Abstract
Introduction:
Ontario delisted high-strength fentanyl, hydromorphone and morphine from the public drug formulary for non-palliative care prescribers on 31 January, 2017. Our aim is to assess the early impact of this policy on prescribing patterns and to examine whether this impact varied by prescriber type, opioid type and opioid strength.
Methods:
We conducted a population-based, cross-sectional study on palliative and non-palliative care patients dispensed fentanyl, hydromorphone or morphine through the Ontario public drug program between 1 January, 2014, and 31 July, 2017. For each month during the study period, we reported the total number of high-strength opioid recipients stratified by prescriber type, and the total volume of each drug dispensed, stratified by strength. We used interventional autoregressive integrated moving average (ARIMA) models to assess the policy’s impact on prescribing patterns.
Results:
We observed a 98% decrease in the total number of publicly funded recipients of high-strength opioids between December 2016 and July 2017 (5930 to 133 recipients) for all prescribers. The policy led to a significant decline in the total volume of all three opioids dispensed: hydromorphone from 20 374 621 to 16 952 097 mg (p < .01); morphine from 40 644 190 to 33 555 480 mg (p < .03); and fentanyl from 9 604 913 to 5 842 405 mcg/h (p < .01). For both fentanyl and hydromorphone, this reduction generally corresponded to an increase in the number of low-strength opioids dispensed.
Conclusion:
Delisting high-strength opioids substantially reduced the number of highstrength opioid recipients and reduced the overall volume of long-acting opioids dispensed in Ontario through the public drug program. Future studies should examine its impact on patient outcomes.
Keywords: fentanyl, morphine, hydromorphone, opioids, policy change, delisting, Ontario, palliative care
Highlights
Delisting of high-strength formulations of fentanyl, hydromorphone and morphine led to reductions in dispensing of these products among all prescribers, despite allowances in the policy for prescribing among palliative care prescribers.
The majority of these changes in dispensing patterns occurred in the months of January and February 2017, while little change occurred between the policy’s announcement in July 2016 and implementation in January 2017.
Despite an increase in dispensing of lower-strength opioid formulations following the policy’s implementation, there was still an overall reduction in the total volume of fentanyl, hydromorphone and morphine dispensed.
Introduction
The use of prescription opioids has increased dramatically over the past 20 years in North America, and recent trends in other countries suggest that overprescribing of opioids is becoming an international phenomenon.1-8 In particular, high doses of opioids are commonly prescribed despite evidence for the risks associated with such practices, including fatal overdoses, motor vehicle collisions and falls and fractures among elderly adults.1,2,9,10 Opioid-prescribing guidelines for chronic non-cancer pain in Canada previously characterized a daily opioid dose above 200 mg morphine equivalents (MME) as a “watchful dose,” whereas recent 2017 guidelines recommend that clinicians avoid doses exceeding 90 MME.11 With the increasing focus on avoiding high daily opioid doses, the broad availability of high-strength formulations that lead to daily doses above 200 MME has been questioned.12 In August 2017, several groups in the United States, including the Physicians for Responsible Opioid Prescribing, the National Safety Council, the Association of State and Territorial Health Officials and the American College of Medical Toxicology, submitted a joint petition to the U.S. Food and Drug Administration to remove high-strength opioids from the commercial market, citing concerns surrounding their safety.13
As part of Ontario’s Strategy to Prevent Opioid Addiction and Overdose, the Ontario Public Drug Programs (OPDP) announced the delisting of high-strength opioids on 20 July, 2016.14,15 These changes eliminated the Ontario Drug Benefit (ODB) reimbursement for high-strength, long-acting opioids, specifically 75 and 100 mcg/h fentanyl patches, 24 and 30 mg hydromorphone controlled-release (CR) capsules, and 200 mg morphine sustained release (SR) tablets. An exception was made for those on the Palliative Care Facilitated Access (PCFA) prescribers list. With the implementation of this policy, eligible recipients of the ODB program (i.e. patients who are ≥ 65 years of age, receive social assistance or home care services, reside in a longterm care home or have high drug costs relative to household income) could no longer have these products reimbursed by the public drug program unless they were receiving palliative care services from a PCFA physician. However, it is still possible to access these high-strength opioids through out-of-pocket or private-payer payments. The policy was implemented on 31 January, 2017, and its impact on publicly funded opioid-prescribing patterns remains unknown.
This paper describes the early impact of delisting high-strength opioid formulations in Ontario. The objective of this study was to quantify the impact of this policy on patterns of opioid prescribing, and to evaluate how this impact differed by prescriber type, opioid type and opioid strength in the first six months following policy implementation.
Methods
Setting
We conducted a population-based, crosssectional study of all individuals who received a prescription for long-acting fentanyl, hydromorphone or morphine that was reimbursed by the OPDP between 1 January, 2014, and 31 July, 2017. This study was approved by the Research Ethics Board of Sunnybrook Health Sciences Centre in Toronto, Ontario.
Data sources
We used administrative health care data from the Institute for Clinical Evaluative Sciences (ICES) in Toronto, Ontario, to conduct this analysis. Specifically, we used the ODB claims database, which captures all opioids dispensed to patients eligible for the ODB programs with an error rate of < 1%.16 In Ontario, physicians registered as PCFA prescribers regularly treat palliative patients and are allowed to prescribe publicly funded prescription medications that are otherwise limited for most physicians practising in Ontario.17 We defined a cohort of physicians registered as PCFA prescribers according to their prescribing history between 2007 (when PCFA was launched) and the end of the study period. Each physician’s PCFA eligibility period was defined as the time between their first and last prescription for a drug claim billed using a specific PIN from the PCFA drug list. We added a 365-day grace period to the date of their last prescription to avoid misclassifying PCFA prescribers as intermittent prescribers of medications on this list. All analyses were performed at the ICES in Toronto, Ontario, using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and a type 1 error rate of .05 as the threshold for statistical significance.
Outcomes
We reported the total number of people dispensed at least one high-strength opioid, stratified by prescriber type (palliative vs. non-palliative care) in each month between 1 January, 2014, and 31 July, 2017. We also reported the total monthly volume of study opioids (morphine, hydromorphone and fentanyl) dispensed by calculating the sum of the quantity of patches (fentanyl) or tablets (hydromorphone or morphine) multiplied by the strength of each formulation for each month of the study period. We included all publicly funded doses of fentanyl patches (25 mcg/h, 50 mcg/h, 75 mcg/h and 100 mcg/h), as well as oral and sustained release formulations of hydromorphone (3 mg, 4.5 mg, 6 mg, 9 mg, 12 mg, 18 mg, 24 mg and 30 mg) and morphine (10 mg, 15 mg, 20 mg, 30 mg, 50 mg, 60 mg, 100 mg and 200 mg). This monthly volume was calculated and reported separately for each drug for the purpose of describing the changes in drug volume dispensed over time. No comparisons were conducted between opioid type, therefore opioid volume was not converted into morphine equivalents. For fentanyl, the volume dispensed reflects the hourly patch strength (i.e. 25 mcg/h) multiplied by the number of patches dispensed. Finally, we reported the total monthly quantity of fentanyl, hydromorphone and morphine dispensed, stratified by opioid strength.
Statistical analysis
We used interventional autoregressive integrated moving average (ARIMA) models to determine the impact of the OPDP’s policy to delist high-strength opioids on the total volume of fentanyl, hydromorphone and morphine prescribed. Our hypothesis was that the policy announcement (20 July, 2016) would lead to a gradual reduction in opioid volumes after the policy announcement as prescribers attempted to taper their patients’ doses, which would continue to accelerate following the policy implementation (31 January, 2017). Therefore, we tested a change in slope from after the announcement until implementation (using a ramp intervention function) and an immediate sustained change after implementation (using a step intervention function). We used augmented Dickey–Fuller tests to assess stationarity of the time series and differenced the time series at the appropriate lags in order to produce stationary time series. We examined autocorrelation function (ACF), partial autocorrelation function (PACF) and inverse correlation function (IACF) plots to determine the appropriate moving average or autoregressive terms for the models. We then assessed the fit of the models using residual ACF, PACF and IACF plots; Ljung–Box chi-square tests to test for white noise; and residual normality diagnostic plots.
Results
Recipients of all high-strength opioids, by prescriber type
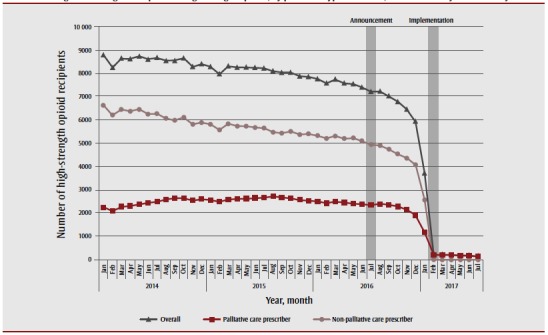
We observed an 18% decrease in the number of recipients of publicly funded, high-strength opioids between July 2016 (the policy announcement) and December 2016 (from 7209 to 5930 recipients) (Figure 1). By the end of February 2017, one month after the policy’s implementation, there were only 197 ODB-eligible recipients of high-strength opioids, all of which were prescribed by palliative care physicians (a 97% reduction from December 2016). This value was generally sustained (range: 133 to 201 recipients monthly) until the end of the study period (there was a 98% reduction between December 2016 and July 2017).
Figure 1. Ontario Drug Benefit–eligible recipients of high-strength opioids, by prescriber type in Ontario, between January 2014 and July 2017.
Volume of opioids dispensed, by opioid type
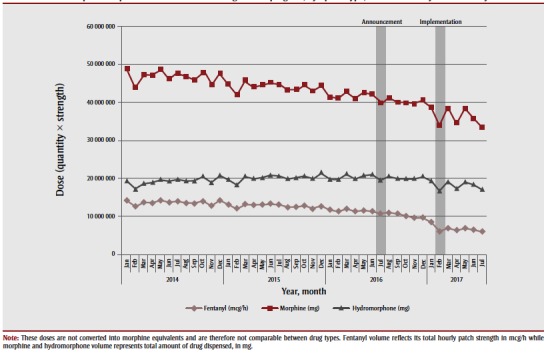
There was no impact of the July 2016 policy announcement on the total volume of fentanyl (p = .17), hydromorphone (p = .71) or morphine (p = .74) dispensed and reimbursed by the ODB program; however, after the implementation of the policy in January 2017, we observed a statistically significant reduction in the total volume of all three opioids dispensed (Figure 2). Specifically, between December 2016 and July 2017, we observed a 17% reduction in the volume of both hydromorphone (from 20 374 621 to 16 952 097 mg; p = .008) and morphine (from 40 644 190 to 33 555 480 mg; p = .028) dispensed, and a 39% reduction in the volume of fentanyl patches dispensed (from 9 604 913 to 5 842 405 mcg/h; p = .007).
Figure 2. Volume of opioids dispensed from the Ontario Drug Benefit program, by opioid type, between January 2014 and July 2017.
Opioid type, by strength
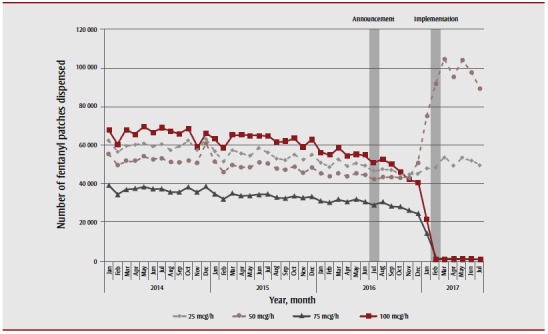
Prior to the announcement of the delisting of high-strength opioids, the most commonly prescribed strength of fentanyl patch was 100 mcg/h, with 54 823 patches dispensed in June 2016. The 75 mcg/h strength was the least commonly prescribed, with 30 616 patches dispensed during the same month (Figure 3). Following the policy’s announcement and subsequent implementation, the number of high-strength fentanyl patches declined dramatically; however, the number of low-strength fentanyl patches prescribed increased in parallel. Specifically, the dispensing of 50 mcg/h fentanyl patches almost doubled (from 50 884 to 89 364 patches—a 75.6% increase) while that of the 25 mcg/h patches increased by 10% (from 45 229 to 49 652 patches) between December 2016 and July 2017.
Figure 3. Volume of fentanyl dispensed from the Ontario Drug Benefit program, by strength, between January 2014 and July 2017.
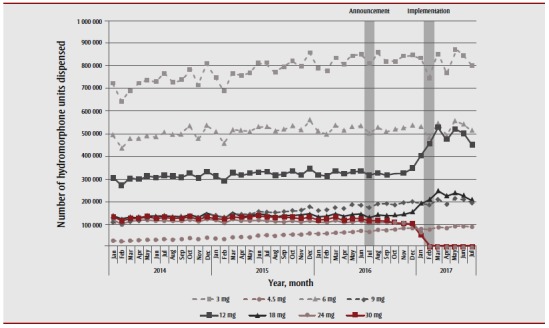
We observed a similar trend in hydromorphone dispensing: high-strength formulations remained stable after the policy’s announcement, and then decreased dramatically in January when the delisting came into effect (Figure 4). By the end of the study period (July 2017), only 5272 tablets for high-strength hydromorphone were dispensed during the month, a decrease of 97% from the 203 012 tablets dispensed in December 2016. Concurrently, there was an increase in 12 mg (30% increase, from 345 742 to 449 584 tablets) and 18 mg (34% increase, from 156 422 to 209 282 tablets) hydromorphone formulations dispensed between December 2016 and July 2017.
Figure 4. Volume of hydromorphone dispensed from the Ontario Drug Benefit program, by strength, between January 2014 and July 2017.
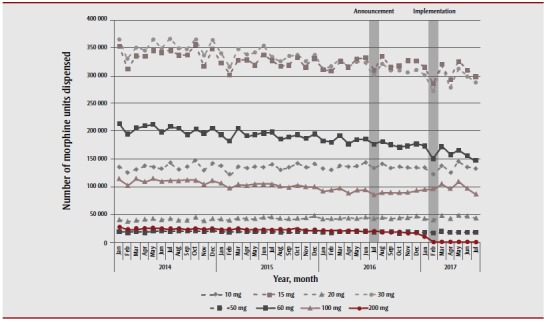
High-strength morphine tablets were among the least commonly dispensed strengths of morphine during the course of the study period (Figure 5). As in the case of the other delisted opioids, we observed no change in high-strength morphine dispensing after the policy announcement, but did observe a reduction immediately after policy implementation (a 100% reduction, from 16 944 units in December 2016 to zero units in July 2017). We also observed a general destabilization in the dispensing trends for many lower-strength morphine formulations, but no consistent pattern of increased dispensing of any of these products.
Figure 5. Volume of morphine dispensed from the Ontario Drug Benefit program, by strength, between January 2014 and July 2017.
Discussion
In this population-based, cross-sectional study we found that delisting highstrength opioid formulations led to a reduction in the dispensing of these products among all prescribers. Our observation that the reduction happened among all prescribers is particularly important because policy makers in Ontario specifically amended the delisting policy to exclude palliative care patients, recognizing the management of pain in palliative care as an important priority.18Therefore, the degree of reduced prescribing of highstrength opioids in this sector is unexpected. This finding may suggest a lack of awareness on the part of palliative care prescribers and pharmacists of this exception to the policy, or a broader impact of the policy on physician decision-making related to the role of high-strength forms of opioids in clinical practice more generally. However, since physicians on the PCFA list may also prescribe medications to non-palliative care patients, it is also possible that these observations are reflective of a reduction in high-strength opioid prescribing to such patients.
We observed an increase in the dispensing of lower-strength opioid formulations following the policy’s implementation, which replaced, to a large degree, the reductions in high-strength opioid dispensing. This result aligns with the notice of the Ontario Ministry of Health and Long-Term Care (MOHLTC) related to the policy, which stated that low-strength, long-acting opioids would remain available through the public drug program and may be combined to form higher doses for patients with a higher opioid tolerance.19 Therefore, it is likely that many patients were transitioned to lower-strength fentanyl, hydromorphone or morphine following the policy implementation. While this increases pill burden for patients who continue on a high daily dose of opioids, the overall reduction in the number of high-strength opioids available in the community may subsequently aid in the prevention of opioid-related adverse events such as accidental overdose and fatality.2,20 Furthermore, despite the increase in the dispensing of low-strength, long-acting opioids, we observed a slight reduction in the overall volume of long-acting opioids dispensed following the policy’s implementation. Therefore, the delisting of high-strength opioids may have encouraged some prescribers to reconsider their patients’ high-dose opioid therapy and begin the process of tapering. Future work is needed to understand how any observed reductions in opioid dose–impacted pain management at the individual level. Given that the population affected by this policy may not have the means to pay for alternative, nonpharmaceutical pain treatment (e.g. physiotherapy, cognitive behavioural therapy), policies considering novel funding mechanisms for these nonpharmaceutical treatment options may be warranted.
It is important to note that the greatest change in dispensing patterns occurred at the end of January 2017, when the policy was implemented. We observed little change in prescribing practice between the policy’s announcement in July 2016 and December of that year, which suggests that clinicians did not use this period to gradually implement prescribing changes.14 This hypothesis is supported by our observation of an increase in lower-strength opioid dispensing following the policy’s implementation. Thus, future studies should explore the impact of this policy on patient-level outcomes including total dose prescribed, changes in payment (e.g. moving to other payers), abrupt dose changes and clinical outcomes such as fatal and nonfatal overdoses.
Strengths and limitations
A major strength of this study is its use of population-based data. Specifically, using records from ICES, we were able to capture prescription records of all patients whose high-strength opioids were reimbursed through the public drug program.
This study has some limitations that merit discussion. First, we studied all highstrength opioid recipients whose prescription opioids were reimbursed by Ontario’s public drug program. However, this excludes those who receive their prescription medications through private insurance or out-of-pocket payments. Therefore, we are unable to draw conclusions about the impact of this policy on broader prescribing patterns to the general public in Ontario. Second, we are only able to capture instances of medication dispensing using administrative claims and are unable to determine whether the recipient used the medication after dispensing. Therefore, it is possible that some of the prescriptions captured may have been unused or diverted to the illicit market. Third, we did not capture sociodemographic information, and therefore could not investigate whether the policy had differential impact on palliative care patients by sex, age or location of residence. Future work could explore these subpopulations. Finally, we categorized physicians as palliative care prescribers if they prescribed medications from the PCFA list. Since these physicians may also treat non-palliative care patients, it is possible that some opioids categorized as “palliative care” in our study may be used by non-palliative care patients.
Conclusion
The delisting of high-strength opioids dramatically reduced the overall number of opioid recipients prescribed these products by both palliative and non-palliative care physicians. This reduction corresponded to an increase in lower-strength opioid dispensing that occurred promptly after the policy’s implementation. We found that this policy led to a small but significant reduction in the volume of long-acting morphine, hydromorphone and fentanyl reimbursed by the public drug program in Ontario. This outcome may indicate that restrictions on highstrength opioid reimbursement created an opportunity for physicians to consider slow, safe tapering of opioids in their patients who are at risk of adverse events from high-dose opioid use. Future research is needed to assess whether this is the case, to confirm these findings over a longer follow-up time and to ensure that this policy did not lead to abrupt cessation of opioids in some patients.
Acknowledgements
This study was supported by grants from the Ontario Ministry of Health and Long- Term Care (MOHLTC), a grant from the Canadian Institutes of Health Research (Grant # 153070), and the Ontario Strategy for Patient-Oriented Research (SPOR) Support Unit, which is supported by the Canadian Institutes of Health Research and the Province of Ontario. The work was also supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario MOHLTC. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES, the Ontario SPOR Support Unit or the Ontario MOHLTC is intended or should be inferred.
Conflicts of interest
The authors declare no conflicts of interest with respect to the publication of this article.
Authors’ contributions and statement
TG, DM, MT and WK contributed to the study concept, research design, data collection and analysis. QG interpreted the findings and wrote the manuscript, with guidance from co-authors. All authors assisted in manuscript revision and have approved the final version.
The content and views expressed in this article are those of the authors and do not necessarily reflect those of the Government of Canada.
References
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171((7)):686–91. doi: 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- Spooner L, Fernandes K, Martins D, et al, et al. High-dose opioid prescribing and opioid-related hospitalization: a population-based study. pone. :e0167479–91. doi: 10.1371/journal.pone.0167479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AP, Becker WC, Schiff GD, et al. Strategies for flipping the script on opioid overprescribing. JAMA Intern Med. 2016;176((1)):7–8. doi: 10.1001/jamainternmed.2015.5946. [DOI] [PubMed] [Google Scholar]
- Clark DJ, Schumacher MA, et al. America’s opioid epidemic: supply and demand considerations. Anesth Analg. 2017:1667–74. doi: 10.1213/ANE.0000000000002388. [DOI] [PubMed] [Google Scholar]
- Makary MA, Overton HN, Wang P, et al. Overprescribing is major contributor to opioid crisis. BMJ. 2017:j4792–74. doi: 10.1136/bmj.j4792. [DOI] [PubMed] [Google Scholar]
- Tran T, Taylor SE, Hardidge A, Findakly D, Aminian P, Elliott RA, et al. Impact of pharmacists assisting with prescribing and undertaking medica¬tion review on oxycodone prescribing and supply for patients discharged from surgical wards. J Clin Pharm Ther. 2017;42((5)):567–72. doi: 10.1111/jcpt.12540. [DOI] [PubMed] [Google Scholar]
- Toth AR, Possidente CJ, Sawyer LM, DiParlo MA, Fanciullo GJ, et al. National and northern New England opioid prescribing patterns, 2013–2014. Toth AR, Possidente CJ, Sawyer LM, DiParlo MA, Fanciullo GJ. 2017;18((9)):1706–14. doi: 10.1093/pm/pnw231. [DOI] [PubMed] [Google Scholar]
- Blake H, Leighton P, Walt G, Ravenscroft A, et al. Prescribing opioid analgesics for chronic non-malignant pain in general practice – a survey of attitudes and practice. Br J Pain. 2015;9((4)):225–32–14. doi: 10.1177/2049463715579284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein M, Bair MJ, et al, et al. Association between opioid prescribing patterns and opioid over¬dose-related deaths. JAMA. 2011:1315–21. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- Gomes T, Redelmeier DA, Juurlink DN, Dhalla IA, Camacho X, Mamdani MM, et al. Opioid dose and risk of road trauma in Canada: a population-based study. JAMA Intern Med. 2013:196–201. doi: 10.1001/2013.jamainternmed.733. [DOI] [PubMed] [Google Scholar]
- Busse JW, Craigie S, Juurlink DN, et al, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017:E659–E666–201. doi: 10.1503/cmaj.170363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Els C, Jackson TD, Hagtvedt R, et al, et al. High-dose opioids for chronic non-cancer pain: an overview of Cochrane Reviews. High-dose opioids for chronic non-cancer pain: an overview of Cochrane Reviews. Cochrane Database Syst Rev (Internet) doi: 10.1002/14651858.CD012509.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J, Hersman DA, Kolodny A, McKay C, Rummler J, et al. Citizen petition (Internet) opioids. Available from: http://i2.cdn.turner.com/cnn/2017/images/08/31/citizen.petition.uhdu.opioids.8.30.17.final.pdf. [Google Scholar]
- Notice from the Executive Officer: changes to the Ontario Drug Benefit (ODB) program funding of opioid medications (Internet) Ontario Public Drug Programs, Ministry of Health and Long-Term Care. Available from: http://www.health.gov.on.ca/en/pro/programs/drugs/opdp_eo/notices/exec_office_20160720.pdf. [Google Scholar]
- Taking action to prevent opioid addiction and overdose (Internet) Ministry of Health and Long-Term Care. Available from: https://news.ontario.ca/mohltc/en/2017/10/taking-action-to-prevent-opioid-addiction-and-overdose.html. [Google Scholar]
- Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D, et al. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10((2)):67–71. [PubMed] [Google Scholar]
- Palliative Care Facilitated Access frequently asked questions (Internet) Ontario Medical Association. Available from: http://www.wwpalliativecare.ca/Uploads/ContentDocuments/PCFA%20FrequentlyAskedQuestions%202017-04-5.pdf. [Google Scholar]
- Changes to the Palliative Care Facilitated Access program (Internet) Ontario Medical Association. Available from: http://wwpalliativecare.ca/Uploads/ContentDocuments/PCFABackgrounder2017-04-05.pdf. [Google Scholar]
- Reminder regarding delisting of high-strength long-acting opioids under the Ontario Drug Benefit (ODB) pro¬gram (Internet) Ministry of Health and Long-Term Care. Available from: http://www.health.gov.on.ca/en/pro/programs/drugs/opdp_eo/notices/exec_office_20170127.pdf. [Google Scholar]
- Korff M, Kolodny A, Deyo RA, Chou R, et al. Long-term opioid therapy reconsidered. Ann Intern Med. 2011:325–8. doi: 10.1059/0003-4819-155-5-201109060-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]


