsive Scale for pathologic gambling; IGD: Internet gaming disorder; ibGD: Internet-based gambling disorder
Discussion
To the best of our knowledge, this study is the first comparison of brain FC between young adults with ibGD and IGD. To summarize the analyses of FC within brain networks: (a) The IGD and ibGD groups shared decreased FC in the DMN, and these groups showed a relationship between the FC coefficient and severity of Internet addiction (measured by YIAS) and pathological gambling (measured by PG-YBOCS), respectively; (b) FC within the cognitive network was increased in the IGD group but not in the ibGD group; (c) FC within the reward circuitry in the ibGD group was increased compared with that observed in the IGD group.
Decreased FC in the default network in the IGD and ibGD groups
The current finding of decreased FC in the DMN in the IGD and ibGD groups has been reported in previous studies (Ding et al., 2013; Jung et al., 2014). Jung et al. (2014) reported that gambling disorder patients demonstrated decreased FC in the DMN relative to healthy control subjects. Ding et al. (2013) found that patients with Internet addiction showed decreased FC from the PCC to bilateral inferior parietal lobules and the right inferior temporal gyrus (DMN). Decreased FC in the DMN suggested deficits in cognitive control, including attention and self-monitoring, in a substance-dependent state (Ma et al., 2011; Tanabe et al., 2011). This study observed that BIS/BAS and K-ARS scores in both the IGD and ibGD groups were higher than those observed in the HC group. K-ARS scores in the ibGD group were higher than those observed in the IGD group. Moreover, FC coefficient values from the right PCC to the right precuneus were negatively correlated with YIAS and PG-YBOCS scores in both the IGD and ibGD groups. Several studies have reported that ADHD is one of the most common psychiatric diseases comorbid with IGD (Yoo et al., 2004). In addition, both IGD and ADHD are associated with deficits in cognition and behavioral problems related to high excitatory personal traits in behavior (Bae, Han, Kim, Shi, & Renshaw, 2016). Probable ADHD was identified in 21.4% of 126 young adults not seeking treatment for problem gambling (Chamberlain, Derbyshire, Leppink, & Grant, 2015). This included gambling behaviors, higher impulsivity, and impaired decision-making. On the whole, these findings suggest that both IGD and ibGD have demonstrated decreased FC within the DMN, which has been associated with high impulsivity and continuous game play or gambling.
Increased FC in the reward circuitry of the ibGD group compared with that in the IGD group
The current results also show the dysfunctional role of reward circuitry in both IGD and ibGD groups. These results are similar to those reported in previous brain studies of IGD and ibGD (Koehler et al., 2013; Meng, Deng, Wang, Guo, & Li, 2015; Peters, Miedl, & Buchel, 2013; Tschernegg et al., 2013). In a meta-analysis of 61 studies, Meng et al. (2015) suggested that patients with IGD have dysfunctional prefrontal reward circuitry and self-regulatory systems. Regarding brain regions associated with reward circuitry, Tschernegg et al. (2013) reported increased FC between frontostriatal regions, and Peters et al. (2013) noted enhanced FC in a striatal-amygdala circuit. Koehler et al. (2013) reported increased FC between the right middle frontal cortex and right striatum in a gambling disorder group. Interestingly, the current results showed that FC in the reward circuitry in the ibGD group increased compared with that observed in the IGD group. Rapid and instant feedback as well as easy access to betting options through online systems in Internet gambling might be associated with the increased connectivity in the reward circuit (Gainsbury et al., 2015; Monaghan, 2009).
Increased FC in the cognitive network in the IGD group
This study found increased FC in the cognitive network in the IGD group. Several studies have found that playing video games can have beneficial effects on cognitive function (Green & Bavelier, 2003, 2007; Li, Polat, Makous, & Bavelier, 2009). For example, action video games enhanced visual-selective attention (Green & Bavelier, 2003) as well as visual-spatial resolution (Green & Bavelier, 2007). Li et al. (2009) reported that video game play enhanced the contrast sensitivity function in vision. Strobach, Frensch, and Schubert (2012) found that executive control skills were improved by video game practice. Kuhn, Gleich, Lorenz, Lindenberger, and Gallinat (2014) reported that playing Super Mario® 30 min/day was associated with increased hippocampal volume. Kuhn, Lorenz et al. (2014) also noted positive relationships between the extent of video game play and cortical thickness in left frontal regions. Anguera et al. (2013) demonstrated that video game training could enhance multitasking ability in an older population. However, the present results did not show increased FC in the cognitive network of the ibGD group. Deficits in cognitive function including inhibition, working memory, cognitive flexibility, planning ability, and sense of time have been reported in prefrontal related areas in patients with gambling disorder (Goudriaan, Oosterlaan, de Beurs, & van den Brink, 2006; Ledgerwood et al., 2012). Grant et al. (2013) reported that increased brain activity induced by an 8-week tolcapone trial was observed within the frontoparietal cognitive network in gambling disorder patients. Yuan et al. (2017) reported decreased FC in the DLPFC-caudate in an IGD group contrary to our results. We interpret that the differences between the two studies are due to different methodologies. On the whole, these findings indicate that playing Internet games can alter brain networks and improve cognitive function. We believe that activation of the cognitive network by Internet game play, but not by Internet gambling, might be a potentially significant finding.
Based on our findings, we suggest that the neurobiology of Internet gaming (cognitive enhancement and less reward dependence) appears potentially different from that of gambling (i.e., no cognitive enhancement and more reward dependence). Future studies should further evaluate the differences between behavioral and chemical addiction, as well as potentially improved cognitive function as a result of Internet game play.
Limitations
There are several limitations to this study. First, the relatively small number of subjects was not large enough to generalize the results to other groups (e.g., female subjects). However, the prevalence of IGD and ibGD is substantially higher in males than in females. In a German nationwide survey of 15,168 adolescents, 3% of the male and 0.3% of the female juvenile participants were diagnosed as being dependent on video and computer games (Rehbein, Psych, Kleimann, Mediasci, & Mößle, 2010). In the case of DSM-IV-defined pathological gambling, 0.64% of men and 0.23% of women had lifetime pathological gambling according to an adult US study of 43,093 participants (Blanco, Hasin, Petry, Stinson, & Grant, 2006). Second, the potential confounders of ADHD, MDD, obsessive–compulsive disorder, impulsivity traits, social alcohol drinking, and smoking might affect FC in brain networks. However, we did our best to reduce these potential confounds by screening patients with the DSM-IV structural clinical interview and administering clinical scales for ADHD, mood, anxiety, and impulsivity. In addition, there were no significant differences in the number of alcohol drinkers and smokers among our three groups. Future studies should include these conditions to identify their impact. Third, due to the small number of subjects, we were underpowered to examine more than one brain region per network of interest. Finally, the correlations between the FC coefficient value and the YIAS/PG-YBOCS scores were exploratory results as we did not consider the multiple comparison issue.
Conclusions
The IGD and ibGD groups shared the characteristics of decreased FC in the DMN. However, there were also different characteristics, including increased FC, in the cognitive network in the IGD group and increased FC in the reward circuitry in the ibGD group.
Funding Statement
Funding sources: This study was supported by a grant from the Korea Creative Content Agency (R2014040055).
Authors’ contribution
SB, DHH, and PFR conceptualized and designed this study. DHH participated in data collection. SB and DHH performed the data analysis and interpreted the results. SB and DHH drafted the manuscript. JJ, KCN, and PFR provided revision of the manuscript for important intellectual content. All authors critically reviewed the content and approved the final manuscript as submitted.
Conflict of interest
The authors report no conflict of interest.
References
- American Psychiatric Association [APA]. (2013). Diagnostic and statistical manual of mental disorders. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Anguera J. A., Boccanfuso J., Rintoul J. L., Al-Hashimi O., Faraji F., Janowich J., Kong E., Larraburo Y., Rolle C., Johnston E., Gazzaley A. (2013). Video game training enhances cognitive control in older adults. Nature, 501(7465), 97–101. doi:10.1038/nature12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S., Han D. H., Kim S. M., Shi X., Renshaw P. F. (2016). Neurochemical correlates of Internet game play in adolescents with attention deficit hyperactivity disorder: A proton magnetic resonance spectroscopy (MRS) study. Psychiatry Research, 254, 10–17. doi:10.1016/j.pscychresns.2016.05.006 [DOI] [PubMed] [Google Scholar]
- Beck A. T., Ward C. H., Mendelson M., Mock J., Erbaugh J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4(6), 561–571. doi:10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- Blanco C., Hasin D. S., Petry N., Stinson F. S., Grant B. F. (2006). Sex differences in subclinical and DSM-IV pathological gambling: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychological Medicine, 36(7), 943–953. doi:10.1017/S0033291706007410 [DOI] [PubMed] [Google Scholar]
- Carbonell X., Guardiola E., Fuster H., Gil F., Panova T. (2016). Trends in scientific literature on addiction to the Internet, video games, and cell phones from 2006 to 2010. International Journal of Prevention Medicine, 7, 63. doi:10.4103/2008-7802.179511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver C. S., White T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67(2), 319–333. doi:10.1037/0022-3514.67.2.319 [Google Scholar]
- Chamberlain S. R., Derbyshire K., Leppink E., Grant J. E. (2015). Impact of ADHD symptoms on clinical and cognitive aspects of problem gambling. Comprehensive Psychiatry, 57, 51–57. doi:10.1016/j.comppsych.2014.10.013 [DOI] [PubMed] [Google Scholar]
- Cole M. W., Schneider W. (2007). The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage, 37(1), 343–360. doi:10.1016/j.neuroimage.2007.03.071 [DOI] [PubMed] [Google Scholar]
- Cox R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. doi:10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Diekhof E. K., Gruber O. (2010). When desire collides with reason: Functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. The Journal of Neuroscience, 30(4), 1488–1493. doi:10.1523/JNEUROSCI.4690-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W. N., Sun J. H., Sun Y. W., Zhou Y., Li L., Xu J. R., Du Y. S. (2013). Altered default network resting-state functional connectivity in adolescents with Internet gaming addiction. PLoS One, 8(3), e59902. doi:10.1371/journal.pone.0059902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling N. A. (2014). Issues raised by the DSM-5 Internet gaming disorder classification and proposed diagnostic criteria. Addiction, 109(9), 1408–1409. doi:10.1111/add.12554 [DOI] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. (1996). Structured clinical interview for DSM-IV axis I disorder. New York, NY: New York State Psychiatric Institute. [Google Scholar]
- Fox M. D., Snyder A. Z., Vincent J. L., Corbetta M., Van Essen D. C., Raichle M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. doi:10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainsbury S. M., Russell A., Hing N., Wood R., Lubman D., Blaszczynski A. (2015). How the Internet is changing gambling: Findings from an Australian Prevalence Survey. Journal of Gambling Studies, 31(1), 1–15. doi:10.1007/s10899-013-9404-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan A. E., Oosterlaan J., de Beurs E., van den Brink W. (2006). Neurocognitive functions in pathological gambling: A comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction, 101(4), 534–547. doi:10.1111/j.1360-0443.2006.01380.x [DOI] [PubMed] [Google Scholar]
- Grant J. E., Odlaug B. L., Chamberlain S. R., Hampshire A., Schreiber L. R., Kim S. W. (2013). A proof of concept study of tolcapone for pathological gambling: Relationships with COMT genotype and brain activation. European Neuropsychopharmacology, 23(11), 1587–1596. doi:10.1016/j.euroneuro.2013.07.008 [DOI] [PubMed] [Google Scholar]
- Grant J. E., Potenza M. N., Weinstein A., Gorelick D. A. (2010). Introduction to behavioral addictions. The American Journal of Drug and Alcohol Abuse, 36(5), 233–241. doi:10.3109/00952990.2010.491884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. S., Bavelier D. (2003). Action video game modifies visual selective attention. Nature, 423(6939), 534–537. doi:10.1038/nature01647 [DOI] [PubMed] [Google Scholar]
- Green C. S., Bavelier D. (2007). Action-video-game experience alters the spatial resolution of vision. Psychological Science, 18(1), 88–94. doi:10.1111/j.1467-9280.2007.01853.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S. N., Knutson B. (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. doi:10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuggi A., Sanz-Arigita E., Gonzalez-Salinas C., Valero-Garcia A. V., Garcia-Santos J. M., Fuentes L. J. (2014). Brain functional connectivity changes in children that differ in impulsivity temperamental trait. Frontiers in Behavioral Neuroscience, 8, 156. doi:10.3389/fnbeh.2014.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez M., Kiehl K. A., Calhoun V. D. (2013). Intrinsic limbic and paralimbic networks are associated with criminal psychopathy. Human Brain Mapping, 34(8), 1921–1930. doi:10.1002/hbm.22037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M. H., Kim J. H., Shin Y. C., Jung W. H., Jang J. H., Choi J. S., Kang D. H., Yi J. S., Choi C. H., Kwon J. S. (2014). Decreased connectivity of the default mode network in pathological gambling: A resting state functional MRI study. Neuroscience Letters, 583, 120–125. doi:10.1016/j.neulet.2014.09.025 [DOI] [PubMed] [Google Scholar]
- Karalunas S. L., Fair D., Musser E. D., Aykes K., Iyer S. P., Nigg J. T. (2014). Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: Toward biologically based nosologic criteria. JAMA Psychiatry, 71(9), 1015–1024. doi:10.1001/jamapsychiatry.2014.763 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim J. K., Yum T. H., Oh K. J., Park Y. S., Lee Y. H. (1992). Factor analysis of K-WAIS revised version. Korean Journal of Clinical Psychology, 11, 1–10. doi:10.1037/1040-3590.2.1.31 [Google Scholar]
- Kim K. H., Kim W. S. (2001). Korean-BAS/BIS scale. The Korean Journal of Health Psychology, 6(2), 19–37. [Google Scholar]
- Kim S. M., Park S. Y., Kim Y. I., Son Y. D., Chung U. S., Min K. J., Han D. H. (2016). Affective network and default mode network in depressive adolescents with disruptive behaviors. Neuropsychiatry Disease and Treatment, 12, 49–56. doi:10.2147/NDT.S95541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler S., Ovadia-Caro S., van der Meer E., Villringer A., Heinz A., Romanczuk-Seiferth N., Margulies D. S. (2013). Increased functional connectivity between prefrontal cortex and reward system in pathological gambling. PLoS One, 8(12), e84565. doi:10.1371/journal.pone.0084565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S., Gleich T., Lorenz R. C., Lindenberger U., Gallinat J. (2014). Playing Super Mario induces structural brain plasticity: Gray matter changes resulting from training with a commercial video game. Molecular Psychiatry, 19(2), 265–271. doi:10.1038/mp.2013.120 [DOI] [PubMed] [Google Scholar]
- Kuhn S., Lorenz R., Banaschewski T., Barker G. J., Buchel C., Conrod P. J., Flor H., Garavan H., Ittermann B., Loth E., Mann K., Nees F., Artiges E., Consortium I. (2014). Positive association of video game playing with left frontal cortical thickness in adolescents. PLoS One, 9(3), e91506. doi:10.1371/journal.pone.0091506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood D. M., Orr E. S., Kaploun K. A., Milosevic A., Frisch G. R., Rupcich N., Lundahl L. H. (2012). Executive function in pathological gamblers and healthy controls. Journal of Gambling Studies, 28(1), 89–103. doi:10.1007/s10899-010-9237-6 [DOI] [PubMed] [Google Scholar]
- Li R., Polat U., Makous W., Bavelier D. (2009). Enhancing the contrast sensitivity function through action video game training. Nature Neuroscience, 12(5), 549–551. doi:10.1038/nn.2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. M., Chau M., Wong P. W., Lai E. S., Yip P. S. (2013). Evaluation of a Web-based social network electronic game in enhancing mental health literacy for young people. Journal of Medical Internet Research, 15(5), e80. doi:10.2196/jmir.2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Liu Y., Fu X. M., Li N., Wang C. X., Zhang H., Qian R. B., Xu H. S., Hu X., Zhang D. R. (2011). Abnormal brain default-mode network functional connectivity in drug addicts. PLoS One, 6(1), e16560. doi:10.1371/journal.pone.0016560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J. A., Laurienti P. J., Kraft R. A., Burdette J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. doi:10.1016/S1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- McCarthy H., Skokauskas N., Mulligan A., Donohoe G., Mullins D., Kelly J., Johnson K., Fagan A., Gill M., Meaney J., Frodl T. (2013). Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry, 70(12), 1329–1337. doi:10.1001/jamapsychiatry.2013.2174 [DOI] [PubMed] [Google Scholar]
- McClure S. M., York M. K., Montague P. R. (2004). The neural substrates of reward processing in humans: The modern role of FMRI. The Neuroscientist, 10(3), 260–268. doi:10.1177/1073858404263526 [DOI] [PubMed] [Google Scholar]
- Meng Y., Deng W., Wang H., Guo W., Li T. (2015). The prefrontal dysfunction in individuals with Internet gaming disorder: A meta-analysis of functional magnetic resonance imaging studies. Addiction Biology, 20(4), 799–808. doi:10.1111/adb.12154 [DOI] [PubMed] [Google Scholar]
- Monaghan S. (2009). Responsible gambling strategies for Internet gambling: The theoretical and empirical base of using pop-up messages to encourage self-awareness. Computers in Human Behavior, 25, 202–207. doi:10.1016/j.chb.2008.08.008 [Google Scholar]
- Pallanti S., DeCaria C. M., Grant J. E., Urpe M., Hollander E. (2005). Reliability and validity of the pathological gambling adaptation of the Yale-Brown Obsessive-Compulsive Scale (PG-YBOCS). Journal of Gambling Studies, 21(4), 431–443. doi:10.1007/s10899-005-5557-3 [DOI] [PubMed] [Google Scholar]
- Peters J., Miedl S. F., Buchel C. (2013). Elevated functional connectivity in a striatal-amygdala circuit in pathological gamblers. PLoS One, 8(9), e74353. doi:10.1371/journal.pone.0074353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E., MacLeod A. M., Snyder A. Z., Powers W. J., Gusnard D. A., Shulman G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. doi:10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehbein F., Psych G., Kleimann M., Mediasci G., Mößle T. (2010). Prevalence and risk factors of video game dependency in adolescence: Results of a German nationwide survey. Cyberpsychology, Behavior, and Social Networking, 13(3), 269–277. doi:10.1089/cyber.2009.0227 [DOI] [PubMed] [Google Scholar]
- Schultz W. (2006). Behavioral theories and the neurophysiology of reward. Annual Review of Psychology, 57, 87–115. doi:10.1146/annurev.psych.56.091103.070229 [DOI] [PubMed] [Google Scholar]
- Shapira N. A., Goldsmith T. D., Keck P. E., Jr., Khosla U. M., McElroy S. L. (2000). Psychiatric features of individuals with problematic Internet use. Journal of Affective Disorders, 57(1–3), 267–272. doi:10.1016/S0165-0327(99)00107-X [DOI] [PubMed] [Google Scholar]
- Sheline Y. I., Price J. L., Yan Z., Mintun M. A. (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America, 107(24), 11020–11025. doi:10.1073/pnas.1000446107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- So Y. K., Noh J. S., Kim Y. S., Ko S. G., Koh Y. J. (2002). The reliability and validity of Korean Parent and Teacher ADHD Rating Scale. Journal of Korean Nueropsychiatry Association, 41, 283–289. [Google Scholar]
- Sohrabi A., Smith A. M., West R. L., Cameron I. (2015). An fMRI study of risky decision making: The role of mental preparation and conflict. Basic and Clinical Neuroscience, 6(4), 265–270. [PMC free article] [PubMed] [Google Scholar]
- Strobach T., Frensch P. A., Schubert T. (2012). Video game practice optimizes executive control skills in dual-task and task switching situations. Acta Psychologica, 140(1), 13–24. doi:10.1016/j.actpsy.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Tanabe J., Nyberg E., Martin L. F., Martin J., Cordes D., Kronberg E., Tregellas J. R. (2011). Nicotine effects on default mode network during resting state. Psychopharmacology, 216(2), 287–295. doi:10.1007/s00213-011-2221-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang R., Razi A., Friston K. J., Tang Y. Y. (2016). Mapping smoking addiction using effective connectivity analysis. Frontiers in Human Neuroscience, 10, 195. doi:10.3389/fnhum.2016.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschernegg M., Crone J. S., Eigenberger T., Schwartenbeck P., Fauth-Buhler M., Lemenager T., Mann K., Thon N., Wurst F. M., Kronbichler M. (2013). Abnormalities of functional brain networks in pathological gambling: A graph-theoretical approach. Frontiers in Human Neuroscience, 7, 625. doi:10.3389/fnhum.2013.00625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. doi:10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Uddin L. Q., Clare Kelly A., Biswal B. B., Xavier Castellanos F., Milham M. P. (2009). Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Human Brain Mapping, 30(2), 625–637. doi:10.1002/hbm.20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Muijden J., Band G. P., Hommel B. (2012). Online games training aging brains: Limited transfer to cognitive control functions. Frontiers in Human Neuroscience, 6, 221. doi:10.3389/fnhum.2012.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H. J., Cho S. C., Ha J., Yune S. K., Kim S. J., Hwang J., Chung A., Sung Y. H., Lyoo I. K. (2004). Attention deficit hyperactivity symptoms and Internet addiction. Psychiatry and Clinical Neurosciences, 58(5), 487–494. doi:10.1111/j.1440-1819.2004.01290.x [DOI] [PubMed] [Google Scholar]
- Young K. S. (1996). Psychology of computer use: XL. Addictive use of the Internet: A case that breaks the stereotype. Psychological Reports, 79, 899–902. doi:10.2466/pr0.1996.79.3.899 [DOI] [PubMed] [Google Scholar]
- Yuan K., Yu D., Cai C., Feng D., Li Y., Bi Y., Liu J., Zhang Y., Jin C., Li L., Qin W., Tian J. (2017). Frontostriatal circuits, resting state functional connectivity and cognitive control in Internet gaming disorder. Addiction Biology, 22(3), 813–822. doi:10.1111/adb.12348 [DOI] [PubMed] [Google Scholar]